NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Clorazepate is a benzodiazepine used as an anticonvulsant as adjunctive therapy in management of epilepsy and as an anxiolytic for therapy of anxiety and alcohol withdrawal. Therapy with clorazepate is not associated with serum aminotransferase elevations, and cases of clinically apparent liver injury from clorazepate have been reported but are very rare.
Background
Clorazepate (klor az' e pate) is a benzodiazepine with particular activity against spread of seizure activity in several animal models. The antiseizure activity of the benzodiazepines is mediated by their ability to enhance gamma-aminobutryic acid (GABA) mediated inhibition of synaptic transmission through binding to the GABA A receptor. Clorazepate is used both as an anticonvulsant and anxiolytic agent. Clorazepate was approved in the United States in 1972 and currently more than 3 million prescriptions are filled yearly. Current indications are as adjunctive therapy in management of partial seizures and for treatment of anxiety disorders and acute alcohol withdrawal. Clorazepate is available in generic forms and under the brand name Tranxene in tablets and capsules in concentrations of 3.75, 7.5, 11.25, 15 and 22.5 mg. Delayed release formulations are also available. The recommended initial dose for adults with seizures is 7.5 mg three times daily, with gradual dose increases generally to an average dose of 30 to 60 mg daily and not in excess of 90 mg daily. Common side effects of clorazepate include drowsiness, lethargy, ataxia, dysarthria and dizziness. Tolerance develops to these side effects, but tolerance can also develop to the therapeutic effects.
Hepatotoxicity
Clorazepate, as with other benzodiazepines, is rarely associated with serum ALT elevations, and clinically apparent liver injury from clorazepate is extremely rare. A few only partially convincing case reports of acute hepatocellular injury from clorazepate have been reported. Rare instances of drug induced liver injury have been reported with other benzodiazepines, such as chlordiazepoxide, diazepam, flurazepam, triazolam and alprazolam. In benzodiazepine related cases of acute liver injury, the latency has ranged from a few weeks to 6 months; the typical pattern of liver enzyme elevations has been cholestatic or mixed, but instances of hepatocellular patterns have also been reported. The injury is usually mild to moderate in severity and self-limited. Fever and rash have not been described nor has autoantibody formation.
Likelihood score: D (possible but rare cause of clinically apparent liver injury).
Mechanism of Injury
The liver injury from benzodiazepines is probably due to a rarely produced intermediate metabolite.
Outcome and Management
The few case reports of hepatic injury due to clorazepate were followed by complete recovery. No cases of acute liver failure or vanishing bile duct injury due to clorazepate have been described. There is no information about cross reactivity with other benzodiazepines (clonazepam, lorazepam or diazepam), but some degree of cross sensitivity should be assumed.
Drug Class: Anticonvulsants, Benzodiazepines
CASE REPORT
Case 1. Acute hepatitis-like injury due to clorazepate.
[Modified from: Parker JLW. Potassium clorazepate (Tranxene)-induced jaundice. Postgrad Med J 1979; 55: 908-910. PubMed Citation]
A 27 year old man developed jaundice and fever, 2 months after starting clorazepate (15 mg increasing 30 mg once daily) for depression. He had poor appetite and had lost 9.5 kilograms since starting the drug. Clorazepate was continued for another two months, at which time he was found to have jaundice and hepatomegaly. Blood tests showed total bilirubin of 8.6 mg/dL, ALT 880 U/L, AST 620 U/L, GGT 104 U/L (normal <45) and alkaline phosphatase 29 KA units (normal <13). He had no rash, fever or eosinophilia and autoantibodies were negative. Clorazepate was stopped. He underwent three liver biopsies over the next five months showing an acute cholestatic hepatitis with gradual resolution, but residual minimal portal lymphocytic infiltrates and thin portal-to-portal fibrosis.
Key Points
| Medication: | Clorazepate |
| Pattern: | Hepatocellular (R=16) |
| Severity: | 3+ (jaundice and hospitalization) |
| Latency: | 2 months |
| Recovery: | Complete recovery over 5 months |
| Other: | None mentioned |
Comment
Acute hepatitis with cholestatic feature arose between 2 and 4 months after starting clorazepate. The pattern of liver enzyme elevations suggested hepatocellular injury. Liver biopsies showed mild fibrosis that was still present five months later. Tests to exclude hepatitis A and B were not done and tests for hepatitis C were not available when this case was reported.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Clorazepate – Tranxene®
DRUG CLASS
Anticonvulsants
COMPLETE LABELING
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Clorazepate Dipotassium | 57109-90-7 | C16-H10-Cl-N4-O3.K.H-K-O |
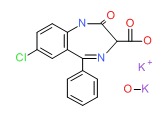 |
REFERENCES
References updated: 25 January 2017
- Zimmerman HJ. Benzodiazepines. Psychotropic and anticonvulsant agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 491-3.(Expert review of benzodiazepines and liver injury published in 1999; mentions rare instances of cholestatic hepatitis have been reported due to alprazolam, chlordiazepoxide, clonazepam, diazepam, flurazepam, and triazolam, and hepatocellular injury with clorazepate, but no reports of hepatic injury with lorazepam, oxazepam or temazepam).
- Larrey D, Ripault M-P. Benzodiazepines. Hepatotoxicity of psychotropic drugs and drugs of abuse. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 455.(Review of drug induced liver injury mentions that there have been rare instances of acute liver injury [usually cholestatic] reported with alprazolam, bentazepam, chlordiazepoxide, clonazepam, clorazepate, clotiazepam, diazepam, flurazepam, and triazolam; a hepatitis-like pattern was reported with alprazolam and diazepam).
- Pirmohamed M, Leeder SJ. Anticonvulsant agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013: pp 423-42.(Review of anticonvulsant induced liver injury; benzodiazepines are not discussed).
- McNamara JO. Pharmacology of the epilepsies. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 583-607.(Textbook of pharmacology and therapeutics).
- Mihic SJ, Harris RA. Hypnotics and sedatives. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 457-80.(Textbook of pharmacology and therapeutics).
- Parker JLW. Potassium clorazepate(Tranxene)-induced jaundice. Postgrad Med J 1979; 55: 908-10. [PMC free article: PMC2425684] [PubMed: 44913](27 year old man developed jaundice, pruritus and fever, 2 months after starting clorazepate [bilirubin 7.6 mg/dL, ALT 880 U/L, Alk P 2.3 times ULN] and had 3 liver biopsies done over 5 months showing intrahepatic cholestasis with thin portal-portal fibrosis septae that persisted as cholestasis and inflammation resolved).
- Bonkowsky HL, Sinclair PR, Emery S, Sinclair JF. Seizure management in acute hepatic porphyria: risks of valproate and clonazepam. Neurology 1980; 30: 588-92. [PubMed: 6770287](38 year old man with acute intermittent porphyria and seizures did not respond to clonazepam, and testing in chicken embryos showed that it increased hepatic prophyrins and ALA synthase activity).
- Døssing M, Andreasen PB. Drug-induced liver disease in Denmark. An analysis of 572 cases of hepatotoxicity reported to the Danish Board of Adverse Reactions to Drugs. Scand J Gastroenterol 1982; 17: 205-11. [PubMed: 6982502](Among 572 cases of hepatotoxicity reported to a Danish registry between 1968 and 1978, 97 were due to psychotropic agents, but only two attributed to benzodiazepines).
- Keränen T, Sivenius J. Side effects of carbamazepine, valproate and clonazepam during long-term treatment of epilepsy. Acta Neurol Scand Suppl 1983; 97: 69-80. [PubMed: 6424398](Clonazepam has many dose related sedative side effects, but compared to carbamazepine and valproate, few serious or long term side effects; no mention of hepatic side effects of clonazepam).
- Davion T, Capron-Chivrac D, Andrejak M, Capron JP. [Hepatitis due to antiepileptic agents] Gastroenterol Clin Biol 1985; 9: 117-26. [PubMed: 3920108](Review of hepatotoxicity of anticonvulsants; among benzodiazepines, cases of cholestatic hepatitis have been linked to chlordiazepoxide and diazepam, but liver injury from this class of drugs is rare).
- Olsson R, Zettergren L. Anticonvulsant-induced liver damage. Am J Gastroenterol 1988; 83: 576-7. [PubMed: 3364416](30 year old man developed fever, rash and jaundice 6 weeks after starting phenytoin; he was switched to carbamazepine and clonazepam, but redeveloped jaundice 3 months later; resolved with stopping, but recurred with restarting clonazepam alone [bilirubin 1.8 mg/dL, ALT 1380 U/L, Alk P 176 U/L] without rash, fever or eosinophilia; resolving in 6 weeks of stopping clonazepam).
- Suzuki A, Aso K, Ariyoshi C, Ishimaru M. Acute intermittent porphyria and epilepsy: safety of clonazepam. Epilepsia 1992; 33: 108-11. [PubMed: 1733741](13 year old girl with acute intermittent porphyria worsened on valproate and on phenytoin therapy, but clonazepam led to control of seizures and no worsening of porphyria).
- Wallace SJ. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf 1996; 15: 378-93. [PubMed: 8968693](Systematic review; ALT elevations occur in 4% of children on phenytoin, 6% on valproate, 1% on carbamazepine; “No child taking… benzodiazepines had raised liver enzyme levels,”).
- Lewis JH, Zimmerman HJ. Drug- and chemical-induced cholestasis. Clin Liver Dis 1999; 3: 433-64, vii. [PubMed: 11291233](Review of drug induced cholestatic syndromes, listing many causes including chlordiazepoxide and flurazepam; “Benzodiazepines may cause cholestatic injury, although this is rare”).
- Selim K, Kaplowitz N. Hepatotoxicity of psychotropic drugs. Hepatology 1999; 29: 1347-51. [PubMed: 10216114](Review of hepatotoxicity of phenothiazines, butyrophenones, tricyclics, MAO inhibitors, acetylcholesterase inhibitors, and psychotropic drugs of abuse; “benzodiazepines…have a very low hepatotoxic potential, with only case reports in the literature, usually with a cholestatic pattern).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl 2004; 10: 1018-23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 137 [0.2%] were done for idiosyncratic drug induced acute liver failure, of which 10 were attributed to phenytoin, 10 to valproate and 1 to carbamazepine, but none to benzodiazepines).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol 2005; 40: 1095-101. [PubMed: 16165719](36 years of reporting to Swedish registry identified 103 cases of acute liver failure due to drugs, of which 1 was attributed to phenytoin, 1 to valproate and 1 to carbamazepine, but none to benzodiazepines).
- Gil-Martin A, Saez-Royuela F, Arias L, Angulo ML, Nogal B. [Hepatic fibrosis after antidepressant treatment] Rev Esp Enferm Dig 2005; 97: 461-2. Spanish. [PubMed: 16048430](32 year old woman developed pruritis and jaundice 1-2 months after starting sertraline, aprazolam and clorazepate, resolving with stopping sertraline, but had persistent minor ALT elevations and biopsy showing mild bridging fibrosis 4 months later).
- Andrade RJ, Lucena MI, Kaplowitz N, García-Muņoz B, Borraz Y, Pachkoria K, García-Cortés M, et al. Outcome of acute idiosyncratic drug-induced liver injury: Long-term follow-up in a hepatotoxicity registry. Hepatology. 2006; 44: 1581-8. [PubMed: 17133470](28 of 493 [5.7%] cases of drug induced liver disease had evidence of chronicity, including 3 cases due to bentazepam and one with clorazepate, the latter with recovery at 25 months).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther 2007; 25: 1401-9. [PubMed: 17539979](Among 126 cases of drug induced liver injury seen in Spain between 1993-2000, 20 were attributed to benzodiazepines including 5 for clorazepate, 5 alprazolam, 6 lorazepam and 4 diazepam, but compared to controls, relative risk of injury was increased only for clorazepate [8.3: estimated frequency 3.4 per 100,000 person-year exposures]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, none were attributed to a benzodiazepine).
- Björnsson E. Hepatotoxicity associated with antiepileptic drugs. Acta Neurol Scand 2008; 118: 281-90. [PubMed: 18341684](Review of hepatotoxicity of all anticonvulsants focusing upon phenytoin, valproate, carbamazepine; “Furthermore, hepatoxicity has not been convincingly shown to be associated with the use of benzodiazepines”).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol 2010; 70: 721-8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children; benzodiazepines were not among the top 40 agents implicated).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were linked to benzodiazepine use).
- Wick JY. The history of benzodiazepines. Consult Pharm 2013; 28: 538-48. [PubMed: 24007886](History of the development, approval, widescale use and eventual restriction and decrease in use of the benzodiazepines).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology 2013; 144: 1419-25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, but none were linked to benzodiazepines).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol 2014; 13: 231-9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, but none were attributed to a benzodiazepine).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 40 [4.5%] were attributed to anticonvulsants, but none to benzodiazepine anticonvulsants).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Clorazepate therapy for intractable epilepsy.[Brain Dev. 1987]Clorazepate therapy for intractable epilepsy.Fujii T, Okuno T, Go T, Ochi J, Hattori H, Kataoka K, Mikawa H. Brain Dev. 1987; 9(3):288-91.
- Review Clonazepam.[LiverTox: Clinical and Researc...]Review Clonazepam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade.[Psychopharmacology (Berl). 2005]Anxiolytic properties of agomelatine, an antidepressant with melatoninergic and serotonergic properties: role of 5-HT2C receptor blockade.Millan MJ, Brocco M, Gobert A, Dekeyne A. Psychopharmacology (Berl). 2005 Feb; 177(4):448-58. Epub 2004 Jul 31.
- Evaluation of clorazepate (Tranxene) as an anticonvulsant--a pilot study.[Neurology. 1979]Evaluation of clorazepate (Tranxene) as an anticonvulsant--a pilot study.Troupin AS, Friel P, Wilensky AJ, Morretti-Ojemann L, Levy RH, Feigl P. Neurology. 1979 Apr; 29(4):458-66.
- Review Clobazam.[LiverTox: Clinical and Researc...]Review Clobazam.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Clorazepate - LiverToxClorazepate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...