NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
The halogenated inhalational anesthetics have been linked to idiosyncratic liver injury for more than 50 years. These agents induce anesthesia by blocking CNS neurotransmission, although the exact mechanism of action is unknown. They are currently the most commonly used inhalational anesthetics in surgery. In the United States, the five halogenated anesthetics in current use are halothane, isoflurane, enflurane, desflurane, and sevoflurane. Halothane is now rarely used outside of pediatrics because of its potential to cause idiosyncratic severe liver injury, which is rare in children.
The classic form of anesthetic induced liver injury was associated with halothane, which was introduced into practice in 1956 and rapidly became the most commonly used agent for general anesthesia. Within a few years of its introduction, however, case reports of liver injury possibly due to halothane began to appear. There was initially great resistance to acceptance of the hepatotoxicity of halothane, largely because of its excellent safety record in other respects and the rarity of the hepatic reaction. Eventually, however, the potential hepatotoxicity of halothane became quite clear. By the 1970s, halothane had become the most common cause of idiosyncratic, drug induced acute liver failure and more than 1000 cases of clinically apparent liver injury had been described in the literature. Furthermore, a reasonable mechanism of hepatic injury by halothane was delineated based upon its extensive metabolism (oxidation) by the major drug metabolizing enzyme system of the liver, specifically the cytochrome P450, CYP 2E1. The metabolism of halothane to a trifluoroacetylated reactive intermediate with subsequent binding to intrahepatic cytosolic proteins was believed to create an antigenic molecule capable of inducing allergic and cytotoxic reactions. This proposed mechanism also matched the allergic, clinical features of halothane hepatitis, marked by a typical incubation period of 7 days after anesthesia, accompanied often by fever, rash, arthralgias and eosinophilia and recurring with reexposure in an accelerated and often more severe form. Thus, the overall rate of occurrence of significant liver injury after halothane was estimated at 1 per 15,000 initial exposures, and probably 1 per 1000 repeated exposures, particularly if the reexposure was within 28 days. Having become the most common drug induced cause of acute liver failure in the United States and most of the world, halothane subsequently became less commonly used and is rarely employed currently.
Other halogenated anesthetics have been introduced and become the major agents currently in use in the United States, including enflurane (1972), isoflurane (1981), desflurane (1993) and sevoflurane (1995). These agents have the theoretical advantage of less metabolism by the CYP 2E1 system (20% by halothane compared to 3% by sevoflurane, 2.4% by enflurane, 0.2% by isoflurane, and 0.01% by desflurane). Nevertheless, rare instances of acute liver injury have been reported with all agents. Furthermore, the pattern of injury described with the newer halogenated anesthetics has resembled that of halothane hepatitis, and trifluoroacetyl adducts have been identified in liver tissue and antibodies to be the adducts in serum of patients with hepatic injury from these other agents. Nevertheless, anesthetic induced acute liver failure is now rare and no longer ranked among the more common causes of drug induced liver disease or acute liver failure.
The various structures of the halogenated anesthetics are shown below. Each agent is discussed separately with individual clinical cases and references.
The following links are to individual drug records.
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Desflurane | 57041-67-5 | C3-H2-F6-O |
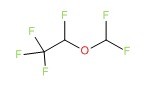 |
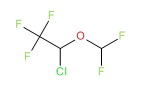
| Enflurane | 13838-16-9 | C3-H2-Cl-F5-O |
 |
| Halothane | 151-67-7 | C2-H-Br-Cl-F3 |
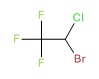 |
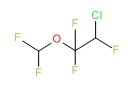
| Isoflurane | 26675-46-7 | C3-H2-Cl-F5-O |
 |
| Sevoflurane | 28523-86-6 | C4-H3-F7-O |
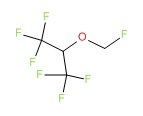 |
- PubChem SubstanceRelated PubChem Substances
- Review Hepatotoxicity of halogenated inhalational anesthetics.[Iran Red Crescent Med J. 2014]Review Hepatotoxicity of halogenated inhalational anesthetics.Safari S, Motavaf M, Seyed Siamdoust SA, Alavian SM. Iran Red Crescent Med J. 2014 Sep; 16(9):e20153. Epub 2014 Sep 5.
- Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury.[Anesth Analg. 1997]Biotransformation of halothane, enflurane, isoflurane, and desflurane to trifluoroacetylated liver proteins: association between protein acylation and hepatic injury.Njoku D, Laster MJ, Gong DH, Eger EI 2nd, Reed GF, Martin JL. Anesth Analg. 1997 Jan; 84(1):173-8.
- Review Anesthetics, General.[LiverTox: Clinical and Researc...]Review Anesthetics, General.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis.[Hepat Mon. 2011]Halothane-induced hepatitis: A forgotten issue in developing countries: Halothane-induced hepatitis.Habibollahi P, Mahboobi N, Esmaeili S, Safari S, Dabbagh A, Alavian SM. Hepat Mon. 2011 Jan; 11(1):3-6.
- Review Clinical pharmacokinetics of the inhalational anaesthetics.[Clin Pharmacokinet. 1987]Review Clinical pharmacokinetics of the inhalational anaesthetics.Dale O, Brown BR Jr. Clin Pharmacokinet. 1987 Mar; 12(3):145-67.
- Halogenated Anesthetics - LiverToxHalogenated Anesthetics - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...