NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Droxidopa is an orally available prodrug of norepinephrine that is used in the treatment of symptomatic orthostatic hypotension due to neurogenic causes of autonomic failure. Droxidopa has had limited clinical use, but has not been linked to serum enzyme elevations nor to instances of clinically apparent acute liver injury.
Background
Droxidopa (drox" i doe' pa) is a prodrug of norepinephrine (noradrenaline) a major adrenergic neurotransmitter important in maintenance of sympathetic autonomic tone and normal blood pressure. Unlike norepinephrine, however, droxidopa does not cross the blood-brain barrier, so that its effects are restricted to the periphery where it acts on the smooth muscle of arteries and arterioles to maintain blood pressure, particularly in response to standing. In short term clinical trials, droxidopa showed modest effects in improving symptoms of postural hypotension in patients with neurogenic causes. The neurogenic causes of primary autonomic failure include Parkinson disease and multiple systemic atrophy (Shy Drager syndrome). Droxidopa was approved for use in the United States in 2014 and current recommendations are limited to treatment of orthostatic hypotension due to neurogenic causes. Droxidopa is available as capsules of 100, 200 and 300 mg. The typical initial dose is 100 mg three times daily, with subsequent titration based upon efficacy and tolerance to as much as 600 mg three times daily. Common side effects include headache, dizziness, nausea and hypertension. Rare instances of neuroleptic malignant syndrome have been reported with its use.
Hepatotoxicity
Liver test abnormalities have not been reported in patients taking droxidopa, but the agent has had limited clinical use. There were no episodes of clinically apparent liver injury reported in the preregistration trials of droxidopa, and since its approval there have been no published reports of droxidopa hepatotoxicity. Thus, liver injury from droxidopa is likely to be rare, if it occurs at all.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
Droxidopa is excreted largely unchanged in the urine and its hepatic metabolism is minimal, perhaps accounting for why hepatotoxicity is rare.
Drug Classes: Antiparkinson Agents, Other (Cardiovascular)
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Droxidopa – Northera®
DRUG CLASS
Antiparkinson Agents
Product labeling at DailyMed, National Library of Medicine, NIH
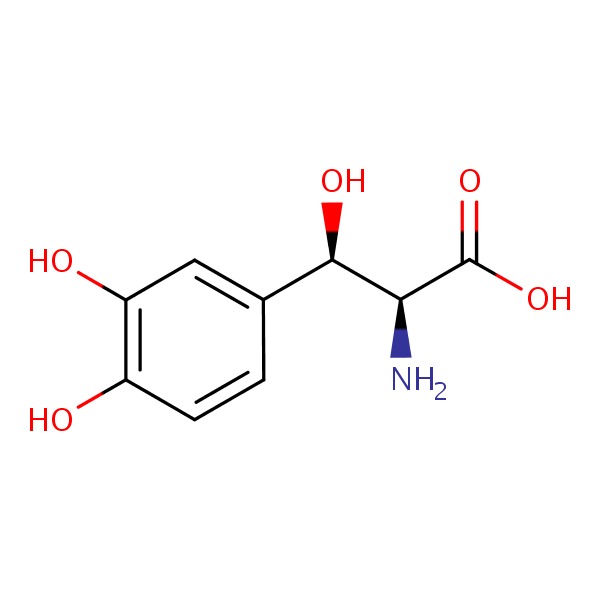
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Droxidopa | 23651-95-8 | C9-H11-N-O5 |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 17 April 2017
- Westfall TC, Westfall DP. Therapeutic uses of sympathomimetic drugs. Adrenergic agonists and antagonists. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 300-4.(Textbook of pharmacology and therapeutics).
- Kaufmann H, Freeman R, Biaggioni I, Low P, Pedder S, Hewitt LA, Mauney J, et al.; NOH301 Investigators. Droxidopa for neurogenic orthostatic hypotension: a randomized, placebo-controlled, phase 3 trial. Neurology 2014; 83: 328-35. [PMC free article: PMC4115605] [PubMed: 24944260](Among 263 patients with neurogenic orthostatic hypotension treated with droxidopa in varying doses [100 to 600 mg], 162 responders then received droxidopa or placebo for 1 week, during which symptoms improved more in droxidopa treated subjects and side effects included headache, dizziness and syncope, while laboratory results showed no “clinically significant trends”).
- Hauser RA, Isaacson S, Lisk JP, Hewitt LA, Rowse G. Droxidopa for the short-term treatment of symptomatic neurogenic orthostatic hypotension in Parkinson's disease (nOH306B). Mov Disord 2015; 30: 646-54. [PubMed: 25487613](Among 171 patients with orthostatic hypotension due to Parkinson disease who were treated with droxidopa or placebo for 10 weeks, symptoms of dizziness were improved with droxidopa therapy and side effects included headache, dizziness, nausea and hypertension; “Overall, …clinical laboratory values… showed no clinically significant trends or differences between treatment groups”).
- Vannorsdall MD, Hariachar S, Hewitt LA. A randomized, placebo-controlled, phase 2 study of the efficacy and safety of droxidopa in patients with intradialytic hypotension. Postgrad Med. 2015; 127: 133-43. [PubMed: 25708022](Among 85 patients on hemodialysis for end stage renal disease who had symptomatic hypotension between dialysis events who were treated with droxidopa [400 or 600 mg three times daily] showed no differences in mean arterial pressure, while side effects were more frequent on droxidopa included nausea, headache, fatigue, sleep disturbance and pruritus).
- Droxidopa (Northera) for neurogenic orthostatic hypotension. Med Lett Drugs Ther 2015; 57 (1471): 92-3. [PubMed: 26079764](Concise review of the mechanism of action, clinical efficacy, safety and costs of droxidopa shortly after its approval for use in the US; mentions side effects of hypertension, headache, dizziness and nausea, and possible severe side effects of neuroleptic malignant syndrome and exacerbation of ischemic heart disease and cardiac arrhythmias; no mention of ALT elevations or hepatotoxicity).
- Isaacson S, Vernino S, Ziemann A, Rowse GJ, Kalu U, White WB. Long-term safety of droxidopa in patients with symptomatic neurogenic orthostatic hypotension. J Am Soc Hypertens 2016; 10: 755-62. [PubMed: 27614923](Among 350 patients with neurogenic orthostatic hypotension treated with droxidopa [100 to 600 mg three times daily] for an average of 1 year, there were no liver related serious adverse events or deaths and “laboratory values showed no trends or consistent changes from baseline that were considered clinically significant”; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Droxidopa: a review of its use in symptomatic neurogenic orthostatic hypotension.[Drugs. 2015]Review Droxidopa: a review of its use in symptomatic neurogenic orthostatic hypotension.Keating GM. Drugs. 2015 Feb; 75(2):197-206.
- Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa.[Hypertension. 2015]Randomized withdrawal study of patients with symptomatic neurogenic orthostatic hypotension responsive to droxidopa.Biaggioni I, Freeman R, Mathias CJ, Low P, Hewitt LA, Kaufmann H, Droxidopa 302 Investigators. Hypertension. 2015 Jan; 65(1):101-7. Epub 2014 Oct 27.
- Droxidopa for Symptomatic Neurogenic Hypotension.[Cardiol Rev. 2017]Droxidopa for Symptomatic Neurogenic Hypotension.Ferguson-Myrthil N. Cardiol Rev. 2017 Sep/Oct; 25(5):241-246.
- Droxidopa and Reduced Falls in a Trial of Parkinson Disease Patients With Neurogenic Orthostatic Hypotension.[Clin Neuropharmacol. 2016]Droxidopa and Reduced Falls in a Trial of Parkinson Disease Patients With Neurogenic Orthostatic Hypotension.Hauser RA, Heritier S, Rowse GJ, Hewitt LA, Isaacson SH. Clin Neuropharmacol. 2016 Sep-Oct; 39(5):220-6.
- Review L-dihydroxyphenylserine (Droxidopa): a new therapy for neurogenic orthostatic hypotension: the US experience.[Clin Auton Res. 2008]Review L-dihydroxyphenylserine (Droxidopa): a new therapy for neurogenic orthostatic hypotension: the US experience.Kaufmann H. Clin Auton Res. 2008 Mar; 18 Suppl 1:19-24. Epub 2008 Mar 27.
- Droxidopa - LiverToxDroxidopa - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...