NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Captopril is an angiotensin-converting enzyme (ACE) inhibitor used in the therapy of hypertension and heart failure. Captopril is associated with a low rate of transient serum aminotransferase elevations and has been linked to rare instances of acute liver injury.
Background
Captopril (kap' toe pril) was the first ACE inhibitor to be approved for use in the United States and is still widely used for therapy of hypertension and heart failure. Like other ACE inhibitors, captopril inhibits the conversion of angiotensin I, a relatively inactive molecule, to angiotensin II which is the major mediator of vasoconstriction and volume expansion induced by the renin-angiotensin system. Other enzymes besides that which converts angiotensin I to II may also be inhibited, which may account for some of the side effects of the ACE inhibitors. Captopril was approved for use in the United States in 1981 and current indications are for hypertension, congestive heart failure, left ventricular dysfunction after myocardial infarction, and treatment and prevention of diabetic nephropathy. Captopril is available in 12.5, 25, 50 and 100 mg tablets in many generic forms and formerly under the trade name Capoten. The typical dose of captopril in adults in 25 to 50 mg two or three times daily, and it is administered long term. Captopril is also available in several fixed combinations with hydrochlorothiazide (Capozide and others). Common side effects of captopril and ACE inhibitors in general include dizziness, fatigue, headache, cough, gastrointestinal upset and skin rash.
Hepatotoxicity
Captopril, like other ACE inhibitors, has been associated with a low rate of serum aminotransferase elevations (<2%) that, in controlled trials, was no higher than with placebo therapy. These elevations were transient and rarely required dose modification. While rare, several dozen cases of clinically apparent liver injury have been reported with captopril therapy. The onset is usually within 2 to 12 weeks of starting therapy and the serum enzyme pattern is typically cholestatic (Case 1). In some instances, cholestasis has been prolonged and relapsing and associated with persistent elevations in serum alkaline phosphatase, suggestive of vanishing bile duct syndrome. Immunoallergic manifestations (rash, fever, eosinophilia) are infrequent and most patients do not develop autoantibodies. Rare instances of captopril injury with a hepatocellular pattern and cases with a long latency (one or more years) have been described as well, a distinctly unusual pattern of drug induced liver injury.
Likelihood score: B (likely but rare cause of clinically apparent liver injury).
Mechanism of Injury
The cause of the minor serum aminotransferase associated with captopril is not known. The clinically apparent acute liver injury from captopril is likely an idiosyncratic reaction to a metabolite. Captopril is hydrolyzed by the liver to its active metabolite captoprilat and has little further hepatic metabolism.
Outcome and Management
Most instances of acute liver injury reported with captopril use have been self limited, but there have been rare reports of acute liver failure due to captopril and several reports of cholestatic hepatitis leading to prolonged jaundice. Patients with severe captopril induced acute liver injury and hypersensitivity reactions should avoid use of other ACE inhibitors, although cross sensitivity to liver injury among the members of this class of agents has not always been shown.
References to the safety and potential hepatotoxicity of captopril are given in the Overview section on the Angiotensin-Converting Enzyme (ACE) Inhibitors.
Drug Class: Antihypertensive Agents, Angiotensin-Converting Enzyme Inhibitors
CASE REPORT
Case 1. Cholestatic hepatitis due to captopril.
[Modified from: Tabibian N, Alpert L, Alpert E. Captopril-induced liver dysfunction. South Med J 1987; 80: 1173-5. PubMed Citation]
A 54 year old man with congestive heart failure and atrial fibrillation on chronic therapy with digoxin, furosemide and isosorbide dinitrate was started on captopril [25 mg three times daily]. One month later he developed mild pruritus, nausea, anorexia and abdominal discomfort but continued taking his prescribed medications. Two months later he noted the onset of jaundice. He had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. He denied fever and rash. Examination showed deep jaundice but no peripheral manifestations of chronic liver disease. Laboratory testing showed total bilirubin of 25 mg/dL with mild elevations in aminotransferases (ALT 73, AST 130 U/L), and marked elevations in alkaline phosphatase (1054 U/L) which had been mildly elevated before captoril therapy (Table). The prothrombin time was normal at 11.8 seconds and eosinophil count at 268/µL. Tests for hepatitis A and B and for autoantibodies were negative. Abdominal ultrasound showed no gallstones or evidence for biliary obstruction, which was confirmed by percutaneous transhepatic cholangiography. A liver biopsy showed intrahepatic cholestasis and changes suggestive of drug induced liver injury. Five days after admission, captopril was stopped while his other medications were continued. Thereafter, he improved rapidly and tests had almost returned to normal when he was last seen a month later.
Key Points
| Medication: | Captopril (75 mg daily) |
|---|---|
| Pattern: | Cholestatic (R=0.7) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 4 weeks to symptoms, 12 weeks to jaundice |
| Recovery: | Partial by 5 weeks |
| Other medications: | Digoxin, furosemide, isosorbide dinitrate |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Pre | Pre | 33 | 338 | 1.3 | Congestive heart failure |
| 12 weeks | 0 | 73 | 1054 | 25.0 | Admission |
| 12.5 weeks | 0 | 61 | 951 | 36.3 | Captopril stopped |
| 13 weeks | 1 day | 60 | 1358 | 31.6 | |
| 5 days | 46 | 683 | 21.8 | Liver biopsy | |
| 14 weeks | 9 days | 35 | 536 | 14.3 | |
| 18 weeks | 5 weeks | 60 | 377 | 3.7 | |
| Normal Values | <40 | <130 | <1.2 | ||
Comment
The patient developed a cholestatic hepatitis 1 to 3 months after adding captopril to a regimen of therapy for chronic congestive heart failure. Typical of cholestatic drug induced liver injury was the relative paucity of symptoms, despite deep jaundice and the slow rate of recovery which was not complete at the time of the last laboratory test. While the patient had mildly abnormal liver tests before being treated, the clinical presentation and improvement on stopping captopril makes this a convincing case. There have been instances in which patients with captopril induced liver injury have been able to tolerate other ACE inhibitors without complications, but others in which there was evidence of cross reactivity. If patients with ACE inhibitor related cholestatic hepatitis are restarted on another ACE inhibitor, informed consent and careful monitoring for reappearance of liver injury for the first few weeks of therapy is warranted.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Captopril – Generic, Capoten®
DRUG CLASS
Angiotensin-Converting Enzyme Inhibitors
Product labeling at DailyMed, National Library of Medicine, NIH
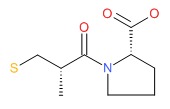
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Captopril | 62571-86-2 | C9-H15-N-O3-S |
 |
- PubChem SubstanceRelated PubChem Substances
- Angiotensin II receptor antagonists and heart failure: angiotensin-converting-enzyme inhibitors remain the first-line option.[Prescrire Int. 2005]Angiotensin II receptor antagonists and heart failure: angiotensin-converting-enzyme inhibitors remain the first-line option.. Prescrire Int. 2005 Oct; 14(79):180-6.
- Review Fosinopril.[LiverTox: Clinical and Researc...]Review Fosinopril.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Ramipril.[LiverTox: Clinical and Researc...]Review Ramipril.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Enalapril.[LiverTox: Clinical and Researc...]Review Enalapril.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Review Lisinopril.[LiverTox: Clinical and Researc...]Review Lisinopril.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Captopril - LiverToxCaptopril - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...