NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Varki A, Cummings RD, Esko JD, et al., editors. Essentials of Glycobiology [Internet]. 3rd edition. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015-2017. doi: 10.1101/glycobiology.3e.058

Essentials of Glycobiology [Internet]. 3rd edition.
Show detailsGlyconanomaterials are interesting biological probes, given the central role of multivalency in glycobiology. Tunable chemical and physical properties such as electronic, photonic, and magnetic properties render nanomaterials that are built on different scaffolds interesting to probe cellular, tissue, and organismal interactions. The attachment of glycans can change the properties of nanomaterials by constructing glycan-containing hybrids with better solubility, biocompatibility, and lower cytotoxicity. Nanomaterials containing glycans have been used as imaging agents, as spectroscopic tools, and to monitor cellular systems, as well as for vaccination and drug delivery.
INTRODUCTION
Glycoproteins or glycolipids are natural glycoconjugates that take part in cellular communication, inflammation, and immune responses using carbohydrate–protein or carbohydrate–carbohydrate interactions. Certain glycan patterns are characteristic markers of diseases such as cancer, asthma, and diabetes. The search for molecular mechanisms requires tools that mimic the presentation of glycans on the cell surface.
Glycans play a unique functional role in biology as individual protein–carbohydrate interactions are often of low affinity and broad specificity, but Nature enhances specificity by utilizing multivalent interactions. The number of carbohydrate residues on a biomolecular construct and the presentation including branching are major determinants of binding avidity of ligands to cell-surface receptors. Because the transition from monovalent to multivalent is associated with a larger variation in affinity/avidity than for some other ligand–receptor interactions, a “thresholding” effect, and in some cases cooperativity, can be exploited by Nature.
To explore and exploit the role of glycans, glycans have to be displayed in a scenario closer to that found on the cellular scale. “Nanotechnology” moves from the angstrom to the nanometer range (from ∼10−10 to ∼10−7 m) to create, manipulate, and characterize structures on those scales.
Larger glycoconjugates bearing multiple copies of carbohydrate on various scaffolds such as glycodendrimers or glycopolymers have been generated and studied to probe carbohydrate–protein interactions. These constructs were expanded to encompass more diverse nanomaterials with inherent high surface/volume ratios to allow for a greater contact surface area and improve any multivalency effects. The integration of nanomaterials in the glycosciences will enable biomedical applications such as drug delivery systems, imaging agents, diagnostic platforms, or precise sensing tools that operate through biological mimicry. Merging the glycosciences and nanotechnology improves our understanding of glycobiology and gives rise to novel glycodevices.
TYPES AND APPLICATIONS OF GLYCONANOMATERIALS
Glyconanomaterials take advantage of the unique physical properties of the nanoscale such as catalytic, photonic, electronic, or magnetic properties that are not seen in the bulk as well as from the properties of glycans such as water solubility, biocompatibility, structural diversity, and targeting properties. Metal, semiconductor, or carbon-based nanomaterials can confer these unique properties to glycans, whereas glycans provide these nanomaterials with an exceptional stability in water and biological buffers, with biocompatibility, and with both passive and active targeting properties.
Additionally, nanoformulations based on polysaccharides have been developed as drug delivery carriers. Superior to some synthetic materials in biocompatibility, biodegradability, low toxicity, low cost, and the ease of chemical modifications, polysaccharides such as chitosan, dextran, hyaluronic acid, and heparin have enabled the preparation of polysaccharide-based nanoparticles for pharmaceutical use. Moreover, in hybrid substructures, polysaccharides were used to coat metallic nanoparticles to create polysaccharide-coated nanoparticles.
INORGANIC NANOPARTICLES
Hybrid materials from inorganic nanostructures and biomolecules are a major focus of nanotechnology. Iron oxide, noble metal, and semiconductor nanoparticles served as synthetic scaffolds to multimerize glycans and enhance the affinity for receptors (Figure 58.1). The physical properties, such as magnetism and fluorescence, of hybrid materials have given rise to applications in sensing, delivery, or imaging.

FIGURE 58.1.
Overview of different types of glyconanomaterials created by coupling glycans to the surface of diverse nanomaterials.
Gold Nanoparticles
The unique optoelectronic properties and facile chemical modification make gold nanoparticles (AuNPs) an important tool to monitor biological binding events. The conjugation of AuNPs with glycans endows them with high aqueous solubility/dispersibility and biocompatibility as the basis for novel strategies in bioanalytical applications. Color changes of AuNPs correlate with the resonance between collective oscillations of electrons (plasmons) and the incident electromagnetic radiation, giving rise to localized surface plasmon resonance (LSPR). Resonance frequencies or surface plasmon bands of gold lie in the visible region (400–750 nm) giving rise to color effects. The high surface area/volume ratio of AuNPs allows particularly enhanced LSPR sensitivity and colorimetric changes. Exploitation of 10–20-nm-sized modified oligonucleotide-bearing AuNPs allows for the colorimetric detection of DNA.
AuNPs are produced by reducing gold salts with sodium citrate and surface modification with “capping” agents. Size, shape, and morphology are tuned by adjusting reaction conditions and allowing access to the near-infrared (NIR) spectrum. Colorimetric carbohydrate–lectin analyses exploiting LSPR of AuNPs have typically used 10-nm particles capped with thiol-poly(ethylene glycol) (thiol-PEG) aldehydes decorated with lactose through reductive amination. Ricinus communis agglutinin (RCA120) induced a reversible color change as did cholera toxin.
Direct visualization is a particularly attractive method in biology. Stable colloidal gold was first described by Faraday in 1857 but applied in biology only in the 1970s when immunogold-staining procedures were used to observe microorganisms by transmission electron microscopy (TEM). Immunogold staining with glyco-AuNPs used mannosylated particles to probe complement activation and opsonization processes in macrophage-mediated endocytosis and to target Escherichia coli–containing type 1 pili mannose-specific receptors. Carbohydrate–protein interactions were visualized via TEM in which AuNPs were seen interacting at the pili tips of E. coli.
Very small gold glyconanoparticles can be prepared by reducing a gold salt in the presence of glycosylated thiol ligands (Figure 58.2). Ligand density and composition can be adjusted precisely. These glyconanoparticles conserve the chemical properties of the ligands and can be advantageously characterized by ultraviolet-visible (UV-Vis) spectroscopy, infrared (IR) spectroscopy, elemental analysis, nuclear magnetic resonance (NMR), TEM, and X-ray photoelectron spectroscopy (XPS).

FIGURE 58.2.
A calculated representation of a 2-nm-sized gold glyconanoparticle formed by 102 gold atoms and coated with 44 molecules of 5-mercaptopentyl α-D-mannopyranoside and the corresponding transmission electron microscopy (TEM) image.
The smaller glyco-clusters lack a LSPR band but can be observed in TEM. Visualization of AuNPs helped to unambiguously demonstrate some weak, previously controversial, effects. Lex-decorated AuNPs provided direct visual evidence for the existence of Ca-mediated sugar–sugar interactions and were used to explore potential mechanisms of sugar-mediated self-assembly of sponge cells.
Small gold glyconanoparticles were helpful in clarifying mechanistic aspects of multivalent carbohydrate recognition and have been exploited as antiadhesion agents to prevent melanoma metastasis, as vaccine candidates, and in cellular and molecular imaging.
Magnetic Nanoparticles
Magnetic nanoparticles (MNPs), including iron oxide and manganese oxide nanoparticles (NPs), are of particular interest as contrast agents for magnetic resonance imaging (MRI). MRI can generate internal tomographic tissue images by using a radio frequency (RF)-induced electromagnetic field; modulation of that field's signal by particles (so-called “contrast”), allows their location to be detected. In clinical practice, small molecule gadolinium complexes are most commonly used as MRI “contrast” agents. MNP-biomolecule hybrids, because of their size and the effect, are, however, typically more sensitive, and because of their potential for the loading of many ligand copies, are expected to be better for receptor targeting and multimodal imaging power (by attaching other labels that allow additional modes).
Functionalization of the MNP surface is the basis for molecule-specific binding interactions and “molecular MRI.” Antibodies have been widely used because of their superb specificity but suffer from high cost, short lifetime due to thermal instability, and their potential immunogenicity. Structurally defined ligands such as glycans in glyco-MNPs provide an attractive alternative.
Glyco-MNPs allow for detection of early stage disease by successfully mimicking leukocyte recruitment during inflammation (see Figure 58.2). By taking advantage of their high surface/volume ratio, glyco-MNPs can display multiple copies of oligosaccharides, thus increasing potential multivalency of binding interactions. Tetrasaccharide sialyl-Lewis x (sLex)-functionalized MNPs successfully targeted E-/P-selectins. Notably, sLex-MNPs detected inflammation events, both in vitro and in vivo without any significant signs of associated cytotoxicity. Studies inside the brain of mouse models of stroke allowed detection by specifically binding to the activated endothelium of blood vessels running through diseased tissue (Figure 58.3). Cross-species efficacy is possible with sugars but harder to achieve with antibody ligands. Thus, glyco-MNPs may be translated more readily from mammalian models to the clinic.
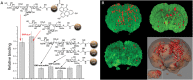
FIGURE 58.3.
(A) In vitro binding studies using sLex-MNPs to rat E-selectin; (B) magnetic resonance images (MRIs) and their 3D reconstruction of sLex magnetic nanoparticles. (Adapted from van Kasteren SI, et al. 2009. Proc Natl Acad Sci 106: 18−23.)
Quantum Dots
Quantum dots (QDs) are luminescent semiconducting nanomaterials. Typically, QDs are made of binary cadmium or zinc selenides or sulfides. QDs can emit light in the entire spectrum and their optical properties are tunable depending on the size. Compared with organic dyes, QDs also have a broader excitation spectrum and sharper emission bands, allowing for multicomponent analysis with a single excitation source. The first glyco-QDs functionalized with carboxymethyldextran and polylysine were used to study carbohydrate–protein interactions. QDs can be stabilized with glycodendrimers. The addition of maltose-modified dendrimers rendered the QDs water soluble and biocompatible while enhancing stability.
Using a host–guest strategy, β-cyclodextrin-quantum dots (β-CD-QDs) were prepared. Synthetic β-CD-QDs behaved much like PEGylated QDs and agglutinated lectins such as ConA, Galanthus nivalis agglutinin (GNA), and peanut agglutinin (PNA).
CARBON-BASED GLYCONANOMATERIALS
Elemental carbon has several allotropes including tetravalent diamond and trivalent graphitic structures, all of which provide potential scaffolds for the arraying of glycans. One allotrope, buckminsterfullerene C60, is the basis for discrete constructs that, although larger than many small molecules, can be manipulated using techniques common to smaller molecules. For the resulting graphene-like sheets, the ratio of boundary carbon atoms/inner carbon atoms increases; and the global reactivity of these structures changes. Carbon nanotubes (CNTs) can be considered to be cylindrical, elongated fullerenes. As a consequence of their curvature, hybridization, and boundary/inner atom ratios both fullerenes and CNTs possess reactivities that differ from those of other carbon allotropes.
[60]Fullerenes
Glycosylated-fullerenes such as α-D-mannosyl [60]fullerenes and fullerenols inhibit erythrocyte aggregation. These “sugar balls” are generated by the introduction of a reactive group such as a terminal alkyne followed by attachment of azido sugars. Thereby, a near-spherical display of glycans is possible. The glycosylated C60s with 0.7-nm diameter are the smallest “nanoparticles” for glyconanotechnology.
Carbon Nanotubes
CNTs are classified based on the number of graphene-like sheets that make up the sidewalls of the cylinder. Single-walled CNTs (SWCNTs) have a typical diameter of 1–2 nm and multiwalled CNTs (MWCNTs) have diameters of ∼2–25 nm. The CNT length can range from tens of nanometers to tens of micrometers or even longer. The inner hollow space and outer surface may be used together to create functionalized CNTs (f-CNTs) to serve as delivery systems.
Broader use of CNTs has been hampered by their perceived cytotoxicity and poor solubility. Cytotoxicity arises from very long needle-like CNTs similar to asbestos that can be avoided by using the right constructs. Noncovalent modification of CNTs relies on physical mixing such as sonication of CNTs with surfactants or polymers. Because the conjugated pi-“scaffolding” of graphene-like sheets is preserved in CNTs, unique physical properties, including characteristic Raman resonances and photoluminescence, are often retained. To mimic mucins, CNTs have been coated, for example, with glycopolymers. A C18-lipid tail “wrapped” the CNT surface through hydrophobic interactions while α-GalNAc residues were incorporated as the cell-surface carbohydrate. Glycopolymer-coated CNTs were nontoxic in vitro, whereas noncoated CNTs induced death in certain cells. The use of glycodendrimers allowed for more uniform CNT coating. Noncovalent modifications have the risk of losing their coating materials once introduced into a biological milieu and the ultimate fate of such noncovalent f-CNTs following loss of surfactant molecules by interaction with cell membranes can be unclear.
Covalent surface glycosylation or glycoconjugation of CNTs creates in vivo biological probes. Covalent modification by oxidation of the CNT surface to introduce carboxylic acids allows for attachment of amino sugars. Galactosylated-SWCNTs created in this way can “capture” pathogenic E. coli. Scanning electron microscope (SEM) images show a strongly bound matrix formed by cells binding to glycosylated nanotubes.
Direct attachment of β-GlcNAc residues via a “one-pot” Staudinger reduction and amidation allows for good control of anomeric configuration. Glycosyltransferases were used for regio- and stereoselective elaboration following monosaccharide attachment. The sugar hydroxyl groups were used as “tagging” sites to visualize the glycans via TEM of heavy element-bearing labels.
Direct 1,3-dipolar cycloaddition of reactive azomethine ylides that were generated in situ from α-amino acids create pyrrolidine derivatives of fullerenes and CNTs. This covalent approach avoids oxidative “cutting” and provided filled-and-functionalized glycosylated CNTs for in vivo applications.
Such nanotubular structures can be considered “1D hollow pores” and display an associated capillarity. In this way, molten salts or their solutions can be encapsulated inside CNTs through capillary action. Filled glyco-CNTs were used for encapsulation of the radioemitter Na125I and in vivo localization of high levels of radionuclide. Multiple copies of GlcNAc improved both water dispersibility and biocompatibility. Thanks to the high aspect ratio and surface area to volume ratio of such CNTs, sugars can be efficiently displayed in a multivalent format (Figure 58.4). These “filled-closed-functionalized” glyco-CNTs are alternative radiotracers for in vivo imaging or radiation-delivery systems with high radioisotope-loading capacity and high sensitivity. The rapid uptake of iodide by the mammalian thyroid served as a test of any potential leakage of radioactive iodide “cargo.” Although “free” iodide 125I rapidly entered the thyroid, iodide encapsulated in the glyco-CNT remained at its target site even after a month.

FIGURE 58.4.
In vivo localization of filled-and-functionalized glyco-single-walled nanotubules (SWNTs). (TH) thyroid; (LU) lungs; (ST) stomach; (LI) liver; (KI) kidney; (BL) bladder. (Adapted from Hong SY, et al. 2010. Nat Mater 9: 485−490.)
Graphene
A supramolecular carbohydrate-functionalized two-dimensional (2D) surface was prepared by decorating thermally reduced graphene sheets with multivalent sugar ligands. Host–guest inclusion on the carbon surface provides a versatile strategy to increase the water solubility of graphene materials and present biofunctional binding groups. The modification with multivalent sugar ligands makes the carbon material an excellent platform for agglutinating and inhibiting the motility of bacteria. Taking advantage of the responsive property of supramolecular interactions, the captured bacteria can then be released partially by adding a competitive guest. The unique thermal IR-absorption properties of graphene allow for the killing of the captured bacteria by IR-laser irradiation of the captured graphene–sugar–E. coli complex.
POLYSACCHARIDE-BASED NANOPARTICLES
Natural polysaccharides are very useful for preparation of nanometric carriers. The low toxicity, biocompatibility, stability, low cost, hydrophilic nature, and availability of reactive sites for chemical modification render polysaccharides attractive building blocks for pharmaceutical applications. Polysaccharides can be used as NP backbone or coating. Polysaccharide-based nanoparticles are prepared by covalent or ionic cross-linking, polyelectrolyte complexation, or self-assembly of hydrophobically modified polysaccharides. Polysaccharide coats improve the water solubility, stability, and long-term circulation of polymeric or metallic nanoparticles.
Chitosan-based NPs were used as drug delivery systems. Positively charged chitosan gives rise to ionic cross-linked particles with polyanions to deliver proteins, oligonucleotides, and plasmid DNA. Multifunctional glycol-chitosan NPs incorporating a NIR fluorophore for fluorescence imaging can encapsulate anticancer drugs or complex small interfering (siRNA) for sequential drug delivery. Chitosan-PEG-coated iron oxide NPs improve intracellular delivery of a DNA repair inhibitor (O6-benzylguanine) to glioblastoma multiform cells and enable treatment monitoring by MRI. Dextran improves the water solubility and stability of iron oxide magnetic nanoparticles. Sulfated dextran electrostatically interacts with positively charged polycations. By functionalization of dextran-coated iron oxide NPs with sLex tetrasaccharide, inflammation events in mouse brain can be monitored both in vitro and in vivo. Hyaluronic acid and heparin-based NPs are promising platforms in cancer therapy. Unlike chitosan- or dextran-based NPs they display inherent targeting properties. Hyaluronic acid binds to CD44, a transmembrane glycoprotein that is overexpressed in many types of cancer. This targeting property has promoted the application of hyaluronic acid-based NPs as theranostic agents. Hyaluronic acid-coated superparamagnetic iron oxide NPs have been used for imaging and drug delivery to cancer cells.
Nanocarriers based on heparin and heparin derivatives have been applied to combat cancer via targeted, magnetic, photodynamic, and gene therapy. Gold and magnetic NPs have been coated or modified with heparin to improve the biocompatibility for applications in heparin-mediated events. Polysaccharide-functionalized gold NPs have given rise to multifunctional NPs with a wide range of applications including imaging, photodynamic therapy, and apoptosis induction of metastatic cells.
GLYCODENDRIMERS
Glycodendrimers allow for fine-tuned control over the number and orientation of glycans on a nanosized molecule. Glycodendrimers with increasing numbers of branching points and glycan content at the surface were used to study lectin-binding properties. “Click” chemistry and amide bond formation have been used to tether sugar molecules to the dendrimer scaffold. Mannose-conjugated glycodendrimers bind specifically to ConA. Supramolecular assemblies have been prepared to display carbohydrates in a defined manner. Dendrimeric arms have also been affixed to β-cyclodextrin (βCD). The βCD was modified to incorporate either a drug such as doxorubicin or a fluorescent dye to monitor the uptake of the dendrimeric structure. Hepatocytes were found to preferentially take up galactose-functionalized materials. Thereby, specific delivery of doxorubicin to hepatocytes was achieved.
Three-dimensional supramolecular scaffolds expressing clusters of sugars were based on Ru(bpy)3. βCDs decorated with seven mannose functional groups each were bound to the ruthenium core via hydrophobic interactions with adamantyl functional groups appended on the core. The resulting supramolecular assemblies bind E. coli that express the mannose receptor in the bacterial pili.
Glycodendrimers can also be combined with particles to create multiple levels of multivalency from both dendrimer and particle. In one example, glycodendrimers displayed on protein-derived particles (virus-like particles) allowed for picomolar inhibition of Ebola virus–related adhesion events.
GLYCONANOMATERIALS IN DIAGNOSIS AND THERAPY
Glycans are suitable biomarkers for medical diagnostics. Glyconanotechnology aids the development of biosensors and methods for the detection of glycans, lectins, or cancer cells and pathogens. Nanoengineered glycan sensors may help with glycoprotein profiling without labeling or glycan liberation steps. A variety of nanomaterials are being explored as specific probes for the label-free lectin or glycan detection. AuNPs and CNTs are the most widely used nanomaterials. Nanoengineered materials can be detected by mass changes with quartz crystal microbalance (QCM) and cantilever sensors, by field-effect transistor (FET) sensors based on carbon nanotubes, or optical sensors based on surface plasmon resonance (SPR) in combination with self-assembled monolayers (SAMs) of glycans or lectins.
Cancer cells and pathogen can be detected using glyconanomaterials. Gold glyconanoparticles and silver signal amplification were used to detect and quantify cell-surface mannose glycans. Gold nanoparticles were covered with mannan and incubated with a human gastric cell line in the presence of the mannose-binding lectin ConA. The specific detection of cancer cells requires further refinement of the system such as the substitution of ConA by a panel of lectins that recognize aberrant glycosylation specific to cancer. ConA-functionalized CNTs were used for electrochemical surface glycan detection. The label-free detection of cells binding to ConA was realized by electrochemical impedance spectroscopy (EIS). Water decontamination was achieved by precipitation of bacteria with glycan-functionalized nanodiamonds as cross-linkers followed by filtration through a 10-mm membrane.
The translation of biomolecular binding events into nanomechanics using a nanosized cantilever array of mannosides was applied to detect and discriminate different strains of E. coli bacteria. Methods exploiting intrinsic optical properties can be explored for biomedical “nonlabeled” imaging applications. QDs and AuNPs allow for spectral tuning, whereas SWCNTs show characteristic Raman peaks as well as photoluminescence in the NIR range. These intrinsic properties have yet to be systematically explored in vivo but hold significant application potential.
CONCLUSIONS
Various glyconanomaterials have been prepared by covering different materials with glycans. Potential applications of these materials range from pathogen detection to diagnostic agents. Given the specific roles of natural, endogenous cellular glycoconjugates, glyconanomaterials that display on similar length scales provide chemical platforms to advance our understanding of carbohydrate-mediated biological events.
By taking advantage of the physical properties and inducible sizes, translation of fundamental studies to biomedical glyconanotechnology appears to be imminent. Biomedical imaging, using multimodal imaging techniques, such as PET/MRI or fluorescence/MRI, is an attractive vision for using glyco-NPs.
ACKNOWLEDGMENTS
The authors appreciate helpful comments and suggestions from Donald Bernsteel, Lingquan Deng, and Patience Sanderson.
FURTHER READING
- Bertozzi CR, Bednarski MD. 1992. Antibody targeting to bacterial cells using receptor-specific ligands. J Am Chem Soc 114: 2242–2245.
- Mammen M, Choi SK, Whitesides GM. 1998. Polyvalent interactions in biological systems: Implications for design and use of multivalent ligands and inhibitors. Angew Chem Int Ed 37: 2755–2794. [PubMed: 29711117]
- de la Fuente JM, Barrientos AG, Rojas TC, Rojo J, Cañada J, Fernández A, Penadés S. 2001. Gold glyconanoparticles as water-soluble polyvalent models to study carbohydrate interactions. Angew Chem Int Ed 113: 2317–2321. [PubMed: 29711834]
- Chen X, Lee GS, Zettl A, Bertozzi CR. 2004. Biomimetic engineering of carbon nanotubes by using cell surface mucin mimics. Angew Chem Int Ed 43: 6111–6116. [PubMed: 15549753]
- Gu L, Elkin T, Jiang X, Li H, Lin Y, Qu L, Tzeng T-RJ, Joseph R, Sun Y-P. 2005. Single-walled carbon nanotubes displaying multivalent ligands for capturing pathogens. Chem Commun 2005: 874–876. [PubMed: 15700066]
- Chen X, Tam UC, Czlapinski JL, Lee GS, Rabuka D, Zettl A, Bertozzi CR. 2006. Interfacing carbon nanotubes with living cells. J Am Chem Soc 128: 6292–6293. [PubMed: 16683774]
- Kiessling LL, Gestwicki JE, Strong LE. 2006. Synthetic multivalent ligands as probes of signal transduction. Angew Chem Int Ed 45: 2348–2368. [PMC free article: PMC2842921] [PubMed: 16557636]
- Hong SY, Tobias G, Ballesteros B, El Oualid F, Errey JC, Doores KJ, Kirkland AI, Nellist PD, Green MLH, Davis BG. 2007. Atomic-scale detection of organic molecules coupled to single-walled carbon nanotubes. J Am Chem Soc 129: 10966–10967. [PubMed: 17696530]
- Wu P, Chen X, Hu N, Tam UC, Blixt O, Zettle A, Bertozzi CR. 2008. Biocompatible carbon nanotubes generated by functionalization with glycodendrimers. Angew Chem Int Ed 47: 5022–5025. [PMC free article: PMC2847391] [PubMed: 18509843]
- Chen X, Wu P, Rousseas M, Okawa D, Gartner Z, Zettl A, Bertozzi CR. 2009. Boron nitride nanotubes are noncytotoxic and can be functionalized for interaction with proteins and cells. J Am Chem Soc 131: 890–891. [PMC free article: PMC2657038] [PubMed: 19119844]
- Csaba N, Köping-Höggård M, Alonso MJ. 2009. Ionically crosslinked chitosan/tripolyphosphate nanoparticles for oligonucleotide and plasmid DNA delivery. Int J Pharm 382: 205–214. [PubMed: 19660537]
- van Kasteren SI, Campbell SJ, Serres S, Anthony DC, Sibson NR, Davis BG. 2009. Glyconanoparticles allow pre-symptomatic in vivo imaging of brain disease. Proc Natl Acad Sci 106: 18–23. [PMC free article: PMC2607245] [PubMed: 19106304]
- Hong SY, Tobias G, Al-Jamal KT, Ballesteros B, Ali-Boucetta H, Lozano-Perez S, Nellist PD, Sim RB, Finucane C, Mather SJ, et al. 2010. Filled and glycosylated carbon nanotubes for in vivo radioemitter localization and imaging. Nat Mater 9: 485–490. [PubMed: 20473287]
- Kikkeri R, Grünstein D, Seeberger PH. 2010. Lectin biosensing using digital analysis of Ru(II)-glycodendrimers. J Am Chem Soc 132: 10230–10232. [PubMed: 20662498]
- Grünstein D, Maglinao M, Kikkeri R, Collot M, Barylyuk K, Lepenies B, Kamena F, Zenobi R, Seeberger PH. 2011. Hexameric supramolecular scaffold orients carbohydrates to sense bacteria. J Am Chem Soc 133: 13957–13966. [PubMed: 21790192]
- El-Dakdouki MH, Zhu DC, El-Boubbou K, Kamat M, Chen J, Li W, Huang X. 2012. Development of multifunctional hyaluronan-coated nanoparticles for imaging and drug delivery to cancer cells. Biomacromolecules 13: 1144–1151. [PMC free article: PMC5475368] [PubMed: 22372739]
- Mizrahy S, Peer D. 2012. Polysaccharides as building blocks for nanotherapeutics. Chem Soc Rev 41: 2623–2640. [PubMed: 22085917]
- Reuel NF, Mu B, Zhang J, Hinckley A, Strano MS. 2012. Nanoengineered glycan sensors enabling native glycoprofiling for medicinal applications: Towards profiling glycoproteins without labeling or liberation steps. Chem Soc Rev 41: 5744–5779. [PubMed: 22868627]
- Ribeiro-Viana R, Sánchez-Navarro M, Luczkowiak J, Koeppe JR, Delgado R, Rojo J, Davis BG. 2012. Virus-like Glycodendrinanoparticles displaying quasi-equivalent nested polyvalency upon glycoprotein platforms potently block viral infection. Nat Commun 3: 1303. [PMC free article: PMC3535419] [PubMed: 23250433]
- Marradi M, Chiodo F, García I, Penadés S. 2013. Glyconanoparticles as multifunctional and multimodal carbohydrate systems. Chem Soc Rev 42: 4728–4745. [PubMed: 23288339]
- Review Glycans in Nanotechnology.[Essentials of Glycobiology. 2022]Review Glycans in Nanotechnology.Delbianco M, Davis BG, Seeberger PH. Essentials of Glycobiology. 2022
- Glyconanomaterials: Emerging applications in biomedical research.[Nano Res. 2014]Glyconanomaterials: Emerging applications in biomedical research.Chen X, Ramström O, Yan M. Nano Res. 2014 Oct; 7(10):1381-1403. Epub 2014 Aug 16.
- Physical and biochemical insights on DNA structures in artificial and living systems.[Acc Chem Res. 2014]Physical and biochemical insights on DNA structures in artificial and living systems.Chen N, Li J, Song H, Chao J, Huang Q, Fan C. Acc Chem Res. 2014 Jun 17; 47(6):1720-30. Epub 2014 Mar 3.
- Review Glyconanotechnology.[Chem Soc Rev. 2013]Review Glyconanotechnology.Reichardt NC, Martín-Lomas M, Penadés S. Chem Soc Rev. 2013 May 21; 42(10):4358-76. Epub 2013 Jan 10.
- Review Viral-based nanomaterials for plasmonic and photonic materials and devices.[Wiley Interdiscip Rev Nanomed ...]Review Viral-based nanomaterials for plasmonic and photonic materials and devices.Petrescu DS, Blum AS. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018 Jul; 10(4):e1508. Epub 2018 Feb 8.
- Glycans in Nanotechnology - Essentials of GlycobiologyGlycans in Nanotechnology - Essentials of Glycobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...

