NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Eslicarbazepine is an aromatic anticonvulsant similar to oxcarbazepine that is used in combination with other antiepileptic agents as therapy of partial onset seizures. Eslicarbazepine is associated with a low rate of transient serum enzyme elevations during therapy and has been implicated in rare instances of clinically apparent liver injury.
Background
Eslicarbazepine (es" li kar baz' e peen) is an aromatic anticonvulsant related in structure and activity to oxcarbazepine. Its mechanism of action is unknown but, like other carboxamides, eslicarbazepine is believed to inhibit voltage-gated sodium channels and thereby slow neurotransmission and interrupt the rapid, repetitive firing that is characteristic of epilepsy. Eslicarbazepine was approved for use in the United States in 2013 as an anticonvulsant to be used alone or in combination with other agents in the therapy of partial onset seizures. Eslicarbazepine is available in tablets of 200, 400, 600 and 800 mg under the brand name Aptiom. The recommended initial dose in adults is 400 mg once daily, which can be increased to 800 to 1600 mg once daily based upon tolerance and effect. The recommended initial doses in children are 200 to 400 mg once daily based upon body weight with maintenance dosages ranging from 400 to 1200 mg. Side effects may include headache, dizziness, ataxia, blurred vision, nausea, fatigue and tremor. Rare, but potentially serious adverse events include hyponatremia, suicidal ideation or behavior and hypersensitivity reactions including DRESS and Stevens Johnson syndrome.
Hepatotoxicity
In prelicensure clinical trials, addition of eslicarbazepine to standard anticonvulsant therapy was reported to be associated with ALT elevations above 3 times the upper limit of normal (ULN) in a small proportion of patients (<1.0%). A single case of hepatitis with jaundice during eslicarbazepine therapy was also reported. Since approval, there have been rare isolated reports of clinically apparent liver injury associated with eslicarbazepine used, but the clinical features suggested that hepatic ischemia or other anticonvulsants combined with eslicarbazepine may have been responsible. The onset was within a few days to several months after starting the anticonvulsant, and the presentation was characterized by a hepatocellular pattern of serum enzyme elevations, one case being asymptomatic and mild and the other severe. In both instances there was rapid recovery.
Likelihood score: D (possible rare cause of clinically apparent liver injury).
Mechanism of Injury
Eslicarbazepine is metabolized by the liver, largely by CYP 2C19, but has not been reported to have significant drug interactions. The mechanism of hepatic injury from eslicarbazepine is not known, but may relate to a toxic or immunogenic metabolite.
Outcome and Management
Most instances of liver injury associated with eslicarbazepine have been transient and mild but more severe cases have been reported. The product label recommends baseline evaluation of liver tests and prompt discontinuation if jaundice or symptoms of liver disease arise. There is no information on the possible cross sensitivity to hepatotoxicity between eslicarbazepine and other anticonvulsants, but its structure would suggest that it would havel cross sensitivity to hypersensitivity reactions with oxcarbazepine and possibly partial cross sensitivity to other aromatic anticonvulsants such as phenytoin, carbamazepine and lamotrigine.
Drug Class: Anticonvulsants
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Eslicarbazepine – Aptiom®
DRUG CLASS
Anticonvulsants
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Eslicarbazepine | 104746-04-5 | C15-H14-N2-O2 |
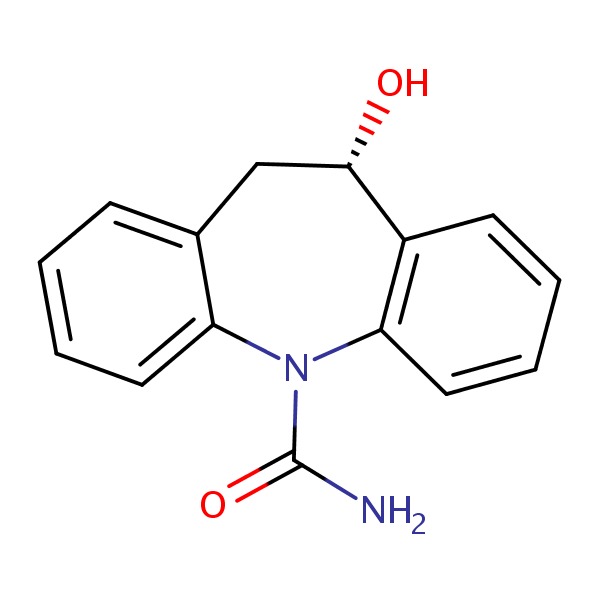 |
| Oxcarbazepine | 28721-07-5 | C15-H12-N2-O2 |
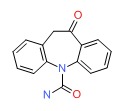 |
ANNOTATED BIBLIOGRAPHY
References updated: 14 February 2018
- Zimmerman HJ. Anticonvulsants. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 498-516.(Expert review of anticonvulsants and liver injury published in 1999 before the availability of eslicarbazepine).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; eslicarbazepine is not discussed).
- McNamara JO. Pharmacotherapy of the epilepsies. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, p. 602.(Textbook of pharmacology and therapeutics).
- Galindo PA, Borja J, Gómez E, Mur P, Gudín M, García R, Encinas C, et al. Anticonvulsant drug hypersensitivity. J Investig Allergol Clin Immunol 2002; 12: 299-304. [PubMed: 12926190](Among 15 patients with cutaneous hypersensitivity reactions to anticonvulsants [9 accompanied by liver test elevations] which were caused by carbamazepine [n=8], phenytoin [5], lamotrigine [4], phenobarbital [4], valproate [1] and felbamate [1], eslicarbazepine and oxcarbazepine were not mentioned).
- Elger C, Halász P, Maia J, Almeida L, Soares-da-Silva P; BIA-2093-301 Investigators Study Group. Efficacy and safety of eslicarbazepine acetate as adjunctive treatment in adults with refractory partial-onset seizures: a randomized, double-blind, placebo-controlled, parallel-group phase III study. Epilepsia 2009; 50: 454-63. [PubMed: 19243424](Among 402 patients with partial onset seizures treated with adjunctive eslicarbazepine [400, 800 or 1200 mg daily] or placebo for 12 weeks, there were no “clinically relevant findings” in the clinical laboratory parameters).
- Halász P, Cramer JA, Hodoba D, Członkowska A, Guekht A, Maia J, Elger C, et al.; BIA-2093-301 Study Group. Long-term efficacy and safety of eslicarbazepine acetate: results of a 1-year open-label extension study in partial-onset seizures in adults with epilepsy. Epilepsia 2010; 51: 1963-9. [PubMed: 20662896](Among 314 patients with poorly controlled partial onset seizures who completed a placebo controlled study of eslicarbazepine and were maintained on therapy [600 to 1600 mg daily] for 52 weeks, common side effects were headache, dizziness, diplopia and somnolence; 3 patients developed rash, and “review of changes in clinical laboratory parameters did not reveal clinically relevant findings”).
- Ben-Menachem E, Gabbai AA, Hufnagel A, Maia J, Almeida L, Soares-da-Silva P. Eslicarbazepine acetate as adjunctive therapy in adult patients with partial epilepsy. Epilepsy Res 2010; 89 (2-3): 278-85. [PubMed: 20299189](Among 395 adults with poorly controlled partial onset seizures given eslicarbazepine [400, 800 or 1200 mg daily] or placebo once daily for 14 weeks, seizure control was greater with the two higher doses of eslicarbazepine and side effects included dizziness, somnolence, headache, ataxia, diplopia, blurred vision, nausea and fatigue; there were no “clinically relevant findings” in clinical laboratory parameters).
- Gil-Nagel A, Elger C, Ben-Menachem E, Halász P, Lopes-Lima J, Gabbai AA, Nunes T, et al. Efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: integrated analysis of pooled data from double-blind phase III clinical studies. Epilepsia 2013; 54: 98-107. [PubMed: 22882018](Among 1049 adults participating in 3 controlled trials of eslicarbazepine [400, 800 or 1200 mg daily] for partial onset seizures, adverse events were dose dependent, the most common being dizziness, somnolence and headache and “very few patients had clinically significant abnormalities in liver function tests” [<1% in any treatment group]).
- Gaitatzis A, Sander JW. The long-term safety of antiepileptic drugs. CNS Drugs 2013; 27: 435-55. [PubMed: 23673774](Review of the long term safety and adverse event profile of anticonvulsants mentions that valproate and felbamate can cause liver failure, but does no mention hepatotoxicity of other anticonvulsants).
- Drugs for epilepsy. Treat Guidel Med Lett 2013; 11: 9-18. [PubMed: 23348233](Concise review of indications and side effects of anticonvulsants; oxcarbazepine [but not eslicarbazepine] is discussed] which is approved as both monotherapy and add-on therapy of adults with partial seizures; discussion of adverse effects does not mention hepatotoxicity).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were attributed to oxcarbazepine or eslicarbazepine).
- Kaniwa N, Saito Y. The risk of cutaneous adverse reactions among patients with the HLA-A* 31:01 allele who are given carbamazepine, oxcarbazepine or eslicarbazepine: a perspective review. Ther Adv Drug Saf 2013; 4: 246-53. [PMC free article: PMC4125310] [PubMed: 25114785](Review of HLA associations [A*31:01 and B*15:02] with hypersensitivity reactions to carbamazepine and the carboxamines, which are metabolized differently and believed to have fewer side effects; cases of A*31:01 associated SJS/TEN have been linked to use of oxcarbazepine, but eslicarbazepine cases have not been reported).
- Kaniwa N, Saito Y. Pharmacogenomics of severe cutaneous adverse reactions and drug-induced liver injury. J Hum Genet 2013; 58: 317-26. [PubMed: 23635947](Review of genetic associations with severe skin and liver injury from drugs, focusing upon allopurinol [B*58:01], abacavir and flucloxacillin [B*57:01] and lapatinib [DQA1*02:01]; the association of B*15:02 with SJS from carbamazepine has not been extended to its liver injury).
- Kaniwa N, Sugiyama E, Saito Y, Kurose K, Maekawa K, Hasegawa R, Furuya H, et al.; Japan Pharmacogenomics Data Science Consortium. Specific HLA types are associated with antiepileptic drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese subjects. Pharmacogenomics 2013; 14: 1821-31. [PubMed: 24236482](Analysis of HLA types among 12 Japanese patients with SJS/TEN due to zonisamide, found five patients [42%] to have HLA-A*02:07 compared to 6.8% of controls).
- Verrotti A, Loiacono G, Rossi A, Zaccara G. Eslicarbazepine acetate: an update on efficacy and safety in epilepsy. Epilepsy Res 2014; 108: 1-10. [PubMed: 24225327](Summary of the mechanism of action, pharmacokinetics, efficacy and safety of eslicarbazepine mentions that “there were no significant changes in clinical laboratory parameters”).
- Eslicarbazepine acetate (Aptiom) for epilepsy. Med Lett Drugs Ther 2014; 56 (1443): 42-3. [PubMed: 24869714](Concise review of the mechanism of action, efficacy, safety, drug interactions and costs of eslicarbazepine shortly after its approval in the US mentions DRESS and Stevens Johnson syndrome [with hepatic toxicity] as potential adverse events).
- Massot A, Gimenez-Arnau A. Cutaneous adverse drug reaction type erythema multiforme major induced by eslicarbazepine. J Pharmacol Pharmacother 2014; 5: 271-4. [PMC free article: PMC4231563] [PubMed: 25422574](41 year old woman with epilepsy developed a painful erythematous rash with oral involvement and lymphadenopathy 25 days after starting eslicarbazepine [bilirubin not given, ALT 226 U/L, GGT 263 U/L, eosinophilia 3900/μL], resolving within a few weeks of stopping therapy and starting prednisone).
- Correia FD, Freitas J, Magalhães R, Lopes J, Ramalheira J, Lopes-Lima J, Chaves J. Two-year follow-up with eslicarbazepine acetate: a consecutive, retrospective, observational study. Epilepsy Res 2014; 108: 1399-405. [PubMed: 25060997](Among 152 patients with refractory epilepsy started on eslicarbazepine at a single referral center and followed for 2 years, adverse events occurred in 42% leading to discontinuation in half, the most common side effects being dizziness, somnolence, nausea, psychiatric syndromes, weight gain, diplopia and rash; no mention of ALT elevations or hepatotoxicity).
- Villanueva V, Serratosa JM, Guillamón E, Garcés M, Giráldez BG, Toledo M, Salas-Puig J, et al. Long-term safety and efficacy of eslicarbazepine acetate in patients with focal seizures: results of the 1-year ESLIBASE retrospective study. Epilepsy Res 2014; 108: 1243-52. [PubMed: 24908564](In a retrospective analysis of 327 patients with focal seizures treated with eslicarbazepine at 12 hospitals in Spain during a 2 year period, rash was reported in 9 [2.7%] and transient ALT elevations in 1 [0.3%] patient).
- Sperling MR, Harvey J, Grinnell T, Cheng H, Blum D; 045 Study Team. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America. Epilepsia 2015; 56: 546-55. [PMC free article: PMC5016771] [PubMed: 25689448](Among 193 adults patients with poorly controlled partial onset seizures who were switched to monotherapy with eslicarbazepine, common side effects were dizziness, headache, fatigue, somnolence and nausea; no mention of ALT elevations or hepatotoxicity).
- Jacobson MP, Pazdera L, Bhatia P, Grinnell T, Cheng H, Blum D; study 046 team. Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a historical-control phase III study. BMC Neurol 2015; 15: 46. [PMC free article: PMC4397697] [PubMed: 25880756](Among 172 patients with poorly controlled partial onset seizures switched to monotherapy with eslicarbazepine and treated for at least 10 weeks, 11 patients developed a serious adverse event including one with DRESS syndrome, but “most clinical laboratory parameters were comparable between treatment groups” and there was no mention of ALT elevations or hepatotoxicity).
- Sperling MR, Abou-Khalil B, Harvey J, Rogin JB, Biraben A, Galimberti CA, Kowacs PA, et al.; 304 Study Team. Eslicarbazepine acetate as adjunctive therapy in patients with uncontrolled partial-onset seizures: Results of a phase III, double-blind, randomized, placebo-controlled trial. Epilepsia 2015; 56: 244-53. [PMC free article: PMC4354260] [PubMed: 25528898](Among 653 patients with poorly controlled partial onset seizures who received adjunctive therapy with eslicarbazepine [800 or 1200 mg daily] or placebo for 12 weeks, the response rate was significantly better with the higher dose than with placebo, and “overall, changes in clinical laboratory parameters [other than serum sodium] were not substantially different between the three groups”).
- Grunze H, Kotlik E, Costa R, Nunes T, Falcão A, Almeida L, Soares-da-Silva P. Assessment of the efficacy and safety of eslicarbazepine acetate in acute mania and prevention of recurrence: experience from multicentre, double-blind, randomised phase II clinical studies in patients with bipolar disorder I. J Affect Disord 2015; 174: 70-82. [PubMed: 25484179](Among 245 patients with acute mania treated with eslicarbazepine or placebo in two randomized controlled, short term trials, there were no liver related serious adverse events reported).
- Ley M, Principe A, Jiménez-Conde J, Rocamora R. Assessing long-term effects of eslicarbazepine acetate on lipid metabolism profile, sodium values and liver function tests. Epilepsy Res 2015; 115: 147-52. [PubMed: 26220393](Among 108 patients treated with eslicarbazepine for an average of 2 years, there were no statistically significant changes in liver tests including ALT and AST).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, but none to oxcarbazepine or eslicarbazepine).
- Holtkamp M, McMurray R, Bagul M, Sousa R, Kockelmann E. Real-world data on eslicarbazepine acetate as add-on to antiepileptic monotherapy. Acta Neurol Scand 2016; 134: 76-82. [PMC free article: PMC5067651] [PubMed: 26915469](Among 247 adults treated in clinical practice with eslicarbazepine for at least 6 months, 57 [26%] had adverse events, 25 [12%] discontinued therapy because of side effects and 8 [4%] had serious adverse events, but none were liver related; no mention of ALT elevations).
- Elger C, Koepp M, Trinka E, Villanueva V, Chaves J, Ben-Menachen E, Kowacs PA, et al. Pooled efficacy and safety of eslicarbazepine acetate as add-on treatment in patients with focal-onset seizures: Data from four double-blind placebo-controlled pivotal phase III clinical studies. CNS Neurosci Ther 2017; 23: 961-72. [PMC free article: PMC5813188] [PubMed: 29030894](Among 1703 patients enrolled in 4 controlled trials, adverse event rates were dose related leading to discontinuation in 9% [400 mg], 13% [800 mg] and 24% [1200 mg] of patients; no mention of ALT elevations or hepatotoxicity).
- Villanueva V, Holtkamp M, Delanty N, Rodriguez-Uranga J, McMurray R, Santagueda P. Euro-Esli: a European audit of real-world use of eslicarbazepine acetate as a treatment for partial-onset seizures. J Neurol 2017; 264: 2232-48. [PMC free article: PMC5656697] [PubMed: 28921040](Among 2058 patients trated with eslicarbazepine in clinical practice, 34% reported adverse events which led to discontinuation in 14%, the most common side effects being dizziness [7%], fatigue [5%], somnolence [5%], and hyponatremia [3.5%]; no mention of ALT elevations or hepatotoxicity).
- Gama H, Vieira M, Costa R, Graça J, Magalhães LM, Soares-da-Silva P. Safety profile of rslicarbazepine acetate as add-on therapy in adults with refractory focal-onset seizures: from clinical studies to 6 years of post-marketing experience. Drug Saf 2017; 40: 1231-40. [PMC free article: PMC5688182] [PubMed: 28752473](Analysis of safety data from 4 controlled trials and global safety databases discusses dizziness, somnolence, headaches, nausea, rash and hyponatremia, but no ALT elevations or hepatotoxicity).
- Toledano R, Jovel CE, Jiménez-Huete A, Bayarri PG, Campos D, Gomariz EL, Giráldez BG, et al. Efficacy and safety of eslicarbazepine acetate monotherapy for partial-onset seizures: Experience from a multicenter, observational study. Epilepsy Behav 2017; 73: 173-9. [PubMed: 28641170](Among 117 Spanish patients with partial onset seizures treated with eslicarbazepine alone, 82% had improvement in seizure control and 18 [15%] had adverse events, but only 1 led to discontinuation, an allergic reaction; no mention of ALT elevations or hepatotoxicity).
- Trinka E, Ben-Menachem E, Kowacs PA, Elger C, Keller B, Löffler K, Rocha JF, et al. Efficacy and safety of eslicarbazepine acetate versus controlled-release carbamazepine monotherapy in newly diagnosed epilepsy: A phase III double-blind, randomized, parallel-group, multicenter study. Epilepsia 2018; 59: 479-91. [PubMed: 29369348](Among 815 patients with new onset of seziures who were treated with esclicarbazepine or carbamazepine for 26 weeks, clinical response rates were similar in the two groups [71% and 76%] as were overall adverse event rates; no mention of ALT elevations or hepatotoxicity with either agent).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Role of eslicarbazepine in the treatment of epilepsy in adult patients with partial-onset seizures.[Ther Clin Risk Manag. 2010]Role of eslicarbazepine in the treatment of epilepsy in adult patients with partial-onset seizures.Brown ME, El-Mallakh RS. Ther Clin Risk Manag. 2010 Apr 15; 6:103-9. Epub 2010 Apr 15.
- Pharmacokinetics of eslicarbazepine acetate at steady-state in adults with partial-onset seizures.[Epilepsy Res. 2011]Pharmacokinetics of eslicarbazepine acetate at steady-state in adults with partial-onset seizures.Perucca E, Elger C, Halász P, Falcão A, Almeida L, Soares-da-Silva P. Epilepsy Res. 2011 Sep; 96(1-2):132-9. Epub 2011 Jun 15.
- Review Lacosamide.[LiverTox: Clinical and Researc...]Review Lacosamide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Pharmacokinetics, drug interactions and exposure-response relationship of eslicarbazepine acetate in adult patients with partial-onset seizures: population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses.[CNS Drugs. 2012]Review Pharmacokinetics, drug interactions and exposure-response relationship of eslicarbazepine acetate in adult patients with partial-onset seizures: population pharmacokinetic and pharmacokinetic/pharmacodynamic analyses.Falcão A, Fuseau E, Nunes T, Almeida L, Soares-da-Silva P. CNS Drugs. 2012 Jan 1; 26(1):79-91.
- Review Eslicarbazepine acetate for the treatment of focal epilepsy: an update on its proposed mechanisms of action.[Pharmacol Res Perspect. 2015]Review Eslicarbazepine acetate for the treatment of focal epilepsy: an update on its proposed mechanisms of action.Soares-da-Silva P, Pires N, Bonifácio MJ, Loureiro AI, Palma N, Wright LC. Pharmacol Res Perspect. 2015 Mar; 3(2):e00124. Epub 2015 Mar 30.
- Eslicarbazepine - LiverToxEslicarbazepine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...