NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Doxylamine is a first generation antihistamine that is used for symptoms of allergic rhinitis and the common cold and as a short acting sedative. Doxylamine has not been linked to instances of clinically apparent acute liver injury.
Background
Doxylamine (dox il' a meen) is a first generation antihistamine that is used to treat the symptoms of allergic rhinitis and the common cold, including sneezing, cough, runny note, watery eyes and itching. Because of its sedating side effects, it is also used as a mild sleeping aid and sedative. Doxylamine belongs to the ethanolamine class of antihistamines (with clemastine and dimenhydrinate) and was approved for use in the United States in 1948. It is still widely used today, largely in combination with other agents such as dextromethorphan, pseudoephedrine, aspirin and acetaminophen in over-the-counter products for relief of symptoms of the common cold and allergic rhinitis. Representative brand names of products that include doxylamine include Alka-Seltzer Plus, Night Time Cold/Flu Relief, NyQuil, and Sleep Aid. The recommended adult oral dose is 25 to 50 mg. Common side effects include sedation, impairment of motor function, confusion, dizziness, blurred vision, dry mouth and throat, palpitations, tachycardia, abdominal distress, constipation and headache. Antihistamines can worsen urinary retention and glaucoma.
Hepatotoxicity
Despite widespread use over many decades, doxylamine has not been linked to liver test abnormalities or to clinically apparent liver injury. The reason for its safety may relate its short half-life and limited duration of use.
Likelihood score: E (unlikely to be a cause of clinically apparent liver injury).
References on the safety and potential hepatotoxicity of antihistamines are given together after the Overview section on Antihistamines.
Drug Class: Antihistamines
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Doxylamine – Generic, Sleep Aid®, Unisom®
DRUG CLASS
Antihistamines
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
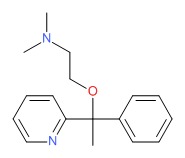
| Doxylamine | 469-21-6 | C17-H22-N2-O |
 |
- Review Triprolidine.[LiverTox: Clinical and Researc...]Review Triprolidine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- A clinical study to evaluate the efficacy of the antihistamine doxylamine succinate in the relief of runny nose and sneezing associated with upper respiratory tract infection.[J Pharm Pharmacol. 1995]A clinical study to evaluate the efficacy of the antihistamine doxylamine succinate in the relief of runny nose and sneezing associated with upper respiratory tract infection.Eccles R, Van Cauwenberge P, Tetzloff W, Borum P. J Pharm Pharmacol. 1995 Dec; 47(12A):990-3.
- Review Phenyltoloxamine.[LiverTox: Clinical and Researc...]Review Phenyltoloxamine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Clemastine.[LiverTox: Clinical and Researc...]Review Clemastine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Carbinoxamine.[LiverTox: Clinical and Researc...]Review Carbinoxamine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Doxylamine - LiverToxDoxylamine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...