NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Lenalidomide is an immunomodulatory and antineoplastic agent that is used in the therapy of multiple myeloma. Lenalidomide is associated with a low rate of serum aminotransferase elevations during therapy and has been implicated in causing rare instances of clinically apparent liver injury which can be severe.
Background
Lenalidomide (len" a lid' oh mide) is a thalidomide derivative that has similar but more potent activity as an antineoplastic agent. Thalidomide and its derivatives have immunomodulatory, antiinflammatory antiangiogenic and antineoplastic activities. The mechanism of action of these agents in the treatment of multiple myeloma is not well defined but may relate to inhibition of tumor necrosis factor (TNF) alpha, a potent proinflammatory cytokine or to stimulation of T and NK cell activity. The thalidomide derivatives have direct cytotoxic effects against myeloma cells in culture and have potent antiangiogenic activities. Lenalidomide was approved for use in multiple myeloma in 2005 and was subsequently approved for use in myelodysplastic syndromes and mantle cell lymphoma. Lenalidomide is available in capsules of 2.5, 5, 10, 15, 20 and 25 mg under the brand name Revlimid. The recommended doses vary by indication and, like thalidomide, its use is restricted because of proven teratogenicity. Side effects of lenalidomide are common and include sedation, dizziness, orthostatic hypotension, neutropenia, lymphopenia, peripheral neuropathy, and arterial and venous thromboembolism (for which reason it is often given with anticoagulation). Rare but potentially severe adverse events include severe cutaneous reactions, seizures, tumor lysis syndrome and hypersensitivity reactions. Lenalidomide is a teratogen and possible cause of severe birth defects and is available only as a part of a strict Risk Evaluation and Mitigation Strategy (REMS), which requires physician training, written patient informed consent, strict birth control measures, regular monitoring and reporting.
Hepatotoxicity
Serum enzyme elevations occur in 8% to 15% of patients taking lenalidomide and are more frequent with higher doses. The enzyme abnormalities are usually mild and self-limited, and only rarely require drug discontinuation. In addition, lenalidomide has been implicated in rare instances of clinically apparent, acute liver injury which can be severe and has led to deaths from acute liver failure. The onset of injury is typically within 1 to 8 weeks of starting therapy. The pattern of serum enzyme elevation at the time of presentation can be either hepatocellular or cholestatic; however, the injury tends to be cholestatic and can be prolonged. Immunoallergic and autoimmune features are not common. Several instances of acute liver injury associated with lenalidomide therapy have occurred in patients with other apparent causes of liver disease or with preexisting chronic hepatitis B or C. If performed during the acute injury, liver biopsy shows hepatocellular necrosis and inflammatory cell infiltration, consistent with acute drug induced injury. In some instances there is bile duct injury and loss resulting in progressive cholestatic liver injury suggestive of vanishing bile duct syndrome. Lenalidomide has also been shown to increase indirect bilirubin levels in patients with underlying Gilbert syndrome, causing a mild hyperbilirubinemia during therapy that soon resolves with stopping treatment and is otherwise benign.
Thalidomide and its derivatives have also been implicated in causing an increased risk of graft-vs-host disease after autologous or allogeneic hematopoietic stem cell transplantation (HSCT) as well as after liver, kidney and heart transplantation. There appears to be cross reactivity to this complication among lenalidomide, pomalidomide and thalidomide. Therapy usually requires discontinuation of the antineoplastic agent as well as treatment with high doses of corticosteroids and tacrolimus or sirolimus. Furthermore, hepatic graft-vs-host disease can occasionally present with an acute hepatitis that resembles hepatocellular drug induced liver injury.
Reactivation of hepatitis B has been reported in patients receiving thalidomide, lenalidomide and pomalidomide, but generally only after HSCT and the role of these agents in causing reactivation is not always clear. Indeed, in studies of large numbers of patients treated for multiple myeloma, the major risk factor for reactivation was found to be HSCT rather than the specific antineoplastic drugs being used. Indeed, lenalidomide therapy is associated with a reduced risk of reactivation in patients with HSCT (although dexamethasone, thalidomide and bortezomib were not), perhaps because of the immune enhancement typically caused by lenalidomide.
Likelihood score: C (probable rare cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism of lenalidomide hepatotoxicity is not clear, but it may be related to its activity in reducing TNF-α production, a potent inflammatory cytokine that activates T cells and promotes inflammation, but is also necessary for normal liver regeneration. Several of the reported cases of hepatotoxicity have occurred in patients with underlying chronic liver disease (hepatitis B, C or nonalcoholic fatty liver), and another possibility is that lenalidomide may worsen preexisting hepatic conditions.
Outcome and Management
The severity of lenalidomide induced liver injury ranges from transient, asymptomatic elevations in serum enzymes to acute liver injury with jaundice to severe acute liver failure and death. The liver injury usually starts to resolve within a week of stopping the medication, but prolonged jaundice with bile duct injury and possible vanishing bile duct syndrome have also been reported. Rechallenge should be reserved for cases of mild liver injury in which the agent is considered very necessary and done with caution and careful monitoring. Nevertheless, instances of reinitiation of therapy without subsequent recurrence of liver injury have been reported.
Drug Class: Antineoplastic Agents, Miscellaneous
Other Related Drugs: Pomalidomide, Thalidomide
CASE REPORT
Case 1. Acute liver injury during lenalidomide therapy of multiple myeloma.(1)
A 50 year old man with refractory and relapsed multiple myeloma was started on a course of lenalidomide after multiple cycles of chemotherapy and both autologous and allogeneic hematopoietic stem cell transplants that provided only temporary remissions. Ten days after starting lenalidomide [10 mg daily], he was found to have abnormal liver tests with a serum ALT of 505 U/L, AST 246 U/L, Alk P 198 U/L, but bilirubin and INR normal. Serum enzyme levels had been normal before starting lenalidomide. He did not drink alcohol and had no risk factors for viral hepatitis. Of note, 3 years earlier he had developed angioedema and a severe skin rash without liver test abnormalities after three months of cyclic lenalidomide therapy. In the interim he had undergone an allogeneic HSCT.
Lenalidomide was discontinued and he was admitted for evaluation and liver biopsy. Blood counts demonstrated eosinophilia (1344/µL). Tests for hepatitis A, B, C and E as well as CMV, EBV, HIV and herpes simplex and zoster infection were negative. Ultrasound showed normal appearing liver without evidence of biliary obstruction. Liver biopsy showed prominent portal and lobular inflammation with a mixed cellular infiltrate rich in eosinophils, lobular areas with confluent necrosis and mild endothelitis and occasional biliary cell injury in portal areas. The liver histology was considered consistent with an acute drug induced hepatitis superimposed upon mild graft-vs-host disease. In further follow up, liver tests improved and were normal 6 weeks later (Table).
Key Points
Laboratory Values
| Time After Starting | Time After Stopping | ALT* (U/L) | Alk P* (U/L) | Bilirubin* (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Normal | Normal | Normal | ||
| 10 days | 0 | 505 | 198 | Normal | |
| 11 days | 1 day | 510 | 230 | ||
| 12 days | 2 days | 515 | 255 | ||
| 17 days | 7 days | 219 | 157 | Normal | Admission, Liver Biopsy |
| 24 days | 14 | 80 | 110 | ||
| 4 weeks | 21 days | 45 | 95 | ||
| 6 weeks | 35 days | 35 | 85 | ||
| Upper limits of normal | <40 | <130 | <1.2 | ||
- *
Some values estimated from Figure 1. Upper limits of normal used standard values
Comment
This man developed asymptomatic and anicteric hepatitis within 10 days of starting lenalidomide. Typical of lenalidomide hepatotoxicity was the rapid latency to onset (less than one month), the hepatocellular pattern of injury, and the rapid improvement with stopping the medication. Also typical was the previous history of autologous or allogeneic HSCT. Indeed, one possibility is that this represented the worsening of an underlying graft-vs-host disease by lenalidomide therapy, an indirect form of drug induced liver injury. In preparation of use of lenalidomide to treat multiple myeloma, maintenance immunosuppressive therapy for prevention of graft-vs-host disease is typically withdrawn. In this case, it was unclear whether maintenance immunosuppression was continued.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAME
Lenalidomide – Revlimid®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Thalidomide | 50-35-1 | C13-H10-N2-O4 |
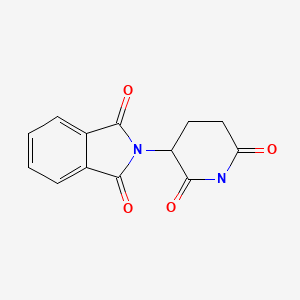
|
| Lenalidomide | 191732-72-6 | C13-H13-N3-O3 |
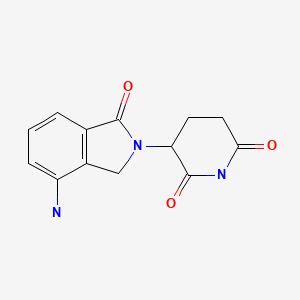
|
| Pomalidomide | 19171-19-8 | C13-H11-N3-O4 |
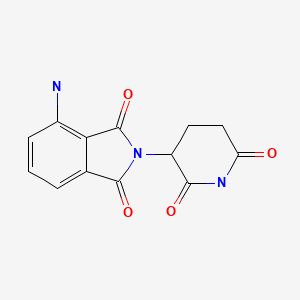
|
CITED REFERENCE
- 1.
- Zanella MC, Rubbia-Brandt L, Giostra E, Chalandon Y, Hadengue A, Spahr L. A case of drug-induced hepatitis due to lenalidomide. Case Rep Gastroenterol. 2011;5:217–22. [PMC free article: PMC3088752] [PubMed: 21552449]
ANNOTATED BIBLIOGRAPHY
References updated: 30 August 2022
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Textbook of hepatotoxicity published in 1999; lenalidomide is not discussed).
- Davern TJ. Hepatotoxicity of immunomodulating agents and the transplant situation. Thalidomide. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 2nd ed. New York: Informa Healthcare USA, 2007, p. 675.(Mentions that thalidomide rarely causes liver injury, but case reports of hepatocellular injury with variable degrees of jaundice have been described, largely in patients with preexisting chronic liver disease; no mention of lenalidomide).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Thalidomide and Lenalidomide. Pathway targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1225-27.(Textbook of pharmacology and therapeutics).
- Hastings RC, Trautman JR, Enna CD, Jacobson RR. Thalidomide in the treatment of erythema nodosum leprosum. With a note on selected laboratory abnormalities in erythema nodosum leprosum. Clin Pharmacol Ther. 1970;11:481–7. [PubMed: 4913866](Results of treating 22 patients with leprosy with thalidomide showed excellent results, often allowing for discontinuation of corticosteroids without significant toxicity).
- Tseng S, Pak G, Washenik K, Pomeranz MK, Shupack JL. Rediscovering thalidomide: a review of its mechanism of action, side effects, and potential uses. J Am Acad Dermatol. 1996;35:969–79. [PubMed: 8959957](Review of history, mechanism of action, side effects and uses of thalidomide; thalidomide has many side effects, but hepatic adverse events were not mentioned).
- Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, et al. Antitumor activity of thalidomide in refractory multiple myeloma. N Engl J Med. 1999;341:1565–71. [PubMed: 10564685](Trial of oral thalidomide in 84 patients with advanced myeloma found some response in 78% and remissions in 10%; adverse events were common, but no mention of ALT elevations or liver injury).
- Clark TE, Edom N, Larson J, Lindsey LJ. Thalomid (Thalidomide) capsules: a review of the first 18 months of spontaneous postmarketing adverse event surveillance, including off-label prescribing. Drug Saf. 2001;24:87–117. [PubMed: 11235821](During first 18 months of postmarketing use of thalidomide in 10,456 patients, 1210 adverse event reports were received, including 4 cases of hepatic failure arising after 1-4 weeks of treatment, although 3 were considered unrelated to therapy).
- Fowler R, Imrie K. Thalidomide-associated hepatitis: a case report. Am J Hematol. 2001;66:300–2. [PubMed: 11279644](Patient with chronic hepatitis C and advanced plasma cell leukemia developed jaundice and nausea 1 week after starting thalidomide [bilirubin 0.4 initially rising to 9.3 mg/dL, ALT 91 to 829 U/L, Alk P 100 to 120 U/L], resolving rapidly upon stopping; high HCV RNA levels noted).
- Grover JK, Uppal G, Raina V. The adverse effects of thalidomide in relapsed and refractory patients of multiple myeloma. Ann Oncol. 2002;13:1636–40. [PubMed: 12377654](Report of adverse effects of thalidomide in 23 patients with myeloma: constipation 100%, sedation 87%, edema 70%, also common were dry skin and mouth, headache, nausea and rash; minor ALT elevations occurred and 1 patient developed acute hepatitis, but it was attributed to hepatitis C).
- Trojan A, Chasse E, Gay B, Pichert G, Taverna C. Severe hepatic toxicity due to thalidomide in relapsed multiple myeloma. Ann Oncol. 2003;14:501–2. [PubMed: 12598363](62 year old woman with multiple myeloma developed acute liver failure after 7 months of thalidomide therapy [bilirubin not given, ALT ~2000 U/L, LDH ~6000 U/L], enzymes falling to normal in 1 week; overall, suggestive of ischemic hepatitis rather than hepatotoxicity).
- Teo SK. Properties of thalidomide and its analogues: implications for anticancer therapy. AAPS J. 2005;7:E14–E19. [PMC free article: PMC2751493] [PubMed: 16146335](Review of the properties and experimental uses of thalidomide as an inhibitor of TNF-α and other cytokines in multiple myeloma and several solid tumors).
- List A, Kurtin S, Roe DJ, Buresh A, Mahadevan D, Fuchs D, Rimsza L, et al. Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med. 2005;352:549–57. [PubMed: 15703420](Open label study of lenalidomide in 43 patients with myelodysplasia and anemia due to myelodysplastic syndrome reported neutropenia in 65% and thrombocytopenia in 74% of patients, and need for dose modification in 25%, but no mention of liver abnormalities).
- Caseiro MM. Treatment of chronic hepatitis C in non-responsive patients with pegylated interferon associated with ribavirin and thalidomide: report of six cases of total remission. Rev Inst Med Trop Sao Paulo. 2006;48:109–12. [PubMed: 16699635](Report of improvement in virological and biochemical responses to peginterferon and ribavirin therapy of chronic hepatitis C by addition of thalidomide [100 mg daily]; no mention of side effects).
- Hanje AJ, Shamp JL, Thomas FB, Meis GM. Thalidomide-induced severe hepatotoxicity. Pharmacotherapy. 2006;26:1018–22. [PubMed: 16803426](Elderly woman with myeloma developed jaundice and marked ALT elevations after 6 weeks of thalidomide therapy [ALT 2205 U/L; bilirubin 5.6 mg/dL], resolving within 3 months of stopping: Case 1 for Thalidomide).
- Hamadani M, Benson DM Jr, Copelan EA. Thalidomide-induced fulminant hepatic failure. Mayo Clin Proc. 2007;82:638. [PubMed: 17493431](64 year old woman with multiple myeloma and HBsAg in serum developed acute liver failure 12 days after starting thalidomide [bilirubin 16.7 mg/dL, ALT 410 U/L, Alk P 101 U/L, no change in HBV DNA], some improvement on stopping drug, but had worsening coagulopathy and renal failure and died 14 days later).
- Hussain S, Browne R, Chen J, Parekh S. Lenalidomide-induced severe hepatotoxicity. Blood. 2007;110:3814. [PubMed: 17984315](57 year old man with multiple myeloma renal dysfunction and previous hematopoietic stem cell transplant developed jaundice 1 week after starting lenalidomide, a derivative of thalidomide [bilirubin 7.2 mg/dL, ALT 90 U/L, Alk P 210 U/L], resolving within 3 weeks of stopping).
- Weber DM, Chen C, Niesvizky R, Wang M, Belch A, Stadtmauer EA, Siegel D, et al. Multiple Myeloma (009) Study Investigators. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–42. [PubMed: 18032763](Controlled trial of lenalidomide in 353 patients with relapsed multiple myeloma showed improvement in response rates and survival time; side effects were common, but no mention of liver abnormalities).
- Dimopoulos M, Spencer A, Attal M, Prince HM, Harousseau JL, Dmoszynska A, San Miguel J, et al. Multiple Myeloma (010) Study Investigators. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–32. [PubMed: 18032762](Controlled trial of lenalidomide in 351 patients with refractory multiple myeloma showed improvement in response rates and survival time; side effects of neutropenia, muscle cramps, constipation, nausea and dizziness were common, but liver abnormalities were not mentioned).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to thalidomide or lenalidomide).
- Dabak V, Kuriakose P. Thalidomide-induced severe hepatotoxicity. Cancer Chemother Pharmacol. 2009;63:583–5. [PubMed: 19083237](2 women with multiple myeloma; 79 year old developed jaundice 7 weeks after starting thalidomide [bilirubin 27.9 mg/dL, ALT 392 U/L, Alk P 1172 U/L], with persistent jaundice, bile duct loss on liver biopsy and death 4 months later; 57 year old developed raised enzymes one month after starting thalidomide [bilirubin not given, ALT 398 U/L, Alk P 175 U/L], resolving within 2 weeks of stopping).
- Levesque E, Bradette M. Hepatotoxicity as a rare but serious side effect of thalidomide. Ann Hematol. 2009;88:183–4. [PubMed: 18665361](36 year old woman with multiple myeloma developed liver test abnormalities 5 weeks after starting thalidomide [bilirubin normal, peak ALT ~1300 U/L], resolving within 20 days of stopping).
- Jain P. Lenalidomide-induced acute liver failure. Blood Transfus. 2009;7:335–6. [PMC free article: PMC2782812] [PubMed: 20011646](93 year old man with myelodysplastic syndrome and HBsAg in serum developed jaundice 10 days after starting lenalidomide [bilirubin 9.2 mg/dL, ALT 2670 U/L, Alk P 342 U/L, IgM anti-HBc positive, but low levels of HBV DNA], resolving over following 4 weeks and patient later tolerated restarting lenalidomide in combination with adefovir, suggesting that the injury was due to acute hepatitis B).
- Castaneda CP, Brandenburg NA, Bwire R, Burton GH, Zeldis JB. Erythema multiforme/Stevens-Johnson syndrome/toxic epidermal necrolysis in lenalidomide-treated patients. J Clin Oncol. 2009;27:156–7. [PubMed: 19047275](After approximately 57,000 patients had received lenalidomide, the sponsor received 12 reports of Stevens-Johnson Syndrome, 3 of erythema multiforme and 1 of toxic epidermal necrolysis, arising 3-112 days after starting; often sparse data were available and there was no mention of liver injury or jaundice).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to thalidomide or lenalidomide).
- Zanella MC, Rubbia-Brandt L, Giostra E, Chalandon Y, Hadengue A, Spahr L. A case of drug-induced hepatitis due to lenalidomide. Case Rep Gastroenterol. 2011;5:217–22. [PMC free article: PMC3088752] [PubMed: 21552449](50 year old man with refractory multiple myeloma and autologous hematopoietic stem cell transplant developed severe skin rash 3 months after starting lenalidomide that resolved upon stopping, but developed serum enzyme elevations one week after restarting lenalidomide 2 years later [bilirubin normal, ALT 509 U/L, Alk P 198 U/L], resolving upon stopping).
- Vilas-Boas F, Gonçalves R, Sobrinho Simões M, Lopes J, Macedo G. Thalidomide-induced acute cholestatic hepatitis: Case report and review of the literature. Gastroenterol Hepatol. 2012;35:560–6. [PubMed: 22789729](77 year old man with multiple myeloma developed jaundice 4 weeks after starting chemotherapy with melphalan, prednisone and thalidomide [bilirubin 11.4 mg/dL, ALT 333 U/L, Alk P 4 times ULN], worsening for a week after stopping thalidomide and then improving; patient later tolerated melphalan, but died of pneumonia shortly thereafter).
- Nojkov B, Signori C, Konda A, Fontana RJ. Lenalidomide-associated hepatotoxicity--a case report and literature review. Anticancer Res. 2012;32:4117–9. [PubMed: 22993370](67 year old man with multiple myeloma developed fatigue within 1 week of starting a second 3-week course of lenalidomide [bilirubin 4.4 mg/dL, ALT 139 U/L, Alk P 190 U/L], with rapid resolution upon stopping [within 8 days]).
- Simondsen KA, Kolesar JM. Lenalidomide-induced elevated bilirubin. J Oncol Pharm Pract. 2012;18:402–5. [PubMed: 22407059](55 year old man with polycythemia vera and myelofibrosis developed hyperbilirubinemia after several cycles of lenalidomide [total bilirubin 1.1 to 2.0 with normal direct bilirubin, ALT and Alk P], which decreased when lenalidomide was held and recurred with subsequent cycles, genetic testing demonstrating 7TA allele in UGT1A1 gene typical of Gilbert syndrome).
- Sockel K, Bornhaeuser M, Mischak-Weissinger E, Trenschel R, Wermke M, Unzicker C, Kobbe G, et al. German MDS and Cooperative Transplant Study Group (GCTSG). Lenalidomide maintenance after allogeneic HSCT seems to trigger acute graft-versus-host disease in patients with high-risk myelodysplastic syndromes or acute myeloid leukemia and del(5q): results of the LENAMAINT trial. Haematologica. 2012;97:e34–5. [PMC free article: PMC3436225] [PubMed: 22952334](Among 10 patients with acute myelogenous leukemia or high risk myelofibrosis who were treated with lenalidomide maintenance therapy 2-4 months after allogenic hematopoietic cell transplantation, 6 developed severe acute graft-vs-host disease within the first 2 cycles of treatment which improved on stopping and recurred in most on restarting lenalidomide).
- Meyers DE, Adu-Gyamfi B, Segura AM, Buja LM, Mallidi HR, Frazier OH, Rice L. Fatal cardiac and renal allograft rejection with lenalidomide therapy for light-chain amyloidosis. Am J Transplant. 2013;13:2730–3. [PubMed: 23914832](68 year old woman with light-chain amyloidosis underwent successful combined heart and kidney transplantation, but 110 days after transplant and 9 days after starting lenalidomide developed severe and ultimately fatal acute cellular rejection of both organs).
- Veluswamy RR, Ward SC, Yum K, Abramovitz RB, Isola LM, Jagannath S, Parekh S. Adverse drug reaction: pomalidomide-induced liver injury. Lancet. 2014;383(9935):2125–6. [PubMed: 24953475](50 year old man with multiple myeloma on lenalidomide and dexamethasone after HCT developed ALT elevations [433 U/L], which resolved on stopping but recurred and worsened when later treated after a second HCT with pomalidomide and dexamethasone [bilirubin 13 mg/dL, ALT 3981 U/L, Alk P 259 U/L], resolving after stopping both drugs, but ultimately requiring corticosteroid therapy).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, none of which were attributed to thalidomide or its derivatives).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 1 case was attributed to thalidomide but none to lenalidomide).
- Mahale P, Thomas SK, Kyvernitakis A, Torres HA. Management of multiple myeloma complicated by hepatitis C virus reactivation: the role of new antiviral therapy. Open Forum Infect Dis. 2015;3:ofv211. [PMC free article: PMC4751339] [PubMed: 26885541](74 year old woman with multiple myeloma and chronic hepatitis C had worsening of serum ALT and increase in HCV RNA levels when treated with thalidomide and dexamethasone, which prevented continued therapy until hepatitis C was successfully treated with sofosbuvir and ribavirin).
- Safran H, Charpentier KP, Kaubisch A, Mantripragada K, Dubel G, Perez K, Faricy-Anderson K, et al. Lenalidomide for second-line treatment of advanced hepatocellular cancer: a Brown University oncology group phase II study. Am J Clin Oncol. 2015;38:1–4. [PubMed: 23648434](Among 40 patients with hepatocellular carcinoma with an inadequate response to sorafenib treated with lenalidomide [25 mg daily for 21 days in 28 day cycles], 6 [15%] had a partial response and toxicities were largely hematologic and rash; 5 patients had ALT elevations above 3 times ULN).
- Kootte RS, Faber LM. Hepatitis E during lenalidomide treatment for multiple myeloma in complete remission. Neth J Med. 2017;75:117–21. [PubMed: 28469048](69 year old woman with multiple myeloma on lenalidomide after HCT was found to have elevations in liver tests [bilirubin 0.5 mg/dL, ALT 328 U/L, Alk P 241 U/L] accompanied by IgM anti-HEV and HEV RNA [suggesting chronic infection sustained by immunosuppression], viremia and ALT elevations resolving with stopping lenalidomide and not returning when it was restarted).
- Bonkovsky HL, Kleiner DE, Gu J, Odin JA, Russo MW, Navarro VM, Fontana RJ, et al. U.S. Drug Induced Liver Injury Network Investigators. Clinical presentations and outcomes of bile duct loss caused by drugs and herbal and dietary supplements. Hepatology. 2017;65:1267–77. [PMC free article: PMC5360519] [PubMed: 27981596](Among 363 patients with drug induced liver injury who underwent liver biopsy, 26 [7%] had bile duct loss, including 1 case attributed to lenalidomide and 1 to thalidomide both marked by severe, and relentlessly progressive cholestatic liver injury resulting in death from liver failure within 3 months of onset).
- Lum EL, Huang E, Bunnapradist S, Pham T, Danovitch G. Acute kidney allograft rejection precipitated by lenalidomide treatment for multiple myeloma. Am J Kidney Dis. 2017;69:701–704. [PubMed: 28189378](65 year old woman developed multiple myeloma 5 years after a successful renal transplant for polycystic kidney disease and developed acute cellular rejection during a second cycle of lenalidomide and dexamethasone that improved with immunosuppression and withdrawal of lenalidomide, later tolerating bortezomib therapy of myeloma).
- Tsukune Y, Sasaki M, Odajima T, Sunami K, Takei T, Moriuchi Y, Iino M, et al. Incidence and risk factors of hepatitis B virus reactivation in patients with multiple myeloma in an era with novel agents: a nationwide retrospective study in Japan. Blood Cancer J. 2017;7:631. [PMC free article: PMC5802507] [PubMed: 29167420](Japanese nationwide analysis of 5078 patients with multiple myeloma identified 760 with resolved hepatitis B [anti-HBc without HBsAg in serum] of whom 7.6% developed reactivation [7.9% at 2 and 14.1% at 5 years], multivariate analysis demonstrating higher rates in those undergoing autologous hematopoietic stem cell transplant [21%:odds ratio=11.6] and lower rates in those receiving lenalidomide [5.2%:odds ratio=0.5], but not thalidomide, bortezomib or dexamethasone).
- Hammami MB, Talkin R, Al-Taee AM, Schoen MW, Goyal SD, Lai JP. Autologous graft-versus-host disease of the gastrointestinal tract in patients with multiple myeloma and hematopoietic stem cell transplantation. Gastroenterology Res. 2018;11:52–57. [PMC free article: PMC5827903] [PubMed: 29511407](Two patients with multiple myeloma who underwent autologous hematopoietic cell transplantation developed autologous graft-vs-host disease with moderately severe gastrointestinal involvement after receiving several cycles of lenalidomide and dexamethasone).
- Azmy V, Neparidze N. Hyperbilirubinemia following lenalidomide administration. Clin Case Rep. 2018;6:875–877. [PMC free article: PMC5930194] [PubMed: 29744077](72 year old man with multiple myeloma developed hyperbilirubinemia after a first cycle of lenalidomide [bilirubin 3.9 with normal direct bilirubin, ALT and Alk P] suspected to be due to Gilbert syndrome).
- Kikuchi T, Kusumoto S, Tanaka Y, Oshima Y, Fujinami H, Suzuki T, Totani H, et al. Hepatitis B virus reactivation in a myeloma patient with resolved infection who received daratumumab-containing salvage chemotherapy. J Clin Exp Hematop. 2020;60:51–54. [PMC free article: PMC7337267] [PubMed: 32404569](72 year old women with multiple myeloma who was negative for HBsAg but positive for anti-HBc without anti-HBs was monitored and had no evidence for reactivation during multiple courses of bortezomib, melphalan, dexamethasone, lenalidomide and pomalidomide over a 3 year period, but then developed HBV DNA [2.2 to 2.6 log10 per mL] and HBsAg after 3rd course of daratumumab with bortezomib and dexamethasone with rapid response upon addition of entecavir).
- Vaxman I, Eaton J, Lee HE, Gertz MA. Acute liver rejection in a multiple myeloma patient treated with lenalidomide. Case Rep Transplant. 2020;2020:8894922. [PMC free article: PMC7749773] [PubMed: 33381347](65 year old woman with primary biliary cirrhosis and liver transplant developed multiple myeloma 9 years later and acute cellular rejection 2 weeks after starting therapy with lenalidomide and dexamethasone [bilirubin 3.3 mg/dL, ALT 306 U/L, Alk P 253 U/L], responding to restarting mycophenolate and starting high dose methylprednisolone and tacrolimus).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Pomalidomide.[LiverTox: Clinical and Researc...]Review Pomalidomide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Thalidomide.[LiverTox: Clinical and Researc...]Review Thalidomide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Thalidomide.[LiverTox: Clinical and Researc...]Review Thalidomide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review A critical evaluation of pembrolizumab in addition to lenalidomide and dexamethasone for the treatment of multiple myeloma.[Expert Rev Hematol. 2020]Review A critical evaluation of pembrolizumab in addition to lenalidomide and dexamethasone for the treatment of multiple myeloma.Oriol A. Expert Rev Hematol. 2020 May; 13(5):435-445. Epub 2020 Mar 21.
- Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study.[Lancet Haematol. 2020]Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study.Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, Dozza L, van der Holt B, Zweegman S, Oliva S, et al. Lancet Haematol. 2020 Jun; 7(6):e456-e468. Epub 2020 Apr 30.
- Lenalidomide - LiverToxLenalidomide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...