NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Enalapril is an angiotensin-converting enzyme (ACE) inhibitor widely used in the therapy of hypertension and heart failure. Enalapril is associated with a low rate of transient serum aminotransferase elevations and has been linked to rare instances of acute liver injury.
Background
Enalapril (en al' a pril) was the second ACE inhibitor to be approved for use in the United States and is still widely used for therapy of hypertension and heart failure. Like other ACE inhibitors, enalapril inhibits the conversion of angiotensin I, a relatively inactive molecule, to angiotensin II which is the major mediator of vasoconstriction and volume expansion induced by the renin-angiotensin system. Other enzymes besides that which converts angiotensin I to II may also be inhibited, which may account for some of the side effects of the ACE inhibitors. Like many other ACE inhibitors, enalapril is a prodrug and must be hydrolyzed in the liver to its active carboxylic metabolite, enalaprilat. Enalapril was approved for use in the United States in 1985 and current indications include hypertension, symptomatic heart failure and prevention of progression of left ventricular dysfunction. Enalapril is available in 2.5, 5, 10 and 20 mg tablets in generic forms and under the trade name Vasotec. The typical initial oral dose of enalapril in adults is 2.5 to 5 mg once or twice daily, which can be increased to a maximum of 40 mg daily. Injectable formulations of enalaprilat, the active metabolic product of enalapril, are available in 1 and 2 mL vials of 1.25 mg/mL for intravenous administration. Enalapril is also available in fixed combinations with hydrochlorothiazide (generically and as Vaseretic) and with felodipine (Lexxel). Common side effects include dizziness, fatigue, headache, cough, gastrointestinal upset and skin rash. Rare, but potentially serious adverse events include hypersensitivity reactions, anaphylaxis and fetal or neonatal mortality.
Hepatotoxicity
Enalapril, like other ACE inhibitors, has been associated with a low rate of serum aminotransferase elevations (<2%) that, in controlled trials, was no higher than with placebo therapy. These elevations were transient and rarely required dose modification. In addition, more than a dozen instances of clinically apparent acute liver injury have been reported with enalapril therapy. The onset is usually within 2 to 12 weeks of starting therapy and the serum enzyme pattern is typically cholestatic. In some instances, cholestasis has been prolonged and relapsing and associated with persistent elevations in serum alkaline phosphatase, suggestive of vanishing bile duct syndrome. Immunoallergic manifestations (rash, fever, eosinophilia) are infrequent and most patients do not develop autoantibodies. There have also been striking instances of enalapril liver injury with a hepatocellular pattern. These cases typically have a long latency (one or more years), some of which have been severe and fatal. The appearance of an acute liver injury more than a year after starting a medication is distinctly unusual, but has been described for several ACE inhibitors.
Likelihood score: B (likely but rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the minor serum aminotransferase elevations associated with enalapril is not known. The clinically apparent cases of hepatotoxicity due to enalapril are clearly idiosyncratic and likely due to a reaction to a metabolite. The instances of enalapril associated liver injury associated with a long latency may be due to a different mechanism. Enalapril is metabolized by the liver to enalaprilat, its active form, but further hepatic metabolism is minimal and enalapril has little interaction with the cytochrome P450 system.
Outcome and Management
Most instances of acute liver injury reported with enalapril use have been self limited, but severe and fatal instances have been reported as have cases of vanishing bile duct syndrome. Patients with severe enalapril induced acute liver injury should avoid use of other ACE inhibitors, although cross sensitivity to liver injury among the members of this class of agents has not always been shown.
References to the safety and potential hepatotoxicity of enalapril are given in the Overview section on the Angiotensin-Converting Enzyme (ACE) Inhibitors (Updated February 2018).
Drug Class: Antihypertensive Agents, Angiotensin-Converting Enzyme Inhibitors
CASE REPORTS
Case 1. Cholestatic hepatitis due to enalapril.
[Modified from: Todd P, Levison D, Farthing MJ. Enalapril-related cholestatic jaundice. J R Soc Med 1990; 83: 271-2. PubMed Citation]
A 67 year old woman was switched from atenolol to enalapril (15 mg daily) in combination with a diuretic (cyclopenthiazide 250 mcg daily) for better control of her essential hypertension. Six weeks later she developed dark urine, jaundice and pruritus. She had no history of liver disease, did not abuse alcohol and had no risk factors for acquiring viral hepatitis. On examination, she was jaundiced but had no fever, rash or peripheral manifestations of chronic liver disease. Laboratory testing showed a total serum bilirubin of 8.9 mg/dL, with moderate elevations of aminotransferase (AST 236 U/L) and alkaline phosphatase levels (437 U/L) (Table). There was no eosinophilia and the prothrombin time was normal. Tests for hepatitis A and B were negative as were autoantibodies. Abdominal ultrasound showed no evidence of biliary obstruction. A liver biopsy showed intrahepatic cholestasis typical of drug induced liver injury. Four days after admission, enalapril was stopped and she began to improve rapidly. When seen 3 months later, she was asymptomatic and laboratory values were normal.
Key Points
| Medication: | Enalapril (15 mg daily) |
|---|---|
| Pattern: | Cholestatic (R=1.4) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 6 weeks to jaundice |
| Recovery: | Partial after 2 weeks, complete after 12 weeks |
| Other medications: | Cyclopenthiazide |
Laboratory Values
* Values and dates in Table estimated from Figure 1.
Comment
The patient developed a cholestatic hepatitis within 6 weeks of starting enalapril therapy and recovery was prompt once the medication was stopped. This is a typical history for enalapril induced acute liver injury. In rare instances of ACE inhibitor associated cholestatic hepatitis, jaundice and pruritus can be prolonged and progress to vanishing bile duct syndrome leading to cirrhosis and end stage liver disease. However, slow but eventually complete recovery is the more common outcome.
Case 2. Cholestatic hepatitis arising after 18 months of enalapril therapy.
[Modified from a case in the database of the Drug-Induced Liver Injury Network]
An 83 year old man with hypertension and coronary artery disease had been taking enalapril and metoprolol for 18 months when he developed fatigue, anorexia, pruritus and jaundice. He had a history of jaundice in his teens which resolved completely. He had also been a moderately heavy drinker much of his life, but quit drinking alcohol 20 years previously. He had no history of drug hypersensitivity or reactions. His other medical problems included hypothyroidism for which he had taken levothyroxine for 7 years, hypercholesterolemia for which he had taken simvastatin for 7 years and fenofibrate for 6 months. On examination, he was jaundiced but had no fever or peripheral manifestations of chronic liver disease. Laboratory tests showed a bilirubin of 5.8 mg/dL, ALT 70 U/L and alkaline phosphatase of 369 U/L; these tests had been repeatedly normal during the previous six months. Tests for hepatitis A, B and C were negative. He had low levels of antinuclear antibody (1:80) and a slight elevation in serum immunoglobulin G (1,734 mg/dL: normal <1,700 mg/dL). Magnetic resonance cholangiopancreatography showed no evidence of gallstones or biliary obstruction. A liver biopsy showed intrahepatic cholestasis suggestive of drug induced liver injury. Simvastatin was stopped and his enalapril, metoprolol and fenofibrate were continued. His serum bilirubin and alkaline phosphatase remained elevated. Three months after onset of jaundice, enalapril was stopped and he rapidly improved, his pruritus resolving and bilirubin falling to normal within two weeks. Serum alkaline phosphatase improved more slowly and was still mildly elevated 6 months later.
Key Points
| Medication: | Enalapril (dose not provided) |
|---|---|
| Pattern: | Cholestatic (R=0.6) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 16 months |
| Recovery: | Partial after 2 weeks |
| Other medications: | Simvastatin, fenofibrate, metoprolol, synthroid |
Laboratory Values
| Time After Starting | Weeks After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | Pre | 25 | 104 | 0.5 | Enalapril started |
| 12 months | 24 | 82 | 0.5 | Fenofibrate started | |
| 14 months | 15 | 114 | 0.4 | ||
| 16 months | 27 | 94 | 0.4 | ||
| 18 months | Pre | 70 | 369 | 5.8 | Simvastatin stopped |
| 19 months | 51 | 425 | 8.7 | ||
| 20 months | 97 | 272 | 4.7 | ||
| 21 months | 0 | 91 | 578 | 7.7 | Enalapril stopped |
| 3 days | 48 | 511 | 0.9 | ||
| 10 days | 40 | 423 | 0.7 | Fenofibrate stopped | |
| 2 weeks | 55 | 356 | 0.6 | ||
| 3 weeks | 44 | 314 | 0.6 | ||
| 22 months | 5 weeks | 71 | 285 | 0.7 | |
| 6 weeks | 42 | 211 | 0.7 | ||
| 23 months | 8 weeks | 52 | 228 | 0.5 | |
| Normal Values | <40 | <100 | <1.2 | ||
Comment
The patient developed a cholestatic hepatitis which was initially attributed to simvastatin. When he had not improved three months later, enalapril was stopped, and jaundice and pruritus improved within a few weeks. Serum ALT and alkaline phosphatase levels remained mildly elevated. Stopping fenofibrate appeared to have no effect. Eight months after stopping enalapril, the patient had no symptoms of liver disease but had mildly elevated serum enzymes, these tests having been repeatedly normal in the past. Had all medications been discontinued when he first developed jaundice, the role of enalapril versus simvastatin (or fenofibrate) would have been difficult to define. The long latency period (18 months) to onset of clinically apparent liver injury is uncommon, but has been described both for the ACE inhibitors such as enalapril and the statins such as simvastatin. The long latency associated cases of hepatotoxicity due to ACE inhibitors is typically, but not invariably, hepatocellular.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Enalapril – Generic, Vasotec®
DRUG CLASS
Angiotensin-Converting Enzyme Inhibitors
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
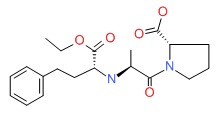
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Enalapril | 75847-73-3 | C20-H28-N2-O5 |
 |
- PubChem SubstanceRelated PubChem Substances
- Review Lisinopril.[LiverTox: Clinical and Researc...]Review Lisinopril.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Interaction between aspirin and angiotensin-converting enzyme inhibitors: should they be used together in older adults with heart failure?[J Am Geriatr Soc. 2002]Interaction between aspirin and angiotensin-converting enzyme inhibitors: should they be used together in older adults with heart failure?Ahmed A. J Am Geriatr Soc. 2002 Jul; 50(7):1293-6.
- Review Fosinopril.[LiverTox: Clinical and Researc...]Review Fosinopril.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ramipril.[LiverTox: Clinical and Researc...]Review Ramipril.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Captopril.[LiverTox: Clinical and Researc...]Review Captopril.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Enalapril - LiverToxEnalapril - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...