NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Dalfampridine is a pyrimidine analogue used in the treatment of relapsing multiple sclerosis. Dalfampridine has had limited clinical use, but has not been linked to serum enzyme elevations during treatment nor to instances of clinically apparent liver injury with jaundice.
Background
Dalfampridine (dal fam' pri deen) is a pyrimidine analogue (4-aminopyridine) and potassium channel blocker that is used to improve mobility and walking speed in patients with multiple sclerosis. Dalfampridine appears to act by prolonging neuronal action potentials and thus improving conduction in demyelinated nerve fibers. Multiple clinical studies have demonstrated that dalfampridine (previously known as 4-aminopyridine) has a rapid onset of action increasing motor strength and mobility in 25% to 40% of patients with multiple sclerosis as well as other neuromuscular conditions. In clinical trials, the effect of dalfampridine has been sustained with long term use. It has no effect on the course of underlying disease in preventing relapses or slowing progression of disability. Dalfampridine was approved for use in the United States in 2010 and current indications are for symptomatic treatment of motor weakness in relapsing multiple sclerosis. Dalfampridine is available in tablets of 10 mg under the brand name Ampyra. The typical dose in adults is 10 mg twice daily. Side effects may include insomnia, dizziness, headache, nausea, fatigue, back pain and ataxia. Uncommon, but serious side effects include seizures, delirium and stupor. Actually, 4-aminopyridine is a well known neurotoxin and is used as an avicide. Overdoses in humans can cause seizures, stupor, coma and death.
Hepatotoxicity
Dalfampridine has been associated with infrequent serum aminotransferase elevations during therapy and has not been convincingly linked to instances of clinically apparent liver injury. In analyses of safety of dalfampridine in pre-registration controlled trials with 1922 patients with multiple sclerosis, there were no reports of hepatic injury or laboratory evidence of a hepatotoxicity signal. Nevertheless, a few instances of clinically apparent liver injury in patients receiving dalfampridine have appeared in the published literature. In each instance, however, attribution to dalfampridine was not convincing.
Likelihood score: E* (unproven although suspected rare cause of clinically apparent liver injury).
Mechanism of Injury
The possible mechanisms by which dalfampridine could be hepatotoxic are not clear. Dalfampridine is eliminated largely unchanged in the urine and appears to have little hepatic metabolism.
Drug Class: Multiple Sclerosis Agents (Symptomatic Therapies)
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Dalfampridine – Generic, Ampyra®
DRUG CLASS
Multiple Sclerosis Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Dalfampridine | 504-24-5 | C5-H6-N2 |
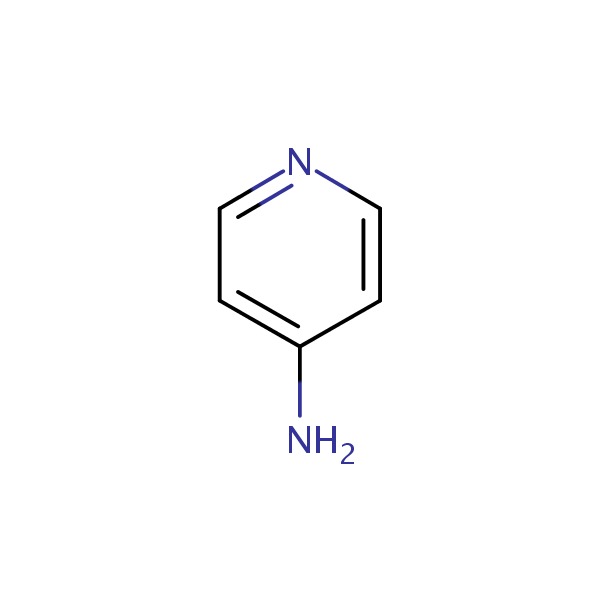 |
ANNOTATED BIBLIOGRAPHY
References updated: 13 November 2017
- Zimmerman HJ. Unconventional drugs. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 731-4.(Expert review of hepatotoxicity published in 1999 before the availability of dalfampridine).
- Larrey D, Ripault M-P. Anxiolytic agents. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 455-6.(Review of hepatotoxicity of hypnotics and sedatives, does not discuss dalfampridine).
- Krensky AM, Bennett WM, Vincenti F. A case study: immunotherapy for multiple sclerosis. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1025-7.(Textbook of pharmacology and therapeutics).
- Davis FA, Stefoski D, Rush J. Orally administered 4-aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol 1990; 27: 186-92. [PubMed: 2317014](Single dose study of 10 or 25 mg of oral dalfampridine or placebo in 20 subjects with multiple sclerosis showed rapid but reversible improvement in motor function in most patients given active drug, without serious adverse effects and no improvement in placebo recipients).
- Stefoski D, Davis FA, Fitzsimmons WE, Luskin SS, Rush J, Parkhurst GW. 4-Aminopyridine in multiple sclerosis: prolonged administration. Neurology 1991; 41: 1344-8. [PubMed: 1891078](Prolonged administration of oral dalfampridine or placebo 26 subjects with multiple sclerosis in different dose regimens for 5 days showed motor and visual improvement with active drug and little change with placebo; no serious adverse events occurred).
- van Diemen HA, Polman CH, van Dongen TM, van Loenen AC, Nauta JJ, Taphoorn MJ, van Walbeek HK, et al. The effect of 4-aminopyridine on clinical signs in multiple sclerosis: a randomized, placebo-controlled, double-blind, cross-over study. Ann Neurol 1992; 32: 123-30. [PubMed: 1510353](Among 70 patients with multiple sclerosis treated with dalfampridine or placebo for 12 weeks and 63 crossed over to opposite treatment, side effects included paresthesias, dizziness, ataxia, nausea and headache, without any changes in laboratory test results).
- Bever CT Jr, Anderson PA, Leslie J, Panitch HS, Dhib-Jalbut S, Khan OA, Milo R, et al. Treatment with oral 3,4 diaminopyridine improves leg strength in multiple sclerosis patients: results of a randomized, double-blind, placebo-controlled, crossover trial. Neurology 1996; 47: 1457-62. [PubMed: 8960727](Among 36 patients with multiple sclerosis treated with dalfampridine or placebo for 16 weeks, neurologic improvements occurred in most patients on active drug, but side effects were frequent, 8 patients withdrew, 3 had episodes of confusion and one had seizures).
- Polman CH, Bertelsmann FW, van Loenen AC, Koetsier JC. 4-aminopyridine in the treatment of patients with multiple sclerosis. Long-term efficacy and safety. Arch Neurol 1994; 51: 292-6. [PubMed: 8129642](Among 23 patients with multiple sclerosis treated with dalfampridine for more than 6 months, one 60 year old woman developed jaundice 6 months after starting dalfampridine but 6 weeks after receiving high doses of intravenous methylprednisolone [bilirubin 2.0, ALT 819 U/L, GGT 199 U/L], which resolved uneventfully within 3 months).
- Johnson NC, Morgan MW. An unusual case of 4-aminopyridine toxicity. J Emerg Med 2006; 30: 175-7. [PubMed: 16567254](56 year old man with diabetic neuropathy rubbed dalfampridine on a sore tooth and rapidly developed delirium and coma, atrial fibrillation and cardiac arrhythmias, which resolved after 3 to 4 days).
- Burton JM, Bell CM, Walker SE, O'Connor PW. 4-aminopyridine toxicity with unintentional overdose in four patients with multiple sclerosis. Neurology 2008; 71: 1833-4. [PubMed: 19029525](3 patients with multiple sclerosis being treated with dalfampridine developed seizures and status epilepticus hours after starting a new, locally compounded prescription, which later was found to have 90-126 mg per tablet instead of the intended 10 mg).
- Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, Marinucci LN, Blight AR; Fampridine MS-F203 Investigators. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet 2009; 373 (9665): 732-8. [PubMed: 19249634](In a placebo controlled trial of 301 patients with multiple sclerosis treated for 14 weeks, walking time improved significantly in 35% of dalfampridine vs 5% of placebo recipients, and "some patients had clinically significant changes during treatment for laboratory values... but there were no clear trends within or differences between treatment groups").
- Traynor K. Dalfampridine approved for MS. Am J Health Syst Pharm 2010; 67: 335. [PubMed: 20172978](News report; in clinical trials conducted in 540 patients with multiple sclerosis, dalfampridine was found to increase walking speed, while the most common side effects were urinary tract infections, dizziness, gastrointestinal upset, headache, insomnia, stuffy nose, throat pain and weakness; no mention of ALT elevations or hepatotoxicity).
- Goodman AD, Brown TR, Edwards KR, Krupp LB, Schapiro RT, Cohen R, Marinucci LN, Blight AR; MSF204 Investigators. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol 2010; 68: 494-502. [PubMed: 20976768](Among 239 patients with multiple sclerosis treated with dalfampridine or placebo for 9 weeks, the average improvement in walking time with therapy was 25% and "there were no notable differences between treatment groups with respect to laboratory values").
- Dalfampridine (Ampyra) for MS. Med Lett Drugs Ther 2010; 52 (1347): 73-4. [PubMed: 20847716](Concise review of the mechanism of action, efficacy, safety and costs of dalfampridine shortly after its approval in the US, mentions common side effects were urinary tract infection, insomnia, dizziness, head nausea, fatigue, back pain and ataxia; no mention of ALT elevations or hepatotoxicity).
- Cornblath DR, Bienen EJ, Blight AR. The safety profile of dalfampridine extended release in multiple sclerosis clinical trials. Clin Ther 2012; 34: 1056-69. [PubMed: 22497693](Review of the literature on the safety of dalfampridine focused on common side effect symptoms; "although a few clinically significant changes in...laboratory values were observed across the studies, there did not appear to be any clinically significant trends within or differences among treatment groups...").
- King AM, Menke NB, Katz KD, Pizon AF. 4-aminopyridine toxicity: a case report and review of the literature. J Med Toxicol 2012; 8: 314-21. [PMC free article: PMC3550165] [PubMed: 22782458](37 year old man with progressive multiple sclerosis developed diaphoresis, delirium, agitation and choreathetoid movements after a suspected overdose of dalfampridine requiring intubation, but ultimately resolving after 5-6 days; no mention of liver test abnormalities).
- Prugger M, Berger T. Assessing the long-term clinical benefit of prolonged-release fampridine tablets in a real-world setting: a review of 67 cases. Patient Relat Outcome Meas 2013; 4: 75-85. [PMC free article: PMC3810492] [PubMed: 24187513](Among 67 patients with multiple sclerosis treated with dalfampridine and followed for up to 6 months, adverse events leading to discontinuation included nausea and insomnia; no mention of ALT elevations or hepatotoxicity).
- McLean MK, Khan S. A review of 29 incidents involving 4-aminopyridine in non-target species reported to the ASPCA Animal Poison Control Center. J Med Toxicol 2013; 9: 418-21. [PMC free article: PMC3846978] [PubMed: 24129835](4-aminopyrine is a bird poison used for bird control in airports and feedlots, but can also poison mammals; report of 29 cases of toxicosis from the poison, mostly in dogs, causing neurologic symptoms and at least 1 death).
- Jara M, Barker G, Henney HR 3rd. Dalfampridine extended release tablets: 1 year of postmarketing safety experience in the US. Neuropsychiatr Dis Treat 2013; 9: 365-70. [PMC free article: PMC3647381] [PubMed: 23662056](Summary of spontaneous adverse event reports attributed to dalfampridine during the first year of marketing included 11,549 adverse events in ~46,000 patients, mostly dizziness, insomnia, ataxia, headache and nausea, but also 85 seizures, 1 case of hepatitis and 1 of hepatic failure; but no details given).
- Ruck T, Bittner S, Simon OJ, Gö K, Wiendl H, Schilling M, Meuth SG. Long-term effects of dalfampridine in patients with multiple sclerosis. J Neurol Sci 2014; 337: 18-24. [PubMed: 24290498](Among 30 patients with multiple sclerosis treated with dalfampridine for 9-12 months, beneficial effects on mobility and fatigue were maintained and no new adverse side effects were identified).
- Yapundich R, Applebee A, Bethoux F, Goldman MD, Hutton GJ, Mass M, Pardo G, et al. Evaluation of dalfampridine extended release 5 and 10 mg in multiple sclerosis: a randomized controlled trial. Int J MS Care 2015; 17: 138-45. [PMC free article: PMC4455866] [PubMed: 26052259](430 patients with multiple sclerosis treated with dalfampridine [5 or 10 mg twice daily] or placebo for 4 weeks showed slight improvements in walking speed during dalfampridine therapy compared with placebo, but only with the higher dose; no mention of ALT elevations or hepatotoxicity).
- Goodman AD, Bethoux F, Brown TR, Schapiro RT, Cohen R, Marinucci LN, Henney HR 3rd, et al; MS-F203, MS-F204, and Extension Study Investigators. Long-term safety and efficacy of dalfampridine for walking impairment in patients with multiple sclerosis: Results of open-label extensions of two Phase 3 clinical trials. Mult Scler 2015 Jan 12 [Epub ahead of print] [PMC free article: PMC4561451] [PubMed: 25583832](Among 483 patients with multiple sclerosis participating in controlled trials of dalfampridine who were enrolled in long term extension studies for up to 5 years, improvements in walking speed tended to decrease over time, and "no new safety signals emerged and no patterns were observed in regard to laboratory findings").
- Goodman AD, Brown TR, Schapiro RT, Klingler M, Cohen R, Blight AR. A pooled analysis of two phase 3 clinical trials of dalfampridine in patients with multiple sclerosis. Int J MS Care 2014; 16: 153-60. [PMC free article: PMC4204376] [PubMed: 25337058](Among 540 patients with multiple sclerosis enrolled in two placebo controlled trials of dalfampridine, "no notable patterns were observed between treatment groups with respect to laboratory values").
- Ballesta Méez M, van Pesch V, Capron A, Hantson P. Prolonged toxic encephalopathy following accidental 4-aminopyridine overdose. Case Rep Neurol Med 2014; 2014: 237064. [PMC free article: PMC4009212] [PubMed: 24822136](58 year old woman with multiple sclerosis developed abdominal pain, stupor and generalized seizures a few minutes after taking 80 mg of dalfampridine [due to an incorrect formulation], ultimately requiring intubation and prolonged ICU stay with prolonged hospitalization and residual neurologic defects).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 7 [0.8%] were attributed to interferon beta, but none were linked to dalfampridine or other drugs for multiple sclerosis).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Ozanimod.[LiverTox: Clinical and Researc...]Review Ozanimod.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ponesimod.[LiverTox: Clinical and Researc...]Review Ponesimod.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Siponimod.[LiverTox: Clinical and Researc...]Review Siponimod.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Dalfampridine improves walking speed, walking endurance, and community participation in veterans with multiple sclerosis: a longitudinal cohort study.[Mult Scler. 2014]Dalfampridine improves walking speed, walking endurance, and community participation in veterans with multiple sclerosis: a longitudinal cohort study.Cameron MH, Fitzpatrick M, Overs S, Murchison C, Manning J, Whitham R. Mult Scler. 2014 May; 20(6):733-8. Epub 2013 Oct 7.
- Review Multiple Sclerosis Agents.[LiverTox: Clinical and Researc...]Review Multiple Sclerosis Agents.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Dalfampridine - LiverToxDalfampridine - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...