NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
Recent evidence from a variety of studies has indicated that gene regulatory mechanisms in multicellular eukaryotes operate in a dosage dependent manner. A consequence of this fact is that new mutations in regulatory loci are available for adaptive selection in the heterozygous state, which is consistent with previous hypotheses that morphological evolution occurs primarily via regulatory genes. Their dosage dependent nature also suggests a common basis for phenomena that result from changes in chromosomal dosage, such as aneuploid syndromes and dosage compensation. Moreover, many quantitative trait loci have been molecularly defined as regulatory genes that exhibit semi-dominant behavior, suggesting also a common basis in the dosage dependent nature of regulatory hierarchies.
Introduction
Recent data from several types of investigations have indicated that gene regulatory systems in multicellular eukaryotes operate in a dosage dependent manner. In other words, the quantity of individual components of regulatory complexes influences target gene expression in the diploid state and ultimately impacts the phenotype. Early evidence came from studies of gene expression in aneuploid individuals relative to the normal euploid. These studies indicated an extensive array of dosage sensitive effects on a single gene. Further realization of this concept came from analysis of developmental control genes and the hierarchies in which they participate. Many genes involved in early development typically operate in a concentration dependent fashion to condition the differential differentiation of various parts of the embryo. Yet another indication of this concept is the identification of the molecular basis of genes responsible for quantitative traits. Many of those elucidated to date exhibit semidominant behavior genetically and produce a dosage effect in those cases that have been tested. Lastly, the realization of the haplo-insufficiency of transcription factors for clinical manifestations in humans has added to the evidence. In this chapter we will review these areas of investigation and discuss the implications of the dosage dependent nature of regulatory gene interactions.
Gene Expression in Aneuploids
One of the first indications of the dosage dependent nature of eukaryotic regulatory hierarchies came from studies of trans-acting chromosomal dosage effects on the expression of target genes throughout the genome. For example, when the long arm of chromosome 1 (1L) in maize was varied in a one to four dosage series, genes located on other chromosomes were modulated in expression.1 Other chromosome arms produced similar effects.2 The magnitude of these changes was within the range of an inverse correlation between the chromosomal dosage and the level of target gene expression. When the dosage of a chromosomal segment was reduced to one copy instead of the normal two, target genes were up-regulated within a two fold limit. However, when the same chromosomal segment was increased from the normal two copies to the trisomic state, target gene expression was reduced to two-thirds of the diploid. Further increase of the chromosomal dosage to four copies caused additional reductions of target gene expression to a lower limit of half the normal level.
In fewer cases, there was a positive correlation between chromosomal dosage and the amount of target gene expression. The monosomic situation caused reductions to nearly half of normal and the trisomic caused increases in gene expression up to 150%. Because the structural gene itself was not varied in copy number, this effect must have been due to an effect of regulatory loci.
Subsequent studies showed that these dosage effects operate on both the protein and RNA levels (Fig. 1).3-7 It was found that any one gene product is sensitive to the trans-acting dosage effects of several but not all chromosomal segments. The effects operate on nuclear genes as well as on those encoded by the organelles.6 Furthermore, the effects on gene expression caused by a particular chromosomal segment can be tissue specific.8
The inverse or direct correlation limits are seldom breached despite the fact that a single gene product can be affected by multiple aneuploidies. This fact suggests that there is little cumulative effect of the various regulatory loci beyond these limits. This conclusion is further supported by the findings of gene expression studies in whole arm (20% of the genome) trisomics of Drosophila.9 In these studies many gene products were assayed from around the genome. The overwhelming gene expression response to the trisomic condition was an inverse effect reducing expression to a lower limit of two-thirds of the normal level. Similarly, a phenotypic screen for dosage effects of the autosomes on the expression of the X linked white eye color gene found numerous regions that modulated white expression with the predominant effect being negative.10
Dosage Compensation
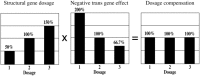
The observation that most trans-acting effects are negative helps to explain the fact that genes encoded on the varied segment often exhibit dosage compensation, i.e., the nearly equal expression of a target locus despite a change in dosage of the chromosome arm on which it resides (Fig. 2). In the original 1L dosage series in maize, the Alcoholdehydrogenase1 gene showed nearly equal expression levels despite the fact that the structural gene was being varied. This compensation resulted from a cancellation of a gene dosage effect by a simultaneous inverse effect on Adh1 produced by another 1L factor. When only a small region that included the Adh1 structural gene was varied, a direct correlation between gene dosage and its expression was observed. However, when another region of 1L, which did not include Adh1, was varied from one to three doses, a negative effect on ADH levels was found.11 In the whole of the chromosome arm, the two effects cancel each other to give dosage compensation.
Similarly, in the aforementioned whole arm trisomics of Drosophila, many genes on the varied arm exhibited compensation.12 Even a segmental trisomic of substantial size surrounding the Adh gene in Drosophila was shown to exhibit dosage compensation for Adh RNA levels. This compensation was also due to a cancellation of the two types of opposing effects.13
Dosage Compensation of the X Chromosome in Drosophila
The same type of inverse dosage effect is exerted by the X chromosome of Drosophila.14-16 The white eye color gene normally resides on the X chromosome and shows dosage compensation with the male having similar expression compared to females even though males have only one X.17-21 A transgene of white was placed in the autosomes in a background in which the endogenous white gene was missing from its normal position. Variation of the X chromosome dosage from one to three in the form of males, females and metafemales (triple X) resulted in a negative effect on the expression of the white transgene, which was held at constant dosage (Fig. 3).22 This result demonstrated that the X chromosome carries at least one negative trans-acting factor for white. Earlier results indicated that the white gene shows a gene dosage effect when only a small region surrounding it is varied.17 Thus, on the normal X chromosome, the combination of a structural gene dosage effect and an inverse effect of regulatory genes acting simultaneously result in dosage compensation.
The dosage effects of the Drosophila X chromosome are more complicated than other chromosomes, probably because this situation has been exposed to natural selection in contrast to experimentally produced aneuploids.23 Given that most trans-acting dosage effects are negative, one might expect that in males, where there is only one X, there would be a general elevation of autosomal gene expression.24,25 Indeed, a tendency for male biased autosomal expression has recently been confirmed in microarray studies.26 Nevertheless, the autosomes are not elevated to the extent that might otherwise be expected, because there is a specific mechanism to ameliorate the inverse dosage effect of the monosomic X upon the autosomes. This is accomplished by sequestrating histone acetylation complexes away from the autosomes in normal males.27-30 The lower autosomal histone acetylation level is associated with lower transcriptional activity. If males carry mutations that eliminate the sequestration process, then the expression is now as predicted, i.e., a widespread up-regulation of autosomal gene expression due to the inverse dosage effect of the X chromosome.31-34 In normal males, the complexes are sequestered to the single X chromosome. In order to prevent overcompensation, the complex overrides the impact of high levels of histone acetylation on the X itself, while still allowing the two-fold up-regulation for the proper level of dosage compensation.32-34
Regulatory Genes Are Responsible for the Inverse and Direct Dosage Effects
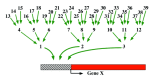
Individual genes that were responsible for the direct and inverse dosage effects were identified in mutagenic screens in Drosophila. The first case involved Inverse regulator-a.35 When heterozygous with a null allele, there is a two-fold up-regulation of the white eye color gene, as well as other loci. The gene maps to a site in the second chromosome that, when normal alleles are varied, shows a negative dosage effect on white pigment and messenger RNA levels. A total of 47 dosage dependent modifiers of white have been identified (Table 1).34 This compilation illustrates the number of dosage dependent factors that include a single target gene within their realm of influence, although they also have varying effects on other genes.
Table 1
Dosage dependent modifiers of the white eye color gene of Drosophila.
Studies involving multiples of these modifiers suggest that the effect of combinations is not cumulative beyond the inverse or direct correlation limits.36 This result is consistent with the chromosomal dosage series. When nonallelic modifiers, each with an approximate two-fold up-regulatory effect on white, were combined, the response was never multiplicative, indicating that the various genes are not acting independently but rather are likely to function in a hierarchy of regulatory networks. Some pairs of modifiers showed epistasis in that the combined effect was more or less equivalent to that of one of the two modifiers.
It is noteworthy in that all characterized white modifiers are regulatory genes of one type or another. They include transcription factors, chromatin proteins, chromatin modifying enzymes and components of signal transduction cascades that are implicated in ultimately affecting gene transcription. The heterogeneous nature of this collection suggests that regulatory genes regardless of type tend to exhibit dosage effects.
Haplo-Insufficiency
The term haplo-insufficiency applies to the phenomenon in which detrimental effects are manifested for short monosomic regions. Recently, the term has been used to describe the fact that tumor suppressors and transcription factors produce a phenotype when present in only a single copy.37 Previous thinking had held that either the uncovering of a mutant form of a tumor suppressor (loss of heterozygosity) or the simultaneous inactivation of both copies was necessary to condition cancer. Certainly these situations foster cancer development, but it has now been realized that hemizygosity of selected tumor suppressor genes also conditions the cancerous state, albeit at a slower rate.38,39 It appears that a dosage effect of tumor suppressor genes is operating.
There is also a growing realization that a single functional copy of many transcription factors will produce human genetic conditions. Seidman and Seidman compiled a list of 32 transcription factor loci that exhibit haplo-insufficient behavior for clinical manifestations.40 These effects are likely the result of a dosage effect of the regulators on their target loci. The recognition of these dosage effects is consistent with work from other organisms and with the fact that aneuploidy produces severe developmental effects.41-43
What Is Dominance?
The question of dominance has been the focus of debate since Sewall Wright and Ronald Fisher introduced the issue in the late 1920s.44-47 At the molecular level, dominance of an allele is likely to involve the presence of a biochemical function encoded by a housekeeping gene, while recessive alleles encode nonfunctional products or none at all.48 Of target genes, only those that occupy the rate-limiting step of a biochemical pathway would produce a semi-dominant effect on the phenotype. The expression of the remaining target genes in a pathway would reflect the action of the regulatory system, whose components would exhibit a dosage effect and thus show semi-dominant behavior in heterozygotes.
The anthocyanin pigment pathway genes in maize provide an example. Most of the structural genes of the biochemical pathway have been identified.49 Functional alleles show complete dominance over null alleles for five of the six known steps. The one exception is the c2 gene that encodes the rate-limiting position in the pathway. In this case, a semi-dominant behavior is exhibited in heterozygotes. In contrast to the behavior of the target loci, the majority of the regulatory genes, namely c1, r1, and in1, show a semi-dominant behavior, at least under most conditions. Another regulator, the vp1 gene, does not have a dosage effect on the phenotype. Thus, as in other cases, the majority of the regulatory genes are dosage dependent. Clearly, the degree of dominance can be quite variable, which contributes to a range of phenotypes.
How Could Multiple Regulators All Produce a Dosage Effect on the Same Target?
There are multiple regulatory genes that, when changed in dosage, alter the level of expression of any one target gene.34,50-52 How could this occur mechanistically? The answer will require further study, but some data are available that shed light on this problem. For example, many helix-loop-helix transcription factors operate in multi-subunit complexes. One of the most thoroughly studied examples involves the regulators of the mammalian muscle differentiation pathway.51 The stoichiometry of the individual transcription factors affects the activity of the complex as a whole. Thus, when the quantity of any one member is altered, the activity of the transcriptional complex is changed. In genetic terms, changing the dosage of a gene encoding any one component of a complex would alter the expression of the battery of target genes. Other examples include the Polycomb and Trithorax complexes of chromatin proteins.53-55 Again, a complex exists composed of many subunits for which varying any individual member alters the effect of the whole.
The stoichiometric effects described above appear to explain why aneuploidy has a greater effect on target gene expression than do changes in whole genome ploidy.2,13,56,57 The “per gene” expression among different ploidy levels usually is nearly equal.57 The effects of aneuploidy are much more extensive, which fits with the concept that an altered stoichiometry of the regulatory genes causes these syndromes. An uncompensated gene dosage effect of some of the regulators on the varied segment relative to the remainder of the genome could produce this stoichiometric shift.
The second aspect of the regulatory mechanisms that helps explain the multiple factor observation is that hierarchies of dosage dependent regulators occur (Fig. 4). If dosage dependent regulators themselves are controlled by dosage dependent regulators, a system is in place such that variation in quantity of any one regulator in the hierarchy will modulate the ultimate
target genes to some degree. A dosage effect at the top of a hierarchy will be transmitted through branching cascades to the targets. Studies of early development in Drosophila indicate that dosage dependent regulators control other transcription factors as targets, which in turn determine the amount and spectrum of the ultimate target genes expressed at any one time and place.58-65 Such a system will contribute to the multi-factorial control of phenotypic traits.
Implications for Aneuploid Syndromes
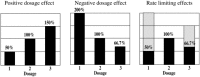
As suggested above, the global effects on target gene expression observed in aneuploids are likely due to the altered stoichiometry in regulatory hierarchies.2 A parallel relationship exists on the phenotypic level in that aneuploid syndromes are more severe than changes in ploidy (Fig. 5).66 The global alterations of gene expression in aneuploids are both positive and negative and operate in both monosomics and trisomics.2,5 Thus, with both increases and decreases of chromosomal dosage, many genes will exhibit lowered expression levels. The monosomic state has lower potential reductions due to those genes subject to positive dosage effects compared to the trisomic reductions for genes subject to the negative dosage effects. Both, of course, are reduced relative to the diploid. This correlation between the severity of the phenotype and the global patterns of gene expression suggest that when many target genes are altered in expression, the phenotype will be affected. There is likely to be a rate limiting aspect of gene expression on phenotypic characteristics and this fact would be reflected in aneuploid syndromes. Because the monosomic and trisomic conditions reduce many gene products, albeit different ones, both situations become rate limiting on the phenotype (Fig. 6).
Implications for the Genetic Basis of Quantitative Traits
Typically quantitative traits are controlled by multiple genes, whose alleles exhibit some degree of semi-dominant behavior.67,68 In other words, alleles affecting quantitative traits often show dosage effects. This aspect of quantitative inheritance suggests a similar mechanism between its control and the dosage effects observed in aneuploids.5 In both cases, multiple regions of the genome exhibit a dosage dependent influence on the phenotype.
In recent years, some quantitative trait loci have been cloned and their molecular nature identified, primarily in plant species. Many of these QTL correspond to regulatory genes and, interestingly, usually exert a negative dosage effect. Examples include teosinte branched in maize, which controls plant morphology;69 two genes in tomato affecting fruit size and shape;70,71 the Dwarf8 gene in maize, which affects stature and flowering time;72FLC in Arabidopsis, which affects flowering time;73 the p1 gene affecting maysin levels in maize;74 and the hairy gene in flies affecting bristle number.75 Thus, expression studies in aneuploids and the elucidation of the genes corresponding to quantitative trait loci suggest at least some common bases.
In addition, developmental processes are affected by regulatory hierarchies that also exhibit dosage effects. Variation in such loci no doubt would contribute to quantitative variation. These mechanisms would likewise be subject to selection at the diploid level. The use of gradients to formulate morphology is consistent with the dosage dependent nature of regulatory hierarchies.58,59,65,76
The Evolution of Regulatory Hierarchies in Multicellular Eukaryotes
In haploid organisms, gene expression levels can be selected directly in the haploid state. In contrast, in diploid organisms, mutations in regulatory processes that require homozygosity to modulate gene expression will not be accessible for natural selection to act under many circumstances. Only in small populations, in which random drift can fix rare mutations, would they become established in a population. Indeed the neutral theory of evolution suggests that adaptive evolution is rare and that most change occurs by drift.77,78 This contention appears to be supported by the fact that comparisons of synonymous versus nonsynonymous base substitutions in protein encoding sequences are consistent with a low level of adaptive selection. Most of these studies, however, have concentrated on genes encoding metabolic or housekeeping functions rather than regulatory loci.78,79 New mutations in metabolic target genes may be more likely to be neutral.
In contrast, the data showing that dosage effects are primarily due to regulatory genes suggest a loose dichotomy between regulatory genes and their target loci in how evolution acts upon them. It appears that regulatory systems in general have been selected to operate in such a manner that the quantity of individual molecules affects the phenotype in the diploid state. Allelic variation would be expected to show some level of semi-dominance. Newly arising mutations in regulatory genes would be immediately available for selection due to their impact in the heterozygote. Emerging evidence from analysis of regulatory factors supports the idea that they are more likely to be under adaptive rather than neutral evolution.80-82
It is also possible that there is balancing selection between alleles of a regulatory gene. The potential for pleiotropy is high for any allelic variant contributing to a regulatory complex that affects target gene expression in multiple tissues. An advantage conferred by an allele's effect on target gene expression in one tissue may be counteracted by a negative effect in another tissue or circumstance. The interplay of adaptive and balancing selection of regulatory variation has not been experimentally explored to any great extent, but is a subject that deserves attention.
Selfish and Cooperative Genes
The writings of George Williams, William Hamilton, Richard Dawkins and Mark Ridley present arguments that genes will evolve characteristics that foster their own perpetuation.83-86 This idea is referred to as the “Selfish Gene” concept. While this term is often denigrated because genes have no motive, there should be little debate that characteristics aiding the survival of particular alleles in the gene pool will be selected. What is often forgotten in such discussions is that characteristics that develop “cooperation” among genes can be the most effective means of self-perpetuation. A system of gene regulation in which multi-component hierarchies are affected by a change in the quantity of any one member allows most regulatory genes the potential to contribute to the phenotype and reproductive success of the individual carrying them. In this manner, alleles of genes in the hierarchy will have the opportunity to foster their own perpetuation.
The evolution of such a multifactorial system also avoids the problem of too few regulatory genes contributing to the phenotype. If only a few genes contribute to the pool of variation, there might be too little available for survival as environmental conditions change. It would seem that those evolutionary lineages with a multifactorial control of phenotypic traits would be more likely to survive over evolutionary time.
Genomic Imprinting As a Nonmutational Means to Modulate Dosage Dependent Genes
Genomic imprinting refers to the phenomenon in which there is monoallelic expression resulting from the inactivation of one copy depending on the parental history. First described in maize for the behavior of the r locus expression in the endosperm,87 there are now many cases known, particularly in mammals. One evolutionary rationale for parental imprinting involves parental conflict.88 Females are postulated to evolve means to disperse resources equally among all progeny, whereas males would evolve mechanisms that foster more resources for their own progeny in competition with other potential fathers. While this concept can explain some facts, it does not appear to be consistent with others.89
Recently another explanation has been proposed to explain imprinting. This concept, referred to as the rheostat model, suggests that imprinting is a means of modulating dosage dependent genes that affect the phenotype.90 An imprinting mechanism can change the expression level of a particular gene without causing a permanent mutation. This explanation fits well with the idea that regulatory hierarchies have evolved to be dosage dependent and provides an additional mechanism by which this can be achieved.
How Many Genes Are Important in Evolution?
There appears to be a loose dichotomy between regulatory genes and their targets in that the former typically show a dosage effect on the phenotype, while the latter do not. This would imply that when new mutations arise in a heterozygote, adaptive evolution is the major mode of evolution on regulatory processes and neutral evolution is the primary mode on the target genes. Positive selection will operate on new favorable alleles of regulatory genes and negative selection will occur against the less favorable alleles. Selection on gene regulatory mechanisms may be the major force in the evolution of multicellular diploid organisms. This concept was first proposed by King and Wilson,91 following their comparison of protein profiles between chimp and human; the profiles were virtually indistinguishable despite the morphological differences between the two species (at least for most individuals).
This argument suggests that the number of genes driving the evolution of multicellular eukaryotes is dramatically smaller than the total. The fraction of the human or Drosophila genomes devoted to regulatory genes of various types approximates 10-15%.92,93 Nevertheless, variation in these few thousand genes is sufficient to provide a unique genotype to each individual in a population of immense size. For example, with the 47 dosage dependent modifiers of the white gene noted above, if there were variation in each (unlikely, but for the sake of discussion) and these were independently assorting (again not true, but for the sake of discussion), then they would produce 140,737,488,835,533 genotypes for the expression level of white. To the extent that the expression levels of the various alleles are not the same and depending on the amount of epistasis, the number of phenotypic classes would approach a similar number. Clearly, this example is grossly oversimplified in terms of gene interactions and the level of variation present in populations, but it illustrates the concept that only a few thousand genes can produce an astounding number of possible genotypes and phenotypes.
No Need for “Hopeful Monsters”
Darwin envisioned gradualism in evolution to explain the changes in morphology and other characteristics of diverging lineages of organisms. In contrast Richard Goldschmidt proposed that dramatic changes could occur by single mutations that alter the characteristics of a species (Hopeful Monsters).94 This suggestion was inspired by the rather significant alterations in developmental processes conditioned by so-called homeotic genes that are now known to control segment identity. The counter argument to Goldschmidt was that such large effect mutations are likely to be detrimental and rapidly selected against.
The realization that many, if not most, regulatory genes can exhibit dosage dependent behavior under at least some circumstances alleviates the need to postulate such Hopeful Monsters. The example of the 47 modifiers of white illustrates that, for even a simple characteristic, the potential exists for selection to change a phenotype dramatically through gradual steps, acting in series on different modifiers. Cryptic variation likely exists for quantitative traits in populations as illustrated in a study by Lauter and Doebley on the progenitor of domesticated maize, teosinte.95 Hidden variation for certain morphological characteristics was present in teosinte populations that became evident only in a different genetic background.
Such cryptic variation may occur if certain regulatory genes are strongly rate limiting on a phenotype in one tissue, thus masking the variation in regulators that are less limiting on the same characteristic. The latter may be more strongly limiting on some other aspect of the total phenotype or on other target genes in the same tissue. In this manner, they would be maintained as dosage dependent. As alternative alleles of a strongly rate limiting regulator become fixed during selection, the rate limiting effects of other regulators in the hierarchy can become more subject to selection. Thus, by shifting which genes are limiting through the hierarchy, the phenotype can be pushed to greater extremes. Although the effect of any one variant allele is small, the cumulative effect of many genes of small effect changing the phenotype in one direction under selection can be much larger.
Hybrid Effects and Incompatibilities
When inbred lines are crossed together, the hybrid progeny in plants and animals are often more vigorous than either parent.96,97 Particularly in plants, the hybrid exceeds the biomass of the better parent. This phenomenon is referred to as heterosis. In preliminary work from our laboratory, in which representative target genes were assayed for their level of expression, it appears that in the hybrid, “per cell” expression is often different from the mid-parent value of the two parents.98 This result suggests that when allelic variation at regulatory loci is brought together in the same genome, novel interactions occur. A reasonable suggestion is that there are incompatibilities of allelic products that contribute to transcriptional regulatory complexes. Inasmuch as a majority of dosage dependent regulators of transcription act in a negative fashion, incompatibilities might lead to a global up-regulation of gene expression. At present it is unclear whether this is the case and what gene products might be responsible for heterosis.
Hybrids between species also often exhibit altered phenotypes not present in either parental species.99-101 When analyzing the differences between Drosophila simulans and mauritiana for the size and shape of the male genitalia, which is the major morphological difference between the two species, there are multiple QTL.102-104 For the most part, these genes exhibit additive or semi-dominant behavior. Thus, these loci appear to represent multiple dosage dependent genes-again showing the control of a specific trait by multiple regulators. In the case of interspecific crosses, sterility or lethality can also occur in hybrids. Some mutations have been identified that override these incompatibilities. To date, those that have been defined molecularly are dosage dependent regulatory genes-suggesting that the incompatibilities occur because of incongruous interactions in regulatory hierarchies.80,105,106 The fact that the various loci function correctly in the respective species, but not when brought together in a hybrid, suggests that the various components of the regulatory hierarchy in any one lineage interact with each other and evolve accordingly to maintain functionality.
Concluding Remarks
No one would deny that global modulations of gene expression can affect the phenotype. Thus, an understanding of the mode of action and interaction of regulatory hierarchies in multicellular organisms is critical to appreciate many aspects of developmental processes and morphological evolution. The realization of the dosage dependent nature of many regulators is a step in that direction, but there is much to be learned about how regulatory complexes do in fact affect the phenotype. One aspect deserving further study involves the interactions of individual components of multimeric complexes. Also, a complete understanding of the impact of regulatory systems on the phenotype will require the elucidation of the pleiotropic effects of any one regulator throughout the organism. Another area deserving attention is how the epistatic interactions of genes contributing to various regulatory complexes operate. Lastly, understanding the means by which regulatory hierarchies are shifted and selected over time is an important goal for elucidating the evolution of regulatory mechanisms and the organisms they control.
Acknowledgements
Research in our laboratory has been supported by the US Department of Energy, National Science Foundation (USA) and the US Department of Agriculture. Nicole Riddle made many helpful suggestions on the manuscript.
References
- 1.
- Birchler JA. A study of enzyme activities in a dosage series of the long arm of chromosome one in maize. Genetics. 1979;92:1211–1229. [PMC free article: PMC1214066] [PubMed: 17248947]
- 2.
- Birchler JA, Newton KJ. Modulation of protein levels in chromosomal dosage series of maize: The biochemical basis of aneuploid syndromes. Genetics. 1981;99:247–266. [PMC free article: PMC1214499] [PubMed: 17249116]
- 3.
- Reichert GH. Two-dimensional gel analysis of proteins from mouse fetuses with trisomy 19 after DEAE-Sepharose chromatography. Genetical Research. 1986;47:193–197. [PubMed: 3744045]
- 4.
- Klose J, Putz B. Analysis of two-dimensional protein patterns from mouse embryos with different trisomics. Proc Natl Acad Sci USA. 1983;80:3753–3753. [PMC free article: PMC394129] [PubMed: 6574515]
- 5.
- Guo M, Birchler JA. Trans-acting dosage effects on the expression of model gene systems in maize aneuploids. Science. 1994;266:1999–2002. [PubMed: 17836517]
- 6.
- Auger DL, Newton KJ, Birchler JA. Nuclear gene dosage effects upon the expression of maize mitochondrial genes. Genetics. 2001;157:1711–1721. [PMC free article: PMC1461607] [PubMed: 11290725]
- 7.
- Wanous MK, Munkvold JD, Kruse JD. et al. Identification of chromosome arms influencing expression of the HMW glutenins in wheat. Theor Appl Genetics. 2003;106:213–220. [PubMed: 12582846]
- 8.
- Cooper JL, Birchler JA. Developmental impact on trans-acting dosage effects in maize aneuploids. genesis. 2001;31:64–71. [PubMed: 11668680]
- 9.
- Devlin RH, Holm DG, Grigliatti TA. The influence of whole-arm trisomy on gene expression in Drosophila. Genetics. 1988;118:87–101. [PMC free article: PMC1203269] [PubMed: 8608935]
- 10.
- Sabl JF, Birchler JA. Dosage dependent modifiers of white alleles in Drosophila melanogaster. Genetical Research. 1993;62:15–22. [PubMed: 8405989]
- 11.
- Birchler JA. The genetic basis of dosage compensation of alcohol dehydrogenase-1 in maize. Genetics. 1981;97:625–637. [PMC free article: PMC1214415] [PubMed: 17249082]
- 12.
- Devlin RH, Holm DG, Grigliatti TA. Autosomal dosage compensation in Drosophila melanogaster strains trisomic for the left arm of chromosome 2. Proc Natl Acad Sci USA. 1982;79:1200–1204. [PMC free article: PMC345929] [PubMed: 6803235]
- 13.
- Birchler JA, Hiebert JC, Paigen K. Analysis of autosomal dosage compensation involving the Alcohol dehydrogenase locus in Drosophila melanogaster. Genetics. 1990;124:677–686. [PMC free article: PMC1203960] [PubMed: 2107122]
- 14.
- Birchler JA, Hiebert JC, Krietzman M. Gene expression in adult metafemales of Drosophila melanogaster. Genetics. 1989;122:869–879. [PMC free article: PMC1203761] [PubMed: 2503426]
- 15.
- Birchler JA. X chromosome dosage compensation in Drosophila. Science. 1996:272–1190. [PubMed: 17792629]
- 16.
- Margolis OS. The effect of a supernumerary X chromosome on members of the Bar series of Drosophila. Genetics. 1934;19:18–84. [PMC free article: PMC1208443] [PubMed: 17246707]
- 17.
- Muller HJ. Further studies on the nature and causes of gene mutations. Proc 6th Int Congr Genetics. 1932;1:213–255.
- 18.
- Muller HJ. Evidence of the precision of genetic adaptation. Harvey Lecture. 1950;43:165–229.
- 19.
- Stern C. Dosage compensation-development of a concept and new facts. Canadian Journal of Genetics and Cytology. 1960;2:105–118.
- 20.
- Baker BS, Gorman M, Marin I. Dosage compensation in Drosophila. Annu Rev Genetics. 1994;28:491–521. [PubMed: 7893138]
- 21.
- Arkhipova I, Li J, Meselson M. On the mode of gene dosage compensation in Drosophila. Genetics. 1997;145:729–736. [PMC free article: PMC1207857] [PubMed: 9055082]
- 22.
- Birchler JA. Expression of cis-regulatory mutants of the white locus in metafemales of Drosophila melanogaster. Genetical Research. 1992;59:11–18. [PubMed: 1572532]
- 23.
- Birchler JA. Inverse effect regions in maize and Drosophila and their possible role in dosage compensation, sexual dimorphism of autosomal genes and sex determination in the latter. Maize Genetics Cooperation News Letter. 1977;51:18–22.
- 24.
- Smith PD, Lucchesi JC. The role of sexuality in dosage compensation in Drosophila. Genetics. 1969;61:607–618. [PMC free article: PMC1212228] [PubMed: 17248430]
- 25.
- Birchler JA. Genetic analysis of a modifier of the sexual dimorphism of glass in Drosophila melanogaster. Genetical Research. 1984;44:125–132.
- 26.
- Parisi M, Nuttall R, Naiman D. et al. Paucity of genes on the Drosophila X chromosome showing male-biased expression. Science. 2003;299:697–700. [PMC free article: PMC1363366] [PubMed: 12511656]
- 27.
- Kuroda MI, Kernan M, Kreber R. et al. The maleless protein associates with the X chromosome to regulate dosage compensation in Drosophila. Cell. 1991;66:935–947. [PubMed: 1653648]
- 28.
- Bone JR, Lavender RJ, Richman R. et al. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes and Development. 1994;8:96–104. [PubMed: 8288132]
- 29.
- Hilfiker AD, Hilfiker-Kleiner D, Pannuti A. et al. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997;16:2054–2060. [PMC free article: PMC1169808] [PubMed: 9155031]
- 30.
- Turner BM, Birley AJ, Lavender J. Histone H4 isoforms acetylated at specific lysine residues define individual chromosomes and chromatin domains in Drosophila polytene nuclei. Cell. 1992;69:376–384. [PubMed: 1568251]
- 31.
- Hiebert JC, Birchler JA. Effects of the maleless mutation on X and autosomal gene expression in Drosophila melanogaster. Genetics. 1994;136:913–926. [PMC free article: PMC1205896] [PubMed: 8005444]
- 32.
- Bhadra U, Pal-Bhadra M, Birchler JA. Role of the male specific lethal (msl) genes in modifying the effects of sex chromosomal dosage in Drosophila. Genetics. 1999;152:249–268. [PMC free article: PMC1460601] [PubMed: 10224258]
- 33.
- Bhadra U, Pal Bhadra M, Birchler JA. Histone acetylation and gene expression analysis of Sex lethal mutants in Drosophila. Genetics. 2000;155:753–763. [PMC free article: PMC1461119] [PubMed: 10835396]
- 34.
- Birchler JA, Bhadra U, Pal Bhadra M. et al. Dosage dependent gene regulation in multicellular eukaryotes: Implications for dosage compensation, aneuploid syndromes and quantitative traits. Developmental Biology. 2001;234:–.
- 35.
- Rabinow L, Nguyen-Huynh AT, Birchler JA. A trans-acting regulatory gene that inversely affects the expression of the white, brown and scarlet loci in Drosophila melanogaster. Genetics. 1991;129:463–480. [PMC free article: PMC1204636] [PubMed: 1743487]
- 36.
- Bhadra U, Pal Bhadra M, Birchler JA. Interactions among dosage-dependent trans-acting modifiers of gene expression and position-effect variegation in Drosophila. Genetics. 1998;150:251–263. [PMC free article: PMC1460319] [PubMed: 9725844]
- 37.
- Veitia RA. Exploring the etiology of haploinsufficiency. Bioessays. 2002;24:175–184. [PubMed: 11835282]
- 38.
- Goss KH, Risinger MA, Kordich JJ. et al. Enhanced tumor formation in mice heterozygous for Blm mutation. Science. 2002;297:2051–2053. [PubMed: 12242442]
- 39.
- Fodde R, Smits R. A matter of dosage. Science. 2002;298:761–763. [PubMed: 12399571]
- 40.
- Seidman JG, Seidman C. Transcription factor haploinsufficiency: When half a loaf is not enough. J Clin Invest. 2002;109:451–455. [PMC free article: PMC150881] [PubMed: 11854316]
- 41.
- Epstein CF. The Consequences of Chromosome Imbalance, Principles, Mechanisms and Models. Cambridge University Press. 1986
- 42.
- Lindsley DL, Sandler L. et al. Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics. 1972;71:157–184. [PMC free article: PMC1212769] [PubMed: 4624779]
- 43.
- Bond DJ, Chandley AC. Aneuploidy. Oxford University Press. 1983
- 44.
- Fisher RA. The possible modifications of the response of the wild type to recurrent mutations. Amer Nat. 1928;62:115–126.
- 45.
- Fisher RA. Two further notes on the origin of dominance. Amer Nat. 1928;62:571–574.
- 46.
- Wright S. Fisher_s theory of dominance. Amer Natl. 1929;63:274–279.
- 47.
- Wright S. Physiological and evolutionary theories of dominance. Amer Natl. 1934;68:24–53.
- 48.
- Orr HA. A test of Fisher_s theory of dominance. Proc Natl Acad Sci USA. 1991;88:11413–11415. [PMC free article: PMC53145] [PubMed: 1763055]
- 49.
- Dooner HK, Robbins TP, Jorgensen RA. Genetic and developmental control of anthocyanin biosynthesis. Ann Rev Genetics. 1991;25:173–. [PubMed: 1839877]
- 50.
- Henikoff S. Dosage-dependent modification of position-effect variegation in Drosophila. Bioesssays. 1996;18:401–409. [PubMed: 8639163]
- 51.
- Weintraub H. The myoD family and myogenesis: Redundancy, networks and thresholds. Cell. 1993;75:1241–1244. [PubMed: 8269506]
- 53.
- Grandori C, Cowley SM, James LP. et al. The MYC/MAX/MAD network and the transcriptional control of cell behavior. Annu Rev Cell Dev Biol. 2000;16:653–699. [PubMed: 11031250]
- 53.
- Kennison JA, Russell MA. Dosage dependent modifiers of homoeotic mutations in Drosophila melanogaster. Genetics. 1987;116:75–86. [PMC free article: PMC1203123] [PubMed: 17246380]
- 54.
- Saurin AJ, Shao Z, Erdjument-Bromage H. et al. A Drosophila Polycomb group complex includes Zeste and dTAFII proteins. Nature. 2001;412:655–660. [PubMed: 11493925]
- 55.
- Bel S, Core N, Djabali M. et al. Genetic interactions and dosage effects of Polycomb group genes in mice. Development. 1998;125:3543–3551. [PubMed: 9716520]
- 56.
- Lucchesi JC, Rawls Jr JM. Regulation of gene function: A comparison of enzyme activity levels in relation to gene dosage in diploids and triploids of Drosophila melanogaster. Biochem Genetics. 1973;9:41–51. [PubMed: 4197884]
- 57.
- Guo M, Davis D, Birchler JA. Dosage effects on gene expression in a maize ploidy series. Genetics. 1996;142:1349–1355. [PMC free article: PMC1207130] [PubMed: 8846910]
- 58.
- Driever W, Nusslein-Volhard C. The bicoid protein determines position in the Drosophila embryo in a concentration-dependent manner. Cell. 1988;54:95–104. [PubMed: 3383245]
- 59.
- Struhl G, Struhl K, K PM. The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell. 1989;57:1259–1273. [PubMed: 2567637]
- 60.
- Warrior R, Levine M. Dose-dependent regulation of pair-rule stripes by gap proteins and the initiation of segment polarity. Development. 1990;110:759–767. [PubMed: 2088718]
- 61.
- Sauer Sauer, Jackle H. Concentration-dependent transcriptional activation or repression by Kruppel from a single binding site. Nature. 1991;353:563–566. [PubMed: 1922363]
- 62.
- Cribb DL, Benassayag C, Randazzo FM. et al. Levels of homeotic protein function can determine developmental identity: Evidence from low-level expression of the Drosophila homeotic gene proboscipedia under Hsp70 control. EMBO J. 1995;14:767–778. [PMC free article: PMC398142] [PubMed: 7882980]
- 63.
- Schulz C, Tautz D. Autonomous concentration-dependent activation and repression of Kruppel by hunchback in the Drosophila embryo. Development. 1994;120:3043–3049. [PubMed: 7607091]
- 64.
- Abrell S, Carrera P, Jackle H. A modifier screen of ectopic Kruppel activity identifies autosomal Drosophila chromosomal sites and genes required for normal eye development. Chromosoma. 2000;109:334–342. [PubMed: 11007492]
- 65.
- Christian JL. BMP, Wnt and Hedgehog signals: How far can they go? Curr Opin Cell Biol. 2000;12:244–249. [PubMed: 10819541]
- 66.
- Blakeslee AF, Belling J, Farnham ME. Chromosomal duplication and Mendelian phenomena in Datura mutants. Science. 1920;52:388–390. [PubMed: 17829955]
- 67.
- Mackay TFC. The genetic basis of quantitative variation: Numbers of sensory bristles of Drosophila melanogaster as a model system. Trends in Genetics. 1995;11:11–470. [PubMed: 8533161]
- 68.
- Tanksley SD. Mapping polygenes. Annu Rev Genetics. 1993;27:205–233. [PubMed: 8122902]
- 69.
- Doebley JF, Stec A, Hubbard L. The evolution of apical dominance in maize. Nature. 1997;386:485–488. [PubMed: 9087405]
- 70.
- Frary A, Nesbitt RC, Frary A. et al. fw2.2: A quantitative trait locus key to the evolution of tomato fruit size. Science. 2000;289:85–88. [PubMed: 10884229]
- 71.
- Liu J, Van Eck J, Cong B. et al. A new class of regulatory genes underlying the cause of pear-shaped tomato fruit. Proc Natl Acad Sci USA. 2002;99:13302–13306. [PMC free article: PMC130628] [PubMed: 12242331]
- 72.
- Thornsberry JM, Goodman MM, Doebley J. et al. 4th. Dwarf8 polymorphisms associate with variation in flowering time. Nature Genetics. 2001;28:286–289. [PubMed: 11431702]
- 73.
- Michaels SD, Amasino RM. Flowering Locus C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell. 1999;11:949–956. [PMC free article: PMC144226] [PubMed: 10330478]
- 74.
- Byrne P, McMullen M, Snook ME. et al. Quantitative trait loci and metabolic pathways: Genetic control of the concentration of maysin, a corn earworm resistance factor, in maize silks. Proc Natl Acad Sci USA. 1996;93:8820–8825. [PMC free article: PMC38551] [PubMed: 11607699]
- 75.
- Robin C, Lyman RF, Long AD. et al. hairy: A quantitative trait locus for Drosophila sensory bristle number. Genetics. 2002;162:155–164. [PMC free article: PMC1462234] [PubMed: 12242230]
- 76.
- Jiang J, Levine M. Binding affinities and cooperative interactions with bHLH activities delimit threshold responses to the dorsal gradient morphogen. Cell. 1993;72:741–752. [PubMed: 8453668]
- 77.
- Kimura M. Evolutionary rate at the molecular level. Nature. 1968;217:624–626. [PubMed: 5637732]
- 78.
- Fay JC, Wu C-I. The neutral theory in the genomic era. Curr Opin Genes Develop. 2001;11:642–646. [PubMed: 11682307]
- 79.
- Sabeti PC, Reich DE, Higgins JM. et al. Detecting recent positive selection in the human genome from haplotype structure. Nature. 2002;419:832–837. [PubMed: 12397357]
- 80.
- Barbash DA, Siino DF, Tarone AM. A rapidly evolving MYB-related protein causes species isolation in Drosophila. Proc Natl Acad Sci (USA). 2003
- 81.
- Barrier M, Robichaux RH, Purugganan MD. Accelerated regulatory gene evolution in an adaptive radiation. Proc Natl Acad Sci USA. 2001;98:10208–10213. [PMC free article: PMC56940] [PubMed: 11517318]
- 82.
- Barrier M, Bustamante CD, Yu J. et al. Selection on rapidly evolving proteins in the Arabidopsis genome. Genetics. 2003;163:723–733. [PMC free article: PMC1462452] [PubMed: 12618409]
- 83.
- Williams GC. Adaptation and Natural Selection. Princeton. Princeton, NJ: University Press. 1966
- 84.
- Hamilton WD. Narrow roads of geneland. Oxford: W. H. Freeman. 1996
- 85.
- Dawkins R. The selfish gene. Oxford, UK: Oxford University Press. 1976
- 86.
- Ridley M. The cooperative gene. Simon and Schuster, New York: The Free Press. 2001
- 87.
- Kermicle JL. Dependence of the R-mottled aleurone phenotype in maize on mode of sexual transmission. Genetics. 1970;66:69–85. [PMC free article: PMC1212486] [PubMed: 17248508]
- 88.
- Moore , Haig D. Genomic imprinting in mammalian development: A parental tug-of-war. Trends in Genetics. 1991;7:45–49. [PubMed: 2035190]
- 89.
- McVean GT, Hurst Hurst. Molecular evolution of imprinted genes: No evidence for antagonistic coevolution. Proc R Soc Lond Biol Sci. 1997;264:739–746. [PMC free article: PMC1688426] [PubMed: 9178545]
- 90.
- Beaudet AL, Jiang Y-h. A rheostat model for a rapid and reversible form of imprinting-dependent evolution. Am J Hum Genet. 2002;70:1389–1397. [PMC free article: PMC379123] [PubMed: 11992247]
- 91.
- King MC, Wilson AC. Evolution at two levels in humans and chimpanzees. Science. 1975;188:107–116. [PubMed: 1090005]
- 92.
- Adams MD, Celniker SE, Holt RA. et al. The Genome Sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. [PubMed: 10731132]
- 93.
- Venter JC, Adams MD, Myers EW. et al. The Sequence of the Human Genome. Science. 2001;291:1304–1351. [PubMed: 11181995]
- 94.
- Goldschmidt RB. Theoretical Genetics. Berkeley, California: University of California Press. 1955
- 95.
- Lauter N, Doebley J. Genetic variation for phenotypically invariant traits detected in teosinte: Implications for the evolution of novel form. Genetics. 2002;160:333–342. [PMC free article: PMC1461939] [PubMed: 11805068]
- 96.
- Bruce AB. The Mendelian theory of heredity and the augmentation of vigor. Science. 1910;32:627–628. [PubMed: 17816706]
- 97.
- Coors JG, Pandey S. Genetics and Exploitation of Heterosis in Crops. Madison, Wisconsin: American Society of Agronomy, Inc. and Crop Science Society of America, Inc. 1999
- 98.
- Osborn TC, Pires JC, Birchler JA. et al. Understanding mechanisms of novel gene expression in polyploids. Trends in Genetics. Trends in Genetics;19:141–147. [PubMed: 12615008]
- 99.
- Dobzhansky T. Genetics and the Origin of Species. New York: Columbia University Press. 1937:–.
- 100.
- Muller HJ. Isolating mechanisms, evolution and temperature. Biol Symp. 1942;6:71–125.
- 101.
- Turelli M, Orr HA. Dominance, epistasis and the genetics of postzygotic isolation. Genetics. 2000;154:1663–1679. [PMC free article: PMC1461023] [PubMed: 10747061]
- 102.
- Liu J, Mercer JM, Stam LF. et al. Genetic analysis of a morphological shape difference in the male genitalia of Drosophila simulans and D. mauritiana. Genetics. 1996;142:1129–1145. [PMC free article: PMC1207113] [PubMed: 8846893]
- 103.
- True JR, Weir BS, Laurie CC. A genome-wide survey of hybrid incompatibility factors by the introgression of marked segments of Drosophila mauritiana chromosomes into Drosophila simulans. Genetics. 1996;142:819–837. [PMC free article: PMC1207021] [PubMed: 8849890]
- 104.
- Laurie CC, True JR, Liu J. et al. An introgression analysis of quantitative trait loci that contribute to a morphological difference between Drosophila simulans and D. mauritiana. Genetics. 1997;145:339–348. [PMC free article: PMC1207799] [PubMed: 9071588]
- 105.
- Ting C-T, Tsaur S-C, Wu M-L. et al. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science. 1998;282:1501–1504. [PubMed: 9822383]
- 106.
- Peres DE, Wu C-I. Further characterization of the Odysseus locus of hybrid sterility in Drosophila: One gene is not enough. Genetics. 1993;140:201–206. [PMC free article: PMC1206547] [PubMed: 7635285]
- Introduction
- Gene Expression in Aneuploids
- Dosage Compensation
- Dosage Compensation of the X Chromosome in Drosophila
- Regulatory Genes Are Responsible for the Inverse and Direct Dosage Effects
- Haplo-Insufficiency
- What Is Dominance?
- How Could Multiple Regulators All Produce a Dosage Effect on the Same Target?
- Implications for Aneuploid Syndromes
- Implications for the Genetic Basis of Quantitative Traits
- The Evolution of Regulatory Hierarchies in Multicellular Eukaryotes
- Selfish and Cooperative Genes
- Genomic Imprinting As a Nonmutational Means to Modulate Dosage Dependent Genes
- How Many Genes Are Important in Evolution?
- No Need for “Hopeful Monsters”
- Hybrid Effects and Incompatibilities
- Concluding Remarks
- Acknowledgements
- References
- Biological Consequences of Dosage Dependent Gene Regulation in Multicellular Euk...Biological Consequences of Dosage Dependent Gene Regulation in Multicellular Eukaryotes - Madame Curie Bioscience Database
Your browsing activity is empty.
Activity recording is turned off.
See more...