NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Cilostazol is a quinolinone derivative that inhibits specific cellular phosphadiesterases, which cause arterial vasodilation and inhibition of platelet function and makes it a valuable as a therapy of intermittent claudication and as a means of secondary prevention of stroke. Cilostazol has not been associated with serum enzyme elevations during therapy or with published instances of clinically apparent liver injury.
Background
Cilostazol (sye loe' sta zol) acts by semi-selective inhibition of phosphodiesterase III, which causes an increase in intracellular cyclic AMP resulting in arterial vasodilation and inhibition of platelet aggregation. Cilostazol is used largely to treat symptoms of intermittent claudication due to peripheral vascular disease where it has been shown to increase pain-free walking distance and improve exercise tolerance. Cilostazol has also been evaluated as an antiplatelet agent and is used off-label as a means of prevention of recurrent stroke. Cilostazol was approved in the United States in 1999 and is widely used with more than one million prescriptions filled yearly. Current indications are limited to treatment of symptoms of intermittent claudication. Cilostazol is available in tablets of 50 and100 mg in several generic forms and under the brand name Pletal. The recommended maintenance dose is 100 mg twice daily. Side effects can include headache, dizziness, tachycardia, palpitations, diarrhea and peripheral edema. Rare, but serious adverse events include thrombocytopenia and agranulocytosis.
Hepatotoxicity
In publications of the multiple, large prospective trials of cilostazol therapy, rates of serum ALT elevations during therapy were not provided. Furthermore, there were no reported instances of clinically apparent acute liver injury. Since its approval and wide scale use, there have been no published reports of hepatotoxicity attributed to cilostazol. Nevertheless, the current product label mentions that instances of serum enzyme elevations and hepatitis have been reported to the sponsor. The time of onset, clinical pattern and course of liver test abnormalities during cilostazol therapy have not been reported.
Likelihood score: E (unlikely cause of clinically apparent liver injury).
Mechanism of Injury
The mechanism by which cilostazol might cause liver injury is not known, but it is metabolized in the liver, largely by cytochrome P450 (CYP) 3A4 and to a lesser extent by CYP 2C19. Thus, cilostazol is potentially susceptible to drug-drug interactions from inhibitors of CYP 3A4 (ketoconazole, erythromycin) and CYP 2C19 (omeprazole), and liver injury may be caused by a toxic or antigen product of its metabolism.
Outcome and Management
The course and outcome of liver injury from cilostazol has not been reported.
Drug Class: Cardiovascular Drugs, Intermittent Claudication Agents
Other Drugs in the Subclass, Intermittent Claudication Agents: Pentoxifylline
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Cilostazol – Generic, Pletal®
DRUG CLASS
Intermittent Claudication Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Cilostazol | 73963-72-1 | C20-H27-N5-O2 |
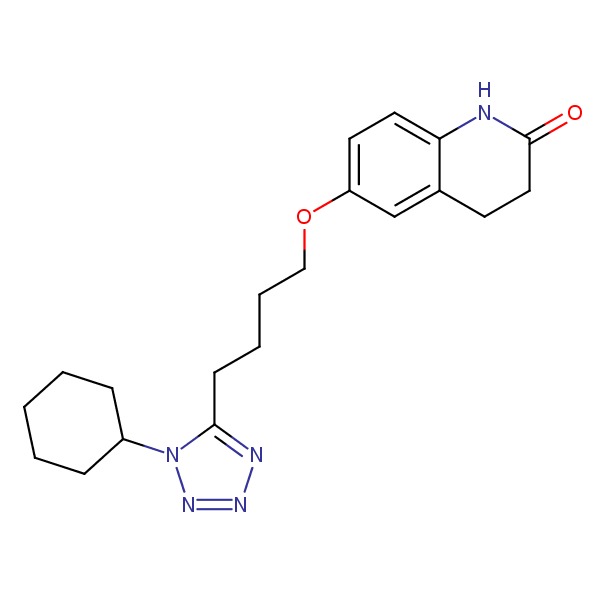 |
ANNOTATED BIBLIOGRAPHY
References updated: 1 December 2017
- Zimmerman HJ. Pentoxifylline. Drugs used in cardiovascular disease. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 654.(Expert review of hepatotoxicity published in 1999, does not discuss cilostazol).
- Michel T, Hoffman BB. Treatment of myocardial ischemia and hypertension. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 746-88.(Textbook of pharmacology and therapeutics).
- Cameron HA, Waller PC, Ramsay LE. Drug treatment of intermittent claudication: a critical analysis of the methods and findings of published clinical trials, 1965-1985. Br J Clin Pharmacol 1988; 26: 569-76 . [PMC free article: PMC1386634] [PubMed: 3061424](Analysis of 75 trials of 33 drugs used to treat intermittent claudication in the English literature [1965-1985: before availability of cilostazol] found design problems in 76% of trials and concluded that available information does not support the efficacy of any agent in exercise performance in intermittent claudication).
- Dawson DL, Cutler BS, Hiatt WR, Hobson RW 2nd, Martin JD, Bortey EB, Forbes WP, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med 2000; 109: 523-30. [PubMed: 11063952](Among 698 patients with intermittent claudication treated with cilostazol [100 mg twice daily], pentoxifylline [400 mg thrice daily] or placebo, maximal walking distance improved more with cilostazol [54%] than pentoxifylline [30%], which was similar in effect to placebo [34%]; adverse events were similar in all 3 groups; no mention of ALT levels or hepatotoxicity).
- Pratt CM. Analysis of the cilostazol safety database. Am J Cardiol 2001; 8: 28D-33D. [PubMed: 11434897](Summary of adverse events from 8 controlled trials of cilostazol [50 to 100 mg twice daily for 12-24 weeks] in 1374 patients with intermittent claudication states that rates of serious adverse events were no different than with placebo; in postmarketing surveillance of 70,430 patients over a 3 year period in the US, 461 adverse events were reported, 34 considered serious, none of which were judged to be drug related; no mention of ALT elevations or hepatotoxicity).
- Strandness DE Jr, Dalman RL, Panian S, Rendell MS, Comp PC, Zhang P, Forbes WP. Effect of cilostazol in patients with intermittent claudication: a randomized, double-blind, placebo-controlled study. Vasc Endovascular Surg 2002; 36: 83-91. [PubMed: 11951094](Among 394 patients with intermittent claudication treated with cilostazol [50 or 100 mg twice daily] or placebo for 24 weeks, the most common adverse events were headache [26% and 41% vs 12%], diarrhea [11% and 17% vs 6%] and there were no "clinically relevant changes in laboratory assessments"; no specific mention of ALT elevations or hepatotoxicity).
- Chapman TM, Goa KL. Cilostazol: a review of its use in intermittent claudication. Am J Cardiovasc Drugs 2003; 3: 117-38. [PubMed: 14727939](Review of the mechanism of action, efficacy and safety of cilostazol based upon 8 controlled trials in 2274 patients mentions that side effects are common, but generally well tolerated and cilostazol has significant drug-drug interactions with CYP 3A4 and 2C19 inducers; no mention of ALT elevations or hepatotoxicity).
- Drugs for intermittent claudication. Med Lett Drugs Ther 2004; 46 (1176): 13-5. [PubMed: 14973403](Concise review of the medical management of intermittent claudication mentions that pentoxifylline is approved for symptomatic therapy of this condition, but that its efficacy is controversial; no mention of ALT elevations or hepatotoxicity).
- Morris DS, Porterfield JR, Sawyer MD. Hemorrhagic cholecystitis in an elderly patient taking aspirin and cilostazol. Case Rep Gastroenterol 2008; 2: 203-7. [PMC free article: PMC3075144] [PubMed: 21490889](91 year old woman with intermittent claudication developed hemorrhagic cholecystitis after having taken both aspirin and cilostazol for 4 years).
- Hiatt WR, Money SR, Brass EP. Long-term safety of cilostazol in patients with peripheral artery disease: the CASTLE study (Cilostazol: A Study in Long-term Effects). J Vasc Surg 2008; 47: 330-6. [PubMed: 18155871](Among 1435 patients with intermittent claudication treated with cilostazol [100 mg twice daily] or placebo for up to 3 years, death rates [1.9 vs 2.2 patients per year] and rates of serious bleeding were not different [2.5 vs 3.1]; no mention of ALT elevations or hepatotoxicity).
- Shinohara Y, Katayama Y, Uchiyama S, Yamaguchi T, Handa S, Matsuoka K, Ohashi Y, et al.; CSPS 2 group. Cilostazol for prevention of secondary stroke (CSPS 2): an aspirin-controlled, double-blind, randomized non-inferiority trial. Lancet Neurol 2010: 959-68. [PubMed: 20833591](Among 2757 patients with stroke treated with cilostazol [100 mg twice daily] or aspirin for up to 5 years [m=29 months], there were no differences in rates of serious adverse events and no mention of ALT elevations or hepatotoxicity).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but none were attributed to cilostazol).
- Stevens JW, Simpson E, Harnan S, Squires H, Meng Y, Thomas S, Michaels J, et al. Systematic review of the efficacy of cilostazol, naftidrofuryl oxalate and pentoxifylline for the treatment of intermittent claudication. Br J Surg 2012; 99: 1630-8. [PubMed: 23034699](Systematic review of the literature on drugs for intermittent claudication concluded that pentoxifylline was less effective than cilostazol, that adverse events were "generally minor" and that serious adverse events were not increased; no mention of ALT elevations or hepatotoxicity).
- Lee WH, Chu CY, Hsu PC, Su HM, Lin TH, Voon WC, Lai WT, Sheu SH. Cilostazol for primary prevention of stroke in peripheral artery disease: a population-based longitudinal study in Taiwan. Thromb Res 2013; 132: 190-5. [PubMed: 23433530](Retrospective cohort study of patients with intermittent claudication in Taiwan treated with cilostazol, aspirin or clopidogrel, found rates for stroke were lower in cilostazol treated subjects; no mention of ALT elevations or hepatotoxicity).
- Perez P, Esteban C, Sauquillo JC, Yeste M, Manzano L, Mujal A, Jiméz Caballero PE, et al.; FRENA Investigators. Cilostazol and outcome in outpatients with peripheral artery disease. Thromb Res 2014; 134: 331-5. [PubMed: 24951338](Among 1317 patients with intermittent claudication followed in a prospective database, patients on cilostazol [n=191] had similar rates of ischemic stroke, major bleeding and death to those not on cilostazol; no mention of ALT elevations or hepatotoxicity).
- Rogers KC, Oliphant CS, Finks SW. Clinical efficacy and safety of cilostazol: a critical review of the literature. Drugs 2015; 75: 377-95. [PubMed: 25758742](Review of the mechanism of action, pharmacology, efficacy and safety of cilostazol; no mention of ALT elevations or hepatotoxicity).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to cilostazol).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Cilostazol for intermittent claudication.[Cochrane Database Syst Rev. 2014]Review Cilostazol for intermittent claudication.Bedenis R, Stewart M, Cleanthis M, Robless P, Mikhailidis DP, Stansby G. Cochrane Database Syst Rev. 2014 Oct 31; 2014(10):CD003748. Epub 2014 Oct 31.
- Cilostazol for intermittent claudication.[Cochrane Database Syst Rev. 2021]Cilostazol for intermittent claudication.Brown T, Forster RB, Cleanthis M, Mikhailidis DP, Stansby G, Stewart M. Cochrane Database Syst Rev. 2021 Jun 30; 6(6):CD003748. Epub 2021 Jun 30.
- Review Cilostazol: a Review of Basic Mechanisms and Clinical Uses.[Cardiovasc Drugs Ther. 2022]Review Cilostazol: a Review of Basic Mechanisms and Clinical Uses.Kherallah RY, Khawaja M, Olson M, Angiolillo D, Birnbaum Y. Cardiovasc Drugs Ther. 2022 Aug; 36(4):777-792. Epub 2021 Apr 16.
- Review [Research and development of cilostazol: an antiplatelet agent].[Yakugaku Zasshi. 2000]Review [Research and development of cilostazol: an antiplatelet agent].Nishi T, Kimura Y, Nakagawa K. Yakugaku Zasshi. 2000 Dec; 120(12):1247-60.
- A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial.[Arch Intern Med. 1999]A new pharmacological treatment for intermittent claudication: results of a randomized, multicenter trial.Beebe HG, Dawson DL, Cutler BS, Herd JA, Strandness DE Jr, Bortey EB, Forbes WP. Arch Intern Med. 1999 Sep 27; 159(17):2041-50.
- Cilostazol - LiverToxCilostazol - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...