This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License which permits noncommercial use and distribution provided the original author(s) and source are credited. (See https://creativecommons.org/licenses/by-nc-nd/4.0/
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Structured Abstract
Background:
Transverse myelitis (TM) is a rare spinal cord inflammatory disorder that can cause paralysis. It can affect both children and adults and can have a variety of causes. Future clinical trials are needed to improve the understanding and treatment of TM, but the outcome data needed to correctly design and power such trials are limited. A prospective study of pediatric TM outcomes at defined time points after symptom onset will provide the necessary information for future interventional trials.
Objectives:
The specific aims of the Collaborative Assessment of Pediatric Transverse Myelitis: Understand, Reveal, Educate (CAPTURE) study were to determine the responses to various treatments for pediatric TM. Furthermore, the study was originally designed to determine which patient-reported outcomes correlated best with clinician-acquired measures. Due to the appearance of a new clinically significant variant of TM, termed acute flaccid myelitis (AFM), post hoc analyses were implemented to understand the impact of treatment on patients diagnosed with AFM.
Methods:
This was a prospective, nonrandomized, observational trial using data from 2 patient cohorts. The cohorts included 1 group of patients who completed the Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric and/or Parent Proxy short forms at designated time points but did not have in-person evaluations at any of the recruiting centers. The second cohort completed the same patient- and/or parent-reported outcome assessments as cohort 1 but were also examined by clinicians at one of the recruiting centers at designated time points to collect predefined clinical measurements. The in-person cohort had neurologic exams and data that were entered into a Research Electronic Data Capture (REDCap) database. For patients who did not complete in-person examinations, their medical records were reviewed at the University of Texas Southwestern Medical Center and the data abstracted. Patients were followed for up to 12 months from TM onset. Clinically distinct subtypes of TM were identified, and the responses to various therapies were analyzed.
Results:
We were unable to adequately assess the planned primary outcome of the study due to underrecruitment but were able to collect several important data sets. All 3 patients (100%) with appropriate available treatment data (ie, those who had Functional Independence Measure for Children [WeeFIM] total scores at onset and 12 months postonset, and were treated with plasma exchange [PLEX] as their first-line therapy at onset) achieved the minimum clinically significant increase in WeeFIM total score of 22 points. Of the 8 patients treated with intravenous immunoglobulin as their first-line therapy at onset, potentially followed by PLEX, 4 (50%) patients achieved the minimum clinically significant increase in WeeFIM total score of 22 points. Those patients treated with PLEX as their first-line therapy did not have an increase in the relative risk of achieving the minimum clinically significant increase in WeeFIM total score compared with those who did not receive PLEX as their first-line therapy (P = .24 by Fisher exact test; 95% CI, 0-4.16 points). Additionally, the PROMIS Parent Proxy Mobility subset and WeeFIM Motor subset were shown to be highly correlated (⍴ = −0.84; 95% CI, −0.91 to −0.74 points; P < .0001). Last, the analysis of the sample of patients with AFM yielded information necessary for the understanding of this condition.
Conclusions:
Despite its limitations, the CAPTURE study had several important accomplishments. First, we quantified demographics and outcomes of patients with classically described TM and the newly recognized variant, AFM. The data collected in this study justify the need for future prospective trials of therapeutic interventions and allow for the appropriate design and powering of those studies. Finally, this report outlines correlations between the patient-reported PROMIS Mobility scale and the Motor section of the clinician-derived WeeFIM scale, indicating that this patient-reported outcome can be used for observational studies and tracks well with the gold standard of clinician-derived motor function.
Limitations:
This study was limited by underrecruitment and missing data. Because of these limitations, it was not possible to complete the preplanned primary end point analysis.
Background
Transverse myelitis (TM) is a rare, acquired condition that can cause damage to a person's spinal cord. The spinal cord is the structure that connects the body and the brain. It contains pathways that are responsible for movement, sensation, and control of bodily functions, such as bowel/bladder control. Anatomically, the spinal cord is composed of gray matter (on the inside of the cord) and white matter (around the outside of the cord, surrounding the gray matter). The white matter contains tracts that carry motor impulses down from the brain through the cord on their way to the muscles or carry sensory information up from the periphery to the brain. The gray matter, located on the inside of the cord, houses a variety of cells, including the cell bodies of motor neurons that transmit signals to muscles. Thus, when a person wants to move a limb, a signal travels from the brain down the white matter tracts of the cord and then connects to a neuron in the gray matter, which conducts the signal to a target muscle. Damage to the white matter or gray matter can lead to loss of function, but the patterns of functional loss will differ between patients based on which part of the spinal cord is affected.
While there are multiple ways to damage a spinal cord (trauma, infarct, etc), TM is defined by inflammation within the spinal cord. Individuals affected by TM have their lives changed abruptly and dramatically. TM commonly leads to weakness, numbness, loss of bowel or bladder function, and, often, paralysis.1-3 It represents one of the most concerning types of medical conditions because it is rare and potentially severe, but it is also potentially treatable if recognized and treated appropriately.
Previous epidemiologic studies have suggested an incidence of approximately 1800 cases per year in the United States, making it a rare condition.4 One in 5 cases are thought to occur in children, making the historical estimate of incident pediatric TM cases 300 per year. These data come from very limited studies and predate the recognition of many clinically important variants of TM.4 Nonetheless, pediatric TM is indeed a rare condition, and as such, there have been limited data about outcomes and response to therapy. Published articles have reported that pediatric patients with TM have been left with significant deficits, but none of these studies were prospective.1 The cohorts included patients referred to tertiary care centers and as such had significant selection biases.1 There is a significant need to quantify outcomes in pediatric TM and determine whether various therapies in the acute setting would impact those outcomes.
Based on the understanding of TM being an inflammatory disorder of the spinal cord, the approach to therapy is focused on anti-inflammatory interventions. Traditionally used therapies have included high-dose corticosteroids, intravenous immunoglobulin, and/or plasma exchange (PLEX).5-7 There are no prospective studies of acute therapies in TM, and most retrospective studies have been focused on adult patients.6 Studies tracking outcomes in pediatric TM were retrospective and included <50 patients. Most of these studies reported outcomes but not relative to the types of treatments used.1 One previously published study reported the safety and efficacy of PLEX in pediatric TM but was limited to 19 patients.7
Historically, the inflammation that causes TM was described as being located within the white matter; thus, patients would have neurologic deficits caused by damage to the ascending sensory tracts and descending motor tracts.8,9 Applying anti-inflammatory therapies would limit damage to the spinal cord and hence lead to better outcomes for patients, but which therapies are most effective has been unknown. Thus, this study was designed to prospectively follow pediatric patients with TM and determine which therapeutic interventions yielded the best outcome for patients.
After the launch of the study, in 2014, a variant of TM, acute flaccid myelitis (AFM), which is characterized by significant damage to the gray matter, was first reported.10 The variant was significant not only because of the portion of the spinal cord targeted for damage but also because AFM occurred as outbreaks and not the sporadic pattern of previously reported TM. The AFM variant followed an epidemiologic pattern significantly different from that of TM.11 Specifically, AFM had seasonal clustering with recognized outbreaks occurring between July and November in 2014, 2016, and 2018.12 Most of the available data suggest enterovirus D68 to be the etiology for AFM.13 Although traditional TM is not an infectious disease, the recognition of a transmissible, viral cause of a TM variant (AFM) transformed what had been a rare disease into a new public health concern. Improving on our abilities to recognize clinically meaningful variants and improving our approach to treatments for TM would provide an immense benefit to health care providers, patients, and families.
Patients with TM are often misdiagnosed or experience a delay in diagnosis.5 Even after a week of symptoms, a third of patients go undiagnosed. Consensus guidelines used to diagnose patients with TM were developed based on adult patient populations.14 Treatment of TM varies among centers. Furthermore, with the recognition of outbreaks of the AFM variant of TM, there has been significant controversy about which treatments should be offered to patients with TM.
Corticosteroids had been used as the standard of care for TM until the recognition of the AFM variant outbreaks in 2014. At that time, due to concern that a virus may trigger AFM, the Centers for Disease Control and Prevention (CDC) counseled clinicians to avoid corticosteroid therapy.15 This caused significant concern among clinicians, patients, and families. Intravenous immunoglobulin (IVIG) is an FDA-approved therapy for a variety of autoimmune disorders. However, its use for conditions such as multiple sclerosis, myasthenia gravis, acute disseminated encephalomyelitis, and TM has been “off label.” In general, IVIG is easy to administer via a peripheral intravenous (IV) line and has few complications. When they occur, complications can include headache from a chemical meningitis, pulmonary edema, kidney injury, venous thrombosis, or anaphylactic reactions. There are no controlled trials of the use of IVIG in TM, but, based on its ease of administration and relative safety profile, it is a commonly used therapy (after steroids) for pediatric TM.
PLEX therapy involves the circulation of a patient's blood through a centrifuge in the presence of an anticoagulant (citrate) to separate and remove a patient's plasma before returning the blood cells to the circulation. It has been used in a variety of autoimmune conditions, including Guillain-Barre syndrome, chronic inflammatory demyelinating polyneuropathy, myasthenia gravis, multiple sclerosis, and TM.6,7,16 The procedures usually require venous access with 2 large-bore IVs (1 IV for the blood to flow into the machine and 1 IV for the blood to return to the patient) or a double-lumen central line (a larger catheter typically inserted into the subclavian or jugular vein). The risks of the procedure include injury during line placement and the PLEX itself. The line placement can result in vascular injury (dissection or clot), pneumothorax, or infection. The procedure can cause hypotension, coagulopathy, hypocalcemia, or citrate reactions. Based on the usual need for a central line and a knowledgeable PLEX team, PLEX is not universally available within community treatment facilities. Nonetheless, many clinicians view PLEX as superior to IVIG therapy.6,7 Indeed, based on published data, guidelines from the American Academy of Neurology recommended that clinicians consider PLEX therapy (in addition to corticosteroids) for patients with TM.17 Complicating matters further, in 2014, aligning with its recommendations about corticosteroids, the CDC counseled against the use of PLEX therapy in patients with AFM.18 Thus, clinicians, patients, and families encounter a dilemma when deciding which therapy to initiate for TM.
There are many obstacles to defining best treatment practices in rare diseases such as TM. First, as outlined above, the rarity of the condition makes prospective randomized trials logistically impractical. Second, the heterogeneity of treatment practices makes single-center retrospective studies biased and difficult to interpret. Third, the heterogeneity of patients makes retrospective data analysis difficult. Thus, there is a significant need for a prospective multicenter study to assess response to therapy in TM. This study was launched to correlate clinician-derived and patient-reported outcomes with functional life measures and to quantify the outcomes of pediatric patients with TM relative to outcomes with various other therapeutic approaches.
TM represents a significant health burden that could be mitigated if more robust data sets relating treatment to clinical outcomes were available to guide therapy. Studies have shown that patients vary in their response to therapies, but the field has not validated a patient-reported outcome that could be used in large-scale national registries. In addition to collecting prospective outcome data relative to treatments, the investigators of the current study recognized a need to interrogate multiple potential outcome measures, because a patient-reported outcome measure in pediatric TM had yet to be defined.
The Collaborative Assessment of Pediatric Transverse Myelitis: Understand, Reveal, Educate (CAPTURE) study was designed to quantify the impact of various acute therapies in the treatment of pediatric TM. As originally conceived, the aims of the study included addressing the following questions:
- Which patient-reported and clinically derived outcomes are most concordant with patient- and family-reported quality of life (QOL) in the pediatric TM patient population? We chose this aim to determine whether subsystem scores (motor vs sensory, etc) would correlate with a measure of overall QOL.
- For pediatric patients with TM, does a standard aggressive treatment protocol using PLEX as the first-line therapy yield better results based on Functional Independence Measure for Children (WeeFIM) scores than do nonaggressive treatment protocols using IVIG and/or corticosteroids as the first-line therapy, potentially followed by PLEX?
- –
Outcomes will include patient-derived and clinical data.
- –
The proportions of patients treated with a single intervention vs multiple interventions will be assessed.
- Can a novel web-based data distribution system improve the collection of research data from future patients?
Due to circumstances outlined in this report, the study required that we make unforeseen amendments during the course of the project. Regarding the first aim, scores on the Patient-Reported Outcomes Measurement Information System (PROMIS) Pediatric version and Parent Proxy and the FIM/WeeFIM were intended to be the patient-reported and clinically derived outcomes, respectively, and a measure such as the Quality of Life in Neurological Disorders (Neuro-QoL) instrument was meant to capture patient and family QOL. However, a QOL measure such as the Neuro-QoL instrument was mistakenly not included in the patients' and families' surveys. Therefore, we were not able to examine this aim as defined without the corresponding QOL data. Additionally, the PROMIS Pediatric version, the patient-reported outcome measure used by our study, is valid only for children aged ≥8 years. Given that a considerable amount of patients included in the CAPTURE study were <8 years old, the patient-reported outcome was not considered, and instead, the PROMIS Parent Proxy was considered due to a lower age limit (5 years old). Similarly, due to such a young population, the WeeFIM results were much greater in the sample collected and were investigated in our analysis instead of the FIM results. Missing baseline data for many patients rendered the second original aim infeasible without making strict assumptions regarding the longitudinal behavior of patient-reported and clinically derived outcomes (eg, strictly nondecreasing from baseline). Additionally, because of lower-than-expected enrollment and respondent rates, adjustments were made to the protocol to bolster enrollment and data quantity. While increasing enrollment and data, this amendment to the protocol introduced more missing longitudinal data, further restricting the applicability of the preplanned analyses. Also, an unexpected outbreak of a previously rare variant of TM, AFM, occurred, and these patients created a large heterogeneity in the patient population. The inclusion of patients with AFM introduced unanticipated subgroups into the preplanned analyses and an additional source of variability in the data. Overall, the lower-than-expected enrollment and respondent rates and the introduction of subgroups rendered preplanned analyses underpowered and infeasible.
Therefore, the primary aim of the CAPTURE study was marginally revised and defined as follows: For pediatric TM, does exposure to PLEX during treatment yield better results based on WeeFIM total scores than does receipt of corticosteroids and/or IVIG only at 6 months and 12 months after symptom onset?
Additionally, because the treatments patients received were not randomized, the data that could be analyzed varied from those expected. Based on the recorded treatment each patient received, it became apparent that there were 2 initial treatment groups represented in the data: initial treatment with corticosteroids and initial treatment with IVIG. Thus, we were able to investigate differences in the outcomes between these 2 groups. Last, with the collection of data from patients with the AFM variant of TM, we were able to investigate potential differences between those patients with classical TM and those with AFM (which became a critically important piece of data for public health officials and clinicians). Thus, we performed the following post hoc, exploratory analyses:
- For pediatric TM, does exposure to PLEX during treatment yield better results than receipt of corticosteroids and/or IVIG only at 6 months and 12 months after symptom onset in terms of 3 secondary outcomes:
- –
WeeFIM Motor subset scores
- –
Parent Proxy PROMIS Mobility subset scores relative to those patients
- –
Parent Proxy PROMIS Upper Extremity subset scores relative to those patients
- For pediatric TM, did initial treatment using corticosteroids yield better results than using IVIG as the initial treatment in terms of several secondary outcomes, including the following:
- –
WeeFIM total scores and WeeFIM Motor subset scores
- –
Parent Proxy PROMIS Motor and Upper Extremity subset scores
- –
Fewer additional treatments
- Do disease presentation and/or clinical outcomes differ between patients with classical TM and those diagnosed with AFM?
- Do disease presentation and/or clinical outcomes differ between patients with different magnetic resonance imaging (MRI) patterns of involvement in AFM?
- Treating the Parent Proxy PROMIS Peer Relationships subset score as a surrogate for patient QOL, we aimed to examine the correlation of this subset to the WeeFIM and other Parent Proxy PROMIS scores obtained.
- Are WeeFIM Motor subset scores and Parent Proxy PROMIS Mobility scores concordant?
Thus, we present results based on the initially planned study aims and then separately report the results of the post hoc analyses.
Patient and Stakeholder Engagement
The CAPTURE study was designed and executed in close consultation with the largest national patient advocacy group for TM, the Transverse Myelitis Association (TMA; now Siegel Rare Autoimmune Association: https://wearesrna.org/). From the stage of protocol development, members of the TMA board, which included families of pediatric patients and adult patients, were consulted about how to balance data collection with the risk of survey fatigue among participants. The executive director of the TMA served as a co-investigator for the study. The lead patient ambassador of the TMA, who was a parent of a patient with pediatric TM, served as a consultant to protocol development and oversaw the TMA's approach to advertising and recruiting for this study.
The TMA partnered with enrollment centers regarding notifications, advertisements, and recruitment. Often, the TMA is the first point of contact (via the website, a phone call, Facebook, etc) for a family with a newly diagnosed child. Patient ambassadors, trained by the scientific and medical advisory board of the TMA, were available to support newly diagnosed families and make them aware of the ongoing CAPTURE study. The TMA hosted symposiums with featured talks about the CAPTURE study, family camps, and podcasts that were distributed to their entire membership and made publicly available. Regular newsletters advertised the study, and the TMA was integral in educating families about the importance of the research. During the course of the study, the TMA was instrumental in providing feedback that led to a study protocol modification and improvement in recruitment efforts. The TMA was present on all steering committee calls and at all steering committee meetings. Furthermore, formal phone-based meetings between the principal investigator and the TMA team occurred 2 to 3 times per year. Finally, there was ongoing communication between the primary site (University of Texas Southwestern [UTSW]) research team and TMA leadership. This frequent and meaningful schedule of interactions allowed us to have real-time recruitment updates and conversations about strategic changes in the protocol. For example, feedback from the TMA early in the course of the study, when recruitment was significantly behind schedule, led to the recognition that many families were not emotionally ready to take part in research in the first 3 months of diagnosis but would be ready by month 6. Thus, the protocol was amended to allow for enrollment through the first 6 months of the study, and this improved our sample size.
From the launch of the study, meetings were held twice a year and continued through study completion. One of the meetings was web based, and the second was in person. The meetings included the investigators, the TMA patient ambassador, the executive director of the TMA, and the study nurses/coordinators who were supporting the study from each enrollment site. These meetings were structured to include an update on the recruitment, data collection, and outreach efforts and to allow for conversations among stakeholders to improve the success of the study. Meetings began with updates from the primary site (UTSW). These updates included individualized site enrollment numbers, missing data, and lost-to-follow-up statistics. Next, technical reviews about updates or changes to data entry processes were provided. Each site was allotted time to raise any concerns or issues. Meetings included time for discussions about advertising and recruitment strategies, including the scheduling of emails, podcasts, and web posts by the TMA. Finally, meetings were used to discuss data analysis plans and manuscript preparation plans.
Methods
Study Overview
The CAPTURE study was a prospective, nonrandomized, observational study to track the outcomes experienced by pediatric patients with TM and to determine the effect of the use of PLEX vs corticosteroids and IVIG on outcomes. The study was designed to account for the unique needs of a geographically diffuse, rare disease community without prospectively validated patient-reported outcomes. These goals had to change during the study for 2 major reasons. First, lower-than-expected enrollment would have a significant impact on the power of the study. Second, it became apparent that an outbreak of a clinically meaningful subtype of TM (ie, AFM) would have to be included in the data analysis plan to account for potential differences between these 2 distinct diseases. Due to these unforeseen circumstances, we were unable to achieve the aims of the study as originally planned but were able to make several contributions to our understanding of TM, clinical subtypes, and response to therapy. The methods of data acquisition and the data points did not change, but additional analyses were required, and the originally planned comparator analysis plan had to be updated to a descriptive approach.
Study Setting
The study had 2 different cohorts. Cohort 1 consisted of 70 patients who were able to obtain in-person assessments at 1 of 7 recruiting centers in the United States (Children's Health in Dallas, TX; University of Colorado in Denver; Toronto Sick Kids in Toronto, Canada; Children's Hospital of Philadelphia in Philadelphia, PA; Johns Hopkins Hospital in Baltimore, MD; Kennedy Krieger Institute in Baltimore, MD; and Cincinnati Children's in Cincinnati, OH). Cohort 2 consisted of 43 patients whose patient-reported outcomes could be accessed via an online Research Electronic Data Capture (REDCap) patient portal. The portal questionnaires were developed at UTSW by adapting PROMIS forms to an online format.
Participants
Participants included children (aged 0-18 years inclusive) diagnosed with TM within 6 months of enrollment and their parents (of note, the inclusion criteria were updated from 3 months to 6 months during the trial due to recruitment shortfalls). Inclusion criteria also required the ability of parents/legal guardians to give consent and the ability of patients (aged ≥10 years) to give assent. Families were required to have internet access so they could complete online questionnaires. Exclusion criteria included a diagnosis of multiple sclerosis or neuromyelitis optica spectrum disorder. Patients were recruited after being treated at 1 of the 7 recruitment centers, being referred to 1 of the 7 centers, or after being contacted by the primary center to participate in the virtual/online cohort. Patients and parents learned about the study from their clinicians or via social media and were directed to contact representatives from the TMA. The representatives then referred the interested family to the primary center (UTSW) to enroll in the study. This involved completing the informed consent process and determining whether the family would be enrolled in the in-person or virtual cohort. The designation of taking part in the in-person cohort was based on a family's ability to seek care at any one of the enrolling centers. Thus, the recruitment of the 2 cohorts happened simultaneously, and cohort assignment was based on family travel capabilities. Patients receiving in-person care from an enrolling center would be confirmed to have evidence of TM (based on diagnostic criteria) and be able to complete study-related visits. Virtual cohort participants had to send medical records and imaging for review at UTSW and complete online questionnaires. Data were reviewed by TM experts to ensure that diagnostic testing, clinical history, and imaging were consistent with a diagnosis of myelitis.
Interventions and Comparators
Interventions, including corticosteroids, IVIG, and PLEX, are considered standard-of-care options for pediatric TM but are applied in various ways at various centers. Furthermore, patients often undergo treatment with >1 therapy. Thus, data analyses have to consider both the different combinations of therapies that could be applied and the sequence of therapeutic interventions. Potential adverse effects of corticosteroids include insomnia, mood changes, hyperphagia, weight gain, hypertension, and/or hyperglycemia. IVIG can cause headaches, nausea, vomiting, and, rarely, thrombotic events. Finally, PLEX can cause hypocalcemia, paresthesias, and coagulopathy. Furthermore, a central line is often used to administer PLEX therapy; thus, patients are closely monitored for line-related complications (eg, thrombosis, infections).5-7
Data were collected about the sequence of treatments and relative responses after each treatment exposure. Traditionally, patients were treated with 30 mg/kg (based on body weight) of IV methylprednisolone for 3 to 5 days at symptom onset. Additionally, patients could receive IVIG, PLEX, or both in combination with the methylprednisolone. IVIG dosing was typically 2 g/kg divided over 4 to 5 days, and PLEX therapy traditionally consisted of 5 to 7 treatments of 1.1 to 1.5 total plasma volume per treatment. These parameters varied by center and by patient.
Data Collection and Sources
The virtual cohort was followed by the lead study center (UTSW). Patients were reminded via email about the need to complete online questionnaires. The in-person cohort was contacted by each site to remind them about follow-up visits and the need for data collection. Patients and parents missed online follow-up time points and/or in-person visits for a variety of reasons. Many indicated significant stressors related to health issues as a reason for missing data entry time points. Study coordinators and research nurses worked to balance gentle reminders with respecting the stressors that families were experiencing after a catastrophic health event. The coordinator from UTSW continues to contact families and tries to obtain any missing data. Contacts with families occurred by email and by phone.
Study Outcomes
The choice of outcomes was deemed to be a critical component of this study, as it would be the first to incorporate patient-reported outcomes and clinically derived data for this patient population. The patient- and family-reported outcome measures relied on those recommended by the NIH PROMIS initiative.19 The instruments selected for this study are detailed below.
Patient-Reported Instruments (Patients Aged ≥8 Years)
Primary outcomes
PROMIS Peds Short Form (SF) v1.0–Mobility 8a
PROMIS Mobility Function instruments measure self-reported capability rather than actual performance of physical activities. This includes the functioning of one's lower extremities (walking or mobility) as well as instrumental activities of daily living, such as running errands. A single Physical Function capability score is obtained from a short form. This was validated for children aged 8 to 17 years. Higher scores correspond to less-severe symptoms.
PROMIS Peds SF v1.0–Upper Extremity 8a
PROMIS Upper Extremity Function instruments measure self-reported capability rather than actual performance of physical activities. This includes the functioning of one's upper extremities (dexterity) as well as instrumental activities of daily living, such as tying shoes. A single Physical Function capability score is obtained from a short form. This was validated for children aged 8 to 17 years. Higher scores correspond to less-severe symptoms.
Secondary outcomes
PROMIS Peds SF v1.0–Anger 6a
The PROMIS Anger instruments assess self-reported angry mood (irritability, frustration), negative social cognitions (interpersonal sensitivity, envy, disagreeableness), and efforts to control anger. Often associated with episodes of frustration that impede goal-directed behavior, anger is marked by attitudes of hostility and cynicism. Specific components relate to verbal and nonverbal evidence of anger. This was validated for children aged 8 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Peds SF v1.0–Anxiety 8a
The PROMIS Anxiety instruments assess self-reported fear (fearfulness, panic), anxious misery (worry, dread), hyperarousal (tension, nervousness, restlessness), and somatic symptoms related to arousal (racing heart, dizziness). Anxiety is best differentiated by symptoms that reflect autonomic arousal and perception of threat. This was validated for children aged 8 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Peds SF v1.0–Depressive Sx 8a
The PROMIS Depression instruments assess self-reported negative mood (sadness, guilt), views of self (self-criticism, worthlessness), and social cognition (loneliness, interpersonal alienation), as well as decreased positive affect and engagement (loss of interest, meaning, and purpose). This was validated for children aged 8 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Peds SF v1.0–Fatigue 10a
The PROMIS Fatigue instruments assess a range of self-reported symptoms, from mild subjective feelings of tiredness to an overwhelming, debilitating, and sustained sense of exhaustion that likely decreases one's ability to execute daily activities and function normally in family or social roles. Fatigue is divided into the experience of fatigue (frequency, duration, and intensity) and the impact of fatigue on physical, mental, and social activities. This was validated for children aged 8 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Peds SF v1.0–Pain Interference 8a
The PROMIS Pain Interference instruments assess self-reported consequences of pain on relevant aspects of one's life. This includes the extent to which pain hinders engagement with social, cognitive, emotional, physical, and recreational activities. Pain Interference also incorporates items probing sleep and enjoyment in life. This was validated for children aged 8 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Peds SF v1.0–Peer Relationships 8a
The PROMIS Pediatric and Parent Proxy Family Relationships item bank assesses the subjective (affective, emotional, cognitive) experience of being involved with one's family, feeling like an important person in the family, feeling accepted and cared for, and feeling that family members, especially parents, can be trusted and depended on for help and understanding. This was validated for children aged 8 to 17 years. Higher scores correspond to less-severe symptoms.
Parent-Reported Measures
The Parent Proxy PROMIS forms mirror the patient-reported forms in content but are structured to collect data from an adult caregiver.
Primary outcomes
PROMIS Parent Proxy SF v1.0–Mobility 8a
This was validated for children aged 5 to 17 years. Higher scores correspond to less-severe symptoms.
PROMIS Parent Proxy SF v1.0–Upper Extremity 8a
This was validated for children aged 5 to 17 years. Higher scores correspond to less-severe symptoms.
Secondary outcomes
PROMIS Parent Proxy SF v1.0–Anger 5a
This was validated for children aged 5 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Parent Proxy SF v1.0–Anxiety 8a
This was validated for children aged 5 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Parent Proxy SF v1.0–Depressive Symptoms 6a
This was validated for children aged 5 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Parent Proxy SF v1.0–Fatigue 10a
This was validated for children aged 5 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Parent Proxy SF v1.0–Pain Interference 8a
This was validated for children aged 5 to 17 years. Lower scores correspond to less-severe symptoms.
PROMIS Parent Proxy SF v1.0–Peer Relationships 7a
This was validated for children aged 5 to 17 years. Higher scores correspond to less-severe symptoms.
Clinically Derived Measures
Patients in cohort 1 (ie, those receiving in-person assessments) were assessed with the practitioner/study coordinator–administered instruments described below.
Primary outcomes
The WeeFIM instrument (version 4.0) contains 18 measurement items divided into 6 areas: self-care (6 items), sphincter control (2 items), transfers (3 items), locomotion (2 items), communication (2 items), and social cognition (3 items). The WeeFIM Motor subscale includes the areas of self-care, sphincter control, transfer, and locomotion; it contains 13 items. The remaining 2 areas (communication, social cognition) comprise the cognitive subscale. A 7-level ordinal rating system ranging from 7 (complete independence) to 1 (total assistance) is used to rate performance. A rating of 1 to 4 indicates that the child requires some level of assistance from another person to complete the activity. A rating of 5 means the child requires supervision or adult cues to complete the activity. A rating of 6 means that the child can complete the activity independently but may require an assistive device or more than a reasonable amount of time to complete it, or that safety is a concern. The WeeFIM measure has been validated as an outcome in spinal cord studies.1,20,21 Scores range from 18 to 126, with changes of 22 points being considered significant.
Secondary outcomes
The 25-foot timed walk
This test measures the time in seconds that it takes an ambulatory participant (aged >6 years) to ambulate 25 feet. It has been validated in demyelinating disease conditions such as multiple sclerosis and has been used as primary end points in clinical trials of therapeutics (in adults).22,23
The 6-minute timed walk
The 6-minute timed walk records the distance that a patient can walk in 6 minutes. While the 25-foot walk only measures walking speed over a short distance, the 6-minute walk quantifies efforts over a longer period of time and accounts for neurologic issues that would impact endurance. It has been validated in children aged 12 to 16 years.24
Hauser Ambulation Index
The Hauser Ambulation Index is a 10-point scale (0-9) that assigns patients a score based on their function. Scores of 0 to 3 include patients who ambulate without assistance, a score of 4 relates to the need for unilateral assistance, scores of 5 to 7 refer to patients in need of bilateral assistance, and scores of 8 to 9 include patients who use a wheelchair for locomotion. The Hauser Ambulation Index has been validated in clinical trials.25
Sample Size Calculations and Power
The original end points of the trial were to determine which patient-reported and clinically derived outcomes are most concordant with patient- and family-reported QOL in the pediatric TM patient population and to determine whether therapy using PLEX was superior to therapy using IVIG relative to change in WeeFIM total scores at 12 months after symptom onset relative to baseline. These outcomes were chosen to explore how patient-reported vs clinician-reported variables would correlate with global QOL. Whether clinician-derived or patient-derived measures would be more meaningful for clinical trials is unknown. The WeeFIM total score was selected as a primary outcome given its prior use in populations with pediatric spinal cord pathologies and because it allowed us to compare various populations with different spinal cord pathologies.20
Powering of the study based on the original aim was based on the following assumptions:
- 180 patients (based on expected diversity of treatments and loss-to-follow-up rate)
- In neurologic literature, the minimum score change in WeeFIM total score that is considered significant is 22.
- Approximately 35% of the patients would be treated only with steroids.
- Approximately 35% of the patients would be treated with combinations of steroids and IVIG.
- Approximately 20% of the patients would be treated with combinations of steroids and PLEX.
- Approximately 10% of the patients would be treated with combinations of steroids, IVIG, and PLEX.
These assumptions were based on limited published data and unpublished data based on referrals to the Children's Health Dallas TM Clinic.1,5 We calculated the sample size and power using the clinically meaningful outcome of a 22-point increase in the WeeFIM total score at 12 months after symptom onset relative to baseline. We anticipated the primary outcome would be that 60% of the steroid-only–treated group would be expected to have a meaningful change in WeeFIM score vs 85% of the PLEX-treated group (PLEX in addition to steroids). A 2-group χ2 test would have 80% power to detect the difference between 60% with significant change and 85% when the sample size was 50 patients per group (this allowed for up to 20% dropout).
The preplanned analyses were not possible because of low overall recruitment and low in-person cohort numbers (n = 70). Thus, the primary analysis was updated to focus on patient-reported outcomes instead of WeeFIM (presented in the “Post Hoc Exploratory Analyses” section of the Results) due to the greater response rate for patient-reported outcomes than for clinically derived outcomes. We did this to augment sample size. PROMIS measures that correlated with WeeFIM measures were selected for analysis and the results reported. Furthermore, the assumptions used to perform the power calculation predated the outbreaks of AFM in 2014 and 2016 and the recognition of various subtypes of AFM; thus, our ultimate data set was underpowered for the primary outcome because the design was based on enrolling patients with 1 phenotype of TM, but the AFM phenotype is expected to have different responses to therapy. Despite this, the CAPTURE study was able to enroll enough patients with AFM to answer important treatment-related questions.
Time Frame for the Study
The study recruited patients from 2014 through 2018. Data were collected from the time of enrollment (between onset and 6 months postonset) until the participant reached the 12-month postonset date.
Original Analytical and Statistical Approaches
The original primary analyses consisted of comparing the simple proportion of patients who improved by a clinically meaningful amount or not between those who received PLEX as their first-line therapy and those who received IVIG as their first-line therapy, potentially followed by treatment with PLEX, coupled with analyses that consist of deriving propensity scores based on patient demographic covariates and presentation and performance of a covariate analysis based on these propensity scores. The distribution of propensity scores was to be examined between the 2 groups, and outliers in either group with no pair mates in the other treatment group were to be eliminated. This trimming is one of the keys that distinguishes propensity score analyses from analysis of covariance, which was to be used to perform a sensitivity analysis of the resultant scores. Repeated-measures logistic regression analysis was to be conducted using the 6- and 12-month scores to enhance power by including participants even if they dropped out between 6 and 12 months. Similar analyses were also to be conducted using continuous-outcome measures and mixed-effects linear regression models with repeated measures.
However, as previously stated, due to lower-than-expected enrollment and respondent rates, as well as the unanticipated additional variability introduced by including patients with AFM, many of the preplanned analyses were rendered infeasible. Therefore, the analysis plan was altered.
Revised Analytical and Statistical Approaches
The original protocol did not specify whether the in-person and virtual cohorts would be combined. To maximize the sample size, we compared the demographic and clinical measurements corresponding to these 2 cohorts by disease to determine whether the pooling of these 2 samples was a valid approach. Because there were no significant differences between the 2 cohorts, the in-person and virtual cohorts were combined. Cohort membership was not a covariate in any of the subsequent analyses due to the limited sample size for each.
Our primary analysis became the comparison of WeeFIM total scores at 6 and 12 months after symptom onset between patients who received PLEX during their treatment at symptom onset, potentially followed by other treatments, and those who did not have exposure to PLEX. To compare the WeeFIM scores between the 2 patient populations, we performed Mann-Whitney U tests independently at each time point. This approach was chosen over t tests due to the skewed distributions of these scores toward higher scores. Due to multiple testing, we chose P = .025 as the threshold type I error rate rather than P = .05, reflecting a Bonferroni-adjusted significance level (for the primary outcome).
Descriptive statistics of measures collected during the CAPTURE study are reported here. When the distribution of numerical data is at least symmetric, the mean and SD are reported. Conversely, for data with skewed distributions, the median and range are reported.
In addition to the primary analysis, many post hoc exploratory analyses were performed. No P value adjustments were made to the resulting P values obtained in the exploratory analysis. However, the results must be interpreted with caution and as directions for future research, given the inflated type I error probability resulting from multiple testing, and must be verified through future studies. The remaining data analysis approaches concern these post hoc exploratory analyses. Aside from the WeeFIM total score, we wanted to investigate differences in WeeFIM Motor subset scores between patients based on treatment exposure. Additionally, 2 patient-reported outcome measures of interest were the Mobility and Upper Extremity subset scores of the PROMIS Parent Proxy questionnaire. Only those patients for whom the PROMIS Parent Proxy surveys are valid (ie, aged 5-17 years) were included in the analysis. Comparisons of WeeFIM Motor subset and PROMIS Parent Proxy Mobility and Upper Extremity scores based on treatment exposure groups were performed using the Mann-Whitney test due to the skewed distributions of these scores.
We used logistic regression to compare the odds of patients receiving subsequent treatment after initial treatment with IV steroid vs IVIG. Subsequent treatment would often be pursued when patients failed to have a clinically adequate response to first-line therapy. Variables included in the analysis were factors corresponding to AFM, gray-matter–isolated AFM, AFM patients treated with IVIG, and gray-matter AFM patients treated with IVIG. These covariates constitute all comparisons of interest.
The ordinal variable corresponding to the degree of improvement after initial treatment as reported by family (1 = no improvement, 2 = minimal improvement, 3 = some improvement, 4 = mostly recovered, and 5 = fully recovered) was analyzed using ordinal logistic regression with a cumulative logit link. Variables included in the analysis were factors corresponding to AFM, initial treatment using IVIG, gray-matter–isolated AFM, AFM patients treated with IVIG, and gray-matter AFM patients treated with IVIG. These covariates constitute all comparisons of interest. The assumption of proportional odds was investigated upon analysis, and all results presented are based on models that do not suggest violation of this assumption.
The Pearson correlation coefficient (⍴) values between the Parent Proxy PROMIS Peer Relationships (or Peer) subset score and WeeFIM subset scores, WeeFIM total score, and remaining Parent Proxy PROMIS subset scores were computed. However, due to the limited number of measurements, the corresponding P values were not computed. Correlations between total score on the WeeFIM Mobility subset and the total score on the PROMIS Parent Proxy Mobility subset from patients with gray-matter–isolated AFM were computed based on rank-transformed data (ie, Spearman correlation coefficient). First, the ranked scores on the PROMIS Parent Proxy Mobility subset are regressed on the ranked WeeFIM Mobility subset scores, the time from baseline (in months), and the interaction of the ranked WeeFIM Mobility subset scores and time from baseline with patient-level random effects included to account for intra-subject correlation. The conditional and marginal R2 values are then reported to provide an indication of the variance in the dependent variable that was explained by covariates included in the model. ⍴ was then computed based on data collected at all time points.
All analyses were performed in R, version 3.5.0 (R Foundation for Statistical Computing). Plots were generated using ggplot2 (version 3.1.0). Ordinal regression was performed using the ordinal package (2019.3-9).
Study Conduct
Two critical changes to the study protocol altered the original data analysis plan. First, based on feedback from patients/families and the TMA, it was suggested that the original inclusion criterion of enrollment within 3 months of onset was too restrictive. Many potential participants expressed interest in the study but reported having “too much going on” to participate so close to symptom onset. The protocol was adjusted to allow for enrollment within 6 months of onset, and the recruitment numbers improved. Furthermore, to increase recruitment numbers, 2 centers (Cincinnati and Colorado) were added halfway through the study (increasing from 5 to 7 centers).
The second change to the protocol occurred because of the unexpected outbreak of AFM, a previously rare variant of TM. The original trial design assumed that the majority of enrollees would be diagnosed with the version of TM that primarily affected white matter. With the recognition of AFM outbreaks came an increased enrollment of patients with AFM, which altered the original data analysis plan. The plan had to be altered because a clinically/pathologically heterogeneous population would be expected to respond differently to therapeutic interventions than would one composed solely of children with the sporadic form of TM. The anti-inflammatory effects of corticosteroids and PLEX might not have the same beneficial impact for a patient with AFM that it would have for a patient with TM, although this possibility is speculative. Finally, the unexpected recognition of patterns of spinal cord damage among patients with AFM further reduced the size of each subpopulation that was analyzed because it introduced another confounding variable for which we must account in our analysis.
Results
A total of 113 participants were enrolled in the study, and 90 had analyzable participant data, including 39 patients with TM and 51 patients with the AFM variant. Because the initial enrollment was expected to be 100 patients with TM, the study is severely underpowered for the original planned analyses. The total enrollment was below that expected for 3 potential reasons. First, the estimated incidence of pediatric TM, on which our original estimates were based, is potentially inaccurate. Second, our ability to identify potential enrollees may have been more limited than expected. Finally, the rate of consent to participate in the study among those asked to consent was lower than expected, although this by itself does not account for the level of underrecruitment. Although the first 2 explanations must account for the underrecruitment, there is no way to know which of the 2 explanations is most meaningful. Figure 1 outlines the reasons for exclusion and the details of the final cohort. Of the patients who had an alternate diagnosis confirmed (n = 10), 2 were diagnosed with acute disseminated encephalomyelitis, 2 were diagnosed with vascular myelopathy, 4 had significant brainstem involvement, 1 was diagnosed with neuromyelitis optica spectrum disorder, and 1 was diagnosed with conversion disorder. In all, 90 patients with analyzable data were included in this analysis.
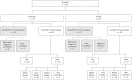
Figure 1
Enrollment Diagram for the CAPTURE Study.
Comparison of In-Person and Virtual Cohorts
The CAPTURE study consisted of 2 cohorts of patients, the in-person cohort and the virtual cohort. The final in-person cohort consisted of 60 patients (26 TM, 34 AFM), and the final virtual cohort consisted of 30 patients (13 TM, 17 AFM). The demographic features of the TM and AFM samples stratified by in-person vs virtual cohort membership are listed in Table 1. The clinical features of the TM and AFM samples stratified by in-person vs virtual cohort membership are listed in Table 2.
Table 1
Demographic Results by Cohort, Diagnosis Within Cohort, and Total Patient Population.
Table 2
Clinical and Laboratory Results by Cohort, Diagnosis Within Cohort, and Total Patient Population.
Before addressing the aims of the study, we conducted preliminary analyses to determine whether any demographic or clinical differences existed between the 2 cohorts (in-person and virtual) that could bias the results. Analyses were conducted within diagnoses (ie, the in-person TM patient sample was compared with the virtual TM patient sample, and the in-person AFM patient sample was compared with the virtual AFM patient sample). Based on these analyses, we determined that the data did not provide sufficient evidence to conclude that the in-person TM patient sample presented with different demographic or clinical characteristics from those of the virtual TM patient sample, or that the in-person AFM patient sample presented with different demographic or clinical characteristics from those of the virtual AFM patient sample. Therefore, we proceeded by combining the 2 cohorts of patients (in-person and virtual) for analysis of the measures we collected. Table 3 provides the demographic and clinical features of the combined in-person and virtual cohorts, stratified by diagnosis (TM vs AFM).
Table 3
Demographic Characteristics, Symptoms at Onset, and Laboratory Results.
Original Aims Addressed
The only original aim of the CAPTURE study we were capable of investigating was a comparison of the change in WeeFIM total scores from symptom onset to 12 months with standard aggressive therapy—defined as “immediate PLEX” following corticosteroids—vs IVIG as first-line therapy, potentially followed by PLEX (ie, “delayed PLEX”). As previously mentioned, due to lower-than-expected enrollment and respondent follow-up rates, this analysis was severely underpowered. Due to the limited number of patients with WeeFIM scores available at symptom onset and 12 months after symptom onset, we had data for only 3 patients who received the immediate PLEX treatment protocol and 8 patients who received the delayed PLEX or IVIG-only treatment protocol. A plot of the longitudinal WeeFIM total scores for the 2 treatment groups is shown in Figure 2.
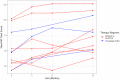
Figure 2
WeeFIM Total Scores for Patients Over Time Stratified by Treatment Regimen.
Of the 3 patients treated with the “aggressive” treatment protocol, all 3 (100%) achieved the minimum clinically significant increase in WeeFIM total score of 22 points. Of the 8 patients treated with the nonaggressive delayed-PLEX treatment protocol, 4 (50%) patients achieved the minimum clinically significant increase in WeeFIM total score of 22 points. We performed a Fisher exact test based on the contingency table of those patients who achieved the minimum clinically significant increase by treatment group. The rate at which the aggressive-treatment group achieved the minimum clinically significant increase in WeeFIM total score was not different from that of the group receiving the nonaggressive treatment (P = .24; 95% CI, 0.00-4.16). However, it must be reiterated that these results are inconclusive due to the limited sample size.
Revised Primary Aim Analysis
The revised primary aim of the CAPTURE study became to examine differences in WeeFIM total scores of patients with TM based on patient exposure to PLEX. Figure 3 provides the longitudinal WeeFIM total scores for the sample of TM patients analyzed, stratified by exposure to PLEX. We examined the outcome data at 6 months after symptom onset and 12 months after symptom onset independently. Table 4 provides the median WeeFIM total scores (as well as scores to be investigated in subsequent post hoc analyses) and their respective ranges, stratified by exposure to PLEX at symptom onset, as well as the results of analysis.
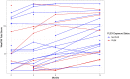
Figure 3
WeeFIM Total Score Longitudinal Measurements by PLEX Exposure for Patients With TM.
Table 4
Median WeeFIM and PROMIS Parent Proxy Scores Analyzed at 6 Months and 12 Months After Symptom Onset for Patients With TM.
Of the 90 analyzed patients, 15 had WeeFIM scores available at 6 months postbaseline. Of these 15 patients, 5 received PLEX treatment. For the 5 patients who received PLEX, even though the median WeeFIM total score was less than that of those who did not receive PLEX, the data do not provide sufficient evidence to conclude that the WeeFIM total scores 6 months after symptom onset are different (or are not different) in those patients who received PLEX from the scores in those who did not (P = .16; Table 4). The study is inconclusive on this point.
Relative to 12-month outcomes, of the 90 analyzed patients, 26 had WeeFIM scores available at 12 months after symptom onset. Of these 26 patients, 10 received PLEX treatment. Again, the data do not provide sufficient evidence to conclude that the WeeFIM total scores at 12 months postbaseline are different in patients who received PLEX from the scores in patients who did not receive PLEX (P = .37). It is critical to recognize that this analysis was completed on a small subset of the enrolled patients, which greatly affects the power, and that the data are relative to PLEX therapy in particular, which differs from the original design of the study. With the smaller sample size, propensity score analyses were not possible.
Post Hoc Exploratory Analyses
Further analyses based on PLEX exposure
In addition to the comparison between those who received PLEX and those who did not receive PLEX addressed in our primary aim, we examined WeeFIM Motor subset scores and PROMIS Parent Proxy Mobility and Upper Extremity subset scores as post hoc exploratory analyses based on PLEX exposure. Based on the results presented in Table 4, the data do not suggest a significant difference in the WeeFIM Motor subset scores between those who received PLEX and those who did not receive PLEX at 6 months after symptom onset (P = .16) or 12 months after symptom onset (P = .15). Similarly, there was no significant difference in the PROMIS Parent Proxy Mobility subset at 6 months after symptom onset (P = .09) or 12 months after symptom onset (P = .06). Last, there was not enough evidence to suggest a difference in the PROMIS Parent Proxy Upper Extremity subset scores between the 2 groups at 6 months after onset (P = .85) or 12 months after onset (P = .25).
Radiographic Features of TM and AFM
Given the apparent differences between subtypes of TM, we felt it important to describe the differences among these populations. A review of the MRIs revealed 1 cohort with pathology only in gray matter and a second cohort with pathology in both gray and white matter, termed mixed matter. The importance of this pattern may reflect 2 types of patients who have differential responses to therapy. Theoretically, the gray-matter–restricted patients may be experiencing virus-mediated death of anterior horn cells, whereas the patients with mixed-matter pathology may have a virus-induced anterior horn cell pathology with a secondary immune response causing damage to the white matter. If this second pattern is immune mediated, patients might uniquely benefit from anti-inflammatory treatments. The MRI findings are summarized in Table 5.
Table 5
MRI Results by Diagnosis.
Exploratory Analysis of the AFM Patient Sample and Its Subtypes
With the inclusion of the AFM cohort, interest developed in examining the outcomes investigated previously relative to the subtype of TM (separating out AFM vs non-AFM). Instead of analyzing the WeeFIM and PROMIS Parent Proxy data relative to PLEX exposure, we investigated the results relative to corticosteroid exposure given the CDC's recommendations against administering corticosteroids to patients presenting with AFM. However, we must reiterate that due to the small sample sizes contained in the AFM sample analyzed, these results must be interpreted with caution. Descriptive statistics corresponding to all WeeFIM subsets and PROMIS Parent Proxy subsets, stratified by diagnosis and matter involvement within the AFM cohort, as well as the changes from the earliest time point collected to 12 months after symptom onset, can be found in the Appendix in Tables A1 through A6.
Table 6 presents the WeeFIM total scores, WeeFIM Motor subset scores, PROMIS Parent Proxy Mobility subset scores, and PROMIS Parent Proxy Upper Extremity subset scores at 6 months and 12 months after symptom onset, stratified by TM and AFM and further classified by exposure to corticosteroids during treatment. Based on the results presented in Table 5, the data do not provide sufficient evidence to conclude that any of the 4 measurements are different between the TM patient cohort treated with corticosteroids and the AFM patient cohort treated with corticosteroids at either 6 months after onset or 12 months after onset. Similarly, the data do not suggest that any of the 4 measurements are different between patients with AFM treated with corticosteroids and those patients with AFM not treated with corticosteroids at either 6 months after onset or 12 months after onset.
Table 6
Median WeeFIM and PROMIS Parent Proxy Scores for TM and AFM Subtypes by Exposure to Corticosteroids.
Table 7 presents the WeeFIM total scores, WeeFIM Motor subset scores, PROMIS Parent Proxy Mobility subset scores, and PROMIS Parent Proxy Upper Extremity subset scores at 6 months and 12 months after symptom onset, stratified by AFM subtypes and further classified by exposure to corticosteroids during treatment. Based on the results presented in Table 6, the data do not provide sufficient evidence to conclude that any of the 4 measurements are different between the gray-matter–isolated AFM patients treated with corticosteroids and those gray-matter–isolated AFM patients not treated with corticosteroids at either 6 months after onset or 12 months after onset. Similarly, the data do not suggest that any of the 4 measurements are different between gray-matter–isolated AFM patients treated with corticosteroids and mixed-matter AFM patients treated with corticosteroids at either 6 months after onset or 12 months after onset.
Table 7
WeeFIM and PROMIS Parent Proxy Scores at 6 Months and 12 Months After Symptom Onset by AFM Subtype.
Treatment Variations
Patients, as expected, underwent a variety of treatments. Table 8 provides the results of the logistic regression modeling of receiving >1 treatment (1 treatment received = 0, >1 treatment received = 1) and the results of the ordinal logistic regression modeling of the degree of improvement. The data used in the logistic regression model are shown in Table 9 in the summary of treatment courses for all TM and AFM patients examined. The data used in the ordinal logistic regression model are depicted in Table 10, which provides the patient-reported degree of recovery for all patients who received IV steroids or IVIG as their initial treatment, stratified by diagnosis and AFM subtypes. It should be noted that no patients received PLEX as their initial treatment.
Table 8
Results of Logistic Regression Modeling of Receiving Additional Treatment and Ordinal Logistic Regression Modeling of Improvement After Initial Treatment Based on Initial Treatment.
Table 9
Treatment History for Patients With TM and AFM.
Table 10
Degree of Improvement After Initial Treatment.
Based on the results shown in Table 8, the data do not provide evidence that diagnosis, initial treatment in the AFM cohort, or matter involvement in the AFM cohort, nor their interactions, significantly impact the odds that a patient received >1 treatment. Similarly, based on the results of the ordinal logistic regression, the data do not suggest that diagnosis, initial treatment, or matter involvement in the AFM cohort, nor their interactions, were significant effects that impacted the odds of greater improvement after initial treatment.
Correlation Between PROMIS Parent Proxy Peer Relationships Subset and WeeFIM and PROMIS Parent Proxy Subsets
The PROMIS Parent Proxy Peer Relationships subset measures social health as a marker of QOL for children. Social health refers to a child's quantity and quality of social interactions. The correlations between WeeFIM subset scores and the PROMIS Parent Proxy Peer Relationships subset are shown in Figures 2 and 3. The data are depicted stratified by months after symptom onset and WeeFIM subset, with the ⍴ value included when at least 2 data values are available. Given that higher PROMIS Parent Proxy Peer Relationships scores correspond with lesser symptom severity, whereas higher WeeFIM scores correspond with greater symptom severity, we would expect negative correlations between the PROMIS Parent Proxy Peer Relationships measure and WeeFIM subset scores if the severity of symptoms is positively correlated between the 2 measures. With very limited data, Figures 4 and 5 suggest the strongest correlations with self-care measures at 12 months after symptom onset instead of overall motor function, and the correlation with the self-care subset is negative, as expected. However, these results must be interpreted with caution due to the sparsity of the available data and must be confirmed in future efforts.

Figure 4
WeeFIM Subset (Self-Care, Sphincter Control, Transfer, Locomotion, and Communication) Scores Relative to PROMIS Parent Proxy Peer Relationships Subset.

Figure 5
WeeFIM Subset (Social Cognition, Motor, Cognitive, and Total) Scores Relative to PROMIS Parent Proxy Peer Relationships Subset.
The correlations between the PROMIS Parent Proxy Peer Relationships subset and the remaining PROMIS Parent Proxy subsets are depicted in Figure 6, using the same approach as in Figures 4 and 5. Regarding the PROMIS Parent Proxy subsets, for the Mobility, Upper Extremity, and Peer Relationships subsets, higher scores correspond to less-severe symptoms, whereas for the Anxiety, Depression, Fatigue, and Pain subsets, higher scores correspond to more-severe symptoms. Therefore, if we expect that symptom severity is positively correlated between the PROMIS Parent Proxy Peer Relationships subset and the remaining PROMIS Parent Proxy subsets, the PROMIS Parent Proxy Peer Relationships subset will be positively correlated with the Mobility and Upper Extremity subset and negatively correlated with the Anxiety, Depression, Fatigue, and Pain subsets. PROMIS Parent Proxy Peer Relationships outcome data at 12 months after symptom onset had stronger inverse correlations between parent-reported Anxiety (⍴ = −0.59), Depression (⍴ = −0.38), Fatigue (⍴ = −0.56), and Pain (⍴ = −0.56) than parent-reported Physical Function (ie, Mobility, ⍴ = −0.11, and Upper Extremity Function, ⍴ = −0.19).

Figure 6
PROMIS Parent Proxy Subset Scores Relative to PROMIS Parent Proxy Peer Relationships Subset.
Correlation Between PROMIS Parent Proxy Mobility Subset and WeeFIM Motor Subset Scores in Gray-Matter–Isolated AFM Patients
For gray-matter–isolated AFM patients, upon regressing the ranked PROMIS Parent Proxy Mobility measure on the ranked WeeFIM Mobility subset scores, time from baseline, and the interaction of the ranked WeeFIM Mobility subset scores and time from baseline, neither time nor the interaction term was significant (P = .11 and P = .21 respectively). This result suggests that the relationship between the ranked WeeFIM Mobility subset scores and the PROMIS Parent Proxy Mobility subset scores is not impacted by the time from follow-up. However, because of the limited sample size and post hoc nature of this analysis, this result should be interpreted with caution. The model was then refit including a single covariate, the ranked WeeFIM Mobility subset scores, and an intercept. Based on the refit model, the data provide sufficient evidence to conclude that as the rank of the WeeFIM Mobility subset score increases by 1, the rank of the PROMIS Parent Proxy Mobility score increases by 0.43 (P = .02). Additionally, there was a strong correlation between the ranked WeeFIM Mobility subset score and the PROMIS Parent Proxy Mobility subset score (⍴ = 0.63; 95% CI, 0.27 to 0.85; P = 0.0007). A plot of the ranked WeeFIM Mobility subset scores and PROMIS Parent Proxy Mobility subset scores is shown in Figure 7.
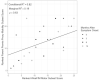
Figure 7
Correlation Between the Rank of the PROMIS Parent Proxy Mobility Score and the Total WeeFIM Mobility Score.
Discussion
Main Results
The CAPTURE study enrolled pediatric patients diagnosed with TM and a subtype of TM, known as AFM, between 2014 and 2018. Of the patients enrolled, the majority had the AFM subtype, and through data collected in the study, 2 subtypes of the AFM subtype were identified. Based on the change in population and lower-than-expected recruitment, the study was not powered to determine statistically significant differences in treatment outcomes. Despite this, however, the CAPTURE study showed that the majority of pediatric patients with TM experience some level of recovery after treatment. These conclusions need to be viewed in the context of the study design, which lacks a no-treatment comparator group. Thus, the total impact of therapies cannot be quantified based on these data. Furthermore, the CAPTURE study was able to find a correlation between PROMIS categories and appropriate WeeFIM subscales.
The study did, however, provide critical data that stand in contrast to public treatment recommendations from the CDC and are being used to update those recommendations. During the initial AFM outbreak, the CDC convened a panel of experts who publicly advised against the use of corticosteroids and PLEX out of concern for potentiating viral replication and worsening spinal cord damage. Since the completion of the CAPTURE study and sharing of these data, the CDC has updated its public statements about treatment to recognize that the evidence is insufficient to summarily dismiss the use of corticosteroids and/or PLEX and that clinicians should consider these treatments on a case-by-case basis.
Lessons Learned
The CAPTURE study was able to demonstrate the need for academic centers to partner with patient advocacy organizations for protocol development and participant recruitment. Although this study did not achieve its recruitment goal, the success it had was related to real-time interaction with the TMA. During this study, it became apparent that representatives from the TMA were well suited for recruitment and counseling about study participation. A significant issue during the study was our limited ability to recruit patients during the very earliest phase of the disease and the challenges patients and families faced completing surveys during the course of the study. While patients and families were enthusiastic to take part in the study, the enthusiasm was tempered by the realities of managing a new and frightening illness. Studies that rely on patient and family participation in the hyperacute setting will always be challenging. Partnering with a patient advocacy organization is helpful for explaining the importance of research from the perspective of an individual who has had shared experiences. Creating data collection systems that are easy for patients to use and integrate into their daily lives would help reduce the rate of missing data. The addition of direct benefits to patients and families for participating in research would also reduce the amount of missing data. The creation of online support systems as part of a research study would incentivize patients and families to spend the time needed to complete surveys. The system used in our study was a web portal that required families to purposely log on and input data. Systems that run on mobile devices and give feedback to the user might augment participation.
Subpopulation Considerations
The CAPTURE study was designed and launched based on previous knowledge about TM, a rare disorder that can affect children. During the study, it became apparent that there were clinically meaningful subpopulations that had not been defined previously. Classically defined TM was described as a white-matter pathology of the spinal cord caused by an immune-mediated attack. During the CAPTURE study, it became apparent that a significant number of pediatric patients had a subtype of TM called AFM, and within the AFM population, there were 2 MRI-defined populations: those with gray-matter–restricted pathology and some patients with mixed-matter pathology. The identification of these distinct subpopulations reduced the sample size in any 1 subtype, which adversely affected the CAPTURE study's ability to achieve its intended goal of measuring differences in response to therapy but did yield data for the design of future clinical trials. The recognition and definition of subpopulations is critical for the design and execution of translational research, and the data generated in this study are being used to support future funding applications to further study these populations.
This study was underpowered for detecting treatment response heterogeneity—the existence of subgroups in which the response to treatment was larger or smaller than in the overall population—and we did not perform analyses to detect it.
Study Limitations
This study had several significant limitations, including underrecruitment, missing follow-up data, and the emergence of a clinically and epidemiologically significant subtype of this already rare condition. The most significant limitation was recruitment. The primary aim of the study required a certain number of enrollees to have in-person evaluations. This did not occur. As discussed, the lack of recruitment was likely a combination of an overestimate of the incidence of this rare disease and families' lack of awareness of the study. Despite partnering with the largest and oldest TM patient advocacy group, we failed to identify and enroll enough patients with TM.
The second significant limitation of the study relates to missing data. Although this is a problem in many studies, the impact on an underrecruiting study is magnified. Despite email reminders and phone calls, families were inconsistent in completing surveys and keeping appointments for follow-up visits.
Finally, the study was limited by the emergence and recognition of previously undefined TM subpopulations. The original study design assumed a single population of pediatric patients with TM and was designed to detect differences in response to therapies. During the study, the recognition of 3 previously unrecognized subtypes of TM divided the study population into smaller-than-anticipated subgroups, which reduced the analytic power to detect statistically significant differences in outcomes from different treatments.
Conclusions
Despite its limitations, the CAPTURE study demonstrated several important considerations relative to conducting patient outcome–focused research in rare disease populations and generated multiple important data sets relative to pediatric TM. The first conclusion of the CAPTURE study is that recruiting for rare disease studies is quite difficult, and success can be augmented by the engagement of patients, families, and patient advocacy organizations. Pursuing these types of arrangements yields better-designed and more successful research studies. Second, the CAPTURE study provided insights into the need to create data capture systems that can enable participants to deal with the challenges they have in finding the time to take part in research when they are under the stress of coping with a catastrophic illness. The emotional, physical, and financial toll that health events take on a family tremendously impact their ability to dedicate precious time and attention to research efforts. Although families recognize the importance of this type of research, it is incumbent upon researchers to find mechanisms to make it easy for them to participate.
Relative to TM, the CAPTURE study described several novel data sets that have changed our understanding of this rare disease. First, the CAPTURE study quantified the contrasting demographics and outcomes of patients with classically described TM relative to the newly recognized variant, AFM. The data collected in this study justify the need for future prospective trials of therapeutic interventions and allow for the appropriate design and powering of those studies. Such studies would benefit from additional physical examination outcomes (eg, separating deficits in upper and lower extremities and differentiating flaccid from spastic weakness), prolonged follow-up, and documentation/quantification of rehabilitation interventions. Finally, this report outlines correlations between the PROMIS Parent Proxy Mobility scale and the Motor section of the clinician-derived WeeFIM scale. This data set indicates that these parent-reported outcomes can be used for observational studies and that they fit with the gold standard of clinician-derived data.
References
- 1.
- Pidcock FS, Krishnan C, Crawford TO, Salorio CF, Trovato M, Kerr DA. Acute transverse myelitis in childhood: center-based analysis of 47 cases. Neurology. 2007;68(18):1474-1480. [PubMed: 17470749]
- 2.
- Harder LL, Holland AA, Frohman E, Graves D, Greenberg BM. Cognitive functioning in pediatric transverse myelitis. Mult Scler. 2013;19(7):947-952. [PubMed: 23166117]
- 3.
- Wolf VL, Lupo PJ, Lotze TE. Pediatric acute transverse myelitis overview and differential diagnosis. J Child Neurol. 2012;27(11):1426-1436. [PubMed: 22914370]
- 4.
- Berman M, Feldman S, Alter M, Zilber N, Kahana E. Acute transverse myelitis: incidence and etiologic considerations. Neurology. 1981;31(8):966-971. [PubMed: 7196523]
- 5.
- Greenberg BM, Krishnan C, Harder L. New onset transverse myelitis diagnostic accuracy and patient experiences. Mult Scler Relat Disord. 2019;30:42-44. [PubMed: 30738277]
- 6.
- Greenberg BM, Thomas KP, Krishnan C, Kaplin AI, Calabresi PA, Kerr DA. Idiopathic transverse myelitis: corticosteroids, plasma exchange, or cyclophosphamide. Neurology. 2007;68(19):1614-1617. [PubMed: 17485649]
- 7.
- Noland DK, Greenberg BM. Safety and efficacy of plasma exchange in pediatric transverse myelitis. Neurol Clin Pract. 2018;8(4):327-330. [PMC free article: PMC6105075] [PubMed: 30140584]
- 8.
- Tavasoli A, Tabrizi A. Acute transverse myelitis in children, literature review. Iran J Child Neurol. 2018;12(2):7-16. [PMC free article: PMC5904733] [PubMed: 29696041]
- 9.
- Wang C, Greenberg B. Clinical approach to pediatric transverse myelitis, neuromyelitis optica spectrum disorder and acute flaccid myelitis. Children (Basel). 2019;6(5):70. doi:10.3390/children6050070 [PMC free article: PMC6560417] [PubMed: 31109018] [CrossRef]
- 10.
- McKay SL, Lee AD, Lopez AS, et al. Increase in acute flaccid myelitis—United States, 2018. MMWR Morb Mortal Wkly Rep. 2018;67(45):1273-1275. [PMC free article: PMC6290805] [PubMed: 30439867]
- 11.
- Bhat A, Naguwa S, Cheema G, Gershwin ME. The epidemiology of transverse myelitis. Autoimmun Rev. 2010;9(5):A395-A399. [PubMed: 20035902]
- 12.
- Messacar K, Asturias EJ, Hixon AM, et al. Enterovirus D68 and acute flaccid myelitis-evaluating the evidence for causality. Lancet Infect Dis. 2018;18(8):e239-e247. doi:10.1016/S1473-3099(18)30094-X [PMC free article: PMC6778404] [PubMed: 29482893] [CrossRef]
- 13.
- Messacar K, Schreiner TL, Van Haren K, et al. Acute flaccid myelitis: a clinical review of US cases 2012-2015. Ann Neurol. 2016;80(3):326-338. [PMC free article: PMC5098271] [PubMed: 27422805]
- 14.
- Krishnan C, Kerr DA. Idiopathic transverse myelitis. Arch Neurol. 2005;62(6):1011-1013. [PubMed: 15956176]
- 15.
- Division of Viral Diseases, National Centers for Immunization and Respiratory Diseases, CDC; Division of Vector-Borne Diseases, Division of High-Consequence Pathogens and Pathology, National Center for Emerging and Zoonotic Infectious Diseases, CDC; Children's Hospital Colorado; Council of State and Territorial Epidemiologists. Notes from the field: acute flaccid myelitis among persons aged ≤21 years—United States, August 1-November 13, 2014. MMWR Morb Mortal Wkly Rep. 2015;63(53):1243-1244. [PMC free article: PMC4646045] [PubMed: 25577990]
- 16.
- Bonastre-Blanco E, Jordan-Garcia Y, Fons-Estupina MC, Medina-Cantillo J, Palomeque-Rico A. Plasmapheresis in a paediatric patient with transverse myelitis and Guillain-Barre syndrome secondary to infection by Mycoplasma pneumoniae [article in Spanish]. Rev Neurol. 2011;53(7):443-444. [PubMed: 21948015]
- 17.
- Scott TF, Frohman EM, De Seze J, Gronseth GS, Weinshenker BG, Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011;77(24):2128-2134. [PubMed: 22156988]
- 18.
- Nelson GR, Bonkowsky JL, Doll E, et al. Recognition and management of acute flaccid myelitis in children. Pediatr Neurol. 2016;55:17-21. [PubMed: 26621554]
- 19.
- Gershon RC, Rothrock N, Hanrahan R, Bass M, Cella D. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas. 2010;11(3):304-314. [PMC free article: PMC3686485] [PubMed: 20847477]
- 20.
- Noh T, Vogt MS, Pruitt DW, Hummel TR, Mangano FT. Pediatric intramedullary spinal cord tumor outcomes using the WeeFIM scale. Childs Nerv Syst. 2018;34(9):1753-1758. [PubMed: 29797065]
- 21.
- Grilli L, Feldman DE, Majnemer A, Couture M, Azoulay L, Swaine B. Associations between a functional independence measure (WeeFIM) and the pediatric quality of life inventory (PedsQL4.0) in young children with physical disabilities. Qual Life Res. 2006;15(6):1023-1031. [PubMed: 16900282]
- 22.
- Motl RW, Cohen JA, Benedict R, et al. Validity of the timed 25-foot walk as an ambulatory performance outcome measure for multiple sclerosis. Mult Scler. 2017;23(5):704-710. [PMC free article: PMC5405807] [PubMed: 28206828]
- 23.
- Goodman AD, Brown TR, Edwards KR, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol. 2010;68(4):494-502. [PubMed: 20976768]
- 24.
- Li AM, Yin J, Yu CC, et al. The six-minute walk test in healthy children: reliability and validity. Eur Respir J. 2005;25(6):1057-1060. [PubMed: 15929962]
- 25.
- Brichetto G, Rinaldi S, Spallarossa P, Battaglia MA, de Carvalho ML. Efficacy of physical therapy in multiple sclerosis as measured with the modified fatigue impact scale and ambulation index: a retrospective study. NeuroRehabilitation. 2013;33(1):107-112. [PubMed: 23949038]
Related Publications
- Acute flaccid myelitis in the United States: long term outcomes recorded in the CAPTURE study. In preparation for submission to JAMA.
- Greenberg B. Acute flaccid myelitis. Presented at: Rare Neuroimmune Disorders Symposium; October 27, 2018; Boston, MA.
- Greenberg B. Acute Flaccid Myelitis CAPTURE Data Set. Presented at: CDC 2018; December 17-19, 2018; Miami, FL.
- Greenberg B. Acute flaccid myelitis. Presented at: American Academy of Neurology Annual Meeting; May 4-10, 2019; Philadelphia, PA.
Acknowledgments
The CAPTURE study group wishes to acknowledge the financial and collaborative support from PCORI. Furthermore, the CAPTURE study group wishes to acknowledge the incredible contributions from patients and families who took part in the study. During a time of immense personal stress, families were able to provide medical records, MRI results, and survey data that made this study possible. Finally, the CAPTURE study could not have been conducted without our partnership with the TMA.
Research reported in this report was funded through a Patient-Centered Outcomes Research Institute® (PCORI®) Award (#CE-1304-7079). Further information available at: https://www.pcori.org/research-results/2013/does-plasma-exchange-help-improve-physical-function-children-transverse
Appendix
Table A1.
Table A2.
Table A3.
Change per month in WeeFIM scores from earliest collected timepoint to 12 months after symptom onset (PDF, 70K)
Table A4.
Parent Proxy PROMIS scores at 6 months after symptom onset (PDF, 95K)
Table A5.
Parent Proxy PROMIS scores at 12 months after symptom onset (PDF, 102K)
Suggested citation:
Greenberg BM, Pardo C, Recio A, et al. (2021). Does Plasma Exchange Help Improve Physical Function in Children with Transverse Myelitis? – The CAPTURE Study. Patient-Centered Outcomes Research Institute (PCORI). https://doi.org/10.25302/02.2021.CE.13047079
Disclaimer
The [views, statements, opinions] presented in this report are solely the responsibility of the author(s) and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute® (PCORI®), its Board of Governors or Methodology Committee.
- Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.[Cochrane Database Syst Rev. 2022]Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, et al. Cochrane Database Syst Rev. 2022 Feb 1; 2(2022). Epub 2022 Feb 1.
- The future of Cochrane Neonatal.[Early Hum Dev. 2020]The future of Cochrane Neonatal.Soll RF, Ovelman C, McGuire W. Early Hum Dev. 2020 Nov; 150:105191. Epub 2020 Sep 12.
- A multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin compared with standard therapy for the treatment of transverse myelitis in adults and children (STRIVE).[Health Technol Assess. 2017]A multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin compared with standard therapy for the treatment of transverse myelitis in adults and children (STRIVE).Absoud M, Brex P, Ciccarelli O, Diribe O, Giovannoni G, Hellier J, Howe R, Holland R, Kelly J, McCrone P, et al. Health Technol Assess. 2017 May; 21(31):1-50.
- Review Novel Augmentation Strategies in Major Depression.[Dan Med J. 2017]Review Novel Augmentation Strategies in Major Depression.Martiny K. Dan Med J. 2017 Apr; 64(4).
- Review Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force[ 2020]Review Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task ForcePatnode CD, Perdue LA, Rossom RC, Rushkin MC, Redmond N, Thomas RG, Lin JS. 2020 Feb
- Does Plasma Exchange Help Improve Physical Function in Children with Transverse ...Does Plasma Exchange Help Improve Physical Function in Children with Transverse Myelitis? – The CAPTURE Study
Your browsing activity is empty.
Activity recording is turned off.
See more...
