NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ergot alkaloids are widely used for therapy of acute migraine headaches and include ergotamine and dihydroergotamine, both of which act by causing vasoconstriction of the carotid artery beds. Ergot alkaloids have multiple side effects, but have little effect on the liver and have not been clearly linked to instances of clinically apparent acute liver injury.
Background
Ergotamine (er got' a meen) and dihydroergotamine are ergot alkaloids that act as vasoconstrictors, probably by stimulating alpha adrenergic receptors particularly in the carotid artery bed. The ergotamines may also have serotoninergic effects which may also be beneficial in migraine. The ergotamines were first reported to alleviate migraine headaches in the 1920s and were introduced into clinical use in the United States in the 1940s. Ergotamine is available in 1 mg tablets in multiple generic forms and under brand names such as Cafergot, Ergomar, Ergostat, Migergot and Wigraine. Various combinations of ergotamine with caffeine (100 mg) and acetaminophen are also available as are rectal suppositories (2 mg with or without caffeine) and sublingual forms (2 mg). The usual recommended dose to abort or treat a vascular headache is 2 mg initially (sublingually or orally) and then 1 to 2 mg every 30 minutes, to a maximum of 6 mg per attack and 10 mg weekly. Dihydroergotamine is available in solution for injection (1 mg/mL) or as a nasal spray (4 mg/mL) in generic forms and under the brand names of DHE 45 and Migranal. The usual recommended dose is 1 mg intramuscularly or intravenously initially, repeated at 1 hour intervals to a total dose of 2 to 3 mg and no more than 6 mg weekly. The nasal spray is given as 0.5 mg to each nostril, repeated every 15 minutes, but to less than 3 mg in 24 hours and 4 mg in one week. The advantage of the nasal and parenteral formulations is the rapid onset of action; the disadvantage is a greater potential for overdose or side effects. Common side effects (ergotism) include nausea, vomiting, light-headedness, numbness and tingling, hypertension, bradycardia, muscle pains and itching. Overdose can cause acute vascular spasm and thrombosis.
Hepatotoxicity
Neither ergotamine nor dihydroergotamine have been implicated in causing liver enzyme elevations, but they are generally used in limited amounts for short and intermittent periods of time. Ergotamine abuse can lead to arterial or venous spastic episodes, and at least one case of portal and splenic vein thrombosis with resultant noncirrhotic portal hypertension has been reported. Ergotamine overdose can lead to ischemic injury to limbs or viscera, including the liver. However, the ergotamines have not been implicated in cases of clinically apparent liver injury and product labels do not mention ALT elevations or liver injury as potential adverse events.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Ergotamine is extensively metabolized by the liver, but it is given in low doses, and intermediates of its metabolism, even if potentially toxic, are likely conjugated and excreted rapidly and without hepatic injury. The vasospastic actions of ergotamines can cause arterial and venous spasms and potentially thromboses to the hepatic artery or portal systems.
Agents used specifically in management of migraines and vascular headaches include: the ergot alkaloids, ergotamine and dihydroergotamine; and, the serotonin receptor agonists (triptans), including almotriptan, eletriptan, frovatriptan, naratriptan, rizatriptan, sumatriptan and zolmitriptan.
Drug Class: Migraine Headache Agents, Vasoconstrictor Agents
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dihydroergotamine – Generic, Migranal®
Ergotamine – Generic, Cafergot®
DRUG CLASS
Migraine Headache Agents
Product labeling at DailyMed, National Library of Medicine, NIH
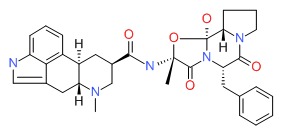
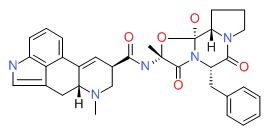
CHEMICAL FORMULAS AND STRUCTURES
REFERENCES
References updated: 10 February 2018
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Expert review of hepatotoxicity published in 1999; ergotamine and triptans are not discussed).
- Larrey D, Ripault MP. Benzodiazepines. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 455. (Review of hepatotoxicity of neuroleptic drugs does not mention.ergotamine or the triptans).
- Sanders-Bush E, Mayer SE. 5-Hydroxytryptamine (Serotonin): receptor agonists and antagonists. In, Brunton LL, Lazo JS, Parker KL, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 11th ed. New York: McGraw-Hill, 2006, pp. 297-315.(Textbook of pharmacology and therapeutics).
- Fisher PE, Silk DB, Menzies-Gow N, Dingle M. Ergotamine abuse and extra-hepatic portal hypertension. Postgrad Med J 1985; 61: 461-3. [PMC free article: PMC2418255] [PubMed: 4022885](Ergotamine abuse can lead to arteriospasm as well as venous complications; 48 year old woman developed hemiparesis after taking ergotamine 6 mg daily for 3 months which resolved on stopping, but she later presented with esophageal varices, believed to be due to splenic and portal vein thromboses).
- Deviere J, Reuse C, Askenasi R. Ischemic pancreatitis and hepatitis secondary to ergotamine poisoning. J Clin Gastroenterol 1987; 9: 350-2. [PubMed: 3112214](29 year old man with suicidal ergotamine overdose presented with hypotension, hypoxia and acidosis and then developed marked ALT [4200 U/L] and amylase [2940 U/L] elevations 2 to 3 days later, resolving rapidly without jaundice; compatible with ischemic injury).
- Block GA, Goldstein J, Polis A, Reines SA, Smith ME. Efficacy and safety of rizatriptan versus standard care during long-term treatment for migraine. Rizatriptan Multicenter Study Groups. Headache 1998; 38: 764-71. [PubMed: 11284464](Among 1831 patients treated for up to 12 months and experiencing 46,773 migraine attacks, pain relief was achieved in 2 hours in 80-90% on rizatriptan and 70% on standard care; side effects were similar and there were “no meaningful differences in the incidences of drug related laboratory adverse events”).
- Geraud G, Compagnon A, Rossi A; COZAM Study Group. Zolmitriptan versus a combination of acetylsalicylic acid and metoclopramide in the acute oral treatment of migraine: a double-blind, randomised, three-attack study. Eur Neurol 2002; 47: 88-98. [PubMed: 11844897](Among 666 patients with migraine treated with either zolmitriptan or aspirin/metoclopramide, side effects of paresthesias and dizziness were more common with zolmitriptan; no mention of any hepatic side effects).
- Diener HC, Jansen JP, Reches A, Pascual J, Pitei D, Steiner TJ; Eletriptan and Cafergot Comparative Study Group. Efficacy, tolerability and safety of oral eletriptan and ergotamine plus caffeine(Cafergot) in the acute treatment of migraine: a multicentre, randomised, double-blind, placebo-controlled comparison. Eur Neurol 2002; 47: 99-107. [PubMed: 11844898](Among 733 patients with migraine treated with either eletriptan or ergotamine/caffeine, side effects were transient and predominately mild or moderate; “No clinically significant laboratory…abnormalities were recorded”).
- Snow V, Weiss K, Wall EM, Mottur-Pilson C; American Academy of Family Physicians; American College of Physicians-American Society of Internal Medicine. Pharmacologic management of acute attacks of migraine and prevention of migraine headache. Ann Intern Med 2002; 137: 840-9. [PubMed: 12435222](Guidelines for management of acute migraine and chronic prevention; recommends use of nasal dihydroergotamine or a triptan in patients whose migraines fail to respond to aspirin, acetaminophen or nonsteroidal antiinflammatory agents).
- Loj J, Solomon GD. Migraine prophylaxis: who, why, and how. Cleve Clin J Med 2006; 73: 793-4, 797, 800-1 passim. [PubMed: 16970133](Preventive therapy of migraine has limited efficacy and may take 2-3 months to have an effect; the most commonly used agents are beta-blockers, calcium channel blockers, anticonvulsants, tricyclic antidepressants and selective serotonin reuptake inhibitors [SSRIs]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to agents used to treat migraine headaches).
- Whyte CA, Tepper SJ. Adverse effects of medications commonly used in the treatment of migraine. Expert Rev Neurother 2009; 9: 1379-91. [PubMed: 19769452](Extensive review of common side effects of medications used to treat migraine; nausea is the most common and dose limiting side effect of ergot alkaloids and can require antiemetics).
- Taylor FR. Acute treatment of migraine headaches. Semin Neurol 2010; 30: 145-53. [PubMed: 20352584](Review of diagnosis and acute management of migraine headaches including use of ergot alkaloids).
- Drugs for migraine. Treat Guidel Med Lett 2011; 9: 7-12. [PubMed: 21304447](Concise review of current medications used for migraine; no discussion of hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America: an analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Among 176 reports of drug induced liver injury from Latin America published between 1996 and 2012, none were attributed to drugs for migraine headaches).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to the triptans or other drugs for migraine headaches).
- Drugs for migraine. Med Lett Drugs Ther 2017; 59 (1514): 27-32. [PubMed: 28170366]Corrected and republished in: JAMA 2017; 317 (21): 2230-1. PubMed Citation (Concise review of current medications used for migraine; no discussion of hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Effects of the ergot alkaloids dihydroergotamine, ergonovine, and ergotamine on growth of Escherichia coli O157:H7 in vitro.[Foodborne Pathog Dis. 2008]Effects of the ergot alkaloids dihydroergotamine, ergonovine, and ergotamine on growth of Escherichia coli O157:H7 in vitro.Looper ML, Edrington TS, Moubarak AS, Callaway TR, Rosenkrans CF Jr. Foodborne Pathog Dis. 2008 Oct; 5(5):599-604.
- Alpha 2-adrenergic agonist and alpha 1-adrenergic antagonist activity of ergotamine and dihydroergotamine in rats.[Arch Int Pharmacodyn Ther. 1986]Alpha 2-adrenergic agonist and alpha 1-adrenergic antagonist activity of ergotamine and dihydroergotamine in rats.Roquebert J, Grenié B. Arch Int Pharmacodyn Ther. 1986 Nov; 284(1):30-7.
- [Ergotamine-caffeine and dihydroergotamine-caffeine in migraine therapy].[Ugeskr Laeger. 1952][Ergotamine-caffeine and dihydroergotamine-caffeine in migraine therapy].DALSGAARD-NIELSEN T. Ugeskr Laeger. 1952 Aug 21; 114(34):1138-42.
- Review The pharmacology of ergotamine and dihydroergotamine.[Headache. 1997]Review The pharmacology of ergotamine and dihydroergotamine.Silberstein SD. Headache. 1997; 37 Suppl 1:S15-25.
- Review Ergotamine and dihydroergotamine: a review.[Curr Pain Headache Rep. 2003]Review Ergotamine and dihydroergotamine: a review.Bigal ME, Tepper SJ. Curr Pain Headache Rep. 2003 Feb; 7(1):55-62.
- Ergot Alkaloids - LiverToxErgot Alkaloids - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...