NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Chenodeoxycholic acid (chenodiol) is a primary bile acid, synthesized in the liver and present in high concentrations in bile that is used therapeutically to dissolve cholesterol gallstones. Chronic therapy is associated with transient elevations in serum aminotransferase levels in up to 30% of patients, but chenodiol has been linked to only rare instances of clinically apparent liver injury with jaundice.
Background
Chenodeoxycholic acid or chenodiol (kee" noe dye' ol) is a naturally occurring bile acid that is used therapeutically to dissolve cholesterol gallstone in patients with a functioning gall bladder who have contraindications to cholecystectomy or refuse surgery. Chenodiol is the major bile acid synthesized by the liver and is usually the bile acid in highest concentration in serum, hepatocytes and bile. When given orally, chenodiol is passively absorbed from the small intestine and taken up by the liver via anion transport proteins. High concentrations of chenodiol in liver decrease the hepatic synthesis of both cholesterol and other bile acids, thereby increasing the cholic acid and decreasing cholesterol concentration in bile. This combination of events results in a decrease in cholesterol saturation of bile, the major impetus to the formation of cholesterol gallstones. When given for 2 years or more, chenodeoxycholic acid can dissolve cholesterol gallstones in 15% to 30% of patients. Chenodiol is most effective on small “floating” gallstones. It is not effective for calcified gallstones or in patients with a non-functioning gallbladder. Even with successful therapy, however, gallstone recurrence is as high as 50% within a few years of stopping chenodiol. Chenodiol was approved for use in patients with radiolucent gallstones in 1983 and remains available, although it has largely been replaced by ursodiol which appears to be more effective and is better tolerated. In addition, the introduction of laparoscopic cholecystectomy has markedly decreased the need for medical therapy of gallstones. Chenodiol is available as tablets of 250 mg generically and under the commercial names Chenix and Chenodal. The recommended dose is 13 to 16 mg/kg daily in two divided doses for up to 2 years. Side effects include gastrointestinal upset with diarrhea, bloating, cramps, dyspepsia, nausea and vomiting.
Hepatotoxicity
In multiple clinical trials of chenodiol therapy for dissolution of gallstones, serum aminotransferase elevations occurred in up to 30% of patients. The elevations generally arose within 2 months of starting therapy and were typically mild, transient and not accompanied by symptoms or jaundice. Liver biopsies done during chenodiol therapy generally showed mild, nonspecific changes. Clinically apparent liver injury with jaundice was not reported. The liver enzyme elevations were generally dose related and usually did not recur on restarting chenodiol at lower doses. While the serum enzyme abnormalities that occurred on chenodiol therapy generated considerable concern, they appeared to be relatively benign. Since the approval of chenodiol and its more widespread use, at least four instances of liver injury with jaundice have been reported to the sponsor, but the clinical features and outcomes of these cases have not been published. Nevertheless, the product label for chenodiol includes a boxed warning about hepatotoxicity although it does not provide advice on the frequency or how to respond to abnormalities. Thus, the reliability of reports of clinically apparent liver injury with chenodiol therapy remains unclear. Once ursodiol was found to be equally as effective as chenodiol, even at lower doses, and was rarely associated with serum enzyme elevations, it rapidly replaced chenodiol as medical therapy for gallstones.
Likelihood score: E* (Suspected but unproven cause of clinically apparent liver injury).
Mechanism of Liver Injury
Chenodiol is thought to cause serum aminotransferase elevations because of conversion to lithocholic acid which has intrinsic, proven hepatotoxicity. In animal models, chenodiol is less hepatotoxic than lithocholic acid, but is more injurious than ursodiol. The more marked hepatotoxicity of chenodiol and lithocholic acid in rodent and primate models compared to humans has been attributed to a more effective sulfation of lithocholic acid in humans that renders it more water soluble, less toxic and more readily excreted.
Outcome and Management
Patients on chenodiol should be monitored with liver tests, including serum bilirubin, ALT, AST and alkaline phosphatase at periodic intervals. Chenodiol should be discontinued for persistent increases in liver test abnormalities, ALT elevations above 8 times the upper limit of normal, elevations of bilirubin more than twice normal or any symptom or sign of liver injury. Replacing chenodiol with ursodiol is probably appropriate in that there does not appear to be cross sensitivity to liver injury or adverse events between chenodiol and other therapeutic bile acids.
Other bile acids used in digestive diseases include cholic acid, obeticholic acid and ursodeoxycholic acid (ursodiol).
Drug Class: Gastrointestinal Agents, Bile Acids
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Chenodiol – Generic, Chenodal®
DRUG CLASS
Gastrointestinal Agents
Product labeling at DailyMed, National Library of Medicine, NIH
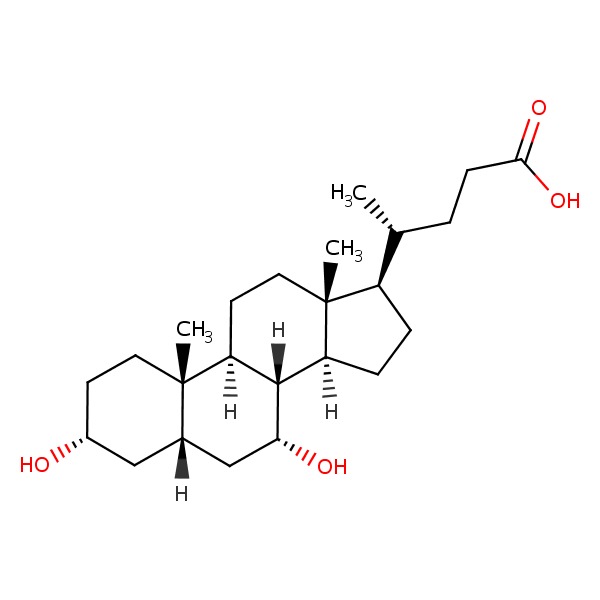
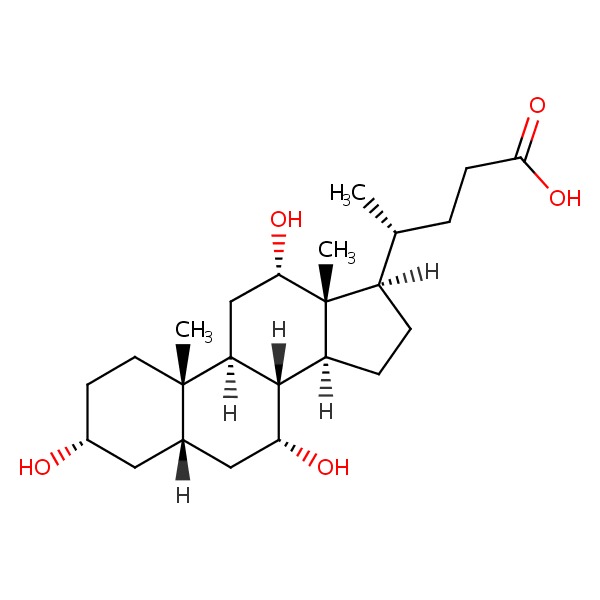
CHEMICAL FORMULAS AND STRUCTURES
ANNOTATED BIBLIOGRAPHY
References updated: 09 September 2016
Abbreviations used: FXR, farnesoid X receptor; NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; OCA, obeticholic acid; PBC, primary biliary cirrhosis (cholangitis).
- Zimmerman HJ. Bile acid derivatives. Miscellaneous drugs and diagnostic chemicals. In, Zimmerman, HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999: pp. 721.(Expert review of hepatotoxicity published in 1999; mentions that chenodiol led to aminotransferase elevations in 30-50% of patients and at least 4 instances of overt hepatitis occurred in recipients, but it has been largely replaced by ursodiol which has been associated with only “trivial” elevations in ALT levels).
- Sharkey KA, Wallace JL. Treatment of disorders of bowel motility and water flux: anti-emetics; agents used in biliary and pancreatic disease. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1323-50.(Textbook of pharmacology and therapeutics).
- Danzinger RG, Hofmann AF, Schoenfield LJ, Thistle JL. Dissolution of cholesterol gallstones by chenodeoxycholic acid. N Engl J Med 1972; 286: 1-8. [PubMed: 5006919](Among 7 women with radiolucent gallstones treated with chenodiol, gallstone dissolution [n=1] or decrease in size [n=3] occurred in 4 patients and side effects included diarrhea, and “mild transient elevations of [AST and Alk P] were seen in several patients but liver biopsies were normal at 6 and 12 months”).
- Thistle JL, Hofmann AF. Efficacy and specificity of chenodeoxycholic acid therapy for dissolving gallstones. N Engl J Med 1973; 289: 655-9. [PubMed: 4580472](Among 53 patients with radiolucent gallstones treated with chenodiol, cholic acid or placebo for 6 months, stone size decreased in 61% of chenodiol, but in none of the cholic acid or placebo recipients; AST elevations above twice ULN occurred in 29% of patients, but levels returned to normal despite continuing treatment).
- Schoenfield LJ. Medical therapy for gallstones. Gastroenterology 1974; 67 (4): 725-9. [PubMed: 4606798](Review of the frequency, epidemiology, pathogenesis and possible medical therapies for gallstone disease mention promising results from small trials of chenodiol, but that up to 25% of patients develop ALT elevations during treatment, although the elevations are usually transient and invariably without jaundice or clinical symptoms).
- Morrissey KP, McSherry CK, Swarm RL, Nieman WH, Deitrick JE. Toxicity of chenodeoxycholic acid in the nonhuman primate. Surgery 1975; 77: 851-60. [PubMed: 1145445](Among 18 baboons treated with chenodiol [18-38 mg/kg daily], 15 [83%] had ALT or AST elevations, most of whom had “significant” histologic changes, similar to those described for lithocholic acid).
- Gadacz TR, Allan RN, Mack E, Hofmann AF. Impaired lithocholate sulfation in the rhesus monkey: a possible mechanism for chenodeoxycholate toxicity. Gastroenterology 1976; 70: 1125-9. [PubMed: 817960](Turnover studies of radiolabeled lithocholate in rhesus monkeys showed minimal sulfation [17%] which may account for the toxicity of chenodiol in this species).
- Levy VG, Bouma ME, Lageron A, Darnis F, Infante R. Hepatic sinusoidal dilatation after chenodeoxycholic-acid therapy. Lancet 1978; 1 (8057): 206. [PubMed: 74627](Among 26 patients with radiolucent gallstones treated with chenodiol for at least 12 months, there was an increase in the proportion with sinusoidal dilatation from 35% at baseline to 58% at 6 and 65% at 12 months, but the significance of this change was unclear).
- Hofmann AF, Thistle JL, Klein PD, Szczepanik PA, Yu PY. Chenotherapy for gallstone dissolution. II. Induced changes in bile composition and gallstone response. JAMA 1978; 239: 1138-44. [PubMed: 628065](Chenodiol but not cholic acid or placebo therapy was associated with disease in cholesterol saturation of bile; the amount of change, however, did not correlate with success of therapy in dissolving gallstones).
- Thistle JL, Hofmann AF, Ott BJ, Stephens DH. Chenotherapy for gallstone dissolution. I. Efficacy and safety. JAMA 1978; 239: 1041-6. [PubMed: 342729](Among 90 patients with radiolucent gallstones treated with chenodiol, response rates increased with time, with higher doses and smaller stones, mild elevations in AST occurred in 16% of patients but were transient, resolving with dose modification and not associated with jaundice or symptoms).
- Schoenfield LJ, Lachin JM. Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety. Ann Intern Med 1981; 95; 257-82. [PubMed: 7023307](Among 916 patients with radiolucent gallstones treated with chenodiol [350 or 750 mg daily] vs placebo for 2 years, gallstone dissolution occurred in 5% and 13.5% vs 0% and ALT elevations in 13-19% vs 13%, which were above 3 times ULN in 5-8% vs 4%, while biopsies showed nonspecific changes only; ALT elevations resolved even without stopping in most and among 17 who stopped therapy, 6 had recurrence on restarting; no patient developed clinically apparent liver injury).
- Miyai K, Javitt NB, Gochman N, Jones HM, Baker D. Hepatotoxicity of bile acids in rabbits: ursodeoxycholic acid is less toxic than chenodeoxycholic acid. Lab Invest 1982; 46: 428-37. [PubMed: 7200166](Feeding rabbits chenodeoxycholic and lithocholic acid led to increases in ALT levels, histologic changes in the liver and significant mortality compared to no treatment or use of ursodiol).
- Fisher RL, Anderson DW, Boyer JL, Ishak K, Klatskin G, Lachin JM, Phillips MJ. A prospective morphologic evaluation of hepatic toxicity of chenodeoxycholic acid in patients with cholelithiasis: the National Cooperative Gallstone Study. Hepatology 1982; 2: 187-201. [PubMed: 7068113](Among 126 patients with cholelithiasis treated with chenodiol for 24 months who underwent liver biopsies at 0, 9 and 24 months, minor nonspecific abnormalities were frequent, but did not correlate with ALT or AST elevations).
- Roda E, Bazzoli F, Labate AM, Mazzella G, Roda A, Sama C, Festi D, et al. Ursodeoxycholic acid vs. chenodeoxycholic acid as cholesterol gallstone-dissolving agents: a comparative randomized study. Hepatology 1982; 2: 804-10. [PubMed: 7141392](Among 223 patients with gallstones treated with ursodiol or chenodiol at 2 doses for 12 months, rates of gallstone dissolution were similar at the higher doses, but were greater with ursodiol even at the lower doses; ALT elevations occurred only in chenodiol groups [14%], but there were no concurrent elevations in Alk P or bilirubin).
- Fromm H, Roat JW, Gonzalez V, Sarva RP, Farivar S. Comparative efficacy and side effects of ursodeoxycholic and chenodeoxycholic acids in dissolving gallstones. A double-blind controlled study. Gastroenterology 1983; 85: 1257-64. [PubMed: 6354826](Among 60 patients with radiolucent gallstones treated with chenodiol [375 or 750 mg] or ursodiol [400 or 800 mg] or placebo daily, complete occurred in 7% on chenodiol versus 30% on ursodiol but none on placebo, while ALT elevations above 3 times ULN occurred in 2 of 26 on chenodiol but none of 24 on ursodiol).
- Phillips MJ, Fisher RL, Anderson DW, Lan SP, Lachin JM, Boyer JL. Ultrastructural evidence of intrahepatic cholestasis before and after chenodeoxycholic acid therapy in patients with cholelithiasis: the national cooperative gallstone study. Hepatology 1983; 3: 209-20. [PubMed: 6832711](Among 103 patients with gallstones treated with chenodiol who underwent liver biopsies before and 9 and 24 months after starting therapy, minor changes in intrahepatic cholestasis were found in “almost every biopsy”, even those taken before treatment, but the degree of change tended to worsen with chenodiol therapy).
- Erlinger S, Le Go A, Husson JM, Fevery J. Franco-Belgian cooperative study of ursodeoxycholic acid in the medical dissolution of gallstones: a double-blind, randomized, dose-response study, and comparison with chenodeoxycholic acid. Hepatology 1984; 4: 308-14. [PubMed: 6706305](Among 197 patients with radiolucent gallstones treated with ursodiol or chenodiol, stone dissolution rates were similar, but diarrhea was less with ursodiol [5% vs 23%]; ALT elevations were transient and mild [less than twice normal] in all except one patient [3%] on chenodiol).
- Fisher MM, Roberts EA, Rosen IE, Shapero TF, Sutherland LR, Davies RS, Bacchus R, Lee SV. The Sunnybrook Gallstone Study: a double-blind controlled trial of chenodeoxycholic acid for gallstone dissolution. Hepatology 1985; 5: 102-7. [PubMed: 3881327](Among 160 patients with radiolucent gallstones treated with chenodiol [375 or 750 mg daily] or placebo for 2 years, stone dissolution occurred in 11-13% vs 0% and no patient developed clinically apparent liver injury).
- Nakashima T, Sano A, Seto Y, Nakajima T, Okuno T, Takino T. A case of hyperbilirubinemia during treatment with chenodeoxycholic acid. Jpn J Med 1987; 26: 404-8. [PubMed: 3694926](A 27 year old woman with acute, symptomatic cholelithiasis developed jaundice within 6 weeks of starting chenodiol [peak values 12.8 mg/dL, ALT normal, Alk P ~3 times ULN], resolving upon stopping but later requiring cholecystectomy).
- Fisher RL, Hofmann AF, Converse JL, Rossi SS, Lan SP. The lack of relationship between hepatotoxicity and lithocholic-acid sulfation in biliary bile acids during chenodiol therapy in the National Cooperative Gallstone Study. Hepatology 1991; 14: 454-63. [PubMed: 1874490](Testing of bile samples from 31 patients with gallstones treated with chenodiol with and without liver test or liver biopsy abnormalities showed no differences in lithocholate levels during treatment).
- Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med 1991; 324: 1548-54. [PubMed: 1674105](Among 146 patients with PBC treated with ursodiol [13-15 mg daily] or placebo for 2 years, ALT, Alk P and bilirubin levels and liver histology [except for fibrosis] improved with ursodiol but not placebo; one patient on ursodiol required discontinuation because of worsening of pruritus; no mention of hepatotoxicity or other toxicities).
- Bashir RM, Lewis JH. Hepatotoxicity of drugs used in the treatment of gastrointestinal disorders. Gastroenterol Clin North Am 1995; 24: 937-67. [PubMed: 8749906](Review of hepatotoxicity of drugs for gastrointestinal disease mentions that ALT elevations occur in up to 30% of patients treated with chenodiol, and are above 3-4 times ULN in 2-3% and that there have been at least 4 cases of clinically apparent liver injury reported to the sponsor whereas no such reports have been linked to ursodiol).
- Bove KE, Heubi JE, Balistreri WF, Setchell KD. Bile acid synthetic defects and liver disease: a comprehensive review. Pediatr Dev Pathol 2004; 7: 315-34. [PubMed: 15383928](Extensive review of the inherited forms of defective bile acid synthesis which are often accompanied by progressive cholestatic liver injury, some of which respond to treatment with oral bile acids, including 3β-OH steroid dehydrogenase, 5β-reductase and mitochondrial C-27 hydroxylase deficiencies as well as several peroxisomal defects [Zellweger syndrome]).
- Paumgartner G. Medical treatment of cholestatic liver diseases: From pathobiology to pharmacological targets. World J Gastroenterol 2006; 12: 4445-51. [PMC free article: PMC4125628] [PubMed: 16874853](Review of the pathogenesis of gallstone formation and medical therapies for gallstone dissolution).
- Chiang JY. Bile acids: regulation of synthesis. J Lipid Res 2009; 50: 1955-66. [PMC free article: PMC2739756] [PubMed: 19346330](Review of the pathways of cholic and chenodeoxycholic acid synthesis and their regulation via FXR, FGF19, FGFR4 and CYP7A1).
- Song P, Zhang Y, Klaassen CD. Dose-response of five bile acids on serum and liver bile acid concentrations and hepatotoxicty in mice. Toxicol Sci 2011; 123: 359-67. [PMC free article: PMC3179674] [PubMed: 21747115](Feeding of various concentrations of 5 different bile acids to mice demonstrated clear toxicity with lithocholic, cholic, deoxycholic and chenodeoxycholic acid, but little or no toxicity with ursodeoxycholic acid).
- Heubi JE, Setchell KD, Jha P, Buckley D, Zhang W, Rosenthal P, Potter C, et al. Treatment of bile acid amidation defects with glycocholic acid. Hepatology 2015; 61: 268-74. [PMC free article: PMC4280294] [PubMed: 25163551](Among 5 children with inborn errors of bile acid synthesis [defective amidation] who were treated with glycocholic acid, all had improvements in fat soluble vitamin absorption and no worsening of liver injury).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, none were attributed to chenodiol or other bile acid therapies).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- The effects of chenodiol on biliary lipids and their association with gallstone dissolution in the National Cooperative Gallstone Study (NCGS).[J Clin Invest. 1984]The effects of chenodiol on biliary lipids and their association with gallstone dissolution in the National Cooperative Gallstone Study (NCGS).Grundy SM, Lan SP, Lachin J. J Clin Invest. 1984 Apr; 73(4):1156-66.
- The lack of relationship between hepatotoxicity and lithocholic-acid sulfation in biliary bile acids during chenodiol therapy in the National Cooperative Gallstone Study.[Hepatology. 1991]The lack of relationship between hepatotoxicity and lithocholic-acid sulfation in biliary bile acids during chenodiol therapy in the National Cooperative Gallstone Study.Fisher RL, Hofmann AF, Converse JL, Rossi SS, Lan SP. Hepatology. 1991 Sep; 14(3):454-63.
- Review Bile Acids.[LiverTox: Clinical and Researc...]Review Bile Acids.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ursodiol: a cholesterol gallstone solubilizing agent.[Drug Intell Clin Pharm. 1988]Review Ursodiol: a cholesterol gallstone solubilizing agent.Rosenbaum CL, Cluxton RJ Jr. Drug Intell Clin Pharm. 1988 Dec; 22(12):941-5.
- Review Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety.[Ann Intern Med. 1981]Review Chenodiol (chenodeoxycholic acid) for dissolution of gallstones: the National Cooperative Gallstone Study. A controlled trial of efficacy and safety.Schoenfield LJ, Lachin JM. Ann Intern Med. 1981 Sep; 95(3):257-82.
- Chenodiol (Chenodeoxycholic Acid) - LiverToxChenodiol (Chenodeoxycholic Acid) - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...