NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Istradefylline is an adenosine receptor antagonist that is used as adjunctive therapy to levodopa/carbidopa in patients with Parkinson disease experiencing difficulty with “off” episodes when motor symptoms breakthrough on treatment. Istradefylline has been associated with a low rate of serum enzyme elevations during therapy, but has not been linked to cases of clinically apparent liver injury with jaundice.
Background
Istradefylline (is” tra def’ i lin) is an adenosine [A2A] receptor antagonist that acts independently of dopaminergic pathways, reducing the overactive striatopallidal output that results in increased GABAergic inhibitory activity. Levodopa/carbidopa is the mainstay of therapy of Parkinson disease increasing brain dopamine levels and typically alleviating the motor symptoms of Parkinson disease for 5 to 6 hours. After the first 2 to 5 years of therapy, however, the duration of benefit becomes shorter (wearing off effect), and patients develop fluctuations between mobility and immobility (on-off effect). Patients with Parkinson disease and motor complications have been found to have overactive striatopallidal output. For this reason, istradefylline was assessed in animal models where it was found to decrease striatopallidal output and the resultant excessive GABAergic activity. In clinical trials, istradefylline when given in combination with levodopa/carbidopa decreased the wake-time “off” episodes in patients with Parkinson disease. Initially, applications for approval of istradefylline were rejected by the FDA because of its modest effects; it was, however, approved in Japan. In 2019, based upon further studies and demonstration of safety, istradefylline was approved in the United States for use as adjunctive therapy to levodopa for adults with Parkinson disease who were experiencing “off” episodes. Istradefylline is currently available as tablets of 20 and 40 mg under the brand name Nourianz. The recommended dose is 20-40 mg once daily. While istradefylline may improve control of symptoms, there is no evidence that it slows the progression of Parkinson disease. Common side effects include dyskinesia, dizziness, constipation, nausea, and insomnia. Less common but potentially severe adverse events include hallucinations, psychotic and compulsive behaviors, hypersexuality, and severe dyskinesia.
Hepatotoxicity
In prelicensure controlled trials, serum ALT elevations occurred in 4% to 11% of istradefylline-treated subjects compared to 5% to 6% of placebo recipients, most of whom were taking multiple other agents for Parkinson disease. The elevations were usually mild-to-moderate in severity, asymptomatic and self-limited in course. ALT elevations above 3 times the ULN occurred in less than 1% recipients and rarely led to discontinuation. None of the aminotransferase elevations were accompanied by symptoms or jaundice. In preregistration clinical trials and subsequently with its more widespread use, istradefylline has not been linked to instances of clinically apparent liver injury. It has, however, had limited clinical use.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Istradefylline is a specific antagonist of the A2A adenosine receptors and a first-in-class agent. The mechanism by which it might cause liver injury is not known. It is metabolized in the liver, largely via CYP 3A4 and 1A1 and is sensitive to drug-drug interactions with strong inducers or inhibitors of the CYP 3A4 microsomal enzyme. Thus, hepatotoxicity might be caused by metabolic idiosyncrasy that produces a toxic or immunogenic byproduct of metabolism.
Outcome and Management
Istradefylline has been linked to mild-to-moderate serum enzyme elevations during therapy. Discontinuation for serum enzyme elevations is rarely necessary, but should be done if the elevations are accompanied by symptoms or jaundice or for ALT elevations of more than 5 times the upper limit of normal (ULN). There is no information on cross sensitivity to liver injury between istradefylline and other agents used in the therapy of Parkinson disease.
Drug Class: Parkinson Disease Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Istradefylline – Nourianz®
DRUG CLASS
Central Nervous System Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
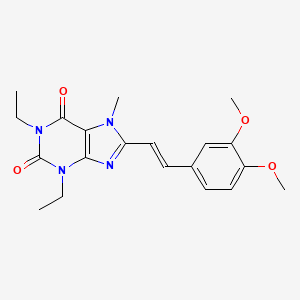
| Istradefylline | 155270-99-8 | C20-H24-N4-O4 |
|
ANNOTATED BIBLIOGRAPHY
References updated: 18 October 2021
Abbreviations used: COMT, catechol O-methyltransferase; MAO, monoamine oxidase.
- Roberson ED. Parkinson Disease. Treatment of central nervous system degenerative disorders. In, Brunton LL, Hilal-Dandan R, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 13th ed. New York: McGraw-Hill, 2018, pp. 328-333.(Textbook of pharmacology and therapeutics; istradefylline and adenosine receptor antagonists are not discussed).
- FDA. https://www
.accessdata .fda.gov/drugsatfda_docs /nda/2019/022075Orig1s000MedR.pdf. (FDA website including product label and clinical review of data submitted in support of approval of istradefylline mentions that therapy is associated with a slight increase rate of ALT elevations compared to placebo [6.9% overall vs 5.9%], but elevations above 3 times ULN were rare and there were no cases with concurrent bilirubin elevations). - Hauser RA, Hubble JP, Truong DD. Istradefylline US-001 Study Group. Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology. 2003;61:297–303. [PubMed: 12913187](Among 83 patients with Parkinson disease and motor fluctuations despite optimal conventional therapy using levodopa who were treated with istradefylline [20 or 40 mg] or placebo daily for 12 weeks, “off” time decreased with istradefylline [-7.1% vs +2.2%], while adverse events included nausea [28% vs 7%], dyskinesia [17% vs 14%], dizziness [13% vs 3%] and lipase elevations; no mention of ALT elevations or hepatotoxicity).
- LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM. 6002-US-005 Study Group. Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces "off" time in Parkinson's disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol. 2008;63:295–302. [PubMed: 18306243](Among 196 patients with Parkinson disease and prominent wearing “off” episodes of motor fluctuations treated with istradefylline [40 mg] or placebo once daily for 12 weeks, there was a greater decrease in daily awake “off” time with istradefylline [-10.8% vs -4.0%], but adverse events were more frequent including dyskinesia [30% vs 15%] and serious adverse events [7.8% vs 1.5%], while “important differences were not observed between the two groups for clinical laboratory” results).
- Stacy M, Silver D, Mendis T, Sutton J, Mori A, Chaikin P, Sussman NM. A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology. 2008;70:2233–40. [PubMed: 18519872](Among 395 levodopa treated patients with Parkinson disease treated with istradefylline [20 or 60 mg] or placebo once daily for 12 weeks, daily wake “off’ time decreased more with istradefylline, and adverse events were generally mild and included dyskinesia, dizziness, headache, nausea, constipation and hallucinations, and “no clinically important differences were observed among the groups for changes in clinical laboratory assessments”; no mention of ALT elevations or hepatoxicity).
- Fernandez HH, Greeley DR, Zweig RM, Wojcieszek J, Mori A, Sussman NM. 6002-US-051 Study Group. Istradefylline as monotherapy for Parkinson disease: results of the 6002-US-051 trial. Parkinsonism Relat Disord. 2010;16:16–20. [PubMed: 19616987](Among 196 patients with Parkinson disease not taking levodopa treated with istradefylline [40 mg] or placebo once daily for 12 weeks, symptom scores improved on istradefylline but the changes were not significantly greater than with placebo, while total adverse event rates were similar [63% vs 65%] as well as “potentially significant changes in laboratory values” [6.4% vs 6.1%]; no mention of ALT elevations or hepatotoxicity).
- Mizuno Y, Hasegawa K, Kondo T, Kuno S, Yamamoto M., Japanese Istradefylline Study Group. Clinical efficacy of istradefylline (KW-6002) in Parkinson's disease: a randomized, controlled study. Mov Disord. 2010;25:1437–43. [PubMed: 20629136](Among 363 Japanese patients with Parkinson disease and motor complications treated with istradefylline or placebo for 12 weeks, there were greater decreases in “off” time with istradefylline [-1.3 and -1.6 vs -0.7 hours], while overall adverse event rates were similar [59% vs 58%] including dyskinesia [7.4% vs 2.5%]; no mention of ALT elevations or hepatotoxicity).
- Kondo T, Mizuno Y., Japanese Istradefylline Study Group. A long-term study of istradefylline safety and efficacy in patients with Parkinson disease. Clin Neuropharmacol. 2015;38:41–6. [PubMed: 25768849](Among 308 Japanese patients with Parkinson disease and motor complications treated with istradefylline or placebo for 52 weeks, istradefylline was associated with a decrease in off time compared to placebo that was sustained for the duration of treatment, while adverse events were similar, and “no clinically meaningful abnormalities were noted in laboratory parameters”).
- Takahashi M, Fujita M, Asai N, Saki M, Mori A. Safety and effectiveness of istradefylline in patients with Parkinson's disease: interim analysis of a post-marketing surveillance study in Japan. Expert Opin Pharmacother. 2018;19:1635–1642. [PubMed: 30281377](Among 476 Japanese patients with Parkinson disease being treated with istradefylline who were followed in a postmarketing surveillance study, adverse events included dyskinesia in 5%, hallucinations [3.4%], somnolence [1.1%], and elevations in ALT [in only 1 subject: 0.2%]).
- Istradefylline (Nourianz) for Parkinson's disease. Med Lett Drugs Ther. 2020;62(1591):20–23. [PubMed: 32022788](Concise review of the mechanism of action, clinical efficacy, safety and costs of istradefylline as an adjunct to carbidopa/levodopa in adults with Parkinson disease who experience “off” episodes, mentions common adverse events of dyskinesia, constipation, dizziness, nausea, hallucinations, and insomnia but does not mention ALT elevations or hepatotoxicity).
- Chen JF, Cunha RA. The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson's disease. Purinergic Signal. 2020;16:167–174. [PMC free article: PMC7367999] [PubMed: 32236790](Review of the mechanism of action and rationale for use of adenosine A2A receptor antagonists, including istradefylline which was not approved based upon an initial FDA application in 2008 because of the relatively modest benefit in reducing “off” time in patients with Parkinson disease and motor complications; eventual approval was supported by consistent beneficial results in multiple clinical trials, substantial safety data in over 4000 patients, and results of long term studies showing modest but sustained improvement).
- Drugs for Parkinson's disease. Med Lett Drugs Ther. 2021;63(1618):25–32. [PubMed: 33647001](Concise review of current medications approved for use in Parkinson disease including levodopa/carbidopa, dopamine agonists, COMT inhibitors, MAO-B inhibitors, anticholinergics, and istradefylline, mentions hepatotoxicity of tolcapone but not of any other of the adjunctive therapies).
- Hauser RA, Hattori N, Fernandez H, Isaacson SH, Mochizuki H, Rascol O, Stocchi F, et al. Efficacy of istradefylline, an adenosine A2A receptor antagonist, as adjunctive therapy to levodopa in Parkinson's disease: a pooled analysis of 8 phase 2b/3 trials. J Parkinsons Dis. 2021;11(4):1663–1675. [PMC free article: PMC8609697] [PubMed: 34486986](In a pooled analysis of 8 randomized placebo-controlled trials of istradefylline in 2719 patients with Parkinson disease and motor complications, changes in “off” time compared to placebo averaged 45 minutes per day with 20 mg and 49 minutes per day with 40 mg of istradefylline, while adverse event rates were similar in the 3 groups [71% and 70% vs 65%] except for dyskinesia [16% and 18% vs 10%], and “no clinically meaningful changes in laboratory parameters…were observed” in any group).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Opicapone.[LiverTox: Clinical and Researc...]Review Opicapone.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Efficacy of Istradefylline, an Adenosine A2A Receptor Antagonist, as Adjunctive Therapy to Levodopa in Parkinson's Disease: A Pooled Analysis of 8 Phase 2b/3 Trials.[J Parkinsons Dis. 2021]Efficacy of Istradefylline, an Adenosine A2A Receptor Antagonist, as Adjunctive Therapy to Levodopa in Parkinson's Disease: A Pooled Analysis of 8 Phase 2b/3 Trials.Hauser RA, Hattori N, Fernandez H, Isaacson SH, Mochizuki H, Rascol O, Stocchi F, Li J, Mori A, Nakajima Y, et al. J Parkinsons Dis. 2021; 11(4):1663-1675.
- Istradefylline: A novel agent in the treatment of "off" episodes associated with levodopa/carbidopa use in Parkinson disease.[Ment Health Clin. 2022]Istradefylline: A novel agent in the treatment of "off" episodes associated with levodopa/carbidopa use in Parkinson disease.Cummins L, Cates ME. Ment Health Clin. 2022 Jan; 12(1):32-36. Epub 2022 Jan 21.
- Evaluating the impact of adjunctive istradefylline on the cumulative dose of levodopa-containing medications in Parkinson's disease: study protocol for the ISTRA ADJUST PD randomized, controlled study.[BMC Neurol. 2022]Evaluating the impact of adjunctive istradefylline on the cumulative dose of levodopa-containing medications in Parkinson's disease: study protocol for the ISTRA ADJUST PD randomized, controlled study.Hatano T, Kano O, Sengoku R, Yoritaka A, Suzuki K, Nishikawa N, Mukai Y, Nomura K, Yoshida N, Seki M, et al. BMC Neurol. 2022 Mar 3; 22(1):71. Epub 2022 Mar 3.
- Review Istradefylline for OFF Episodes in Parkinson's Disease: A US Perspective of Common Clinical Scenarios.[Degener Neurol Neuromuscul Dis...]Review Istradefylline for OFF Episodes in Parkinson's Disease: A US Perspective of Common Clinical Scenarios.Isaacson SH, Betté S, Pahwa R. Degener Neurol Neuromuscul Dis. 2022; 12:97-109. Epub 2022 Jul 23.
- Istradefylline - LiverToxIstradefylline - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...