NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Disopyramide is an oral antiarrhythmic agent that has been in wide use for several decades. Long term disopyramide therapy is associated with a low rate of serum enzyme elevations and is a rare cause of clinically apparent acute liver injury.
Background
Disopyramide (dye" soe pir' a mide) is a pyridine derivative and has electrophysiological effects that resemble quinidine (antiarrhythmic Class IA). Disopyramide decreases cardiac automaticity, increases refractory periods and slows conduction. Disopyramide is considered a myocardial depressant and can depress contractility. It also has anticholinergic effects. Disopyramide was approved for use in the United States in 1977, and current indications include suppression of symptomatic premature ventricular contractions and life threatening ventricular tachycardia. It is also effective in conversion as well as maintenance of normal sinus rhythm in patients with atrial fibrillation or flutter, but is not approved for this indication in the United States. Disopyramide is available in capsules of 100 and 150 mg generically and under the brand name Norpace. Extended release formulations are also available. The usual maintenance dose in adults is 400 to 800 mg daily in 2 to 4 divided doses. The most common side effects include dry mouth, urinary hesitancy, fatigue, headache, dizziness and anxiety.
Hepatotoxicity
In clinical trials, disopyramide was associated with a low rate of serum aminotransferase and alkaline phosphatase elevations. Despite wide scale use, disopyramide has only rarely been linked to cases of clinically apparent liver injury. Two types of hepatic injury have been described. The first is an acute hepatocellular injury that arises within 1 to 3 days of starting disopyramide and resembles acute hepatic ischemia with marked early rises in serum aminotransferase levels (and LDH), with minimal increase in alkaline phosphatase and subsequent rise in serum bilirubin. The prothrombin time may be abnormal initially. The injury resolves rapidly and may relate to sudden worsening of congestive heart failure due to the myocardial depressant effects of disopyramide.
The second type of injury is a cholestatic hepatitis that arises after 1 to 3 weeks of therapy and is characterized by jaundice and pruritus, with prominent elevations in serum alkaline phosphatase and mild-to-moderate increases in serum aminotransferase levels (Case 1). This injury may be prolonged, but reported cases have been self limited. Immunoallergic and autoimmune features are rare.
Likelihood score: B (rare but likely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which disopyramide causes liver injury is not clear but the early, acute hepatocellular injury is probably caused by ischemia due to sudden worsening of heart failure. The more delayed cholestatic hepatitis attributed to disopyramide is an idiosyncratic reaction and may relate to hypersensitivity to a product of its metabolism by the cytochrome P450 system (CYP 3A4).
Outcome and Management
The hepatic injury caused by disopyramide ranges from asymptomatic and transient serum enzyme elevations, to acute hepatic necrosis to cholestatic hepatitis. Disopyramide has not been linked to cases of acute liver failure, chronic hepatitis or vanishing bile duct syndrome. Following the cholestatic, idiosyncratic hepatic injury due to disopyramide, recurrence with reexposure is typical and should be avoided. Withdrawal of disopyramide therapy may be associated with relapse in the arrhythmia for which it is used and is often done in the hospital under careful monitoring. There is no evidence that there is cross sensitivity to hepatic injury between disopyramide and other oral antiarrhythmic agents.
Drug Class: Antiarrhythmic Agents
CASE REPORT
Case 1. Mild cholestatic hepatitis due to disopyramide.
[Modified from: Edmonds ME, Hayler AM. A case of intra-hepatic cholestasis after disopyramide therapy. Eur J Clin Pharmacol 1980; 18: 285-6. PubMed Citation]
A 74 year old woman developed jaundice 13 days after starting disopyramide (100 mg daily) for difficult-to-control atrial fibrillation. She gave no history of liver disease, alcohol abuse or risk factors for viral hepatitis. However, she had a complex medical history which included chronic obstructive pulmonary disease, right heart failure, gout and diverticulitis. Six weeks before the onset of jaundice, she had undergone an emergency colostomy for large bowel obstruction after perforation of a sigmoid diverticulum. The surgery was carried out under general anesthesia with thiopentone, fentanyl and nitrous oxide. Postoperatively, she received several antibiotics including ampicillin, metronidazole and cefuroxime. The medications that she took chronically were digoxin (0.125 mg), furosemide (240 mg), prednisolone (3 mg/day), and allopurinol (300 mg/day). On examination, she was jaundiced but had no fever, rash or clinical evidence of heart failure. Laboratory tests showed a serum bilirubin of 2.2 mg/dL, AST 30 U/L, alkaline phosphatase 840 U/L and GGT 840 U/L (normal 4-18 U/L). These tests had been normal earlier during her prolonged hospital admission (Table). Tests for hepatitis B and serum autoantibodies were negative. Plasma levels of disopyramide had been in the normal therapeutic range. Disopyramide and the antibiotics were discontinued. Her serum bilirubin improved over the next week, but alkaline phosphatase levels rose to above 2000 U/L and then began to fall slowly. Six weeks after stopping disopyramide, all liver tests were normal.
Key Points
| Medication: | Disopyramide (100 mg daily) |
| Pattern: | Cholestatic (R=0.2) |
|---|---|
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 2 weeks |
| Recovery: | 6 weeks |
| Other medications: | Ampicillin, metronidazole, cefuroxime, allopurinol, furosemide, digoxin, prednisolone, diazepam |
Laboratory Values
* Values estimated from Figure 1.
Comment
Despite the complexity of the medical history and exposure to multiple medications, the mild cholestatic hepatitis was entirely compatible with disopyramide induced liver injury which typically arises within 1-3 weeks of starting the medication and is characterized by a mild cholestatic course. Most of the other medications (except for ampicillin and metronidazole) were continued or restarted without recurrence of jaundice or liver test abnormalities.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Disopyramide – Generic, Norpace®
DRUG CLASS
Antiarrhythmic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Disopyramide | 3737-09-5 | C21-H29-N3-O |
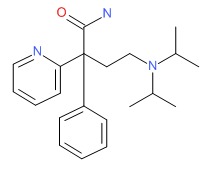 |
ANNOTATED BIBLIOGRAPHY
References updated: 04 January 2018
- Zimmerman HJ. Antiarrhythmics. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 642-4.(Expert review of hepatotoxicity published in 1999 mentions that disopyramide has been linked to at least 10 cases of drug-induced liver disease).
- De Marzio DH, Navarro VJ. Hepatotoxicity of cardiovascular and antidiabetic drugs. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 524.(Review of hepatotoxicity of cadriovascular drugs including antiarrhythmics; disopyramide is not discussed).
- Sampson KJ, Kass RS. Antiarrhythmic drugs. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 815-60.(Textbook of pharmacology and therapeutics).
- Riccioni N, Bozzi L, Susini N, Roni P. Disopyramide-induced intrahepatic cholestasis. Lancet 1977; 2: 1362-3. [PubMed: 74773](61 year old man developed pruritus 10 days after starting disopyramide followed by jaundice [3.0 mg/dL, ALT 410 U/L, Alk P 171 U/L, 55% eosinophils], resolving several months after stopping).
- Meinertz T, Langer KH, Kasper W, Just H. Disopyramide-induced intrahepatic cholestasis. Lancet 1977; 2: 828-9. [PubMed: 71640](38 year old woman with recurrent atrial fibrillation developed nausea within 3 days of starting disopyramide followed by jaundice [bilirubin 5.7 mg/dL, ALT 242 U/L, Alk P 1381 U/L], resolving several months later).
- Koch-Weser J. Disopyramide. N Engl J Med 1979; 300: 957-62. [PubMed: 431563](Review of disopyramide; mechanism of action is unclear, myocardial depressant and has anticholinergic activity which accounts for side effects of urinary retention, visual blurring, constipation and nausea; acute psychoses, cholestatic jaundice, hypoglycemia and agranulocytosis have occurred in rare patients).
- Tonkin AM, Joel SE, Reynolds JL. Unusual hepatocellular and cardiovascular complications of disopyramide. Chest 1980; 77:125. [PubMed: 7351137](74 year old male with ventricular arrhythmia developed abnormal liver enzymes 3 days after starting disopyramide [ALT 6200 U/L, Alk P 125 U/L, bilirubin 4.0 mg/dL, INR 2.6], resolving in 2 weeks).
- Craxi A, Gatto G, Maringhini A, Orsini S, Pinzello G, Pagliaro L. Disopyramide and cholestasis. Ann Intern Med 1980; 93: 150-1. [PubMed: 7396302](2 men and 1 woman, ages 60-79 years, developed cholestatic hepatitis 10 to 15 days after starting disopyramide [bilirubin 0.9, 14.6 and 16.9 mg/dL, ALT peak 65-120 U/L, Alk P 280-300 U/L], all resolving, but two had jaundice that lasted 4 and 7 months).
- Edmonds ME, Hayler AM. A case of intra-hepatic cholestasis after disopyramide therapy. Eur J Clin Pharmacol 1980; 18: 285-6. [PubMed: 7439249](74 year old woman developed jaundice 13 days after starting disopyramide [bilirubin 2.3 mg/dL, Alk P 840 rising to 2000 U/L, AST 30 U/L], resolving 6 weeks after stopping: Case 1).
- Scheinman SJ, Poll DS, Wolfson S. Acute cardiac failure and hepatic ischemia induced by disopyramide phosphate. Yale J Biol Med 1980; 53: 361-6. [PMC free article: PMC2595921] [PubMed: 7222741](2 men, ages 61 and 62 years, developed heart failure and raised serum enzymes within 1-2 days of starting disopyramide [AST 4780 and 1495 U/L, bilirubin peak 2.1 mg/dL, Alk P normal], resolving within 7-8 days).
- Doody PT. Disopyramide hepatotoxicity and disseminated intravascular coagulation. South Med J 1982; 75: 496-8. [PubMed: 7071649](55 year old woman developed nausea and cyanosis within 24 hours of starting disopyramide followed by confusion by day 4, ALT rising from 28 to 3090 U/L, Alk P 92 to 138 U/L, direct bilirubin to 1.6 mg/dL, protime to 40 seconds, with recovery in 10 days).
- Strathman I, Schubert EN, Cohen A, Nitzberg DM. Hypoglycemia in patients receiving disopyramide phosphate. Drug Intell Clin Pharm 1983; 17: 635-8. [PubMed: 6413187](Analysis of 32 reports of hypoglycemia in patients on disopyramide, 14 had liver disease; studies of glucose metabolism done in volunteers demonstrated a glucose lowering effect of disopyramide).
- Bakris GL, Cross PD, Hammarsten JE. Disopyramide-associated liver dysfunction. Mayo Clin Proc 1983; 58: 265-7. [PubMed: 6834895](61 year old man developed jaundice 21 days after starting disopyramide [bilirubin 7.7 mg/dL, Alk P 2274, ALT not given], resolving within next 3 months).
- Arif M, Laidlaw JC, Oshrain C, Willis PW 3rd, Nissen CH, McDermott DJ, Smith WS, et al. A randomized, double-blind, parallel group comparison of disopyramide phosphate and quinidine in patients with cardiac arrhythmias. Angiology 1983; 34: 393-400. [PubMed: 6408949](124 patients with ventricular arrhythmias received disopyramide or quinidine for 8 weeks; AST elevations occurred in 3 on quinidine and 1 on disopyramide but none had symptoms or jaundice).
- Antonelli D, Koltun B, Barzilay J. Acute hepatotoxic effect of disopyramide. Chest 1984; 86: 274. [PubMed: 6744970](46 year old developed abdominal pain 1 day after starting disopyramide [ALT 770 U/L, normal bilirubin and Alk P], resolving in 2 weeks).
- Bauman JL, Gallastegui J, Strasberg B, Swiryn S, Hoff J, Welch WJ, Bauernfeind RA. Long-term therapy with disopyramide phosphate: side effects and effectiveness. Am Heart J 1986; 111: 654-60. [PubMed: 3082173](Among 40 patients with arrhythmias treated with disopyramide long term, most common side effects were xerostomia, constipation, blurred vision and urinary hesitancy; no mention of liver injury or ALT elevations).
- Libersa C, Caron J, Pladys A, Beuscart R, Kacet S, Wajman A, Connell C, et al. Propafenone versus disopyramide: a double-blind randomized crossover trial in patients presenting chronic ventricular arrhythmias. Clin Cardiol 1987; 10: 405-10. [PubMed: 2440632](Ten patients with ventricular arrhythmias were treated with disopyramide vs propafenone vs placebo in a crossover study for 6 days each; no change in chemical parameters).
- Jonason T, Ringqvist I, Bandh S, Nilsson G, Nilsson H, Lidell C, Bjerle P, et al. Propafenone versus disopyramide for treatment of chronic symptomatic ventricular arrhythmias. A multicenter study. Acta Med Scand 1988; 223: 515-23. [PubMed: 3291557](38 patients with symptomatic ventricular arrhythmias were treated with either propafenone or disopyramide for 28 days; propafenone had fewer side effects; no mention of liver injury or ALT elevations).
- Roden DM. Antiarrhythmic drugs: from mechanisms to clinical practice. Heart 2000; 84: 339-46. [PMC free article: PMC1760959] [PubMed: 10956304](Overview of antiarrhythmic drugs which are separated in four classes based upon molecular target: I being sodium channel blockers; II beta blockers; III potassium channel blockers; and, IV calcium channel blockers; some agents having multiple targets).
- McNamara RL, Tamariz LJ, Segal JB, Bass EB. Management of atrial fibrillation: review of the evidence for the role of pharmacologic therapy, electrical cardioversion, and echocardiography. Ann Intern Med 2003; 139: 1018-33. [PubMed: 14678922](Systematic review of literature on efficacy of antiarrhythmic agents for different forms of arrhythmia; disopyramide has shown efficacy for maintenance of sinus rhythm after conversion from atrial fibrillation: hepatic side effects are not discussed).
- Drugs for cardiac arrhythmias. Treat Guidel Med Lett 2007; 5: 51-8. [PubMed: 17505408](Concise review of drugs for arrhythmias; disopyramide can aggravate heart failure and anticholinergic effects are often prominent; no mention of adverse effects on the liver).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease collected between 2004 and 2008 in the US, none were attributed to disopyramide).
- Treatment of atrial fibrillation. Treat Guidel Med Lett 2010; 8 (97): 65-70. [PubMed: 20733547](Concise review of efficacy and safety of drugs for atrial fibrillation; disopyramide is effective in maintaining sinus rhythm after cardioversion; side effects resemble those of anticholinergic drugs; no mention of hepatotoxicity or ALT elevations).
- Camm J. Antiarrhythmic drugs for the maintenance of sinus rhythm: risks and benefits. Int J Cardiol 2012; 155: 362-71. [PubMed: 21708411](Review of the safety and efficacy of antiarrhythmic drugs used to maintain normal sinus rhythm; no discussion of hepatotoxicity).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury from Latin American countries published between 1996 and 2012 identified 176 cases, but none attributed to disopyramide).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to disopyramide).
- Verlinden NJ, Coons JC. Disopyramide for hypertrophic cardiomyopathy: a pragmatic reappraisal of an old drug. Pharmacotherapy 2015; 35: 1164-72. [PubMed: 26684556](Review of the potential role of chronic disopyramide therapy for hypertrophic cardiomyopathy based upon analyses of 3 retrospective studies in 372 patients mentions common side effects including dry mouth, urinary retention and blurred vision due to the anitcholinergic effects of disopyramide, and cardiac arrhythmias attributed to drug induced prolongation of the QTc interval; no mention of ALT elevations or hepatotoxicity).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Flecainide.[LiverTox: Clinical and Researc...]Review Flecainide.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation.[Cochrane Database Syst Rev. 2019]Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation.Valembois L, Audureau E, Takeda A, Jarzebowski W, Belmin J, Lafuente-Lafuente C. Cochrane Database Syst Rev. 2019 Sep 4; 9(9):CD005049. Epub 2019 Sep 4.
- Review Mexiletine.[LiverTox: Clinical and Researc...]Review Mexiletine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide.[Drug Saf. 1990]Review Poisoning due to class IA antiarrhythmic drugs. Quinidine, procainamide and disopyramide.Kim SY, Benowitz NL. Drug Saf. 1990 Nov-Dec; 5(6):393-420.
- Effects of diet-induced hypokalaemia on the antiarrhythmic and electrophysiological actions of prolonged oral treatment with either amiodarone or disopyramide in the anaesthetised rat.[J Cardiovasc Pharmacol. 1987]Effects of diet-induced hypokalaemia on the antiarrhythmic and electrophysiological actions of prolonged oral treatment with either amiodarone or disopyramide in the anaesthetised rat.Winslow E, Marshall RJ, Hope FG. J Cardiovasc Pharmacol. 1987 Mar; 9(3):267-75.
- Disopyramide - LiverToxDisopyramide - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...