NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The loop diuretics are potent and widely used agents in the therapy of edematous states and congestive heart failure and less commonly for hypertension. Clinically apparent acute liver injury due to the loop diuretics is exceeding rare, if it occurs at all.
Background
The loop diuretics act by inhibition of the sodium-potassium-chloride symporter present in the thick ascending limb of the loop of Henle causing an inhibition of sodium reuptake. The increase in delivery of sodium to the distal convoluted loop overwhelms its capacity for sodium reabsorption and a brisk sodium diuresis ensues. The loop diuretics are grouped together because of shared mechanism of action, but they have distinct chemical structures. The loop diuretics are more potent than the typical thiazide diuretics and usually have a shorter duration of action. As a result, the loop diuretics are used more for the therapy of edema than long term therapy of hypertension. Common and shared side effects of the loop diuretics include dizziness, headache, gastrointestinal upset, hyponatremia, hypokalemia and dehydration. Uncommon but potentially severe adverse events include profound electrolyte and water loss, dehydration leading to hypotension and syncope, electrolyte depletion with hypokalemia, hypomagnesemia, and hyponatremia, increases in serum creatinine and uric acid with worsening of renal failure and precipitation of hepatic encephalopathy in patients with cirrhosis, hyperuricemia, gout, ototoxicity, thrombocytopenia and hypersensitivity reactions.
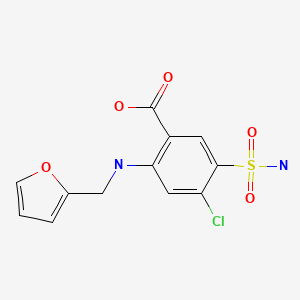
Furosemide (fure oh' se mide) was the first loop diuretic to be approved in the United States (1966) and is still widely used with more than 37 million prescriptions filled yearly. Furosemide is available in tablets of 20, 40 and 80 mg in generic forms and under the brand name Lasix. Furosemide is also available as an oral solution and as a liquid solution for injection. The usual adult dose of furosemide is 20 to 320 mg daily, given in one to three divided doses.
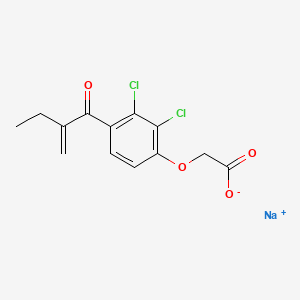
Ethacrynic (eth a krin' ik) acid was the second loop diuretic to be approved for use in the United States (1967), but is now rarely used; it remains available in 25 mg tablets and a solution for intravenous use generically and under the brand name Edecrin. The usual oral adult dose is 25 to 100 mg in one to three divided doses daily.
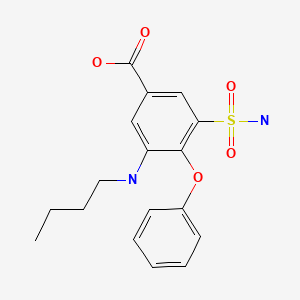
Bumetanide (bue met' a nide) is a potent loop diuretic that was approved for use in the United States in 1983 and continues to be used for the treatment of edema. Bumetanide is available as tablets of 0.5, 1 and 2 mg in generic forms and under the trade name of Bumex as well as a solution for parenteral administration. The usual oral adult dose is 0.5 to 2 mg once daily.
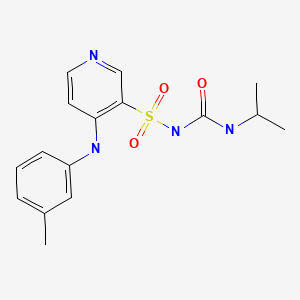
Torsemide (tor' se mide) was approved for use in edema in the United States in 1993 and is still in common use used for both edema and hypertension. Torsemide is available in tablets of 5, 10, 20 and 100 mg in generic forms and under the brand name of Demadex. Solutions are available for intravenous use as well. The usual oral adult dose is 5 to 20 mg once daily.
Hepatotoxicity
Use of the loop diuretics has not been associated with an increased rate of serum aminotransferase elevations. There have been only rare, reported cases of clinically apparent liver injury associated with loop diuretics and most of these reports were not very convincing. Interestingly, furosemide causes a direct hepatotoxicity in mice and has been used as an animal model of drug induced liver injury. This injury does not appear to occur in humans. Instances of liver injury in patients on furosemide usually present with ischemic hepatitis (shock liver) caused by heart failure with diuretic induced dehydration and hypotension. Thus, idiosyncratic, clinically apparent liver injury from the loop diuretics must be exceeding rare, if it occurs at all.
Likelihood score, all loop diuretics: E (unlikely causes of clinically apparent liver injury).
Mechanism of Injury
The cause of the rare occurrence of clinically apparent liver injury associated with the loop diuretics is not known. These agents are metabolized minimally by the liver and generally have rapid renal excretion.
Outcome and Management
Cases of clinically apparent liver injury due to the loop diuretics have been too few to characterize their severity and course. There have been no published instances of acute liver failure or chronic liver injury attributed to any of the loop diuretics. Cross reactivity among the four agents is unlikely because of the variability of their chemical structure.
Drug Class: Diuretics
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Bumetanide – Generic, Bumex®
Ethacrynic Acid – Generic, Edecrin®
Furosemide – Generic, Lasix®
Torsemide – Generic, Demadex®
DRUG CLASS
Diuretics
COMPLETE LABELING (Bumetanide)
COMPLETE LABELING (Ethacrynic Acid)
COMPLETE LABELING (Furosemide)
COMPLETE LABELING (Torsemide)
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Bumetanide | 28395-03-1 | C17-H20-N2-O5-S |
|
| Ethacrynic Acid | 58-54-8 | C13-H12-Cl2-O4 |
|
| Furosemide | 54-31-9 | C12-H11-Cl-N2-O5-S |
|
| Torsemide | 56211-40-6 | C16-H20-N4-O3-S |
|
ANNOTATED BIBLIOGRAPHY
References updated: 13 October 2021
- Zimmerman HJ. Diuretic drugs. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 662-4.(Expert review of hepatotoxicity of diuretics published in 1999 mentions that clinically apparent liver injury due to diuretics is rare; mentions that furosemide is hepatotoxic in rats but has been linked to only a few instances of jaundice in humans).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 519-40.(Review of hepatotoxicity of cardiovascular agents, mentions that thiazide diuretics can rarely cause cholestatic hepatitis but no mention of other types of diuretics).
- Jackson EK. Drugs affecting renal excretory function. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 445-70.(Textbook of pharmacology and therapeutics).
- Datey KK, Deshmukh SN, Dalvi CP, Purandare NM. Hepatocellular damage with ethacrynic acid. Br Med J. 1967;3:152–3. [PMC free article: PMC1842848] [PubMed: 6028103](25 year old with severe rheumatic heart disease developed jaundice 2 weeks after starting ethacrynic acid [bilirubin 6.8 mg/dL, ALT 360 U/L], improving on stopping and recurring on restarting, patient dying of congestive heart failure).
- Mitchell JR, Potter WZ, Hinson JA, Jollow DJ. Hepatic necrosis caused by furosemide. Nature. 1974;251:508–11. [PubMed: 4424638](In mice, furosemide is converted to a reactive arylating metabolite that causes massive hepatic necrosis within 24 hours).
- Walker RM, McElligott TF. Furosemide induced hepatotoxicity. J Pathol. 1981;135:301–14. [PubMed: 7328448](Mice given furosemide develop liver injury similar to acetaminophen toxicity but have centrilobular congestion and necrosis within 3 hours with congestion and occlusion of sinusoidal lumens caused by detachment of endothelial cells).
- Mousson C, Justrabo E, Tanter Y, Chalopin JM, Rifle G. Nephrologie. 1986;7:199–203. [Acute granulomatous interstitial nephritis and hepatitis caused by drugs. Possible role of an allopurinol-furosemide combination] [PubMed: 3822042](58 year old woman developed fever, rash and renal insufficiency after 6 week therapy with allopurinol [bilirubin 2.2 mg/dL, ALT 56 U/L, Alk P 780 U/L, eosinophils 1250/μL], resolving rapidly on stopping medications; patient was also taking furosemide, but injury pattern was typical of allopurinol).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants reported to UNOS between 1990 and 2002, 137 [0.5%] were done for idiosyncratic drug induced acute liver failure, none were attributed to a diuretic).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish Registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Reports of drug induced liver injury to a Spanish network found 570 cases; diuretics not mentioned among listed causes).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports [89% from the US]; no diuretic found in the 20 most commonly implicated agents).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Population based survey of 126 cases of acute liver injury [24 with acute liver failure] due to drugs between 1993-1999 in Spain calculated relative risk of injury compared to the general population: hydrochlorothiazide was being taken by 7 and furosemide by 8 patients, but relative risk was not increased in comparison to a control group).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, no case was attributed to a diuretic).
- Drugs for hypertension. Treat Guidel Med Lett. 2009;7:1–10. [PubMed: 19107095](Brief overview of currently available drugs for hypertension with guidelines on their use and information on prices and toxicities: “thiazide diuretics are the first-line therapy for many patients with hypertension”).
- Wargo KA, Banta WM. A comprehensive review of the loop diuretics: should furosemide be first line? Ann Pharmacother. 2009;43:1836–47. [PubMed: 19843838](Systematic review of literature on comparing efficacy and safety of the loop diuretics; no discussion of hepatotoxicity or ALT elevations).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](Among 313 cases of drug induced liver injury seen over a 12 year period at a large hospital in Bangalore, India, none were attributed to a diuretic).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Among 624,673 adverse event reports in children between 2000 and 2006 in the WHO VigiBase, no diuretic was mentioned among the 30 most common causes of liver injury).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, of which none were attributed to a diuretic).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, none of which were attributed to a diuretic).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to a diuretic).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to a diuretic).
- Pizzarossa AC, Rebella M. Hypoxic hepatitis and furosemide. BMJ Case Rep. 2018;2018:bcr2018225497. [PMC free article: PMC6169621] [PubMed: 30275022](60 year old woman with heart failure treated with beta blockers and furosemide [40 mg twice daily] and was found to be hypotensive and dehydrated after a syncopal episode with evidence of hypoxic hepatic injury [bilirubin 0.7 mg/dL, ALT 343 rising to 952 U/L, AST 343 rising to 1152 U/L, Alk P 110 U/L], responding to hydration with rapid recovery).
- Drugs for hypertension. Med Lett Drugs Ther. 2020;62(1598):73–80. [PubMed: 32555118](Concise summary of efficacy, safety and costs of drugs used to treat hypertension including the diuretics, focusing upon relative usefulness; no mention of hepatic adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Diuretics.[LiverTox: Clinical and Researc...]Review Diuretics.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Principles of diuretic therapy.[Dis Mon. 1998]Review Principles of diuretic therapy.Antes LM, Fernandez PC. Dis Mon. 1998 Jun; 44(6):254-68.
- Interaction of diuretics and electrolytes in congestive heart failure.[Am J Cardiol. 1990]Interaction of diuretics and electrolytes in congestive heart failure.Nicholls MG. Am J Cardiol. 1990 Mar 6; 65(10):17E-21E; discussion 22E-23E.
- Review Diuretics in the treatment of hypertension. Part 2: loop diuretics and potassium-sparing agents.[Expert Opin Pharmacother. 2014]Review Diuretics in the treatment of hypertension. Part 2: loop diuretics and potassium-sparing agents.Tamargo J, Segura J, Ruilope LM. Expert Opin Pharmacother. 2014 Apr; 15(5):605-21. Epub 2014 Jan 23.
- Review Piretanide: a loop-active diuretic. Pharmacology, therapeutic efficacy and adverse effects.[Pharmacotherapy. 1984]Review Piretanide: a loop-active diuretic. Pharmacology, therapeutic efficacy and adverse effects.Marsh JD, Smith TW. Pharmacotherapy. 1984 Jul-Aug; 4(4):170-80.
- Loop Diuretics - LiverToxLoop Diuretics - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...