NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Ceritinib is a small molecule tyrosine kinase receptor inhibitor and antineoplastic agent that is used in the therapy of selected forms of advanced non-small cell lung cancer (NSCLC). Ceritinib is associated with a moderate rate of serum aminotransferase elevations during therapy and rare instances of clinically apparent acute liver injury.
Background
Ceritinib (se ri' ti nib) is a small molecule tyrosine kinase receptor inhibitor with potent activity against anaplastic lymphoma kinase (ALK) that is rearranged and mutated in selected cancers including approximately 5% of non-small cell lung cancer (NSCLC). The mutated, rearranged ALK promotes unregulated cell growth and proliferation and is overexpressed in some cancer cells. Ceritinib has been found to inhibit mutated ALK in cell culture and in several clinical trials was found to induce objective responses in a proportion of patients with advanced ALK-positive NSCLC. Ceritinib received accelerated approval for use crizotinib-refractory, ALK-positive NSCLC in the United States in 2014. Ceritinib is available in capsules of 150 mg under the brand name Zykadia. The recommended initial dose is 750 mg (5 capsules) once daily, continued until progressive disease or intolerable toxicity occurs. Side effects are common and include diarrhea, nausea and vomiting, abdominal pain, fatigue, anorexia and constipation. Uncommon, but potentially severe side effects include severe diarrhea leading to dehydration and renal failure, interstitial lung disease, prolongation of the QTc interval, bradycardia, hyperglycemia, and embryo-fetal toxicity.
Hepatotoxicity
Elevations in serum aminotransferase levels are common during ceritinib therapy occurring in 20% to 50% of patients, but rising above 5 times the upper limit of the normal range in only 1% to 2%. Hepatic failure is said to have occurred in 0.2% of patients and to have resulted in several fatalities. Hepatotoxicity appears to be a class effect among ALK inhibitors, although liver injury appears to be more frequent and more severe with crizotinib than ceritinib or alectinib. Specific details of the liver injury associated with ceritinib such as latency, serum enzyme pattern, clinical features and course, have not been published. Other ALK inhibitors typically cause liver injury arising within days or weeks of starting therapy, and presenting abruptly with hepatocellular enzyme elevations and a moderate-to-severe course. Immunoallergic and autoimmune features are not common. The rate of clinically significant liver injury and hepatic failure is increased in patients with preexisting cirrhosis or hepatic impairment due to liver tumor burden. Recurrence upon reexposure has been reported.
Likelihood score: D (possible cause of clinically apparent liver injury).
Mechanism of Injury
The latency until onset and the recurrence of injury with reexposure suggests that the clinically apparent liver injury attributed to ALK inhibitors is immunologically mediated. In contrast, the transient serum enzyme elevations that are not uncommon during therapy suggest a direct, intrinsic hepatotoxicity, perhaps caused by off-target inhibition of critical tyrosine kinase receptors in hepatocytes. Ceritinib is metabolized in the liver largely by the cytochrome P450 system (largely CYP 3A) and is susceptible to drug-drug interactions with inhibitors or inducers of CYP 3A.
Outcome and Management
Liver injury due to ceritinib varies in severity from minor, transient serum enzyme elevations to acute symptomatic hepatitis and acute liver failure. Serum aminotransferase elevations above 5 times the upper limit of normal (if confirmed) should lead to temporary discontinuation, which should be permanent if laboratory values do not improve significantly or resolve within a few weeks or if symptoms or jaundice arise. Restarting therapy is usually, but not always followed by recurrence of the serum enzyme elevations. There does not appear to be cross reactivity with other tyrosine kinase receptor inhibitors and, in some situations, switching to another protein kinase inhibitor may be appropriate.
Drug Class: Antineoplastic Agents, Protein Kinase Inhibitors
Related Drugs: Alectinib, Crizotinib
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Ceritinib – Zykadia®
DRUG CLASS
Antineoplastic Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NO. | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Ceritinib | 1032900-25-6 | Not available |
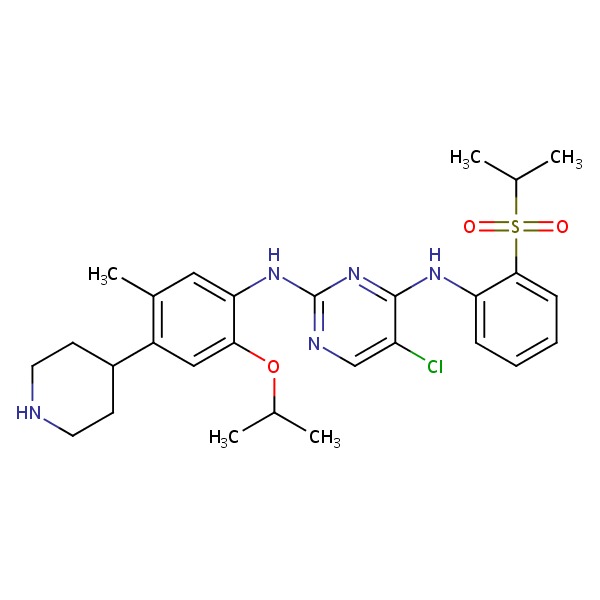 |
ANNOTATED BIBLIOGRAPHY
References updated: 01 June 2017
Abbreviations used: ALK, anaplastic lymphoma kinase; NSCLC, non-small cell lung cancer;
- Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999.(Review of hepatotoxicity published in 1999 before the availability of tyrosine kinase receptor inhibitors).
- DeLeve LD. Erlotinib. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 556.(Review of hepatotoxicity of cancer chemotherapeutic agents published in 2013; discusses the hepatotoxicity of crizotinib, but not alectinib or ceritinib).
- Chabner BA, Barnes J, Neal J, Olson E, Mujagic H, Sequist L, Wilson W, et al. Targeted therapies: tyrosine kinase inhibitors, monoclonal antibodies, and cytokines. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1731-54.(Textbook of pharmacology and therapeutics).
- Shaw AT, Engelman JA. ALK in lung cancer: past, present, and future. J Clin Oncol 2013; 31: 1105-11. [PMC free article: PMC4209068] [PubMed: 23401436](Review of the history of discovery of ALK mutations and development of crizotinib as therapy of NSCLC patients with this mutation as well as next-generation ALK inhibitors in development; no discussion of hepatotoxicity or ALT elevations).
- Shah RR, Morganroth J, Shah DR. Hepatotoxicity of tyrosine kinase inhibitors: clinical and regulatory perspectives. Drug Saf 2013; 36: 491-503. [PubMed: 23620168](Review of the hepatotoxicity of 18 tyrosine kinase inhibitors approved for use in cancer in the US as of 2013; crizotinib is listed as causing liver enzyme elevations in up to 57% of patients [ ≥5 times ULN in 6%] and to having been linked cases of hepatitis and fatal hepatic failure; no mention of alectinib or ceritinib).
- Shaw AT, Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, Camidge DR, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014; 370: 1189-97. [PMC free article: PMC4079055] [PubMed: 24670165](Among 59 patients with ALK-rearranged NSCLC enrolled in dose escalation studies and 71 patients treated in open label studies at the optimal dose of ceritinib, overall objective response rates were 58% at the higher doses and adverse events included ALT elevations in 35%, which were above 5 times the ULN in 21%, both rates being greater with higher doses).
- Ceritinib (Zykadia) for non-small cell lung cancer. Med Lett Drugs Ther 2014; 56 (1447): 62-3. [PubMed: 25046419](Concise review of the mechanism of action, clinical efficacy, safety and costs of ceritinib, the second oral tyrosine kinase inhibitor with activity against ALK-positive metastatic NCSLC; mentions that ALT elevations are common during therapy and hepatotoxicity has been reported).
- Chabner BA. Approval after phase I: ceritinib runs the three-minute mile. Oncologist 2014; 19: 577-8. [PMC free article: PMC4041677] [PubMed: 24789171](Editorial in response to the accelerated approval of ceritinib for ALK-positive metastatic NSCLC mentioning its rapid, 3 year development and approval based upon limited, open label, phase 1 and 2 studies only and, as a consequence, the incomplete knowledge about side effects).
- Gainor JF, Tan DS, De Pas T, Solomon BJ, Ahmad A, Lazzari C, de Marinis F, et al. Progression free and overall survival in ALK-positive NSCLC patients treated with sequential crizotinib and ceritinib. Clin Cancer Res 2015; 21: 2745-52. [PMC free article: PMC4470734] [PubMed: 25724526](In a retrospective analysis of 73 patients with ALK-positive NSCLC who were treated sequentially with crizotinib and then ceritinib, median overall survival was 49 months; no discussion of adverse event rates).
- Cooper MR, Chim H, Chan H, Durand C. Ceritinib: a new tyrosine kinase inhibitor for non-small-cell lung cancer. Ann Pharmacother 2015; 49: 107-12. [PubMed: 25258420](Systematic review of the literature on ceritinib therapy of NSCLC identified only one article and two abstracts; listing, but no discussion of ALT elevations and hepatotoxicity).
- Crinò L, Ahn MJ, De Marinis F, Groen HJ, Wakelee H, Hida T, Mok T, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol 2016; 34: 2866-73. [PubMed: 27432917](Among 140 patients with crizotinib resistant, ALK-positive NSCLC treated with ceritinib [750 mg daily], the overall objective response rate was 39% and all patients reported adverse events that were "grade 3 or 4" in 71% with ALT elevations in 44% of patients that were above 5 times ULN in 17%; no mention of clinically apparent liver injury or hepatic failure).
- Sassier M, Mennecier B, Gschwend A, Rein M, Coquerel A, Humbert X, Alexandre J, et al. Successful treatment with ceritinib after crizotinib induced hepatitis. Lung Cancer 2016; 95: 15-6. [PubMed: 27040846](Two patients with ALK-positive NSCLC who developed ALT elevations while on crizotinib [ALT 1825 U/L after 10 days and ALT 531 U/L after 1 month] both had recurrent ALT elevations within days of restarting crizotinib at a lower dose, yet both later tolerated ceritinib [for 7 months or more] without recurrence of ALT abnormalities).
- Kim DW, Mehra R, Tan DS, Felip E, Chow LQ, Camidge DR, Vansteenkiste J, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol 2016; 17: 452-63. [PMC free article: PMC5063047] [PubMed: 26973324](Among 255 patients with NSCLC treated with ceritinib in an extension of phase 1 studies [median follow up 11 months], the overall objective response rate was 67% and side effects included ALT elevations above 5 times ULN in 30%, with 3 patients discontinuing drug due to liver test abnormalities and one due to "cholestatic hepatitis").
- Soria JC, Tan DS, Chiari R, Wu YL, Paz-Ares L, Wolf J, Geater SL, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet 2017; 389 (10072): 917-29. [PubMed: 28126333](Among 376 previously untreated patients with ALK-positive NSCLC treated with ceritinib or platinum based chemotherapy, progression free survival rates were greater with ceritinib [16.6 vs 8.1 months] while overall adverse event rates were similar, although ALT elevations were more frequent with ceritinib [60% vs 22%] as were ALT elevations above 5 times ULN [31% vs 3%], although there were no instances of clinically apparent liver injury in either group).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Osimertinib.[LiverTox: Clinical and Researc...]Review Osimertinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Alectinib.[LiverTox: Clinical and Researc...]Review Alectinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Brigatinib.[LiverTox: Clinical and Researc...]Review Brigatinib.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer.[EBioMedicine. 2016]P-glycoprotein Mediates Ceritinib Resistance in Anaplastic Lymphoma Kinase-rearranged Non-small Cell Lung Cancer.Katayama R, Sakashita T, Yanagitani N, Ninomiya H, Horiike A, Friboulet L, Gainor JF, Motoi N, Dobashi A, Sakata S, et al. EBioMedicine. 2016 Jan; 3:54-66. Epub 2015 Dec 12.
- Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial.[Lancet Oncol. 2016]Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial.Kim DW, Mehra R, Tan DSW, Felip E, Chow LQM, Camidge DR, Vansteenkiste J, Sharma S, De Pas T, Riely GJ, et al. Lancet Oncol. 2016 Apr; 17(4):452-463. Epub 2016 Mar 11.
- Ceritinib - LiverToxCeritinib - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...