NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
Busulfan is an orally administered anticancer alkylating agent used in the treatment of chronic myelogenous leukemia, as well as a parenterally administered myeloablative agent used in preparation of hematopoietic cell transplantation (HCT). Busulfan has been linked to transient serum enzyme elevations during therapy, to rare cases of cholestatic hepatitis, instances of nodular regenerative hyperplasia and, when given in high doses, to sinusoidal obstruction syndrome which can be severe and fatal.
Background
Busulfan (bue sul' fan) is an alkylating agent of the alkylsulfonate type containing several methanesulfonate groups, which are hydrolyzed in aqueous solution and released to form reactive carbonium ions that alkylate DNA causing inhibition of DNA, RNA and protein synthesis and triggering cell death in rapidly dividing cells. Busulfan is effective in the palliative treatment of chronic myelogenous leukemia and as part of a myeloablative regimen in preparation of bone marrow or stem cell transplantation. Busulfan was approved for clinical use in the United States in 1954 and is still in wide use. Busulfan is available in tablets of 2 mg under the brand name of Myleran and in solution for intravenous infusion (6 mg/mL) as Busulflex. The recommended dosage is dependent on the disease process and patient age, body weight and comorbidities. Common side effects of busulfan include fatigue, weakness, dizziness, oral ulcers, pharyngitis, nausea, anorexia, abdominal discomfort and bone marrow suppression.
Hepatotoxicity
Oral busulfan therapy is associated with a low rate of serum enzyme elevations that are typically transient, mild and usually do not require dose adjustment. Rare instances of clinically apparent acute liver disease have been described in patients taking oral busulfan long term. The onset of injury is usually after years of therapy, and the pattern of serum enzyme elevations is usually cholestatic (Case 1). At least one case of cholestatic injury was reported to lead to hepatic failure.
Perhaps complicating the issue of interpretation of liver enzyme elevations during busulfan therapy is that chronic use of busulfan has been linked to cases of nodular regenerative hyperplasia. This process also arises at least six months if not years of therapy with busulfan or antimetabolites (azathioprine, thioguanine) and typically presents with signs of portal hypertension (varices, variceal hemorrhage, ascites) and minimal, nonspecific symptoms and serum enzyme elevations. Thrombocytopenia is almost always present and is often the first sign of evolving portal hypertension. Nodular regeneration usually starts to improve within a few weeks to months of stopping the antineoplastic or immunosuppressive therapy. However, in some cases the complications of portal hypertension are severe and can result in hepatic failure particularly if complicated by septicemia or other organ failure (Case 2).
Finally, and most importantly, busulfan given in high doses intravenously combined with total body irradiation, cyclophosphamide, or other alkylating agents in preparation for either bone marrow or stem cell transplantation (hematopoietic cell transplantation, HCT) has been linked to sinusoidal obstruction syndrome (SOS, formerly referred to as veno-occlusive disease). The onset of injury is usually within 10 to 20 days of HCT and presents with abdominal pain, liver tenderness, weight gain due to fluid accumulation, and jaundice. With some newer conditioning regimens, sinusoidal obstruction syndrome can present later, 30 to 75 days after HCT. The serum enzymes are usually elevated, typically with marked increases in serum aminotransferase levels (and lactic dehydrogenase), but minimal increases in alkaline phosphatase. In severe cases, there is hepatomegaly and ascites and signs of hepatic failure arise. Sinusoidal obstruction syndrome tends to be severe and the fatality rate can be as high as 50%. Poor prognostic signs are marked increases in serum ALT levels and high bilirubin levels. In fatal instances, death from multiorgan failure arises within days to weeks of onset. In cases with spontaneous recovery, there may be residual fibrosis or nodular regeneration. Autoantibody formation and allergic manifestations are uncommon, although fever may be present at onset. Risk factors for the development of sinusoidal obstruction syndrome are higher doses of busulfan, combination with cyclophosphamide and total body irradiation, pharmacokinetics that favor higher busulfan or cyclophosphamide exposure, and preexisting liver disease, particularly chronic hepatitis C. The frequency of sinusoidal obstruction syndrome after HCT ranges from 20% to 50%, but its incidence has decreased markedly in recent years with use of less aggressive conditioning regimens, better control over drug dosing, and lower frequency of hepatitis C (with elimination of posttransfusion hepatitis after anti-HCV testing of all blood donors).
Likelihood score: A (well known cause of clinically apparent liver injury, generally as a result of direct toxicity from high doses given for myeloablation in preparation for hematopoietic cell transplantation and rarely due to idiosyncratic liver injury).
Mechanism of Injury
Although the mechanism of idiosyncratic hepatotoxicity from busulfan is not clear, the drug undergoes extensive hepatic metabolism. Nodular regeneration is thought to be due to damage of small vasculature in the liver. Sinusoidal obstruction syndrome appears to be the result of direct cytotoxicity of busulfan and other agents to the hepatic sinusoidal lining cells, causing their extrusion and obstruction of sinusoids, congestion and centrolobular hepatic necrosis.
Outcome and Management
In cases of idiosyncratic liver injury, symptoms and liver enzymes abnormalities resolve within 1 to 2 months of stopping busulfan. Sinusoidal obstruction syndrome tends to be severe and the fatality rate is high. In patients who recover from sinusoidal obstruction syndrome, residual fibrosis and nodular regeneration may be present. Rechallenge with busulfan after clinically apparent liver injury should be avoided.
Drug Class: Antineoplastic Agents, Alkylating Agents
CASE REPORTS
Case 1. Cholestatic liver disease attributed to long term busulfan therapy.
[Modified from: Morris LE, Guthrie TH Jr. Busulfan-induced hepatitis. Am J Gastroenterol 1988; 83: 682-3. PubMed Citation]
A 61 year old man with chronic myelocytic leukemia developed fatigue, abdominal pain and fever after having been on busulfan in varying doses for 8 years. He had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. His only other medication was hydrochlorothiazide which he took for hypertension. On examination, he was febrile (38.5 oC), but had no jaundice, rash or hepatomegaly. Laboratory tests showed serum bilirubin of 0.9 mg/dL, ALT 77 U/L, AST 47 U/L, GGT 682 U/L, and alkaline phosphatase 449 U/L. Abdominal ultrasound showed no evidence of gallstones or biliary obstruction. Tests for hepatitis A and B were negative. A liver biopsy showed intrahepatic cholestasis and mild hepatocellular necrosis with minimal inflammation. His abdominal pain resolved rapidly but he continued to have low grade fevers. The pattern of liver enzyme elevations did not change and serum bilirubin remained normal. Two weeks after admission, busulfan and hydrochlorthiazide were discontinued and the fevers rapidly abated. In follow up two weeks later, he was asymptomatic and liver enzymes had decreased. One year later serum enzymes were normal. He had been switched to another thiazide diuretic.
Key Points
| Medication: | Busulfan |
|---|---|
| Pattern: | Cholestatic (R=~0.5) |
| Severity: | 1+ (serum enzyme elevations and symptoms without jaundice) |
| Latency: | 8 years |
| Recovery: | 2 weeks |
| Other medications: | Hydrochlorothiazide |
Comment
This patient developed a drug-fever accompanied by cholestatic serum enzyme elevations after 8 years of variable doses of busulfan. Such a long latency to onset is unusual for drug induced liver injury, but the patient had probably stopped and started the medication multiple times and was taking busulfan three times weekly at the time of onset of symptoms. The most convincing evidence for the role of busulfan was the persistence of fever while the medication was continued and its prompt resolution when busulfan was stopped. The only complicating issue is that hydrochlorthiazide was also being taken and was stopped at the same time.
Case 2. Nodular regenerative hyperplasia due to chronic therapy with busulfan and thioguanine.
[Modified from Case B in: Key NS, Kelly PM, Emerson PM, Chapman RW, Allan NC, McGee JO. Oesophageal varices associated with busulphan-thioguanine combination therapy for chronic myeloid leukaemia. Lancet 1987; 2: 1050-2. PubMed Citation]
A 58 year old woman with chronic myelogenous leukemia was treated with the combination of busulfan and thioguanine with an excellent palliative response. However, 16 months after starting combination therapy, she had acute upper gastrointestinal hemorrhage due to previously unsuspected esophageal varices. She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. Physical examination revealed splenomegaly and ascites. Serum bilirubin was 2.2 mg/dL, AST 65 U/L, Alk P 398 U/L, and INR was elevated at 1.5. Abdominal ultrasound showed a coarse liver texture, splenomegaly and ascites, but patent hepatic and portal veins. She continued to have variceal hemorrhage and underwent esophageal transection under general anesthesia. Postoperatively, she developed pneumonia, and progressive hepatic and respiratory failure and died. Autopsy showed a large liver (2110 grams) with a nodular surface. The hepatic veins were patent, but the portal vein contained a recent thrombus. Histologically, the liver showed diffuse regenerative hyperplasia with minimal fibrosis. There was sinusoidal infiltration by leukemic cells.
Key Points
| Medication: | Busulfan and thioguanine |
|---|---|
| Pattern: | Cholestatic (R=0.7) |
| Severity: | 5+ (hepatic failure and death) |
| Latency: | 16 months |
| Recovery: | None |
| Other medications: | None |
Comment
Chronic busulfan therapy, particularly when combined with thioguanine, has been linked to cases of noncirrhotic portal hypertension due to nodular regeneration hyperplasia. The process is often silent and serum enzymes are minimally and nonspecifically elevated. The first sign of impending portal hypertension is usually a fall in platelet count, but such changes are often attributed to the underlying condition or to bone marrow suppression by the alkylating agent or antimetabolite. The cause of nodular regeneration is unknown, but it is likely due to injury to the hepatic vasculature, and close inspection of portal areas in liver tissue usually shows injury or paucity of small portal veins. While the nodular regeneration leads to portal hypertension, hepatic function is usually preserved until there is a severe complication such as bleeding, hemodynamic instability and septicemia, at which time jaundice and other signs of hepatic failure can arise. This complication has led to the avoidance of the combination of thioguanine and busulfan as palliative therapy for chronic leukemia.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Busulfan – Myleran®
DRUG CLASS
Antineoplastic Agents, Alkylating Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULA AND STRUCTURE
| DRUG | CAS REGISTRY NUMBER | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Busulfan | 55-98-1 | C6-H14-O6-S2 |
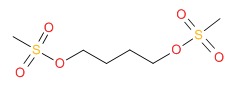 |
ANNOTATED BIBLIOGRAPHY
References updated: 02 October 2017
- Zimmerman HJ. Alkylating Agents. Oncotherapeutic and immunosuppressive agents. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 678-80.(Expert review of alkylating agents published in 1999; mentions that busulfan had been implicated in cholestatic jaundice, nodular regenerative hyperplasia [with thioguanine] and sinusoidal obstruction syndrome [SOS]).
- DeLeve LD. Cancer chemotherapy. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, p. 551.(Review of hepatotoxicity of cancer chemotherapeutic agents mentions that busulfan has been linked to rare cases of cholestatic hepatitis, nodular regeneration [NRH] and sinusoidal obstruction syndrome [SOS]).
- Chabner BA, Bertino J, Clearly J, Ortiz T, Lane A, Supko JG, Ryan DP. Cytotoxic agents. Chemotherapy of neoplastic diseases. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1677-730.(Textbook of pharmacology and therapeutics; busulfan is an alkyl sulfonate, its major adverse effect is myelosuppression; high doses cause SOS in up to 10% of patients).
- Kyle RA, Dameshek W. Porphyria cutanea tarda associated with chronic granulocytic leukemia treated with busulfan (Myleran). Blood 1964; 23: 776-85. [PubMed: 14161411](55 year old man with chronic myelogenous leukemia developed blast crisis, hyperpigmentation and elevations in urinary porphyrins while on busulfan therapy, liver tests were normal; autopsy showed iron overload).
- Underwood JC, Shahani RT, Blackburn EK. Cholestatic jaundice following treatment of chronic granulocytic leukemia with busulphan. J Clin Pathol 1970; 23: 827. [PMC free article: PMC476917] [PubMed: 5278972](25 year old man with chronic granulocytic leukemia developed jaundice after 6 years of maintenance busulfan [bilirubin 2.3 mg/dL, ALT 32 U/L, Alk P 3 times ULN], with progressive hepatic failure and death one month later).
- Shulman HM, McDonald GB, Matthews D, Doney KC, Kopecky KJ, Gauvreau JM, Thomas ED. An analysis of hepatic venocclusive disease and centrilobular hepatic degeneration following bone marrow transplantation. Gastroenterology 1980; 79: 1178-91. [PubMed: 7002704](Analysis of liver biopsies from 204 patients who had undergone HCT for various malignancies, finding SOS in 27 patients, early lesions being centrilobular congestion, hepatocyte degeneration and subintimal edema within small central venules and later lesions fibrous obliteration of central venule lumina and fibrosis; correlated with more aggressive conditioning regimens including those with busulfan).
- Farthing MJG, Clark MI, Sloan JP, Powles RI, McElwain TJ. Liver disease after bone marrow transplantation. Gut 1982; 23: 465-74. [PMC free article: PMC1419702] [PubMed: 7042484](Among 43 patients with HCT, 83% had elevations in liver tests and 7 died of liver disease; diagnoses being graft-vs-host disease in 48%, SOS in 5% [2 fatal cases] and multifactorial liver injury).
- Santos GW, Tutschka PJ, Brookmeyer R, Saral R, Beschorner WE, Bias WB, Braine HG, et al. Marrow transplantation for acute nonlymphocytic leukemia after treatment with busulfan and cyclophosphamide. N Engl J Med 1983; 309: 1347-53. [PubMed: 6355849](Pilot study of HCT for nonlymphocytic leukemia using a conditioning regimen of cyclophosphamide and busulfan; SOS occurred in only 3 patients which was considered lower than occurs with busulfan alone or with total body irradiation combined with cyclophosphamide).
- McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. Venocclusive disease of the liver after bone marrow transplantation: diagnosis, incidence, and predisposing factors. Hepatology 1984; 4: 116-22. [PubMed: 6363247](Among 255 patients undergoing HCT, 53 [21%] met criteria for SOS, its occurrence associated with older age, underlying liver disease, AST levels and diagnosis).
- McDonald GB, Sharma P, Matthews DE, Shulman HM, Thomas ED. The clinical course of 53 patients with venocclusive disease of the liver after marrow transplantation. Transplantation 1985; 39: 603-8. [PubMed: 3890288](Among 255 patients undergoing HCT for malignant disease, 53 [21%] developed SOS, marked by weight gain [93%] starting ~6 days after transplant followed by jaundice [98%: bilirubin 3.6-29.4 mg/dL], abdominal pain [75%], liver enlargement [68%]; 24 [45%] had serious progressive disease with ascites and hepatic encephalopathy and 17 [32%] died).
- Hartmann O, Benhamou E, Beaujean F, Pico JL, Kalifa C, Patte C, Flamant F, et al. High-dose busulfan and cyclophosphamide with autologous bone marrow transplantation support in advanced malignancies in children: a phase II study. J Clin Oncol 1986; 4: 1804-10. [PubMed: 3537217](Results of using busulfan and cyclophosphamide for HCT in 20 children: AST elevations occurred in 6 but severe SOS in only one child).
- Key NS, Kelly PM, Emerson PM, Chapman RW, Allan NC, McGee JO. Oesophageal varices associated with busulphan-thioguanine combination therapy for chronic myeloid leukaemia. Lancet 1987; 2: 1050-2. [PubMed: 2889964](Case series of 5 patients who developed abnormal liver tests 6 to 45 months after starting busulfan and thioguanine for chronic myelocytic leukemia with varices found after 16 to 98 months, biopsies showing nodular regeneration: Case 2).
- Peters WP, Henner WD, Grochow LB, Olsen G, Edwards S, Stanbuck H, Stuart A, et al. Clinical and pharmacologic effects of high dose single agent busulfan with autologous bone marrow support in the treatment of solid tumors. Cancer Res 1987; 47: 6402-6. [PubMed: 2824032](6 patients with advanced cancer received 4 days of high dose busulfan [16 mg/kg]; had limited efficacy and all developed bilirubin elevations and 2 had transient immunological syndromes: lupus and chronic active hepatitis, but these syndromes resolved without residal evidence of liver injury).
- Jones RJ, Lee KS, Beschorner WE, Vogel VG, Grochow LB, Braine HG, Vogelsang GB, et al. Veno-occlusive disease of the liver following bone marrow transplantation. Transplantation 1987; 4: 778-83. [PubMed: 3321587](Among 235 patients undergoing HCT between 1982 and 1985, sinusoidal obstruction syndrome [SOS] developed in 52 [22%] of whom half died, making SOS the third most common cause of death in this population).
- Morris LE, Guthrie TH Jr. Busulfan-induced hepatitis. Am J Gastroenterol 1988; 83: 682-3. [PubMed: 3376924](61 year old man with chronic myelocytic leukemia developed fever and alkaline phosphatase elevations after 8 years of busulfan therapy [bilirubin 0.9 mg/dL, ALT 77 U/L, Alk P 449 U/L], which resolved only once busulfan was stopped: Case 1).
- Brugieres L, Hartmann O, Benhamou E, Patte C, Kalifa C, Lemerle J. [Hepatic complications after high-dose chemotherapy and bone marrow autograft in solid tumors in children]. Presse Med 1988; 17: 1305-8. French. [PubMed: 2969580](Among 236 children receiving HCT between 1979 and 1987, liver disease arose after 43 courses [20%] in 39 patients, including SOS in 11 [5%] which was fatal in 4; risk factors included use of busulfan and previous high dose chemotherapy).
- Brugieres L, Hartmann O, Benhamou E, Zafrani ES, Caillaud JM, Patte C, Kalifa C, et al. Veno-occlusive disease of the liver following high-dose chemotherapy and autologous bone marrow transplantation in children with solid tumors: incidence, clinical course and outcome. Bone Marrow Transplant 1988; 3: 53-8. [PubMed: 3048471](Among 173 children undergoing 236 courses of high dose chemotherapy and HCT, 39 children had hepatic abnormalities and 11 met the criteria for having SOS; syndrome marked by weight gain [decrease in sodium excretion] with weight gain of 5-12% followed by hepatomegaly and jaundice [bilirubin 4.5-26.9 mg/dL, ALT 60-1850 U/L, Alk P 1.5-2.5 times ULN]; 4 patients [2%] died).
- Grochow LB, Jones RJ, Brundrett RB, Braine HG, Chen T-L, Saral R, Santos GW, et al. Pharmacokinetics of busulfan: correlation with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother Pharmacol 1989; 25: 55-61. [PubMed: 2591002](Analysis of busulfan pharmacokinetics in 15 patients undergoing HCT after conditioning with busulfan and cyclophosphamide showed marked variability in disposition and higher area under the curve plasma levels in 6 patients developing SOS).
- Adang RP, Breed WP. [Liver damage during busulfan therapy]. Ned Tijdschr Geneeskd 1989; 133: 1515-8. Dutch. [PubMed: 2797253](57 year old man with chronic megakaryocytic granulocytic myelosis, who was treated with intermittent low dose busulfan for 2 years, developed fatigue and elevations in liver enzymes [peak ALT 27 U/L, Alk P 602 U/L, bilirubin normal], improving but not resolving completely upon stopping busulfan).
- Peters WG. [Liver damage during busulfan therapy]. Reply by authors. Ned Tijdschr Geneeskd 1989; 133:1849-50. Dutch. [PubMed: 2797298](Letter in response to Adang [1989] suggesting that the cause of the abnormalities was SOS [although nodular regeneration was more likely]).
- Snover DC, Weisdorf S, Bloomer J, McGlave P, Weisdorf D. Nodular regenerative hyperplasia of the liver following bone marrow transplantation. Hepatology 1989; 9: 443-8. [PubMed: 2646196](Retrospective review of liver histology from 101 patients after HCT found SOS in 9% vs nodular regeneration in 23%, which were often indistinguishable clinically).
- Vassal G, Hartmann O, Benhamou E. Busulfan and veno-occlusive disease of the liver. Ann Intern Med 1990; 112: 881. [PubMed: 2344115](Among 403 children receiving busulfan for HCT, 28 [7%] developed SOS and 3 died; occurrence associated with higher doses).
- Shepherd P, Harrison DJ. Idiopathic portal hypertension associated with cytotoxic drugs. J Clin Pathol 1990; 43: 206-10. [PMC free article: PMC502331] [PubMed: 2332518](4 cases of portal hypertension developing in patients on thioguanine and busulfan for leukemia or chlorambucil for Hodgkin disease after 21-70 months, often with variable elevations in alkaline phosphatase).
- Shepherd PC, Fooks J, Gray R, Allan NC. Thioguanine used in maintenance therapy of chronic myeloid leukaemia causes non-cirrhotic portal hypertension. Results from MRC CML. II. Trial comparing busulphan with busulphan and thioguanine. Br J Haematol 1991; 79: 185-92. [PubMed: 1958475](Among 674 patients with chronic leukemia, 18 of 337 [5%] treated with busulfan and thioguanine developed portal hypertension after 1 to 8 years [median 2 years], compared to none of 338 given busulfan alone, whereas liver test abnormalities [~50%] and jaundice [~3%] occurred in similar proportions of both groups).
- Morgan M, Dodds A, Atkinson K, Szer J, Downs K, Biggs J. The toxicity of busulphan and cyclophosphamide as the preparative regimen for bone marrow transplantation. Br J Haematol 1991; 77: 529-34. [PubMed: 2025579](Retrospective analysis found SOS occurring in 12 of 67 patients [19%: one fatality] receiving busulfan and irradiation in preparation of HCT, compared to only 2 of 166 [1.3%] receiving cyclophosphamide and irradiation).
- Ozkaynak MF, Weinberg K, Kohn D, Sender L, Parkman R, Lenarsky C. Hepatic veno-occlusive disease post-bone marrow transplantation in children conditioned with busulfan and cyclophosphamide: incidence, risk factors, and clinical outcome. Bone Marrow Transplant 1991; 7: 467-74. [PubMed: 1908340](Retrospective analysis found SOS in 14 of 50 [36%] children given busulfan and cyclophosphamide in preparation for HCT, the injury arising 13-30 days after transplant and ultimately resolving in all).
- Kasai M, Kiyama Y, Watanabe M, Seto K, Matsuura A, Tanaka J, Takeda H, et al. Toxicity of high-dose busulfan and cyclophosphamide as a preparative regimen for bone marrow transplantation. Transplant Proc 1992; 24: 1529-30. [PubMed: 1496647](Among 16 patients given a regimen which included busulfan and cyclophosphamide in preparation of HCT, 4 developed SOS and died within 1-2 months).
- Méresse V, Hartmann O, Vassal G, Benhamou E, Valteau-Couanet D, Brugieres L, Lemerle J. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant 1992; 10: 135-41. [PubMed: 1525602](Among 136 children undergoing autologous HCT after conditioning with busulfan [with or without melphalan, thiotepa, or cyclophosphamide], 30 [22%] developed SOS by clinical criteria and 4 died [3%]; predictive risk factors included dose of alkylating agents in conditioning regimen).
- McDonald GB, Hinds MS, Fisher LD, Schoch HG, Wolford JL, Banaji M, Hardin BJ, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255-67. [PubMed: 8420443](Prospective analysis of 355 patients undergoing HCT; SOS developed in 54% and was severe in 16%, higher rates were associated with the combination of busulfan with cyclophosphamide [32%] and with total body irradiation [23%]; mortality rate being 98% in those with severe injury).
- Rosenthal MA, Grigg AP, Sheridan WP. High dose busulphan/cyclophosphamide for autologous bone marrow transplantation is associated with minimal non-hemopoietic toxicity. Leuk Lymphoma 1994; 14: 279-83. [PubMed: 7524888](Among 69 patients receiving busulfan and cyclophosphamide in preparation of HCT, 16 [23%] developed liver toxicity but most cases were mild; 6 patients developed SOS [9%] but only one died).
- Bearman SI. The syndrome of hepatic veno-occlusive disease after marrow transplantation. Blood 1995; 85: 3005-20. [PubMed: 7756636](Review of hepatic SOS after HCT; usually presents with painful hepatomegaly, weight gain [fluid and ascites] and jaundice within 3 weeks of myeloablation, histology showing occlusion of small central veins and sinusoids and extensive zone 3 [centrolobular] injury).
- Dix SP, Wingard JR, Mullins RE, Jerkunica I, Davidson TG, Gilmore CE, York RC, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant 1996; 17: 225-30. [PubMed: 8640171](Pharmacokinetic study of busulfan during myeloablative preparation of patients for HCT found higher rate of SOS with the combination of cyclophosphamide and busulfan [4 of 10: 40%] compared to busulfan with etoposide [7 of 38] or Ara-C [1 of 18], despite similar drug levels).
- Styler MJ, Crilley P, Biggs J, Moul J, Copelan E, Topolsky D, Avalos B, et al. Hepatic dysfunction following busulfan and cyclophosphamide myeloablation: a retrospective, multicenter analysis. Bone Marrow Transplant 1996; 18: 171-6. [PubMed: 8832011](Among 350 patients treated with busulfan and cyclophosphamide in preparation of HCT, 27% developed SOS, 11% being severe).
- Atra A, Whelan JS, Calvagna V, Shankar AG, Ashley S, Shepherd V, Souhami RL, et al. High-dose busulphan/melphalan with autologous stem cell rescue in Ewing's sarcoma. Bone Marrow Transplant 1997; 20: 843-6. [PubMed: 9404924](Among 18 patients given busulfan and melphalan in preparation for HCT, only one patient developed SOS, which was not fatal).
- Diaz MA, Vicent MG, Madero L. High-dose busulfan/melphalan as conditioning for autologous PBPC transplantation in pediatric patients with solid tumors. Bone Marrow Transplant 1999; 24: 1157-9. [PubMed: 10642802](Among 30 children given busulfan and melphalan in preparation for HCT, 3 developed transient liver test abnormalities and one developed fatal SOS).
- Lee JL, Gooley T, Bensinger W, Schiffman K, McDonald GB. Veno-occlusive disease of the liver after busulfan, melphalan, and thiotepa conditioning therapy: incidence, risk factors, and outcome. Biol Blood Marrow Transplant 1999; 5: 306-15. [PubMed: 10534061](Among 253 patients who received a regimen of busulfan, melphalan and thiotepa in preparation for HCT, 70 [28%] developed SOS, which was severe in 11 and fatal in 9).
- Hassan M, Ljungman P, Ringden O, Hassan Z, Oberg G, Nilsson C, Bekassy A, et al. The effect of busulphan on the pharmacokinetics of cyclophosphamide and its 4-hydroxy metabolite: time interval influence on therapeutic efficacy and therapy-related toxicity. Bone Marrow Transplant 2000; 25: 915-24. [PubMed: 10800057](Cyclophosphamide given sooner rather than later after busulfan in preparation for HCT was associated with a higher rate of side effects including SOS [58% vs 14%]).
- Olavarria E, Hassan M, Eades A, Nilsson C, Timms A, Matthews J, Craddock C, et al. A phase I/II study of multiple-dose intravenous busulfan as myeloablation prior to stem cell transplantation. Leukemia 2000; 14: 1954-9. [PubMed: 11069031](In a pilot study of an intravenous preparation of busulfan, none of 12 patients undergoing HCT developed SOS).
- Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W, Blume K, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant 2002; 8: 493-500. [PubMed: 12374454](Retrospective analysis found a higher rate of SOS after oral [33%: 10 of 30] vs intravenous [8%: 5 of 61] busulfan with cyclophosphamide in preparation of HCT).
- Fernandez HF, Tran HT, Albrecht F, Lennon S, Caldera H, Goodman MS. Evaluation of safety and pharmacokinetics of administering intravenous busulfan in a twice-daily or daily schedule to patients with advanced hematologic malignant disease undergoing stem cell transplantation. Biol Blood Marrow Transplant 2002; 8: 486-92. [PubMed: 12374453](Among 12 patients receiving intravenous busulfan either as one or two infusions daily for 4 days followed by cyclophosphamide in preparation for HCT, only one developed SOS).
- Bornhauser M, Storer B, Slattery J, Appelbaum FR, Deeg HJ, Hansen J, Martin PJ, et al. Conditioning with fludarabine and targeted busulfan for transplantation of allogeneic hematopoietic stem cells. Blood 2003; 102: 820-6. [PubMed: 12676781](Assessment of conditioning regimen of busulfan and fludarabine in preparation for HCT in 42 patients found low rate of toxicity and excellent survival rates).
- De Lima M, Couriel D, Thall PF, Wang X, Madden T, Jones R, Shpall EJ, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 2004; 104: 857-64. [PubMed: 15073038](Assessment of busulfan combined with fludarabine as a conditioning regimen for HCT found low rate of toxicity and improved survival).
- Foschi FG, Savini P, Marano G, Musardo G, Bedeschi E, Girelli F, Emiliani F, et al. Focal nodular hyperplasia after busulfan treatment. Dig Liver Dis 2005; 37: 619-21. [PubMed: 15886082](48 year old woman was found to have a 2.9 by 2.5 cm focal nodular hyperplasia after a year of therapy with busulfan for thrombocytosis, which gradually decreased in size upon stopping busulfan and was no longer detectable 2 years later).
- Kusumi E, Kami M, Kanda Y, Murashige N, Seki K, Fujiwara M, Koyama R, et al. Hepatic injury following reduced intensity unrelated cord blood transplantation for adult patients with hematological diseases. Biol Blood Marrow Transplant 2006; 12: 1302-9. [PubMed: 17162212](Among 104 patients who underwent reduced intensity cord blood transplantation using melphalan/fludarabine and total body irradiation, liver injury occurred in 36 [35%] which was mainly due to graft-vs-host disease and septicemia; no cases of SOS).
- McDonald GB. Review article: management of hepatic disease following haematopoietic cell transplant. Aliment Pharmacol Ther 2006; 24: 441-52. [PubMed: 16886910](Sinusoidal obstruction syndrome arises in first 20-30 days after HCT, case fatality rate is 15-20%, frequency ~38%, but frequency varies by risk factors [cyclophosphamide pharmacokinetics, preexisting liver disease, irradiation dose and use of gemtuzumab], no therapy has proven efficacious but defibrotide and repletion of glutathione deserve evaluation).
- Carreras E, Rosiñol L, Terol MJ, Alegre A, de Arriba F, García-Laraña J, Bello JL, et al.; Spanish Myeloma Group/PETHEMA. Veno-occlusive disease of the liver after high-dose cytoreductive therapy with busulfan and melphalan for autologous blood stem cell transplantation in multiple myeloma patients. Biol Blood Marrow Transplant 2007; 13: 1448-54. [PubMed: 18022574](Among 734 patients undergoing autologous HCT for multiple myeloma, SOS occurred in 8% [19 of 240] with 2% mortality [median onset 29 days] receiving busulfan and melphalan, but in only 0.4% [2 of 494: 0.2% mortality, onset 9-13 days] receiving melphalan alone).
- McCune JS, Batchelder A, Deeg HJ, Gooley T, Cole S, Phillips B, Schoch HG, et al. Cyclophosphamide following targeted oral busulfan as conditioning for hematopoietic cell transplantation: pharmacokinetics, liver toxicity, and mortality. Biol Blood Marrow Transplant 2007; 13: 853-62. [PubMed: 17580264](Cyclophosphamide pharmacokinetics were determined in 222 patients receiving cyclophosphamide and either busulfan or total body irradiation in preparation of HCT; there was considerable variability in cyclophosphamide levels, being higher in busulfan regimens and higher levels correlating with risk of SOS).
- Gekkurt E, Stoehlmacher J, Stueber C, Wolschke C, Eiermann T, Iacobelli S, Zander AR, et al. Pharmacogenetic analysis of liver toxicity after busulfan/cyclophosphamide-based allogeneic hematopoietic stem cell transplantation. Anticancer Res 2007; 27: 4377-80. [PubMed: 18214047](Retrospective analysis of methylene-tetrahydrofolate-reductase [MTHFR] and glutathione S-transferase [GST] polymorphisms among 84 adults receiving busulfan and cyclophosphamide in preparation for HCT found weak association between a MTHFR polymorphism and sinusoidal obstruction syndrome [86% rate in those who were homozygous compared to 39% of those who were heterozygous or wild type]).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J; Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology 2008; 135: 1924-34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected between 2004 and 2008, antineoplastic agents were rarely implicated; 3 were considered due to mercaptopurine and 1 each due to bortezombin, cyclophosphamide, docetaxel, and temozolomide).
- Ulrickson M, Aldridge J, Kim HT, Hochberg EP, Hammerman P, Dube C, Attar E, Ballen KK, Dey BR, McAfee SL, Spitzer TR, Chen YB. Busulfan and cyclophosphamide (Bu/Cy) as a preparative regimen for autologous stem cell transplantation in patients with non-Hodgkin lymphoma: a single-institution experience. Biol Blood Marrow Transplant 2009; 15: 1447-54. [PubMed: 19822305](Among 78 patients with non-Hodgkin lymphoma undergoing autologous HCT after conditioning with busulfan and cyclosphosphamide, 3 [4%] developed SOS, none fatal).
- McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology 2010; 51: 1450-60. [PMC free article: PMC2914093] [PubMed: 20373370](Review of liver complications of HCT, which have become less frequent with better understanding of their cause and means of prevention; the rate of SOS has decreased because of avoidance of more aggressive ablative therapies [total body irradiation and high dose cyclophosphamide], better understanding of pharmacokinetics of the alkylating agents, and lower frequency of hepatitis C in HCT recipients).
- O'Donnell PH, Artz AS, Undevia SD, Pai RK, Del Cerro P, Horowitz S, Godley LA, et al. Phase I study of dose-escalated busulfan with fludarabine and alemtuzumab as conditioning for allogeneic hematopoietic stem cell transplant: reduced clearance at high doses and occurrence of late sinusoidal obstruction syndrome/veno-occlusive disease. Leuk Lymphoma 2010; 51: 2240-9. [PMC free article: PMC4477684] [PubMed: 20919852](Safety study of busulfan based therapy for HCT recipients; the risk of SOS appeared to correlate with high exposures to busulfan).
- Reuben A, Koch DG, Lee WM; Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology 2010; 52: 2065-76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury, but few anticancer drugs were implicated [1 case each for melphalan and gemtuzumab]).
- Cantoni N, Gerull S, Heim D, Halter J, Bucher C, Buser A, Tsakiris DA, et al. Order of application and liver toxicity in patients given BU and CY containing conditioning regimens for allogeneic hematopoietic SCT. Bone Marrow Transplant 2011; 46: 344-9. [PubMed: 20548339](Retrospective analysis of liver toxicity after conditioning regimens of busulfan followed by cyclosphospamde versus the reverse order found higher rate of SOS when busulfan was given first [12.5%: 2 of 16 patients] than when it was given after cyclosphosphamide [0 of 59 patients]).
- Pai RK, van Besien K, Hart J, Artz AS, O'Donnell PH. Clinicopathologic features of late-onset veno-occlusive disease/sinusoidal obstruction syndrome after high dose intravenous busulfan and hematopoietic cell transplant. Leuk Lymphoma 2012; 53: 1552-7. [PMC free article: PMC4482341] [PubMed: 22280517](Among 36 patients with advanced malignancies undergoing HCT after high escalating doses of busulfan with fludarabine and alemtuzumab, 8 developed late onset SOS, arising 33 to 77 days after HCT with ascites, hepatomegaly and jaundice [bilirubin 1.3-22.9 mg/dL]; four died).
- Perkins JB, Kim J, Anasetti C, Fernandez HF, Perez LE, Ayala E, Kharfan-Dabaja MA, et al. Maximally tolerated busulfan systemic exposure in combination with fludarabine as conditioning before allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2012; 18: 1099-107. (Among 72 patients with malignancies undergoing HCT after a conditioning regimen of fludarabine with different levels of systemic exposures to busulfan, SOS occurred in 0 of 40 given the standard exposure, but 2 of 29 with the intermediate high level and 3 of 3 at the highest level; ALT elevations [>3 times ULN] occurring in 20%, 31% and 100% of subjects). [PubMed: 22198540]
- Dignan FL, Wynn RF, Hadzic N, Karani J, Quaglia A, Pagliuca A, Veys P, et al.; Haemato-oncology Task Force of British Committee for Standards in Haematology; British Society for Blood and Marrow Transplantation. BCSH/BSBMT guideline: diagnosis and management of veno-occlusive disease (sinusoidal obstruction syndrome) following haematopoietic stem cell transplantation. Br J Haematol 2013; 163: 444-57. [PubMed: 24102514](Guidelines for diagnosis, prophylaxis and treatment of SOS after HCT from a British task force recommends using clinical criteria for diagnosis and defibrotide for prophylaxis and treatment combined with judicious clinical care, and mentions that busulfan particularly in combination with cyclophosphamide is a risk factor).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al.; United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology 2015; 148: 1340-52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 49 [5%] were attributed to antineoplastic agents, but none were due to busulfan).
- Chen S, Osborn JD, Chen X, Boyer MW, McDonald GB, Hildebrandt GC. Subacute hepatic necrosis mimicking veno-occlusive disease in a patient with HFE H63D homozygosity after allogeneic hematopoietic cell transplantation with busulfan conditioning. Int J Hematol 2015; 102: 729-31. [PubMed: 26497867](31 year old man with myelogenous leukemia who underwent allogenic hematopoietic cell transplantation after myeloablation with busulfan and fludarabine developed fever, rash, abdominal pain and jaundice with ascites [bilirubin 1.3 rising to 5.3 mg/dL, ALT 330 to 1339 U/L, and Alk P 84 U/L], biopsy being interpreted as showing hepatitis rather than sinusoidal obstruction or graft-vs-host disease).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Melphalan.[LiverTox: Clinical and Researc...]Review Melphalan.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Retrospective study of the digestive tract mucositis derived from myeloablative and non-myeloablative/reduced-intensity conditionings with busulfan in hematopoietic cell transplantation patient.[Support Care Cancer. 2019]Retrospective study of the digestive tract mucositis derived from myeloablative and non-myeloablative/reduced-intensity conditionings with busulfan in hematopoietic cell transplantation patient.Eduardo FP, Bezinelli LM, Gobbi M, Rosin FCP, Carvalho DLC, Ferreira MH, da Silva CC, Hamerschlak N, Corrêa L. Support Care Cancer. 2019 Mar; 27(3):839-848. Epub 2018 Aug 14.
- Reduced-Intensity Conditioning with Busulfan, Fludarabine, and Antithymocyte Globulin for Hematopoietic Cell Transplantation from Unrelated or Haploidentical Family Donors in Patients with Acute Myeloid Leukemia in Remission.[Biol Blood Marrow Transplant. ...]Reduced-Intensity Conditioning with Busulfan, Fludarabine, and Antithymocyte Globulin for Hematopoietic Cell Transplantation from Unrelated or Haploidentical Family Donors in Patients with Acute Myeloid Leukemia in Remission.Lee KH, Lee JH, Lee JH, Kim DY, Park HS, Choi EJ, Ko SH, Seol M, Lee YS, Kang YA, et al. Biol Blood Marrow Transplant. 2017 Sep; 23(9):1555-1566. Epub 2017 May 25.
- Association Between the Magnitude of Intravenous Busulfan Exposure and Development of Hepatic Veno-Occlusive Disease in Children and Young Adults Undergoing Myeloablative Allogeneic Hematopoietic Cell Transplantation.[Transplant Cell Ther. 2022]Association Between the Magnitude of Intravenous Busulfan Exposure and Development of Hepatic Veno-Occlusive Disease in Children and Young Adults Undergoing Myeloablative Allogeneic Hematopoietic Cell Transplantation.Bognàr T, Bartelink IH, Egberts TCG, Rademaker CMA, Versluys AB, Slatter MA, Kletzel M, Nath CE, Cuvelier GDE, Savic RM, et al. Transplant Cell Ther. 2022 Apr; 28(4):196-202. Epub 2022 Jan 19.
- Review Azathioprine.[LiverTox: Clinical and Researc...]Review Azathioprine.. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. 2012
- Busulfan - LiverToxBusulfan - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...