NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Baba AI, Câtoi C. Comparative Oncology. Bucharest (RO): The Publishing House of the Romanian Academy; 2007.
Mammary tumors, at least in dogs and to a certain extent in cats, have many similarities to breast neoplasms in women. In human breast neoplasms, a highly invasive and treatment-resistant protein, responsible for malignant evolution, has been identified. This protein, called stromelysin-3 (ST3) appears in the case of malignant tumors, having extremely high levels, and is not found in cultured malignant tumor cells, but is detected around the tumor. So, the healthy cells adjacent to the tumor, having the property to change the extracellular matrix, the stroma, favoring the invasion of metastatic cells, produce it. It seems that cancer cells produce substances that cause healthy cells to produce ST3. Attempts are made to obtain anti-ST3 drugs [25].
Mammary gland tumors are frequent in dogs and cats, appearing extremely sporadically in other domestic animals. In bitches, the incidence of mammary tumors is estimated at 50% of all neoplasms in this species, of which approximately 60% are benign and 40% malignant [8]. The mean onset age for mammary tumors is between 10 and 11 years. Incidence is higher in certain breeds, such as: Poodle, English Spaniel, Brittany Spaniel, English Setter, Pointer, Fox Terrier, Boston Terrier and Cocker Spaniel; incidence is lower in Chihuahua and Boxer breeds [6].
Mammary tumors in the bitch are hormone dependent, and the risk of appearance increases after each estrous cycle. Mammary tumor cells, either benign or malignant, have estrogen and progesterone receptors. These hormones induce the hypertrophy of the mammary parenchyma, which is obvious after estrus [8, 37]. The higher tumor incidence in the posterior mammary gland pairs is correlated with the higher gland volume and abundant secretion during the lactation period. If before the first cycle the risk of tumor appearance is 0.5%, it reaches 8% after the first cycle and over 26% after the second or more estrous cycles [40]. In dogs, approximately 40% of all mammary tumors are located in the inguinal mammary glands and appear shortly after estrus. The incidence of the different histological forms can be the following: benign tumors, 51.0%, of which 45.5% are fibroadenomas, 5.0% simple adenomas, and 0.5% benign mesenchymal tumors; carcinomas 45.5%, of which solid carcinomas 16.9%, tubular adenocarcinomas 15.4%, papillary adenocarcinomas 8.6%, and anaplastic carcinomas 4.0%; sarcomas and malignant mixed tumors 3.5% [6].
GILBERTSON et al. (1983) propose a classification of mammary carcinomas in the bitch, depending on biological behavior, taking into consideration the histological aspects:
- – stage 0: malignant proliferation is limited to the anatomical structure of the mammary ducts, carcinoma in situ;
- – stage 1: malignant proliferations extend beyond the anatomical limits of the mammary ducts, invading the adjacent stroma, invasive carcinoma, with vascular and lymph node identification;
- – stage 2: carcinoma with vascular or lymphatic invasion or metastases in the regional lymph nodes;
- – stage 3: obvious metastatic disease.
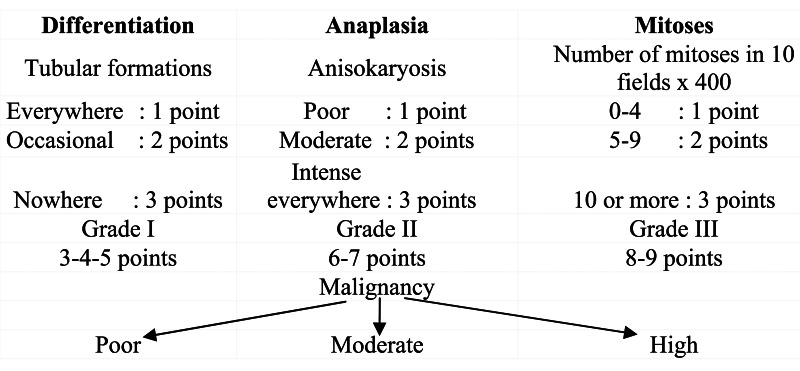
In this system, tumors are evaluated by: the nuclear differentiation degree (high, moderate and poor differentiation) and the lymphoid cellular reaction. Lymphoid reaction occurs diffusely in the tumor and perivascularly.
Mammary tumors can be evaluated by: speed of evolution; growth pattern (infiltrative or non-infiltrative); tumor size; extension according to the TNM system; presence of metastases in lymph nodes and/or viscera. Malignant epithelial tumors can be grouped as follows:
- – non-infiltrating tumors: intracanalicular carcinoma and lobular carcinoma in situ;
- – infiltrating tumors: simple or complex infiltrating canalicular carcinoma; infiltrating lobular carcinoma; epithelioid carcinoma; spindle cell carcinoma; anaplastic carcinoma and other carcinoma types [23]. The authors remark the necessity of establishing prognosis based on clinical data and histological grading, having a predictive value. The same authors propose the use of the histological grading for mammary tumors in dogs, adopted by WHO for human breast cancer:
This grading does not apply to in situ tumors or to particular forms (spindle cell, epidermoid, anaplastic or other forms).
A similarity between human breast tumors and mammary tumors in bitches is found regarding incidence, behavior and histological origin. The appearance of mammary tumors in bitches corresponds to the development of these tumors in women. The risk of developing these tumors increases starting with the age of 6 years in bitches and 40 years in women (the age of 2 years in dogs is equivalent to the age of 24 years in humans, then every year after the age of 2 corresponds to 4 years in humans) [37].
Typical and atypical lobular and ductal hyperplastic lesions have been present in 73.63% of cases in dogs and 56.25% in cats, at the periphery of neoplastic mammary nodules. These changes can be considered precancerous; benign precancerous lesions from the periphery of malignant neoplasms suggest multicentric histogenesis, and evolution is in successive stages, with a continuity in time of the oncogenic system. In conclusion, benign hyperplastic lesions, associated with both benign and malignant tumors, are considered as precancerous stages. Histologically, benign and/or hyperplastic lesions are found in the proximity of a malignant tumor [4].
Benign tumors (51%): fibroadenomas (benign mixed tumors), 45.5%; simple adenomas, 5%; benign mesenchymal tumors, 0.5%.
Malignant tumors (49%): solid carcinomas, 16.9%; tubular adenocarcinomas, 15.4%; papillary carcinomas, 8.6%; anaplastic carcinomas, 4%; sarcomas, 3.1%; carcinosarcomas (malignant mixed tumors), 0.6% [6].
Clinically, mammary tumors appear as single or multiple nodules in the parenchyma, with or without the involvement of nipples. They can be located in any of the eight glands, and benign and malignant forms can coexist. Approximately 2/3 of mammary tumors are found in glands 4 and 5, probably due to the more abundant parenchyma at this level. On clinical examination, tumors are either benign or malignant, they can be small, firm, with well circumscribed nodules, which makes impossible the differentiation between benignity and malignancy. Rapid growth, local tissue invasion and ulceration are characteristic of malignancy. Inflamed mammary carcinomas appear as diffuse swellings, with the multiple involvement of the congeneric gland, being painful and warm. Peripheral edema is present due to the obstruction of lymphatic vessels and retrograde growth.
Metastases frequently develop by lymphatic route, and also, by venous route. In the case of carcinomas, the lymphatic route is the most important, towards the axillary and inguinal lymph nodes, and fine lymphatic vessels enter the thoracic cavity, facilitating lung metastases, as well as the abdominal cavity, with the possible dissemination of neoplasms in the liver and other parenchymatous organs. Less affected sites by metastasis are the long bones and the skeleton. It is noteworthy that lung metastases can be evidenced several months before the mammary neoplasm can be clinically detected. Radiographically, lung neoplasms can be identified in a proportion of 65 up to 97%, depending on the size and delimitation of nodules [49].
Diagnosis is established based on clinical examination, supplemented by blood and serological profile. Radiographic thoracic and abdominal examination can evaluate the presence of metastases in the lungs and the increase in volume of lymph nodes. Fine needle aspiration for cytologic examination is not recommended, since it cannot differentiate with certainty a benign from a malignant tumor [40]
Prognosis and therapeutic approach will be established according to classic criteria: history, clinical signs, histological type and everything that is related to the subject’s condition.
Mammary tumors in cats are particularly frequent, especially at the mean age of 10–14 years. Siamese cats have a higher risk, and they can develop mammary tumors even at a young age. In this species, mammary tumors are less hormone-dependent, early ovariohysterectomy being only partially protective. The incidence of mammary tumors is estimated at 31.8 and 20.4 in 100000 uncastrated cats, ovariohysterectomized cats, respectively.
Malignant neoplasms are found in a much higher proportion compared to neoplasms in bitches. In decreasing order, incidence is appreciated as follows: adenocarcinomas, then carcinomas and sarcomas. Metastases appear early and in a high proportion in lymph nodes and lungs, being also detected in the pancreas, kidneys, central nervous system and, more rarely, in the heart, liver and jejunum [11].
Clinically, the tumor may be single or multiple, having a smooth surface and diffuse growth. It frequently ulcerates, eliminating a lactescent secretion with sanguinolent striations.
Prognosis is directly related to the tumor size. Cats with tumors under 2 cm or 2–3 cm in diameter have a 3 year, 2 year 6 month survival, respectively. Radical mastectomy, compared to conservative operation, does not increase survival.
Treatment, in both dogs and cats, in the absence of metastases and inflammation, consists of surgical removal. The surgical option consists in the removal of tumor nodules, mammectomy, regional mastectomy (lumpectomy), unilateral or bilateral mastectomy. In all cases, the basic principle is surgical intervention as early as possible, taking into consideration the tumor limits, the local infiltrative growth, the adherence to adjacent tissues, the presence of ulceration or infection. Before surgery, it is important to establish the existence of metastases in the lymph nodes and/or at distance, as well as the patient’s general condition, including a hematologic and biochemical profile.
Cytologic examination by fine needle aspiration or the examination of the mammary fluid from the affected gland can provide data regarding the development of a neoplasm or mastitis. Biopsy by partial incision should be avoided; total excision is preferred, followed by histopathological examination. In the case of multiple tumors, the microscopic examination of each tumor will be performed.
Surgery should respect several basic principles:
- – the tumor excision will include a 2 cm margin around the tumor;
- – the veins from the proximity of the tumor will be ligatured before surgery, in order to prevent the diffusion of emboli;
- – surgery will aim to minimize the trauma;
- – the sectioning of the tumor or the capsule, when present, should be avoided, and in the case of such an accident, the instruments used will be immediately replaced;
- – in the case of lymphatic drainage in the proximity of the tumor (vessels and lymph nodes), this will be excised with the tumor;
- – surgery should be differentiated into: simple nodulectomy; uni- or bilateral mastectomy, in the case of multiple tumors; partial mastectomy, in the case of tumors more than 1 cm in diameter;
- – excision is not indicated in the case of diffuse invasive anaplastic carcinoma [8].
Chemotherapy in mammary neoplasms can be applied under the form of drug combinations, such as cyclophosphamide, vincristine and methotrexate or as postoperative adjuvant treatment.
Mixed therapy in mammary neoplasms in dogs and cats has been less used, compared to breast tumors in women. In human breast cancer, radiotherapy is used as a postoperative adjuvant or in the case of neoplasms that cannot be operated or in that of intensely developed metastases. There are few data regarding this aspect in veterinary medicine. Antiestrogenic compounds, such as tamoxifen, have been used in women, but only sporadically in bitches. The same data concerning mixed therapy are reported for cats [40].
The treatment of mammary tumors in dogs and cats cannot be considered a solved problem, as long as approximately 80% of subjects with carcinomas have recurrences that result in death.
We consider useful the presentation of some general principles and therapeutic schemes suggested by different authors experienced in this field. Thus, MAGNOL and ACHACHE (1983) propose surgery in the first place, then complementary cytoreductive therapy (chemotherapy, hormone therapy), eradication by immunotherapy, which is still in an experimental phase; radiotherapy can also be attempted.
Surgical therapy can be performed under the form of tumorectomy, mammectomy and monoblock resection. Monoblock resection should be applied depending on the tumor location, the involvement of the adjacent mammary gland or glands, superficial drainage lymph nodes, and tissues from the mammary parenchyma and lymph nodes.
Ovariectomy or ovariohysterectomy will be carried out prophylactically in bitches before the first cycle and as curative therapy in young cats with multicentric fibroadenomatosis.
According to BOSTOCK (1986), a therapeutic protocol of mammary neoplasms that can be operated in dogs includes the following stages:
Chemotherapeutic protocol:
Vincristine sulfate 0.5 mg/m2, i.v., once per week;
Cyclophosphamide 50.0 mg/m2, per os, every other day;
5-Fluorouracyl 100.0 mg/m2, i.v., once per week.
The combination will be administered every month for 6 cycles. Treatment for 1 year.
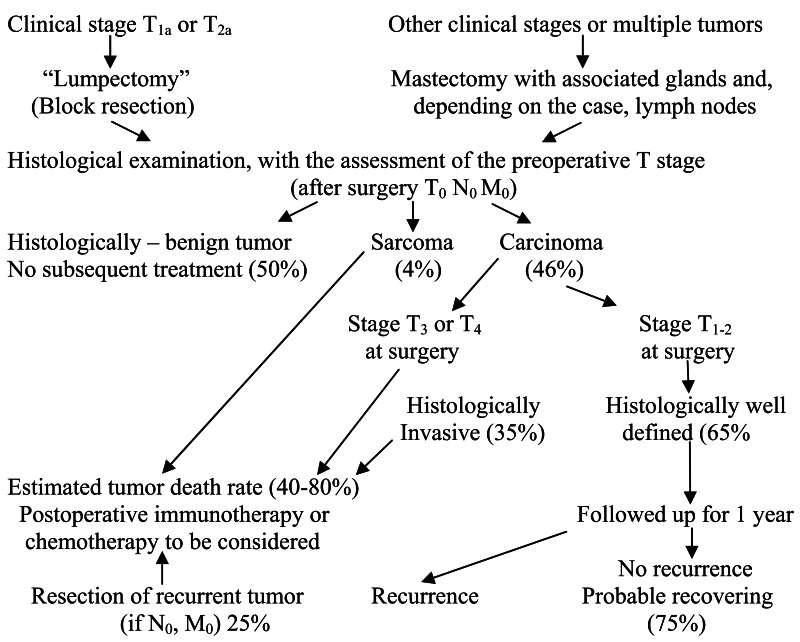
According to BOSTOCK (1975), survival of dogs with mammary neoplasms following surgery varies depending on histological diagnosis; during surgery, all subjects were N0 and M0, but immediately post-operation, they were T0.
| Histological diagnosis | Mortality % through neoplasm | Survival time in weeks (mortality of all causes) | |
|---|---|---|---|
| After 1 year | After 2 years | ||
| Complete adenoma | 0 | 0 | 125 |
| Mixed mammary tumor | 6 | 6 | 114 |
| Well differentiated carcinoma | 21 | 25 | 110 |
| Invasive tubular and papillary carcinoma | 35 | 47 | 40 |
| Solid and anaplastic invasive carcinoma | 73 | 75 | 20 |
Histological Classification of Mammary Tumors of the Dog and the Cat (Misdorp et al. 1999)
CANINE
- Malignant Tumors
- 1.1 Noninfiltrating (in situ) carcinoma
- 1.2 Complex carcinoma
- 1.3 Simple carcinoma
- 1.3.1 Tubulopapillary carcinoma
- 1.3.2 Solid carcinoma
- 1.3.3 Anaplastic carcinoma
- 1.4 Special types of carcinomas
- 1.4.1 Spindle cell carcinoma
- 1.4.2 Squamous cell carcinoma
- 1.4.3 Mucinous carcinoma
- 1.4.4 Lipid-rich carcinoma
- 1.5 Sarcoma
- 1.5.1 Fibrosarcoma
- 1.5.2 Osteosarcoma
- 1.5.3 Other sarcomas
- 1.6 Carcinosarcoma
- 1.7 Carcinoma or sarcoma in benign tumor
- Benign Tumors
- 2.1 Adenoma
- 2.1.1 Simple adenoma
- 2.1.2 Complex adenoma
- 2.1.3 Basaloid adenoma
- 2.2 Fibroadenoma
- 2.2.1 Low-cellularity fibroadenoma
- 2.2.2 High-cellularity fibroadenoma
- 2.3 Benign mixed tumor
- 2.4 Duct papilloma
- Unclassified Tumors
- Mammary Hyperplasias/Dysplasias
- 4.1 Ductal hyperplasia
- 4.2 Lobular hyperplasia
- 4.2.1 Epithelial hyperplasia
- 4.2.2 Adenosis
- 4.3 Cysts
- 4.4 Duct ectasia
- 4.5 Focal fibrosis (fibrosclerosis)
- 4.6 Gynecomastia
FELINE
- Malignant Tumors
- 1.1 Noninfiltrating (in situ) carcinoma
- 1.2 Tubulopapillary carcinoma
- 1.3 Solid carcinoma
- 1.4 Cribriform carcinoma
- 1.5 Squamous cell carcinoma
- 1.6 Mucinous carcinoma
- 1.7 Carcinosarcoma
- 1.8 Carcinoma or sarcoma in benign tumor
- Benign Tumors
- 2.1 Adenoma
- 2.1.1 Simple adenoma
- 2.1.2 Complex adenoma
- 2.2 Fibroadenoma
- 2.2.1 Low-cellularity fibroadenoma
- 2.2.2 High-cellularity fibroadenoma
- 2.3 Benign mixed tumor
- 2.4 Duct papilloma
- Unclassified Tumors
- Mammary Hyperplasias/Dysplasias
- 4.1 Ductal hyperplasia
- 4.2 Lobular hyperplasia
- 4.2.1 Epithelial hyperplasia
- 4.2.2 Adenosis
- 4.2.3 Fibroadenomatous change (feline mammary hypertrophy, fibro-epithelial hypertrophy)
- 4.3 Cysts
- 4.4 Duct ectasia
- 4.5 Focal fibrosis (fibrosclerosis)
For a differential diagnosis of certainty between benign and malignant epithelial tumors, more or less elaborated methods have been attempted, among which immunochemical differentiation of basal membranes in different types of neoplasms, compared to intact mammary tissue. This is of major importance, especially for human breast neoplasms. WILLEBRAND et al. (1986) have found in normal tissue, as well as in benign tumors, the presence of intact basal membranes, with a continuous appearance. In this sense, the authors remark the possibility of easy differentiation of sclerosing adenosis, in which tubules have a continuous basal membrane, from tubular carcinoma, in which the basal membrane is almost completely absent. Fragments of basal membranes have been detected in infiltrating lobular carcinoma of alveolar type, while in the classic and trabecular type the basal membrane is absent, suggesting that the alveolar type could be an intermediate phase in the development of lobular carcinoma in situ. The cited authors mention the possibility of differentiation between epitheliosis and papillomatosis, between adenosclerosis and tubular carcinoma, as well as other lesional tumoral or non-tumoral forms of the mammary parenchyma. In conclusion, the immunochemical study of basal membranes, using type IV collagen antibodies, is useful for the differentiation of benign and malignant lesions of the mammary parenchyma.
The diagnosis of mammary neoplasms in bitches has used morphometric methods, such as nuclear perimeter, nuclear surface, number of nuclei per millimeter and the minimal distance of cells from the basal membrane. The conclusion of CIUREA et al. (1992) is that morphometric analyses can be used for the grading and classification of mammary neoplasms.
The study of the regional organization of nucleoli has shown wide variations between the different types of mammary tumors, in the bitch. This method allows the differentiation between invasive mammary carcinoma and other tumor forms, as well as a prognostic estimation [7]. The Ag NOR calculation in breast cancer in women has no prognostic value [50]. The combined use of cytologic examination and DNA analysis in mammary tumors provides information in benign or malignant nature of these, especially preoperatively [21, 22]. The combined use of cytologic and histological examination of tumors increases the certainty index in the assessment of malignancy or benignity of mammary tumors. By cytologic examination, a 90–100% rate was obtained in the establishment of tumor malignancy, while for malignant forms the proportion was between 59 and 75%, and non-conclusive diagnoses represented 44–49% [1]. The increase of polyploid DNA is in relation to the malignancy grade of mammary carcinomas, especially in metastatic forms [46]. The nuclear DNA amount is correlated with the malignancy grade, and it can be a landmark in the prognosis of breast tumors [35]. The study of translocation (4; 27) in the cells of mammary adenocarcinomas in dogs has shown a normal karyotype in 75% (2n=78) and a reciprocal translocation 4; 27 in 25% of cases. The break point on chromosome 4 was near the centromere, and on chromosome 27, near the telomere.
The presence of local infiltration with eosinophilic cells in anaplastic mammary carcinomas signifies a delay in tumor progression, while eosinophilia in peripheral blood shows the possibility of dissemination and unfavorable prognosis [27].
11.1. CARCINOMA
Carcinoma in dogs and cats is characterized by different stromal reactions depending on the subject. A simpler classification that can allow histopathologists to establish rapid diagnosis and, to a certain extent, prediction, includes in the group of epithelial tumors: papillary, tubular, solid and anaplastic carcinomas. Precancerous lobules and ductal hyperplasia are found at the periphery of carcinogenic nodules, in adjacent tissues, in both dogs (73.6%) and cats (56.3%) [4].
Macroscopically, carcinoma is characterized by rapid growth, doubling its volume in a short time, with local invasion, infiltrating the surrounding normal tissue. Sometimes, mammary neoplasms are delimited, a capsule appearing on palpation, but they present fibrous adhesions to the epidermis and the muscle fasciae or the neighboring muscles, and they cannot be freely moved. The skin is frequently ulcerated and the tumor invades the lymphatic vessels and lymph nodes, skin and the adjacent gland on the same side.
Mammary neoplasms can have diameters between 2 and 20 cm, and round, ovoid, discoid, fungiform or poorly defined shapes. The tumor extends rapidly and occupies the whole gland, as well as adjacent glands, and carcinomas can coexist with benign tumors in the same gland and/or the neighboring glands.
Adenocarcinomas are usually soft, and in section, a diffuse or lobular structure of homogeneous tissue of white-cream color appears. Papillary carcinomas have a firm or soft-bloody structure. Scirrhous carcinomas have irregular shapes, they are poorly delimited and occasionally invade adjacent mammary glands. They are of a dense-ligneous, even hard consistency, white-gray or brown-yellow color, and they adhere to skin or the basic musculature. Solid carcinomas are soft and in section they have a lobular structure, of white or gray color. Squamous cell carcinomas are of irregular shapes, hard, in section their structure is lobular, color being gray or white, with yellow spots [37].
Carcinomas generally develop rapidly, and they are detected by the owner in 2–6 months. If, following diagnosis, the mammary tumor is not removed, tumor growth lasts for a longer time before the dog’s death. Carcinomas may develop over a period of 3 months to 6 years. The conclusions drawn by FOWLER et al. (1974), based on a large number of mammary neoplasms in bitches, regarding the behavior of carcinomas after diagnosis and treatment, are extremely interesting. The mean survival time after the diagnosis of infiltrating papillary carcinomas was 5.6 months, less than for other carcinomas. The mean duration after the diagnosis of infiltrating solid carcinoma was 7.3 months. Scirrhous carcinoma had a longer duration than non-scirrhous carcinoma, 9.7 months. Most dogs with infiltrating papillary carcinoma died following metastases. The behavior of scirrhous carcinoma was similar to that of papillary carcinoma.
Epithelial carcinomas may develop from intralobular ductal or alveolar epithelium, appearing as non-infiltrating or infiltrating tumor structures. The evolution duration of non-infiltrating carcinomas was 36 months and that of infiltrating carcinomas 13 months.
Metastases of mammary carcinomas occur in 25% of cases. The metastatic process is facilitated by both blood flow and lymphatic flow, distributed in the mammary parenchyma.
11.1.1. Adenocarcinoma
Simple tubular adenocarcinoma is a relatively frequent tumor in dogs, the tubular type being predominant. The microscopic structure is dominated by tubular epithelial cells, in which pleomorphism and mitotic activity is estimated from low to high, and necrotic foci are frequent. The connective stroma can be in limited or moderate amounts. Tumor proliferation is surrounded by variable lymphocytic infiltration. The histological differentiation between tubular adenocarcinoma and benign lesion forms is difficult. Benign lesions can have in some cases a high mitotic activity or they can stimulate local invasive growth. In cats, these tumors represent the most frequent type. Tubular adenocarcinoma has in 70% of cases an invasive character, or expansive nodular character in 30%. The lymphocytic infiltration of the tumor is frequent, in approximately 50% of cases (Fig. 11.1, 11.2, 11.4, 11.5, 11.6, 11.30).
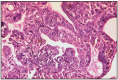
Fig. 11.1
Tubular adenocarcinoma, simple type.

Fig. 11.2
Tubular adenocarcinoma, scirrhous type.

Fig. 11.4
Tubular adenocarcinoma, simple type.
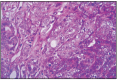
Fig. 11.5
Tubular adenocarcinoma, simple type.

Fig. 11.6
Tubular adenocarcinoma, simple type.
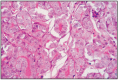
Fig. 11.30
Tubular adenocarcinoma, cat.
Complex tubular carcinoma in dogs has a predominantly tubular cell arrangement. Microscopically, tubular epithelial cells and myoepithelial cells form tumors of this type. Cuboid or fusiform cells, the latter with frequently vacuolated cytoplasm, line tumor tubules. Sometimes, the surrounding neoplastic myoepithelial cells are arranged more or less in a mass of stellate reticular cells. Tumor growth may be expansive, nodular or lobular. The histological difference between the highly differentiated carcinoma of this type and complex adenoma is extremely difficult. Malignancy is characterized by numerous mitoses, cellularity and large necrotic foci. Complex tubular adenocarcinoma has not been diagnosed in cats [19] (Fig. 11.7. and 11.8).
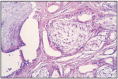
Fig. 11.7
Tubular adenocarcinoma, complex type.
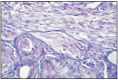
Fig. 11.8
Tubular adenocarcinoma, complex type.
Papillary adenocarcinomas arise de novo or by the evolution towards malignization of the benign form, papillary adenoma. Papillary adenocarcinomas are usually multicentric, with simultaneous neoplastic changes in distinct and unrelated parts of the mammary gland. Papillary carcinomas can develop: when an alveolar structure or a canaliculus dilates and forms intraluminal papillae; when multiple mammary ducts dilate, thin stromal septa break, transforming the lobe into a cystic mass, in which papillae develop; when interlobular ducts become papillomatous. Approximately 18% of papillary carcinomas develop from mammary lobules, the rest from interlobular ducts [37].
Simple papillary adenocarcinoma is formed by columnar or cuboid cells, arranged in tubules under a sessile papillary form, or with a pedunculated structure, with scant connective stroma. The tumor is found in particular in dogs (Fig. 11.3, 11.9–11.12). These tumor types are almost exclusively papillary, but solid areas may also appear. About 80% of these neoplasms have infiltrative extension in the skin and the surrounding tissues, also invading the lymphatic system. A high pleomorphism and numerous mitoses are noted. Differential diagnosis is made with certain difficulty compared to benign papilliferous proliferation lesions.

Fig. 11.3
Papillary adenocarcinoma, simple type.
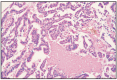
Fig. 11.9
Papillary adenocarcinoma, simple type.

Fig. 11.10
Papillary adenocarcinoma, simple type, pulmonary metastasis.

Fig. 11.11
Cystic papillary adenocarcinoma, simple type.
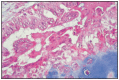
Fig. 11.12
Papillary adenocarcinoma and chondroma.
In addition to cell pleomorphism and a high mitotic index, necrotic foci, lymphocytic and plasmacytic infiltration is found in the tumor mass, and granulocytes are present in the duct lumen.
Complex papillary adenocarcinoma in dogs has a low incidence. The cells of neoplastic ducts are arranged in sessile or pedunculated papillary structures. Extensive areas of myoepithelial cells surround cuboid or columnar epithelial cells. The tumor is well delimited, without lymphocytic infiltration.
Simple cystic papillary adenocarcinoma is common in bitches, appearing as papillary or cystic structures, formed by cuboid epithelial cells, with low to moderate pleomorphism and mitotic activity.
Papillary cystadenocarcinoma is well differentiated, which makes difficult differential diagnosis from papillary cystadenoma and cystadenopapillomatosis. In cats, this tumor type has invasive growth, with lymphocytic infiltration. Pleomorphism and the mitotic index can be considered as moderate.
Complex cystic papillary adenocarcinoma has a low incidence in dogs, a papillary and cystic structure. The tumor is formed by epithelial and myoepithelial cells, which makes it extremely similar to simple cystic papillary adenocarcinoma, the difference consisting in the presence of microfoci of fusiform myoepithelial cells with cytoplasmic vacuoles. This type of neoplasm produces metastases, although it shows a high differentiation, which makes difficult differential diagnosis from the benign form. The tumor has not been diagnosed in cats.
11.1.2. Solid carcinoma
Solid carcinomas may arise through an extensive intralobular invasion in adenocarcinomas, in which lobules are changed into solid tumor cell masses with a reduced glandular structure and inapparent stroma; epithelial excrescences can form and invasion may occur in the papillary carcinoma, with papillary projections transformed into solid cellular masses, or from diffuse infiltrating carcinomas, with invading cell bundles, so compact that they form solid cellular masses.
Solid carcinoma is probably a more advanced form of other types of carcinoma, being found much more frequently when the neoplasm has developed over a long time period, without the intervention of surgery or other therapeutic form.
Simple solid carcinoma is frequently found in dogs and is formed by epithelial or myoepithelial cells. Cells are arranged in solid foci, cords or masses, forming tubes or other structures with a lumen. Sometimes there are few solid foci, along with differentiated adenomatous formations. Pleomorphism and the mitotic index are moderate to high, and the connective stroma is in a moderate amount. This type of solid carcinoma has an infiltrative growth and a 60% penetration rate in the regional lymph nodes.
Some solid carcinomas are formed by cells with vacuolated cytoplasm, being similar to myoepithelial cells, which indicates an exclusive extension of poorly differentiated myopithelial carcinoma (Fig. 11.13).
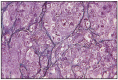
Fig. 11.13
Solid carcinoma, simple type.
In cats, solid carcinoma appears almost exclusively, which develops with intense infiltrative growth and penetration in lymphatic vessels. The presence of lymphocytic and plasmacytic infiltration can be evaluated as moderate to intense.
Complex solid carcinoma has only been diagnosed in bitches. Microscopically, it is formed by epithelial and myoepithelial cells. Epithelial cells have vacuolated cytoplasm, they are arranged as tubes and apparently with squamous metaplasia. In most cases, neoplastic cells are structured under the form of layers, cords or masses that present a lumen. The growth of this carcinoma is frequently expansive, and penetration in lymphatic vessels and lymph nodes is exceptional (Fig. 11.14).
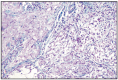
Fig. 11.14
Solid carcinoma, complex type.
Differential diagnosis between complex solid carcinoma and complex adenoma is extremely difficult.
11.1.3. Spindle cell carcinoma
Spindle cell carcinoma in dogs appears most frequently as a solid tumor. This carcinoma has an expansive or infiltrative growth, with penetration in the lymphatic system. Histologically, it is similar to fibrosarcoma, which has made some authors include this carcinoma in the group “malignant myoepithelioma”. Differential diagnosis between spindle cell carcinoma and fibrosarcoma is difficult, but reticulin fibers can be evidenced by special staining.
11.1.4. Anaplastic carcinoma
Anaplastic carcinoma is a neoplasm that cannot be classified in one of the following types: adenocarcinoma, solid carcinoma, squamous cell carcinoma or mucinous carcinoma. Anaplastic carcinoma appears as an infiltrative neoplasm, formed by large pleomorphic cells, frequently with bizarre chromatin-rich nuclei, with the presence of multinucleated cells. Anaplastic giant cell mammary carcinoma is not frequent and has only been reported in female dogs and cats [34]. By special histochemical methods, antibodies and ultrastructural examination, the origin of canine mammary tumors has been proved to be in the stem cell [22, 47]. The authors mention the possibility of the coexistence of malignant epithelium and giant cells in anaplastic carcinoma. SALDA et al. (1993) have electron microscopically identified the presence of disseminated tonofibril bundles, but not of lysosomal formations. The conclusion is that canine mammary tumors originate from the stem cell. Mitoses are present in a considerable number. Cells have eosinophilic and vacuolated cytoplasm, sometimes being extremely small, poorly defined, with tubular structures or extremely small solid cords. Polynuclear granulocytes are present among tumor cells and in the stroma of most anaplastic carcinomas. This will be taken into consideration, as there is a risk for confusion with mastitis. The collagenous stroma is abundant, the carcinoma is scirrhous and contains lymphocytic foci. The infiltration of neoplastic cells in the lymphatic system is frequent. Some anaplastic carcinomas can be recognized as epithelial tumors (Fig. 11.17).
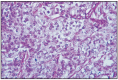
Fig. 11.17
Anaplastic carcinoma.
11.1.5. Squamous cell carcinoma
Squamous cell carcinoma is formed by stratified squamous epithelium, spinous and/or keratinized cells. This neoplasm can be simple or complex, with the presence of extremely varied histological structures. Approximately half of squamous cell carcinomas appear as adenomatous structures, with the presence of keratohyalin granules and keratin lamellae. Other squamous cell carcinomas appear as solid layers and cords with cornified areas. Basal cells are dominant at the periphery of the neoplasm, and keratohyalin granules are absent or rare. In the central area, non-lamellar keratin is mixed with necrosis, and the neoplasm is frequently abundantly infiltrated with lymphocytes. Primary squamous cell mammary carcinoma should be differentiated from primary squamous cell tumors derived from the skin, dermal adnexae and epidermal cysts. Squamous cell mammary carcinoma has not been diagnosed in cats (Fig. 11.18).

Fig. 11.18
Squamous carcinoma.
11.1.6. Mucinous carcinoma
Mucinous carcinoma, in its typical form, is rarely found in female dogs and cats. Microscopically, the predominance of myoepithelial cells has been found, without these being related to mucus secretion. Three types can be theoretically identified: mucin that can arise from secretory epithelial cells, from connective tissue cells or myoepithelial cells. Cystic adenoid carcinoma can be included in this category. Few mucinous carcinomas have been reported in dogs and cats (Fig. 11.15, 11.16).

Fig. 11.15
Mucinous carcinoma.
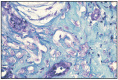
Fig. 11.16
Mucinous carcinoma.
11.2. SARCOMA
Mammary sarcomas are relatively frequent in bitches, but not in cats. Sarcoma metastases have only been diagnosed in dogs.
11.2.1. Myoepithelioma
Malignant myoepithelioma appears as a round or ovoid formation, up to 4 cm in diameter, of soft consistency, poorly circumscribed and frequently ulcerated. Microscopically, cells arranged in bundles or spirals, with myoid aspect and the loss of the lobular structure form it. Cells have vacuolated or clear cytoplasm, round or elongated nuclei, and multinucleated giant cells can be sometimes identified. Necrotic foci are frequent in the tumor mass. Lung metastases have been reported.
11.2.2. Osteosarcoma
Cells that only produce osteoid and/or bone form osteosarcoma. Bone and osteoid trabeculae appear in various proportions in the structure of the tumor, in different areas of the neoplastic mass. The tumor center is occupied by a dense compact mass, while the cellular area is at the periphery. Necrotic cysts and hemorrhagic foci frequently appear. Neoplastic cells are pleomorphic and frequently polyhedral, while in other tumors, fusiform cells predominate. In the majority of tumors, the mitotic index is high, as well as the number of multinucleated cells similar to osteoclasts, situated in the proximity of neoplastic bone tissue (Fig. 11.19, 11.20).
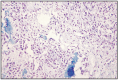
Fig. 11.19
Osteoblastic osteosarcoma.

Fig. 11.20
Chondroblastic osteosarcoma.
11.2.3. Fibrosarcoma
Fibrosarcoma is characterized by fusiform cells of different types, with intercellular reticulin and collagen fibers that vary from one tumor to another. In the bitch, these fibers are arranged in parallel bundles or at random. Sometimes, fibers and cells are concentric around blood vessels, which requires differentiation from hemangiopericytoma.
Edema or mucinous material occurs in some tumors. Pleomorphism and the number of mitoses vary significantly from one fibrosarcoma to another. In cats, mammary sarcoma with myofibroblast-like cells has been diagnosed, in which multinucleated giant cells and mitoses have been identified. Electron microscopically, cells have a rich rough endoplasmic reticular content with dilated cisternae, prominent Golgi complex, mitochondria, intracytoplasmic filament bundles with densifications in foci, discontinuous basal membrane and cell junctions. The lack of cytokeratin, by immunoreactivity, confirms the non-epithelial origin of neoplastic cells [20]. In the case of complications, such as abscesses or extensive hemorrhage, diagnosis is based on peripheral structure in which the characteristics of the neoplasm are maintained.
11.2.4. Osteochondrosarcoma
Osteochondrosarcoma can also appear as fibrolipoosteochondrosarcoma or combined sarcoma; it is structurally composed of bone and cartilaginous tissue, and it sometimes contains neoplastic fibrous and/or adipose tissue. The neoplasm may develop directly as Osteochondrosarcoma or, indirectly, when enchondral ossification occurs in cartilaginous tissue. In most cases, metastases with differentiated tissue develop. Differential diagnosis, even from benign tumors, is sometimes extremely difficult.
11.3. CARCINOSARCOMA
Carcinosarcoma is a malignant mixed tumor, formed by epithelium-like cells (epithelial, myoepithelial or both cells type) and connective tissue-like cells. Histological structure varies widely, from one tumor to another. A high percentage (30%) of mixed tumors contains fusiform cells, which are considered to originate in myoepithelial cells or connective tissue. The osteosarcomatous component is considered to originate from enchondral ossification (secondary bone) and from fibrous cells, primary bone.
Infiltrative growth and lymphatic metastases occur more rarely; it seems that these aspects only involve the carcinomatous component, less the sarcomatous component. Histological differentiation of carcinosarcoma is required from benign mixed tumors, and is sometimes difficult (Fig. 11.24.).

Fig. 11.24
Fibroadenoma.
Malignant mixed tumors grow more slowly than carcinomas, with limits from 1 month to 1 year or more, the majority during of at least 6 months. Some of the mixed tumors histologically diagnosed as benign can be potentially and evolutionally malignant.
Metastases in malignant mixed tumors usually appear under the form of carcinomas and exceptionally as osteosarcomas, chondrosarcomas or myoepitheliomas. Metastases have been found in the lymph nodes, lungs, liver, kidneys and exceptionally in other organs.
11.4. BENIGN TUMORS
Benign tumors have been classified according to the classification known in women. In most cases, in female dogs and cats, these lesions do not evolve independently, but some of them correspond to those described in women. Benign mammary tumors, of adenoma type, are difficult to differentiate histologically from the physiological hyperplasia of the mammary gland. In both cases, large mammary lobules appear, with the dilation of acini and mammary ducts, papillary concretions being occasionally present, both in benign and malignant forms. This lesion is extremely rare in dogs and cats. Nodules formed by fusiform cells with little stroma may be found, being considered as solid myoepithelial adenoma.
Complex adenomas are extremely frequent in dogs and cats and are formed by the proliferation of secretory epithelial cells, myoepithelial cells and insignificant stroma. These tumors partially correspond to cellular fibroadenomas, benign mixed tumors, and sometimes to complex (non-tumoral) lobular hyperplasia. Inflammation and metaplastic changes are described as non-tumoral inflammatory nodular hyperplasia.
Differential diagnosis between complex adenoma and complex adenocarcinoma is difficult.
11.4.1. Papilloma
Duct papilloma is infrequent in dogs and cats, but it can frequently be identified in a complex tumor, especially in the complex type.
Duct papillomatosis. Papillary duct covering and cysts should be differentiated from duct papilloma. The presence of adenosis in the duct lumen with ectasia and the appearance of secondary papillary formations have led to the term duct papillomatosis. The differentiation between duct papillomatosis and papillary adenocarcinoma is difficult, especially in cats. Papillary proliferations develop in both acinar lobules and the intralobular duct system, in the sinus of the mammary glands, by duct dilation or septal breaking and formation of cysts, in which papilliferous proliferations develop. Papilliferous expansions can be sessile, pedunculated or polypous. The epithelium that covers these proliferations is mono-or pluristratified, flat and atrophied, simple cuboid or columnar. The lumen of the duct or cyst contains homogeneous acidophilic fluid. Papillary proliferations develop from well vascularized connective tissue filaments, covered by cuboid or columnar cells (Fig. 11.27).

Fig. 11.27
Duct papilloma, dog.
11.4.2. Fibroadenoma
Fibroadenoma can be pericanalicular and intracanalicular, the latter being of either non-cellular or cellular type.
Pericanalicular fibroadenoma is characterized by the dominance of fusiform, myoepithelial cells and/or stromal tissue.
Benign mixed tumors appear as cartilage, bone and fat tissue. It is difficult, sometimes impossible, to differentiate the tumor stroma from cartilaginous metaplasia or the myoepithelial component. Benign mixed tumors originate in several germ layers, including a wide variety of tissues, for example epithelial cells, fibrous connective tissue, cartilage and bone. Myoepithelial cells can be identified in all tumor forms. The origin of cartilaginous and bone tissue can be metaplastic, by the transformation of epithelial cells, but more likely, it is in the stromal connective tissue. Cartilaginous tissue is supposed to be derived from myoepithelial cell metaplasia.
Macroscopically, mixed tumors have sizes that range from several millimeters to 20 cm, firm and frequently cartilaginous or of bone consistency. In section, they appear lobulated, of white-gray color, and cysts with unclear or red-brown liquid are frequent. Papillary changes frequently occur on the internal side of cysts. The tumor has a spherical, ovoid shape, or appears as a pendulous mass, affecting one or more mammary glands, sometimes with the involvement of the whole gland. Mixed tumors are well encapsulated, being non-adherent to neighboring tissues, and more rarely to skin.
Microscopically, the aspects of the development of a mixed tumor are preceded by myoepithelial cell changes and cartilage formation. In the early proliferation stage, a separation of myoepithelial cells is found in the basal layer of the ducts. In this stage, the basal membrane is intact, myoepithelial cells are grouped in spherical masses of stellate or elongated cells, separated by a colorless or slightly basophilic substance. These myoepithelial cell masses penetrate the stroma, causing a severe disorganization of epithelial elements, compressing the canaliculi and the acinar structure in thin structures. Myoepithelial cells, being at the same time a precursor of hyaline cartilage, secrete the intercellular substance. In order to differentiate myoepithelial cells from fibroblasts and from epithelial cells, the alkaline phosphatase reaction is performed, which is positive in the cytoplasm of myoepithelial cells and negative in the other cells (Fig. 11.25).
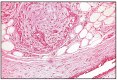
Fig. 11.25
Benign mixed tumor.
Electron microscopically, the dominant aspect of neoplastic myoepithelial cells is the high number of cytoplasmic filaments. These filaments are more separated, larger and more rectilinear in tumor cells than in normal cells and are much easier to distinguish. An extensive Golgi apparatus that frequently occupies a large cytoplasm area represents another characteristic of neoplastic myoepithelial cells. As myoepithelial cells proliferate and organize into neoplasms, the tissue resembles more and more hyaline cartilage, the Golgi apparatus extends, while the number of cytoplasmic filaments diminishes [44].
The cartilage formed in mixed tumors appears as nodules or plaques of various sizes. Osteoblasts actively participate in bone formation, under the form of osteoid or well mineralized bone tissue. The connective stroma is frequently hyalinized.
Benign mixed tumors that reach large sizes may become infected and/or ulcerated, having foci and diffuse collections that contain fluids and tissue debris, and hyalinization and calcification are usual aspects.
Total fibroadenomatous change may appear in one or all mammary glands, in young cats, being considered a dysplastic benign lesion. It is difficult to establish a equivalence with andromastosis in women, considered as a bilateral juvenile pseudohypertrophy (Fig. 11.32).
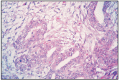
Fig. 11.32
Fibroadenomatous change, cat.
Benign soft tissue tumors. The group of these tumors includes lipomas and hemangiomas, to which benign mixed tumors that mimic chondroma, osteoma or fibroma are added.
Unclassified tumors. This group includes benign and malignant tumors that cannot be included in the previously described categories.
11.5. BENIGN DYSPLASIAS
Non-papillary cysts are found in women; in bitches they appear in most cases under the form of multiple cysts, in one or more mammary glands, and differentiation from duct dilation is arbitrary.
Papillary cysts are most frequently found in the bitch, under a complex form. Differentiation from intracystic, intraductal papillomas and from papillomatosis is arbitrary. The oncocytic transformation of epithelial cells towards papillary growth is accompanied by cytonuclear pleomorphism.
Adenosis is rarely found in dogs and cats, formations are little obvious, being clinically detected as irregular lesions. Microscopy identifies confluent, multilobular or multiductal foci of adenotic type, forming complex adenoma: intralobular adenosis and complex lobular hyperplasia are not distinct and can be confused in some cases with duct papillomatosis (Fig. 11.29). Usually, adenosis resembles other proliferation types.
Fig. 11.29
Lobular hyperplasia, adenosis, simple type.
Typical epithelial proliferation in ducts or lobules. This hyperplasia type is also known as epitheliosis and appears as an epithelial or hyperplastic proliferation in preexisting structures, not as a growth in cysts or dilated ducts, adenosis, etc. (Fig. 11.28, 11.31). The proliferation contains a single cellular type, but the presence of fusiform cells is suggestive of a mixture of myoepithelial cells. Proliferated epithelial cells have the shape of duct cells, being columnar, with hyperchromatic nuclei. This has led to the term “mural epitheliosis”.
Fig. 11.28
Ductal hyperplasia.
Fig. 11.31
Lobular hyperplasia, adenosis, epitheliosis, cat.
Normal epithelial proliferation is found in bitches, predominantly in ductolobular areas, more rarely organized as nodules. Epitheliosis in the bitch has a uniform growth, with unchanged cells, which makes difficult the subdivision into types.
Duct ectasia is frequent in female dogs and cats, which determines a spongy change of the mammary parenchyma. Ectasia affects terminal, intralobular tubules and some acini. Lobular hyperplasia includes the inflammatory type that is complicated by ectasia. Tubules are dilated, containing protein fluid with cell remnants. Ectatic formations are lined by columnar cells, and occasional papilliferous proliferations may appear. Spontaneous regressions or regressions following ovariectomy as well as progesterone administration may occur [51].
Fibrosclerosis is frequent in bitches, being associated with the persistence of the ovarian corpus albicans. The lesion appears under the form of small nodules, in which irregular collagenous bands are identified, with regressive changes or smooth scars.
Gynecomastia has been diagnosed in male dogs with active testicular tumors.
Non-inflammatory lobular hyperplasia is found in bitches and cats and appears as foci or nodules that can be sometimes large, in which secretory or myoepithelial epithelium is dominant. It is characterized by an extremely varied lesional picture, acinar and duct ectasia, sclerosis and secretion, with the presence of inflammatory or metaplastic cells. In order to be correctly interpreted, these lesions should be correlated with the physiological condition of the bitch, since they may lend to confusion with the lactating condition. In bitches that are not gestating or lactating, hyperplastic foci are histologically identified around the age of 3 years. The most frequent location is in the last two mammary gland pairs. Lobular hyperplasia could be considered as a preneoplastic condition [51].
Feline fibroepithelial hyperplasia is more rarely reported, almost all cases appearing in females under 2 years, even 1 year of age, exceptionally in old cats. Usually, all mammary glands are affected concomitantly, although some nodules can be larger. Formations appear as hard, non-painful masses, 2–5 cm in diameter, sometimes even larger. In section, they have a multilobular structure of white to slightly red color.
Microscopically, the lesion is clearly circumscribed, without being encapsulated, with a multilobular aspect, and an extremely marked proliferation of the intralobular duct system should be mentioned. Interlobular proliferation is less obvious. The proliferation of the intralobular duct system is structurally identical to physiological hyperplasia from the early gestational stage. Ramified canals have an envelope of epithelial cells, sometimes arranged in 3–4 layers. These cells are hyperchromatic, with the presence of mitotic figures. Intralobular connective tissue is intensely proliferated, edematous, and cells present mitoses. Hormonal factors are etiologically incriminated [37] (Fig. 11.19).
Inflammatory lobular hyperplasia. In dogs and cats, foci or inflammatory type nodules appear, which are frequently the result of (mucinous, squamous or oncocytic) metaplasia, with pigment accumulation in macrophages, lymphoplasmacytic infiltration of the stroma and granulocytes in the duct lumen (Fig. 11.21 and 11.22).
Fig. 11.21
Simple adenoma.

Fig. 11.22
Simple myoepithelial adenoma.
11.6. MAMMARY TUMORS IN OTHER SPECIES
Mammary tumors, which are rare or even very rare in other species, have been reported extremely sporadically in ruminants, horses and swine.
In mares, the few mammary tumors reported have been solid scirrhous carcinomas, with local invasive growth, and in all cases metastases have been identified [48].
In cows, fibropapillomas have been described in the galactophorous duct or in the large ducts, in all cases fibroma, squamous cell carcinoma and osteoma-fibrosarcoma being found [51]. Acinar and duct carcinoma was diagnosed in an 8-year-old Simental cow, under the form of multiple nodules and metastases in the mesenchymal serosae, liver, spleen, diaphragm, thoracic cavity, reproduction and gastrointestinal systems. Microscopically, neoplastic acinar and duct cells formed aberrant acini [43]. A multicentric papillary cystadenoma was diagnosed in a 9-year-old ovariectomized Holstein cow [45].
The experimental neoplasms of the mammary glands allow to understand carcinogenesis and implicitly, to prevent and/or fight mammary carcinoma. The mouse mammary tumor model has been used in order to study the characteristics of paraneoplastic lesions. The possibility of transplanting tissue and neoplastic cells from mouse mammary tumors has opened the way to studies whose results can be extrapolated to other neoplasms and even generalized for tumor disease. There is a possibility to elucidate oncogenic and cloning activities during tumor genesis, with the development of transgenic mice technology, opening the perspective of in vivo carcinogenesis studies. Transgenic mice and transgenic mammary glands provide a controlled system capable of answering the numerous questions of cancer disease. Experimental studies in mice have proved the existence of protoneoplastic lesions and increased malignant potential [9].
The problem of the implication of some viruses in the carcinogenesis of mammary neoplasms has also been studied in mice. The mouse mammary tumor virus is transmitted by milk and by sexual cells. It has been demonstrated that in order to be active, the mouse mammary tumor virus needs a certain hormonal context. The virus is of RNA type, it integrates into chromosomes, a reverse-transcriptase being needed to copy the retrovirus in the DNA, which in its turn integrates in chromosomal DNA. Mouse mammary cell cultures treated with corticosteroids stimulate viral RNA synthesis [12]. Viral particles have also been identified in spontaneous neoplasms, in carcinosarcomas in bitches [39].
Chemical substances, such as medroxyprogesterone acetate, capable of inducing mammary adenocarcinoma in female mice, have been experimented. Following the subcutaneous administration of 40 mg medroxyprogesterone acetate, mammary carcinomas have developed in both non-pubertal and gestating females, some subjects also developing metastases [24]. Medroxyprogesterone acetate is also used as contraceptive treatment in precocious puberty.
The in vitro study of mammary tumors in bitches has proved that 17-β-estradiol and, to a lower extent, progesterone induce the replication of mammary tumor cells, results being similar to those obtained in mammary cancer cells. Cells present specific hormone receptors. It can be supposed that the mitogenic effects of estradiol and progesterone on mammary cancer might be the result of an indirect stimulating mechanism, such as the production of autocrine or paracrine growth factors. Estrogen stimulation of tumor proliferation determines a shortening of the S phase of the cell cyle DNA [26].
Fig. 11.23
Complex adenoma.
Fig. 11.26
Basaloid adenoma, mammary gland, dog.
BIBLIOGRAPHY
- 1.
- Allen SW, Prasse KW, Mahaffey EA. Cytologic Differentiation of Benign from Malignant Canine Mammary Tumors. Vet. Pathol. 1986;23:649–655. [PubMed: 3811129]
- 2.
- Allen SW, Mahaffey EA. Canine mammary neoplasia: prognostic indicators and response to surgical therapy. J. Am. Anim. Hos. Ass. 1989;25 (5):540–546.
- 3.
- Baba AI, Rotaru O, Cristea I, Elefterescu H. Diagnosticul histopatologic postoperator al tumorilor mamare la căţea. Bul. Inst. Agronomic, Zoot. -Med. Vet. Cluj-Napoca. 1985:43–50.
- 4.
- Benazzi C, Marcato PS, Piacini A. Lesioni associate alle neoplasie della mammella de cane e del gatto. Boll. Ass. Italiana Vet. Pice. Anim. 1989;28 (3):245–252.
- 5.
- Boitor I, Baba AI. Cancerul sferei genitale la animalele domestice, Vol. Cancerul, oncologie comparată Prima consfătuire de oncologie comparată, Cluj-Napoca. 1981:147–165.
- 6.
- Bostock DE. Canine and feline mammary neoplasm. Br. Vet. J. 1986;142:506–515. [PubMed: 3594183]
- 7.
- Bostock DE, Moriarty J, Crocker J. Correlation betwen Histologic Diagnosis Mean Nucleolar Organizer Region Count and Prognosis in Canine Mammary Tumours. Vet. Pathol. 1992;29:381–385. [PubMed: 1413404]
- 8.
- Brearley MJ. Mammary gland tumours in the dog. Practice, Nov. 1989:248–253.
- 9.
- Cardiff RD, Aguilar-Cordova E. Proto-Neoplasia Revidited: The Molecular Biology of Mouse Mammary Hyperplasia. Anticancer Res. 1988;8:925–934. [PubMed: 2845853]
- 10.
- Ciurea D, Wilkins RJ, Shalev M, Liu Z, Barba J, Gil J. Use of computerized interactive morphometry in the diagnosis of mammary adenoma and adenocarcinoma in dogs. Am. J. Vet. Res. 1992;53 (3):300–303. [PubMed: 1595955]
- 11.
- Chen HC. A case of feline pappiliferous mammary adenocarcinoma with widespread metastases. Can. J. Comp. Med. 1968;32:465–467. [PMC free article: PMC1319277] [PubMed: 15846894]
- 12.
- Crépin M. Cancer du sein: qui est responsable? Recherche. 1982:772–774.
- 13.
- Delisee F. Démarche diagnostique en cancérologie. Rec. Méd. Vét. 1990;166 (11):949–954.
- 14.
- Destexhe E, Lespagnard L, Degeyter M, Heymann R, Coignoul F. Immunohistochemical identification of myoepithelial, epithelial and connective tissue cells in canine mammary tumors. Vet. Pathol. 1983;30 (2):146–154. [PubMed: 7682367]
- 15.
- Destexhe E. Morphologie et biologie des tumeurs mammaires de la chienne. Thèse, Univ; Liège: 1994.
- 16.
- Else RW, Hannant D. Some epidemiologic aspects of mammary neoplasia in the bitch. Vet. Rec. 1979;104:296–304. [PubMed: 575967]
- 17.
- Fowler EH, Wilson GP, Koestner A. Biologic Behavior of Canine Mammary neoplasms Based on a Histogenetic Classification. Vet. Pathol. 1974;11:212–229. [PubMed: 4377052]
- 18.
- Gilbertson SR, Kurzman ID, Zachrau RE, Hurvitz AI, Black MM. Canine mammary epithelial neoplasms: biological implications of morphologic characteristics assessed in 232 dogs. Vet. Pathol. 1983;20:127–142. [PubMed: 6836870]
- 19.
- Hampe JF, Misdorp W. Tumors and dysplasias of the mammary gland. Bull. World Health Org. 1974:111–133. [PMC free article: PMC2481221] [PubMed: 4371737]
- 20.
- Hayden DW, Ghobrial HK, Johnson KH, Buoen LC. Feline Mammary Sarcoma Composed of Cells Resembling Myofibroblasts. Vet. Pathol. 1986;23:118–124. [PubMed: 3962079]
- 21.
- Hellmén E, Lindgren A, Linell F, Matsson P, Nilsson A. Comparison of histology and clinical variables to DNA ploidy in canine mammary tumors. Vet. Pathol. 1988;25 (3):219–226. [PubMed: 3394213]
- 22.
- Hellmén E, Lindgren A. The accuracy of cytology in diagnosis and DNA analysis of canine mammary tumours. J. Comp. Pathol. 1989;101 (4):443–450. [PubMed: 2607016]
- 23.
- Lagadic M, Estrada M, Camadro JP, Durand P, Goebel J. Tumeurs mammaires de la chienne: critères du pronostic histologique et intérêt d’un granding. Rec. Méd. Vét. 1990;166 (4):1035–1042.
- 24.
- Lanari C, Molinolo AA, Pasqualini CD. Induction of mammary adenocarcinomas by medroxyprogesterone acetate in balb/c female mice. Cancer Letters. 1986;33:215–223. [PubMed: 2947675]
- 25.
- Lantiéri Marie-Françoise. Cancer du sein: premier soupçon de la cause. Scie. Vie. 1991;882:24–27. mars.
- 26.
- Lespagnard L, Kiss R, Danguy A, Legros N, Lenglet G, de Vleeschouwer N, Paridaens R. In vitro Studies of Canine Mammary Tumors. Influence of 17 Beta-Estradiol and Progesterone on Cell Kinetics Parameters, Oncology. 1987;44:292–301. [PubMed: 3670796]
- 27.
- Losco PE. Local and Peripheral Eosinophilia in a Dog with Anaplastic Mammary Carcinoma. Vet. Pathol. 1986;23:536–538. [PubMed: 3750747]
- 28.
- Magnol JP, Achache S. Cancerologie vétérinaire et comparée. Maloine Editeur; Paris: 1983. pp. 346–350.
- 29.
- Mandelli G, Scanziani E, Cairoli F. “Granding” istologico delle neoplasie mammaire dei carnivori. Clinica Veterinaria. 1987;110:347–358.
- 30.
- Mayr B, Swidersky W, Schleger W. Translocation (4; 27) in a canin mammary complex adenocarcinoma. Vet. Rec. 1990;126 (29):42–45. [PubMed: 2301114]
- 31.
- Mialot M, Lagadic M. Epidémiologie descriptive des tumeurs du Chien et du Chat. Rec. Méd. Vét. 1990;166 (11):937–947.
- 32.
- Misdorp W, Cotchin E, Hampe JF, Jabara GA. Canine malignant mammary tumor. Vet. Pathol. 1971;8:99–112. [PubMed: 4367432]
- 33.
- Misdorp W. Quelques aspects comparatifs de cancers de la mammelle chez la chienne, la chatte et la femme. Rec. Méd. Vét. 1972;148:583–590.
- 34.
- Misdorp W, Cotchin E, Hampe JF, Jabara AG, Von Sanderssleben J. Canine malignant mammary tumors III. Special types of carcinomas, malignant mixed tumors. Vet. Pathol. 1973;10:241–256. [PubMed: 4360454]
- 35.
- Möllermark G, Kängström LE, Eliasson I, Azawedo E, Barrios C, Larsson O, Engström W. Distribution of nuclear DNA-content in canine mammary tumours. J. Small Anim. Proc. 1988;29:309–314.
- 36.
- Moulton IE, Tayler DON, Dorn CR. Canine mammary tumours. Vet. Pathol. 1970;7:289–320. [PubMed: 4328309]
- 37.
- Moulton JE. Tumors in Domestic Animals. University of California Press; Berkeley: 1978. Tumors of the Mammary Gland; pp. 346–371.
- 38.
- Mulligan RM. Mammary cancer in the dog, 120 cases. Amer. J. Vet. Res. 1975;36:1391–1396. [PubMed: 169716]
- 39.
- Nerurkar VR, Chitale RA, Jalnapurkar BV. Comparative pathology of canine mammary tumors. J. Comp. Pathol. 1989;101 (4):389–397. [PubMed: 2558128]
- 40.
- O’Keefe DA. Tumors of the Genital System and Mammary Glands. In: Ettinger, Feldman, editors. Veterinary Internal Medicine. Vol. 2. W.B. Saunders Company; Philadelphia: 1995. pp. 1699–1704.
- 41.
- Parodi AL. Tumeurs mammaires de la chienne et de la chatte, epidemiologie, caractères lesionels et evolution. Rec. Méd. Vét. 1975;153:835–839.
- 42.
- Parodi AL. Epidemiologie de cancer du chienne et de la chatte. Rec. Méd. Vét. 1977;153:723–731.
- 43.
- Petrites-Murphy MB. Mammary Carcinoma with Peritoneal Metastasis in a Cow. Vet. Pathol. 1992;29:552–553. [PubMed: 1333111]
- 44.
- Pulley LT. Ultrastructural and Histochemical demonstration of Myoepithelium in Mixed Tumors of the Canine Mammary Gland. Amer. J. Vet. Res. 1973;34:1513–1522. [PubMed: 4357706]
- 45.
- Reimer JM, Sweeney RW, Saik J. Multicentric papillary cystadenoma in the udder of cow. J. Am. Vet. Med. Ass. 1988;192:1297–1298. [PubMed: 3391855]
- 46.
- Rutteman GR, Cornelisse CJ, Dijkshoorn NJ, Poortman J, Misdorp W. Flow Cytometric Analysis of DNA Ploidy in Canine Mammary Tumors. Cancer Res. 1988;48:3411–3417. [PubMed: 3370639]
- 47.
- Della Salda L, Sarli G, Benazzi C, Marcato PS. Giant Cells in Anaplastic Mammary Carcinoma of the Dog and Cat. J. Comp. Pathol. 1993;109:345–360. [PubMed: 8106667]
- 48.
- Schmahl W. Solides Karzinom der Mamma bei einem Pferd. Berl. Münch. Tierärztl. Wochenschr. 1972;85:141–142. [PubMed: 5063662]
- 49.
- Tiemessen I. Thoracic metastases of canine mammary gland tumors. A radiographic study. Vet. Rad. 1989;30 (6):249–252.
- 50.
- Toikkanen S, Joensuu H. Ag NOR counts have no prognostic value in breast cancer. J. Pathol. 1993;169:251–254. [PubMed: 8445490]
- 51.
- Wilcock BP. Neoplastic Disease of Skin and Mammary Gland. In: Jubb, Kennedy, Palmer, editors. Pathology of Domestic Animals. I. Academic Press; New York: 1993. pp. 733–738.
- 52.
- Willebrand D, Bosman FT, de Goeij AFPM. Patterns of basement membrane deposition in benign and malignant breast tumours. Histopathology. 1986;10:1231–1241. [PubMed: 3817761]
- 53.
- Misdorp W, Else RW, Hellmén E, Lipscomb TP. Histological Classification of Mammary Tumors of the Dog and the Cat. Second Series. VII. WHO, Armed Forces Institute of Pathology, American Registry of Pathology; Washington, D.C: 1999.
- 54.
- Baba AI. Oncologie comparată. Acad. Române; Bucureşti: 2002.
List of Figures 11.1-11.32
- Fig. 11.1 Tubular adenocarcinoma, simple type.
- Fig. 11.2 Tubular adenocarcinoma, scirrhous type.
- Fig. 11.3 Papillary adenocarcinoma, simple type.
- Fig. 11.4 Tubular adenocarcinoma, simple type.
- Fig. 11.5 Tubular adenocarcinoma, simple type.
- Fig. 11.6 Tubular adenocarcinoma, simple type.
- Fig. 11.7 Tubular adenocarcinoma, complex type.
- Fig. 11.8 Tubular adenocarcinoma, complex type.
- Fig. 11.9 Papillary adenocarcinoma, simple type.
- Fig. 11.10 Papillary adenocarcinoma, simple type, pulmonary metastasis.
- Fig. 11.11 Cystic papillary adenocarcinoma, simple type.
- Fig. 11.12 Papillary adenocarcinoma and chondroma.
- Fig. 11.13 Solid carcinoma, simple type.
- Fig. 11.14 Solid carcinoma, complex type.
- Fig. 11.15 Mucinous carcinoma.
- Fig. 11.16 Mucinous carcinoma.
- Fig. 11.17 Anaplastic carcinoma.
- Fig. 11.18 Squamous carcinoma.
- Fig. 11.19 Osteoblastic osteosarcoma.
- Fig. 11.20 Chondroblastic osteosarcoma.
- Fig. 11.21 Simple adenoma.
- Fig. 11.22 Simple myoepithelial adenoma. *)
- Fig. 11.23 Complex adenoma.
- Fig. 11.24 Fibroadenoma.
- Fig. 11.25 Benign mixed tumor. *)
- Fig. 11.26 Basaloid adenoma, mammary gland, dog.
- Fig. 11.27 Duct papilloma, dog.
- Fig. 11.28 Ductal hyperplasia.
- Fig. 11.29 Lobular hyperplasia, adenosis, simple type.
- Fig. 11.30 Tubular adenocarcinoma, cat.
- Fig. 11.31 Lobular hyperplasia, adenosis, epitheliosis, cat.
- Fig. 11.32 Fibroadenomatous change, cat.
Footnotes
- *)
Courtesy of W.H.O.
- MAMMARY GLAND TUMORS - Comparative OncologyMAMMARY GLAND TUMORS - Comparative Oncology
Your browsing activity is empty.
Activity recording is turned off.
See more...