NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Larotrectinib is a selective inhibitor of neurotrophin receptor kinase (NTRK) that is used in the therapy of solid tumors harboring NTRK gene fusions. Larotrectinib is associated with a high rate of serum aminotransferase elevations during therapy but has not been linked to instances of clinically apparent liver injury with jaundice.
Background
Larotrectinib (lar" oh trek' ti nib) is an orally available, small molecule inhibitor of the tropomyosin receptor kinases (TRK-A, -B and -C). The genes of TRK are found to be oncogenic drivers in many solid tumors including gliomas and sarcomas. The tumors harbor a fusion product of the proto-oncogene family and overexpress the neurotrophin receptor kinases which results in abnormal cell growth. Larotrectinib has activity against all three forms of TRK and has been shown to be effective in prolonging progression free survival in children and adults with various solid tumors harboring an NTRK gene fusion. Larotrectinib received accelerated approval for use in the United States in 2018 for the treatment of advanced or metastatic solid tumors that have NTRK gene fusion. Larotrectinib is available in capsules of 25 and 100 mg and as an oral solution of 20 mg/mL under the brand name Vitrakvi. The recommended dose in adults is 100 mg orally twice daily, the dose in children and small adults being adjusted to body surface area. Side effects are common and include fatigue, nausea, vomiting, constipation, diarrhea, dizziness and cough. Uncommon, but potentially serious side effects include neurotoxicity (delirium, dysarthria, dizziness, gait disturbance, tremor), hepatotoxicity (ALT and AST elevations) and embryo-fetal toxicity.
Hepatotoxicity
In early clinical trials in a total of 176 patients with various forms of solid tumors which had an NTRK gene fusion, elevations in serum aminotransferase levels occurred in 45% of patients treated with larotrectinib. Serum aminotransferase levels rose to above 5 times ULN in 6% of patients and led to early discontinuation in 2%. Serum aminotransferase elevations typically arose after 4 to 12 weeks of treatment, but usually without jaundice or alkaline phosphatase elevations. Most elevations resolved within 4 to 8 weeks and discontinuations were uncommon. Restarting larotrectinib at a reduced dose after resolution of the aminotransferase abnormalities was generally well tolerated and did not lead to recurrence of liver injury. Cases with jaundice and symptoms during larotrectinib therapy have not been reported, but the clinical experience with this kinase inhibitor has been limited and prelicensure clinical trials were carried out with careful clinical monitoring.
Likelihood score: E* (unproven but suspected cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the liver injury due to larotrectinib is unknown. Larotrectinib is metabolized in the liver largely via CYP 3A4 and is highly susceptible to drug-drug interactions with CYP 3A modulators, such that the dose modifications are recommended if there is concurrent use of CYP 3A inducers (dose increase) or inhibitors (dose decrease by half).
Outcome and Management
Routine monitoring of liver tests is recommended for patients starting larotrectinib, including serum ALT, AST and bilirubin every 2 weeks for the first month and monthly thereafter and as clinically indicated. Serum aminotransferase elevations above 5 times the upper limit of normal should lead to dose interruption. If changes persist, are severe, or reoccur on restarting, larotrectinib should be discontinued. There have been no reports of acute liver failure, chronic hepatitis or vanishing bile duct syndrome due to larotrectinib.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Larotrectinib – Vitrakvi®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
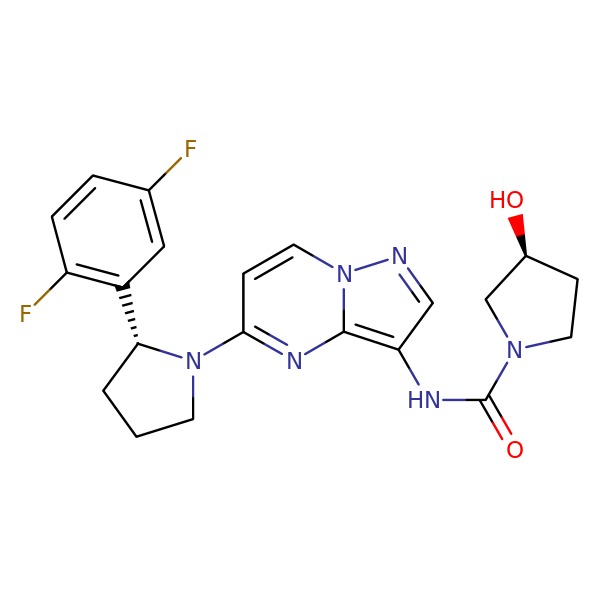
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Larotrectinib | 1223403-58-4 | C21-H22-F2-N6-O2 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 15 April 2019
Abbreviations: NTKR, neurotrophic tyrosine kinase receptor.
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors).
- DeLeve LD. Kinase inhibitors. Gefitinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents published in 2013, before the availability of larotrectinib).
- Wellstein A, Giaccone G, Atkins MB, Sausville EA. Pathway-targeted therapies: monoclonal antibodies, protein kinase inhibitors, and various small molecules. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 1203-36.(Textbook of pharmacology and therapeutics).
- https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2018/210861Orig1s000 _211710Orig1s000MultidisciplineR.pdf . (FDA website with the formal review of the safety and efficacy of larotrectinib based upon results in 176 adults and children with solid tumors and NTRK gene fusions, 45% of whom had ALT or AST elevations during therapy, which were above 5 times ULN in 6% and resulted in drug discontinuations in 2%, but no patient developed clinically apparent liver injury with jaundice). - Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 cases [6%] were attributed to antineoplastic agents, including 9 due to kinase inhibitors such as imatinib and lapatinib, but none were attributed to larotrectinib).
- DuBois SG, Laetsch TW, Federman N, Turpin BK, Albert CM, Nagasubramanian R, Anderson ME, et al. The use of neoadjuvant larotrectinib in the management of children with locally advanced TRK fusion sarcomas. Cancer 2018; 124: 4241-7. [PMC free article: PMC6263791] [PubMed: 30204247](5 children with advanced refractory sarcomas harboring a NTRK fusion were treated with larotrectinib, and all had an objective response and were able to undergo surgical resection, 3 with a complete response without evidence of recurrence and on no therapy 7-15 months later).
- Ziegler DS, Wong M, Mayoh C, Kumar A, Tsoli M, Mould E, Tyrrell V, et al. Brief report: Potent clinical and radiological response to larotrectinib in TRK fusion-driven high-grade glioma. Br J Cancer 2018; 119: 693-6. [PMC free article: PMC6173734] [PubMed: 30220707](A 3 year old girl with high grade glioma refractory to conventional chemotherapy was found to have a chromosomal translocation resulting in an NTRK3 fusion gene and subsequently had a dramatic clinical and radiological response to larotrectinib therapy with excellent tolerance).
- Landman Y, Ilouze M, Wein S, Neiman V, Yerushalmi R, Yakimov M, Ku N, et al. Rapid response to larotrectinib (LOXO-101) in an adult chemotherapy-naive patients with advanced triple-negative secretory breast cancer expressing ETV6-NTRK3 fusion. Clin Breast Cancer 2018; 18: e267-e270. [PubMed: 29233640](37 year old woman with advanced metastatic "triple negative" breast cancer with NTRK3 fusion gene detected in tumor tissue had a dramatic clinical response to larotrectinib therapy).
- Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, Nagasubramanian R, et al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet Oncol 2018; 19: 705-14. [PMC free article: PMC5949072] [PubMed: 29606586](Among 24 children or adolescents with advanced or metastatic solid tumors treated with larotrectinib, 14 of 15 with NTRK fusions had an objective response compared to none of those without such mutations, and side effects were mostly mild, ALT or AST elevations occurred in 42% but only one patient required discontinuation because of ALT elevations above 5 times ULN).
- Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018; 378: 731-9. [PMC free article: PMC5857389] [PubMed: 29466156](Among 55 children and adults with advanced or metastatic solid tumors harboring TRK fusions treated with larotrectinib, the overall response rate was 75% and adverse events were predominantly mild, with increased ALT or AST in 42%, but values above 5 times ULN in only 7% and no patient had to discontinue therapy because of treatment related adverse events).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Evaluating larotrectinib for the treatment of advanced solid tumors harboring an NTRK gene fusion.[Expert Opin Pharmacother. 2021]Review Evaluating larotrectinib for the treatment of advanced solid tumors harboring an NTRK gene fusion.Filippi R, Depetris I, Satolli MA. Expert Opin Pharmacother. 2021 Apr; 22(6):677-684. Epub 2021 Feb 12.
- Larotrectinib efficacy and safety in adult patients with tropomyosin receptor kinase fusion sarcomas.[Cancer. 2023]Larotrectinib efficacy and safety in adult patients with tropomyosin receptor kinase fusion sarcomas.Kummar S, Shen L, Hong DS, McDermott R, Keedy VL, Casanova M, Demetri GD, Dowlati A, Melcón SG, Lassen UN, et al. Cancer. 2023 Dec 1; 129(23):3772-3782. Epub 2023 Sep 28.
- Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study.[Lancet Oncol. 2018]Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study.Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, Nagasubramanian R, Davis JL, Rudzinski E, Feraco AM, et al. Lancet Oncol. 2018 May; 19(5):705-714. Epub 2018 Mar 29.
- Antitumor activity of larotrectinib in tumors harboring NTRK gene fusions: a short review on the current evidence.[Onco Targets Ther. 2019]Antitumor activity of larotrectinib in tumors harboring NTRK gene fusions: a short review on the current evidence.Ricciuti B, Genova C, Crinò L, Libra M, Leonardi GC. Onco Targets Ther. 2019; 12:3171-3179. Epub 2019 Apr 30.
- Review Belgian expert consensus for tumor-agnostic treatment of NTRK gene fusion-driven solid tumors with larotrectinib.[Crit Rev Oncol Hematol. 2022]Review Belgian expert consensus for tumor-agnostic treatment of NTRK gene fusion-driven solid tumors with larotrectinib.Awada A, Berghmans T, Clement PM, Cuppens K, De Wilde B, Machiels JP, Pauwels P, Peeters M, Rottey S, Van Cutsem E. Crit Rev Oncol Hematol. 2022 Jan; 169:103564. Epub 2021 Nov 30.
- Larotrectinib - LiverToxLarotrectinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...