NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Dasatinib is a selective tyrosine kinase receptor inhibitor that is used in the therapy of chronic myelogenous leukemia (CML) positive for the Philadelphia chromosome. Dasatinib is commonly associated with transient elevations in serum aminotransferase levels during treatment, but with only rare instances of clinically apparent acute liver injury.
Background
Dasatinib is an orally available, small molecule inhibitor of the unique BCR-ABL tyrosine kinase receptor, which is the product of a fusion gene resulting from the translocation between chromosomes 9 and 22 that underlies the Philadelphia chromosome of chronic myelogenous leukemia (CML). The abnormal tyrosine kinase receptor is constitutively expressed and causes abnormal cell growth and proliferation. Inhibition of the enzyme can lead to dramatic reversal of progression of leukemia and is highly effective, although limited by the development of tumor resistance caused by mutations in the kinase. Dasatinib is actually a multi-kinase inhibitor and also has activity against scr, c-Kit and ephrin receptors, among others. Dasatanib received approval for use in the United States in 2006 and is one of five such specific inhibitors of BCR-ABL approved for clinical use, the others being imatinib [2001], nilotinib [2007], bosutinib [2012] and ponatinib [2012]. Dasatinib is available in tablets of 20, 50, 70, 80, 100 and 140 mg under the brand name Sprycel. Current indications are for treatment of Philadelphia chromosome-positive CML in the chronic, accelerated or blast phase usually after failure of a previous treatment. It is also approved for use in adutls with Philadelphia chromcsome-positive acute lymphoblastic leukemia. The typical dose is 100 to 140 mg daily. Common side effects include bone marrow suppression, fluid retention, diarrhea, headache, muscle cramps, arthralgias, fatigue, dyspnea, cough, pleural effusions and rash. Rare, but potentially severe side effects include bone marrow suppression, QTc prolongation, heart failure and pulmonary artery hypertension.
Hepatotoxicity
In large clinical trials, elevations in serum aminotransferase levels during dasatinib therapy occurred in up to 50% of patients, but were usually mild and self-limited. Elevations above 5 times the upper limit of normal (ULN) occurred in 1% to 9% of patients and generally responded to dose adjustment or temporary discontinuation and restarting at a lower dose, which is recommended if liver test results are markedly elevated (ALT or AST persistently greater than 5 times ULN or bilirubin more than 3 times ULN). While episodes of marked serum aminotransferase elevations with symptoms have been reported, there have been no published reports of clinically apparent liver injury with jaundice attributed to dasatinib therapy. Certainly other tyrosine kinase receptor inhibitors used in the therapy of CML such as imatinib, nilotinib and ponatinib have been associated with cases of acute liver injury with jaundice. With these agents, the liver injury typically arises after several months of therapy and the pattern of serum enzyme elevations is typically hepatocellular. Immunoallergic features (rash, fever and eosinophilia) and autoantibody formation are usually not present.
Reactivation of hepatitis B has been reported with dasatinib as well as imatinib and nilotinib therapy. Reactivation typically occurs in an HBsAg positive person treated with the tyrosine kinase inhibitor for 3 to 6 months, presenting with jaundice, marked serum aminotransferase elevations and an increase in HBV DNA levels. Reactivation of hepatitis B can be severe and fatal instances have been reported after imatinib and nilotinib therapy. Screening of patients for HBsAg and anti-HBc is sometimes recommended before starting cancer chemotherapy and those with HBsAg offered prophylaxis with oral antiviral agents, such as lamivudine, tenofovir or entecavir.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
Dasatinib is metabolized in the liver largely through the CYP 3A4 pathway and liver injury may be related to production of a toxic intermediate. Because of this pathway of metabolism, dasatinib is susceptible to drug-drug interactions when used with agents that induce or inhibit CYP 3A4.
Outcome and Management
Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to dose reduction or temporary cessation. In some situations, therapy can be restarted, particularly with concurrent prednisone (10 to 20 mg daily). In patients with clinically apparent liver injury and jaundice, restarting therapy should be done with caution. There does not appear to be cross reactivity with other tyrosine kinase inhibitors and switching to another tyrosine kinase receptor inhibitor may be the most appropriate approach. No cases of acute liver failure have occurred in patients receiving dasatinib, but instances have been reported with imatinib, nilotinib and ponatinib.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
Other Drugs in the Subclass, Chronic Myeloid Leukemia Agents: Bosutinib, Imatinib, Nilotinib, Omacetaxine, Ponatinib
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dasatinib – Sprycel®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
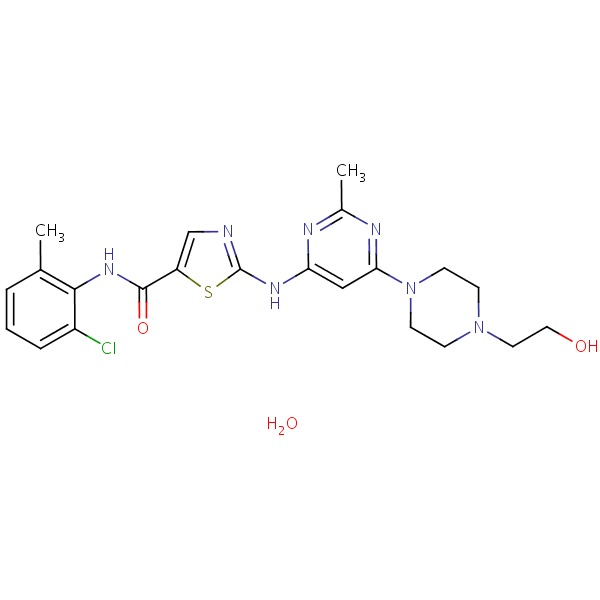
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Dasatinib | 863127-77-9 | C22-H26-Cl-N7-O2-S.H2-O |
 |
ANNOTATED BIBLIOGRAPHY
References updated: 05 December 2017
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of dasatinib and the tyrosine kinase inhibitors).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 541-67.(Review of hepatotoxicity of cancer chemotherapeutic agents; imatinib, gefitinib, erlotinib and crizotinib are discussed, but not dasatinib).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- Talpaz M, Shah NP, Kantarjian H, Donato N, Nicoll J, Paquette R, Cortes J, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med 2006; 354: 2531-41. [PubMed: 16775234](Among 84 patients with CML resistant or intolerant of imatinib therapy who were treated with dasatinib, hematologic responses occurred in 68 [81%]; side effects included diarrhea, nausea, dyspnea, pleural effusion and myelosuppression; no mention of ALT elevations or hepatotoxicity).
- Guilhot F, Apperley J, Kim DW, Bullorsky EO, Baccarani M, Roboz GJ, Amadori S, et al. Dasatinib induces significant hematologic and cytogenetic responses in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in accelerated phase. Blood 2007; 109: 4143-50. [PubMed: 17264298](Among 107 patients with CML in accelerated phase and resistant or intolerant of imatinib who were treated with dasatinib, common side effects were diarrhea [50%], nausea [22%], peripheral edema [22%], pleural effusions [23%] and fatigue [19%]; no mention of ALT elevations or hepatotoxicity).
- Dasatinib (Sprycel) for CML and Ph + ALL. Med Lett Drugs Ther 2007; 49 (1252): 6-7. [PubMed: 17220814](Concise summary of the mechanism of action, efficacy and safety of dasatinib shortly after its approval in the US).
- Bonvin A, Mesnil A, Nicolini FE, Cotte L, Michallet M, Descotes J, Vial T. Dasatinib-induced acute hepatitis. Leuk Lymphoma 2008; 49: 1630-2. [PubMed: 18608866](43 year old man with CML intolerant to imatinib therapy developed worsening fatigue and serum enzyme elevations 6 months after starting dasatinib therapy [bilirubin 1.5 mg/dL, ALT 758 U/L, Alk P 1432 U/L], which resolved within 3 weeks of stopping).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, one case was attributed to imatinib, but none to other tyrosine kinase inhibitors).
- Apperley JF, Cortes JE, Kim DW, Roy L, Roboz GJ, Rosti G, Bullorsky EO, et al. Dasatinib in the treatment of chronic myeloid leukemia in accelerated phase after imatinib failure: the START a trial. J Clin Oncol 2009; 27: 3472-9. [PMC free article: PMC4979080] [PubMed: 19487385](Among 174 patients with CML in accelerated phase who were resistant or intolerant of imatinib and were treated with dasatinib, hematologic responses occurred in 64%; common side effects were cytopenias and pleural effusions occurred in 27%; no mention of ALT elevations or hepatotoxicity).
- Shayani S. Dasatinib, a multikinase inhibitor: therapy, safety, and appropriate management of adverse events. Ther Drug Monit 2010; 32: 680-7. [PubMed: 20864900](Review of efficacy and safety of dasatinib mentions that "liver abnormalities, which typically are associated with imatinib intolerance, rarely were reported in patients taking dasatinib").
- Kantarjian H, Shah NP, Hochhaus A, Cortes J, Shah S, Ayala M, Moiraghi B, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2010; 362: 2260-70. [PubMed: 20525995](Among 519 newly diagnosed patients with CML in chronic phase treated with either dasatinib or imatinib, cytogenetic responses were more frequent with dasatinib and safety profiles were similar, pleural effusions occurring only with dasatinib and ALT elevations causing discontinuation in 2 patients on imatinib, but none on dasatinib).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, none of which were attributed to tyrosine kinase inhibitors).
- Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf 2013; 36: 491-503. [PubMed: 23620168](Review of the hepatotoxicity of 18 tyrosine kinase inhibitors approved for use in cancer in the US as of 2013; dasatinib is associated with ALT elevations in up to 50% of patients [above 5 times ULN in 1-9%], but has not been linked to cases of acute hepatitis or liver failure).
- Harbaum L, Marx A, Goekkurt E, Schafhausen P, Atanackovic D. Treatment with dasatinib for chronic myeloid leukemia following imatinib-induced hepatotoxicity. Int J Hematol 2014; 99: 91-4. [PubMed: 24264834](32 year old woman with CML developed jaundice 6 months after starting imatinib [bilirubin 2.8 rising to 9 mg/dL, ALT 1638 U/L, Alk P 94 U/L, INR 1.33], recovered on prednisolone within 6 weeks of stopping imatinib and did not have a recurrence when subsequently treated successfully with dasatinib).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [6%] were attributed to antineoplastic agents including 9 to kinase inhibitors such as imatinib, lapatinib and regorafinib).
- Sasaki K, Lahoti A, Jabbour E, Jain P, Pierce S, Borthakur G, Daver N, et al. Clinical safety and efficacy of nilotinib or dasatinib in patients with newly diagnosed chronic-phase chronic myelogenous leukemia and pre-existing liver and/or renal dysfunction. Clin Lymphoma Myeloma Leuk 2016; 16: 152-62. [PMC free article: PMC4769134] [PubMed: 26796981](Among 215 patients with CML treated with nilotinib or dasatinib for an median of 49 months, the presence of mild liver dysfunction had no apparent effect on response rates or adverse events including liver toxicity; ALT and AST levels "remained generally unchanged" with elevations above 5 times ULN occurring only in the nilotinib treatment arm [11% vs 0%]).
- Bunchorntavakul C, Reddy KR. Drug hepatotoxicity: newer agents. Clin Liver Dis 2017; 21: 115-34. [PubMed: 27842767](Overview of hepatotoxicity of newer agents including tyrosine kinase inhibitors such as imatinib, bosutinib, nilotinib, ponatinib and dasatinib, all of which have been implicated in causing ALT and AST elevations [in 23-50% of patients] as well as clinically apparent liver injury).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.[Clin Ther. 2007]Review Dasatinib: a tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia and philadelphia chromosome-positive acute lymphoblastic leukemia.Steinberg M. Clin Ther. 2007 Nov; 29(11):2289-308.
- Review Ponatinib.[LiverTox: Clinical and Researc...]Review Ponatinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Imatinib.[LiverTox: Clinical and Researc...]Review Imatinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Bosutinib.[LiverTox: Clinical and Researc...]Review Bosutinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Outcome of patients with chronic myeloid leukemia in lymphoid blastic phase and Philadelphia chromosome-positive acute lymphoblastic leukemia treated with hyper-CVAD and dasatinib.[Cancer. 2021]Outcome of patients with chronic myeloid leukemia in lymphoid blastic phase and Philadelphia chromosome-positive acute lymphoblastic leukemia treated with hyper-CVAD and dasatinib.Morita K, Kantarjian HM, Sasaki K, Issa GC, Jain N, Konopleva M, Short NJ, Takahashi K, DiNardo CD, Kadia TM, et al. Cancer. 2021 Aug 1; 127(15):2641-2647. Epub 2021 Apr 6.
- Dasatinib - LiverToxDasatinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...