NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-.

LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].
Show detailsOVERVIEW
Introduction
The combination of amoxicillin and clavulanate is an oral antibiotic widely used in the treatment of mild-to-moderate bacterial infections including sinusitis, bronchitis, otitis media, cellulitis and community acquired pneumonia. Amoxicillin-clavulanate is currently the most common cause of clinically apparent, drug induced acute liver injury both in the United States and Europe.
Background
The combination of amoxicillin and clavulanate is a commonly used antibiotic which is active against many bacterial organisms that cause sinusitis, bronchitis, otitis media, skin and tissue infections and community acquired pneumonia. The combination consists of amoxicillin which is a semisynthetic, third generation penicillin and clavulanate which is a beta lactam that acts as an inhibitor of beta lactamase, the major bacterial enzyme responsible for penicillin resistance. Amoxicillin-clavulanate was approved for use in the United States in 1984 and, currently, approximately 6 million prescriptions are filled yearly, making it one of the most common antibiotic regimens used. Current indications are for mild-to-moderate bacterial infections due to known or suspected penicillinase resistant gram positive or gram negative organisms. This combination is provided in multiple dose combinations, typically as 250 to 875 mg amoxicillin with 125 mg of clavulanate, given two to three times daily for 7 to 10 days. Amoxicillin-clavulanate is available in multiple generic formulations and under the brand name Augmentin. Side effects are usually mild and self-limited and can include diarrhea, nausea and vomiting, fatigue, headache, and rash. Rare but potentially serious adverse events include hypersensitivity reactions, anaphylaxis, severe skin rash, Stevens Johnson syndrome, C. difficile diarrhea, interstitial nephritis, neutropenia, aplastic anemia and thrombocytopenic purpura.
Hepatotoxicity
Amoxicillin-clavulanate has been implicated in hundreds of cases of clinically apparent acute liver injury and this combination is currently the most common cause of drug induced liver disease in most large case series from the United States and Europe. The onset of injury is typically a few days to as long as 8 weeks (average ~3 weeks) after initiation of therapy and often occurs after the course of antibiotic is completed, the delay being a few days to as long as six weeks. The onset is typically with fatigue, low grade fever, nausea and abdominal pain, followed by pruritus and jaundice. The pattern of liver enzyme elevations is typically cholestatic with marked elevations in alkaline phosphatase and gamma glutamyl transpeptidase (Case 1). In some instances, aminotransferase levels are markedly elevated giving a mixed (Case 2) or hepatocellular pattern (Case 3), particularly in younger patients with earlier onset of injury. In children, amoxicillin-clavulanate hepatotoxicity is typically anicteric and presents with nausea, vomiting and abdominal pain rather than jaundice and itching. The pattern of serum enzyme elevations is also much more likely to be hepatocellular in children, but the course of illness is typically benign. Because the liver injury may present days or weeks after stopping therapy, the association of the liver injury with receipt of amoxicillin-clavulanate may be missed. Immunoallergic features (fever, rash, eosinophilia) can occur, but are not invariably present and are usually not prominent. Autoantibody formation is not common. The hepatic injury is idiosyncratic and is estimated to occur after ~1 in 2,500 prescriptions. The injury is more common in men than women, in the elderly and after multiple courses. Genetic studies indicate a link with HLA types, particularly the extended haplotype: DRB1*15:01-DRB5*01:01-DQB1*06:02.
Likelihood score: A (well established cause of clinically apparent liver injury).
Mechanism of Injury
The cause of amoxicillin-clavulanate hepatotoxicity is unknown, but is probably immunoallergic in origin. Allergic manifestations can occur and include rash, fever, arthralgias and eosinophilia. Several studies have reported an HLA Class II association with DRB1*15:01 and the extended haplotype DRB1*15:01-DRB1*01:01-DQB1*06:02. An independent HLA Class I association has also been made with HLA-A*02:01. The liver injury appears to be due to the clavulanate rather than amoxicillin, as reexposure to amoxicillin alone has not been associated with recurrence (Case 5), whereas reexposure to the combination is usually followed by a more rapid onset of a more severe hepatic injury, which can include prolonged cholestasis and development of cirrhosis. Other beta lactamase inhibitors (tazobactam and sulbactam) have not been reported to cause a similar hepatic injury, although it has been reported with other penicillins when combined with clavulanate (ticarcillin/clavulanate).
Outcome and Management
The liver injury caused by amoxicillin-clavulanate is typically associated with jaundice and can be severe and prolonged (with jaundice lasting 4 to 24 weeks), but rarely results in lasting injury or death. Deaths due to amoxicillin-clavulanate hepatic injury have been described, but largely in patients with other comorbidities including cirrhosis or with multiple exposures. In addition, rare instances of prolonged cholestasis and vanishing bile duct syndrome have been reported after acute amoxicillin-clavulanate injury. Corticosteroids have been used in patients with marked or prolonged cholestasis, but their efficacy has not been shown and their use cannot be recommended routinely. Cholestyramine or ursodiol may help alleviate symptoms but probably do not speed recovery. Rechallenge with amoxicillin-clavulanate results in recurrence and should be avoided. Amoxicillin alone, on the other hand, is safe and does not cause recurrence of liver injury except in the rare instance in which the penicillin rather than clavulanate is responsible for the liver injury.
Drug Class: Antiinfective Agents, Aminopenicillins
Other Drugs in the Subclass, Aminopenicillins: Ampicillin, Ampicillin-Sulbactam, Amoxicillin, Bacampicillin, Pivampicillin, Ticarcillin-Clavulanate
CASE REPORTS
Case 1. Cholestatic hepatitis from amoxicillin-clavulanate.(1)
A 75 year old man with a history of prostate cancer and regular alcohol use (2 to 3 drinks daily) was given amoxicillin-clavulanate (500 mg/125 mg) for chronic maxillary sinusitis. Because of persistent symptoms, the antibiotic was continued for 31 days. When seen in follow up two weeks later, he complained of jaundice and was admitted to the hospital for evaluation. He had symptoms of dark urine, weakness, and poor appetite. Blood test results showed a total bilirubin of 42.7 mg/dL, ALT 194 U/L, AST 107 U/L, and alkaline phosphatase 257 U/L. Tests for acute hepatitis A, B, C and E were negative. Ultrasound of the abdomen showed no evidence of biliary obstruction or gallstones. During the hospitalization he developed profound anemia and thrombocytopenia requiring blood and platelet transfusions, and was further treated with corticosteroids and cyclophosphamide. His serum bilirubin peaked at 48.8 mg/dL and remained elevated for several months while aminotransferase and alkaline phosphatase levels were only modestly elevated (Table). The prothrombin time was elevated (INR 1.4 to 1.6) transiently and he developed mild confusion that was believed to be due to hepatic encephalopathy; he was treated with lactulose. During the hospitalization he developed renal failure and required dialysis. A liver biopsy was performed and findings were consistent with amoxicillin-clavulanate hepatotoxicity. He was hospitalized for 2 months and required another several months to recover fully. However, when seen 4 months after onset of the jaundice, he was back to his usual state of health and had normal laboratory tests, including normal aminotransferase and alkaline phosphatase levels, normal serum bilirubin and creatinine, and normal hemoglobin and platelet counts.
Key Points
| Medication: | Amoxicillin-clavulanate 500/125 mg thrice daily for 31 days |
|---|---|
| Pattern: | Cholestatic (R=1.9) |
| Severity: | 4+ (jaundice, hospitalization, severe thrombocytopenia and acute renal failure) |
| Latency: | 45 days, 14 days after stopping |
| Recovery: | Slowly over 4 months |
| Other medications: | Guaifenesin |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 31 days | 0 | Amoxicillin-clavulanate stopped | |||
| 40 days | 9 days | Jaundice | |||
| 6 weeks | 2 weeks | 126 | 195 | 43.8 | Admission, platelets 2,000 |
| 7 weeks | 3 weeks | 58 | 165 | 48.6 | |
| 2 months | 4 weeks | 59 | 248 | 48.8 | |
| 5 weeks | 44 | 198 | 42.4 | Acute renal failure | |
| 7 weeks | 40 | 195 | 19.4 | Liver biopsy | |
| 3 months | 8 weeks | 40 | 267 | 7.4 | |
| 98 | 67 | 47 | 242 | 3.6 | Discharged, platelets 376,000 |
| 158 | 127 | 11 | 81 | 0.6 | Creatinine 2.5 |
| Normal Values | <42 | <115 | <1.2 | ||
Comment
This case is an example of severe amoxicillin-clavulanate hepatotoxicity. Liver histology showed central lobular retention of bile with mixed infiltrates of lymphocytes, neutrophils and eosinophils in portal areas and in areas of focal spotty necrosis in the parenchyma. There was minimal steatosis, no fibrosis and no biliary damage or peribiliary fibrosis or edema. The findings were consistent with a drug induced intrahepatic cholestasis. The case was typical of amoxicillin-clavulanate hepatotoxicity in its onset 1 to 2 weeks after stopping therapy in an elderly man without other history or risk factors for liver or biliary disease. The severe thrombocytopenia and anemia were atypical, but similar nonhepatic manifestations of immunoallergic injury have been reported. Important conditions to exclude were biliary obstruction due to malignancy or gallstone disease and viral hepatitis. Therapy should be limited to symptomatic management of pruritus and avoidance of further hepatic injury. Appropriate management calls for follow up documentation of resolution of liver injury. The patient should be warned to avoid any further exposure to amoxicillin-clavulanate. In view of the severity of the injury, use of amoxicillin alone might also be best avoided.
Case 2. Mixed cholestatic-hepatocellular injury due to amoxicillin-clavulanate.(1)
A 52 year old man without major medical illnesses developed an upper respiratory infection and was given a 14 day course of amoxicillin-clavulanate. One and two months later, because of similar symptoms of fever and congestion, he was given a 2nd and 3rd 14 day course along with antihistamines and decongestants. Two weeks after the 3rd course of antibiotics, he developed abdominal pain, nausea, poor appetite, and itching, followed soon after by jaundice and dark urine. He was seen and blood tests revealed total bilirubin of 3.9 mg/dL with marked elevations in both ALT and alkaline phosphatase (Table). He was managed as an outpatient, did not undergo liver biopsy, and recovered symptomatically over the next few weeks. When seen one year later, he was asymptomatic and laboratory tests had returned to normal.
Key Points
| Medication: | Amoxicillin-clavulanate 500/125 mg twice daily for 42 days |
|---|---|
| Pattern: | Mixed cholestatic-hepatocellular (R=2.3) |
| Severity: | 2+ (jaundice, never hospitalized) |
| Latency: | 58 days, 16 days after stopping |
| Recovery: | Six months after stopping |
| Other medications: | None |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 2 weeks | 0 | Amoxicillin-clavulanate stopped | |||
| 4 weeks | 16 days | Nausea, jaundice, arthralgia | |||
| 16 days | 879 | 731 | 3.9 | Albumin 4.3 g/dL | |
| 5 weeks | 3 weeks | 629 | 503 | 1.7 | |
| 7 weeks | 5 weeks | 141 | 182 | 0.8 | |
| 10 weeks | 8 weeks | 90 | 103 | 0.8 | |
| 14 weeks | 3 months | 88 | 95 | 0.6 | INR 0.9 |
| ~1 year | 57 | 76 | 0.7 | Albumin 4.5 g/dL | |
| Normal Values | <65 | <126 | <1.2 | ||
Comment
This case is a typical example of mild-to-moderate amoxicillin-clavulanate hepatotoxicity. The early appearance of itching and high alkaline phosphatase levels (~6 fold elevated) indicated that the injury was “cholestatic,” while the high AST and ALT levels (peak at 10-15 fold elevated) provided the evidence that the injury was “mixed”. The repeated courses of the combination within a short period of time may have predisposed to the hepatotoxicity. The jaundice and itching lasted only two weeks, and he eventually had full recovery.
Case 3. Hepatocellular injury due to amoxicillin-clavulanate.(1)
A woman in her 20s received a ten day course of amoxicillin-clavulanate (500 mg/125 mg) twice daily for suspected sinusitis. She felt somewhat nauseated during therapy, but was then without symptoms until 8 to 9 weeks after stopping therapy when she developed anorexia, nausea, vomiting and abdominal pain. She noted dark urine and jaundice and went to an emergency room where blood tests were taken showing a total bilirubin of 12.5 mg/dL, ALT 3500 U/L, AST 2554 U/L, and alkaline phosphatase 187 U/L. She was also on birth control pills and had intermittently taken cold and sinus remedies and loratadine for allergic sinusitis during the previous two months. She received a one week course of nabumetone (a nonsteroidal antiinflammatory agent) and prednisone for acute neck spasms one month previously. She denied taking acetaminophen in the recent past and had no history of exposure to viral hepatitis or known risk factors for hepatitis or liver disease. She drank little alcohol and had never received amoxicillin-clavulanate previously. Tests for acute hepatitis A, B, C and E were negative as were tests for antinuclear and smooth muscle antibody. An ultrasound of the abdomen showed no evidence of biliary tract disease or gallstones. She did not undergo liver biopsy. Serum aminotransferase levels decreased while serum bilirubin rose to 27.2 mg/dL before falling (Table). Serum alkaline phosphatase levels were never very elevated. Prothrombin time was abnormal for a short period (peak INR 1.7). She recovered slowly and remained symptomatic with fatigue and abdominal discomfort for several months. When seen six months after onset of jaundice, she had no symptoms and serum bilirubin, aminotransferase and alkaline phosphatase levels had returned to normal.
Key Points
| Medication: | Amoxicillin-clavulanate unknown dose twice daily for 14 days |
|---|---|
| Pattern: | Hepatocellular (R=66) |
| Severity: | 4+ (jaundice, hospitalization and INR >1.5) |
| Latency: | 80 days, 66 days since stopping medication |
| Recovery: | <2 months after onset of jaundice |
| Other medications: | Oral contraceptives for 4 years |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Comments |
|---|---|---|---|---|---|
| 14 days | 0 | Amoxicillin-clavulanate stopped | |||
| 11 weeks | 66 days | Jaundice followed by fatigue and severe itching | |||
| 12 weeks | 75 days | 3500 | 187 | 12.5 | Admission |
| 13 weeks | 78 days | 955 | 156 | 16.4 | |
| 14 weeks | 84 days | 438 | 159 | 16.3 | |
| 89 days | 308 | 134 | 11.6 | ||
| 15 weeks | 91 days | 210 | 145 | 7.0 | Discharge |
| 19 weeks | 4 months | 21 | 65 | 1.9 | Asymptomatic |
| Normal Values | <42 | <115 | <1.2 | ||
Comment
This case is an example of moderate-to-severe amoxicillin-clavulanate hepatotoxicity. Somewhat atypical was the long incubation period between stopping the antibiotic and onset of jaundice as well as the prominent elevations in serum aminotransferase levels, the heights of which suggested viral hepatitis or acetaminophen overdose. The prominent ALT elevations and symptoms of fatigue and nausea rather than itching indicate a hepatocellular rather than cholestatic pattern of injury. Amoxicillin-clavulanate is typically associated with cholestatic injury but hepatocellular patterns can occur, particularly in younger patients such as this young woman. The potential role of other medications taken at the time (including unacknowledged receipt of acetaminophen) should be considered. While self-limited, the course of illness was severe and protracted. Further follow up is warranted to exclude a relapsing, acute onset of autoimmune hepatitis.
Case 4. Mixed cholestatic-hepatocellular injury due to amoxicillin-clavulanate.(1)
A 60 year old man was hospitalized with cellulitis and was discharged on a 10 day course of amoxicillin-clavulanate (500 mg/125 mg) twice daily. At the time of hospitalization, he had normal serum bilirubin, alkaline phosphatase and aminotransferase levels. Five days after stopping the antibiotic, he developed itching followed by poor appetite. After ten days of symptoms, he noted dark urine and pale stools and was seen in an emergency room. He was given intravenous fluids for dehydration and hydroxyzine for itching. When laboratory tests showed a total bilirubin of 7.7 mg/dL, alkaline phosphatase 286 U/L, ALT 387 U/L, and AST 134 U/L, he was admitted for evaluation. Prothrombin time and albumin levels were normal. He had no previous history of liver disease and drank little alcohol. Over the previous week he had taken acetaminophen (up to 4 grams daily) for back pain and itching. On a chronic basis, he took montelukast (Singular) and used inhalers (with albuterol, fluticasone and salmeterol) for asthma; was prescribed losartan (Cozaar) for hypertension; and, used several topical lotions for psoriasis. Tests for acute hepatitis A, B and C were negative. He had IgG antibody to HEV but IgM anti-HEV was negative. Autoantibodies including antinuclear and smooth muscle antibodies were not detected. An abdominal ultrasound showed no evidence of biliary obstruction and no gallstones. A liver biopsy was obtained which showed a mixed hepatocellular-cholestatic pattern of injury compatible with drug induced liver disease. During the next several days serum bilirubin levels rose to 8.1 mg/dL, but then started to decline even as ALT and AST levels rose to 8 to 20 fold above normal and alkaline phosphatase to 2.5 times normal (Table). He was discharged after a week on symptomatic therapy with cholestyramine. He remained symptomatic with itching and fatigue for another month, but on a return visit two months after presentation he felt well and laboratory tests had returned almost to baseline values.
Key Points
| Medication: | Amoxicillin-clavulanate 875/125 mg twice daily for 10 days |
|---|---|
| Pattern: | Mixed cholestatic-hepatocellular (R=3.9) |
| Severity: | 3+ (jaundice, hospitalization) |
| Latency: | 24 days, 14 days after stopping |
| Recovery: | Within 2 months of stopping |
| Other medications: | Montelukast, fluticasone-salmeterol, albuterol, losartan, and Synthroid chronically |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| Baseline | 44 | 65 | 0.6 | ||
| 10 days | 0 | Amoxicillin-clavulanate stopped | |||
| 24 days | 14 days | 371 | 301 | 7.8 | Pain, itching |
| 4 weeks | 19 days | 336 | 286 | 6.4 | Severe itching |
| 5 weeks | 23 days | 695 | 244 | 3.7 | |
| 5 weeks | 27 days | 724 | 188 | 2.3 | Biopsy |
| 7 weeks | 5 weeks | 250 | 142 | 1.3 | |
| 8 weeks | 6 weeks | 58 | 101 | 0.9 | |
| Normal Values | <42 | <115 | <1.2 | ||
Comment
The history and presentation of this case were typical of moderately severe amoxicillin-clavulanate hepatotoxicity with onset of itching followed by jaundice occurring 1 to 2 weeks after stopping a ten day course of treatment in an older man with no other obvious cause of liver disease. The pattern of serum enzyme results with prominent elevations in serum aminotransferase and alkaline phosphatase levels suggested mixed hepatocellular-cholestatic injury which was also shown by liver histology. While heavy acetaminophen use may have contributed to the initial aminotransferase elevations, the liver biopsy did not show evidence of typical acetaminophen injury. Other medications being taken (montelukast, albuterol, fluticasone, salmeterol, losartan) had been used chronically, have not or only rarely been linked to liver injury and were restarted without recurrence of liver disease. The drug induced liver injury was fully reversible, but symptoms and illness lasted for almost two months.
Case 5. Cholestatic hepatitis due to clavulanate.(2)
A 20 year old woman developed jaundice 10 days after starting an intravenous regimen of amoxicillin-clavulanate (2 grams daily) for bacterial pneumonia and pleurisy. She had no history of liver disease, alcohol abuse or risk factors for viral hepatitis. Serum bilirubin, alkaline phosphatase and aminotransferase levels were normal on admission, but rose gradually during the hospitalization and antibiotic therapy (Table). When jaundice was first noted laboratory testing showed a total bilirubin of 2.6 mg/dL (direct 1.9 mg/dL), ALT 112 U/L, AST 58 U/L, Alk P 425 U/L, and GGT 212 U/L. The amoxicillin-clavulanate was discontinued, but antibiotic therapy was still needed and she was treated with amoxicillin (3 grams daily) and metronidazole (1.5 grams daily). On this regimen, her pulmonary symptoms and fever improved rapidly and liver tests gradually improved and were in the normal range 30 days after stopping amoxicillin with clavulanate.
Key Points
| Medication: | Amoxicillin-clavulanate intravenously for 10 days |
|---|---|
| Pattern: | Cholestatic (R=0.6) |
| Severity: | 3+ (jaundice, hospitalization prolonged) |
| Latency: | 10 days, still on therapy |
| Recovery: | Within 1 month of stopping |
| Other medications: | Amoxicillin, metronidazole |
Laboratory Values
| Time After Starting | Time After Stopping | ALT (U/L) | Alk P (U/L) | Bilirubin (mg/dL) | Other |
|---|---|---|---|---|---|
| 0 | 0 | 39 | 112 | 0.5 | Admission |
| Intravenous amoxicillin-clavulanate started | |||||
| 1 day | 0 | 96 | 146 | 0.9 | |
| 2 days | 0 | 75 | 206 | .. | |
| 5 days | 0 | 117 | 417 | .. | |
| 7 days | 0 | 112 | 425 | 2.6 | |
| 10 days | 0 | Amoxicillin-clavulanate stopped | |||
| 11 days | 1 day | 58 | 285 | 2.2 | |
| 14 days | 4 day | 27 | 296 | 2.1 | Amoxicillin started |
| 17 days | 7 day | 17 | 222 | 1.5 | |
| 6 weeks | 4 weeks | 10 | 95 | 0.5 | |
| Normal Values | <54 | <133 | <1.2 | ||
Comment
The history and presentation of this case were typical of mild amoxicillin-clavulanate hepatotoxicity with onset within a week of starting therapy. Of interest was that routine laboratory testing demonstrated a rapid onset of hepatic injury that was subclinical for the first week. The rapidity of onset suggests previous exposure to amoxicillin-clavulanate, although no such history was obtained. Once the antibiotic combination was stopped, recovery was prompt. Some form of antibiotic therapy was considered necessary and the attending physicians felt comfortable using amoxicillin as the injury was thought to be due to clavulanate rather than the penicillin. The subsequent course supported their conclusions. Several other cases in the literature have supported the belief that the injury in typical amoxicillin-clavulanate hepatotoxicity is due to clavulanate. However, in an individual case, there is always the possibility that the hepatic injury is actually due to the amoxicillin; although in that situation the clinical syndrome should be dominated by signs and symptoms of hypersensitivity (rash, fever, eosinophilia) usually arising within 1 to 2 weeks of starting therapy, features that can occur with typical amoxicillin-clavulanate liver injury but are generally mild and self-limited.
PRODUCT INFORMATION
REPRESENTATIVE TRADE NAMES
Amoxicillin-Clavulanate – Generic, Augmentin®
DRUG CLASS
Antiinfective Agents
Product labeling at DailyMed, National Library of Medicine, NIH
CHEMICAL FORMULAS AND STRUCTURES
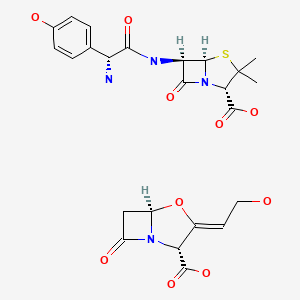
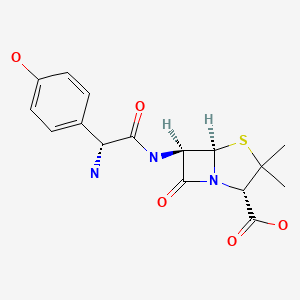
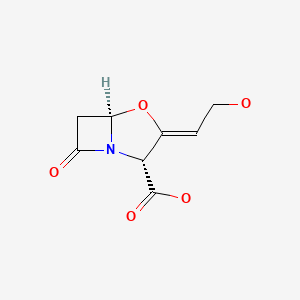
| DRUG | CAS REGISTRY NO | MOLECULAR FORMULA | STRUCTURE |
|---|---|---|---|
| Amoxicillin-Clavulanate | 79198-29-1 | C16-H19-N3-O5-S.C8-H9-N-O5 |
|
| Amoxicillin Anhydrous | 26787-78-0 | C16-H19-N3-O5-S |
|
| Clavulanic Acid | 58001-44-8 | C8-H9-N-O5 |
|
CITED REFERENCES
- 1.
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159]
- 2.
- Peroux JL, Peroux E, Jais F, Philit F, Chichmanian RM. Gastroenterol Clin Biol. 1992;16:102–3. [Augmentin hepatotoxicity: responsibility of clavulanic acid? Apropos of a case] French. [PubMed: 1537473]
ANNOTATED BIBLIOGRAPHY
References updated: 20 October 2020
- Zimmerman HJ. Hepatic injury from the treatment of infectious and parasitic diseases. In, Zimmerman HJ. Hepatotoxicity: the adverse effects of drugs and other chemicals on the liver. 2nd ed. Philadelphia: Lippincott, 1999, pp. 589-638.(Expert review of hepatotoxicity published in 1999; amoxicillin-clavulanate is capable of causing acute liver injury with jaundice that is typically cholestatic in pattern but can be hepatocellular, onset is delayed as long as 35 days after the drug is stopped, etiology is idiosyncrasy likely to be immunologic, most likely due to the clavulanate, recovery in 30 to 74 days, rarely causes fatality).
- Moseley RH. Hepatotoxicity of antimicrobials and antifungal agents. In, Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier, 2013, pp. 463-82.(Review of hepatotoxicity of antibiotics; amoxicillin-clavulanate typically causes a cholestatic or mixed pattern of injury with a preponderance in elderly men and with consecutive uses; features of hypersensitivity occur in up to 60% of patients; fatal, severe and prolonged cases have been described; mechanism is likely immunologic and due to the clavulanate rather than amoxicillin).
- Petri WA Jr. Penicillins, cephalosporins, and other β-lactam antibiotics. In, Brunton LL, Chabner BA, Knollman BC, eds. Goodman & Gilman’s the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill, 2011, pp. 1477-1504.(Textbook of pharmacology and therapeutics).
- Brogden RN, Carmine A, Heel RC, Morley PA, Speight TM, Avery GS. Amoxycillin/ clavulanic acid: a review of its antibacterial activity, pharmacokinetics and therapeutic use. Drugs. 1981;22:337–62. [PubMed: 7037354](Review of structure, pharmacology, mechanism of action, therapeutic efficacy and safety of oral amoxicillin-clavulanate: "there have been occasional reports of mild increases in serum transaminase levels during treatment").
- van den Broek JW, Buennemeyer BL, Stricker BH. Ned Tijdschr Geneeskd. 1988;132:1495–7. [Cholestatic hepatitis caused by a combination of amoxicillin and clavulanic acid (Augmentin)] Dutch. [PubMed: 3173514](66 year old man developed fatigue and jaundice shortly after a 2nd course of amoxicillin-clavulanate [bilirubin 7.1 rising to 35 mg/dL, ALT 325 U/L, GGT 272 U/L], resolving in 3 months and recurring 4 days after restarting the combination but not with two courses of amoxicillin alone).
- Verhamme M, Ramboer C, Van de Bruaene P, Inderadjaja N. Cholestatic hepatitis due to an amoxycillin/clavulanic acid preparation. J Hepatol. 1989;9:260–4. [PubMed: 2809168](Two cases: 60 and 53 year old men developed jaundice and pruritus 3 weeks after starting 10 day course of amoxicillin-clavulanate [bilirubin 13.0 and 13.8 mg/dL, ALT 356 and 314 U/L, Alk P 647 and 273 U/L], resolving within 2 to 3 months).
- Schneider JE, Kleinman MS, Kupiec JW. Cholestatic hepatitis after therapy with amoxicillin/clavulanate potassium. N Y State J Med. 1989;89:355–6. [PubMed: 2739957](44 year old woman developed abdominal pain and jaundice 20 days after starting a 10 day course of amoxicillin-clavulanate [bilirubin 1.9 rising to 4.6 mg/dL, AST 129 U/L, Alk P 259 U/L], resolving within 2 months).
- Dowsett JF, Gillow T, Heagerty A, Radcliffe M, Toadi R, Isle I, Russell RCG. Amoxycillin/clavulanic acid (Augmentin)-induced intrahepatic cholestasis. Dig Dis Sci. 1989;34:1290–3. [PubMed: 2752877](75 year old man developed nausea followed by jaundice ~1 week after finishing a 10 day course of amoxicillin-clavulanate [bilirubin 4.7 rising to 16 mg/dL, ALT 225 U/L, Alk P 316 U/L, eosinophilia], biopsy showing cholestatic hepatitis, resolving in 14 weeks).
- Reddy KR, Brillant P, Schiff ER. Amoxicillin-clavulanate potassium-associated cholestasis. Gastroenterology. 1989;96:1135–41. [PubMed: 2925057](Between 1984-87, 18 cases of possible amoxicillin-clavulanate hepatotoxicity with adequate documentation were reported to the sponsor, including 7 cholestatic, 6 mixed and 4 hepatocellular cases; 16 with jaundice, 14 pruritus, 2 fever; eosinophilia in 60%; latency 2-43 days, 14 occurring after stopping; bilirubin 2.7-44 mg/dL, ALT 43-440 U/L, Alk P 63-1330 U/L, resolving in 30-74 days, no fatalities).
- Stricker BH, Van den Broek JW, Keuning J, Eberhardt W, Houben HGJ, Johnson M, Blok APR. Cholestatic hepatitis due to antibacterial combination of amoxicillin and clavulanic acid (Augmentin). Dig Dis Sci. 1989;34:1576–80. [PubMed: 2791808](5 cases of suspected amoxicillin-clavulanate hepatotoxicity; all were men with jaundice arising 6-25 days after starting antibiotic [bilirubin 9-32 times ULN, ALT 2 to 6.3 times ULN, Alk P 1 to 2.6 times ULN], two were rechallenged and both had recurrence).
- Cleau D, Jobard JM, Alves T, Gury S, Rey B, Vuillemard M, Noirot A, et al. Gastroenterol Clin Biol. 1990;14:1007–9. [Cholestatic hepatitis induced by the amoxicillin-clavulanic acid combination. A case and review of the literature] French. [PubMed: 2289658](50 year old man developed pruritus and jaundice a week after completing a 7 day course of amoxicillin-clavulanate [bilirubin 14.2 mg/dL, ALT 76 U/L, Alk P 526 U/L], resolving within 2 months).
- Pelletier G, Ink O, Fabre M, Hagège H. Gastroenterol Clin Biol. 1990;14:601. [Hepatic cholestasis probably due to the combination of amoxicillin and clavulanic acid] French. [PubMed: 2397873](79 year old woman developed pruritus and jaundice 4 weeks after starting an 8 day course of amoxicillin-clavulanate [bilirubin 12.2 mg/dL, ALT 5 times ULN, Alk P 8.5 times ULN], resolving within 4 months).
- Reddy KR, Schiff ER. Hepatitis and Augmentin. Dig Dis Sci. 1990;35:1045–6. [PubMed: 2384035](Letter in response to Sticker commenting on the rarity of the injury in children despite wide scale use).
- Michielsen PP, Van Outryve MJ, Van Marck EA, De Maeyer MH, Pelckmans PA, Van Maercke YM. Amoxycillin/clavulanic acid induced cholestasis. J Hepatol. 1990;11:392. [PubMed: 2290034](24 year old man developed jaundice 23 days after starting 7 day course of amoxicillin-clavulanate [bilirubin 6.8 mg/dL, ALT 119 U/L, Alk P 275 U/L], resolving within 2 months).
- Escallier F, Dalac S, Caillot D, Boulitrop C, Collet E, Lambert D. Rev Med Interne. 1990;11:73–5. [Erythema multiforme, aplasia, cholestatic hepatitis during treatment with Augmentin (amoxicillin + clavulanic acid)] French. [PubMed: 2326558](82 year old man developed rash 5 days after starting amoxicillin-clavulanate, which was continued for 3 more days when he developed fever, erythema multiforme, bone marrow aplasia, [platelets 13,000, leukocytes 200] and liver test abnormalities [ALT 88 U/L, Alk P 367 U/L], with complex course but ultimate recovery within next six months).
- Alexander P, Roskams T, Van Steenbergen W, Peetermans W, Desmet V, Yap SH. Intrahepatic cholestasis induced by amoxicillin/clavulanic acid (Augmentin): a report on two cases. Acta Clin Belg. 1991;46:327–32. [PubMed: 1661553](Two cases: 61 year old man developed pruritus and jaundice at end of 28 day course of amoxicillin-clavulanate [bilirubin 3.8 mg/dL, ALT 1.5 times ULN, Alk P 4 times ULN, eosinophils 9%], resolving within 12 weeks; 78 year old woman developed pruritus and jaundice 2 weeks after a course of amoxicillin-clavulanate [bilirubin 18.8 mg/dL, ALT 60 U/L, Alk P 682 U/L], resolving within 8 weeks).
- Wong FS, Ryan J, Dabkowski P, Dudley FJ, Sewell RB, Smallwood RA. Augmentin-induced jaundice. Med J Aust. 1991;154:698–701. [PubMed: 2034154](8 cases of amoxicillin-clavulanate associated jaundice, ages 45 to 77 years, 6 men, 2 women; onset 0.5 to 6 weeks after stopping [peak bilirubin 6.4 to 34.7 mg/dL, ALT 77 to 921 U/L, Alk P 188 to 1420 U/L], resolving in 2-21 weeks).
- Silvain C, Fort E, Levillain P, Labat-Labourdette J, Beauchant M. Granulomatous hepatitis due to combination of amoxicillin and clavulanic acid. Dig Dis Sci. 1992;37:150–2. [PubMed: 1728522](79 year old man developed jaundice 20 days after finishing 14 day course of amoxicillin-clavulanate [bilirubin 16.9 mg/dL, ALT 350 U/L, Alk P 1870 U/L, eosinophils 610], resolving within 11 weeks).
- Ryan J, Dudley FJ. Cholestasis with ticarcillin-potassium clavulanate (Timentin). Med J Aust. 1992;156:291. [PubMed: 1738336](75 year old man developed pruritus and jaundice 31 days after completing a 9 day course of intravenous ticarcillin/clavulanate and shortly after a 28 day course of induction chemotherapy for lymphoma [bilirubin 8.1 mg/dL, ALT 448 U/L, Alk P 1330 U/L], with persistent jaundice and death 4 weeks later while liver tests were improving).
- Cabelleria Rovira E, Masso Ubeda RM, Arago López JV, Sanchís Closa A. An Med Interna. 1992;9:360–1. [Cholestatic hepatitis from amoxicillin-clavulanic acid] Spanish. [PubMed: 1633249](71 year old man developed pruritus and jaundice which resolved in 3 weeks and then recurred after a 3 day course of amoxicillin-clavulanate [bilirubin 17 mg/dL, ALT 126 U/L, Alk P 577 U/L], at which point a history of taking this antibiotic before the first episode was obtained).
- Larrey D, Vial T, Micaleff A, Babany G, Morichau-Beauchant M, Michel H, Benhamou JP. Hepatitis associated with amoxycillin-clavulanic acid combination report of 15 cases. Gut. 1992;33:368–71. [PMC free article: PMC1373830] [PubMed: 1568657](15 cases of amoxicillin-clavulanate hepatotoxicity reported to French regional centers including 12 men [80%], ages 39 to 82 years [mean=64], with latency of 7-60 days [mean=18], 11 arising after stopping, all had jaundice and 12 pruritus; no fatalities).
- Hebbard GS, Smith KG, Gibson PR, Bhathal PS. Augmentin-induced jaundice with a fatal outcome. Med J Aust. 1992;156:285–6. [PubMed: 1738330](81 year old man developed jaundice and pruritus 1 week after completing a 4 week course of amoxicillin-clavulanate [bilirubin 21.9 mg/dL, ALT 97 U/L, Alk P 231 U/L], with prolonged jaundice [bilirubin 35.8 mg/dL], drowsiness and death 5 weeks later).
- Peroux JL, Peroux E, Jais F, Philit F, Chichmanian RM. Gastroenterol Clin Biol. 1992;16:102–3. [Augmentin hepatotoxicity: responsibility of clavulanic acid? Apropos of a case] French. [PubMed: 1537473](20 year old woman developed jaundice 7 days after starting amoxicillin-clavulanate [bilirubin 3.7 mg/dL, ALT 112 U/L, Alk P 425 U/L], resolving within 30 days despite continuing treatment with amoxicillin).
- Yap I, Gwee KA, Wee A. Augmentin-induced cholestatic jaundice--a case report. Singapore Med J. 1993;34:464–5. [PubMed: 8153703](55 year old man with alcoholic pancreatitis developed jaundice after 6 days of amoxicillin-clavulanate [bilirubin 4.0 mg/dL, AST 56 U/L, Alk P 324 U/L], resolving within 5 weeks).
- Horsmans Y, Geubel AP. Amoxycillin-clavulanic acid-erythromycin cross-liver toxicity: a case report. J Hepatol. 1994;21:911–2. [PubMed: 7890912](67 year old man developed fatigue and pruritus 6 days after completing 5 day course of amoxicillin-clavulanate [bilirubin unclear, ALT 185 U/L, Alk P 235 U/L], and enzyme elevations later rose upon starting erythromycin [twice]).
- Hanssens M, Mast A, Van Maele V, Pauwels W. Ned Tijdschr Geneeskd. 1994;138:1481–3. [Cholestatic jaundice caused by amoxicillin-clavulanic acid in 4 patients] Dutch. [PubMed: 8052321](4 cases of amoxicillin-clavulanate hepatotoxicity: 2 men and 2 women, ages 72 to 84 years, treated for 7-10 days with onset after 25-35 days with jaundice, fever and pruritus or abdominal pain [bilirubin 7.2-18.8 mg/dL, ALT 77-320 U/L, Alk P 195-630 U/L], resolving in 40-60 days).
- Thomson JA, Fairley CK, Ugoni AM, Forbes AB, Purcell PM, Desmond PV, Smallwood RA, McNeil JJ. Risk factors for the development of amoxycillin-clavulanic acid associated jaundice. Med J Aust. 1995;162:638–40. [PubMed: 7603374](Analysis of 34 cases of amoxicillin-clavulanate associated jaundice compared to 136 controls found male sex [65% vs 46%], older age [60 vs 40 years], longer treatment [5.7 vs 7 days], but not dose or other medical conditions, were more frequent in cases with liver injury).
- Watteeuw G, Vasilevski D, Hautekeete M, Taton G, Lambilliotte JP, François E, Adler M. Rev Med Brux. 1995;16:391–3. [Cholestatic hepatitis and amoxicillin-clavulanic acid combination. Personnel case report and literature review] French. [PubMed: 8570979](58 year old man developed jaundice and pruritus 36 days after starting a 10 day course of amoxicillin-clavulanate, resolving within 2 months).
- Galindo C, Buenestado J, Reñé JM, Piñol MC. Rev Esp Enferm Dig. 1995;87:597–600. [Acute pancreatitis associated with hepatotoxicity induced by amoxicillin-clavulanic acid] Spanish. [PubMed: 7577112](25 year old man developed abdominal pain and jaundice 5 weeks after starting 6 day course of amoxicillin-clavulanate [bilirubin 7.0 mg/dL, ALT 268 U/L, Alk P 2287 U/L, amylase 878 U/L], resolving within 1 month).
- Hautekeete ML. Hepatotoxicity of antibiotics. Acta Gastroenterol Belg. 1995;58:290–6. [PubMed: 7491842](Review of hepatotoxicity of antibiotics including amoxicillin-clavulanate occurring after 1:10,000 to 1:100,000 prescriptions, particularly in older men who are treated repeatedly or with prolonged courses typically with cholestatic pattern of injury and with fever, rash and arthralgias in a minority of patients).
- Hautekeete ML, Brenard R, Horsmans Y, Henrion J, Verbist L, Derue G, Druez P, et al. Liver injury related to amoxycillin-clavulanic acid: interlobular bile-duct lesions and extrahepatic manifestations. J Hepatol. 1995;22:71–7. [PubMed: 7751590](Analysis of liver histology from 7 cases of amoxicillin-clavulanate hepatotoxicity; intralobular bile duct abnormalities found in all biopsies but no ductopenia).
- Reddy KR, Schiff ER. Hepatotoxicity of antimicrobial, antifungal, and antiparasitic agents. Gastroenterol Clin North Am. 1995;24:923–36. [PubMed: 8749905](Review of hepatotoxicity of antibiotics including amoxicillin-clavulanate; histologically centrizonal cholestasis is characteristic as well as mild-to-moderate inflammation and bile duct abnormalities).
- Pillans PI. Drug associated hepatic reactions in New Zealand: 21 years’ experience. N Z Med J. 1996;109:315–9. [PubMed: 8816722](Survey of adverse drug reaction reports found 943 causes of liver injury; amoxicillin-clavulanate was listed only for the last 7 year period [1988-94], during which it accounted for 32 cases [8%]).
- García Rodríguez LA, Stricker BH, Zimmerman HJ. Risk of acute liver injury associated with the combination of amoxicillin and clavulanic acid. Arch Intern Med. 1996;156:1327–32. [PubMed: 8651842](Analysis of General Practitioners Database of 93,433 users of amoxicillin-clavulanate [A/C] and 360,333 users of amoxicillin alone [A] identified 35 subsequent cases of idiopathic liver injury found 21 with A/C [1.7 per 10,000 prescriptions] compared to 17 with A [0.3 per 10,000], rate with A/C increasing with age, male sex and repeated use).
- Pedro-Botet J, Supervía A, Barranco C, Solá R, Bruguera M. Intrahepatic cholestasis without hepatitis induced by amoxycillin/clavulanic acid. J Clin Gastroenterol. 1996;23:137–8. [PubMed: 8877644](73 year old woman developed jaundice 5 days after starting amoxicillin-clavulanate [bilirubin 6.2 mg/dL, ALT 140 U/L, Alk P 1783 U/L], resolving within one month).
- Bralet MP, Zafrani ES. Ann Pathol. 1996;16:425–9. [Hepatitis caused by the amoxicillin-clavulanic acid combination. An example of drug-induced biliary hepatotoxicity] French. [PubMed: 9090930](Five cases of amoxicillin-clavulanate hepatotoxicity, biopsies showing centrolobular cholestasis in all and bile duct injury without ductopenia in 4).
- Bustamante Balén M, Pérez Aguilar F, Rayón Martín M, García Herola A, Berenguer Lapuerta J. Gastroenterol Hepatol. 1997;20:187–9. [Cholestatic hepatitis caused by amoxycillin-clavulanic acid. Report of a new case] Spanish. [PubMed: 9280613](47 year old man developed pruritus and jaundice one month after starting amoxicillin-clavulanate [bilirubin 11.6 mg/dL, ALT 159 U/L, Alk P 195 U/L, 4% eosinophils], resolving within 3 months).
- Reñe JM, Buenestado J, Piñol MC. Gastroenterol Hepatol. 1997;20:337–8. [Hepatotoxicity caused by amoxicillin-clavulanic acid] Spanish. [PubMed: 9296855](Letter in response to Bustamante Balen discussing the link between amoxicillin and clavulanate and hypersensitivity liver injury).
- Vial T, Biour M, Descotes J, Trepo C. Antibiotic-associated hepatitis: update from 1990. Ann Pharmacother. 1997;31:204–20. [PubMed: 9034423](Systematic review of drug induced liver injury due to antibiotics from 1990 to 1995, several hundred cases of amoxicillin-clavulanate associated liver injury have been published occurring 1-4 weeks after stopping therapy with cholestatic or mixed enzyme elevations with jaundice and pruritus, typically in older men with longer courses of therapy; immunoallergic features are common and recurrence with shortened latency on rechallenge suggests immunologic basis, most likely due to clavulanate as some patients have tolerated amoxicillin by itself but had recurrence with combination; no apparent cross sensitivity with other beta lactamase inhibitors).
- Caballero Plasencia AM, Valenzuela Barranco M, Martin Ruiz JL, Guilarte Lopez-Manas J. Gastroenterol Hepatol. 1997;20:45–6. [Hepatotoxicity caused by amoxicillin, clavulanic acid or both?] Spanish. [PubMed: 9072200](31 year old man developed pruritis and jaundice the day after finishing a 5 day course of amoxicillin-clavulanate [bilirubin 2.9 mg/dL, ALT 291 U/L, Alk P 509 U/L], yet had tolerated amoxicillin alone and later had recurrence after 2 days of reexposure to amoxicillin-clavulanate [bilirubin 3.8 mg/dL, ALT 396 U/L, Alk P 616 U/L], resolving within 3 months of stopping).
- de Haan F, Stricker BH. Ned Tijdschr Geneeskd. 1997;141:1298–301. [Liver damage associated with the combination drug amoxicillin-clavulanic acid (Augmentin)] Dutch. [PubMed: 9380177](Summary of 40 cases of amoxicillin-clavulanate hepatotoxicity reported to Dutch Drug Safety Unit between 1982-96, mean age 61 years, 70% men, latency mean=3 weeks from starting [maximum 55 days], resolved in all [mean=6 weeks, range 10 to 168 days], 5 had recurrence on reexposure).
- Barrio J, Castiella A, Lobo C, Indart A, López P, García-Bengoechea M, Cosme A, et al. Rev Esp Enferm Dig. 1998;90:523–6. [Cholestatic acute hepatitis induced by amoxycillin-clavulanic acid combination. Role of ursodeoxycholic acid in drug-induced cholestasis] Spanish. [PubMed: 9741209](Two cases: 68 year old man developed jaundice and pruritus 7 days after finishing 10 day course of amoxicillin-clavulanate [bilirubin 14.8 mg/dL, ALT 378 U/L, Alk P 542 U/L], resolving in 2 months; 95 year old man developed abdominal pain and jaundice 33 days after starting 2 week course [bilirubin 7.7 mg/dL, ALT 225 U/L, Alk P 1229 U/L], resolving within 2 months).
- Nathani MG, Mutchnick MG, Tynes DJ, Ehrinpreis MN. An unusual case of amoxicillin/clavulanic acid-related hepatotoxicity. Am J Gastroenterol. 1998;93:1363–5. [PubMed: 9707067](40 year old woman developed rash and jaundice 4 weeks after finishing 18 day course of amoxicillin-clavulanate [bilirubin 10.3 mg/dL, ALT 199 U/L, Alk P 362 U/L, eosinophils 7%], resolving and then recurring within 5 days of restarting [bilirubin 1.2 mg/dL, ALT 199 U/L, Alk P 362 U/L], resolving in 4 weeks).
- Ballester Fayos J, Rodríguez Gil FJ, Paredes Arquiola JM, García del Castillo G, Antón-Conejero MD, Añón Rodríguez R, Moreno-Osset E. Gastroenterol Hepatol. 1998;21:114–5. [Amoxicillin-clavulanic acid hepatotoxicity] Spanish. [PubMed: 9580395]
- Julve R, García A, Gómez A, Primo J, Molés JR, Hinojosa J. Gastroenterol Hepatol. 1998;21:92–4. [Acute hepatocellular lesion induced by amoxicillin-clavulanic acid] Spanish. [PubMed: 9549187](21 year old man developed itching and jaundice 8 days after stopping a 10 day course of amoxicillin-clavulanate [bilirubin 11.8 rising to 33 mg/dL, ALT 840 U/L, Alk P 390 U/L], resolving within 10 weeks of onset).
- Maggini M, Raschetti R, Agostinis L, Cattaruzzi C, Troncon MG, Simon G. Use of amoxicillin and amoxicillin-clavulanic acid and hospitalization for acute liver injury. Ann Ist Super Sanita. 1999;35:429–33. [PubMed: 10721209]
- Beurton I, Germanese JC, Becker MC, Koch S, Carbillet JP, Miguet JP, Bresson-Hadni S. Gastroenterol Clin Biol. 1999;23:1097–8. [Acute hepatitis and destructive cholangitis probably induced by amoxicillin-clavulanic acid combination] French. [PubMed: 10592885](73 year old developed jaundice 5 weeks after starting 2 week course of amoxicillin-clavulanate [bilirubin 20.1 mg/dL, ALT 12.5 times ULN, Alk P 3.5 times ULN], resolving within 6 weeks).
- Soza A, Riquelme F, Alvarez M, Duarte I, Glasinovic JC, Arrese M. Rev Med Chil. 1999;127:1487–91. [Hepatotoxicity by amoxicillin/clavulanic acid: case report] Spanish. [PubMed: 10835757](72 year old man developed jaundice and pruritus 36 days after starting 20 day course of amoxicillin-clavulanate [bilirubin 4.3 rising to 16 mg/dL, ALT 470 U/L, Alk P 400 U/L], resolving in 11 weeks with apparent clinical response to ursodiol therapy).
- Hautekeete ML, Horsmans Y, Van Waeyenberge C, Demanet C, Henrion J, Verbist L, Brenard R, et al. HLA association of amoxicillin-clavulanate--induced hepatitis. Gastroenterology. 1999;117:1181–6. [PubMed: 10535882](35 patients with amoxicillin-clavulanate hepatotoxicity were tested for HLA A and B class associations: DRB1*1501-DRB5*0101-DQB1*0602 haplotype found in 57% of cases vs 15% of controls; less common with hepatocellular injury pattern).
- Limauro DL, Chan-Tompkins NH, Carter RW, Brodmerkel GJ Jr, Agrawal RM. Amoxicillin/clavulanate-associated hepatic failure with progression to Stevens-Johnson syndrome. Ann Pharmacother. 1999;33:560–4. [PubMed: 10369618](37 year old man developed jaundice, rash and pruritus 3 weeks after stopping 10 day course of amoxicillin-clavulanate [bilirubin 27.7 mg/dL, ALT 53 U/L, Alk P 562 U/L, eosinophils 10%], with progression of rash to Stevens Johnson syndrome, septicemia and death).
- Maggini M, Raschetti R, Agostinis L, Cattaruzzi C, Troncon MG, Simon G. Use of amoxicillin and amoxicillin-clavulanic acid and hospitalization for acute liver injury. Ann Ist Super Sanita. 1999;35:429–33. [PubMed: 10721209](Among 118 cases of acute liver injury seen at Italian regional hospitals, 2 were attributed to amoxicillin-clavulanate and 3 to amoxicillin alone).
- O'Donohue J, Oien KA, Donaldson P, Underhill J, Clare M, MacSween RN, Mills PR. Co-amoxiclav jaundice: clinical and histological features and HLA class II association. Gut. 2000;47:717–20. [PMC free article: PMC1728095] [PubMed: 11034591](22 cases of amoxicillin-clavulanate hepatotoxicity tested for HLA class II associations: DRB1*1501 found in 70% vs 20% of 134 controls: all had extended haplotype of DRB1*1501-DRB5*0101-DQA1*0102-DQB1*0602).
- Bolzan H, Spatola J, Castelletto R, Curciarello J. Gastroenterol Hepatol. 2000;23:237–9. [Intrahepatic cholestasis induced by amoxicillin alone] Spanish. [PubMed: 10902278](24 year old woman developed pruritus at the end of a 10 day course of amoxicillin-clavulanate and jaundice 10 days later [bilirubin 10.5 mg/dL, ALT 30 U/L, Alk P 314 U/L], resolving within 2 months).
- Chawla A, Kahn E, Yunis EJ, Daum F. Rapidly progressive cholestasis: An unusual reaction to amoxicillin/clavulanic acid therapy in a child. J Pediatr. 2000;136:121–3. [PubMed: 10636987](2 year old boy developed rash 1 day after a 10 day course of amoxicillin-clavulanate with jaundice 3 days later [bilirubin 3.5 rising to 11 mg/dL, ALT 246 U/L, Alk P 630 U/L, GGT 555 U/L], with persistence of cholestasis requiring liver transplant 8 months later, explant showed vanishing bile duct syndrome).
- Katsinelos P, Vasiliadis T, Xiarchos P, Patakiouta F, Christodoulou K, Pilpilidis I, Eugenidis N. Ursodeoxycholic acid (UDCA) for the treatment of amoxycillin-clavulanate potassium (Augmentin)-induced intra-hepatic cholestasis: report of two cases. Eur J Gastroenterol Hepatol. 2000;12:365–8. [PubMed: 10750660](Two cases: 71 year old man developed jaundice 10 days after 7 day course of amoxicillin-clavulanate [bilirubin 6.4 mg/dL, ALT 77 U/L, Alk P 258 U/L], with slow resolution and apparent response to ursodiol; 81 year old man developed jaundice 1 week after a 1 week course [bilirubin 18.6 mg/dL, ALT 87 U/L, Alk P 427 U/L], resolving in 3 months and apparent response to ursodiol).
- Gresser U. Amoxicillin-clavulanic acid therapy may be associated with severe side effects--review of the literature. Eur J Med Res. 2001;6:139–49. [PubMed: 11309226](Systematic review of reports of adverse reactions to amoxicillin-clavulanate found 208 cases of liver injury, 158 could be evaluated: 70% men, mean age 60 years, mean duration of therapy 2 weeks, latency to onset averaging 25 days and mean time to resolution 12 weeks; 55% had mixed pattern of injury; 3 fatalities).
- Berg P, Hahn EG. Hepatotoxic reactions induced by beta-lactamase inhibitors. Eur J Med Res. 2001;6:535–42. [PubMed: 11772541](Systematic review of hepatotoxicity of beta lactamase inhibitors: convincing evidence for hepatotoxicity found only for combinations with clavulanate: case report of 55 year old man developing jaundice 2 weeks after a 14 day course of amoxicillin-clavulanate [bilirubin rising to ~22 mg/dL, Alk P ~800 U/L]).
- Schey R, Avni Y, Bruck R, Shirin H. History of drug-induced hepatitis and risk of amoxicillin/clavulanate-induced hepatotoxicity. Ann Pharmacother. 2001;35:1142–3. [PubMed: 11573870](Two cases: 62 year old man developed pruritus 2.5 weeks after finishing a 1 week course of amoxicillin-clavulanate [bilirubin 29.0 mg/dL, ALT 470 U/L, Alk P 271 U/L, eosinophils 8%], resolving within 5 weeks; 58 year old man developed pruritus 3 weeks after finishing 1 week course of therapy [bilirubin 5.5 mg/dL, ALT 248 U/L, Alk P 255 U/L, no eosinophilia], resolving within 6 weeks).
- Boyd IW. Comment: history of drug-induced hepatitis and risk of amoxicillin/clavulanate-induced hepatotoxicity. Ann Pharmacother. 2001;35:1677. [PubMed: 11793648](Letter in response to Schey [2001] mentioning that frequency of amoxicillin-clavulanate hepatotoxicity is estimated at 1.7 per 10,000 prescriptions and more than 300 cases have been reported from Australia).
- Andrade RJ, Lucena MI, Fernández MC, Vega JL, Camargo R. Hepatotoxicity in patients with cirrhosis, an often unrecognized problem: lessons from a fatal case related to amoxicillin/clavulanic acid. Dig Dis Sci. 2001;46:1416–9. [PubMed: 11478492](61 year old woman with cirrhosis developed jaundice 54 days after finishing a 21 day course of amoxicillin-clavulanate [bilirubin 27.1 mg/dL, ALT 73 U/L, Alk P normal], with progressive liver failure and death 11 days later).
- Nicholson SC, Webb CD, Moellering RC Jr. Antimicrobial-associated acute hepatitis. Pharmacotherapy. 2002;22:794–6. [PubMed: 12066973](Discussion of case described by Henann [2001] which was attributed to gatifloxacin, raising issues of possible role of hepatitis C, amoxicillin-clavulanate, clarithromycin and levofloxacin).
- Jordán T, González M, Casado M, Suárez JF, Pulido F, Guerrero E, Esteban J. Gastroenterol Hepatol. 2002;25:240–3. [Amoxicillin-clavulanic acid induced hepatotoxicity with progression to cirrhosis.] Spanish. [PubMed: 11975871](42 year old man developed pruritus and jaundice 5 weeks after starting 4 day course of amoxicillin-clavulanate, with prolonged course and worsening upon reexposure, liver biopsies showing progression to cirrhosis).
- Sgro C, Clinard F, Ouazir K, Chanay H, Allard C, Guilleminet C, Lenoir C, et al. Incidence of drug-induced hepatic injuries: a French population-based study. Hepatology. 2002;36:451–5. [PubMed: 12143055](All adverse drug reactions from French region from 1997-2000 found 34 cases of liver injury, 40 drugs involved, most common being amoxicillin-clavulanate [n=4]).
- Ibáñez L, Pérez E, Vidal X, Laporte JR. Grup d'Estudi Multicènteric d'Hepatotoxicitat Aguda de Barcelona (GEMHAB). Prospective surveillance of acute serious liver disease unrelated to infectious, obstructive, or metabolic diseases: epidemiological and clinical features, and exposure to drugs. J Hepatol. 2002;37:592–600. [PubMed: 12399224](Prospective study of acute serious liver disease over 6 years found 107 cases of drug induced liver injury, 13 [12%] attributed to clavulanate).
- Thiim M, Friedman LS. Hepatotoxicity of antibiotics and antifungals. Clin Liver Dis. 2003;7:381–99. vi-vii. [PubMed: 12879990](Review of antibiotic induced liver injury, including amoxicillin-clavulanate).
- Zaidi SA. Hepatitis associated with amoxicillin/clavulanic acid and/or ciprofloxacin. Am J Med Sci. 2003;325:31–3. [PubMed: 12544082](80 year old man developed rash 8 days after starting amoxicillin-clavulanate and 5 days after starting ciprofloxacin and found to have abnormal liver tests 5 days later [bilirubin 0.8 rising to 1.8 mg/dL, ALT 154 to 972 U/L, Alk P 120 to 358 U/L], resolving in 1 month).
- Martí J. Enferm Infecc Microbiol Clin. 2003;21:322–3. [Cholestatic hepatitis due to amoxicillin-clavulanic acid with positive re-exposure] Spanish. [PubMed: 12809593](86 year old man developed jaundice after an 8 day course of amoxicillin-clavulanate [bilirubin 8.5 mg/dL, ALT 388 U/L, Alk P 337 U/L], resolving within 4 weeks; had a previous history of similar response).
- Andrade RJ, Lucena MI, Alonso A, García-Cortes M, García-Ruiz E, Benitez R, Fernández MC, et al. HLA class II genotype influences the type of liver injury in drug-induced idiosyncratic liver disease. Hepatology. 2004;39:1603–12. [PubMed: 15185301](Analysis of HLA Class II associations in 140 patients with drug induced liver injury: DRB1*1501 was increased in cholestatic cases compared to controls [35% vs 19%], among 27 cases due to amoxicillin-clavulanate, DRB1*1501 present in 33% suggesting association is with cholestatic liver injury rather than a specific drug).
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug-induced liver injury in the United States. Liver Transpl. 2004;10:1018–23. [PubMed: 15390328](Among ~50,000 liver transplants done in the US between 1990 and 2002, 137 were for non-acetaminophen drug induced acute liver failure, but only one [~1%] was attributed to amoxicillin-clavulanate).
- de Abajo FJ, Montero D, Madurga M, García Rodríguez LA. Acute and clinically relevant drug-induced liver injury: a population based case-control study. Br J Clin Pharmacol. 2004;58:71–80. [PMC free article: PMC1884531] [PubMed: 15206996](Population based study of 1.6 million persons in UK and 128 valid cases of drug induced liver disease; amoxicillin-clavulanate was the most common cause [n=13] and had an incidence rate of 8.6 per 100,000 users).
- Andrade RJ, Lucena MI, Fernández MC, Pelaez G, Pachkoria K, García-Ruiz E, García-Muñoz B, et al. Spanish Group for the Study of Drug-Induced Liver Disease. Drug-induced liver injury: an analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–21. [PubMed: 16083708](Reports to a Spanish network found 461 cases of drug induced liver disease, most common cause being amoxicillin-clavulanate [n=59: 13%]).
- Björnsson E, Jerlstad P, Bergqvist A, Olsson R. Fulminant drug-induced hepatic failure leading to death or liver transplantation in Sweden. Scand J Gastroenterol. 2005;40:1095–101. [PubMed: 16165719](Summary of 25 years of adverse drug reaction reporting in Sweden identified 103 cases of drug induced acute liver failure: none were attributed to amoxicillin-clavulanate).
- Fontana RJ, Shakil AO, Greenson JK, Boyd I, Lee WM. Acute liver failure due to amoxicillin and amoxicillin/clavulanate. Dig Dis Sci. 2005;50:1785–90. [PubMed: 16187174](Patient found to have abnormal liver tests [ALT 141 U/L, Alk P 98 U/L, normal bilirubin] 19 days after starting a 12 day course of amoxicillin-clavulanate, and admitted with acute liver failure 1 month later with bilirubin 27.6 mg/dL, ALT 620 U/L, Alk P 170 U/L, INR 2.0; dying 10 days later).
- Andrade RJ, Lucena MI, Kaplowitz N, García-Munoz B, Borraz Y, Pachkoria K, García-Cortés M, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology. 2006;44:1581–8. [PubMed: 17133470](At least 6 months follow up on 406 patients with drug induced liver injury in Spanish database identified 28 with evidence of chronic liver injury; amoxicillin-clavulanate accounted for 4 cases, rate of 6%; 3 had cholestatic enzyme pattern).
- Lucena MI, Andrade RJ, Fernández MC, Pachkoria K, Pelaez G, Durán JA, Villar M, et al. Spanish Group for the Study of Drug-Induced Liver Disease. (Grupo de Estudio para las Hepatopatías Asociadas a Medicamentos (GEHAM)). Determinants of the clinical expression of amoxicillin-clavulanate hepatotoxicity: a prospective series from Spain. Hepatology. 2006;44:850–6. [PubMed: 17006920](Series of 69 patients with amoxicillin-clavulanate hepatotoxicity which represented ~14% of drug induced liver injury; mean time to onset of jaundice 16 days [range 1-71], 2-55 days after stopping; 52% males; mean age 56 years; 31% cholestatic, 33% mixed and 36% hepatocellular; 1 patient died, 4 developed chronicity; hepatocellular cases were younger in age and had lower bilirubin and higher ALT levels).
- Björnsson E, Olsson R. Suspected drug-induced liver fatalities reported to the WHO database. Dig Liver Dis. 2006;38:33–8. [PubMed: 16054882](Survey of drug induced liver fatalities reported to WHO database between 1968-2003 revealed 4690 reports; 21 drugs were associated with >50 cases, but not amoxicillin-clavulanate).
- Cundiff J, Joe S. Amoxicillin-clavulanic acid-induced hepatitis. Am J Otolaryngol. 2007;28:28–30. [PubMed: 17162128](Patient developed jaundice and pruritus 1 week after stopping a 4 week course of amoxicillin-clavulanate; laboratory results not given; resolution of jaundice in 3 weeks and enzyme elevations in 10 weeks).
- Hussaini SH, O'Brien CS, Despott EJ, Dalton HR. Antibiotic therapy: a major cause of drug-induced jaundice in southwest England. Eur J Gastroenterol Hepatol. 2007;19:15–20. [PubMed: 17206072](Analysis of 800 cases of jaundice presenting to a referral center in UK between 1998-2004, found 28 cases of drug induced liver injury; amoxicillin-clavulanate was the most common cause [n=9: 32%]).
- Sabaté M, Ibáñez L, Pérez E, Vidal X, Buti M, Xiol X, Mas A, et al. Risk of acute liver injury associated with the use of drugs: a multicentre population survey. Aliment Pharmacol Ther. 2007;25:1401–9. [PubMed: 17539979](Population based survey of 126 cases of acute liver injury [24 with acute liver failure] due to drugs between 1993-1999 in Spain calculated relative risk of injury from amoxicillin-clavulanate to be 67 compared to the general population, and with an incidence rate of 27 per 100,000 person years of exposure).
- Jakab SS, West AB, Meighan DM, Brown RS Jr, Hale WB. Mycophenolate mofetil for drug-induced vanishing bile duct syndrome. World J Gastroenterol. 2007;13:6087–9. [PMC free article: PMC4250896] [PubMed: 18023105](69 year old man developed rash 3 weeks after starting amoxicillin-clavulanate with subsequent jaundice [bilirubin 1.0 rising to 7.4 mg/dL, ALT 82 GGT 360 U/L], with prolonged jaundice and vanishing bile duct syndrome but subsequent resolution with mycophenolate mofetil therapy).
- Chalasani N, Fontana RJ, Bonkovsky HL, Watkins PB, Davern T, Serrano J, Yang H, Rochon J., Drug Induced Liver Injury Network (DILIN). Causes, clinical features, and outcomes from a prospective study of drug-induced liver injury in the United States. Gastroenterology. 2008;135:1924–34. [PMC free article: PMC3654244] [PubMed: 18955056](Among 300 cases of drug induced liver disease in the US collected from 2004 to 2008, amoxicillin-clavulanate was the most common single cause accounting for 23 cases [8%]).
- Domínguez Jiménez JL, Marín Moreno M, Bernal Blanco E, Puente Gutiérrez JJ, Guiote Malpartida S, de la Mata García M. Gastroenterol Hepatol. 2008;31:46. [Acute cholestatic hepatitis induced by amoxicillin-clavulanic acid] Spanish. [PubMed: 18218281](36 year old former alcoholic developed jaundice 10 days after stopping fluconazole and 10 days after stopping 3 day course of amoxicillin-clavulanate [bilirubin 18.7 mg/dL, ALT 128 U/L, AST 125 U/L, Alk P 539 U/L, 26% eosinophils], with subsequent worsening jaundice and ascites but eventual recovery in 2 months: acute on chronic injury).
- Daly AK, Day CP. Genetic association studies in drug-induced liver injury. Semin Liver Dis. 2009;29:400–11. [PubMed: 19826974](Review of genetic associations reported with drug induced liver injury; linkage of DRB1*1501 and amoxicillin-clavulanate liver toxicity found in two of three studies).
- Hunt CM. Mitochondrial and immunoallergic injury increase risk of positive drug rechallenge after drug-induced liver injury: a systematic review. Hepatology. 2010;52:2216–22. [PubMed: 21105110](Review of safety of rechallenge after clinically apparent drug induced liver injury including discussion of amoxicillin-clavulanate).
- Donaldson PT, Daly AK, Henderson J, Graham J, Pirmohamed M, Bernal W, Day CP, Aithal GP. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J Hepatol. 2010;53:1049–53. [PubMed: 20800921](HLA testing in 61 patients with amoxicillin-clavulanate liver injury found increase in HLA-DRB1*15 [53% vs 30%] and reduction in DRB1*07 [10% vs 29%], but no correlations with clinical features).
- Invernizzi P. Drug-induced liver injury: is it time for genetics to change our clinical practice? J Hepatol. 2010;53:993–4. [PubMed: 20851491](Editorial accompanying article by Donaldson [2010]).
- Devarbhavi H, Dierkhising R, Kremers WK, Sandeep MS, Karanth D, Adarsh CK. Single-center experience with drug-induced liver injury from India: causes, outcome, prognosis, and predictors of mortality. Am J Gastroenterol. 2010;105:2396–404. [PubMed: 20648003](313 cases of drug induced liver injury were seen over a 12 year period at a large hospital in Bangalore, India: amoxicillin-clavulanate accounted for only 3 cases [1%]).
- Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, Sturkenboom MC. Drug-induced hepatic injury in children: a case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. [PMC free article: PMC2997312] [PubMed: 21039766](Worldwide pharmacovigilance database contained 9036 hepatic adverse drug reactions in children, amoxicillin-clavulanate accounting for 38 cases [0.4%] for an adjusted odds ratio of 1.7).
- Reuben A, Koch DG, Lee WM., Acute Liver Failure Study Group. Drug-induced acute liver failure: results of a U.S. multicenter, prospective study. Hepatology. 2010;52:2065–76. [PMC free article: PMC3992250] [PubMed: 20949552](Among 1198 patients with acute liver failure enrolled in a US prospective study between 1998 and 2007, 133 were attributed to drug induced liver injury none of which were attributed to the combination of amoxicillin and clavulanate, although two were attributed to amoxicillin alone).
- Herrero-Herrero JI, García-Aparicio J. Corticosteroid therapy in a case of severe cholestasic hepatitis associated with amoxicillin-clavulanate. J Med Toxicol. 2010;6:420–3. [PMC free article: PMC3550477] [PubMed: 20237968](76 year old man developed fever, pruritus and jaundice 4 weeks after starting a 3 week course of amoxicillin-clavulanate [bilirubin 2.2 rising to 50.1 mg/dL, ALT 352 U/L, Alk P 266 U/L, eosinophils 8%], with prompt clinical response to prednisone [1 mg/kg/day] and ursodiol and recovery in 10 weeks).
- Lucena MI, Molokhia M, Shen Y, Urban TJ, Aithal GP, Andrade RJ, Day CP, et al. Spanish DILI Registry; EUDRAGENE; DILIN; DILIGEN; International SAEC. Susceptibility to amoxicillin-clavulanate-induced liver injury is influenced by multiple HLA class I and II alleles. Gastroenterology. 2011;141:338–47. [PMC free article: PMC3129430] [PubMed: 21570397](Genome wide association study of 201 patients with amoxicillin-clavulanate liver injury from Europe and the US [Caucasians only] found an association with the class II extended allele HLA-DRB1*15:01-DQB1*06:02, which had been previously shown, as well as a separate but interacting linkage to a class 1 region: HLA-A*02:01).
- Urban TJ, Shen Y, Stolz A, Chalasani N, Fontana RJ, Rochon J, Ge D, et al. Drug-Induced Liver Injury Network. DILIGEN; EUDRAGENE; Spanish DILI Registry; International Serious Adverse Events Consortium. Limited contribution of common genetic variants to risk for liver injury due to a variety of drugs. Pharmacogenet Genomics. 2012;22:784–95. [PMC free article: PMC3636716] [PubMed: 22968431](Genome wide association study done on 783 patients with suspected drug induced liver injury found an association of hepatocellular injury with a single nucleotide polymorphism in the vicinity of STAT4).
- Hita EO, García JA, Gonzalez JC, Molina AA, Cordero MA, Escobar JS, Ruiz-Extremera A. Amoxicillin-clavulanic acid hepatotoxicity in children. J Pediatr Gastroenterol Nutr. 2012;55:663–7. [PubMed: 22710999](Among 24 cases of drug induced liver injury enrolled in a prospective database from 8 pediatric centers in Spain [2008-2011], 11 were due to amoxicillin-clavulanate; ages 1 to 11 years, 11 boys and 2 girls, 3 to 29 days after starting [4 were still taking drug when symptoms arose], 64% of cases were hepatocellular and bilirubin was normal in all except one, children presented with nausea, vomiting and abdominal pain rather than itching and jaundice).
- Studniarz M, Czubkowski P, Cielecka-Kuszyk J, Jankowska I, Teisseyre M, Kamińska D, Markiewicz M, et al. Amoxicillin/clavulanic acid-induced cholestatic liver injury after pediatric liver transplantation. Ann Transplant. 2012;17:128–31. [PubMed: 22466919](8 year old boy with biliary atresia and liver transplant on corticosteroids and tacrolimus developed jaundice 2 days after finishing a 2 week course of amoxicillin-clavulanate [bilirubin 5.3 mg/dL, ALT 227 U/L, Alk P not provided], resolving within 12 weeks).
- Kwon H, Lee SH, Kim SE, Lee JH, Jee YK, Kang HR, Park BJ, Park JW, Hong CS. Spontaneously reported hepatic adverse drug events in Korea: multicenter study. J Korean Med Sci. 2012;27:268–73. [PMC free article: PMC3286773] [PubMed: 22379337](Summary of 2 years of adverse event reporting in Korea; of 9360 reports, 567 were liver related, only one of which was attributed to amoxicillin-clavulanate).
- Sánchez-Ruiz-Granados E, Bejarano-García A, Uceda-Torres E. Recurrent cholestasis by amoxicillin-clavulanic acid: the importance of a correct diagnosis of hepatotoxicity. Rev Esp Enferm Dig. 2012;104:616–7. [PubMed: 23368660](62 year old man with a hepatic hydatid cyst and previous episode of unexplained jaundice, redeveloped jaundice and pruritus 20 days after starting amoxicillin-clavulanate [bilirubin 12 mg/dL, ALT and Alk P normal]).
- Stephens C, López-Nevot MÁ, Ruiz-Cabello F, Ulzurrun E, Soriano G, Romero-Gómez M, Moreno-Casares A, et al. HLA Alleles Influence the Clinical Signature of Amoxicillin-Clavulanate Hepatotoxicity. PLoS One. 2013;8:e68111. [PMC free article: PMC3706603] [PubMed: 23874514](Analysis of HLA alleles in 75 cases of amoxicillin-clavulanate hepatotoxicity found several associations of specific alleles with clinical features).
- Girelli F, Bernardi S, Gardelli L, Bassi B, Parente G, Dubini A, Serra L, Nizzoli M. A new case of DRESS syndrome induced by sulfasalazine and triggered by amoxicillin. Case Rep Rheumatol. 2013;2013:409152. [PMC free article: PMC3723001] [PubMed: 23936716](53 year old woman with uveitis developed sore throat, fever and lymphadenopathy 6 weeks after starting sulfasalazine which was treated with amoxicillin-clavulanate whereupon she developed worsening fever, rash and was found to have liver injury [bilirubin 2.7 mg/dL, ALT 350 U/L, Alk P 2959 U/L, INR normal], responding to methylprednisolone and stopping drugs; role of amoxicillin-clavulanate being uncertain in this onset of DRESS syndrome with sulfasalazine therapy).
- Ferrajolo C, Verhamme KM, Trifirò G, 't Jong GW, Giaquinto C, Picelli G, Oteri A, et al. Idiopathic acute liver injury in paediatric outpatients: incidence and signal detection in two European countries. Drug Saf. 2013;36:1007–16. [PubMed: 23591830](Analysis of 3 large pediatric healthcare databases form Italy and the Netherlands [2000-2008] identified 785 cases of idiopathic acute liver injury in children: 73/100,000 in Italy and 21/100,000 in Holland, linked to 110 possible medications, adjusted relative risks was greatest for clarithromycin [26], amoxicillin-clavulanate [19], amoxicillin [7.5] and for combinations of isoniazid and rifampin [4852] and acetaminophen [94]).
- Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419–25. [PubMed: 23419359](In a population based study of drug induced liver injury from Iceland, 96 cases were identified over a 2 year period, including 15 due to amoxicillin-clavulanate making it the most common cause, estimated to occur after 1 per 2350 prescriptions; 13 cases were symptomatic, but only 6 were jaundiced and none were fatal).
- Sistanizad M, Peterson GM. Drug-induced liver injury in the Australian setting. J Clin Pharm Ther. 2013;38:115–20. [PubMed: 23350857](Among 17 cases of suspected drug induced liver injury seen at a single referral hospital in Tasmania over a 12 month period, 11 were due to antibiotics including flucloxacillin in 4, amoxicillin in 2, amoxicillin-clavulanate in 2, and rifampin, moxifloxacin and ciprofloxacin in 1 each).
- Beraldo DO, Melo JF, Bonfim AV, Teixeira AA, Teixeira RA, Duarte AL. Acute cholestatic hepatitis caused by amoxicillin/clavulanate. World J Gastroenterol. 2013;19:8789–92. [PMC free article: PMC3870529] [PubMed: 24379601](63 year old man developed jaundice and pruritus arising 45 days after a course of amoxicillin-clavulanate [bilirubin 8.3 mg/dL, ALT 200 U/L, Alk P 60 U/L], with severe course but ultimate recovery).
- Devarbhavi H, Andrade RJ. Drug-induced liver injury due to antimicrobials, central nervous system agents, and nonsteroidal anti-inflammatory drugs. Semin Liver Dis. 2014;34:145–61. [PubMed: 24879980](Review of drug induced liver injury from various classes of agents, mentions that amoxicillin-clavulanate is the leading cause of drug induced liver injury, marked by a latency of several days to weeks, often after stopping the antibiotic, the injury varying from cholestatic to hepatocellular and the mortality rate being as high as 7%).
- Björnsson ES. Epidemiology and risk factors for idiosyncratic drug-induced liver injury. Semin Liver Dis. 2014;34:115–22. [PubMed: 24879977](Estimates of the incidence of drug induced liver injury have ranged from 2 to 19 cases per 100,000 inhabitants, probably because of variation in medication use, definitions used and rigor of capturing all patients in a population; in recent studies, amoxicillin-clavulanate has been the most frequently implicated drug).
- Kaye JA, Castellsague J, Bui CL, Calingaert B, McQuay LJ, Riera-Guardia N, Saltus CW, et al. Risk of acute liver injury associated with the use of moxifloxacin and other oral antimicrobials: a retrospective, population-based cohort study. Pharmacotherapy. 2014;34:336–49. [PMC free article: PMC4260122] [PubMed: 24865821](In a US healthcare database with 1.3 million antimicrobial users there were 607 cases of liver injury and 11 cases of liver failure, the highest relative risk for current single use being 3.2 for levofloxacin, 2.5 for amoxicillin-clavulanate, 2.5 for doxycycline, 2.3 for moxifloxacin and 2.3 for amoxicillin).
- Hernández N, Bessone F, Sánchez A, di Pace M, Brahm J, Zapata R, A, Chirino R, et al. Profile of idiosyncratic drug induced liver injury in Latin America. An analysis of published reports. Ann Hepatol. 2014;13:231–9. [PubMed: 24552865](Systematic review of literature of drug induced liver injury in Latin American countries published from 1996 to 2012 identified 176 cases, 3 of which were caused by amoxicillin-clavulanate).
- Fontana RJ. Pathogenesis of idiosyncratic drug-induced liver injury and clinical perspectives. Gastroenterology. 2014;146:914–28. [PMC free article: PMC4031195] [PubMed: 24389305](Review of clinical phenotypes and pathogenesis of different forms of drug induced liver injury including antibiotics and amoxicillin-clavulanate).
- Leise MD, Poterucha JJ, Talwalkar JA. Drug-induced liver injury. Mayo Clin Proc. 2014;89:95–106. [PubMed: 24388027](Review of drug induced liver injury mentions that antibiotics are the most common cause and amoxicillin-clavulanate the most common single cause in Europe and the US, accounting for 8-22% of cases).
- Chalasani N, Bonkovsky HL, Fontana R, Lee W, Stolz A, Talwalkar J, Reddy KR, et al. United States Drug Induced Liver Injury Network. Features and outcomes of 899 patients with drug-induced liver injury: The DILIN Prospective Study. Gastroenterology. 2015;148:1340–52.e7. [PMC free article: PMC4446235] [PubMed: 25754159](Among 899 cases of drug induced liver injury enrolled in a US prospective study between 2004 and 2013, 91 [12%] were attributed to amoxicillin-clavulanate, making it rank first among 177 implicated agents and well ahead of the second ranked drug isoniazid with 49 cases [6.5%]).
- Yazici C, Mutlu E, Bonkovsky HL, Russo MW. Risk factors for severe or fatal drug-induced liver injury from amoxicillin-clavulanic acid. Hepatol Res. 2015;45:676–82. [PubMed: 25163514](Systematic review of amoxicillin-clavulanate associated liver injury literature found exposure to hepatotoxic co-medications was most common in fatal cases [21%: 3 of 14) than in cases with subsequent recovery [2.3%: 2 of 89]).
- Pavlos R, Mallal S, Ostrov D, Buus S, Metushi I, Peters B, Phillips E. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med. 2015;66:439–54. [PMC free article: PMC4295772] [PubMed: 25386935](Review of HLA associations of drug hypersensitivity reactions and 3 hypothetical models to explain T cell responses to drugs: mediated by haptens, pharmacological interactions or altered peptide repertoires).
- Guéant JL, Romano A, Cornejo-Garcia JA, Oussalah A, Chery C, Blanca-López N, Guéant-Rodriguez RM, et al. HLA-DRA variants predict penicillin allergy in genome-wide fine-mapping genotyping. J Allergy Clin Immunol. 2015;135:253–9. [PubMed: 25224099](In a genome wide association study of 387 patients with immediate allergic reactions to beta-lactam antibiotics, several class 2 HLA associations [HLA-DRA regions] were found for penicillin responses, but they did not apply to cephalosporin cases).
- Björnsson ES. Drug-induced liver injury: an overview over the most critical compounds. Arch Toxicol. 2015;89:327–34. [PubMed: 25618544](Review of the most common causes of drug induced liver injury in 3 recent surveys all of which listed amoxicillin-clavulanate as the most frequent cause).
- Mengual-Moreno E, Lizarzábal-García M, Ruiz-Soler M, Silva-Suarez N, Andrade-Bellido R, Lucena-González M, Bessone F, et al. Invest Clin. 2015;56:3–12. [Case reports of drug-induced liver injury in a reference hospital of Zulia state, Venezuela] Spanish. [PubMed: 25920181](Among 13 patients with drug induced liver injury presenting at a single referral hospital in Venezuela over a 1 year period, the most common causes were ibuprofen (n=3), acetaminophen ([n=3], isoniazid [n=2] and Herbalife products [n=2]; 1 self-limited but slow to recover case was attributed to amoxicillin-clavulanate in a 64 year old man [bilirubin 36.1 mg/dL, ALT 66 U/L, Alk P 629 U/L]).
- Yaseen FS, Saide K, Kim SH, Monshi M, Tailor A, Wood S, Meng X, et al. Promiscuous T-cell responses to drugs and drug-haptens. J Allergy Clin Immunol. 2015;136:474–6.e8. [PubMed: 25910715](T cell responses to either soluble or dendritic cell processed amoxicillin or clavulanate were detected in selected patients with a history of amoxicillin-clavulanate induced liver injury and were HLA restricted).
- Suzuki A, Yuen NA, Ilic K, Miller RT, Reese MJ, Brown HR, Ambroso JI, et al. Comedications alter drug-induced liver injury reporting frequency: Data mining in the WHO VigiBase™. Regul Toxicol Pharmacol. 2015;72:481–90. [PMC free article: PMC4548888] [PubMed: 25988394](Analysis of the WHO VigiBase for possible effects of co-medications in drug induced liver injury using acetaminophen, isoniazid, valproate and amoxicillin-clavulanate found evidence of a decrease or an increase in case incidence for different co-medications).
- Kim SH, Saide K, Farrell J, Faulkner L, Tailor A, Ogese M, Daly AK, et al. Characterization of amoxicillin- and clavulanic acid-specific T cells in patients with amoxicillin-clavulanate-induced liver injury. Hepatology. 2015;62:887–99. [PubMed: 25998949](Lymphocytes from 6 of 7 patients with a history of amoxicillin-clavulanate induced liver injury showed significant proliferation or cytokine release in response to amoxicillin or clavulanate in vitro, and T cell clones responding to each drug were produced that were HLA-resisted, but not cross reactive).
- Moreno L, Sánchez Delgado J, Vergara M, Casas M, Miquel M, Dalmau B. Recurrent drug-induced liver injury (DILI) with ciprofloxacin and amoxicillin/clavulanic. Rev Esp Enferm Dig. 2015 Dec;107:767–8. [PubMed: 26671593](Case report of a patient who developed drug induced liver injury attributed to ciprofloxacin and subsequently a separate episode due to amoxicillin-clavulanate).
- Ferrer P, Amelio J, Ballarín E, Sabaté M, Vidal X, Rottenkolber M, Schmiedl S, et al. PROTECT Work Package 2. Systematic review and meta-analysis: macrolides and amoxicillin/clavulanate-induced acute liver injury. Basic Clin Pharmacol Toxicol. 2016;119:3–9. [PubMed: 26707367](Metaanalysis of placebo controlled trials of macrolides and amoxicillin-clavulanate found significant increased risk of ALT elevations with erythromycin but not clarithromycin, telithromycin or amoxicillin-clavulanate; no mention of azithromycin).
- Björnsson ES. Hepatotoxicity by drugs: the most common implicated agents. Int J Mol Sci. 2016;17:224. [PMC free article: PMC4783956] [PubMed: 26861310](Review of the most common causes of drug induced liver injury based upon categorization from LiverTox).
- deLemos AS, Ghabril M, Rockey DC, Gu J, Barnhart HX, Fontana RJ, Kleiner DE, et al. Drug-Induced Liver Injury Network (DILIN). Amoxicillin-clavulanate-induced liver injury. Dig Dis Sci. 2016;61:2406–16. [PMC free article: PMC4945382] [PubMed: 27003146](Among 1038 cases of drug induced liver injury enrolled in a US database between 2004 and 2014, 117 were attributed to amoxicillin-clavulanate [9%]; mean age 60 years, 62% men, mean latency 29 days, mostly jaundiced and cholestatic [median bilirubin 6 mg/dL, ALT 362 U/L, Alk P 288 U/L, R value 2.7], 3 underwent liver transplant with resolution in almost all cases, although sometimes delayed).
- Meng X, Earnshaw CJ, Tailor A, Jenkins RE, Waddington JC, Whitaker P, French NS, et al. Amoxicillin and clavulanate form chemically and immunologically distinct multiple haptenic structures in patients. Chem Res Toxicol. 2016;29:1762–72. [PubMed: 27603302](Analysis of amoxicillin and clavulanate adducts produced in vitro and detected in vivo in patients with amoxicillin-clavulanate hepatotoxicity, the adducts were also present in culture median used to detect reactivity of specific T cell clones from patients ).
- Lee J, Ji SC, Kim B, Yi S, Shin KH, Cho JY, Lim KS, Lee SH, Yoon SH, et al. Exploration of biomarkers for amoxicillin/clavulanate-induced liver injury: multi-omics approaches. Clin Transl Sci. 2017;10:163–71. [PMC free article: PMC5421739] [PubMed: 27785887](Among 30 Korean, male healthy volunteers treated with amoxicillin-clavulanate twice daily for 14 days, serum ALT levels doubled in 6 becoming abnormal in only one, these changes accompanied by increases in miRNA122 and with a positive lymphocyte stimulation response to clavulanate in one subject but not with higher plasma levels of either agent).
- Munz M, Grummich H, Birkmann J, Wilhelm M, Holzgrabe U, Sörgel F. Severe drug-Induced liver injury as an adverse drug event of antibiotics: a case report and review of the literature. Chemotherapy. 2017;62:367–73. [PubMed: 28934748](20 year old woman received 4 antibiotics [amoxicillin, ciprofloxacin, cefazolin and clindamycin] and acetaminophen within weeks of developing rash, fever and jaundice [bilirubin 3.7 mg/dL, ALT 1219 U/L, Alk P 143 U/L, GGT 201 U/L, INR 1.6], with worsening and signs of hepatic failure but subsequent spontaneous and complete resolution, the specific cause being obscure because of the many drug exposures).
- Visentin M, Lenggenhager D, Gai Z, Kullak-Ublick GA. Drug-induced bile duct injury. Biochim Biophys Acta Mol Basis Dis. 2018;1864 4 Pt B:1498–506. [PubMed: 28882625](Review of drug induced bile duct injury focusing on agents flucloxacin and amoxicillin-clavulanate that injure small ducts and fluorouracil [given by hepatic artery], ketamine, scolicidal agents [for Hydatid disease given into the cysts] that injure larger ducts causing a sclerosing cholangitis like picture).
- Takeuchi Y, Shinozaki T, Kumamaru H, Hiramatsu T, Matsuyama Y. Analyzing intent-to-treat and per-protocol effects on safety outcomes using a medical information database: an application to the risk assessment of antibiotic-induced liver injury. Expert Opin Drug Saf. 2018;17:1071–9. [PubMed: 30252549](Cohort matching of cases with vs without antibiotic therapy in a large electronic medical record database from the University of Tokyo Hospital from 2011 to 2015 with adjustments found rates of liver test abnormalities within 30 days of starting penicillins [25.2 per 1000] was higher than that of fluoroquinolones [11.4] and macrolide antibiotics [8.1] as well as controls [6.5 to 7.1]).
- Vilà-Nadal G, Lleonart Bellfill R, Martí Garrido J, Baliellas Comellas C, Corominas Sánchez M. Recurrent drug-induced liver injury with cetirizine and amoxicillin-clavulanate potassium. J Investig Allergol Clin Immunol. 2019;29:321–2. [PubMed: 30957759](35 year old woman developed fatigue and jaundice shortly after finishing a 12 day course of antibiotics consisting of 8 days of amoxicillin and 4 of amoxicillin-clavulanate [bilirubin 6.3 mg/dL, ALT 2139 U/L, and Alk P132 U/L], having had a similar episode 13 years earlier that arose 1 month after starting cetirizine [bilirubin 8.8 mg/dL, ALT 1873 U/L, Alk P 120], recovering rapidly and completely from both episodes).
- Cirulli ET, Nicoletti P, Abramson K, Andrade RJ, Björnsson ES, Chalasani N, Fontana RJ, et al. Drug-Induced Liver Injury Network (DILIN) investigators. International DILI consortium (iDILIC). A missense variant in PTPN22 is a risk factor for drug-induced liver injury. Gastroenterology. 2019;156:1707–16.e2. [PMC free article: PMC6511989] [PubMed: 30664875](Genome wide association studies on 2048 patients with drug induced liver injury and 12,439 controls identified a variant in PTPN22 which was highly associated with liver injury, allele frequency being 0.12 among cases and 0.08 among controls with highest association in Northern Europeans and in cases of amoxicillin clavulanate, PTPN22 being a cellular kinase involved in modulation of immune reactions).
- Li L, Zheng S, Chen Y. Stevens-Johnson syndrome and acute vanishing bile duct syndrome after the use of amoxicillin and naproxen in a child. J Int Med Res. 2019;47:4537–43. [PMC free article: PMC6753534] [PubMed: 31448655](6 year old boy developed rash and jaundice shortly after receiving amoxicillin and naproxen [bilirubin 3.1 mg/dL, ALT 942 U/L, Alk P 318 U/L, GGT 150 U/L, INR 1.1], with progression of rash to Stevens Johnson syndrome requiring high dose corticosteroid therapy and worsening of liver disease [peak bilirubin 20.4 mg/dL, peak GGT 981 U/L], which persisted for 4 months but eventually resolved although Alk P and GGT remained slightly abnormal at 6 months).
- Alshabeeb MA, Aithal GP, Daly AK. Investigation of oxidative stress-related candidate genes as risk factors for drug-induced liver Injury due to co-amoxiclav. DNA Cell Biol. 2020;39:349–54. [PubMed: 31905014](Among 165 patients with amoxicillin-clavulanate liver injury there was no increase frequency of variants in SOD2, GPX1, GSTM1 and GSTT1, which had previously been implicated in playing a role in pathogenesis).
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Augmentin (amoxicillin/clavulanate) in the treatment of community-acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent.[J Antimicrob Chemother. 2004]Review Augmentin (amoxicillin/clavulanate) in the treatment of community-acquired respiratory tract infection: a review of the continuing development of an innovative antimicrobial agent.White AR, Kaye C, Poupard J, Pypstra R, Woodnutt G, Wynne B. J Antimicrob Chemother. 2004 Jan; 53 Suppl 1:i3-20.
- Review Amoxicillin/clavulanate potassium extended release tablets: a new antimicrobial for the treatment of acute bacterial sinusitis and community-acquired pneumonia.[Expert Opin Pharmacother. 2003]Review Amoxicillin/clavulanate potassium extended release tablets: a new antimicrobial for the treatment of acute bacterial sinusitis and community-acquired pneumonia.Benninger MS. Expert Opin Pharmacother. 2003 Oct; 4(10):1839-46.
- Comparative effectiveness and safety of cefdinir and amoxicillin-clavulanate in treatment of acute community-acquired bacterial sinusitis. Cefdinir Sinusitis Study Group.[Antimicrob Agents Chemother. 1...]Comparative effectiveness and safety of cefdinir and amoxicillin-clavulanate in treatment of acute community-acquired bacterial sinusitis. Cefdinir Sinusitis Study Group.Gwaltney JM Jr, Savolainen S, Rivas P, Schenk P, Scheld WM, Sydnor A, Keyserling C, Leigh A, Tack KJ. Antimicrob Agents Chemother. 1997 Jul; 41(7):1517-20.
- Randomized, investigator-blinded, multicenter study of gatifloxacin versus amoxicillin/clavulanate treatment of recurrent and nonresponsive otitis media in children.[Pediatr Infect Dis J. 2005]Randomized, investigator-blinded, multicenter study of gatifloxacin versus amoxicillin/clavulanate treatment of recurrent and nonresponsive otitis media in children.Sáez-Llorens X, Rodriguez A, Arguedas A, Hamed KA, Yang J, Pierce P, Echols R. Pediatr Infect Dis J. 2005 Apr; 24(4):293-300.
- Double-blind, randomized study of the efficacy and safety of oral pharmacokinetically enhanced amoxicillin-clavulanate (2,000/125 milligrams) versus those of amoxicillin-clavulanate (875/125 milligrams), both given twice daily for 7 days, in treatment of bacterial community-acquired pneumonia in adults.[Antimicrob Agents Chemother. 2...]Double-blind, randomized study of the efficacy and safety of oral pharmacokinetically enhanced amoxicillin-clavulanate (2,000/125 milligrams) versus those of amoxicillin-clavulanate (875/125 milligrams), both given twice daily for 7 days, in treatment of bacterial community-acquired pneumonia in adults.File TM Jr, Lode H, Kurz H, Kozak R, Xie H, Berkowitz E, 600 Study Group. Antimicrob Agents Chemother. 2004 Sep; 48(9):3323-31.
- Amoxicillin-Clavulanate - LiverToxAmoxicillin-Clavulanate - LiverTox
Your browsing activity is empty.
Activity recording is turned off.
See more...