This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
A large proportion of patients experiencing acute low back pain (LBP) initially seek care from their primary care providers (PCPs). Clinical practice guidelines recommend that patients with acute (recent-onset) LBP be provided with nonpharmacological treatment and discourage early diagnostic and intervention procedures. However, the implementation of clinical practice guidelines at the PCP level and in physical therapy care continues at suboptimal rates. Stratification tools, which include questionnaires that divide patients into categories of high, moderate, or low risk, can help identify patients who, because they are at high risk for persistent disabling LBP, may have the most to benefit from patient-centered interventions. Our research question was, therefore, for high-risk patients presenting with acute LBP, should prompt referral to a stratified approach to physical therapy care be recommended to prevent a transition to chronic LBP?
Objectives:
Our original objectives were (1) to compare the rate of transition to chronic LBP when high-risk patients receive usual care (UC) or a stratified approach to care; and (2) to estimate the rate and predictors of transition to chronic LBP among patients deemed low to medium risk. Due to the higher-than-expected nonresponse for the primary outcome, PCORI imposed a modification of the study objectives and, hence, the contractual agreement between PCORI and the study sponsor, the University of Pittsburgh. The modified objectives were to estimate the rate of transition to chronic LBP and average back-related physical function with UC and with a stratified approach to care (UC plus referral to psychologically informed physical therapy [UC+PIPT]) among patients presenting to primary care clinics with acute LBP and deemed high risk. For the patients deemed medium and low risk, we sought to estimate the rate of transition to chronic LBP and health care use and to determine the factors associated with these outcomes. PCORI officials believed that the limitations of (1) lower-than-expected response rates coupled with (2) limited treatment integrity did not warrant the use of inferential statistical approaches, a stance with which the Targeted Interventions to Prevent Chronic Low Back Pain in High-Risk Patients (TARGET) trial investigators disagreed. Thus, the aims were modified to reflect the descriptive approach imposed by PCORI.
Methods:
This study used 2 simultaneous research designs in primary care clinics of 5 large US academic health care systems to meet the objectives among patients presenting to primary care for acute LBP. Patients with acute LBP at all clinics were risk stratified (high, medium, low) using the Subgroups for Targeted Treatment (STarT) Back Screening Tool. For the first objective among patients deemed high risk for persistent disabling symptoms, we used a cluster randomized controlled trial (RCT) where, ultimately, 76 primary care clinics were randomized to UC+PIPT or UC. To address the second objective, we used an observational cohort for those patients who screened low to medium risk on the STarT Back Screening Tool. Originally, the planned study sample size was 2880, which was based on a nonresponse rate of 8%, 60 randomized clinics, and 90% power to detect a relative reduction of chronic LBP of 40%. During recruitment, we revised the expected sample size to an average of 31 enrolled high-risk patients per clinic (60 × 31 = 1860), 80% power, and assuming a 40% nonresponse rate at 6 months. In the intervention group (UC+PIPT), we assumed that the best-practice alert, which is a clinician decision support tool available in the electronic medical record (EMR) when a particular element of a patient's care needs additional attention, would be executed as a rule (we did not hypothesize a percentage adherence to the alert). The primary outcomes were the transition from acute to chronic LBP and LBP-related functional disability as determined by the Oswestry Disability Index (Oswestry) at 6 months. Secondary outcomes were LBP-related processes of health care and use of services over 12 months, as obtained from EMRs. Generalized linear mixed models were used to estimate the proportion of patients who transitioned to chronic LBP and mean functional disability at 6 months, along with corresponding confidence intervals adjusting for the clustered design. These models were also used to assess factors associated with the transition to chronic LBP and resource use among low- to medium-risk patients.
Results:
The nonresponse rate for the primary outcomes at 6 months was 40%. Among high-risk patients in the cluster RCT, approximately half reported chronic LBP at 6 months (UC+PIPT group [n = 658]: 47%; 95% CI, 42%-51%; UC group [n = 635]: 51%; 95% CI, 46%-56%). Thirty-nine percent of patients in the UC+PIPT group received a referral for PIPT; <1% of patients in the UC group received a referral for PIPT. Both groups reported moderate disability at 6 months, with average Oswestry scores of 27 (95% CI, 25-29) and 30 (95% CI, 28-32) for UC+PIPT and UC, respectively. About 1 in 4 patients were prescribed opioids at the index visit. Nearly half of both groups had primary care visits for LBP over 12 months, 15% to 18% had a visit to a specialist, 17% to 19% received imaging, and <5% had interventional pain procedures, such as epidural steroid injections. Fewer than 3% had surgery in either group. In the observational cohort of low- and medium-risk patients, we recruited 3524 and 3847 patients, respectively. About 1 in 5 low-risk patients reported chronic LBP at 6 months (19%; 95% CI, 16%-21%) compared with 33% of the medium-risk patients (95% CI, 30%-35%). Health insurance (eg, Medicaid vs all others), obesity, smoking, baseline disability, and several processes of care at the index visit were associated with higher rates of chronic LBP at 6 months in the low- and medium-risk patients. About 9% of low-risk and 15% of medium-risk patients underwent imaging during the 12 months.
Conclusions:
In a multisite US health care setting, a comprehensive intervention (UC+PIPT) to support stratified care for LBP targeting high-risk patients that included numerous implementation strategies in primary care and physical therapy did not reduce the transition from acute to chronic LBP. Patients in both groups had a higher-than-expected transition to chronic LBP. The rates of diagnostic testing and interventional pain procedures were relatively low. The stratification tool successfully identified groups at lowest and highest risk for transition to chronic LBP.
Limitations:
This study has 2 major limitations: (1) limited treatment fidelity reflected in the low rate of PIPT referrals and (2) high nonresponse of the primary outcome at 6 months that was similar across risk strata. Although the nonresponse rate was about 40% at 6 months, there were no clinically meaningful differences in baseline characteristics between those who responded and those who did not.
Background
Internationally, low back pain (LBP) is one of the most prevalent, potentially disabling, and costly conditions for which people seek health care.1-3 In the United States, up to 80% of adults will experience at least 1 episode of LBP in their lifetime,4,5 with close to a quarter reporting an acute episode of LBP (eg, an incident within the previous month).6 In terms of cost, spinal pain represents the third most costly health care condition in the United States, with estimates exceeding $87 billion.7
A large proportion of patients experiencing acute LBP initially seek care from primary care providers (PCPs).8-10 The prognosis of acute LBP is generally purported to be good,11 though epidemiologic studies in primary care settings report that up to 44% of patients with LBP have no improvement or worsen,12 suggesting that the prevailing attitude of spontaneous recovery may be overestimated.
Recent studies have demonstrated equivocal results using an LBP-specific, stratified approach in primary care, which entails using a 9-item prognostic screening tool (STarT Back Screening Tool) to assess risk factors for disabling chronic LBP, coupled with matched intervention pathways that include combined physical and biobehavioral interventions. The approach has shown promise in European studies13-15 but has been less successful in a study based in the United States.16 Regarding the latter, an intensive qualitative analysis suggested that the negative result is attributable to clinicians failing to implement the recommended treatments.17
The Targeted Interventions to Prevent Chronic Low Back Pain in High-Risk Patients (TARGET) trial was designed to assess whether the stratified approach to LBP was effective in the United States. Specifically, we sought to (1) test whether usual care (UC) plus a referral to psychologically informed physical therapy (UC+PIPT) is superior to UC alone, as demonstrated by lower rates of transition to chronic LBP and functional disability among patients with acute LBP identified as high risk for chronicity; and (2) determine the rates and factors associated with the transition to chronic LBP among low- to medium-risk patients seen in primary care for acute LBP.
Summary of Original Study Plan and Modification
The original study plan included a cluster randomized controlled trial (RCT) designed to compare the rates of transition to chronic LBP at 6 months between UC and UC+PIPT for patients presenting with acute LBP who were deemed high risk for transition to chronic LBP. The original study plan also included a comparison in the same group for back-related function, measured by the Oswestry Disability Index (Oswestry) and comparisons of LBP-related medical use. The original study plan had a secondary aim to follow patients with acute LBP who were deemed low and medium risk for transition to chronic LBP in a nonrandomized, observational cohort to determine the proportion of patients who transition to chronic LBP, functional disability at 6 and 12 months, and rates of LBP-related medical use. Originally, we had planned to recruit from a minimum of 12 PCP practices from each of 5 geographical locations (a total of 60 PCP practices to cluster randomize). The original sites included the University of Pittsburgh Medical Center (UPMC; Pittsburgh, Pennsylvania), Intermountain Healthcare (IHC; Salt Lake City, Utah), Boston Medical Center (BMC; Boston, Massachusetts), Johns Hopkins Medicine (JHM; Baltimore, Maryland), and the Medical University of South Carolina (MUSC; Charleston).
We made several modifications to the study plan. One occurred before recruitment (change 1), and the remainder occurred around the end of enrollment in June/July 2018. In the latter case, changes were mutually agreed on by the investigative team (changes 4-7 and 9 listed below). However, some changes were mandated by PCORI program officers (changes 2, 3, and 8). We have provided a more detailed description of changes along with timelines later in this report. A summary of changes to the original study plan entailed the following:
- Naming of the intervention and comparator groups
- Revision of the aims to remove wording related to comparisons between intervention groups
- Alignment of the analysis plan with the revised aims to focus on estimation rather than inferential statistics
- Decreases in sample size for both the trial and prospective observational cohort
- Elimination of 12-month patient-reported outcomes (PROs)
- Elimination of qualitative assessments with patients and providers
- Addition of participant incentives for completed 6-month follow-up
- Addition of clinical sites to meet targeted enrollment
- Exclusion of data from 1 study site due to problems with study implementation (We did not recruit from MUSC and adjusted the number of PCP practices from 60 to a total of 76. The details of the changes can be found at the end of the Methods section.)
Specific Aims
The revised specific aims of our project were as follows:
- Primary aim 1. In patients with acute LBP who are deemed “high risk” for transition to chronic LBP, as determined from Subgroups for Targeted Treatment (STarT) Back Screening Tool scores, to estimate the proportions and confidence intervals of high-risk patients who transitioned to chronic LBP at 6 months in the UC group and the UC+PIPT group. Our primary outcome measure was the proportion of high-risk patients who transitioned to chronic LBP during the 6-month follow-up period.
- Primary aim 2. To estimate the average back-related physical function using the Oswestry Disability Index and its confidence intervals for high-risk patients in the UC group and the UC+PIPT group at 6 months after the index visit for acute LBP.
- Secondary aim 1. To compare the 2 groups (UC and UC+PIPT) on LBP-related medical use (eg, opioid prescriptions, referral to specialty consults and diagnostic imaging, epidural steroid injections, referral to PT). Our outcome measures were the proportions of high-risk patients who were prescribed or underwent each of these items during the initial 12-month follow-up period.
- Secondary aim 2. To follow patients with acute LBP who are deemed low and medium risk in a nonrandomized observational cohort and to determine the proportion who transition to chronic LBP and/or have worse back-related physical function at 6 months and have higher LBP-related medical use at 12 months.
- Purpose of the observational cohort study. The purpose of this ancillary observational study was to compare and contrast rates of transition from acute LBP to chronic LBP and resource use in the treatment of LBP among low- vs medium-risk patients as determined from STarT Back Screening Tool scores. The TARGET trial is the first to (1) investigate stratified care for acute LBP whereby all patients admitted to the study are not already experiencing chronic LBP; and (2) determine precise estimates of the transition to chronic LBP across 4 geographically diverse areas of the United States.
Patient and Stakeholder Engagement
We engaged with stakeholders from the onset of the TARGET trial planning periods and continue to communicate with them as we move into the dissemination phase of the TARGET trial. The primary stakeholders were patients with LBP, providers, and payers/insurers. In addition to having 3 patient co-investigators from Boston, Pittsburgh, and Salt Lake City as members of the steering committee and participants in monthly teleconferences with co-investigators and annual in-person meetings, we had an 8-member advisory board of stakeholders who met annually to guide the project (Table 1).
Table 1
Members of the Advisory Board.
The strategy of bidirectional partnership was most often used in the TARGET trial. Patients, providers, and payers were involved in (1) topic solicitation, agenda setting, and development of research questions; (2) proposal development; (3) methods and study design; (4) recruitment; and (5) data collection.
From our first advisory board meeting (September 30, 2015), we were able to document the following takeaways:
- The conversation around LBP needs to be reshaped to put patients in active control of self-management and living with pain.
- Foundations have already created materials that are effective for promoting patient self-management (peer groups, social media, videos, graphical materials, workbooks); the challenge is getting these materials to patients in the format that is most helpful for them.
- Involvement of family and caregivers in the care of patients with LBP can be effective for improving communication and supporting ongoing self-management.
- Patients want the right information at the right time: Make the care plan clear, make it accessible (ie, via patient portal), and let them know their progress.
- Communication and coordination among members of the LBP care team (PCP, PT, specialists, pharmacy, family, faith-based organizations) is important for patients; patients want a consistent plan across care settings.
- Reducing PT co-pays and making PT more accessible and flexible can improve patient attendance and adherence.
- PCPs view LBP as a condition that resolves on its own with minimal intervention (nonsteroidal anti-inflammatory drugs [NSAIDs]) despite national data that show significant reoccurring visits and resource use.
- Despite the biopsychosocial causes of LBP, the treatment is often overmedicalized, with overtreatment of low-risk patients and undertreatment of high-risk patients.
- PCPs perceive that they do not have the time or resources in their offices to treat all the underlying causes of LBP.
- Appealing to PCP professionalism and providing additional data for efficient, targeted treatment of LBP embedded within the workflow is a viable approach.
- Training PCPs about stratified LBP treatments could be achieved through implementation webinars, short videos to boost confidence, or written materials; the challenge is finding the best approach for this stakeholder group.
- Integration of tools, language, reporting, and the electronic medical records (EMRs) between PCPs and PTs can help bridge the communication gap.
- Insurance solutions could be in the form of bundled payments, incentives for evidence-based LBP approaches, prior authorizations, or reduced co-pays for PT.
- Patient education about the low value and potentially high cost of LBP treatments in a high-deductible setting will also change behavior.
The TARGET trial had 3 patient co-investigators: 1 from Pittsburgh, 1 from Boston, and 1 from Salt Lake City. The patient co-investigators are still in communication with the investigative team as we plan to disseminate the results of the TARGET trial. They also attended all advisory board meetings, the last of which occurred in September 2019.
In the early stages of proposal development, the choice of outcomes that were important to patients was primarily directed by patients with LBP. Patients consistently endorsed function-related outcomes instruments, including the Oswestry, which was ultimately used as one of the primary outcomes for the TARGET trial. Though the Oswestry is a legacy instrument that is being replaced both clinically and in research endeavors with Patient-Reported Outcomes Measurement Information System (PROMIS) instruments, it still has a strong presence in EMRs, having already been incorporated in EMRs of 2 geographical sites: UPMC and IHC. The Oswestry is also an accepted outcome instrument in the LBP research community, thus increasing the generalizability of the study. Patients also directed the choice against using pain scales or numeric pain ratings, stating that although their use was widespread, they were confusing in that they were inconsistently administered (eg, different stem phrases and different anchor points). Consequently, we did not incorporate numeric pain ratings in the TARGET trial. In addition, we have used patient stakeholder engagement experiences to influence other initiatives (eg, the NIH Chronic Low Back Pain Consortium,18 The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop19) about the limitations of numeric pain rating approaches to quantify pain in clinical trials.
Throughout the TARGET trial, patient co-investigators were instrumental in improving patient-facing materials by providing specific feedback regarding readability and acceptability. For example, in the early stages of implementation, all communications targeting patients were reviewed and edited by patient co-investigators.
Provider stakeholders at each of the sites offered direction for working within the primary care and PT clinics. We were able to gather the minimum of what was necessary to conduct the trial (screening information, stratification by risk, automated referral through a best-practice alert, electronic distribution of the referral), all of which were designed to seamlessly fit into the workflow of very busy primary care clinics. We were also able to modify and slightly tailor the data collection for each environment without compromising the standardization of the approach. For example, tablets were the norm for data input at the UPMC, IHC, and JHM sites; however, we found alternatives to the use of the tablet at BMC by using patient navigators. All these methods resulted in efficient patient screening and successful input of baseline data into the EMR. The choice of cluster vs individual randomization was also heavily influenced by our provider stakeholder engagement. The logistics of individual randomization, given the limited resources and extreme time challenges in primary care, were deemed highly improbable of success. Time challenges and the risk of cross-contamination led to the decision to use cluster randomization.
The bidirectional partnership model was challenging in the provider sphere largely because of a lack of stakeholder time. This was particularly true in primary care engagement. One comment from a PCP representative on our advisory board was particularly telling when she stated:
If I were to do all of the required screening and adhered to all of the best-practice alerts and followed up with all of the patients, 100% of the time, I'd have 4 hours to sleep every night.
We could see the strain of time at every step of primary care engagement despite our efforts to accommodate the providers' schedules. Invariably, meetings were sparsely attended by PCPs who instead would send their administrators and office staff. Attendance was sparse despite prior agreements, many enthusiastic, that the providers “would be thrilled” to attend. Thus, we were largely dependent on recruitment strategies that administrators and office staff could implement, such as identifying potentially eligible patients to be screened via prior visit record reviews and reminding PCPs of pending referrals. The TARGET trial had buy-in from the leadership of primary care practices; however, we were not able to reach a point where the frontline PCPs consistently engaged fully with patients and study personnel.
In the initial stages of the study design, we proposed recruiting from a wide variety of portals of entry for acute LBP. Payer/insurer feedback strongly suggested that we focus exclusively on primary care. As researchers, we were well aware of the time challenges in primary care environments and certainly realized the high degree of difficulty of implementing a study with a primary care focus. The data that payers shared with us strongly suggested that (1) across the United States, primary care was a common portal of entry for people with acute LBP and (2) care for episodes of back pain care originating in primary care was often low value. In addition, through our stakeholder engagement with our payers/insurers, we decided that the focus of the TARGET trial should be exclusively primary care.
We were also encouraged by successful implementation and effectiveness of the stratified approach to LBP in European countries, all of which took place in primary care.14,15 Our goal was to see whether the favorable results of a stratified approach to acute LBP could be replicated in the United States; however, we did not seek to replicate exactly the study design of our European counterparts. First, the 2 European studies used different methodologies. Second, we were bound by the following requirements of the PCORI funding announcement (PFA) (1) to conduct a pragmatic RCT; (2) to focus on acute LBP (the European studies recruited patients with acute LBP and chronic LBP); and (3) to use a definitive measure of “transition to chronic” (the European studies looked at functional outcome and claims-based resource use).
We were instrumental in persuading payers to address a long-standing barrier to PT access: the requirement for co-payments. National payers who were represented on our advisory board changed their payment policies to eliminate or drastically reduce out-of-pocket expenses for PT services. We believe that TARGET trial engagement with stakeholders was instrumental in such changes; this is evidenced by the principal investigator (A.D.) co-presenting with payer stakeholders the case for changes at the Optum Forum (2017), along with subsequent co-presentations of alternative payment models by the principal investigator (A.D.), co-principal investigator (R.B.S.), and payer/insurer stakeholders (David Elton, Optum; Michael Parkinson, UPMC Health Plan).
Methods
Study Overview
TARGET was designed to inform the effectiveness of UC+PIPT compared with UC using a multisite, pragmatic, cluster RCT for primary care patients with acute LBP identified as high risk for persistent disabling symptoms and to determine the rates of transition to chronic LBP among patients deemed low and medium risk in a US setting. The clusters were the primary care clinics at 5 distinct US health care systems in diverse geographical areas: UPMC, BMC, IHC, JHM, and MUSC. Patients who presented to primary care with acute LBP were screened using the STarT Back Screening Tool20 and deemed high, medium, or low risk for persistent disabling LBP symptoms. Only those deemed high risk were identified for the cluster RCT. Patients deemed low or medium risk were identified for the observational cohort; for these patients, the clinic allocation to intervention or control would have no influence on their outcomes.
Clinics were randomized (1:1) to UC or UC+PIPT. Clinics in the UC group were not given any specific instructions to alter their normal clinical procedures beyond baseline data collection. Educational outreach covering the latest acute LBP guidelines was offered through grand rounds, staff meetings, and online modules to all participating clinics without monitoring or incentivizing adherence to the guidelines. Clinics randomized to the UC+PIPT group were provided the same outreach and instructions as were the UC clinics, enhanced or electronic instructions for immediate referral of high-risk patients to a standardized regimen of PIPT (a stratified approach to care), and PIPT training of physical therapists in the referral area. The primary outcomes were transition to chronic LBP and LBP-related functional disability at 6 months. Secondary outcomes were referrals for and use of health care services outside the recommended LBP practice guidelines (eg, lumbar imaging, opioids, invasive procedures), which were evaluated at the index visit and at 12 months using EMR data.
The study was funded by a contract between PCORI and the University of Pittsburgh. The contract was to run from July 1, 2015, to December 31, 2019. Study enrollment began in May 2016 and concluded in June 2018; a 6-month follow-up was completed in February 2019, and a 12-month follow-up for resource use was completed at the end of June 2019. All IRBs representing the 5 health systems concurred with study investigators that both groups of the study were comparing 2 different standards of care; therefore, all procedures carried out in the enrolled clinics were designated as parts of a quality improvement initiative. The quality improvement initiative focused on 3 changes in the health system to improve the treatment and management of acute LBP: (1) implementing standardized measures to identify and risk-stratify patients with acute back pain; (2) providing education to PCPs on current guidelines for care for LBP; and (3) establishing a mechanism to facilitate early referral from the intervention clinics to a stratified approach using PIPT for the treatment of high-risk acute LBP. The study was approved by each site's IRB, with varying approaches to obtaining consent for the 6-month survey follow-up and 12-month EMR review, as follows:
- BMC required consent for 6-month follow-up and for 12-month EMR review. BMC obtained consent at the time of the 6-month follow-up. EMR data after the index visit up to 12 months were extracted only for those who consented to the study.
- JHM required consent at enrollment for the 6-month follow-up but had a HIPAA waiver and waiver of consent for the 12-month limited data set from the EMR for all patients screened as acute.
- The University of Pittsburgh was the IRB of record for UPMC and IHC, which required consent for the 6-month follow-up but had a waiver of HIPAA authorization and consent for the 12-month EMR review. Both sites obtained consent for the 6-month follow-up at the time of the follow-up.
- MUSC obtained consent for the 6-month follow-up only, 1 month after the baseline visit. No EMR data were to be extracted for these patients.
The overview of the TARGET trial is shown in Figure 1.

Figure 1
Overview of TARGET Trial.
The TARGET trial is characterized as “rather pragmatic” to “very pragmatic” across the Pragmatic Explanatory Continuum Indicator Summary-2 (PRECIS-2)25 domains, with the exception of flexibility in the intervention delivery (Figure 2), and is registered with ClinicalTrials.gov (identifier NCT02647658). The study did not have and was not required to have an independent data and safety monitoring board. The study protocol was published in Contemporary Clinical Trials.26

Figure 2
TARGET Trial Scored on PRECIS-2.
Study Design
Two study designs were used in TARGET to address the objectives. The first was a cluster RCT where clinics were randomized with 1:1 allocation to the UC+PIPT or UC group. Findings from the cluster RCT apply only to the high-risk patients. Randomization was stratified by site, number of patients with LBP, and percentage of patients who had Medicaid or self-paid for insurance. The randomization by site was conducted by one of the study statisticians. The second study design was an observational cohort of the patients who screened low to medium risk. Both studies included a 6-month follow-up for the primary outcome and analysis of 12-month use data sourced from the EMR.
Study Setting
The clusters were the primary care clinics at 5 distinct US health care systems in diverse geographical areas: UPMC, BMC, IHC, JHM, and MUSC. We selected these 5 US sites because they were large health systems with an extensive network of primary care clinics in both urban and rural environments, with integrated EMRs to facilitate data collection. Because the main study was designed to be a pragmatic RCT, the office/clinical personnel in the individual PCP clinics screened for eligible patients; collected baseline measures; and provided UC, which could include UC and/or referral of patients to the PIPT intervention (in the intervention clinics).
Participants
The population of interest was adult patients aged >18 years who presented to primary care with a complaint of acute LBP or a confirmatory primary encounter (eg, an accompanying LBP ICD-10 code) or ordering diagnosis of back pain reflected in the EMR based on the ICD-9 or ICD-10-CM codes. Staff at the clinical sites used clinic-based electronic tablets or verbally administered questionnaires to determine eligibility with the 2-item Chronic Low Back Pain Questionnaire (cLBP-Q)18 and the 9-item STarT Back Screening Tool to stratify risk (low, medium, or high) for persistent disabling symptoms20 and to obtain the baseline assessment of LBP-related functional disability with the 10-item Oswestry Disability Index, version 2.1a (http://www.rehab.msu.edu/_files/_docs/Oswestry_Low_Back_Disability.pdf).27,28 The cLBP-Q consists of 2 questions (Figure 3) pertaining to the duration of LBP interference with the ability to perform regular activities (question 1: duration of LBP) and the frequency of LBP interference with the ability to perform regular activities in the past 6 months (question 2: frequency of LBP). Based on the responses, chronic is defined as a response of “greater than 3 months” to question 1 and a response of “at least half the days in the past 6 months” to question 2. Patients were considered to have acute LBP if they were not chronic—that is, if LBP interfered with their ability to perform regular daily activities for 3 months or less or for fewer than half the days during the past 6 months.
Figure 3
Chronic Low Back Pain Questionnaire.
Patients with acute LBP who were identified as high risk for persistent disabling symptoms on the STarT Back Screening Tool (Figure 4; subscore ≥4) served as the population of interest for the cluster RCT. Patients identified as low to medium risk for persistent disabling symptoms (low risk = total score ≤3; medium risk = total score ≥4 and subscore ≤3) served as the population of interest for the observational cohort. Patients were excluded if they presented with indications of a specific nonmusculoskeletal cause of LBP, commonly referred to as “red flag” signs and symptoms, documented in the EMR. Examples of excluded conditions include cauda equina syndrome, cancer-related back pain, epidural abscess, or vertebral fracture.29 No other exclusions were made.
Figure 4
STarT Back Screening Tool for Risk Stratification.
Interventions and Comparators for the Cluster RCT
In the original research plan, we proposed “usual care” vs “cognitive behavioral therapy provided by physical therapists.” That is, our approach was to allow PCPs to do what they think is best, termed usual care, and to compare it with PCPs teaming up with physical therapists to deliver cognitive behavioral therapy for selected patients who have LBP along with risk factors for persistent LBP. Subsequent discussion with PCORI program officers as well as consultation with the study team eventually changed the group designations to guideline-based care (GBC) vs GBC plus PIPT. The change from usual care to GBC was primarily driven by PCORI program officers, who were opposed to using the term usual care primarily because of its nonspecificity. In response to external peer review of the draft final research report (DFRR), we have subsequently changed all references to GBC in this report to UC. The UC component of the experimental group transferred to the UC+PIPT group. The PIPT designation of the experimental group was largely driven by the experts on the TARGET trial investigative team, including physical therapists and a clinical psychologist, who have studied and published in the cognitive behavioral topical area. Their suggestion was to use the term psychologically informed physical therapy because (1) the physical therapy portion of the name was included because the care was administered by physical therapists and (2) the psychologically informed portion of the name included key components addressing psychological obstacles to recovery, such as communication, pain coping skills, and activity-based treatments, along with more typical physical interventions (aerobic exercise, strengthening, etc).
Usual Care
As part of the quality improvement initiative, we provided UC sites with educational outreach covering the latest acute LBP guidelines through grand rounds, staff meetings, and an online module (Updates in the Treatment of Low Back Pain: Challenges and Opportunities, available at https://pittrstce.adobeconnect.com/p5r2u6d9o3l/?launcher=false&fcsContent=true&pbMode=normal) to all participating clinics; we did not monitor or incentivize adherence. UC typically starts with a focused history and physical examination, with diagnostic imaging and testing indicated only if there are signs of severe or progressive neurologic deficits or a serious underlying condition (eg, red flags). First-line medication options are acetaminophen or NSAIDs; alternatively, spinal manipulation can be considered. Patient education should include advice to stay active and avoid bed rest, with an emphasis on giving a positive prognosis for full recovery. Other than the descriptions given in the educational materials, clinics in the UC-only group were not given any specific instructions to alter their normal clinical procedures beyond collecting baseline data. PCPs in the UC group treated high-risk patients according to their individual clinical judgment. We did not monitor adherence to guidelines once recruitment commenced or provide feedback regarding guideline adherence; there was no audit or checklist for the UC group.
UC Plus PIPT
Clinics randomized to the intervention group had 2 intervention-specific components: (1) specific instructions for immediate referral of high-risk patients to a standardized regimen of PIPT and (2) PIPT training for physical therapists in the immediate proximity of the clinics. Our intention was to enhance the application of a stratified approach to treating LBP in primary care environments while minimizing cross-study group contamination between patients. Thus, the unit of intervention allocation was the clinic.
Implementation of PIPT referral
In all study locations except BMC, we used a best-practice alert in the EMR, with an automatic electronic referral for PIPT. The alert was generated only if a patient was identified as having acute LBP based on responses to the cLBP-Q and considered high risk based on the STarT Back Screening Tool. Patients deemed low or moderate risk were not eligible for this referral. The best-practice alert notified the treating provider that the patient was high risk and provided the option to order PT using a stratified approach for LBP. Depending on the clinic, the data were either input by the patient via an EMR portal, transferred from the tablet over a wireless network to the EMR, or input by hand by medical assistants directly into the EMR. For 3 study sites (UPMC, IHC, MUSC), the order was electronically connected to a central PT department or referred to an administrator, who then contacted patients after their physician visit to schedule PT. At JHM, this customized referral was printed on patients' after-visit summary for hand delivery to the referral physical therapist for treatment. At BMC, the patient navigator generated the PIPT referral (coded as “stratified approach to LBP”) for the PCP who was treating the patient. If the order was not declined by the PCP, the patient navigator contacted the patient for subsequent scheduling of PT with a physical therapist who had attended and completed the PIPT training course. From a PRECIS-2 perspective, the process of automating the PIPT referral, pending the referral, and linking the referral directly to PT environments goes well beyond UC procedures, thus making the trial less pragmatic.
PIPT physical therapist training
The PIPT training program was delivered to physical therapists who commonly receive referrals from the primary care clinics randomized to UC+PIPT. Extensive details on the PIPT training have been published.30 Briefly, the PIPT course provided an overview of the theoretical rationale and supporting data for an approach focused on educating patients about their condition and reducing fear of movement as well as addressing physical impairments, such as mobility deficits and pain. The course also covered specific PIPT management principles and skills with demonstration and practice, including the principles of cognitive behavioral training, motivational interviewing, patient-centered communication styles, pain coping skills, activity-based treatments, impairment-based interventions, and treatment monitoring components. The format included 12 brief online educational modules that were recommended for review before the course but were not mandatory and 1 face-to-face 8-hour session led by a physical therapist and a clinical psychologist. Ongoing training via telephone, email access to trainers/mentors, booster and refresher training, and online discussion groups were available to promote adherence to the PIPT skills in clinical treatment. The amount and frequency of follow-up communication and training maintenance were different in each geographical region because of the pragmatic nature of the trial. From a PRECIS-2 perspective, PIPT training directed specifically to the physical therapists who commonly dealt with the PCPs in the UC+PIPT group of the trial was not customary and thus made the trial less pragmatic. To allow for increased pragmatism, we kept the training time commitment equivalent to that of more typical continuing education requirements for physical therapists in the participating states.
Delivery of PIPT
Physical therapists receiving PIPT referrals for patients from the study were expected to implement PIPT treatment with the high-risk patients using their clinical judgment to determine the number of PIPT treatment sessions. Physical therapists were trained to document specific PIPT treatment components that were delivered during patient care by completing self-report checklists (Figure 5). The PIPT checklist was to be completed at the initial encounter as part of the patient's plan of care. The checklist was to be shared with the primary care clinic via the shared EMR or by fax when not sharing the same EMR. Faxed checklists were to be scanned by primary care clinic staff and placed in the patient's EMR to allow tracking and receipt of prescribed PIPT.

Figure 5
PIPT Checklist.
The PIPT checklist was modified from that proposed in the original research plan to include categories of PIPT care (communication, pain coping skills taught and reinforced, activity-based treatment), along with more specific techniques under each category. These changes were the result of consultation with our team of co-investigators whose expertise was in behavioral management of people with chronic pain conditions, as well as an expert clinical psychologist, who was brought onto the TARGET trial investigative team and helped design and implement the PIPT training. This team of investigators was included in our physical therapist training sessions, which included guidance on how to document PIPT care (eg, use the PIPT checklist). The PIPT checklist was designed as a reminder to incorporate PIPT principles in treatment interventions; it was not designed to allow for treatment fidelity checks. Even though the checklist was designed to be returned to PCP offices, there was no way to enforce its return. Furthermore, for those checklists that were retuned, the data were ultimately not retrievable and auditable due to a high degree of variability in how the checklists interfaced with the EMRs within and between sites. For example, sometimes the checklists were scanned and placed as PDFs in the EMR. Even if they could be found and retrieved, the process required manual data input.
Expanding PIPT treatment integrity: documentation solutions
As the study progressed, there continued to be concerns about treatment integrity, some of which centered on documentation of the PIPT approach throughout the PT episode of care. We explored ways to enhance the PIPT checklist to encompass the entire episode of care by working with the EMR programmers at both UPMC and IHC. Specifically, we explored ways to document the treatment principles of PIPT to the EMR's visit documentation. Both Epic representatives at UPMC and Cerner representatives at IHC have offered solutions whereby use of the PIPT principles can be added to the visit documentation.
Figure 6 shows how physical therapists in the Cerner system currently document different PT procedures, practices, and exercises administered or taught to the patient in a given PT visit.

Figure 6
Screenshot of Cerner Treatment List Filled Out at Each Treatment Session.
Similarly, Figure 7 shows how physical therapists in the Pittsburgh Epic system currently document different PT procedures, practices, and exercises administered or taught to the patient at a given PT visit.

Figure 7
Screenshot of Present Epic Treatment List Filled Out at Each Treatment Session.
Advantages of Adding Discrete Items Related to PIPT to the EMR
Though PCORI questioned the adequacy of the PIPT checklist in accurately representing the delivery of PIPT throughout the episode of care, the program officers rejected our inclusion of this information in the contract. Adding elements of PIPT to the PT EMR at both UPMC and IHC directly addresses the concern of capturing elements of PIPT treatment throughout the episode of care. Having the PIPT items as discrete elements in the EMR also allows for more accessible data and easier tracking. Finally, Epic and Cerner are extensively deployed EMR systems, which will contribute to the generalizability of the method and enhance dissemination efforts. Both IHC and UPMC implemented these additions in January 2018.
Hypothesized causal pathways and their theoretical basis
In the TARGET trial, the hypothesized causal pathway that would explain the transition of acute LBP to chronic LBP is the presence of psychological obstacles to recovery concomitant to the physical insult that precipitated the back pain incident. Thus, patients were risk stratified based on the presence of symptoms such as excessive catastrophizing, fear avoidance, and reduced activity levels, all believed to be negative contributors to recovery from an acute LBP episode. For patients who present with the physical insult causing the acute LBP and concomitant psychological obstacles to recovery, referral and receipt of PIPT should have a direct and positive impact on recovery, given the theoretical framework of PIPT that focuses on educating patients about their condition; reduces fear of movement; and provides interventions designed to improve physical impairments, mobility deficits, and pain. Our dependent variables in this study were the presence of chronic LBP per the NIH consensus definition.18 Alternative explanations for the transition from acute LBP to chronic LBP include the presence of obesity, symptoms of depression, and other comorbid conditions known to be factors in LBP.
Study Outcomes
The primary outcomes of both the cluster RCT and the observational cohort study were (1) the proportion of patients who transition to chronic LBP at 6 months and (2) back-related physical function at 6 months. Chronicity was determined in an identical manner as in the baseline assessment—that is, through the patients' answers to a simple 2-item questionnaire (the cLBP-Q) adapted from the NIH Task Force on Research Standards for chronic LBP.18 Back-related physical functional outcome was measured with the Oswestry,27 which is a reliable, well-validated patient-centered legacy instrument that assesses the functional impact of back pain on the patient. Items include how back pain affects patients' ability to care for themselves (washing, dressing, etc), lift, walk, sit, stand, and sleep; it also assesses the effect of the pain on the patient's sex life (if applicable), social life, and ability to travel.
The secondary outcomes of the study were LBP-related processes of care and medical use over 12 months from the index visit, as documented in the EMR. Process-of-care measures were defined as referral to PT or PIPT, referral to specialty physicians, orders for diagnostic imaging, orders for opioid prescriptions, and orders for other LBP-related pain medications. Medical use outcomes were defined as outpatient visits (PCPs and specialists), receipt of diagnostic imaging, interventional pain procedures (eg, epidural injections), electrodiagnostic tests (eg, nerve conduction velocity), surgeries, hospitalizations, and emergency department (ED) visits. In our analyses of guideline-concordant care, we defined nonconcordant care as early referral of diagnostic imaging, opioid prescription, referral for specialty care (eg, surgeons), and interventional pain procedures (eg, epidural injections).
Sample Size Calculations and Power
Cluster RCT (High Risk)
The original proposed sample size was 2880 participants, which was based on an attrition rate of 8%, 60 randomized clinics, relatively high variability in sample sizes across clinics (coefficient of variation, 0.4), and 90% power to detect a relative reduction of transition to chronic LBP in 40% of participants. In late 2017, PCORI requested that we consider whether we could reach our primary aim for the cluster RCT based on past and projected enrollment and the higher-than-expected nonresponse at 6 months (∼35%-40%). We revised our quality improvement enrollment sample size to demonstrate that we would have approximately 80% power to detect a 40% reduction in transition to chronic LBP with n = 30 clinics per intervention strategy and fixed n = 10 to 14 patient completers per PCP clinic for the 6-month assessment. We assumed patients in the UC clinics would have a transition rate of 25% to 30%, and the intraclass correlation coefficient (ICC) would be 0.05, an upper bound compared with other cluster RCTs of interventions for either pain or LBP (original sample size assumptions). We aimed for an average of n = 31 enrolled high-risk patients per clinic (60 × 31 = 1860) in the quality improvement phase of the study to account for the high variation in cluster sizes (assuming a coefficient of variation of 0.65) and a 40% nonresponse rate at 6 months.32 Thus, we were able to provide an acceptable power analysis to PCORI in which the major changes were a 40% nonresponse rate (from 8%); reduction of the β from .90 to .80 while maintaining original assumptions about variability in sample sizes across clinics; and effect size.
Observational Cohort (Low and Medium Risk)
We assumed that low- to medium-risk patients would represent about 80% of those screened; therefore, we aimed for an average of n = 115 patients to be enrolled per clinic, for a total sample of N = 6900 low- or medium-risk patients with acute LBP. This sample size would allow precise determination of the 6-month transition rates for low- and medium-risk patients with acute LBP in primary care and testing for subgroup differences—specifically, underserved compared with not underserved (Medicaid as payer as a proxy population), obese vs nonobese, depressed vs nondepressed, and smokers vs nonsmokers. To estimate power for the subgroup comparisons, we assumed that low- and medium-risk patients with acute LBP would have an expected 20% transition to chronic LBP, an ICC of 0.01, and a 60% response rate at 6 months. For the underserved, obesity, and smoking subgroups, the expected sample sizes for the observational cohort would allow us to detect at least a 30% relative difference (6% absolute difference) from 20%, with power ranging from 79% to 87%. We also had 65% power to detect a 40% relative or 8% absolute difference for the depression subgroup. The estimated prevalence percentages for the subgroups of obesity (26%), smoking (20%), depression (7%), and underserved (∼27%) were based on national surveys and previously published rates in primary care.
Randomization, Allocation Concealment, and Masking
In total, 77 eligible primary care clinics were randomized to UC+PIPT or UC using a stratified permuted block randomization, with a block size of 4 and 1:1 allocation ratio. Clinics were the unit of allocation instead of patients because of our concerns about cross-contamination of the interventions within the same clinic. Primary care clinics were considered eligible if they were in a participating health care system and agreed to implement screening procedures. The study biostatistician generated the list in Rx64 version 3.3.2 and was blinded to clinic names. The clinics were stratified by study site, and then sorted by the number of annual back pain encounters and percentage of patients covered by Medicaid or self-pay insurance. Once sorted, the allocation list was applied and sent to the lead coordinator, who added the clinic names, and then sent the lists to their respective site coordinator. The site coordinators were responsible for communicating with the clinics corresponding to their site assignment, the training teams, and the information technology specialists to ensure that the correct training and referrals were available. After randomization, any patient presenting with LBP was eligible for screening. After allocation of the interventions to clinics, the site personnel, clinical staff at the clinics, and the TARGET trial team were not blinded to the intervention groups. Staff conducting the 6-month follow-ups and personnel retrieving the EMR data were masked to the intervention groups.
Time Frame for the Study
The RCT was divided into a quality improvement component, which included (1) the initial cluster randomization and all activities that occurred in the primary care clinics on the initial visit; and (2) the research component, which included the 6-month follow-up assessment. Quality improvement enrollment began in May 2016 and ended on June 30, 2018. The 6-month follow-up ended in February 2019, and 12-month use outcomes were retrieved for data through June 30, 2019.
Data Sourcing and Linkages
Data sources
Data for the study were obtained from 3 major sources: the EMR systems of the local health systems at each study site, the Patient-Centered Outcomes Research Clinical Data Research Network (PCORnet) for UPMC and JHM only, and primary data collection from participants. PCORnet is a collaboration among 9 large clinical research networks and 2 health plan research networks; it consists of EMR and billing data from various health systems that are curated through a rigorous process and structured into a common data model. UPMC and JHM are part of the PaTH PCORnet (https://www.pcori.org/research-results/2015/path-towards-learning-health-system-path). Baseline screening surveys (cLBP-Q, STarT Back Screening Tool, and Oswestry) and secondary outcomes of primary care processes of care were extracted from the local EMRs at each study site except for the baseline screen surveys for nonconsented patients JHM. These baseline data were extracted from the PaTH PCORnet common data elements that included JHM and UPMC data. Twelve-month all-source use data were extracted directly from the EMRs for the BMC, IHC, and JHM systems, while 12-month use data for UPMC were extracted from PCORnet. The primary outcomes of transition to chronic LBP and LBP-related functional disability at 6 months were captured by the University Center for Social and Urban Research (UCSUR) at the University of Pittsburgh for study quality improvement enrollees in the UPMC, BMC, and JHM health systems; the research teams at IHC and MUSC obtained 6-month follow-up data locally.
Data security plan
We developed a comprehensive data security plan to minimize the risk of patient privacy and confidentiality breaches and to prevent improper use or disclosure of patient protected health information (PHI). Data from all the study sites were routed into a centralized repository at the University of Pittsburgh Health Services Research Data Center (HSRDC) via Secure File Transfer Protocol (SFTP). At the HSRDC, an honest broker was solely responsible for segregating, securely storing, and managing identifiable and deidentified data sets. Data were maintained on secure servers in separate, password-protected databases and tables to manage research staff members' rights and access to PHI based on their role within the study. Research staff were required to complete a robust set of research integrity, data security, and HIPAA privacy trainings. Last, research staff were required to use virtual desktops and work queues accessed via virtual private networks (VPNs) to perform their work on secure servers. All final data sets were stored in the HSRDC.
EMR data extraction processes, data linkages, and data transfer
The TARGET trial data integrity team, honest broker at HSRDC, and system analysts at each local health system were involved in the data extraction and transfer processes. The data integrity team coordinated with the systems analysts at each local health system to develop standardized data extraction specifications and procedures necessary to capture study quality improvement enrollees and outcome measures. The data integrity team provided each site with the data dictionaries, file structure, and the Structured Query Language (SQL) program extraction codes. Data from the local EMRs were then securely transferred to the HSRDC. Sites were asked to extract enrollment data and to transfer the data weekly. These files were received by the honest broker at the HSRDC, who segregated the files into identifiable and deidentifiable data sets. The honest broker removed patient PHI, provided a deidentifiable TARGET study ID for each patient, and then made these deidentifiable files available to the study data integrity team. The honest broker was responsible for maintaining a separate crosswalk file that linked the patient's identifiable information with their TARGET study ID. The data in the PCORnet common data model are deidentified (limited data set with dates) to allow for sharing across the clinical research networks. For these data, the honest broker was responsible for maintaining a separate crosswalk file that linked the patient's PCORnet deidentified ID with their TARGET study ID. The study data integrity team coordinated with the data personnel responsible for maintaining the PCORnet common data model at UPMC and JHM to securely transfer these files into the HSRDC. These linkages between the patient's identifiable information and their study ID were necessary to facilitate the 6-month follow-up and 12-month EMR data collection processes and to track deaths. The deidentifiable data sets underwent rigorous quality assurance processes and restructuring to allow for data analysis. The final restructured analytic data sets were then made available to the study statistician team.
Primary (6-month) outcomes data collection
Six-month data collection processes were standardized across the 5 sites and collected by 1 of 3 methods: (1) trained interviewers using a computer-assisted telephone interview version of the questionnaires, (2) web-based responses sent via an email link, and (3) return-mail responses to questionnaires mailed at 6 months to the participant's home address. We developed an algorithm based on the contact information available for the patients starting with email, and then telephone, with simultaneous hard copy. We recorded the final contact modality so that we could monitor the method of follow-up among responders. The algorithm was the same for patients from intervention and control clinics. The final modality of contact was documented to allow for regular monitoring of responses by method of contact. Using similar data set structures and specifications, the system analysts at UCSUR, IHC, and MUSC then transferred 6-month survey data files to the HSRDC (via SFTP), where they were then deidentified and made available to the study data integrity team.
In an effort to improve our 6-month follow-up response rate, the following changes were made to our initial approach: (1) Reminder letters were mailed to quality improvement patients at 1, 3, and 5 months after baseline assessment; and (2) the 5-month letter included a self-addressed, stamped envelope whereby the patient was given the option of completing the assessments as a self-report and mailing it back.
Analytical and Statistical Approaches
We specified all plans for data analysis a priori in the PCORI-approved revised research plan (approved June 20, 2018).
Cluster RCT (High Risk)
We compared distributions of baseline characteristics for clinics and patients between the UC+PIPT and UC groups to assess the effectiveness of the randomization. All analyses for treatment group comparisons used an intention-to-treat approach. We estimated the rates (and corresponding 95% CIs) of transition to chronic LBP at 6 months for high-risk patients in each intervention group using a generalized linear mixed model (GLMM), with a logit link with fixed effects for the intervention group and site accounting for the cluster RCT design with a random clinic effect. From these models, we estimated the ICC to assess our assumptions for sample size analyses and for future investigations of LBP in primary care settings. We estimated the average back-related functional status on the Oswestry at 6 months (and corresponding 95% CIs) stratified by intervention group using GLMMs, controlling for baseline Oswestry and study site, with random clinic effects to account for clustering.
Secondary outcomes were processes of care and medical use outcomes up to 12 months after the index visit. We estimated the proportions of high-risk patients for each of these outcomes within intervention group and their 95% CIs, adjusting for clustering at the clinic level using Taylor series linearization for variance estimation (design based). We had originally planned to use GLMMs for these secondary outcomes, but many of the outcomes were very rare, causing convergence problems with the models.
Prospective Observational Cohort (Low and Medium Risk)
We estimated the proportion (and corresponding 95% CIs) of patients who transitioned to chronic LBP stratified by low and medium risk at baseline using GLMMs with a logit link, adjusting for clustering with random clinic effects. We used GLMMs to estimate mean functional disability on the Oswestry stratified by low and medium risk.
Secondary outcomes were processes of care and medical use outcomes up to 12 months after the index visit. As with the high-risk group, we estimated the proportions of low- and medium-risk patients for each of these outcomes and their 95% CIs, adjusting for clinics by using Taylor series linearization for variance estimation.
The GLMMs with a logit link were used to assess baseline factors associated with the transition to chronic LBP at 6 months and guideline-nonconcordant medical use over 12 months; the latter is defined as any imaging or professional encounters for orthopedics, neurology, physical medicine and rehabilitation, pain management, or anesthesiology. For the modeling, we first looked at factors in certain domains (demographics, LBP-related factors, processes of care at the index visit) that were associated with each outcome at P < .15, and then combined those factors into a final model and retained variables that were significant at P < .15. Our goal was to potentially identify subpopulations at risk for worse outcomes in the presence of the multitude of other factors that could be involved.
Subpopulations
Subpopulations of interest were defined by smoking, obesity, health insurance (eg, Medicaid vs self-pay insurance), and a diagnosis of depression or anxiety. The rationale for these subpopulations was included in the original research proposal—namely, that the presence of these characteristics has been linked to chronic LBP. In addition, we added risk stratification to the list of subpopulations of interest. We conducted stratified analyses for subpopulations of interest for the trial in the high-risk patients, with the exception of depression/anxiety, as the numbers were too small. We focused on the estimation of chronic LBP at 6 months within intervention groups stratified by subpopulation. We did not formally test for heterogeneity of treatment effects (HTE). For the low- and medium-risk cohorts, we determined the rates of transition to chronic LBP at 6 months for each subpopulation level (complete-case analysis).
Missing Data
We monitored response rates monthly throughout the study by site and used multiple modalities to encourage patients to complete surveys to optimize the response rates. For the primary outcomes at 6 months, we documented the final disposition as consented/completed, refused, unable to contact, opted out, and using complete-case analysis. All secondary outcomes were obtained from the EMR and had no missing data except for use outside the health care systems, which we do not have a method to measure. For all 3 risk groups (low, medium, and high), we compared baseline characteristics, such as age, sex, health insurance status, and processes of care at the index visit, between patients with and without 6-month follow-up data to assess potential biases that may exist in the complete-case analysis. In these comparisons, we adjusted for clustering at the clinic level using Taylor series linearization for variance estimation.
Changes to the Original Study Protocol
The following changes were made to the study research plan with final approval by PCORI on June 20, 2018.
Stakeholder-Engaged Changes in the Protocol
During the preapproval phase of the study, we had considerable discussion with stakeholder representatives about the pragmatic nature of the trial. We designed the trial per the Spring 2014 funding cycle PFA, “Pragmatic clinical studies and large simple trials to evaluate patient-centered outcomes.” Table 2 illustrates items from the PFA and how the TARGET trial investigative team worked with relevant stakeholders to develop the final proposal as submitted.
Table 2
Development of Final Proposal: PFA Compared With TARGET Trial Accomplishments.
PCORI Program Officer-Initiated Changes to the Protocol
In November 2017, the study team was notified by PCORI program officers that changes to the protocol should be considered. At this time, enrollment was still ongoing at all sites and continued through June/July 2018. There were program officer-initiated changes to the protocol, some of which were not mutually agreed upon. In the cases of changes in aims and statistical analyses, the changes were mandated (eg, continued funding was predicated on making the changes) by PCORI program officers. In the case of the changed aims and statistical analyses, the TARGET trial investigative team's compromise was to include PCORI changes (aims, statistical analyses) in the contract language (and hence, the DFRR) but to preserve the right to revert to original aims and statistical analyses in peer-reviewed submissions. Final PCORI acceptance of the protocol changes occurred shortly after enrollment ended in approximately June/July 2018. We continued all follow-up assessments through February 2019 for the primary outcomes at 6 months. The changes mandated by PCORI program officers are summarized below, including those mandated and those with mutual agreement:
- All prior references to usual care were changed to guideline-based care, and all prior references to physical therapy coupled with cognitive behavioral coaching were replaced by psychologically informed physical therapy. Therefore, our intervention groups for the trial were referred to throughout the original report as guideline-based care and guideline-based care plus psychologically informed physical therapy. Our original intent was to use the term usual care to describe our comparator group. Before proposal submission, we were provided data from a variety of sources that demonstrated significant unwarranted variability and guideline-based inconsistency in all of our primary care centers. Therefore, in our approach, usual care would capture care resulting from variability in care delivery. PCORI representatives indicated that the term usual care “was not sufficiently descriptive and did not really capture what was happening,” and thus were opposed to using this term to characterize the comparator group of the trial. Thus, we compromised on guideline-based care as a descriptor. In retrospect and based on our data analyses of process-of-care measures in PCP environments, the term guideline-based care remains a misnomer for this group in that PCP behavior fell far short of LBP guidelines, which was fully predictable.33 For this reason, we fully expected the term guideline-based care to be rejected as an accurate descriptor by peer reviewers in submitted manuscripts and may well opt back to the term usual care. After peer review of the DFRR, the overwhelming consensus of the peer reviewers was that usual care was a better descriptor than was guideline-based care, and in the final research report, we will refer to the groups compared as UC vs UC+PIPT.
- The 6-month assessments were originally proposed to be administered by primary care office personnel, but before the start of the study, we changed to centralized follow-up administered by UCSUR at the University of Pittsburgh for the Pittsburgh, Boston, and Baltimore sites. IHC and MUSC obtained their own PRO data. The decision to have IHC and MUSC obtain their own outcome data was pragmatic and the result of extensive discussion among the site principal investigators, advisory board, and PCORI personnel; it was ultimately based on a combination of existing structures and the culture in the Salt Lake City and Charleston areas that would ultimately allow for more successful follow-up with less attrition. Our original intent was to use primary care sites largely because we thought patients were more likely to be responsive to correspondence originating from a familiar source (eg, their primary care office) as opposed to a previously unknown research group that was not readily identified with a representative of their health care delivery team. We were not able to convince PCORI representatives of the merits of our strategy of a local approach vs a centralized one, largely because of the fear that primary care environments, strapped for time, would not have the capacity to perform the follow-ups on a consistent basis, which we also agreed was a legitimate concern. Alternatively, we proposed transferring the task of obtaining 6-month outcome data to UCSUR, thus moving the task of obtaining 6-month outcome data from the primary care environment to a research-based environment, which satisfied PCORI representatives. Moving from a local to a centralized process was a PCORI-mandated change.
- The original research aims and analysis plans were modified to remove wording about direct comparisons between intervention groups. The decision not to use inferential statistics to analyze TARGET trial data was mandated by program officers at PCORI because of concerns about the nonresponse rates at 6 months and PIPT treatment integrity. The study team did not agree with PCORI representatives about this point. Program officers claimed that the use of inferential statistics was inappropriate, mainly because of inadequate follow-up and lack of evidence related to treatment integrity. First, our trial had internal validity with respect to randomization, minimal contamination because of allocation at the cluster level, and blinded assessments of the 6-month outcomes. We also conducted analyses comparing patients with and without a 6-month follow-up, which show similar baseline characteristics. Most importantly, we had very few exclusion criteria for this study. Although the attrition rate was high, we have large numbers of patients with 6-month data who are representative of high-risk patients because their measurable baseline characteristics do not differ from those with missing data in a clinically meaningful way. We believe the likelihood of extreme cases of nonignorable missingness (ie, that all missing data in the control group are nonchronic and all missing data in the intervention group are chronic [or vice versa]) is 0. These possibilities could have been explored with missing data analysis and sensitivity analyses to allow the reader to judge the results and inference. In terms of treatment integrity, we delivered the interventions exactly as described in our proposal with minimal changes and were well into recruitment when these concerns were raised. The TARGET trial investigative team stands by the pragmatic nature of the treatment delivery as being consistent with the original PFA, and the intervention was delivered in a way that was consistent with the original and revised proposals. Having presented the case for inferential statistics with the above-described justification (including an external consultant) to PCORI program officers, our approach was denied by program officers, and descriptive analyses were mandated with the specific instructions of not including any inferential statistical analyses. Thus, we have presented the results without direct comparisons, as required.
- We removed sensitivity analysis using missing data methods as well as analyses of proposed testing of HTE, per PCORI's mandate.
- We ceased obtaining the 12-month PROs on July 1, 2018, because of the limitations of only conducting these among patients who responded at 6 months as well as the low response rate. The decision to eliminate the 12-month PROs was mandated by PCORI and eventually agreed upon by the investigators. Though peer-reviewed journals traditionally desire 12-month outcomes in RCTs and cohort studies involving chronic musculoskeletal management, we agreed that for the purposes of the TARGET trial aims, 6-month outcomes were adequate.
- PCORI representatives and the study investigative team mutually agreed that the target sample size for the randomized trial cohort would be decreased to 1860 high-risk patients enrolled in the cluster RCT at baseline, and the sample size for the observational cohort was decreased to 6900 low- to medium-risk patients enrolled at baseline. This sample size analysis was conducted assuming the original n = 60 clusters (primary care clinics). The original proposed sample sizes were 2880 and 9780. The nonresponse at 6 months was higher than the original sample size analysis assumptions (44% vs 8%, respectively). In hindsight, the original 8% was not a realistic assumption given that these patients were not enrolled in an efficacy trial, in which these attrition rates are normally seen in studies of PT. Rather, these patients were enrolled in a quality improvement project upon presenting to the primary care clinic for care. Unfortunately, the patient-centered primary outcomes were not stored in the medical record but rather had to be retrieved using a survey methodology approach (phone, email, mail), resulting in higher nonresponse rates.
- Qualitative assessments with patients and providers were removed from the protocol after discussions with PCORI and the TARGET trial advisory board, leading to a decision to reallocate funds designated for qualitative assessments to participant payment for completing the 6-month assessments. Through stakeholder engagement with our advisory board as well as consultation with PCORI representatives, all were in agreement that transference of resources from the qualitative aims to remuneration for patients at follow-up was highly desirable. The study team would emphasize that this strategic redistribution of TARGET trial funds was driven by our patient co-investigators and patient stakeholders.
- The number of participating clinics was higher (76) than originally proposed (60), and PCORI approved this change to ensure sufficient recruitment.
- Recruitment at 1 site (MUSC) was terminated early, and the data collected from this site were not included in analyses. Reasons for not including MUSC were as follows. The site was the last one to begin recruitment and thus had to be up to speed without much room for delay. Implementation of the most basic components of the trial encountered logistical barriers that the TARGET trial investigative team believed could not be overcome in the original time frame we had proposed. These failures occurred despite weekly interaction with the site team and the TARGET trial investigative team in Pittsburgh and a comprehensive site visit in Charleston. Simultaneously, PCORI officials were interested in shortening the time frame of TARGET from the original 5-year time frame to 4.5 years. Another factor in favor of eliminating MUSC was the fact that in considering the 4 remaining sites, we had adequate primary care sites from an internal validity (eg, power) perspective to answer the question we had proposed. These considerations and the TARGET trial investigative team's doubts that the site could overcome the barriers to implementation led to the decision to abandon the site. Though a small sample of data were made available from South Carolina, when the data were assessed, it was not possible to ensure their completeness and accuracy; thus, we decided not to include even what little data had been collected from the site.
- Claims data were not used or analyzed to determine medical resource use; EMR data were used instead. The overriding desire from PCORI representatives to reduce the time commitment of the TARGET trial from 5 years to 4.5 years precluded any attempt to analyze the limited claims data (eg, UPMC Health Plan and Select Health) as originally proposed. This decision was mutually agreed upon by PCORI and the TARGET trial study team.
Results
Enrollment and Flow of Participants
Patients were screened and enrolled from 76 clinics across 4 of the 5 intended sites. Screening, enrollment, and implementation of the intervention were not successful at MUSC because of multiple technical and logistical problems.
Of the nearly 10 000 patients with acute LBP who were screened, 36% were classified as low risk, 40% as medium risk, and 24% as high risk for developing chronic LBP. For the high-risk group, 55% had 6-month outcomes; in these participants, we were able to capture 98% of 12-month use data, which did not differ by intervention group (Figure 8). Study enrollment occurred for 25 months, from May 2016 to June 2018 (Figure 9). Information for the low- and medium-risk patients can be found in Figure 10 in the section for the observational cohort.
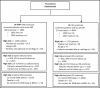
Figure 8
Flow of Participants in the Study.
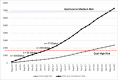
Figure 9
Cumulative Enrollment of Clinics and Patients (Stratified by Risk of Developing Chronic LBP), by Month.

Figure 10
Flow of Participants in the Observational Cohort.
Results of the Cluster RCT for High-Risk Patients
Baseline Characteristics of Clinics and Patients
The volume of LBP in the past 12 months and percentage of patients who had Medicaid or self-pay insurance were similar across clinics randomized to UC+PIPT and UC alone (Table 3). One clinic in the UC+PIPT group had missing information on volume and percentage of Medicaid and self-pay insurance and thus is not included in Table 3.
Table 3
Clinic Characteristics by Intervention Group.
The baseline characteristics of the patients were similar between intervention groups (Table 4). The average age was 50 years; 59% were female; among those with health insurance information, 49% had private insurance; 75% were White; and 50% were obese.
Table 4
High-Risk Patients: Baseline Patient Characteristics by Intervention Group.
The average back-related functional disability indicated severe disability as measured by the Oswestry (http://www.rehab.msu.edu/_files/_docs/Oswestry_Low_Back_Disability.pdf). A majority of patients said their LBP had interfered with their ability to perform regular daily activities for less than 1 month, and about 1 in 10 reported that it was more than 3 months. All reported fewer than half the days with respect to frequency. Those who reported more than half the days were considered chronic and were excluded due to our focus on acute LBP. The average STarT Back Screening Tool total score and subscores used to identify the high-risk group were similar between the 2 intervention groups (Table 5).
Table 5
High-Risk Patients: Baseline Patient Back Pain and Function Characteristics by Intervention Group.
Process of Care (0-21 Days) at the Index Visit
Nearly 4 out of 10 participants in the UC+PIPT group received a referral to the stratified approach to care (PIPT), and 2 out of 10 received a referral to PT (Table 6). About 30% received a referral to PT in the UC group. Very few patients were referred to a specialist in either group, with the highest percentage being referred to pain management (4.6%) or spine surgery (5.2%-6.9%). Pain management includes physical medicine and rehabilitation, anesthesiology, pain clinic, sports medicine, pain treatment, or epidural steroid injection. About 1 in 5 participants received a referral for plain radiographs, and only 7% to 8% received a referral for magnetic resonance imaging (MRI)/computed tomography (CT). With respect to medication, 25% of participants received a prescription for opioids, around 38% to 45% received a prescription for muscle relaxants, and 30% received a prescription for NSAIDs. Other medication prescriptions were rare (acetaminophen, benzodiazepines, topical NSAIDs).
Table 6
Processes of Care at the Index Visit Up to 21 Days.
Primary Outcomes at 6-Month Follow-up (Primary Aim 1 and Primary Aim 2)
About half of patients in both groups reported a transition to chronic LBP at 6 months. Just over 70% stated that LBP had interfered with their ability to perform regular daily activities for more than 3 months, and among them, about 70% responded that the frequency of LBP interference was half the days or more than half the days. Both groups were lower on functional disability, showing scores indicative of moderate disability rather than severe disability, which was the average at baseline (Table 7).
Table 7
Primary Outcomes for TARGET Trial High-Risk Patients.
Comparison of Participants With 6-Month Follow-up vs Participants Without 6-Month Follow-up
The 6-month follow-up nonresponse rate was higher (44%) than the originally planned attrition (8%). To determine whether there were any potential biases in the estimates from those who responded, we investigated whether there were any differences between those with follow-up data and those without. We investigated differences in demographics and baseline clinical characteristics as well as differences in processes of care at the index visit. With respect to demographics, those with follow-up were 2 years younger and were more likely than the nonresponse group to be women (61% vs 56%, respectively), have private health insurance (50% vs 44% among those with information), be obese (51% vs 46%), and be White (77% vs 71%). No other differences were detected for baseline demographics (Table 8a).
Table 8a
Baseline Patient Characteristics by 6-Month Follow-up for TARGET Trial High-Risk Patients.
For the baseline back pain characteristics, there were no differences detected on the Oswestry between those with and without 6-month follow-up data. Those with follow-up data were slightly less likely to report back pain duration of less than 1 month than were those without follow-up data (58% vs 64%, respectively; Table 8b). The average STarT Back Screening Tool scores and subscores were identical between the groups.
Table 8b
Baseline Back Pain Characteristics by 6-Month Follow-up for TARGET Trial High-Risk Patients.
Participants who responded to the 6-month follow-up were less likely to have had MRI/CT and more likely to have received a prescription for muscle relaxants than were those who did not respond (Table 8c). No other differences were detected between the 2 groups with respect to processes of care at the index visit.
Table 8c
Processes of Care at the Index Visit by 6-Month Follow-up for TARGET Trial High-Risk Patients.
Process of Care: 3 Weeks to 12 Months (Secondary Aim 1)
From 3 weeks to 12 months after the index visit, fewer than 10% of patients received referral to PIPT or PT (Table 9). Very few patients received referral to any specialists except pain management (2%-3%) and spine surgery (3%-4%). Roughly 7% to 8% of patients received an opioid prescription, 6% to 8% received a prescription for muscle relaxants, and 5% to 8% received a prescription for NSAIDs.
Table 9
Processes of Care From 3 Weeks to 12 Months for TARGET Trial High-Risk Patients.
Use From 3 Weeks to 12 Months After the Index Visit (Secondary Aim 1)
After the index visit, about half of participants in both groups saw a PCP for their LBP, 15% to 18% saw a specialist, and 17% to 19% underwent imaging (Table 10). Roughly 2 in 100 underwent surgery, and fewer than 1% were hospitalized for LBP. Between 5% and 8% had an ED visit for back pain.
Table 10
Use Outcomes From 3 Weeks to 12 Months for TARGET Trial High-Risk Patients.
Results of the Observational Cohort for Low- and Medium-Risk Patients
Clinical Characteristics
Patients from 76 clinics are included in this analysis for the cohort study (Table 11). These clinics had a median volume intake of nearly 500 patients with LBP in the 12 months before the study, and just under 10% of their patient population had Medicaid or were considered self-pay insured.
Table 11
Clinic Characteristics for Low- and Medium-Risk Patients.
Flow Diagram for the Observational Cohort
Of all patients with acute LBP who were screened, 36% were deemed low risk to transition to chronic LBP, and 40% were deemed medium risk on the STarT Back Screening Tool (Figure 10). About 52% of low-risk and 57% of medium-risk patients had a 6-month follow-up. A total of 48 patients died during the 12-month follow-up (21 low risk, 27 medium risk) but were still included in the analysis because they had some follow-up data after baseline.
Baseline Demographics for the Observational Cohort
Patients presenting with acute LBP and stratified as low and medium risk were about 51 years of age, just over half were women, a majority had private health insurance, and 80% were White. The average body mass index (BMI) was 30, with 41% and 47% classified as obese for low- and medium-risk patients, respectively (Table 12).
Table 12
Baseline Patient Characteristics for Low- and Medium-Risk Patients.
Baseline LBP Characteristics for the Observational Cohort
The low-risk group had minimal disability on the Oswestry, whereas the medium-risk group had moderate disability. Just over 60% of patients said their LBP had interfered with their ability to perform regular activities for less than 1 month. By definition, the STarT Back Screening Tool scores were low for the low-risk group and midrange for the medium-risk group on both the totals and subscores (Table 13).
Table 13
Baseline Patient Back Pain and Function Characteristics for Low- and Medium-Risk Patients.
Process of Care (0-21 Days) at the Index Visit for the Observational Cohort
Referral to PT was relatively low for both groups, at only 26% in the low-risk group and 33% in the medium-risk group (Table 14). Very few participants were referred to specialists. Approximately 18% to 21% were referred for x-ray and 2% to 5% for MRI/CT. One in 5 medium-risk participants were prescribed opioids, which is double the percentage prescribed opioids in the low-risk group. Between 25% and 29% of participants in the low-risk group were prescribed NSAIDs and muscle relaxants, respectively. A much higher percentage of participants in the medium-risk group received a prescription for muscle relaxants (43%).
Table 14
Processes of Care at the Index Visit for TARGET Trial Low- and Medium-Risk Patients.
Primary Outcome at 6 Months Among Low- and Medium-Risk Patients (Secondary Aim 2)
One in 5 low-risk patients reported transitioning to chronic LBP at 6 months compared with about a third in the medium-risk group (33%). About 50% of the low-risk group stated that their LBP interfered with regular daily activities for more than 3 months and 37% for at least half the days. These percentages were higher in the medium-risk group, at 61% and 54% for those groups, respectively. The low-risk group reported minimal disability on average, and the medium-risk group reported moderate disability, but both groups had lower average disability than that at baseline at the index visit (Table 15).
Table 15
Primary Outcome at 6 Months for TARGET Trial Low- and Medium-Risk Patients.
Comparison of Participants With 6-Month Follow-up vs Participants Without 6-Month Follow-up Among Those at Low and Medium Risk
Because of the high percentage of participants without 6-month follow-up, we ran additional analyses to compare those with follow-up vs those without within each risk group. Briefly, we detected no differences in baseline demographics (Table 16a), baseline back pain (Table 16b), and processes of care at the index visit (Table 16c), with the exception of race, where participants with 6-month follow-up were slightly more likely to be White.
Table 16a
Baseline Patient Characteristics by 6-Month Follow-up for TARGET Trial Low- and Medium-Risk Patients.
Table 16b
Baseline Back Pain by 6-Month Follow-up for TARGET Trial Low- and Medium-Risk Patients.
Table 16c
Baseline Processes of Care by 6-Month Follow-up for TARGET Trial Low- and Medium-Risk Patients.
Process of Care: 3 Weeks to 12 Months for the Observational Cohort (Secondary Aim 2)
Very few observational cohort patients received a referral to PT up to 12 months after the index visit (Table 17). Fewer than 1% received a referral to a specialist, with the exception of pain management (1%-2%; includes physical medicine and rehabilitation, anesthesiology, pain clinic, sports medicine, pain treatment, or epidural steroid injection) and spine surgery (2%-3%). About 4% of low-risk and 6% of medium-risk patients received a prescription for opioids. Similar rates were observed for muscle relaxants and NSAIDs.
Table 17
Processes of Care Up to 12 Months for TARGET Trial Low- and Medium-Risk Patients: Observational Cohort.
Use (12 Months) for the Observational Cohort (Secondary Aim 2)
After the index visit, 30% of low-risk patients and 40% of medium-risk patients saw a PCP for their LBP, and much fewer saw a specialist (8% and 12%, respectively). About half as many low-risk patients underwent imaging and interventional procedures than did medium-risk patients (imaging, 9% vs 16%; interventional procedures, 1.4% vs 3%). Very few underwent surgery or were hospitalized for LBP (<2%), but a higher number (2%-4%) visited the ED for LBP (Table 18).
Table 18
Use Outcomes Up to 12 Months for TARGET Trial Low- and Medium-Risk Patients: Observational Cohort.
Factors Associated With Transition to Chronic LBP at 6 Months Among Patients Classified as Low Risk (Secondary Aim 2)
Patients with Medicaid had a higher rate of transition to chronic LBP at 6 months, as did patients with depression or anxiety diagnoses. Surprisingly, those who were obese or were current smokers were less likely to transition to chronic LBP (Table 19a).
Table 19a
Baseline Patient and Clinic Characteristics Associated With Transition to Chronic LBP at 6 Months for Low-Risk Patients.
Patients who reported chronic LBP at 6 months had higher baseline functional disability and STarT Back Screening Tool scores. Patients with back pain and leg symptoms were more likely to report chronic LBP at 6 months, as were those who reported interference for greater than 3 months (Table 19b). There was significant variability in the transition to chronic LBP across sites.
Table 19b
Baseline Back Pain Characteristics Associated With Transition to Chronic LBP at 6 Months for Low-Risk Patients.
Low-risk patients who were referred to pain management were more likely to transition to chronic LBP than were those who were not referred (40% vs 18%, respectively). Similar trends were seen for those with referral to spine surgery and MRI/CT and prescription for acetaminophen (Table 19c).
Table 19c
Baseline Processes of Care Associated With Transition to Chronic LBP at 6 Months for Low-Risk Patients.
Final Model for Transition to Chronic LBP at 6 Months for Low-Risk Patients (Secondary Aim 2)
In a final model, several patient-level and process-of-care measures were associated with chronic LBP at 6 months (Table 20). Patients with Medicaid, smokers, patients referred to pain management or MRI/CT, and those prescribed acetaminophen were much more likely to transition to chronic LBP, controlling for other factors.
Table 20
Final Model for Transition to Chronic LBP at 6 Months for Low-Risk Patients.
Factors Associated With Guideline-Nonconcordant Medical Use Over 12 Months in Patients Classified as Low Risk (Secondary Aim 2)
Older patients were more likely to have guideline-nonconcordant medical use, defined as any imaging or professional encounters for orthopedics, neurology, physical medicine and rehabilitation, pain management, or anesthesiology. Health insurance was associated with use, whereby those with Medicare had the highest rate (Table 21a).
Table 21a
Baseline Patient Characteristics Associated With Guideline-Nonconcordant Medical Use for Low-Risk Patients.
Low-risk patients with worse disability and higher STarT Back Screening Tool scores were more likely to have guideline-nonconcordant medical use over 12 months after their visit for acute LBP. Outcome rates ranged from 5% to 15% across sites (Table 21b).
Table 21b
Baseline Back Pain Characteristics Associated With Guideline-Nonconcordant Medical Use for Low-Risk Patients.
Few processes of care at the baseline visit were associated with guideline-nonconcordant medical use over 12 months (Table 21c). Referrals for imaging were most highly associated with future use, even with the relatively small numbers of patients who were referred for x-ray or MRI/CT. In total, 15% of those referred for x-ray had guideline-nonconcordant medical use over 12 months compared with 9% of those without x-ray referral. Nearly 1 in 4 of those referred for MRI/CT had guideline-nonconcordant medical use over 12 months compared with only 10% among those not referred for MRI/CT. Opioid prescription was associated with a higher percentage of use, as well.
Table 21c
Baseline Processes of Care Associated With Guideline-Nonconcordant Medical Use Over 12 Months for Low-Risk Patients.
Final Model for Guideline-Nonconcordant Medical Use at 12 Months for Low-Risk Patients (Secondary Aim 2)
The final factors most strongly associated with guideline-nonconcordant medical use over 12 months were older age, site, higher STarT Back Screening Tool score, referral to spine surgery, order for x-ray or MRI/CT, and opioid prescription (Table 22). Patients prescribed muscle relaxants at the index visit were less likely to have guideline-nonconcordant medical use over 12 months than were those not prescribed muscle relaxants, controlling for age, site, baseline disability, and other processes of care.
Table 22
Final Model for Guideline-Nonconcordant Medical Use Over 12 Months for Low-Risk Patients.
Factors Associated With Transition to Chronic LBP at 6 Months for Patients Classified as Medium Risk (Secondary Aim 2)
Medium-risk patients who transitioned to chronic LBP were older. Patients with Medicare and Medicaid were more likely to transition to chronic LBP than were patients with self-pay insurance, as were those who had a diagnosis of depression or anxiety, were obese, were current smokers, and were Black (Table 23a).
Table 23a
Baseline Patient Characteristics Associated With Transition to Chronic LBP at 6 Months for Medium-Risk Patients.
Patients with back and leg symptoms were more likely to transition to chronic LBP, as were those who at the index visit reported longer duration of interference with activities. Patients who transitioned to chronic LBP at 6 months had higher STarT Back Screening Tool scores at the index visit (Table 23b).
Table 23b
Baseline Back Pain Characteristics Associated With Transition to Chronic LBP at 6 Months for Medium-Risk Patients.
For processes of care, patients referred to pain management, spine surgery, and MRI/CT had higher rates of transition to chronic LBP than did those without referrals. Patients with prescriptions for opioids and muscle relaxants had higher rates of transition than did those without those prescriptions (Table 23c).
Table 23c
Baseline Processes of Care Associated With Transition to Chronic LBP at 6 Months for Medium-Risk Patients.
Final Model for Transition to Chronic LBP at 6 Months for Medium-Risk Patients (Secondary Aim 2)
The final model for assessing factors associated with transition to chronic LBP among medium-risk patients included many patient-level and process-of-care measures (Table 24). Participants with Medicare and Medicaid, who were obese, who were smokers, and who had worse STarT Back Screening Tool scores had higher rates of chronic LBP. Process of care, including referral to pain management, spine surgery, MRI/CT, and prescriptions for opioids and muscle relaxants, remained significant, controlling for patient-level characteristics and back pain measures.
Table 24
Final Model for Transition to Chronic LBP at 6 Months for Medium-Risk Patients.
Factors Associated With Guideline-Nonconcordant Medical Use Over 12 Months Among Patients Classified as Medium Risk (Secondary Aim 2)
Patient characteristics associated with guideline-nonconcordant medical use included age, sex, health insurance, race, and ethnicity (Table 25a). Patients of older age were more likely to receive guideline-nonconcordant medical use, as were men, patients covered by Medicare, White patients, and non-Hispanic patients.
Table 25a
Baseline Patient Characteristics and Guideline-Nonconcordant Medical Use Over 12 Months for Medium-Risk Patients.
Patients with worse disability, back pain, and leg symptoms; those with interference for 1 to 3 months; and those with higher STarT Back Screening Tool scores were more likely to have guideline-nonconcordant medical use over 12 months (Table 25b). There was significant variability across sites.
Table 25b
Baseline Back Pain Characteristics and Guideline-Nonconcordant Medical Use Over 12 Months for Medium-Risk Patients.
Patients who were referred to PIPT, pain management, spine surgery, or imaging had higher rates of guideline-nonconcordant medical use over 12 months (Table 25c). Patients prescribed opioids and benzodiazepines had higher rates of guideline-nonconcordant medical use, whereas those prescribed muscle relaxants had lower rates.
Table 25c
Baseline Processes of Care and Guideline-Nonconcordant Medical Use Over 12 Months for Medium-Risk Patients.
Final Model for Guideline-Nonconcordant Medical Use at 12 Months for Medium-Risk Patients (Secondary Aim 2)
Among medium-risk patients, many patient-level characteristics and processes of care were associated with the receipt of guideline-nonconcordant medical use over the 12 months after the index visit. Factors most strongly related included site, type of back pain (axial vs back and leg symptoms), and referral to either spine surgery or pain management (Table 26).
Table 26
Final Model for Guideline-Nonconcordant Use at 12 Months for Medium-Risk Patients.
Discussion
We were able to cluster randomize 77 primary care clinics across 4 sites, ultimately losing 1 primary care clinic and leaving 76 contributing clinics remaining. A total of 9730 patients were identified as having acute LBP; 24% were deemed high risk, 40% medium risk, and 36% low risk, thus making the TARGET trial one of the largest studies ever conducted on acute LBP in primary care environments. The percentages of people in each of these categories were similar to those obtained using the STarT Back Screening Tool in both European14,15 and North American populations.16
Among high-risk patients in the UC+PIPT group (n = 1207), 55% (95% CI, 48%-61%) were referred to PT or PIPT, compared with 31% of patients in the UC-only group (n = 1093; 95% CI, 25%-36%). Thus, in the UC+PIPT group, nearly twice as many patients were given referrals to a nonpharmacological provider in their initial encounter as in the UC group, which is a step toward more guideline-concordant care. Still, 40% of patients not being referred to PT in the UC+PIPT group represents a nonadherence level on the part of PCPs that was not expected. Low adherence to the PIPT best-practice alert represents a lack of follow-through on almost half of the patients in the experimental group (UC+PIPT), which was far below the expectations of the study team and the primary care stakeholders. Our implementation plan sought to facilitate the referral and receipt of the intervention in the UC+PIPT group via a 2-fold process: (1) triggering a best-practice alert in the EMR for the medical assistants and/or PCPs for all high-risk patients, with specific instructions for immediate referral to PIPT; and (2) once the referrals were executed and processed by the PCP, establishing mechanisms to expedite notification with the PT clinics. PIPT orders were not completed unless the PCP acted on the best-practice alert, a situation that failed to happen in almost half of the high-risk patients. The inability to implement the best-practice alert-PIPT referral linkage was a major limitation of the TARGET trial and demonstrates the implementation challenges in present primary care environments.
Our approach is novel and included the use of the definition of chronic LBP, defined as back pain that has persisted at least 3 months and has resulted in pain on at least half the days in the past 6 months, advanced by the NIH Task Force on Research Standards for Chronic Low Back Pain. To our knowledge, we are the first to use this definition in a study of this size. Importantly, this definition of chronic LBP allowed us to eliminate those patients with existing chronic LBP from admission and follow the cohort to precisely measure the transition to chronic LBP using the NIH definition. We supplemented our primary aim with another aim related to function using the Oswestry Disability Index. Again, we found no difference in functional outcomes at 6 months between the groups. Interestingly, the 6-month outcome of the functional outcome (measured by the Oswestry) averaged 27% and 30% in the UC+PIPT and UC groups, respectively, indicating that patients were still experiencing significant self-reported functional limitations.
At our inaugural TARGET trial annual advisory board meeting in September 2015, we reported the low rates of referral to PIPT in response to the best-practice alert to primary care stakeholders in the study practices. In response, we spurred significant discussion expressing the magnitude of the task of implementation in primary care. For example, when told that almost half the time the best-practice alert was ignored, a common response was, “Actually, 50% adherence to a best-practice alert is pretty good considering all the best-practice alerts that we face on a daily basis.” Surprisingly, there is evidence that this statement has veracity, which has been attributed to “alert fatigue,” whereby clinicians become less likely to accept alerts as they receive more of them.34 However, the overwhelming response from the primary care stakeholders for the failure to implement best practices was related to time constraints and the overburdening amount of work associated with the EMR. When we discussed potential solutions with primary care stakeholders, one idea to improve best-practice alert execution was the “opt-out” approach (ie, the best-practice alert is executed unless the PCP specifically declines it). The opt-out strategy suggested to our primary care stakeholders at the initiation of the study was not acceptable, largely because they were uncomfortable with the chance of having an order potentially go out under their name without their knowledge.
Presumably by choice, patients with LBP continue to use primary care as the portal of entry to an episode of LBP care. Adherence to best-care principles for LBP at primary care sites remains suboptimal.33 We saw clear evidence of nonadherence, as evidenced by 1 in 4 high-risk patients with acute LBP receiving opioid prescriptions at the index visit. In the TARGET trial, we attempted to change patterns of guideline-noncompliant care using strategies suggested by others, including automated data collection and survey scoring, best-practice alerts, and removal of the administrative chore of referral follow-through from primary care office staff, all with suboptimal success. Ultimately, approximately half of the high-risk patients reported transition to chronic LBP at 6 months (UC+PIPT group [n = 658]: 47%; 95% CI, 42%-51%; UC group [n = 635]: 51%; 95% CI, 46%-56%), a figure that did not vary much between the UC and UC+PIPT groups. Unfortunately, in the TARGET trial, we are not able to definitively determine whether the failure to show a change in transition rate between groups was due to failure to implement the stratified approach to a degree that adequately perturbed the system or whether it was due to the lack of effectiveness of the stratified approach itself. However, in future trials of the stratified approach in primary care environments in the United States, preliminary work focusing first on implementation strategies is crucial before any attempt at a study of effectiveness.
Once patients were referred for PIPT, another implementation challenge was ensuring that PIPT was actually administered. From our patient stakeholder group, we knew that substantial co-payments for PT were the norm across the country. PCORI made it clear from the onset that it would not pay for treatment in its pragmatic trial funding initiative. We did have limited funds to cover co-payments for underserved patients (eg, those patients covered by Medicaid); however, through patient stakeholder engagement and feedback from our study coordinators, we found that the co-payment burden was greater with patients under commercial plans than was the burden under Medicaid. Most state Medicaid plans cover PT with no or minimal co-payments, though access is severely limited in some of these states (eg, Pennsylvania, where PT for patients in Medicaid plans must take place in hospital systems). Conversely, in many cases, commercial benefit plans required co-payments of $30 to $50 per visit. In addition, in many of the high-deductible plans, patients had to pay the entire cost of the PIPT. In talking with our European PT colleagues, it does not appear that the out-of-pocket expense for PT is such a barrier to care delivery and to research, which speaks to the difficulty in comparing the delivery and outcomes of the behavioral component of the stratified approach in PT environments in Europe and the United States.
Attempts to incorporate the stratified approach to acute LBP in the United States were undertaken by Cherkin et al16 in their Matching Appropriate Treatments to Consumer Healthcare needs (MATCH) study. Some key comparisons between TARGET and MATCH include the following:
- In MATCH, approximately 50% of patients with LBP seen in intervention clinics were risk stratified over the study period, which is higher than the 38% achieved at the UPMC clinics; however, we observed rates as high as 65% at the individual clinic level.
- Like TARGET, MATCH trained all clinic personnel using upfront group training and focused “chair side” training; however, the MATCH team did not provide any other ongoing implementation support throughout the study. MATCH's educational approach placed a greater emphasis on PCP training than did TARGET, and this may partially explain differences in screening rates, as a lack of physician engagement was frequently cited as a barrier in the TARGET study. The Implementation to improve Patient Care through Targeted treatment (IMPaCT)14 study took a very different approach.
- Risk stratification is another core component of acute LBP management, and all 3 studies integrated the STarT Back Screening Tool and automated its scoring within the EMR. In MATCH, the STarT Back Screening Tool could be completed verbally or on paper. In TARGET, we sought to leverage technology further by providing all clinics with study-related tablets that could be handed to the patient. However, technology was not purely facilitatory. At the UPMC site of the TARGET trial, barriers related to technology were encountered. For example, clinic staff faced with multiple competing priorities did not have time to problem-solve technology issues. Although some clinics had developed alternative strategies or solutions, others had not, which impacted risk-stratification rates. This inconsistency in identifying solutions highlights the need for effective strategies for spreading knowledge throughout organizations. Furthermore, many UPMC clinics adapted the implementation strategy and opted to administer the STarT Back Screening Tool using paper forms, even though patient responses could be entered directly using the tablet or verbal administration (eg, reading instrument items to the participant) for scoring within the EMR. Overall, 31% of the clinics had abandoned the tablets completely and used paper forms or verbally administered the risk-stratification questionnaires, 23% relied exclusively on tablets for risk stratification, and 62% used a mixed strategy and based the decision to use the tablet, use paper forms, or verbally administer the questionnaire on the current situation and patient. Clearly, a technological solution does not efficiently integrate into all clinic workflows equally and highlights the need for adaptability on the part of the clinic.
- TARGET and MATCH relied on all personnel for risk stratification, and IMPaCT relied on the physician. In TARGET, risk-stratification rates varied across points in the workflow. Risk-stratification rates were lowest (11%) when back pain was not identified until the patient was with the physician. The TARGET trial was designed to minimize burden on physicians, and they were not expected to perform the risk-stratification process with patients.
- In TARGET, we used many strategies to ensure that risk stratification occurred in the intervention clinics, including best-practice alerts for medical assistants and physicians, allowing medical assistants to “pend” PIPT orders for physician review, audit and feedback reports, practice facilitation, and financial incentives for the clinic staff. At the UPMC site, 42% of patients with LBP who were classified as high risk in the stratification process were referred to PIPT. This result is promising considering that in the MATCH trial, the risk-stratification process had limited impact on physicians' referral of patients to recommended treatments. A potential reason for the difference may have been the number and complexity of the referral options recommended in MATCH.
The MATCH study report noted a lack of implementation of a stratified approach in the primary care sites of one health system.16 In TARGET, implementation shortfalls prevailed across 5 health systems covering 77 primary care clinics in 5 geographically diverse US health systems.
The Pragmatic-Explanatory Continuum
As per the original PFA, we designed a trial that was largely pragmatic, erring on the side of generalizability for most the categories of the PRECIS-2. The TARGET trial investigative team worked extensively with stakeholders from provider groups (hospital and practice administrators, PCPs, and physical therapists) to incorporate many implementation strategies that became part of everyday care in primary care and PT settings. Many of these strategies were only marginally successful. These implementation strategies were, by design and necessity, highly pragmatic; by design, they were to keep the trial pragmatic by approximating real-life processes per the PFA, and by necessity, due to logistical concerns (eg, very busy primary care and PT environments). More importantly, the approach we used is highly generalizable and based on provider stakeholder engagement from hospital administrators, PCPs, and physical therapists. All decisions regarding our implementation approaches were vetted by our stakeholder group and were assumed to have a high probability of success. If we have learned anything from the TARGET trial, it is that education- and EMR-based approaches (eg, best-practice alerts, automated referrals) are not sufficient for successful implementation of the stratified approach to LBP.
One might suggest that simply adding more “explanatory” strategies to a trial would lead to greater penetration and assurance of treatment adherence and integrity (eg, greater oversight by PCPs in managing the care of people with chronic conditions, assigning case managers to assist in oversight, implementing “warm handoffs” [transfer of care between 2 members of the health care team, where the handoff occurs in front of the patient and family]). All are intriguing ideas that, in one way or another, were proposed to our primary care stakeholder group early in the trial. On the contrary, adding more things to do in a primary care environment or a PT environment was met with an overwhelming negative response. Current primary care and PT environments simply do not have the time and resources to add these strategies to their workflow.
In the United States, the TARGET trial's highly generalizable approach demonstrates that despite numerous implementation strategies that our stakeholders initially predicted would have some degree of success, there was insufficient uptake of the stratified approach in primary care. Thus, we are left with an unresolved question: Did the trial show little group effect due to shortfalls in implementation, or are the successes of the stratified approach in the European trials not generalizable to the United States?
Complex Intervention
The PIPT in this study provides a very good example of a complex intervention. In PIPT, cognitive behavioral principles and education are packaged with exercises for back pain. PIPT involves many separate but interacting components that are likely to be important to the success of the intervention, although the “active ingredients” are often difficult to specify. In developing a novel, complex intervention comprising separate elements and implementing these in a pragmatic trial, rigorous adherence to a process of evaluation was largely precluded. Specifically, we erred on the side of generalizability by allowing flexibility in (1) intervention delivery, (2) the expertise of those delivering treatment, (3) the degree of standardization of the intervention protocol, and (4) efforts to ensure compliance. Therefore, the trial was not designed to assess adherence to each element of the intervention and thus to explain which elements failed in an evaluation process for complex interventions:
- Intervention and delivery. Our ability to perform the most detailed analyses for intervention integrity will be in the UPMC and IHC settings, both of which have integrated PT operations that are on the same EMR system as primary care. We were able to determine whether a PIPT referral matched up with a patient who was high risk; in the UC+PIPT group, the referral did not guarantee that the patient went to PT, but we were able to verify PT attendance in the integrated PT settings (UPMC and IHC). We also incorporated checklists that could be used to show completion of the steps of the PIPT intervention and that were designed to be sent back to primary care offices for input into the EMR. In addition, the EMRs of the PT clinics at UPMC and IHC were being developed as the study was being conducted, and we included the key elements of the PIPT intervention in the PT health record. However, we would not have comprehensive data (eg, from the start of the study) for the PIPT elements of the health record. Our preliminary analyses of these data demonstrated that approximately 60% of patients deemed high risk had a referral to PIPT (ie, 40% of the time, the PCP ignored the best-practice alert). Following through, about 40% of the patients who received a PIPT referral actually received PT, either PIPT or standard PT.
- Expertise. Physical therapists who received the PIPT training represented a wide range of experience with varying credentials (eg, specialist certifications). We did not match physical therapists with advanced training to high-risk study patients. We did highly infiltrate each physical location with training sessions, however, and targeted marketing for the PIPT courses to the PT clinics to which the PCPs referred their patients.
- Degree of standardization. Though coursework provided a standardized approach to treatment and instructors modeled this approach in the role-playing that was part of the course, we did not provide assessments of adherence to the standardized approach other than the PIPT checklist. We did not prescribe dosing (eg, numbers of visits, duration of sessions), as typical PT care does not prescribe specific dosing, and mandating the number of sessions may have led to patients' angst because of additional out-of-pocket expenses (co-payments). Our patient stakeholders suggested that out-of-pocket expenses were a major and often insurmountable obstacle to achieving a prescribed amount of PT.
- Effort to ensure compliance. In keeping with the pragmatic nature of the trial and enhancing generalizability, we did not employ methods to ensure compliance.
The limitations of our approach correspond to the limitations that are present in any pragmatic trial. We were well aware of the limitations of the pragmatic trial approach in assessing the effectiveness of an intervention and were deliberately less pragmatic with intervention and delivery. For example, checklists, specifically guided referrals for PIPT, and strong encouragement from the sources of physician referrals to PT to obtain the PIPT training offered at the geographical site were certainly not part of UC and warranted a “3” on the PRECIS-2 scale (equally pragmatic and explanatory). However, that level of emphasis on elements of an explanatory trial may still be inadequate to perform an analysis of complex interventions to the level prescribed by the PCORI standard. Similarly, because of the pragmatic nature of the study, it is not possible to describe treatments actually received in either group with greater granularity.
In considering the choice of a comparator, we could not stop the UC group from ordering generic PT. However, we were able to keep the referral specific to PIPT away from the comparator group by offering it only at the sites that were cluster randomized to PIPT. In addition, none of the sites encouraged checklists or any other forms of reminders or guidance toward the behavioral component of PIPT. Still, PT referral occurred close to 30% of the time, which represents a 3-fold increase compared with PT referral rates documented by our sites before the study, perhaps because the opioid crisis was ongoing during the trial, and there were strong recommendations to use nonpharmacological approaches to pain management, including PT.
We were able to reduce bias by geographically stratified cluster randomizing by clinical sites, which we believe prevented site-specific crossover between groups of the trial. The best evidence for the success of this design strategy was the lack of PIPT referrals in the UC group.
Observational Cohort
Approximately 26% (95% CI, 23%-29%) and 33% (95% CI, 30%-37%) of low- and medium-risk patients, respectively, received referral to PT. The 26% PT referral rate was an increase from the <15% historical PT referral rates from our prestudy data at all 4 sites. The increased referral rate to PT in primary care was not surprising given the recent opioid crisis. In addition, in the middle of the TARGET trial, the American College of Physicians released its low back pain guidelines that recommended beginning the treatment of LBP with nonpharmacological approaches.35 Given the national opioid crisis, all participating health systems were imposing protocols to reduce opioid use, some of which included encouragement of nonpharmacological approaches, such as PT.
Similar to other studies, type of health insurance (Medicaid/Medicare vs private payer), obesity, smoking, baseline disability, and several processes of care at the index visit were associated with higher rates of chronic LBP at 6 months in the low- and medium-risk patients. About 9% of low-risk and 16% of medium-risk individuals underwent imaging during the 12 months following the initial visit, and fewer than 2% of patients had surgery.
About 1 in 5 low-risk patients reported chronic LBP at 6 months (19%; 95% CI, 16%-21%), compared with 1 in 3 medium-risk patients (33%; 95% CI, 30%-35%). In this study, we used the NIH Task Force on Chronic Low Back Pain's definition of chronic LBP.18 Looking across risk strata, the STarT Back Screening Tool successfully stratified patients by risk of developing chronic LBP. However, even though the proportion of patients transitioning to chronic LBP were highest in the high-risk group, followed by the medium-risk group, a nontrivial proportion of patients transitioned from acute LBP to chronic LBP in the low-risk group as well. A 19% rate of transition to chronic LBP in the low-risk group is still very high and should be alarming, particularly at a time when the financial burden of LBP warrants a “call to arms” to deal effectively with strategies to prevent the transition of acute LBP to chronic LBP. The STarT Back Screening Tool is useful, but perhaps combining the results of the tool with other patient characteristics would inform possible interventions to prevent the transition to chronic LBP. For example, we found that patient characteristics related to health insurance, comorbidities (eg, obesity, smoking), and higher initial Oswestry scores were all associated with increased transition to chronic LBP in the low-risk group.
Several processes of care—referral to a pain management specialist and ordering an MRI or CT scan—were also highly associated with low-risk individuals transitioning to chronic LBP. It could be argued that PCPs are recognizing differences in the severity of acute LBP that predict adverse outcomes, which prompts their referrals to specialists (eg, pain management) or advanced imaging. Concerning the latter, there is considerable consensus opinion that imaging in acute LBP has a limited role in the absence of red flag findings, as the imaging findings correlate poorly with symptoms.31 An alternative explanation is that the imaging, with its propensity for spurious findings, serves to “medicalize” the LBP condition, which may contribute to the transition to chronicity.32
We found similar results in our analysis of the medium-risk cohort's transition to chronic LBP. For example, predictors again included patient variables (eg, initial high Oswestry score, comorbidities, and process variables [referral for pain management and spine surgery]).
Overall, the transition to chronic LBP in the TARGET trial was patently high and exceeded epidemiologically based estimates in primary care that we cited in the Background section of this report. Originally, we assumed an overall transition rate of 20% based on previously published studies that documented rates of transition ranging from 7% to 30%.12,37-40 However, we found a transition rate of close to 20% in the low-risk group alone. Some of the disparity between our findings and previous studies may be related to our use of the NIH Task Force definition for chronic LBP,18 which has not been rigorously tested until now. The differing rates of transition to chronic LBP across risk strata as defined by the STarT Back Screening Tool would suggest some degree of validity to the NIH definition, however. We would also suggest that the definition may be more sensitive to the transition to chronic LBP, given the much-higher-than-expected rates of transition. In either case, our results suggest that the higher-than-expected rates of transition to LBP would indicate that the task of addressing the challenges of preventing acute LBP from becoming chronic may be even greater than previously thought, thus justifying the recent “call to action” approach that will be needed.2 Our implementation challenges, however, also further illustrate the complexity of addressing the problem, which will not only require changes within the health care environment, but also workplace systems, legal frameworks, personal beliefs, politics, and the overall societal context in which we experience health.33,41
Subpopulation Considerations
Additional analyses were conducted in high-risk patients stratified by subgroups of interest purely as a supplemental exercise, as we stated our interest in these groups a priori (Appendix A). We see highly variable rates of transition to chronic LBP and levels of back-related physical function across all the groups. There appears to be little to no impact of the PIPT intervention vs UC, with the exception of current smokers, but these results should be interpreted with caution given the small sample sizes in the subpopulations. For the observational cohort, we consistently observed that our subpopulations of smokers, obese patients, and those with Medicaid were at the highest risk of transition to chronic LBP but not necessarily at highest risk for guideline-nonconcordant medical use over 12 months.
Study Limitations
This study has 2 major limitations: (1) limited treatment fidelity reflected in the low rate of PIPT referrals and (2) high rates of missing primary outcome findings at 6 months. Although the nonresponse rate was about 40% at 6 months, there were no clinically meaningful differences in baseline characteristics between those who responded and those who did not.
We also provided multiple exploratory assessments of process-of-care and use outcomes but did not adjust them for multiple comparisons. Any inference between intervention groups using the confidence intervals should be interpreted with caution.
Conclusions
In a cluster RCT in US primary care settings, we compared a comprehensive intervention to support stratified care for acute LBP (UCT+PIPT) with UC. The stratification tool successfully identified groups at lowest and highest risk for transition to chronic LBP. The UC+PIPT intervention, which targeted patients with a high risk of transitioning to chronic LBP and included numerous implementation strategies in primary care and PT, did not appreciably reduce the rates of transition from acute LBP to chronic LBP. Patients in both study groups had higher-than-expected rates of transition to chronic LBP. The rates of diagnostic testing and interventional pain procedures were relatively low.
The implementation shortfalls of the TARGET trial suggest that we will need to rethink our approach to best-practice implementation in primary care and not be so dependent on approaches oriented to the EMR. In future studies, additional strategies might include (1) alternatives to PT environments in which to deliver the behavioral portion of the PIPT care (eg, telehealth, incorporation of alternative providers such as occupational therapists and counselors); (2) incorporation and integration of behavioral health into primary care settings using a more team-based approach for LBP, a strategy that has been effective in treating depression in primary care settings34; and (3) incorporation of shared savings or other incentive programs in which LBP is included along with other diagnoses, such as diabetes and hypertension.35
References
- 1.
- WHO Scientific Group on the Burden of Musculoskeletal Conditions at the Start of the New Millennium. The burden of musculoskeletal conditions at the start of the new millennium. World Health Organ Tech Rep Ser. 2003;919:i-x, 1-218. [PubMed: 14679827]
- 2.
- Buchbinder R, van Tulder M, Öberg B, et al. Low back pain: a call for action. Lancet. 2018;391(10137):2384-2388. [PubMed: 29573871]
- 3.
- Clark S, Horton R. Low back pain: a major global challenge. Lancet. 2018;391(10137):2302. [PubMed: 29573869]
- 4.
- Freburger JK, Holmes GM, Agans RP, et al. The rising prevalence of chronic low back pain. Arch Intern Med. 2009;169(3):251-258. [PMC free article: PMC4339077] [PubMed: 19204216]
- 5.
- Rubin DI. Epidemiology and risk factors for spine pain. Neurol Clin. 2007;25(2):353-371. [PubMed: 17445733]
- 6.
- Hoy D, Bain C, Williams G, et al. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012;64(6):2028-2037. [PubMed: 22231424]
- 7.
- Dieleman JL, Baral R, Birger M, et al. US spending on personal health care and public health, 1996-2013. JAMA. 2016;316(24):2627-2646. [PMC free article: PMC5551483] [PubMed: 28027366]
- 8.
- Institute of Medicine Committee on Advancing Pain Research, Care, and Education. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. National Academies Press; 2011. [PubMed: 22553896]
- 9.
- Shekelle PG, Markovich M, Louie R. An epidemiologic study of episodes of back pain care. Spine (Phila Pa 1976). 1995;20(15):1668-1673. [PubMed: 7482015]
- 10.
- Kosloff TM, Elton D, Shulman SA, Clarke JL, Skoufalos A, Solis A. Conservative spine care: opportunities to improve the quality and value of care. Popul Health Manag. 2013;16(6):390-396. [PMC free article: PMC3870576] [PubMed: 23965043]
- 11.
- Bigos S, Bower O, Braen G, et al. Quick Reference Guide for Clinicians Number 14: Acute Low Back Problems in Adults: Assessment and Treatment. Agency for Health Care Policy and Research. December 1994. Accessed May 6, 2021. https:
//centerforinquiry .org/wp-content/uploads /sites/33/quackwatch/clinicians .pdf [PubMed: 7987418] - 12.
- Jayson MI. Why does acute back pain become chronic? BMJ. 1997;314(7095):1639-1640. [PMC free article: PMC2126849] [PubMed: 9193284]
- 13.
- Somerville S, Hay E, Lewis M, et al. Content and outcome of usual primary care for back pain: a systematic review. Br J Gen Pract. 2008;58(556):790-797. [PMC free article: PMC2573978] [PubMed: 19000402]
- 14.
- Foster NE, Mullis R, Hill JC, et al. Effect of stratified care for low back pain in family practice (IMPaCT Back): a prospective population-based sequential comparison. Ann Fam Med. 2014;12(2):102-111. [PMC free article: PMC3948756] [PubMed: 24615305]
- 15.
- Hill JC, Whitehurst DG, Lewis M, et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): a randomised controlled trial. Lancet. 2011;378(9802):1560-1571. [PMC free article: PMC3208163] [PubMed: 21963002]
- 16.
- Cherkin D, Balderson B, Wellman R, et al. Effect of low back pain risk-stratification strategy on patient outcomes and care processes: the MATCH randomized trial in primary care. J Gen Intern Med. 2018;33(8):1324-1336. [PMC free article: PMC6082187] [PubMed: 29790073]
- 17.
- Hsu C, Evers S, Balderson BH, et al. Adaptation and implementation of the STarT Back risk stratification strategy in a US health care organization: a process evaluation. Pain Med. 2018;20(6):1105-1119. [PMC free article: PMC6934440] [PubMed: 30272177]
- 18.
- Deyo RA, Dworkin SF, Amtmann D, et al. Report of the NIH Task Force on Research Standards for Chronic Low Back Pain. J Pain. 2014;15(6):569-585. [PMC free article: PMC4128347] [PubMed: 24787228]
- 19.
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Global Health, et al. The Role of Nonpharmacological Approaches to Pain Management: Proceedings of a Workshop. National Academies Press; 2019. [PubMed: 31145569]
- 20.
- Williams CM, Maher CG, Hancock MJ, et al. Low back pain and best practice care: a survey of general practice physicians. Arch Intern Med. 2010;170(3):271-277. [PubMed: 20142573]
- 21.
- Østbye T, Yarnall KS, Krause KM, Pollak KI, Gradison M, Michener JL. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3(3):209-214. [PMC free article: PMC1466884] [PubMed: 15928223]
- 22.
- Reiss-Brennan B, Brunisholz KD, Dredge C, et al. Association of integrated team-based care with health care quality, utilization, and cost. JAMA. 2016;316(8):826-834. [PubMed: 27552616]
- 23.
- Hayen AP, van den Berg MJ, Meijboom BR, Struijs JN, Westert GP. Incorporating shared savings programs into primary care: from theory to practice. BMC Health Serv Res. 2015;15:580. doi:10.1186/s12913-015-1250-0 [PMC free article: PMC4696086] [PubMed: 26715151] [CrossRef]
- 24.
- Hill JC, Dunn KM, Lewis M, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59(5):632-641. [PubMed: 18438893]
- 25.
- Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE, Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. [PubMed: 25956159]
- 26.
- Delitto A, Patterson CG, Stevans JM, et al. Study protocol for targeted interventions to prevent chronic low back pain in high-risk patients: a multi-site pragmatic cluster randomized controlled trial (TARGET Trial). Contemp Clin Trials. 2019;82:66-76. [PubMed: 31136834]
- 27.
- Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25(22):2940-2952; discussion 2952. [PubMed: 11074683]
- 28.
- Barth M, Diepers M, Weiss C, Thomé C. Two-year outcome after lumbar microdiscectomy versus microscopic sequestrectomy: part 2: radiographic evaluation and correlation with clinical outcome. Spine (Phila PA 1976). 2008;33(3):273-279. [PubMed: 18303459]
- 29.
- Tsiang JT, Kinzy TG, Thompson N, et al. Sensitivity and specificity of patient-entered red flags for lower back pain. Spine J. 2019;19(2):293-300. [PubMed: 29959102]
- 30.
- Beneciuk JM, George SZ, Greco CM, et al. Targeted interventions to prevent transitioning from acute to chronic low back pain in high-risk patients: development and delivery of a pragmatic training course of psychologically informed physical therapy for the TARGET trial. Trials. 2019;20(1):256. doi:10.1186/s13063-019-3350-3 [PMC free article: PMC6501335] [PubMed: 31060589] [CrossRef]
- 31.
- Fairbank JC, Couper J, Davies JB, O'Brien JP. The Oswestry Low Back Pain Disability Questionnaire. Physiotherapy. 1980;66(8):271-273. [PubMed: 6450426]
- 32.
- Eldridge SM, Ashby D, Kerry S. Sample size for cluster randomized trials: effect of coefficient of variation of cluster size and analysis method. Int J Epidemiol. 2006;35(5):1292-1300. [PubMed: 16943232]
- 33.
- Slade SC, Kent P, Patel S, Bucknall T, Buchbinder R. Barriers to primary care clinician adherence to clinical guidelines for the management of low back pain: a systematic review and metasynthesis of qualitative studies. Clin J Pain. 2016;32(9):800-816. [PubMed: 26710217]
- 34.
- Ancker JS, Edwards A, Nosal S, et al. Effects of workload, work complexity, and repeated alerts on alert fatigue in a clinical decision support system. BMC Med Inform Decis Mak. 2017;17(1):36. doi:10.1186/s12911-017-0430-8 [PMC free article: PMC5387195] [PubMed: 28395667] [CrossRef]
- 35.
- Qaseem A, Wilt TJ, McLean RM, Forciea MA; Clinical Guidelines Committee of the American College of Physicians. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514-530. [PubMed: 28192789]
- 36.
- Lateef H, Patel D. What is the role of imaging in acute low back pain? Curr Rev Musculoskelet Med. 2009;2(2):69-73. [PMC free article: PMC2697333] [PubMed: 19468875]
- 37.
- Deyo RA, Mirza SK, Martin BI. Back pain prevalence and visit rates: estimates from U.S. national surveys, 2002. Spine (Phila PA 1976). 2006;31(23):2724-2727. [PubMed: 17077742]
- 38.
- Von Korff M, Saunders K. The course of back pain in primary care. Spine (Phila Pa 1976). 1996;21(24):2833-2837; discussion 2838-2839. [PubMed: 9112707]
- 39.
- Mehling WE, Gopisetty V, Bartmess E, et al. The prognosis of acute low back pain in primary care in the United States: a 2-year prospective cohort study. Spine (Phila Pa 1976). 2012;37(8):678-684. [PMC free article: PMC3335773] [PubMed: 22504516]
- 40.
- Traeger AC, Buchbinder R, Elshaug AG, Croft PR, Maher CG. Care for low back pain: can health systems deliver? Bull World Health Organ. 2019;97(6):423-433. [PMC free article: PMC6560373] [PubMed: 31210680]
Related Publications
- Delitto A, Patterson CG, Stevans JM, et al. Stratified care to prevent chronic low back pain in high-risk patients: the TARGET trial. A multi-site pragmatic cluster randomized trial. EClinicalMedicine. 2021;34:100795. doi:10.1016/j.eclinm.2021.100795 [PMC free article: PMC8040279] [PubMed: 33870150] [CrossRef]
- Stevans JM, Delitto A, Khoja SS, et al. Risk factors associated with transition from acute to chronic low back pain in US patients seeking primary care. JAMA Netw Open. 2021;4(2):e2037371. doi:10.1001/jamanetworkopen.2020.37371 [PMC free article: PMC7887659] [PubMed: 33591367] [CrossRef]
- Beneciuk JM, George SZ, Greco CM, et al. Targeted interventions to prevent transitioning from acute to chronic low back pain in high-risk patients: development and delivery of a pragmatic training course of psychologically informed physical therapy for the TARGET trial. Trials. 2019;20(1):256. doi:10.1186/s13063-019-3350-3 [PMC free article: PMC6501335] [PubMed: 31060589] [CrossRef]
- Delitto A, Patterson CG, Stevans JM, et al. Study protocol for targeted interventions to prevent chronic low back pain in high-risk patients: a multi-site pragmatic cluster randomized controlled trial (TARGET Trial). Contemp Clin Trials. 2019;82:66-76. [PubMed: 31136834]
Acknowledgments
We are grateful to our patient co-investigators, Jewel Cash, D. Scott Lake, and Marie Tamasy, and to the advisory board members for the important perspectives they provided throughout the project. We also thank the members of the research team who served as co-investigators, project directors, coordinators, and data managers and programmers: William G. Adams, MD; Michael C. Albert, MD; Kelly N. Daley, PT, MBA; Salvatore D'Amico; Daniel L. Brinton, PhD; Patti L. Ephraim, MPH; Janet K. Freburger, PT, PhD; Jennifer A. Freel, PhD, MBA; Michael Friedman, PT, MBA; Carol M. Greco, PhD; Linda J. Hough, MPH; Stephen J. Hunter, PT, DPT; Todd Jahangiri; Jong-Hyeon Jeong, PhD; Samannaaz S. Khoja, PT, PhD; Chelsey M. Lemaster, MS, MPHD; LaPricia Lewis-Boyer; Kate I. Minick, PT, DPT, PhD; Rebecca G. Mishuris, MD; Dorothy N. Plumb, MA; Linda Rosen, MS; Michael J. Schneider, DC, PhD; Kit N. Simpson, DrPH; Annie N. Simpson, PhD; Gwendolyn A. Sowa, MD, PhD; Wendy A. Spigle; Ajay D. Wasan, MD, MSc; Charles T. Williams, MD; Devyn Woodfield, MS; Bonnie J. Woods, MS; and Guan Yu.
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (PCS-1402-10867). Further information available at: https://www.pcori.org/research-results/2015/comparing-ways-treat-low-back-pain-and-prevent-chronic-pain-and-disability
Appendix
Suggested citation:
Delitto A, Patterson CG, Stevans JM, et al. (2021). Comparing Ways to Treat Low Back Pain and Prevent Chronic Pain and Disability—The TARGET Trial. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/05.2021.PCS.140210867
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Comparing Ways to Treat Low Back Pain and Prevent Chronic Pain and Disability—Th...Comparing Ways to Treat Low Back Pain and Prevent Chronic Pain and Disability—The TARGET Trial
Your browsing activity is empty.
Activity recording is turned off.
See more...
