NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
1. Step 4 treatment
1.1. Review question: What is the most clinically and cost-effective sequence for step 4 treatment for hypertension?
1.2. Introduction
Antihypertensive treatment is usually very effective in lowering blood pressure to within normal limits. However, in some individuals, blood pressure remains elevated despite being prescribed multiple antihypertensive medications, and these individuals remain at an elevated risk of cardiovascular events. The term ‘resistant hypertension’ is commonly applied to individuals who are prescribed 3 antihypertensive medications including a diuretic, but their blood pressure remains above the target. Those with resistant hypertension have double the risk of cardiovascular events than those without resistant hypertension, thus making them an important group to study. Estimates vary as to what proportion of those with hypertension have ‘resistant hypertension’, but it is generally thought to be around 5%.
Current clinical practice when selecting a step 4 treatment is to choose 1 of a number of medications based on the person’s and the clinician’s preference without robust evidence as to which might lower blood pressure the most effectively. During the guideline scoping process, a number of recently published clinical studies were highlighted that were designed to identify which medication(s) would be the optimal choice as step 4 treatment. In this chapter, the evidence for choosing a step 4 medication was reviewed.
1.3. PICO table
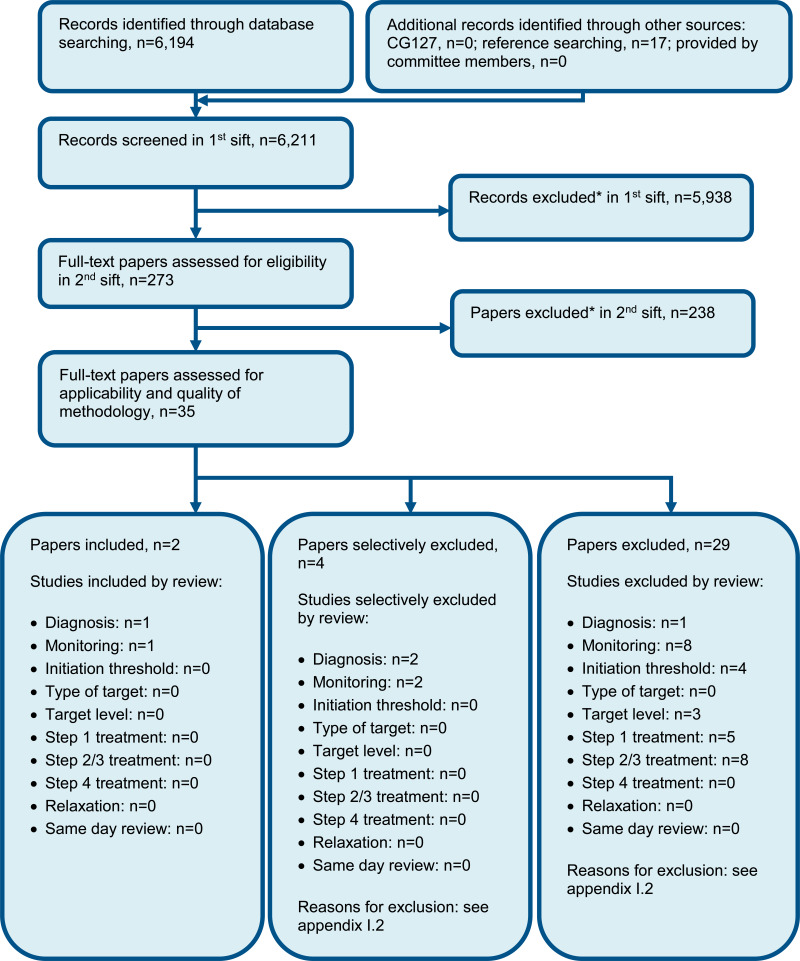
For full details, see the review protocol in appendix A.
1.4. Clinical evidence
1.4.1. Included studies
No relevant clinical studies comparing step 4 antihypertensive pharmacological treatment received for a minimum of 1 year were identified.
See also the study selection flow chart in appendix C, study evidence tables in appendix D, forest plots in appendix E and GRADE tables in appendix F.
1.4.2. Excluded studies
One Cochrane review relevant to this review question was identified.44 This was excluded because it included crossover studies without the minimum washout period of 4 weeks as required for inclusion within this review. The references were checked for any relevant studies.
See the excluded studies list in appendix I. Table 14 outlines the full excluded studies list, and Table 13 provides additional detail of studies that were included in the previous guideline iteration (CG127) but excluded from this update.
See the excluded studies list in appendix I.
1.5. Economic evidence
1.5.1. Included studies
No relevant health economic studies were identified.
1.5.2. Excluded studies
No health economic studies that were relevant to this question were excluded due to assessment of limited applicability or methodological limitations.
See also the health economic study selection flow chart in appendix G.
1.5.3. Resource costs
Costs are illustrated below for average doses of the most commonly used drugs from each class listed on the review protocol, based on committee opinion.
1.6. Evidence statements
1.6.1. Clinical evidence statements
No relevant published evidence was identified.
1.6.2. Health economic evidence statements
No relevant economic evaluations were identified.
1.7. The committee’s discussion of the evidence
1.7.1. Interpreting the evidence
1.7.1.1. The outcomes that matter most
The committee considered all-cause mortality, quality of life, stroke and myocardial infarction to be critical outcomes for decision-making. Heart failure, vascular procedures, angina and discontinuation or dose reduction due to side effects were also considered important for decision-making.
No relevant clinical studies were identified therefore no evidence was available for any of these outcomes.
1.7.1.2. The quality of the evidence
No clinical studies relevant to the review question were identified.
1.7.1.3. Benefits and harms
No clinical studies relevant to this review protocol were identified.
The committee discussed the use of different step 4 antihypertensive treatments. It agreed that there was very little evidence within this area, so the committee formed consensus recommendations based on their clinical experience. They were aware of a trial (PATHWAY-2) that assessed the step 4 treatment in resistant hypertension. Afterdiscussing the findings of the PATHWAY-2 trial, they agreed that this study did not meet the inclusion criteria for this review due to having a short follow-up and no outcomes relevant to the agreed protocol. Nevertheless, it suggested that adding spironolactone could be effective at reducing blood pressure as a step 4 treatment. It was noted that higher doses of spironolactone were used (25 mg–50 mg), and the 50 mg dose was noted to lower blood pressure more. However, it was unclear what proportion of people were receiving the 50 mg dose. The study also suggested that amiloride could be as effective as spironolactone in lowering blood pressure. However, the committee noted that amiloride is more expensive, and it is taken twice a day, whereas spironolactone is taken only once daily making it a more convenient option for people who are already taking multiple medications. The committee agreed that changes in blood pressure alone, without information on cardiovascular events was not very informative to patient important outcomes, however they agreed that there was no evidence to suggest a better treatment option was available than spironolactone, which was now part of common clinical practice, and so it should still be recommended as step 4 treatment for those who had an inadequate response to 3 previous treatments.
It was discussed that the previous spironolactone dose recommendation of 25 mg once daily was too specific given the limited evidence base; instead, the committee decided to leave this more open as a ‘low-dose’ if the potassium level was 4.5 mmol/l or lower. The committee suggested that they were aware of recent evidence, outside of the remit for this reviwew, which suggested a smaller dose of 12.5 mg could be effective as a step 4 treatment. The committee also agreed that there was no evidence with hard outcomes data to warrant recommending a higher dose thiazide in people with higher potassium levels, and it was agreed that in this case alpha- or beta-blockers should be considered instead, as higher dose thiazide diuretics are not more effective than lower dose thiazide diuretics.
The need for further research to inform choice of step 4 treatment was discussed; however, the committee considered this would be unlikely to be funded, as the PATHWAY-2 trial had addressed this question previously, despite not including the hard cardiovascular outcomes this committee considered necessary to make a strong recommendation on the topic.
The committee discussed the need to seek specialist advice in order to investigate alternative reasons for a lack of response to treatment, such as adherence issues or secondary causes of hypertension to better manage treatment. The previous guideline recommendation stated that specialist advice should be sought regardless of whether a fourth antihypertensive drug was already added. The committee agreed that its clinical experience suggested the decision to seek specialist advice would be made on a case-by-case basis, but generally, it would either be appropriate to treat a person with resistant hypertension or to seek specialist advice. The committee highlighted the importance of taking the person’s preference into account, particularly where people might be concerned that they are already on 3 drugs and hadn’t responded well to these. The committee therefore agreed to reword the previous recommendation to clarify that either option should be considered.
The committee discussed the long-term implications of spironolactone treatment. Although there was no evidence identified for this within the review, including a lack of information on adverse events, the committee agreed that the multiple known harms of consistently high blood pressure outweighed this uncertainty. They did agree, however, that further evidence was required in order for healthcare professionals and people with hypertension to understand the choice of drugs available and the benefit and harms associated with each of these.
The committee discussed the use of ambulatory or home blood pressure measurement to confirm elevated blood pressure levels based on their experience and current practice. It was agreed that this is generally the method used in current practice to confirm resistant hypertension. Although there could be some variation in current practice, the committee agreed that this is the best and most accurate method of identifying people with resistant hypertension. Screening for postural hypotension was also considered an important factor to include in a recommendation, as it could affect whether additional treatment could be harmful.
1.7.2. Cost effectiveness and resource use
No economic evidence was identified for this question.
The drugs that could be used for resistant hypertension can vary in price; for example, amiloride hydrochloride is more expensive than spironolactone. The population affected with resistant hypertension, although being a small proportion of those with hypertension (around 6%), still results in a large amount of people given the size of the hypertensive population.
It was discussed how the measurement method to confirm resistant hypertension is important and best practice would be to confirm elevated measurements using ambulatory or home blood pressure recordings. This has been added as a recommendation and is generally already believed to be current practice. But where it is not, it will be of benefit because it will more accurately identify those with resistant hypertension. The committee considered that the population on 3 drugs who actually have resistant hypertension is likely to be smaller than those labelled as having resistant hypertension. This could mean a reduction in treatment as there might be some overtreatment of resistant hypertension in practice due to inappropriate measurement (overtreatment can however also be because people are not properly adhering to their medication, rather than their medication is not working – although this is more difficult to identify). There might be some additional diagnostic costs involved if some areas do not currently confirm resistant hypertension in this way, but this depends on the measurement method; for example, if someone is already using home monitoring with their own device then that person could use the same method to diagnose if the hypertension is resistant.
There was no clinical evidence identified; therefore, the committee agreed to carry forward previous recommendations with some minor amendments. These included deleting a recommendation on considering higher dose thiazide-like diuretic therapy for those with high blood potassium levels, as this was not considered to be current practice and people would generally go onto step 4 of alpha or beta-blockers.
It was also discussed how the recommendation around seeking specialist advice for those in whom blood pressure was uncontrolled on 3 drugs was unclear, as it stated specialist advice should be sought even if a step 4 treatment was already added. The committee’s opinion was that not all clinicians would seek specialist advice, as some would be more comfortable trying a step 4 treatment and some would prefer to seek advice first. The recommendation was changed to make it clearer that step 4 treatment could be considered or specialist advice could be sought. As the previous recommendation was a consider recommendation, practice was variable as to whether people were seeking specialist advice; therefore, this wording change is unlikely to have an impact on practice. It was also discussed whether it should be specified if seeking advice means referring a person to a more specialist service, or if it should be stated who this individual might be. However, the committee agreed that asking for advice is more flexible because the advice may well be to refer the person, or it may be more of an informal discussion between clinicians. Additionally, specifying whether the specialist should be a hypertension specialist was thought to be too restrictive because the specialist could also be another role such as a cardiologist, nephrologist or endocrinologist and would really depend on local services.
On balance, the recommendations are not expected to have a resource impact.
References
- 1.
- Abarquez RF, Jr., Sy RG, Castillo RR. Efficacy of slow-release oral isradipine in moderate-to-severe hypertension with add-on spirapril. American Journal of Hypertension. 1993; 6(3 Pt 2):77S–79S [PubMed: 8466734]
- 2.
- Abascal VM, Larson MG, Evans JC, Blohm AT, Poli K, Levy D. Calcium antagonists and mortality risk in men and women with hypertension in the Framingham Heart Study. Archives of Internal Medicine. 1998; 158(17):1882–6 [PubMed: 9759683]
- 3.
- Abe H, Minatoguchi S, Ohashi H, Murata I, Minagawa T, Okuma T et al. Renoprotective effect of the addition of losartan to ongoing treatment with an angiotensin converting enzyme inhibitor in type-2 diabetic patients with nephropathy. Hypertension Research. 2007; 30(10):929–35 [PubMed: 18049024]
- 4.
- Abe M, Okada K, Matsumoto K. Clinical experience in treating hypertension with fixed-dose combination therapy: Angiotensin II receptor blocker losartan plus hydrochlorothiazide. Expert Opinion on Drug Metabolism & Toxicology. 2009; 5(10):1285–303 [PubMed: 19761411]
- 5.
- Abetel G, Mérier G, Karly M, Genoud A, Bousquet JC. Value of a blood pressure profile in evaluating 2 antihypertensive agents. Schweizerische Medizinische Wochenschrift. 1984; 114(48):1746–9 [PubMed: 6151741]
- 6.
- Adir J, Janda SM, Curry CL, Taylor RE, Poku CD, Rotenberg KS. Comparative efficacy and safety of immediate-release and controlled-release hydralazine in black hypertensive patients. Clinical Therapeutics. 1987; 9(6):640–50 [PubMed: 3326679]
- 7.
- Adolphe AB, Vlachakis ND, Rofman BA, Brescia D, Zellner SR. Long-term open evaluation of amlodipine vs hydrochlorothiazide in patients with essential hypertension. International Journal of Clinical Pharmacology Research. 1993; 13(4):203–10 [PubMed: 8150546]
- 8.
- Agabiti-Rosei E, Ambrosioni E, Finardi G, Folino P, Gambassi G, Malini P et al. Perindopril versus captopril: Efficacy and acceptability in an Italian multicenter trial. American Journal of Medicine. 1992; 92(4B):79S–83S [PubMed: 1580285]
- 9.
- Agabiti-Rosei E, Trimarco B, Muiesan ML, Reid J, Salvetti A, Tang R et al. Cardiac structural and functional changes during long-term antihypertensive treatment with lacidipine and atenolol in the European Lacidipine Study on Atherosclerosis (ELSA). Journal of Hypertension. 2005; 23(5):1091–8 [PubMed: 15834297]
- 10.
- Agarwal R, Weir MR. Blood pressure response with fixed-dose combination therapy: Comparing hydrochlorothiazide with amlodipine through individual-level meta-analysis. Journal of Hypertension. 2013; 31(8):1692–701 [PubMed: 23697963]
- 11.
- Ahola TL, Kantola IM, Maki J, Reunanen A, Jula AM. Adding a low-dose antihypertensive regimen would substantially improve the control of hypertension and reduce cardiovascular morbidity among uncomplicated hypertensive patients. European Journal of Preventive Cardiology. 2012; 19(4):712–22 [PubMed: 21609976]
- 12.
- Ahrens K, Bramlage P. Importance of a fixed combination of telmisartan and amlodipine for the treatment of hypertension. Drugs of Today. 2010; 46(5):339–50 [PubMed: 20517535]
- 13.
- Akanabe H, Ishiguro M, Yagi Y, Ohshima S, Ohmae M, Mori H et al. Effect of diltiazem hydrochloride in essential hypertension. International Journal of Clinical Pharmacology, Therapy, and Toxicology. 1985; 23(2):63–9 [PubMed: 3886566]
- 14.
- Akioyamen L, Levine M, Sherifali D, O’Reilly D, Frankfurter C, Pullenayegum E et al. Cardiovascular and cerebrovascular outcomes of long-term angiotensin receptor blockade: Meta-analyses of trials in essential hypertension. Journal of the American Society of Hypertension. 2016; 10(1):55–69 [PubMed: 26684588]
- 15.
- Akram J, Sheikh UE, Mahmood M, Donnelly R. Antihypertensive efficacy of indapamide SR in hypertensive patients uncontrolled with a background therapy: The NATIVE study. Current Medical Research and Opinion. 2007; 23(12):2929–36 [PubMed: 17931463]
- 16.
- Alderman MH. Evaluation of the efficacy of prazosin versus propranolol as initial antihypertensive therapy. American Journal of Medicine. 1989; 86(1B):45–9 [PubMed: 2643865]
- 17.
- Alici G, Aliyev F, Bellur G, Okcun B, Turkoglu C, Karpuz H. Effect of seven different modalities of antihypertensive therapy on pulse pressure in patients with newly diagnosed stage I hypertension. Cardiovascular Therapeutics. 2009; 27(1):4–9 [PubMed: 19207474]
- 18.
- ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). JAMA. 2000; 283(15):1967–1975 [PubMed: 10789664]
- 19.
- ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002; 288(23):2981–97 [PubMed: 12479763]
- 20.
- Alviar CL, Devarapally S, Nadkarni GN, Romero J, Benjo AM, Javed F et al. Efficacy and safety of dual calcium channel blockade for the treatment of hypertension: A meta-analysis. American Journal of Hypertension. 2013; 26(2):287–97 [PubMed: 23382415]
- 21.
- Amar J, Joire JE, Salvador M. Study of the efficacity and tolerance of diltiazem LP 300 mg in 2000 hypertensive patients (alone or combined with an angiotensin converting enzyme inhibitor). Annales de Cardiologie et d’Angeiologie. 1999; 48(1):69–75 [PubMed: 12555361]
- 22.
- Ames R, Griffing G, Marbury T, Miller E, Schoenberger J, Glenn B et al. Effectiveness of indapamide versus enalapril as second-step therapy of systemic hypertension. American Journal of Cardiology. 1992; 69(3):267–70 [PubMed: 1731472]
- 23.
- Amir M, Cristal N, Bar-On D, Loidl A. Does the combination of ACE inhibitor and calcium antagonist control hypertension and improve quality of life? The LOMIR-MCT-IL study experience. Blood Pressure Supplement. 1994; 1:40–2 [PubMed: 8205297]
- 24.
- Andersen H, Botta G, Galasse R, Hill JF. Efficacy of captopril and hydrochlorothiazide administered once a day. Postgraduate Medical Journal. 1986; 62:(Suppl 1):146–9 [PubMed: 3534851]
- 25.
- Andersen NH, Knudsen ST, Poulsen PL, Poulsen SH, Helleberg K, Eiskjaer H et al. Dual blockade with candesartan cilexetil and lisinopril in hypertensive patients with diabetes mellitus: Rationale and design. Journal of the Renin-Angiotensin-Aldosterone System. 2003; 4(2):96–9 [PubMed: 12806591]
- 26.
- Andersen NH, Poulsen PL, Knudsen ST, Poulsen SH, Eiskjaer H, Hansen KW et al. Long-term dual blockade with candesartan and lisinopril in hypertensive patients with diabetes: The CALM II study. Diabetes Care. 2005; 28(2):273–7 [PubMed: 15677778]
- 27.
- Ando K, Ohtsu H, Uchida S, Kaname S, Arakawa Y, Fujita T et al. Anti-albuminuric effect of the aldosterone blocker eplerenone in non-diabetic hypertensive patients with albuminuria: A double-blind, randomised, placebo-controlled trial. The Lancet Diabetes & Endocrinology. 2014; 2(12):944–53 [PubMed: 25466242]
- 28.
- Andreadis EA, Tsourous GI, Marakomichelakis GE, Katsanou PM, Fotia ME, Vassilopoulos CV et al. High-dose monotherapy vs low-dose combination therapy of calcium channel blockers and angiotensin receptor blockers in mild to moderate hypertension. Journal of Human Hypertension. 2005; 19(6):491–6 [PubMed: 15759025]
- 29.
- Andren L, Svensson A, Hansson L. Captopril or atenolol in essential hypertension. Acta Medica Scandinavica Supplementum. 1983; 677:115–8 [PubMed: 6367371]
- 30.
- Andreucci VE, Usberti M, Federico S, Pecoraro C, Balletta M, Meccariello S. Long-term follow-up of minoxidil therapy in refractory hypertension. A prospective trial in patients with various degrees of renal insufficiency. Clinical Nephrology. 1983; 19(2):55–60 [PubMed: 6340875]
- 31.
- Angeli F, Verdecchia P, Reboldi GP, Gattobigio R, Bentivoglio M, Staessen JA et al. Calcium channel blockade to prevent stroke in hypertension: A meta-analysis of 13 studies with 103,793 subjects. American Journal of Hypertension. 2004; 17(9):817–22 [PubMed: 15363825]
- 32.
- Anonymous. The Nordic Diltiazem Study (NORDIL): A prospective intervention trial of calcium antagonist therapy in hypertension. Blood Pressure. 1993; 2(4):312–21 [PubMed: 8173702]
- 33.
- Anonymous. Efficacy and tolerability of losartan versus enalapril alone or in combination with hydrochlorothiazide in patients with essential hypertension. Cardiovascular Reviews and Reports. 1996; 17(1):57–58
- 34.
- Anonymous. Randomized double-blind comparison of a calcium antagonist and a diuretic in elderly hypertensives. National Intervention Cooperative Study in Elderly Hypertensives Study Group. Hypertension. 1999; 34(5):1129–33 [PubMed: 10567194]
- 35.
- Applegate WB, Byington RP. MIDAS, the Multicenter Isradipine/Diuretic Atherosclerosis Study. Design features and baseline data. American Journal of Hypertension. 1991; 4(2 Pt 2):114S–117S [PubMed: 1827000]
- 36.
- Arima H, Anderson C, Omae T, Woodward M, MacMahon S, Mancia G et al. Degree of blood pressure reduction and recurrent stroke: The PROGRESS trial. Journal of Neurology, Neurosurgery and Psychiatry. 2014; 85(11):1284–5 [PubMed: 24828894]
- 37.
- Arriaga-Gracia J, Sanchez-Garcia JL, Gonzalez-Garcia CA. Nicardipine or propranolol combined with hydrochlorothiazide in patients with essential hypertension. Proceedings of the Western Pharmacology Society. 1993; 36:39–43 [PubMed: 8378396]
- 38.
- Bakris G, Briasoulis A, Dahlof B, Jamerson K, Weber MA, Kelly RY et al. Comparison of benazepril plus amlodipine or hydrochlorothiazide in high-risk patients with hypertension and coronary artery disease. American Journal of Cardiology. 2013; 112(2):255–9 [PubMed: 23582626]
- 39.
- Bakris GL, Cooper-Dehoff RM, Zhou Q, Kupfer S, Champion A, Pepine CJ et al. Dual therapy in hypertensive patients with coronary artery disease: The role of calcium channel blockers and beta-blockers. American Journal of Cardiovascular Drugs. 2007; 7:(Suppl 1):25–9 [PubMed: 19845074]
- 40.
- Balamuthusamy S, Molnar J, Adigopula S, Arora R. Comparative analysis of beta-blockers with other antihypertensive agents on cardiovascular outcomes in hypertensive patients with diabetes mellitus: A systematic review and meta-analysis. American Journal of Therapeutics. 2009; 16(2):133–42 [PubMed: 19145207]
- 41.
- Baldwin SP, Harless WT, Lacy CA, Motley JF, Rietbrock MJ, Sehy JT. A comparative study of the efficacy and side effects of metolazone 1/2 mg tablets (Microx) vs triamterene 50 mg plus hydrochlorothiazide 25 mg in the treatment of mild hypertension. Advances in Therapy. 1987; 4(6):265–278
- 42.
- Bang CN, Soliman EZ, Simpson LM, Davis BR, Devereux RB, Okin PM et al. Electrocardiographic left ventricular hypertrophy predicts cardiovascular morbidity and mortality in hypertensive patients: The ALLHAT study. American Journal of Hypertension. 2017; 30(9):914–922 [PMC free article: PMC5861536] [PubMed: 28430947]
- 43.
- Bangalore S, Wild D, Parkar S, Kukin M, Messerli FH. Beta-blockers for primary prevention of heart failure in patients with hypertension insights from a meta-analysis. Journal of the American College of Cardiology. 2008; 52(13):1062–72 [PubMed: 18848139]
- 44.
- Batterink J, Stabler SN, Tejani AM, Fowkes CT. Spironolactone for hypertension. Cochrane Database of Systematic Reviews 2010, Issue 8. Art. No.: CD008169. DOI: 10.1002/14651858.CD008169.pub2. [PubMed: 20687095] [CrossRef]
- 45.
- Benjamin N, Phillips RJ, Robinson BF. Verapamil and bendrofluazide in the treatment of hypertension: A controlled study of effectiveness alone and in combination. European Journal of Clinical Pharmacology. 1988; 34(3):249–53 [PubMed: 3294021]
- 46.
- Berger A, Chima P, Dawes M, Davey NB, Grundy PF, Lee PS et al. A fixed combination of felodipine 5 mg and metoprolol 50 mg compared with double doses of the individual components as antihypertensive therapy. Journal of Drug Development. 1992; 4(4):199–206
- 47.
- Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB et al. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003; 289(16):2073–2082 [PubMed: 12709465]
- 48.
- Blumenthal JA, Ekelund LG, Emery CF. Quality of life among hypertensive patients with a diuretic background who are taking atenolol and enalapril. Clinical Pharmacology and Therapeutics. 1990; 48(4):447–54 [PubMed: 2225705]
- 49.
- BMJ Group and the Royal Pharmaceutical Society of Great Britain. British National Formulary. Available from: https://www
.evidence .nhs.uk/formulary/bnf/current Last accessed: 08 November 2018 - 50.
- Boissel JP, Collet JP, Lion L, Ducruet T, Moleur P, Luciani J et al. A randomized comparison of the effect of four antihypertensive monotherapies on the subjective quality of life in previously untreated asymptomatic patients: Field trial in general practice. Journal of Hypertension. 1995; 13(9):1059–67 [PubMed: 8586825]
- 51.
- Borgmastars H, Forsen B, Tuomilehto J, Hellebo R, Walle PO, Nielsen HM et al. Felodipine versus hydrochlorothiazide as an addition to a beta-blocker in the treatment of hypertension. Drugs. 1987; 34:(Suppl 3):136–8 [PubMed: 2894972]
- 52.
- Bremner AD, Mehring GH, Meilenbrock S. Long-term systemic tolerability of valsartan compared with lisinopril in elderly hypertensive patients. Advances in Therapy. 1997; 14(5):245–253
- 53.
- Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. New England Journal of Medicine. 2001; 345(12):861–9 [PubMed: 11565518]
- 54.
- Brown MJ, Palmer CR, Castaigne A, De Leeuw PW, Mancia G, Rosenthal T et al. Principal results from the international nifedipine GITS Study: Intervention as a goal in hypertension treatment (INSIGHT). European Heart Journal, Supplement. 2001; 3(Suppl B):B20–B26
- 55.
- Byrd JB, Zeng C, Tavel HM, Magid DJ, O’Connor PJ, Margolis KL et al. Combination therapy as initial treatment for newly diagnosed hypertension. American Heart Journal. 2011; 162(2):340–6 [PMC free article: PMC3153357] [PubMed: 21835296]
- 56.
- Byyny RL. Antihypertensive efficacy of the angiotensin II AT1-receptor antagonist losartan: Results of a randomized, double-blind, placebo-controlled, parallel-group trial using 24-hour blood pressure monitoring. Blood Pressure Supplement. 1996; 5:71–7 [PubMed: 8913544]
- 57.
- Castano G, Mas R, Gamez R, Fernandez J, Illnait J, Fernandez L et al. Concomitant use of policosanol and beta-blockers in older patients. International Journal of Clinical Pharmacology Research. 2004; 24(2–3):65–77 [PubMed: 15689053]
- 58.
- Celis H, Yodfat Y, Thijs L, Clement D, Cozic J, De Cort P et al. Antihypertensive therapy in older patients with isolated systolic hypertension: The Syst-Eur experience in general practice. Family Practice. 1996; 13(2):138–43 [PubMed: 8732324]
- 59.
- Cesaris R, Ranieri G, Chiarappa R. Nadolol vs. chlortalidone in hypertensive patients unresponsive to treatment with captopril alone. Clinica Terapeutica. 1986; 116(6):465–471 [PubMed: 3522061]
- 60.
- Chapman N, Dobson J, Wilson S, Dahlof B, Sever PS, Wedel H et al. Effect of spironolactone on blood pressure in subjects with resistant hypertension. Hypertension. 2007; 49(4):839–45 [PubMed: 17309946]
- 61.
- Chatellier G, Sassano P, Amiot AM, Corvol P, Menard J. Efficacy and influence on quality of life of enalapril as a first step treatment of hypertension. Clinical and Experimental Hypertension Part A: Theory and Practice. 1987; 9(2–3):513–9 [PubMed: 3038415]
- 62.
- Chi C, Tai C, Bai B, Yu S, Karamanou M, Wang J et al. Angiotensin system blockade combined with calcium channel blockers is superior to other combinations in cardiovascular protection with similar blood pressure reduction: A meta-analysis in 20,451 hypertensive patients. Journal of Clinical Hypertension. 2016; 18(8):801–8 [PMC free article: PMC8032162] [PubMed: 26778747]
- 63.
- Chrysant SG, Cohen M. Long-term antihypertensve effects with chronic administration of isradipine controlled release. Current Therapeutic Research, Clinical and Experimental. 1997; 58(1):1–9
- 64.
- Circelli M, Nicolini G, Egan CG, Cremonesi G. Efficacy and safety of delapril/indapamide compared to different ACE-inhibitor/hydrochlorothiazide combinations: A meta-analysis. International Journal of General Medicine. 2012; 5:725–34 [PMC free article: PMC3459665] [PubMed: 23049265]
- 65.
- Coope J, Warrender TS. Randomised trial of treatment of hypertension in elderly patients in primary care. BMJ. 1986; 293(6555):1145–51 [PMC free article: PMC1341855] [PubMed: 3094811]
- 66.
- Correa A, Rochlani Y, Khan MH, Aronow WS. Pharmacological management of hypertension in the elderly and frail populations. Expert Review of Clinical Pharmacology. 2018; 11(8):805–817 [PubMed: 30004797]
- 67.
- Cowley AJ, Wynne RD, Hampton JR. Flosequinan as a third agent for the treatment of hypertension: A placebo controlled, double-blind study. European Journal of Clinical Pharmacology. 1987; 33(2):203–4 [PubMed: 2891533]
- 68.
- Cranston WI, Juel-Jensen BE. The effects of spironolactone and chlorthalidone on arterial pressure. The Lancet. 1962; 1(7240):1161–1164 [PubMed: 13882033]
- 69.
- Curb JD, Pressel SL, Cutler JA, Savage PJ, Applegate WB, Black H et al. Effect of diuretic-based antihypertensive treatment on cardiovascular disease risk in older diabetic patients with isolated systolic hypertension. Systolic Hypertension in the Elderly Program Cooperative Research Group. JAMA. 1996; 276(23):1886–92 [PubMed: 8968014]
- 70.
- Daae LN, Westlie L. A 5-year comparison of doxazosin and atenolol in patients with mild-to-moderate hypertension: Effects on blood pressure, serum lipids, and coronary heart disease risk. Blood Pressure. 1998; 7(1):39–45 [PubMed: 9551876]
- 71.
- Dahlöf B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): A multicentre randomised controlled trial. The Lancet. 2005; 366(9489):895–906 [PubMed: 16154016]
- 72.
- Dahlof B, Zanchetti A, Diez J, Nicholls MG, Yu CM, Barrios V et al. Effects of losartan and atenolol on left ventricular mass and neurohormonal profile in patients with essential hypertension and left ventricular hypertrophy. Journal of Hypertension. 2002; 20(9):1855–64 [PubMed: 12195129]
- 73.
- Daien V, Duny Y, Ribstein J, du Cailar G, Mimran A, Villain M et al. Treatment of hypertension with renin-angiotensin system inhibitors and renal dysfunction: A systematic review and meta-analysis. American Journal of Hypertension. 2012; 25(1):126–32 [PubMed: 21993366]
- 74.
- De Rosa ML, Cardace P, Rossi M, Baiano A, De Cristofaro A. Evaluation of long-term efficacy and tolerability of irbesartan in elderly hypertensive patients with renal impairment in an open-label study. Current Therapeutic Research, Clinical and Experimental. 2002; 63(3):201–215
- 75.
- de Souza F, Muxfeldt E, Fiszman R, Salles G. Efficacy of spironolactone therapy in patients with true resistant hypertension. Hypertension. 2010; 55(1):147–52 [PubMed: 19858405]
- 76.
- Degl’Innocenti A, Elmfeldt D, Hofman A, Lithell H, Olofsson B, Skoog I et al. Health-related quality of life during treatment of elderly patients with hypertension: Results from the Study on COgnition and Prognosis in the Elderly (SCOPE). Journal of Human Hypertension. 2004; 18(4):239–245 [PubMed: 15037872]
- 77.
- Destro M, Cagnoni F, D’Ospina A, Ricci AR, Demichele E, Peros E et al. Role of valsartan, amlodipine and hydrochlorothiazide fixed combination in blood pressure control: an update. Vascular Health and Risk Management. 2010; 6:253–60 [PMC free article: PMC2856580] [PubMed: 20407632]
- 78.
- Devereux RB, de Faire U, Fyhrquist F, Harris KE, Ibsen H, Kjeldsen SE et al. Blood pressure reduction and antihypertensive medication use in the losartan intervention for endpoint reduction in hypertension (LIFE) study in patients with hypertension and left ventricular hypertrophy. Current Medical Research and Opinion. 2007; 23(2):259–70 [PubMed: 17288679]
- 79.
- Dews I, VandenBurg M. A 52-week, open-label, dose-titration safety study of imidapril in the treatment of mild to moderate hypertension. Current Therapeutic Research - Clinical and Experimental. 2001; 62(2):167–176
- 80.
- Diao D, Wright JM, Cundiff DK, Gueyffier F. Pharmacotherapy for mild hypertension. Cochrane Database of Systematic Reviews 2012, Issue 8. Art. No.: CD006742. DOI: 10.1002/14651858.CD006742.pub2. [PMC free article: PMC8985074] [PubMed: 22895954] [CrossRef]
- 81.
- Du LP, Cheng ZW, Zhang YX, Li Y, Mei D. The impact of fixed-dose combination versus free-equivalent combination therapies on adherence for hypertension: A meta-analysis. Journal of Clinical Hypertension. 2018; 20(5):902–907 [PMC free article: PMC8030969] [PubMed: 29700923]
- 82.
- Ekbom T, Dahlof B, Hansson L, Lindholm LH, Schersten B, Wester PO. Antihypertensive efficacy and side effects of three beta-blockers and a diuretic in elderly hypertensives: A report from the STOP-Hypertension study. Journal of Hypertension. 1992; 10(12):1525–30 [PubMed: 1338084]
- 83.
- Ekbom T, Linjer E, Hedner T, Lanke J, De Faire U, Wester PO et al. Cardiovascular events in elderly patients with isolated systolic hypertension. A subgroup analysis of treatment strategies in STOP-Hypertension-2. Blood Pressure. 2004; 13(3):137–41 [PubMed: 15223721]
- 84.
- Estacio RO, Schrier RW. Antihypertensive therapy in type 2 diabetes: Implications of the appropriate blood pressure control in diabetes (ABCD) trial. American Journal of Cardiology. 1998; 82(9 Suppl 1):9–14 [PubMed: 9822137]
- 85.
- Family Physicians Hypertension Study Group, Cajochen C, Krauchi K, Von AMA, Mori D, Graw P et al. A multicenter comparison of the antihypertensive effects of atenolol and chlorthalidone given alone and in combination. Current Therapeutic Research, Clinical and Experimental. 1984; 35(1):31–39
- 86.
- Fariello R, Dal Palu C, Pessina A, Semplicini A, Pirrelli A, Vulpis V et al. Antihypertensive efficacy of urapidil versus hydrochlorothiazide alone in patients with mild to moderate essential hypertension and of their combination in nonresponders to monotherapy. Drugs. 1990; 40:(Suppl 4):60–2 [PubMed: 2092970]
- 87.
- Farsang C, Lengyel M, Borbas S, Zorandi A, Szoradi Dienes B. Value of rilmenidine therapy and its combination with perindopril on blood pressure and left ventricular hypertrophy in patients with essential hypertension (VERITAS). Current Medical Research and Opinion. 2003; 19(3):205–217 [PubMed: 12803735]
- 88.
- Fasano ML, Soro S, Ferrara LA. Long-term antihypertensive efficacy of ketanserin plus chlorthalidone. Drugs Under Experimental and Clinical Research. 1989; 15(11–12):587–90 [PubMed: 2700322]
- 89.
- Faust G. One-year study of nilvadipine administered once a day. Efficacy and long-term tolerability in hypertensives. Fortschritte der Medizin. 1993; 111(11):46–50 [PubMed: 8508996]
- 90.
- Faust G. A one year study of single administration of nilvadipine. Results of the effectiveness and long-term tolerance in hypertension. Fortschritte der Medizin. 1993; 111(11):188–192 [PubMed: 8508996]
- 91.
- Ferdinand KC. Advances in antihypertensive combination therapy: benefits of low-dose thiazide diuretics in conjunction with omapatrilat, a vasopeptidase inhibitor. Journal of Clinical Hypertension. 2001; 3(5):307–12 [PMC free article: PMC8101848] [PubMed: 11588409]
- 92.
- Fernandes LA, Cestario ED, Cosenso-Martin LN, Vilela-Martin JF, Yugar-Toledo JC, Fuchs FD. Chlorthalidone plus amiloride reduces the central systolic blood pressure in stage 1 hypertension patients. Cardiology Research. 2016; 7(6):196–201 [PMC free article: PMC5295510] [PubMed: 28197292]
- 93.
- Fernandez R, Puig JG, Rodriguez-Perez JC, Garrido J, Redon J, Group TS. Effect of two antihypertensive combinations on metabolic control in type-2 diabetic hypertensive patients with albuminuria: A randomised, double-blind study. Journal of Human Hypertension. 2001; 15(12):849–56 [PubMed: 11773987]
- 94.
- Ferrara LA, de Simone G, Mancini M, Fasano ML, Pasanisi F, Vallone G. Changes in left ventricular mass during a double-blind study with chlorthalidone and slow-release nifedipine. European Journal of Clinical Pharmacology. 1984; 27(5):525–8 [PubMed: 6394350]
- 95.
- Finnerty FA, Jr., Gyftopoulos A, Berry C, McKenney A. Step 2 regimens in hypertension. An assessment. JAMA. 1979; 241(6):579–81 [PubMed: 762813]
- 96.
- Fogari R, Derosa G, Zoppi A, Lazzari P, D’Angelo A, Mugellini A. Comparative effect of canrenone or hydrochlorothiazide addition to valsartan/amlodipine combination on urinary albumin excretion in well-controlled type 2 diabetic hypertensive patients with microalbuminuria. Expert Opinion on Pharmacotherapy. 2014; 15(4):453–9 [PubMed: 24410484]
- 97.
- Fogari R, Mugellini A, Zoppi A, Lazzari P, Destro M, Rinaldi A et al. Effect of telmisartan/hydrochlorothiazide vs lisinopril/hydrochlorothiazide combination on ambulatory blood pressure and cognitive function in elderly hypertensive patients. Journal of Human Hypertension. 2006; 20(3):177–85 [PubMed: 16306998]
- 98.
- Fogari R, Zoppi A, Corradi L, Mugellini A, Lazzari P, Preti P et al. Long-term effects of ramipril and nitrendipine on albuminuria in hypertensive patients with type II diabetes and impaired renal function. Journal of Human Hypertension. 1999; 13(1):47–53 [PubMed: 9928752]
- 99.
- Fogari R, Zoppi A, Lusardi P, Mugellini A. Efficacy and tolerability of manidipine hydrochloride in the long-term treatment of mild-moderate hypertension. Manidipine Efficacy in Long-Term Treatment Group. Blood Pressure Supplement. 1996; 5:24–8 [PubMed: 8973789]
- 100.
- Fogari R, Zoppi A, Mugellini A, Maffioli P, Preti P, Derosa G. Effects of valsartan or ramipril addition to amlodipine/hydrochlorothiazide combination on left ventricular mass in diabetic hypertensive patients with left ventricular hypertrophy. Expert Opinion on Pharmacotherapy. 2012; 13(8):1091–9 [PubMed: 22515416]
- 101.
- Fogari R, Zoppi A, Tettamanti F, Malamani G, Pasotti C. The effect of celiprolol on the blood lipid profile in hypertensive patients with high cholesterol levels. Cardiovascular Drugs and Therapy. 1991; 4:(Suppl 6):1287–90 [PubMed: 1826213]
- 102.
- Forette F, Seux ML, Staessen JA, Thijs L, Babarskiene MR, Babeanu S et al. The prevention of dementia with antihypertensive treatment: New evidence from the Systolic Hypertension in Europe (Syst-Eur) study. Archives of Internal Medicine. 2002; 162(18):2046–2052 [PubMed: 12374512]
- 103.
- Forrest WA, Bridgman KM, Ebbutt AF. “24-hour blood pressure control” with sustained release oxprenolol 160mg plus cyclopenthiazide 0.25mg (Trasidrex) in general practice. British Journal of Clinical Practice. 1983; 37(11–12):385–8 [PubMed: 6367794]
- 104.
- Fossum E, Olsen MH, Hoieggen A, Wachtell K, Reims HM, Ibsen H et al. Long-term plasma catecholamines in patients with hypertension and left ventricular hypertrophy treated with losartan or atenolol: ICARUS, a LIFE substudy. Journal of Human Hypertension. 2004; 18(6):375–80 [PubMed: 15057253]
- 105.
- Franco RJ, Sampaio M, Balbi AL, Martin LC, Luna RL. An open comparative study of captopril + hydrochlorothiazide versus chlorthalidone for the treatment of mild and moderate primary hypertension. Arquivos Brasileiros de Cardiologia. 1992; 59(5):423–427 [PubMed: 1340743]
- 106.
- Franse LV, Pahor M, Di Bari M, Somes GW, Cushman WC, Applegate WB. Hypokalemia associated with diuretic use and cardiovascular events in the Systolic Hypertension in the Elderly Program. Hypertension. 2000; 35(5):1025–30 [PubMed: 10818057]
- 107.
- Frewin DB, Aldons P, Wilson LL, O’Sullivan EF, Wyndham RN, Karrasch J et al. Felodipine in combination with a beta-adrenoceptor blocker as an effective substitute for triple therapy in severe hypertension. The Australian Felodipine Multicentre Study Group. European Journal of Clinical Pharmacology. 1991; 41(5):393–6 [PubMed: 1684748]
- 108.
- Frick MH, Cox DA, Himanen P, Huttunen M, Pitkajarvi T, Porsti P et al. Serum lipid changes in a one-year, multicenter, double-blind comparison of doxazosin and atenolol for mild to moderate essential hypertension. American Journal of Cardiology. 1987; 59(14):61G–67G [PubMed: 2884854]
- 109.
- Frick MH, Halttunen P, Himanen P, Huttunen M, Porsti P, Pitkajarvi T et al. A long-term double-blind comparison of doxazosin and atenolol in patients with mild to moderate essential hypertension. British Journal of Clinical Pharmacology. 1986; 21:(Suppl 1):55S–62S [PMC free article: PMC1400748] [PubMed: 2939868]
- 110.
- Gaddam K, Pimenta E, Thomas SJ, Cofield SS, Oparil S, Harding SM et al. Spironolactone reduces severity of obstructive sleep apnoea in patients with resistant hypertension: a preliminary report. Journal of Human Hypertension. 2010; 24(8):532–7 [PMC free article: PMC2891919] [PubMed: 20016520]
- 111.
- Gao D, Ning N, Niu X, Wei J, Sun P, Hao G. Aliskiren vs. angiotensin receptor blockers in hypertension: meta-analysis of randomized controlled trials. American Journal of Hypertension. 2011; 24(5):613–21 [PubMed: 21293386]
- 112.
- Gasowski J, Staessen JA, Celis H, Fagard RH, Thijs L, Birkenhager WH et al. Systolic Hypertension in Europe (Syst-Eur) trial phase 2: Objectives, protocol, and initial progress. Systolic Hypertension in Europe Investigators. Journal of Human Hypertension. 1999; 13(2):135–45 [PubMed: 10100063]
- 113.
- Gazdick LP, Maxwell M, Ruff D, Goldberg AI, Nelson EB, Berman R et al. A double-blind, randomized, parallel, active-controlled study to evaluate the antihypertensive efficacy and safety of losartan in patients with severe hypertension. American Journal of Hypertension. 1994; 7(4 Pt 2):100A
- 114.
- George C, Grippat J, Safar M. Second line treatment of essential hypertension after beta-blockade. A randomised trial in 558 patients initially treated with bisoprolol 10mg. Drug Investigation. 1990; 2(3):150–154
- 115.
- Ghiadoni L, Bruno RM, Cartoni G, Stea F, Magagna A, Virdis A et al. Combination therapy with lercanidipine and enalapril reduced central blood pressure augmentation in hypertensive patients with metabolic syndrome. Vascular Pharmacology. 2017; 92:16–21 [PubMed: 26070528]
- 116.
- Giles TD, Sander GE, Roffidal LE, Quiroz AC, Mazzu AL. Comparative effects of nitrendipine and hydrochlorothiazide on calciotropic hormones and bone density in hypertensive patients. American Journal of Hypertension. 1992; 5(12 Pt 1):875–9 [PubMed: 1285936]
- 117.
- Gillespie EL, White CM, Kardas M, Lindberg M, Coleman CI. The impact of ACE inhibitors or angiotensin II type 1 receptor blockers on the development of new-onset type 2 diabetes. Diabetes Care. 2005; 28(9):2261–6 [PubMed: 16123505]
- 118.
- Girerd X, Genes N. Comparison of an early or a late drug titration on the efficacy and safety of Irbesartan/HCTZ combination in uncontrolled hypertensive patients: Results of the actual study. Journal of Hypertension. 2010; 28(Suppl A):e18–9
- 119.
- Gitt AK, Baumgart P, Bramlage P, Mahfoud F, Potthoff SA, Senges J et al. EARLY Treatment with azilsartan compared to ACE-inhibitors in anti-hypertensive therapy--rationale and design of the EARLY hypertension registry. BMC Cardiovascular Disorders. 2013; 13:46 [PMC free article: PMC3706336] [PubMed: 23819631]
- 120.
- Glorioso N, Argiolas G, Filigheddu F, Troffa C, Cocco F, Bulla E et al. Conceptual basis and methodology of the SOPHIA study. Pharmacogenomics. 2007; 8(11):1497–509 [PubMed: 18034615]
- 121.
- Goicolea I, Fernández González R, Piniés J, Garrido J, Martínez JM, Armenteros S et al. Effect of antihypertensive combinations on arterial pressure, albuminuria, and glycemic control in patients with type II diabetic nephropathy: A randomized study. Nefrologia. 2002; 22(2):170–178 [PubMed: 12085418]
- 122.
- Gosse P, Dubourg O, Gueret P, De Simone G, Schmieder R, De Leeuw PW et al. Efficacy of very low dose perindopril 2 mg/indapamide 0.625 mg combination on left Review protocols ventricular hypertrophy in hypertensive patients: The P.I.C.X.E.L. study rationale and design. Journal of Human Hypertension. 2002; 16(9):653–9 [PubMed: 12214263]
- 123.
- Grimm RH, Jr., Flack JM, Schoenberger JA, Gonzalez NM, Liebson PR. Alpha-blockade and thiazide treatment of hypertension. A double-blind randomized trail comparing doxazosin and hydrochlorothiazide. American Journal of Hypertension. 1996; 9(5):445–54 [PubMed: 8735175]
- 124.
- Guo JH, Lu YH, Shi GP, Guo ZP, Xu Q, Shen JH et al. Protective effect of spironolactone on myocardium during perioperation period of percataneous coronary intervention. Academic Journal of Second Military Medical University. 2011; 32(8):889–892
- 125.
- Guo JZ, Gong YC, Zhang JL, Qing YW, Dai QY, Wang YC et al. A clinical intervention study among 463 essential hypertensive patients with metabolic syndrome. Chinese Journal of Cardiovascular Diseases. 2005; 33(2):132–136 [PubMed: 15924807]
- 126.
- Gupta A, Mackay J, Whitehouse A, Godec T, Collier T, Pocock S et al. Long-term mortality after blood pressure-lowering and lipid-lowering treatment in patients with hypertension in the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Legacy study: 16-year follow-up results of a randomised factorial trial. Lancet. 2018; 392(10153):1127–1137 [PubMed: 30158072]
- 127.
- Gyntelberg F, Jacobsen K, Martlev A, Backer P. 2 antihypertensive therapeutic regimes compared in a controlled clinical trial in general practice. Cyclopenthiazide + KC1/methyldopa versus oxprenolol/hydralazine. Ugeskrift for Laeger. 1977; 139(11):641–646 [PubMed: 320734]
- 128.
- Hall J, Marbury T, Gray J, Chaudhery S, Chen S, James D et al. Long term safety, tolerability and efficacy of valsartan: Results from one and two year trials. Journal of Clinical Research. 1998; 1:147–159
- 129.
- Hamada T, Kuwabara M, Watanabe A, Mizuta E, Ohtahara A, Omodani H et al. A comparative study on the effectiveness of losartan/hydrochlorothiazide and telmisartan/hydrochlorothiazide in patients with hypertension. Clinical and Experimental Hypertension. 2014; 36(4):251–7 [PubMed: 23865441]
- 130.
- Hamada T, Mizuta E, Kondo T, Hirai M, Yamada K, Kato M et al. Effects of a low-dose antihypertensive diuretic in combination with losartan, telmisartan, or candesartan on serum urate levels in hypertensive patients. Arzneimittel-Forschung. 2010; 60(2):71–5 [PubMed: 20329654]
- 131.
- Hamed AT, Taha MM. Comparative study on renoprotective effect of aliskiren-pentoxifylline combination, valsartan and enalapril among patients with hypertension, type 2 diabetes mellitus and diabetic nephropathy. Jordan journal of pharmaceutical sciences. 2014; 7(1):1–14
- 132.
- Hanon O, Boully C, Caillard L, Labouree F, Cochiello S, Chaussade E. Treatment of hypertensive patients with diabetes and microalbuminuria with combination indapamide sr/amlodipine: Retrospective analysis of NESTOR. American Journal of Hypertension. 2015; 28(8):1064–71 [PubMed: 25628416]
- 133.
- Hanon O, Caillard L, Chaussade E, Hernandorena I, Boully C. Blood pressure-lowering efficacy of indapamide SR/amlodipine combination in older patients with hypertension: A post hoc analysis of the NESTOR trial (Natrilix SR vs Enalapril in Hypertensive Type 2 Diabetics With Microalbuminuria). Journal of Clinical Hypertension. 2017; 19(10):965–972 [PMC free article: PMC8030879] [PubMed: 28721700]
- 134.
- Hansson L, Hedner T, Lund-Johansen P, Kjeldsen SE, Lindholm LH, Syvertsen JO et al. Randomised trial of effects of calcium antagonists compared with diuretics and beta-blockers on cardiovascular morbidity and mortality in hypertension: the Nordic Diltiazem (NORDIL) study. The Lancet. 2000; 356(9227):359–65 [PubMed: 10972367]
- 135.
- Hansson L, Lindholm LH, Ekbom T, Dahlof B, Lanke J, Schersten B et al. Randomised trial of old and new antihypertensive drugs in elderly patients: Cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. The Lancet. 1999; 354(9192):1751–6 [PubMed: 10577635]
- 136.
- Hansson L, Lindholm LH, Niskanen L, Lanke J, Hedner T, Niklason A et al. Effect of angiotensin-converting-enzyme inhibition compared with conventional therapy on cardiovascular morbidity and mortality in hypertension: The Captopril Prevention Project (CAPPP) randomised trial. The Lancet. 1999; 353(9153):611–6 [PubMed: 10030325]
- 137.
- Hansson L, Lithell H, Skoog I, Baro F, Banki CM, Breteler M et al. Study on COgnition and Prognosis in the Elderly (SCOPE). Blood Pressure. 1999; 8(3):177–83 [PubMed: 10595696]
- 138.
- Hasegawa H, Takano H, Narumi H, Ohtsuka M, Mizuguchi T, Namiki T et al. Effects of telmisartan and losartan on cardiovascular protection in Japanese hypertensive patients. Hypertension Research. 2011; 34(11):1179–84 [PubMed: 21796128]
- 139.
- Helgeland A. Treatment of mild hypertension: A five year controlled drug trial. The Oslo study. American Journal of Medicine. 1980; 69(5):725–32 [PubMed: 7001898]
- 140.
- Helgeland A. Double-blind comparison of trimazosin and propranolol in essential hypertension. American Heart Journal. 1983; 106(5 Pt 2):1253–8 [PubMed: 6356854]
- 141.
- Himmelmann A, Hansson L, Hansson BG, Hedstrand H, Skogstrom K, Ohrvik J et al. ACE inhibition preserves renal function better than beta-blockade in the treatment of essential hypertension. Blood Pressure. 1995; 4(2):85–90 [PubMed: 7599759]
- 142.
- Hosie J, Jones JC, Clifford PD. Long term usage of Prestim (timolol/bendrofluazide) in the management of mild to moderate hypertension in general practice. British Journal of Clinical Practice. 1983; 37(11–12):393–6 [PubMed: 6367796]
- 143.
- Hradec J, Zamorano J, Sutradhar S. Post hoc analysis of the Cluster Randomized Usual Care versus Caduet Investigation Assessing Long-term risk (CRUCIAL) trial. Current Medical Research and Opinion. 2013; 29(6):589–96 [PubMed: 23464930]
- 144.
- Hughes AD, Stanton AV, Jabbar AS, Chapman N, Martinez-Perez ME, Mc GTSA. Effect of antihypertensive treatment on retinal microvascular changes in hypertension. Journal of Hypertension. 2008; 26(8):1703–7 [PubMed: 18622251]
- 145.
- Hulley SB, Furberg CD, Gurland B, McDonald R, Perry HM, Schnaper HW et al. Systolic Hypertension in the Elderly Program (SHEP): Antihypertensive efficacy of chlorthalidone. American Journal of Cardiology. 1985; 56(15):913–20 [PubMed: 4072925]
- 146.
- Ibsen H, Lindholm LH, Pedersen OL, Dahlöf B, Kjeldsen S. The effect of losartan versus atenolol on cardiovascular morbidity and mortality in patients with diabetes mellitus in the LIFE-study. Ugeskrift for Laeger. 2003; 165(5):459–462 [PubMed: 12599844]
- 147.
- Ibsen H, Westberg B. The efficacy and tolerability of long-term felodipine treatment in hypertension. The Scandinavian Multicenter Group. Cardiovascular Drugs and Therapy. 1990; 4(3):641–7 [PubMed: 1981681]
- 148.
- Ichihara A, Kaneshiro Y, Takemitsu T, Sakoda M. Effects of amlodipine and valsartan on vascular damage and ambulatory blood pressure in untreated hypertensive patients. Journal of Human Hypertension. 2006; 20(10):787–94 [PubMed: 16810279]
- 149.
- J. Elan Investigators. Effect of losartan and amlodipine on left ventricular diastolic function in patients with mild-to-moderate hypertension (J-ELAN): Rationale and design. Circulation Journal. 2006; 70(1):124–8 [PubMed: 16377936]
- 150.
- Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. New England Journal of Medicine. 2008; 359(23):2417–28 [PubMed: 19052124]
- 151.
- Johnson JA, Gong Y, Bailey KR, Cooper-DeHoff RM, Chapman AB, Turner ST et al. Hydrochlorothiazide and atenolol combination antihypertensive therapy: Effects of drug initiation order. Clinical Pharmacology and Therapeutics. 2009; 86(5):533–9 [PMC free article: PMC2765524] [PubMed: 19571804]
- 152.
- Johnston GD, Wilson R, McDermott BJ, McVeigh GE, Duffin D, Logan J. Low-dose cyclopenthiazide in the treatment of hypertension: A one-year community-based study. Quarterly Journal of Medicine. 1991; 78(286):135–43 [PubMed: 2031076]
- 153.
- Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: The VALUE randomised trial. The Lancet. 2004; 363(9426):2022–31 [PubMed: 15207952]
- 154.
- Kaku K, Enya K, Sugiura K, Totsuka N. Efficacy and safety of combination therapy with candesartan cilexetil and pioglitazone hydrochloride in patients with hypertension and type 2 diabetes mellitus. Current Medical Research and Opinion. 2011; 27:(Suppl 3):73–84 [PubMed: 22106979]
- 155.
- Katayama S, Kawamori R, Iwamoto Y, Saito I, Kuramoto K, Group AS. In half of hypertensive diabetics, co-administration of a calcium channel blocker and an angiotensin-converting enzyme inhibitor achieved a target blood pressure of <130/80 mmHg: The azelnidipine and temocapril in hypertensive patients with type 2 diabetes (ATTEST) study. Hypertension Research. 2008; 31(8):1499–508 [PubMed: 18971523]
- 156.
- Kawalec P, Holko P, Gawin M, Pilc A. Effectiveness of fixed-dose combination therapy in hypertension: Systematic review and meta-analysis. Archives of Medical Science. 2018; 14(5):1125–1136 [PMC free article: PMC6111352] [PubMed: 30154897]
- 157.
- Kereiakes DJ, Chrysant SG, Izzo JL, Littlejohn T, Oparil S, Melino M et al. Long-term efficacy and safety of triple-combination therapy with olmesartan medoxomil and amlodipine besylate and hydrochlorothiazide for hypertension. Journal of Clinical Hypertension. 2012; 14(3):149–57 [PMC free article: PMC8108825] [PubMed: 22372774]
- 158.
- Kerfoot BP, Turchin A, Breydo E, Gagnon D, Conlin PR. An online spaced-education game among clinicians improves their patients’ time to blood pressure control: A randomized controlled trial. Circulation: Cardiovascular Quality and Outcomes. 2014; 7(3):468–74 [PMC free article: PMC4040124] [PubMed: 24847084]
- 159.
- Kim JH, Zamorano J, Erdine S, Pavia A, Al-Khadra A, Sutradhar S et al. Reduction in cardiovascular risk using proactive multifactorial intervention versus usual care in younger (< 65 years) and older (>= 65 years) patients in the CRUCIAL trial. Current Medical Research and Opinion. 2013; 29(5):453–63 [PubMed: 23448581]
- 160.
- Kim JH, Zamorano J, Erdine S, Pavia A, Al-Khadra A, Sutradhar S et al. Proactive cardiovascular risk management versus usual care in patients with and without diabetes mellitus: CRUCIAL trial subanalysis. Postgraduate Medicine. 2012; 124(4):41–53 [PubMed: 22913893]
- 161.
- Kjeldsen SE, Dahlof B, Devereux RB, Julius S, Aurup P, Edelman J et al. Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: A Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002; 288(12):1491–8 [PubMed: 12243636]
- 162.
- Kjeldsen SE, Dzongowski P, Li N, Wang L, Radlmaier A. Fixed-dose combination of nifedipine gastrointestinal therapeutic system and candesartan cilexetil in patients with moderate-to-severe essential hypertension: An open-label, long-term safety and efficacy study. Journal of Clinical Pharmacy and Therapeutics. 2016; 41(6):695–702 [PubMed: 27670639]
- 163.
- Kjeldsen SE, Jamerson KA, Bakris GL, Pitt B, Dahlof B, Velazquez EJ et al. Predictors of blood pressure response to intensified and fixed combination treatment of hypertension: The ACCOMPLISH study. Blood Pressure. 2008; 17(1):7–17 [PubMed: 18568687]
- 164.
- Kjeldsen SE, Julius S, Mancia G, McInnes GT, Hua T, Weber MA et al. Effects of valsartan compared to amlodipine on preventing type 2 diabetes in high-risk hypertensive patients: The VALUE trial. Journal of Hypertension. 2006; 24(7):1405–12 [PubMed: 16794491]
- 165.
- Ko GT, Chan HC, Chan CH. Blood pressure reduction and tolerability of amlodipine versus nifedipine retard in Chinese patients with type 2 diabetes mellitus and hypertension: A randomized 1-year clinical trial. International Journal of Clinical Pharmacology and Therapeutics. 2001; 39(8):331–5 [PubMed: 11515707]
- 166.
- Kohlmann O, Jr., Roca-Cusachs A, Laurent S, Schmieder RE, Wenzel RR, Fogari R. Fixed-dose manidipine/delapril versus losartan/hydrochlorothiazide in hypertensive patients with type 2 diabetes and microalbuminuria. Advances in Therapy. 2009; 26(3):313–24 [PubMed: 19330493]
- 167.
- Kostis JB, Wilson AC, Freudenberger RS, Cosgrove NM, Pressel SL, Davis BR et al. Long-term effect of diuretic-based therapy on fatal outcomes in subjects with isolated systolic hypertension with and without diabetes. American Journal of Cardiology. 2005; 95(1):29–35 [PubMed: 15619390]
- 168.
- Kuwajima I, Kuramoto K, Ogihara T, Iimura O, Abe K, Saruta T et al. Tolerability and safety of a calcium channel blocker in comparison with a diuretic in the treatment of elderly patients with hypertension: secondary analysis of the NICS-EH. Hypertension Research. 2001; 24(5):475–80 [PubMed: 11675939]
- 169.
- Lacourciere Y, Belanger A, Godin C, Halle JP, Ross S, Wright N et al. Long-term comparison of losartan and enalapril on kidney function in hypertensive type 2 diabetics with early nephropathy. Kidney International. 2000; 58(2):762–9 [PubMed: 10916100]
- 170.
- Lane DA, Shah S, Beevers DG. Low-dose spironolactone in the management of resistant hypertension: a surveillance study. Journal of Hypertension. 2007; 25(4):891–4 [PubMed: 17351384]
- 171.
- Laufer E, Reid C, Qi XL, Jennings GL. Absence of detectable regression of human hypertensive left ventricular hypertrophy following drug treatment for 1 year. Clinical and Experimental Pharmacology and Physiology. 1998; 25(3–4):208–15 [PubMed: 9590570]
- 172.
- Laurent S, Boutouyrie P, Vascular Mechanism Collaboration. Dose-dependent arterial destiffening and inward remodeling after olmesartan in hypertensives with metabolic syndrome. Hypertension. 2014; 64(4):709–16 [PubMed: 25001274]
- 173.
- Lavenius B, Hansson L. A double-blind comparison of spironolactone and hydrochlorothiazide in hypertensive patients treated with metoprolol. International Journal of Clinical Pharmacology, Therapy, and Toxicology. 1982; 20(6):291–5 [PubMed: 7049966]
- 174.
- Leonetti G, Magnani B, Pessina AC, Rappelli A, Trimarco B, Zanchetti A et al. Tolerability of long-term treatment with lercanidipine versus amlodipine and lacidipine in elderly hypertensives. American Journal of Hypertension. 2002; 15(11):932–40 [PubMed: 12441211]
- 175.
- Levine B. Eprosartan provides safe and effective long-term maintenance of blood pressure control in patients with mild to moderate essential hypertension. Current Medical Research and Opinion. 2001; 17(1):8–17 [PubMed: 11464450]
- 176.
- Licata G, Ganguzza A, Corrao S, Merlino G, Chiara T, D’Aubert MD et al. Effects of cilazapril on renal haemodynamics in mild to moderate hypertensive subject: randomized controlled trial vs. hydrochlorothiazide. American Journal of Hypertension. 1994; 7(4 Pt 2):49A
- 177.
- Lim PO, Donnan PT, MacDonald TM. Does the Dundee Step Test predict outcome in treated hypertension? A sub-study protocol for the ASCOT trial. Anglo-Scandinavian Cardiac Outcome Trial. Journal of Human Hypertension. 2000; 14(1):75–8 [PubMed: 10673735]
- 178.
- Lin M, Chiang HT, Chen CY. Comparisons of long-term effects between converting enzyme inhibitors and conventional therapy in treating mild to moderate hypertension. Chinese Medical Journal. 1991; 48(5):339–50 [PubMed: 1659934]
- 179.
- Lin M, chiang HT, Yang YF, Lin SL, Chen CY, Kong CW et al. Long-term beneficial effects of converting enzyme inhibitors in patients with moderate hypertension and left ventricular hypertrophy. Acta Cardiologica Sinica. 1993; 9(4):254–263
- 180.
- Lin M, Yang YF, Chiang HT, Lee D, Wang SP, Chang MS et al. Beneficial effects of angiotensin-converting enzyme inhibitors on cardiovascular and renal functions in patients with hypertension and diabetes. Acta Cardiologica Sinica. 1995; 11(1):30–38
- 181.
- Lind L, Pollare T, Berne C, Lithell H. Long-term metabolic effects of antihypertensive drugs. American Heart Journal. 1994; 128(6 Pt 1):1177–83 [PubMed: 7985599]
- 182.
- Lindholm LH, Anderson H, Ekbom T, Hansson L, Lanke J, Dahlof B et al. Relation between drug treatment and cancer in hypertensives in the Swedish Trial in Old Patients with Hypertension 2: A 5-year, prospective, randomised, controlled trial. The Lancet. 2001; 358(9281):539–44 [PubMed: 11520524]
- 183.
- Lindholm LH, Hansson L, Dahlof B, Ekbom T, Hedner T, De Faire U et al. The Swedish Trial in old patients with hypertension-2 (STOP-hypertension-2): A progress report. Blood Pressure. 1996; 5(5):300–4 [PubMed: 8879603]
- 184.
- Lindholm LH, Hansson L, Ekbom T, Dahlof B, Lanke J, Linjer E et al. Comparison of antihypertensive treatments in preventing cardiovascular events in elderly diabetic patients: results from the Swedish Trial in Old Patients with Hypertension-2. STOP Hypertension-2 Study Group. Journal of Hypertension. 2000; 18(11):1671–5 [PubMed: 11081782]
- 185.
- Lindholm LH, Ibsen H, Borch-Johnsen K, Olsen MH, Wachtell K, Dahlof B et al. Risk of new-onset diabetes in the Losartan Intervention For Endpoint reduction in hypertension study. Journal of Hypertension. 2002; 20(9):1879–86 [PubMed: 12195132]
- 186.
- Lindholm LH, Ibsen H, Dahlof B, Devereux RB, Beevers G, de Faire U et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): A randomised trial against atenolol. The Lancet. 2002; 359(9311):1004–10 [PubMed: 11937179]
- 187.
- Lindner UK. Ergometric diagnosis and therapy control of hypertension in out-patients. Medizinische Klinik. 1984; 79(11):326–328
- 188.
- Lindroos M, Lehtonen A. Timolol and a hydrochlorothiazide-amiloride combination in the treatment of essential hypertension in young and middle-aged patients: A comparative study with once-daily administration. International Journal of Clinical Pharmacology, Therapy, and Toxicology. 1984; 22(12):643–5 [PubMed: 6396239]
- 189.
- Littlejohn T, Punzi H, Webster D, Majul CR, Oigman W, Olvera R. Telmisartan plus amlodipine combination is effective in both treatment-nave and previously treated hypertensive patients: Sub-analysis from a factorial design study. Journal of Clinical Hypertension. 2009; 11(Suppl A):A36
- 190.
- Liu JC, Zhou DX, Li ZS. A comparison of amlodipine with benazepril in treatment of elderly primary hypertension. Journal of Railway Medical University. 2000; 21(9):28–30
- 191.
- Liu L, Wang JG, Celis H, Staessen JA. Implications of the Systolic Hypertension in China trial. Clinical and Experimental Hypertension. 1999; 21(5–6):499–505 [PubMed: 10423076]
- 192.
- Lombardo M, Alli C, Broccolino M, Ferrari S, Montemurro L, Zaini G et al. Long-term effects of angiotensin-converting enzyme inhibitors and calcium antagonists on the right and left ventricles in essential hypertension. American Heart Journal. 1997; 134(3):557–64 [PubMed: 9327716]
- 193.
- López NC, Corral JL, Perozo M, García P, Bustillo N, Arreaza MR et al. Nifedipine in the treatment of moderate and severe arterial hypertension. Long-term effect on arterial pressure and on the left ventricle. Revista Española de Cardiología. 1997; 50(8):567–572 [PubMed: 9340698]
- 194.
- Lu Z, Chen Y, Li L, Wang G, Xue H, Tang W. Combination therapy of renin-angiotensin system inhibitors plus calcium channel blockers versus other two-drug combinations for hypertension: A systematic review and meta-analysis. Journal of Human Hypertension. 2017; 31(1):1–13 [PubMed: 26740336]
- 195.
- Ludwig M, Stapff M, Ribeiro A, Fritschka E, Tholl U, Smith RD et al. Comparison of the effects of losartan and atenolol on common carotid artery intima-media thickness in patients with hypertension: Results of a 2-year, double-blind, randomized, controlled study. Clinical Therapeutics. 2002; 24(7):1175–93 [PubMed: 12182261]
- 196.
- Luno J, Varas J, Ramos R, Merello I, Aljama P, MartinMalo A et al. The combination of beta blockers and renin-angiotensin system blockers improves survival in incident hemodialysis patients: A propensity-matched study. KI Reports. 2017; 2(4):665–675 [PMC free article: PMC5678679] [PubMed: 29142984]
- 197.
- Lynch AI, Boerwinkle E, Davis BR, Ford CE, Eckfeldt JH, Leiendecker-Foster C et al. Pharmacogenetic association of the NPPA T2238C genetic variant with cardiovascular disease outcomes in patients with hypertension. JAMA. 2008; 299(3):296–307 [PubMed: 18212314]
- 198.
- Lynch AI, Eckfeldt JH, Davis BR, Ford CE, Boerwinkle E, Leiendecker-Foster C et al. Gene panels to help identify subgroups at high and low risk of coronary heart disease among those randomized to antihypertensive treatment: The GenHAT study. Pharmacogenetics and Genomics. 2012; 22(5):355–66 [PMC free article: PMC3325375] [PubMed: 22388798]
- 199.
- M’Buyamba-Kabangu JR, Fagard R, Lijnen P, Staessen J, Lissens W, Ditu M et al. Calcium entry blockade or beta-blockade in long-term management of hypertension in blacks. Clinical Pharmacology and Therapeutics. 1987; 41(1):45–54 [PubMed: 3802705]
- 200.
- Maclean D, Elton RA, Muir AL, Readman AS, Vallance BD, Wilcox RG. Felodipine compared with hydralazine as third line therapy in hypertension. British Journal of Clinical Pharmacology. 1986; 21:577P–8P
- 201.
- Maclean D, Vallance BD, Wilcox RG. Felodipine vs hydralazine: A controlled trial as third line therapy in hypertension. British Journal of Clinical Pharmacology. 1986; 21(6):621–626 [PMC free article: PMC1400980] [PubMed: 2874821]
- 202.
- Mahmud A, Mahgoub M, Hall M, Feely J. Does aldosterone-to-renin ratio predict the antihypertensive effect of the aldosterone antagonist spironolactone? American Journal of Hypertension. 2005; 18(12 Pt 1):1631–5 [PubMed: 16364838]
- 203.
- Malacco E, Mancia G, Rappelli A, Menotti A, Zuccaro MS, Coppini A et al. Treatment of isolated systolic hypertension: The SHELL study results. Blood Pressure. 2003; 12(3):160–7 [PubMed: 12875478]
- 204.
- Malminiemi K. Long-term celiprolol therapy lowers fasting plasma leptin levels. Cardiovascular Drugs and Therapy. 2000; 14(1):67–75 [PubMed: 10755203]
- 205.
- Mancia G, Parati G, Bilo G, Maronati A, Omboni S, Baurecht H et al. Assessment of long-term antihypertensive treatment by clinic and ambulatory blood pressure: data from the European Lacidipine Study on Atherosclerosis. Journal of Hypertension. 2007; 25(5):1087–94 [PubMed: 17414674]
- 206.
- Mann J, Julius S. The Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial of cardiovascular events in hypertension. Rationale and design. Blood Pressure. 1998; 7(3):176–83 [PubMed: 9758088]
- 207.
- Marfatia R, White WB, Schumacher H. Effects of telmisartan with hydrochlorothiazide versus valsartan with hydrochlorothiazide in patients with moderate-to-severe hypertension. International Journal of Hypertension. 2012; 2012:976828 [PMC free article: PMC3400348] [PubMed: 22844584]
- 208.
- Marre M, Puig JG, Kokot F, Fernandez M, Jermendy G, Opie L et al. Equivalence of indapamide SR and enalapril on microalbuminuria reduction in hypertensive patients with type 2 diabetes: the NESTOR Study. Journal of Hypertension. 2004; 22(8):1613–22 [PubMed: 15257186]
- 209.
- Martinez-Martin FJ, Rodriguez-Rosas H, Peiro-Martinez I, Soriano-Perera P, Pedrianes-Martin P, Comi-Diaz C. Olmesartan/amlodipine vs olmesartan/hydrochlorothiazide in hypertensive patients with metabolic syndrome: The OLAS study. Journal of Human Hypertension. 2011; 25(6):346–53 [PMC free article: PMC3099035] [PubMed: 21107432]
- 210.
- Mason JM, Dickinson HO, Nicolson DJ, Campbell F, Ford GA, Williams B. The diabetogenic potential of thiazide-type diuretic and beta-blocker combinations in patients with hypertension. Journal of Hypertension. 2005; 23(10):1777–81 [PubMed: 16148594]
- 211.
- Matsuno Y, Minatoguchi S, Fujiwara H, Gifu Substudy Group of The Case-J Trial. Effects of candesartan versus amlodipine on home-measured blood pressure, QT dispersion and left ventricular hypertrophy in high-risk hypertensive patients. Blood Pressure Supplement. 2011; 1:12–9 [PubMed: 21247247]
- 212.
- Matsushita K, Muramatsu T, Kondo T, Maeda K, Shintani S, Murohara T et al. Rationale and design of the NAGOYA HEART Study: Comparison between valsartan and amlodipine regarding morbidity and mortality in patients with hypertension and glucose intolerance. Journal of Cardiology. 2010; 56(1):111–7 [PubMed: 20409690]
- 213.
- Matsuzaki M, Ogihara T, Umemoto S, Rakugi H, Matsuoka H, Shimada K et al. Prevention of cardiovascular events with calcium channel blocker-based combination therapies in patients with hypertension: A randomized controlled trial. Journal of Hypertension. 2011; 29(8):1649–59 [PubMed: 21610513]
- 214.
- Mazza A, Schiavon L, Zuin M, Lenti S, Ramazzina E, Rubello D et al. Effects of the antihypertensive fixed-dose combinations on an early marker of hypertensive cardiac damage in subjects at low cardiovascular risk. American Journal of Hypertension. 2016; 29(8):969–75 [PubMed: 27053407]
- 215.
- McAreavey D, Ramsay LE, Latham L, Lorimer AR, McLaren D, Reid JL et al. The ‘third drug’ trial: a comparative study of anti-hypertensive agents added to treatment when blood pressure is uncontrolled by a beta-blocker plus thiazide diuretic. Journal of Hypertension Supplement. 1983; 1(2):S116–S9 [PubMed: 6400110]
- 216.
- Mende CW, Giles TD, Bharucha DB, Ferguson WG, Mallick M, Patel MD. Efficacy of nebivolol-valsartan single-pill combination in obese and nonobese patients with hypertension. Journal of Clinical Hypertension. 2017; 19(6):632–639 [PMC free article: PMC5484387] [PubMed: 28075064]
- 217.
- Metelitsa VI, Filatova NP. Comparative trial of nadolol, propranolol, prazosin and hydrochlorothiazide in patients with arterial hypertension after 12 months of treatment using the stepwise protocol. Cooperative study in the USSR: II. Results of the combined treatment, side effects, factors affecting drop-out rate and conclusion. Journal of Drug Development. 1991; 4(1):15–23
- 218.
- Metelitsa VI, Filatova NP. The results of the comparative study of nadolol, propranolol, prazosin and hydrochlorothiazide in patients with arterial hypertension in a 12-month stepped-plan treatment (cooperative research). The Working Group of the Cooperative Program to Study New Preparations in the Prevention of Arterial Hypertension. I. The research protocol and results of monotherapy. Terapevticheskii Arkhiv. 1991; 63(8):30–34 [PubMed: 1792612]
- 219.
- Middeke M, Richter WO, Schwandt P, Beck B, Holzgreve H. Normalization of lipid metabolism after withdrawal from antihypertensive long-term therapy with beta blockers and diuretics. Arteriosclerosis. 1990; 10(1):145–7 [PubMed: 2297344]
- 220.
- Middeke M, Richter WO, Schwandt P, Holzgreve H. The effects of antihypertensive combination therapy on lipid and glucose metabolism: Hydrochlorothiazide plus sotalol vs. hydrochlorothiazide plus captopril. International Journal of Clinical Pharmacology and Therapeutics. 1997; 35(6):231–4 [PubMed: 9208337]
- 221.
- Misson R, Merkel T, Cutler RE. Comparison of blood pressure, plasma lipid and cardiac performance responses to prazosin versus propranolol in thiazide-treated hypertensive patients. American Journal of Cardiology. 1984; 53(3):51A–54A [PubMed: 6364760]
- 222.
- Mizuno H, Hoshide S, Tomitani N, Kario K. Comparison of ambulatory blood pressure-lowering effects of higher doses of different calcium antagonists in uncontrolled hypertension: The Calcium Antagonist Controlled-Release High-Dose Therapy in Uncontrolled Refractory Hypertensive Patients (CARILLON) Study. Blood Pressure. 2017; 26(5):284–293 [PubMed: 28524699]
- 223.
- Morgan TO. Efficacy of cilazapril compared with hydrochlorothiazide in the treatment of mild-to-moderate essential hypertension. Multicentre Study Group. American Journal of Medicine. 1989; 87(6B):37S–41S [PubMed: 2532459]
- 224.
- Mroczek WJ, Burris JF, DeQuattro V. A multicenter evaluation of guanadrel sulfate and methyldopa in hypertensive patients receiving a diuretic. Current Therapeutic Research, Clinical and Experimental. 1984; 36(5 I):1004–1015
- 225.
- Muller FB, Bolli P, Linder L, Kiowski W, Erne P, Buhler FR. Calcium antagonists and the second drug for hypertensive therapy. American Journal of Medicine. 1986; 81(6A):25–9 [PubMed: 2879444]
- 226.
- Nakae S, Taniguchi I, Suzuki K, Yoshida H, Kunou M, Saito Y. Comparison of an angiotensin II receptor blocker and an angiotensin converting enzyme inhibitor on the aldosterone breakthrough during antihypertensive treatment. Tokyo jikeikai medical journal. 2006; 121(4):165–176
- 227.
- National Institute for Health and Care Excellence. Developing NICE guidelines: the manual. London. National Institute for Health and Care Excellence, 2014. Available from: http://www
.nice.org.uk /article/PMG20/chapter /1%20Introduction%20and%20overview [PubMed: 26677490] - 228.
- Nct. A Prospective Randomised Study to Compare a Fixed Dose Combination of Telmisartan 80 mg Plus Hydrochlorothiazide 25 mg With a Fixed Dose Combination of Telmisartan 80 mg Plus Hydrochlorothiazide 12.5 mg in Patients With Uncontrolled Hypertension Who Fail to Respond Adequately to Treatment With a Fix. Available from: https:
//clinicaltrials .gov/ct2/show/NCT00239369?cond =A+Prospective+Randomised+Study+to+Compare+a+Fixed+Dose+Combination+of+Telmisartan+80+mg+Plus+Hydrochlorothiazide+25+mg+With+a+Fixed+Dose+Combination+of+Telmisartan+80+mg+Plus+Hydrochlorothiazide+12 .5+mg+in+Patients+With+Uncontrolled+Hypertension+Who&rank=1 Last accessed: 10/10/2018 - 229.
- Neutel JM, Cushman WC, Lloyd E, Barger B, Handley A. Comparison of long-term safety of fixed-dose combinations azilsartan medoxomil/chlorthalidone vs olmesartan medoxomil/hydrochlorothiazide. Journal of Clinical Hypertension. 2017; 19(9):874–883 [PMC free article: PMC8031288] [PubMed: 28681550]
- 230.
- Neutel JM, Frishman WH, Oparil S, Papademitriou V, Guthrie G. Comparison of telmisartan with lisinopril in patients with mild-to-moderate hypertension. American Journal of Therapeutics. 1999; 6(3):161–6 [PubMed: 10423659]
- 231.
- Oberman A, Pool PE. Trimazosin for the treatment of hypertensive patients failing to respond to thiazides. American Heart Journal. 1983; 106(5 Pt 2):1258–64 [PubMed: 6356855]
- 232.
- Ocón J, Oliván J, Garrido Peralta M, Ruilope L, Rodicio JL, Seco Vasco J et al. Multicenter study of the efficacy of 3 antihypertensive regimens: captopril + hydrochlorothiazide, oxprenolol + hydrochlorothiazide, and alphamethyldopa + hydrochlorothiazide. Medicina Clínica. 1985; 85(15):617–621 [PubMed: 3908851]
- 233.
- Ogawa H, Kim-Mitsuyama S, Matsui K, Jinnouchi T, Jinnouchi H, Arakawa K et al. Angiotensin II receptor blocker-based therapy in Japanese elderly, high-risk, hypertensive patients. American Journal of Medicine. 2012; 125(10):981–90 [PubMed: 22503610]
- 234.
- Ogihara T, Kuramoto K. Effect of long-term treatment with antihypertensive drugs on quality of life of elderly patients with hypertension: A double-blind comparative study between a calcium antagonist and a diuretic. NICS-EH Study Group. National Intervention Cooperative Study in Elderly Hypertensives. Hypertension Research. 2000; 23(1):33–7 [PubMed: 10737133]
- 235.
- Ogihara T, Matsuzaki M, Umemoto S, Rakugi H, Matsuoka H, Shimada K et al. Combination therapy for hypertension in the elderly: A sub-analysis of the Combination Therapy of Hypertension to Prevent Cardiovascular Events (COPE) Trial. Hypertension Research. 2012; 35(4):441–8 [PubMed: 22278623]
- 236.
- Ogihara T, Saruta T, Rakugi H, Saito I, Shimamoto K, Matsuoka H et al. Combinations of olmesartan and a calcium channel blocker or a diuretic in elderly hypertensive patients: A randomized, controlled trial. Journal of Hypertension. 2014; 32(10):2054–63 [PMC free article: PMC4166009] [PubMed: 24999799]
- 237.
- Ogihara T, Saruta T, Rakugi H, Saito I, Shimamoto K, Matsuoka H et al. Combination therapy of hypertension in the elderly: A subgroup analysis of the Combination of OLMesartan and a calcium channel blocker or diuretic in Japanese elderly hypertensive patients trial. Hypertension Research. 2015; 38(1):89–96 [PMC free article: PMC4287656] [PubMed: 25253583]
- 238.
- Ohnishi K, Kohno M, Yukiiri K, Masugata H, Wada Y, Takagi Y et al. Influence of the angiotensin II receptor antagonist losartan on diuretic-induced metabolic effects in elderly hypertensive patients: Comparison with a calcium channel blocker. International Journal of Clinical Pharmacology and Therapeutics. 2001; 39(10):417–22 [PubMed: 11680666]
- 239.
- Okin PM, Kjeldsen SE, Lindholm LH, Dahlof B, Devereux RB. The relationship of electrocardiographic left ventricular hypertrophy to decreased serum potassium. Blood Pressure. 2012; 21(3):146–52 [PubMed: 22243363]
- 240.
- Oshikawa J, Toya Y, Morita S, Taguri M, Hanaoka K, Hasegawa T et al. Angiotensin receptor blocker (ARB)-diuretic versus ARB-calcium channel blocker combination therapy for hypertension uncontrolled by ARB monotherapy. Clinical and Experimental Hypertension. 2014; 36(4):244–50 [PubMed: 23848219]
- 241.
- Ostergren J, Poulter NR, Sever PS, Dahlof B, Wedel H, Beevers G et al. The Anglo-Scandinavian Cardiac Outcomes Trial: blood pressure-lowering limb: effects in patients with type II diabetes. Journal of Hypertension. 2008; 26(11):2103–11 [PubMed: 18854748]
- 242.
- Park C, Wang G, Durthaler JM, Fang J. Cost-effectiveness analyses of antihypertensive medicines: A systematic review. American Journal of Preventive Medicine. 2017; 53(6 Suppl 2):S131–S142 [PMC free article: PMC5836308] [PubMed: 29153114]
- 243.
- Patay B. Series of single patient trials comparing the efficacy between the most commonly prescribed thiazide diuretic in the us, hydrochlorothiazide, and lisinopril for the treatment of stage 1 hypertension. 2010. Available from: https:
//clinicaltrials .gov/ct2/show/NCT01258764?term =Series+of+Single+Patient+Trials+Comparing+the+Efficacy+Between+the+Most+Commonly+Prescribed+Thiazide+Diuretic+in+the+US %2C+Hydrochlorothiazide %2C+and+Lisinopril+for+the+Treatment+of+Stage+1+Hypertension&rank=1 Last accessed: 11/10/2018 - 244.
- Persson B, Granerus G, Hedner T, Wysocki M, Andersson O. Systemic and renal hemodynamic effects of single oral doses of cadralazine and long term antihypertensive effects of cadralazine in patients receiving therapy with beta-blockers and diuretics. Acta Medica Scandinavica Supplementum. 1986; 714:177–82 [PubMed: 2883832]
- 245.
- Philip IG, Qureshi SM, Richards HH, Sharma SK, Wright FG, Young PH et al. A comparison of a hydrochlorothiazide plus triamterene combination (Dyazide) and atenolol in the treatment of patients with mild hypertension: A multicentre study in general practice. British Journal of Clinical Practice. 1987; 41(10):947–53 [PubMed: 3333144]
- 246.
- Pierini D, Anderson KV. Azilsartan medoxomil/chlorthalidone: A new fixed-dose combination antihypertensive. Annals of Pharmacotherapy. 2013; 47(5):694–703 [PubMed: 23585646]
- 247.
- Piller LB, Ford CE, Davis BR, Nwachuku C, Black HR, Oparil S et al. Incidence and predictors of angioedema in elderly hypertensive patients at high risk for cardiovascular disease: A report from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). Journal of Clinical Hypertension. 2006; 8(9):649–56; quiz 657–8 [PMC free article: PMC8109552] [PubMed: 16957427]
- 248.
- Remonti LR, Dias S, Leitao CB, Kramer CK, Klassman LP, Welton NJ et al. Classes of antihypertensive agents and mortality in hypertensive patients with type 2 diabetes-Network meta-analysis of randomized trials. Journal of Diabetes and Its Complications. 2016; 30(6):1192–200 [PubMed: 27217022]
- 249.
- Ritter J. A phase I randomized, placebo-controlled, double-blind study in hypertensive patients of the blood pressure interaction between TC-5214 and anti-hypertensive medications (Calcium Channel Blockers, Beta Blockers, and ACE Inhibitors). 2013. Available from: https://www
.hra.nhs.uk /planning-and-improving-research /application-summaries /research-summaries /to-investigate-interaction-of-tc-5214-with-antihypertensive-medication/ Last accessed: 10/10/2018 - 250.
- Rodilla E, Costa JA, Perez-Lahiguera F, Baldo E, Gonzalez C, Pascual JM. Spironolactone and doxazosin treatment in patients with resistant hypertension. Revista Española de Cardiología. 2009; 62(2):158–66 [PubMed: 19232189]
- 251.
- Roush GC, Abdelfattah R, Song S, Ernst ME, Sica DA, Kostis JB. Hydrochlorothiazide vs chlorthalidone, indapamide, and potassium-sparing/hydrochlorothiazide diuretics for reducing left ventricular hypertrophy: A systematic review and meta-analysis. Journal of Clinical Hypertension. 2018; 24:24 [PMC free article: PMC8030834] [PubMed: 30251403]
- 252.
- Ruoff G. Comparative trials of terazosin with other antihypertensive agents. American Journal of Medicine. 1986; 80(5 Suppl 2):42–8 [PubMed: 2872806]
- 253.
- Russell GI, Pohl JE, Baldwin J, Bing RF, Heagerty AM, Thurston H et al. Treatment of essential hypertension: Changes in blood pressure, echocardiography and electrocardiography on three therapeutic regimes. European Journal of Clinical Pharmacology. 1985; 28(2):119–24 [PubMed: 3157577]
- 254.
- Safar M, Zanchetti A, Sever PS. Perindopril and indapamide as a combination in the treatment of mild to moderate hypertension: A double-blind randomized placebo-controlled european multicenter study. American Journal of Hypertension. 1994; 7(4):43A
- 255.
- Saha C, Eckert GJ, Ambrosius WT, Chun TY, Wagner MA, Zhao Q et al. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005; 46(3):481–7 [PubMed: 16116042]
- 256.
- Saini R, Romanini M, Mos L. Tolerability and efficacy of fosinopril and hydrochlorothiazide compared with amiloride and hydrochlorothiazide in patients with mild to moderate hypertension. Clinical Drug Investigation. 1998; 15(2):91–9 [PubMed: 18370473]
- 257.
- Saku K, Zhang B, Okamoto T, Takeda Y, Liu K, Jimi S et al. Medium-term effects of betaxolol monotherapy and combination therapy with nitrendipine on lipoprotein and apolipoprotein metabolism in patients with mild to moderate essential hypertension. Journal of Human Hypertension. 1996; 10(4):263–268 [PubMed: 8736459]
- 258.
- Sano T, Kawamura T, Matsumae H, Sasaki H, Nakayama M, Hara T et al. Effects of long-term enalapril treatment on persistent microalbuminuria in well-controlled hypertensive and normotensive NIDDM patients. Diabetes Care. 1994; 17(5):420–424 [PubMed: 8062609]
- 259.
- Saruta T, Ogihara T, Saito I, Rakugi H, Shimamoto K, Matsuoka H et al. Comparison of olmesartan combined with a calcium channel blocker or a diuretic in elderly hypertensive patients (COLM Study): Safety and tolerability. Hypertension Research. 2015; 38(2):132–6 [PMC free article: PMC4322201] [PubMed: 25253582]
- 260.
- Sato A, Hayashi M, Saruta T. Relative long-term effects of spironolactone in conjunction with an angiotensin-converting enzyme inhibitor on left ventricular mass and diastolic function in patients with essential hypertension. Hypertension Research. 2002; 25(6):837–42 [PubMed: 12484506]
- 261.
- Sato N. Combination of Antihypertensive therapy in the elderly, Multicenter Investigation Multi-center randomized controlled study on efficacy of ARB/Diuretic mixture versus ARB/Ca channel blocker combination therapy to blood pressure or cognitive function in the elderly patients who have insufficient controlled hypertension in ARB monotherapy. 2009. Available from: https://upload
.umin.ac .jp/cgi-open-bin/ctr_e/ctr_view .cgi?recptno=R000002036 Last accessed: 08/01/2019 - 262.
- Sato N, Saijo Y, Hasebe N, Camui Investigators. Combination of antihypertensive therapy in the elderly, multicenter investigation (CAMUI) trial. International Heart Journal. 2012; 53(4):244–8 [PubMed: 22878803]
- 263.
- Sato N, Saijo Y, Sasagawa Y, Morimoto H, Takeuchi T, Sano H et al. Combination of antihypertensive therapy in the elderly, multicenter investigation (CAMUI) trial: Results after 1 year. Journal of Hypertension. 2013; 31(6):1245–55 [PubMed: 23492647]
- 264.
- Seedat YK, Parag KB. Comparison of fosinopril and propranolol combined with chlorthalidone in the treatment of moderately to severely hypertensive black and Indian patients. Current Therapeutic Research, Clinical and Experimental. 1992; 51(6):830–838
- 265.
- Seedat YK, Randeree IG. Antihypertensive effect and tolerability of perindopril in indian hypertensive and type 2 diabetic patients: 1-year randomised, double-blind, parallel study vs atenolol. Clinical Drug Investigation. 1998; 16(3):229–40 [PubMed: 18370544]
- 266.
- Soucek M, Plachý M. The FEVER (Felodipine EVEnt Reduction) trial; a randomised, double-blind, placebo-controlled trial in Chinese hypertensive patients. Vnitrni Lekarstvi. 2007; 53(1):63–70 [PubMed: 17472017]
- 267.
- Spoelstra-de Man AM, van Ittersum FJ, Schram MT, Kamp O, van Dijk RA, Ijzerman RG et al. Aggressive antihypertensive strategies based on hydrochlorothiazide, candesartan or lisinopril decrease left ventricular mass and improve arterial compliance in patients with type II diabetes mellitus and hypertension. Journal of Human Hypertension. 2006; 20(8):599–611 [PubMed: 16673014]
- 268.
- Stamler R, Stamler J, Gosch FC, Berkson DM, Dyer A, Hershinow P. Initial antihypertensive drug therapy: Alpha blocker or diuretic: Interim report of a randomized, controlled trial. American Journal of Medicine. 1986; 80(2 Suppl 1):90–3 [PubMed: 2868659]
- 269.
- Swales JD, Bing RF, Heagerty A, Pohl JE, Russell GI, Thurston H. Treatment of refractory hypertension. The Lancet. 1982; 1(8277):894–6 [PubMed: 6122111]
- 270.
- Thomopoulos C, Katsimagklis G, Archontakis S, Skalis G, Makris T. Optimizing the management of uncontrolled hypertension: What do triple fixed-dose drug combinations add? Current Vascular Pharmacology. 2017; 16(1):61–65 [PubMed: 28413969]
- 271.
- Trimarco V, Izzo R, Migliore T, Rozza F, Marino M, Manzi MV et al. Should thiazide diuretics be given as first line antihypertensive therapy or in addition to other medications? High Blood Pressure & Cardiovascular Prevention. 2015; 22(1):55–9 [PubMed: 24956971]
- 272.
- Umemoto S, Ogihara T, Matsuzaki M, Rakugi H, Shimada K, Kawana M et al. Effects of calcium-channel blocker benidipine-based combination therapy on cardiac events-subanalysis of the COPE trial. Circulation Journal. 2017; 82(2):457–463 [PubMed: 28867690]
- 273.
- Wallin JD, Wilson D, Winer N, Maronde RF, Michelson EL, Langford H et al. Treatment of severe hypertension with labetalol compared with methyldopa and furosemide. Results of a long-term, double-blind, multicenter trial. American Journal of Medicine. 1983; 75(4 Pt 1):87–94 [PubMed: 6356903]
- 274.
- White WB, Murwin D, Chrysant SG, Koval SE, Davidai G, Guthrie R. Effects of the angiotensin II receptor blockers telmisartan versus valsartan in combination with hydrochlorothiazide: A large, confirmatory trial. Blood Pressure Monitoring. 2008; 13(1):21–7 [PubMed: 18199920]
Appendices
Appendix A. Review protocols
Table 3Review protocol: Step 4 treatment
| Field | Content |
|---|---|
| Review question | What is the most clinically and cost-effective step 4 antihypertensive drug treatment for hypertension in adults? |
| Type of review question |
Intervention review A review of health economic evidence related to the same review question was conducted in parallel with this review. For details, see the health economic review protocol for this NICE guideline. |
| Objective of the review | To establish which step 4 treatment is most clinically and cost effective in adults with hypertension that remains uncontrolled following step 3 treatment. |
| Eligibility criteria – population / disease / condition / issue / domain |
Population: Adults (over 18 years) with primary hypertension are taking the maximally tolerated doses of at least 3 drugs (including a diuretic) and their blood pressure is still uncontrolled. Stratify by:
|
| Eligibility criteria – intervention(s) / exposure(s) / prognostic factor(s) | Step 4 antihypertensive pharmacological treatment received for a minimum of 1 year.
|
| Eligibility criteria – comparator(s) / control or reference (gold) standard |
|
| Outcomes and prioritisation | All outcomes to be measured at a minimum of 12 months. Where multiple time points are reported within each study, the longest time point only will be extracted. Critical
Important
|
| Eligibility criteria – study design | RCTs and SRs |
| Other inclusion exclusion criteria | Minimum follow up time: 1 year Exclusions:
|
| Proposed sensitivity / subgroup analysis, or metaregression | Subgroups to explore heterogeneity:
|
| Selection process – duplicate screening / selection / analysis | A senior research fellow will undertake quality assurance prior to completion. |
| Data management (software) |
Pairwise meta-analyses will be performed using Cochrane Review Manager (RevMan5). GRADEpro will be used to assess the quality of evidence for each outcome. Endnote will be used for bibliography, citations, sifting and reference management. |
| Information sources – databases and dates |
Medline, Embase, the Cochrane Library Language: Restrict to English only Key papers: PATHWAY-2 trial (2015) http://www |
| Identify if an update | Yes, 2011 |
| Author contacts |
https://www |
| Highlight if amendment to previous protocol | For details, please see section 4.5 of Developing NICE guidelines: the manual. |
| Search strategy – for 1 database | For details, please see appendix B |
| Data collection process – forms / duplicate | A standardised evidence table format will be used, and published as appendix D of the evidence report. |
| Data items – define all variables to be collected | For details, please see evidence tables in appendix D (clinical evidence tables) or H (health economic evidence tables). |
| Methods for assessing bias at outcome / study level |
Standard study checklists were used to appraise individual studies critically. For details, please see section 6.2 of Developing NICE guidelines: the manual The risk of bias across all available evidence was evaluated for each outcome using an adaptation of the ‘Grading of Recommendations Assessment, Development and Evaluation (GRADE) toolbox’ developed by the international GRADE working group http://www |
| Criteria for quantitative synthesis | For details, please see section 6.4 of Developing NICE guidelines: the manual. |
| Methods for quantitative analysis – combining studies and exploring (in)consistency | For details, please see the separate Methods report for this guideline. |
| Meta-bias assessment – publication bias, selective reporting bias | For details, please see section 6.2 of Developing NICE guidelines: the manual. |
| Confidence in cumulative evidence | For details, please see sections 6.4 and 9.1 of Developing NICE guidelines: the manual. |
| Rationale / context – what is known | For details, please see the introduction to the evidence review. |
| Describe contributions of authors and guarantor | A multidisciplinary committee developed the evidence review. The committee was convened by the National Guideline Centre (NGC) and chaired by Anthony Wierzbicki in line with section 3 of Developing NICE guidelines: the manual. Staff from the NGC undertook systematic literature searches, appraised the evidence, conducted meta-analysis and cost-effectiveness analysis where appropriate, and drafted the evidence review in collaboration with the committee. For details, please see Developing NICE guidelines: the manual. |
| Sources of funding / support | The NGC is funded by NICE and hosted by the Royal College of Physicians. |
| Name of sponsor | The NGC is funded by NICE and hosted by the Royal College of Physicians. |
| Roles of sponsor | NICE funds the NGC to develop guidelines for those working in the NHS, public health and social care in England. |
| PROSPERO registration number | Not registered |
Table 4Health economic review protocol
| Review question | All questions – health economic evidence |
|---|---|
| Objectives | To identify health economic studies relevant to any of the review questions. |
| Search criteria |
|
| Search strategy | A health economic study search will be undertaken using population-specific terms and a health economic study filter – see appendix B below. No date cut-off from the previous guideline was used. |
| Review strategy | Studies not meeting any of the search criteria above will be excluded. Studies published before 2002, abstract-only studies and studies from non-OECD countries or the US will also be excluded. Studies published after 2002 that were included in the previous guideline(s) will be reassessed for inclusion and may be included or selectively excluded based on their relevance to the questions covered in this update and whether more applicable evidence is also identified. Each remaining study will be assessed for applicability and methodological limitations using the NICE economic evaluation checklist which can be found in appendix H of Developing NICE guidelines: the manual (2014).227 Inclusion and exclusion criteria
Where there is discretion The health economist will make a decision based on the relative applicability and quality of the available evidence for that question in discussion with the guideline committee if required. The ultimate aim is to include health economic studies that are helpful for decision-making in the context of the guideline and the current NHS setting. If several studies are considered of sufficiently high applicability and methodological quality that they could all be included, then the health economist, in discussion with the committee if required, may decide to include only the most applicable studies and to exclude selectively the remaining studies. All studies excluded based on applicability or methodological limitations will be listed with explanation in the excluded health economic studies appendix below. The health economist will be guided by the following hierarchies. Setting:
Health economic study type:
Year of analysis:
Quality and relevance of effectiveness data used in the health economic analysis:
|
Appendix B. Literature search strategies
The literature searches for this review are detailed below and complied with the methodology outlined in Developing NICE guidelines: the manual 2014, updated 2017.
For more detailed information, please see the Methodology Review. [Add cross reference]
B.1. Clinical search literature search strategy
Searches were constructed using a PICO framework where population (P) terms were combined with Intervention (I) and in some cases Comparison (C) terms. Outcomes (O) are rarely used in search strategies for interventions as these concepts may not be well described in title, abstract or indexes and therefore difficult to retrieve. Search filters were applied to the search where appropriate.
Table 5Database date parameters and filters used
| Database | Dates searched | Search filter used |
|---|---|---|
| Medline (OVID) | 1946–02 October 2018 |
Exclusions Randomised controlled trials Systematic review studies |
| Embase (OVID) | 1974–02 October 2018 |
Exclusions Randomised controlled trials Systematic review studies |
| The Cochrane Library (Wiley) |
Cochrane Reviews to Issue 8 of 12, August 2018 CENTRAL to Issue 7 of 12, July 2018 DARE and NHSEED to Issue 2 of 4, April 2015 HTA to Issue 4 of 4, October 2016 | None |
Table 6Medline (Ovid) search terms
| 1. | exp Hypertension/ |
| 2. | hypertens*.ti,ab. |
| 3. | (elevat* adj2 blood adj pressur*).ti,ab. |
| 4. | (high adj blood adj pressur*).ti,ab. |
| 5. | (increase* adj2 blood pressur*).ti,ab. |
| 6. | ((systolic or diastolic or arterial) adj2 pressur*).ti,ab. |
| 7. | or/1–6 |
| 8. | exp pregnancy/ |
| 9. | exp Hypertension, Pregnancy-Induced/ not exp Hypertension/ |
| 10. | (pre eclampsia or pre-eclampsia or preeclampsia).ti,ab. |
| 11. | exp Hypertension, Portal/ not exp Hypertension/ |
| 12. | exp Hypertension, Pulmonary/ not exp Hypertension/ |
| 13. | exp Intracranial Hypertension/ not exp Hypertension/ |
| 14. | exp Ocular Hypertension/ not exp Hypertension/ |
| 15. | exp Diabetes Mellitus, Type 1/ not exp Diabetes Mellitus, Type 2/ |
| 16. | or/9–15 |
| 17. | 7 not 16 |
| 18. | letter/ |
| 19. | editorial/ |
| 20. | news/ |
| 21. | exp historical article/ |
| 22. | Anecdotes as Topic/ |
| 23. | comment/ |
| 24. | case report/ |
| 25. | (letter or comment*).ti. |
| 26. | or/18–25 |
| 27. | randomized controlled trial/ or random*.ti,ab. |
| 28. | 26 not 27 |
| 29. | animals/ not humans/ |
| 30. | exp Animals, Laboratory/ |
| 31. | exp Animal Experimentation/ |
| 32. | exp Models, Animal/ |
| 33. | exp Rodentia/ |
| 34. | (rat or rats or mouse or mice).ti. |
| 35. | or/28–34 |
| 36. | 17 not 35 |
| 37. | (exp child/ or exp pediatrics/ or exp infant/) not (exp adolescent/ or exp adult/ or exp middle age/ or exp aged/) |
| 38. | 36 not 37 |
| 39. | limit 38 to English language |
| 40. | exp Angiotensin-Converting Enzyme Inhibitors/ |
| 41. | Angiotensin-converting enzyme inhibitor*.ti,ab. |
| 42. | (ACE inhibitor* or ACEI).ti,ab. |
| 43. | (Captopril or Enalapril or Fosinopril or Imidapril or Lisinopril or Moexipril or Perindopril or Quinapril or Ramipril or Trandolapril or Capoten or Ecopace or Noyada or Innovace or Tanatril or Zestril or Perdix or Coversil or Accupro or Tritace).ti,ab. |
| 44. | Captopril/ or Enalapril/ or Fosinopril/ or Lisinopril/ or Perindopril/ or Ramipril/ |
| 45. | exp Angiotensin Receptor Antagonists/ |
| 46. | (Angiotensin II adj3 (antagonist* or blocker*)).ti,ab. |
| 47. | ARB.ti,ab. |
| 48. | (Azilsartan or Candesartan or Eprosartan or Irbesartan or Losartan or Olmesartan or Telmisartan or Valsartan or Edarbi or Amias or Teveten or Aprovel or Ifirmasta or Sabervel or Cozaar or Olmetec or Tolura or Micardis or Diovan).ti,ab. |
| 49. | Losartan/ or Valsartan/ or Olmesartan Medoxomil/ |
| 50. | exp Calcium Channel Blockers/ |
| 51. | Calcium channel blocker*.ti,ab. |
| 52. | CCB.ti,ab. |
| 53. | (Amlodipine or Clevidipine or Diltiazem or Felodipine or Isradipine or Lacidipine or Lercanidipine or Nicardipine or Nifedipine or Verapamil or Amlostin or Istin or Adizem or Angitil or Dilcardia or Dilzem or Slozem or Tildiem or Viazem or Zemtard or Kenzem or Cardioplen or Felendil or Neofel or Parmid or Plendil or Pinefeld or Vascalpha or Molap or Motens or Zanidip or Cardene or Adalat or Adipine or Coracten or Fortipine or Nifedipress or Tensipine or Valni or Securon or Verapress or Vertab or Univer or Zolvera or Cleviprex).ti,ab. |
| 54. | Amlodipine/ or Diltiazem/ or Felodipine/ or Isradipine/ or Nicardipine/ or Nifedipine/ or Verapamil/ |
| 55. | Diuretics/ |
| 56. | Diuretics, Thiazide/ |
| 57. | ((thiazide or thiazide-like or non-thiazide or conventional or potassium sparing) adj3 diuretic*).ti,ab. |
| 58. | Mineralocorticoid Receptor Antagonists/ |
| 59. | ((mineralocorticoid or aldosterone) adj3 antagonist*).ti,ab. |
| 60. | (Amiloride or Cyclopenthiazide or Spironolactone or Eplerenone or Bendroflumethiazide or Hydrochlorothiazide or Co-amilozide or Co-triamterzide or Co-zidocapt or Chlortalidone or Indapamide or Metolazone or Xipamide or Carace or Zestoretic or Coversyl or Accuretic or Cozaar or Sevikar or Olmetec or Actelsar or Tolucombi or Co-Diovan or Hygroton or Co-tenidone or Kalspare or Natrilix or Cardide or Indipam or Rawel or Tensaid or Alkapamid or Zaroxolyn or Diurexan or Aprinox or Neo-Naclex or CoAprovel or Lisoretic or Dyazide or Navispare or Lasilactone).ti,ab. |
| 61. | Amiloride/ or Cyclopenthiazide/ or Spironolactone/ or Bendroflumethiazide/ or Hydrochlorothiazide/ or Chlortalidone/ or Indapamide/ or Metolazone/ or Xipamide/ or Chlorthalidone/ or Metolazone/ |
| 62. | Adrenergic beta-Antagonists/ |
| 63. | (adrenergic beta antagonist* or beta blocker* or b blocker*).ti,ab. |
| 64. | (Carvedilol or Labetalol or Atenolol or Nadolol or Oxprenolol or Pindolol or Propranolol or Timolol or Acebutolol or Bisoprolol or Celiprolol or Esmolol or Metoprolol or Nebivolol or Tenormin or Tenif or Corgard or Slow-Trasicor or Visken or Viskladix or Bedranol or Beta-Prograne or Syprol or Betim or Sectral or Cardicor or Congescor or Celectol or Breviblock or Betaloc or Lopresor or Nebilet).ti,ab. |
| 65. | Labetalol/ or Nadolol/ or Oxprenolol/ or Pindolol/ or Propranolol/ or Timolol/ or Acebutolol/ or Bisoprolol/ or Celiprolol/ or Metoprolol/ or Nebivolol/ |
| 66. | exp Adrenergic alpha-Antagonists/ |
| 67. | (adrenergic alpha antagonist* or alpha adrenoreceptor blocker* or alpha blocker*).ti,ab. |
| 68. | (Doxazosin or Prazosin or Terazosin or Cardura or Doxadura or Raporsin or Slocinx or Doxzogen or Larbex or Hypovase or Hytrin).ti,ab. |
| 69. | Doxazosin/ or Prazosin/ |
| 70. | Antihypertensive Agents/ |
| 71. | centrally acting antihypertensive*.ti,ab. |
| 72. | (Clonidine or Moxonidine or Minoxidil or Methyldopa or Catapres or Dixarit or Aldomet or Physiotens).ti,ab. |
| 73. | Clonidine/ or Minoxidil/ or Methyldopa/ |
| 74. | renin inhibitor*.ti,ab. |
| 75. | (Aliskiren or Rasilez).ti,ab. |
| 76. | or/40–75 |
| 77. | 39 and 76 |
| 78. | randomized controlled trial.pt. |
| 79. | controlled clinical trial.pt. |
| 80. | randomi#ed.ti,ab. |
| 81. | placebo.ab. |
| 82. | randomly.ti,ab. |
| 83. | Clinical Trials as topic.sh. |
| 84. | trial.ti. |
| 85. | or/78–84 |
| 86. | Meta-Analysis/ |
| 87. | exp Meta-Analysis as Topic/ |
| 88. | (meta analy* or metanaly* or metaanaly* or meta regression).ti,ab. |
| 89. | ((systematic* or evidence*) adj3 (review* or overview*)).ti,ab. |
| 90. | (reference list* or bibliograph* or hand search* or manual search* or relevant journals).ab. |
| 91. | (search strategy or search criteria or systematic search or study selection or data extraction).ab. |
| 92. | (search* adj4 literature).ab. |
| 93. | (medline or pubmed or cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or science citation index or bids or cancerlit).ab. |
| 94. | cochrane.jw. |
| 95. | ((multiple treatment* or indirect or mixed) adj2 comparison*).ti,ab. |
| 96. | or/86–95 |
| 97. | 77 and (85 or 96) |
Table 7Embase (Ovid) search terms
| 1. | exp Hypertension/ |
| 2. | hypertens*.ti,ab. |
| 3. | (elevat* adj2 blood adj pressur*).ti,ab. |
| 4. | (high adj blood adj pressur*).ti,ab. |
| 5. | (increase* adj2 blood pressur*).ti,ab. |
| 6. | ((systolic or diastolic or arterial) adj2 pressur*).ti,ab. |
| 7. | or/1–6 |
| 8. | exp pregnancy/ |
| 9. | exp Maternal Hypertension/ |
| 10. | (pre eclampsia or pre-eclampsia or preeclampsia).ti,ab. |
| 11. | exp Hypertension, Portal/ not exp Hypertension/ |
| 12. | exp Hypertension, Pulmonary/ not exp Hypertension/ |
| 13. | exp Intracranial Hypertension/ |
| 14. | exp Ocular Hypertension/ not exp Hypertension/ |
| 15. | exp Diabetes Mellitus, Type 1/ not exp Diabetes Mellitus, Type 2/ |
| 16. | or/8–15 |
| 17. | 7 not 16 |
| 18. | letter.pt. or letter/ |
| 19. | note.pt. |
| 20. | editorial.pt. |
| 21. | case report/ or case study/ |
| 22. | (letter or comment*).ti. |
| 23. | or/18–22 |
| 24. | randomized controlled trial/ or random*.ti,ab. |
| 25. | 23 not 24 |
| 26. | animal/ not human/ |
| 27. | nonhuman/ |
| 28. | exp Animal Experiment/ |
| 29. | exp Experimental Animal/ |
| 30. | animal model/ |
| 31. | exp Rodent/ |
| 32. | (rat or rats or mouse or mice).ti. |
| 33. | or/25–32 |
| 34. | 17 not 33 |
| 35. | (exp child/ or exp pediatrics/) not (exp adult/ or exp adolescent/) |
| 36. | 34 not 35 |
| 37. | limit 36 to English language |
| 38. | exp *Angiotensin-Converting Enzyme Inhibitors/ |
| 39. | Angiotensin-converting enzyme inhibitor*.ti,ab. |
| 40. | (ACE inhibitor* or ACEI).ti,ab. |
| 41. | (Captopril or Enalapril or Fosinopril or Imidapril or Lisinopril or Moexipril or Perindopril or Quinapril or Ramipril or Trandolapril or Capoten or Ecopace or Noyada or Innovace or Tanatril or Zestril or Perdix or Coversil or Accupro or Tritace).ti,ab. |
| 42. | *Captopril/ or *Enalapril/ or *Fosinopril/ or *Imidapril/ or *Lisinopril/ or *Moexipril/ or *Perindopril/ or *Quinapril/ or *Ramipril/ or *Trandolapril/ or *enalapril maleate/ |
| 43. | *angiotensin receptor antagonist/ |
| 44. | (Angiotensin II adj3 (antagonist* or blocker*)).ti,ab. |
| 45. | ARB.ti,ab. |
| 46. | (Azilsartan or Candesartan or Eprosartan or Irbesartan or Losartan or Olmesartan or Telmisartan or Valsartan or Edarbi or Amias or Teveten or Aprovel or Ifirmasta or Sabervel or Cozaar or Olmetec or Tolura or Micardis or Diovan).ti,ab. |
| 47. | *Azilsartan/ or *Candesartan/ or *Eprosartan/ or *Irbesartan/ or *Losartan/ or *Valsartan/ or *Olmesartan Medoxomil/ or *Telmisartan/ |
| 48. | exp *Calcium Channel Blockers/ |
| 49. | Calcium channel blocker*.ti,ab. |
| 50. | CCB.ti,ab. |
| 51. | (Amlodipine or Clevidipine or Diltiazem or Felodipine or Isradipine or Lacidipine or Lercanidipine or Nicardipine or Nifedipine or Verapamil or Amlostin or Istin or Adizem or Angitil or Dilcardia or Dilzem or Slozem or Tildiem or Viazem or Zemtard or Kenzem or Cardioplen or Felendil or Neofel or Parmid or Plendil or Pinefeld or Vascalpha or Molap or Motens or Zanidip or Cardene or Adalat or Adipine or Coracten or Fortipine or Nifedipress or Tensipine or Valni or Securon or Verapress or Vertab or Univer or Zolvera or Cleviprex).ti,ab. |
| 52. | *Amlodipine/ or *Diltiazem/ or *Felodipine/ or *Isradipine/ or *Nicardipine/ or *Nifedipine/ or *Verapamil/ |
| 53. | *Diuretics/ |
| 54. | *thiazide diuretic agent/ |
| 55. | ((thiazide or thiazide-like or non-thiazide or conventional or potassium sparing) adj3 diuretic*).ti,ab. |
| 56. | *mineralocorticoid antagonist/ |
| 57. | ((mineralocorticoid or aldosterone) adj3 antagonist*).ti,ab. |
| 58. | (Amiloride or Cyclopenthiazide or Spironolactone or Eplerenone or Bendroflumethiazide or Hydrochlorothiazide or Co-amilozide or Co-triamterzide or Co-zidocapt or Chlortalidone or Indapamide or Metolazone or Xipamide or Carace or Zestoretic or Coversyl or Accuretic or Cozaar or Sevikar or Olmetec or Actelsar or Tolucombi or Co-Diovan or Hygroton or Co-tenidone or Kalspare or Natrilix or Cardide or Indipam or Rawel or Tensaid or Alkapamid or Zaroxolyn or Diurexan or Aprinox or Neo-Naclex or CoAprovel or Lisoretic or Dyazide or Navispare or Lasilactone).ti,ab. |
| 59. | *Amiloride/ or *Cyclopenthiazide/ or *Spironolactone/ or *Bendroflumethiazide/ or *Hydrochlorothiazide/ or *Chlortalidone/ or *Indapamide/ or *Metolazone/ or *Xipamide/ |
| 60. | *Adrenergic beta-Antagonists/ |
| 61. | (adrenergic beta antagonist* or beta blocker* or b blocker*).ti,ab. |
| 62. | (Carvedilol or Labetalol or Atenolol or Nadolol or Oxprenolol or Pindolol or Propranolol or Timolol or Acebutolol or Bisoprolol or Celiprolol or Esmolol or Metoprolol or Nebivolol or Carvedilol or Tenormin or Tenif or Corgard or Slow-Trasicor or Visken or Viskladix or Bedranol or Beta-Prograne or Syprol or Betim or Sectral or Cardicor or Congescor or Celectol or Breviblock or Betaloc or Lopresor or Nebilet).ti,ab. |
| 63. | *Carvedilol/ or *Labetalol/ or *Nadolol/ or *Oxprenolol/ or *Pindolol/ or *Propranolol/ or *Timolol/ or *Acebutolol/ or *Bisoprolol/ or *Celiprolol/ or *Metoprolol/ or *Nebivolol/ |
| 64. | exp *Adrenergic alpha-Antagonists/ |
| 65. | (adrenergic alpha antagonist* or alpha adrenoreceptor blocker* or alpha blocker*).ti,ab. |
| 66. | (Doxazosin or Prazosin or Terazosin or Cardura or Doxadura or Raporsin or Slocinx or Doxzogen or Larbex or Hypovase or Hytrin).ti,ab. |
| 67. | *doxazosin/ or *Prazosin/ or *Terazosin/ |
| 68. | *Antihypertensive Agents/ |
| 69. | centrally acting antihypertensive*.ti,ab. |
| 70. | (Clonidine or Moxonidine or Methyldopa or Catapres or Dixarit or Aldomet or Physiotens).ti,ab. |
| 71. | *clonidine/ or *moxonidine/ or *Methyldopa/ |
| 72. | renin inhibitor*.ti,ab. |
| 73. | (Aliskiren or Rasilez).ti,ab. |
| 74. | *Aliskiren/ |
| 75. | or/38–74 |
| 76. | 37 and 75 |
| 77. | random*.ti,ab. |
| 78. | factorial*.ti,ab. |
| 79. | (crossover* or cross over*).ti,ab. |
| 80. | ((doubl* or singl*) adj blind*).ti,ab. |
| 81. | (assign* or allocat* or volunteer* or placebo*).ti,ab. |
| 82. | crossover procedure/ |
| 83. | single blind procedure/ |
| 84. | randomized controlled trial/ |
| 85. | double blind procedure/ |
| 86. | or/77–85 |
| 87. | systematic review/ |
| 88. | meta-analysis/ |
| 89. | (meta analy* or metanaly* or metaanaly* or meta regression).ti,ab. |
| 90. | ((systematic* or evidence*) adj3 (review* or overview*)).ti,ab. |
| 91. | (reference list* or bibliograph* or hand search* or manual search* or relevant journals).ab. |
| 92. | (search strategy or search criteria or systematic search or study selection or data extraction).ab. |
| 93. | (search* adj4 literature).ab. |
| 94. | (medline or pubmed or cochrane or embase or psychlit or psyclit or psychinfo or psycinfo or cinahl or science citation index or bids or cancerlit).ab. |
| 95. | cochrane.jw. |
| 96. | ((multiple treatment* or indirect or mixed) adj2 comparison*).ti,ab. |
| 97. | or/87–96 |
| 98. | 76 and (86 or 97) |
Table 8Cochrane Library (Wiley) search terms
| #1. | MeSH descriptor: [Hypertension] explode all trees |
| #2. | hypertens*:ti,ab |
| #3. | (elevat* near/2 blood next pressur*):ti,ab |
| #4. | (high near/1 blood near/1 pressur*):ti,ab |
| #5. | (increase* near/2 blood pressur*):ti,ab |
| #6. | ((systolic or diastolic or arterial) near/2 pressur*):ti,ab |
| #7. | (or #1-#6) |
| #8. | MeSH descriptor: [Angiotensin-Converting Enzyme Inhibitors] explode all trees |
| #9. | Angiotensin-converting enzyme inhibitor*:ti,ab |
| #10. | (ACE inhibitor* or ACEI):ti,ab |
| #11. | (Captopril or Enalapril or Fosinopril or Imidapril or Lisinopril or Moexipril or Perindopril or Quinapril or Ramipril or Trandolapril or Capoten or Ecopace or Noyada or Innovace or Tanatril or Zestril or Perdix or Coversil or Accupro or Tritace):ti,ab |
| #12. | MeSH descriptor: [Captopril] explode all trees |
| #13. | MeSH descriptor: [Angiotensin Receptor Antagonists] explode all trees |
| #14. | (AngiotensinII near/3 (antagonist* or blocker*)):ti,ab |
| #15. | ARB:ti,ab |
| #16. | (Azilsartan or Candesartan or Eprosartan or Irbesartan or Losartan or Olmesartan or Telmisartan or Valsartan or Edarbi or Amias or Teveten or Aprovel or Ifirmasta or Sabervel or Cozaar or Olmetec or Tolura or Micardis or Diovan):ti,ab |
| #17. | MeSH descriptor: [Losartan] explode all trees |
| #18. | MeSH descriptor: [Calcium Channel Blockers] explode all trees |
| #19. | Calcium channel blocker*:ti,ab |
| #20. | CCB:ti,ab |
| #21. | (Amlodipine or Clevidipine or Diltiazem or Felodipine or Isradipine or Lacidipine or Lercanidipine or Nicardipine or Nifedipine or Verapamil or Amlostin or Istin or Adizem or Angitil or Dilcardia or Dilzem or Slozem or Tildiem or Viazem or Zemtard or Kenzem or Cardioplen or Felendil or Neofel or Parmid or Plendil or Pinefeld or Vascalpha or Molap or Motens or Zanidip or Cardene or Adalat or Adipine or Coracten or Fortipine or Nifedipress or Tensipine or Valni or Securon or Verapress or Vertab or Univer or Zolvera or Cleviprex):ti,ab |
| #22. | MeSH descriptor: [Amlodipine] explode all trees |
| #23. | MeSH descriptor: [Diuretics] this term only |
| #24. | MeSH descriptor: [Sodium Chloride Symporter Inhibitors] this term only |
| #25. | ((thiazide* or thiazide-like or non-thiazide or conventional or potassium sparing) near/3 diuretic*):ti,ab |
| #26. | MeSH descriptor: [Mineralocorticoid Receptor Antagonists] explode all trees |
| #27. | ((mineralocorticoid or aldosterone) near/3 antagonist*):ti,ab |
| #28. | (Amiloride or Cyclopenthiazide or Spironolactone or Eplenerone or Bendroflumethiazide or Hydrochlorothiazide or Co-amilozide or Co-triamterzide or Co-zidocapt or Chlortalidone or Indapamide or Metolazone or Xipamide or Carace or Zestoretic or Coversyl or Accuretic or Cozaar or Sevikar or Olmetec or Actelsar or Tolucombi or Co-Diovan or Hygroton or Co-tenidone or Kalspare or Natrilix or Cardide or Indipam or Rawel or Tensaid or Alkapamid or Zaroxolyn or Diurexan or Aprinox or Neo-Naclex or Co-Aprovel or Lisoretic or Dyazide or Navispare or Lasilactone):ti,ab |
| #29. | MeSH descriptor: [Amiloride] explode all trees |
| #30. | MeSH descriptor: [Adrenergic beta-Antagonists] this term only |
| #31. | (adrenergic beta antagonist* or beta blocker* or b blocker*):ti,ab |
| #32. | (Carvedilol or Labetalol or Atenolol or Nadolol or Oxprenolol or Pindolol or Propranolol or Timolol or Acebutolol or Bisoprolol or Celiprolol or Esmolol or Metoprolol or Nebivolol or Carvedilol or Tenormin or Tenif or Corgard or Slow-Trasicor or Visken or Viskladix or Bedranol or Beta-Prograne or Syprol or Betim or Sectral or Cardicor or Congescor or Celectol or Breviblock or Betaloc or Lopresor or Nebilet):ti,ab |
| #33. | MeSH descriptor: [Labetalol] explode all trees |
| #34. | MeSH descriptor: [Adrenergic alpha-Antagonists] explode all trees |
| #35. | (adrenergic alpha antagonist* or alpha adrenoreceptor blocker* or alpha blocker*):ti,ab |
| #36. | (Doxazosin or Prazosin or Terazosin or Cardura or Doxadura or Raporsin or Slocinx or Doxzogen or Larbex or Hypovase or Hytrin):ti,ab |
| #37. | MeSH descriptor: [Doxazosin] explode all trees |
| #38. | MeSH descriptor: [Antihypertensive Agents] this term only |
| #39. | centrally acting antihypertensive*:ti,ab |
| #40. | (Clonidine or Moxonidine or Methyldopa or Catapres or Dixarit or Aldomet or Physiotens):ti,ab |
| #41. | MeSH descriptor: [Clonidine] explode all trees |
| #42. | renin inhibitor*:ti,ab |
| #43. | (Aliskiren or Rasilez):ti,ab |
| #44. | (or #8-#43) |
| #45. | #7 and #44 |
B.2. Health Economics literature search strategy
Health economic evidence was identified by conducting a broad search relating to hypertension in adults population in NHS Economic Evaluation Database (NHS EED – this ceased to be updated after March 2015) and the Health Technology Assessment database (HTA) with no date restrictions. NHS EED and HTA databases are hosted by the Centre for Research and Dissemination (CRD). Additional searches were run on Medline and Embase for health economics, economic modelling and quality of life studies.
Table 9Database date parameters and filters used
| Database | Dates searched | Search filter used |
|---|---|---|
| Medline | 2014–28 August 2018 |
Exclusions Health economics studies |
| Embase | 2014–28 August 2018 |
Exclusions Health economics studies |
| Centre for Research and Dissemination (CRD) |
HTA - Inception–28 August 2018 NHSEED - Inception to March 2015 | None |
Table 10Medline (Ovid) search terms
| 1. | exp Hypertension/ |
| 2. | hypertens*.ti,ab. |
| 3. | (elevat* adj2 blood adj pressur*).ti,ab. |
| 4. | (high adj blood adj pressur*).ti,ab. |
| 5. | (increase* adj2 blood pressur*).ti,ab. |
| 6. | ((systolic or diastolic or arterial) adj2 pressur*).ti,ab. |
| 7. | or/1–6 |
| 8. | letter/ |
| 9. | editorial/ |
| 10. | news/ |
| 11. | exp historical article/ |
| 12. | Anecdotes as Topic/ |
| 13. | comment/ |
| 14. | case report/ |
| 15. | (letter or comment*).ti. |
| 16. | or/8–15 |
| 17. | randomized controlled trial/ or random*.ti,ab. |
| 18. | 16 not 17 |
| 19. | animals/ not humans/ |
| 20. | exp Animals, Laboratory/ |
| 21. | exp Animal Experimentation/ |
| 22. | exp Models, Animal/ |
| 23. | exp Rodentia/ |
| 24. | (rat or rats or mouse or mice).ti. |
| 25. | or/18–24 |
| 26. | 7 not 25 |
| 27. | limit 26 to English language |
| 28. | Economics/ |
| 29. | Value of life/ |
| 30. | exp “Costs and Cost Analysis”/ |
| 31. | exp Economics, Hospital/ |
| 32. | exp Economics, Medical/ |
| 33. | Economics, Nursing/ |
| 34. | Economics, Pharmaceutical/ |
| 35. | exp “Fees and Charges”/ |
| 36. | exp Budgets/ |
| 37. | budget*.ti,ab. |
| 38. | cost*.ti. |
| 39. | (economic* or pharmaco?economic*).ti. |
| 40. | (price* or pricing*).ti,ab. |
| 41. | (cost* adj2 (effective* or utilit* or benefit* or minimi* or unit* or estimat* or variable*)).ab. |
| 42. | (financ* or fee or fees).ti,ab. |
| 43. | (value adj2 (money or monetary)).ti,ab. |
| 44. | or/28–43 |
| 45. | 27 and 44 |
Table 11Embase (Ovid) search terms
| 1. | exp Hypertension/ |
| 2. | hypertens*.ti,ab. |
| 3. | (elevat* adj2 blood adj pressur*).ti,ab. |
| 4. | (high adj blood adj pressur*).ti,ab. |
| 5. | (increase* adj2 blood pressur*).ti,ab. |
| 6. | ((systolic or diastolic or arterial) adj2 pressur*).ti,ab. |
| 7. | or/1–6 |
| 8. | letter.pt. or letter/ |
| 9. | note.pt. |
| 10. | editorial.pt. |
| 11. | case report/ or case study/ |
| 12. | (letter or comment*).ti. |
| 13. | or/8–12 |
| 14. | randomized controlled trial/ or random*.ti,ab. |
| 15. | 13 not 14 |
| 16. | animal/ not human/ |
| 17. | nonhuman/ |
| 18. | exp Animal Experiment/ |
| 19. | exp Experimental Animal/ |
| 20. | animal model/ |
| 21. | exp Rodent/ |
| 22. | (rat or rats or mouse or mice).ti. |
| 23. | or/15–22 |
| 24. | 7 not 23 |
| 25. | limit 24 to English language |
| 26. | health economics/ |
| 27. | exp economic evaluation/ |
| 28. | exp health care cost/ |
| 29. | exp fee/ |
| 30. | budget/ |
| 31. | funding/ |
| 32. | budget*.ti,ab. |
| 33. | cost*.ti. |
| 34. | (economic* or pharmaco?economic*).ti. |
| 35. | (price* or pricing*).ti,ab. |
| 36. | (cost* adj2 (effective* or utilit* or benefit* or minimi* or unit* or estimat* or variable*)).ab. |
| 37. | (financ* or fee or fees).ti,ab. |
| 38. | (value adj2 (money or monetary)).ti,ab. |
| 39. | or/26–38 |
| 40. | 25 and 39 |
Table 12NHS EED and HTA (CRD) search terms
| #1. | MeSH DESCRIPTOR Hypertension EXPLODE ALL TREES IN NHSEED,HTA |
| #2. | (Hypertens*) IN NHSEED, HTA |
| #3. | (elevat* adj2 blood adj pressur*) IN NHSEED, HTA |
| #4. | (high adj blood adj pressur*) IN NHSEED, HTA |
| #5. | (increase* adj2 blood pressur*) IN NHSEED, HTA |
| #6. | ((systolic or diastolic or arterial) adj2 pressur*) IN NHSEED, HTA |
| #7. | #1 OR #2 OR #3 OR #4 OR #5 OR #6 |
Appendix C. Clinical evidence selection
Appendix D. Clinical evidence tables
None.
Appendix E. Forest plots
None.
Appendix F. GRADE tables
None.
Appendix G. Health economic evidence selection
Appendix H. Health economic evidence tables
None.
Appendix I. Excluded studies
I.1. Excluded clinical studies
Table 13Studies excluded from the clinical review that were included in the previous guideline (CG127)
| Study | Exclusion details |
|---|---|
| Chapman 200760 | Follow up from the ASCOT trial consisting of only 1,790 of the original 19,257 participants. Incorrect comparison; follow up of those who took spironolactone only. |
| de Souza 201075 | Incorrect comparison; not a comparative study. The uncontrolled group received spironolactone and there were no details of the comparator. |
| Gaddam 2010110 | Less than minimum duration; 8 weeks follow up, less than the 12 month minimum follow up specified by the protocol. |
| Lane 2007170 | Less than minimum duration; 3–6 months follow up, less than the 12 month minimum follow up specified by the protocol. |
| Mahmud 2005202 | Incorrect intervention, incorrect population, less than minimum duration. One group consisted of people not having any previous antihypertensive treatment and were given either spironolactone or bendroflumethiazide as step 1 treatment. There was a 4 week follow up with a 4 week washout period and another 4 weeks of intervention. The second group were followed up 3 to 4 months later, less than the 12 month minimum follow up specified by the protocol. |
| Rodilla 2009250 | Less than minimum duration. The follow up was for a median of 3 months for the spironolactone group, and 5 months for the doxazosin group, not meeting the 12 month minimum follow up specified by the protocol. |
Table 14Studies excluded from the clinical review
| Study | Exclusion reason |
|---|---|
| Abarquez 19931 | Less than minimum duration |
| Abascal 19982 | Incorrect study design |
| Abe 20073 | Not review population |
| Abe 20094 | Less than minimum duration |
| Abetel 19845 | Not in English |
| Adir 19876 | Inappropriate comparison |
| Adolphe 19937 | Less than minimum duration |
| Agabiti-Rosei 19928 | Less than minimum duration. Inappropriate comparison |
| Agabiti-Rosei 20059 | No relevant outcomes |
| Agarwal 201310 | Less than minimum duration |
| Ahola 201211 | Incorrect study design |
| Ahrens 201012 | Incorrect study design |
| Akanabe 198513 | Less than minimum duration |
| Akioyamen 201614 | Systematic review, references checked |
| Akram 200715 | Less than minimum duration |
| Alderman 198916 | Inappropriate comparison |
| Alici 200917 | Less than minimum duration. Inappropriate comparison |
| ALLHAT officers 200219 | Inappropriate comparison |
| ALLHAT Collaborative Research Group 200018 | Inappropriate comparison |
| Alviar 201320 | Inappropriate comparison |
| Amar 199921 | Article not in English |
| Ames 199222 | Less than minimum duration |
| Amir 199423 | No relevant outcomes |
| Andersen 198624 | Inappropriate comparison |
| Andersen 200325 | Inappropriate comparison |
| Andersen 200526 | Inappropriate comparison |
| Ando 201427 | Incorrect population |
| Andreadis 200528 | Less than minimum duration |
| Andren 198329 | Less than minimum duration |
| Andreucci 198330 | Incorrect study design. Incorrect interventions |
| Angeli 200431 | Not review population |
| Anonymous 199934 | Inappropriate comparison |
| Anonymous 199332 | Inappropriate comparison |
| Anonymous 199633 | Less than minimum duration |
| Applegate 199135 | No relevant outcomes. Incorrect study design |
| Arima 201436 | Not review population |
| Arriaga-gracia 199337 | Less than minimum duration |
| Bakris 200739 | Not review population |
| Bakris 201338 | Not review population |
| Balamuthusamy 200940 | Systematic review - references checked |
| Baldwin 198741 | Inappropriate comparison |
| Bang 201742 | Incorrect interventions |
| Bangalore 200843 | Systematic review, references checked. Inappropriate comparison |
| Batterink 201044 | Incorrect study design |
| Benjamin 198845 | Incorrect study design |
| Berger 199246 | Less than minimum duration |
| Black 200347 | Inappropriate comparison |
| Blumenthal 199048 | Less than minimum duration |
| Boissel 199550 | Inappropriate comparison |
| Borgmastars 198751 | No relevant outcomes |
| Bremner 199752 | Incorrect interventions |
| Brenner 200153 | Not review population |
| Brown 200154 | Less than minimum duration |
| Byrd 201155 | Not review population |
| Byyny 199656 | Less than minimum duration. Inappropriate comparison |
| Castano 200457 | Inappropriate comparison |
| Celis 199658 | Inappropriate comparison |
| Cesaris 198659 | Article not in English |
| Chapman 200760 | Incorrect study population, incorrect intervention |
| Chatellier 198761 | Less than minimum duration |
| Chi 201662 | Systematic review, references checked. Less than minimum duration |
| Chrysant 199763 | Incorrect study design. Inappropriate comparison |
| Circelli 201264 | Less than minimum duration |
| Coope 198665 | Inappropriate comparison |
| Correa 201866 | Incorrect study design |
| Cowley 198767 | Less than minimum duration |
| Cranston 196268 | Incorrect study design |
| Curb 199669 | Inappropriate comparison |
| Daae 199870 | Incorrect interventions |
| Dahlof 200272 | Less than minimum duration |
| Dahlöf 200571 | Incorrect study design |
| Daien 201273 | Systematic review, references checked |
| De rosa 200274 | Inappropriate comparison |
| de Souza 201075 | Incorrect study design |
| Degl’innocenti 200476 | Inappropriate comparison |
| Destro 201077 | Incorrect study design |
| Devereux 200778 | Inappropriate comparison |
| Dews 200179 | Incorrect study design |
| Diao 201280 | Inappropriate comparison |
| Du 201881 | Incorrect study design |
| Ekbom 199282 | Incorrect study design |
| Ekbom 200483 | Incorrect interventions. Incorrect study design |
| Estacio 199884 | Not review population |
| Family Physicians Hypertension Study Group 198485 | Less than minimum duration |
| Fariello 199086 | Less than minimum duration |
| Farsang 200387 | Incorrect study design |
| Fasano 198988 | Incorrect study design. Incorrect interventions |
| Faust 199390 | Article not in English |
| Faust 199389 | Article not in English |
| Ferdinand 200191 | Incorrect study design |
| Fernandes 201692 | Less than minimum duration |
| Fernandez 200193 | Less than minimum duration |
| Ferrara 198494 | No relevant outcomes |
| Finnerty 197995 | Incorrect interventions |
| Fogari 1991101 | No relevant outcomes |
| Fogari 199699 | Incorrect study design. Incorrect interventions |
| Fogari 199998 | Inappropriate comparison |
| Fogari 200697 | Less than minimum duration |
| Fogari 2012100 | Less than minimum duration |
| Fogari 201496 | Less than minimum duration |
| Forette 2002102 | Inappropriate comparison |
| Forrest 1983103 | Less than minimum duration |
| Fossum 2004104 | No relevant outcomes. Inappropriate comparison |
| Franco 1992105 | Article not in English |
| Franse 2000106 | Incorrect interventions. Inappropriate comparison |
| Frewin 1991107 | Incorrect study design. Incorrect interventions |
| Frick 1986109 | Inappropriate comparison |
| Frick 1987108 | No relevant outcomes. Inappropriate comparison |
| Gaddam 2010110 | Less than minimum duration |
| Gao 2011111 | Systematic review, references checked |
| Gasowski 1999112 | Incorrect study design. Incorrect interventions |
| Gazdick 1994113 | Incorrect study design |
| George 1990114 | Less than minimum duration |
| Ghiadoni 2017115 | Less than minimum duration |
| Giles 1992116 | Inappropriate comparison. No relevant outcomes |
| Gillespie 2005117 | Systematic review, references checked |
| Girerd 2010118 | Incorrect study design |
| Gitt 2013119 | Incorrect study design |
| Glorioso 2007120 | Incorrect study design. Less than minimum duration |
| Goicolea 2002121 | Article not in English |
| Gosse 2002122 | Inappropriate comparison |
| Grimm 1996123 | Incorrect study design |
| Guo 2005125 | Article not in English |
| Guo 2011124 | Article not in English |
| Gupta 2018126 | Incorrect interventions |
| Gyntelberg 1977127 | Article not in English |
| Hall 1998128 | Inappropriate comparison |
| Hamada 2010130 | No relevant outcomes |
| Hamada 2014129 | No relevant outcomes |
| Hamed 2014131 | Less than minimum duration. Incorrect study design |
| Hanon 2015132 | Inappropriate comparison |
| Hanon 2017133 | Inappropriate comparison |
| Hansson 1999136 | Inappropriate comparison |
| Hansson 1999135 | Inappropriate comparison |
| Hansson 1999137 | Inappropriate comparison |
| Hansson 2000134 | Inappropriate comparison |
| Hasegawa 2011138 | Inappropriate comparison |
| Helgeland 1980139 | Inappropriate comparison |
| Helgeland 1983140 | Less than minimum duration |
| Himmelmann 1995141 | Inappropriate comparison |
| Hosie 1983142 | Inappropriate comparison |
| Hradec 2013143 | Inappropriate comparison |
| Hughes 2008144 | Incorrect interventions. No relevant outcomes |
| Hulley 1985145 | Inappropriate comparison |
| Ibsen 1990147 | Incorrect interventions |
| Ibsen 2003146 | Article not in English |
| Ichihara 2006148 | Inappropriate comparison |
| J. Elan investigators 2006149 | Inappropriate comparison |
| Jamerson 2008150 | Incorrect study design |
| Johnson 2009151 | No relevant outcomes |
| Johnston 1991152 | Inappropriate comparison |
| Julius 2004153 | Not review population |
| Kaku 2011154 | Inappropriate comparison |
| Katayama 2008155 | Inappropriate comparison |
| Kawalec 2018156 | Incorrect study design |
| Kereiakes 2012157 | Less than minimum duration |
| Kerfoot 2014158 | Incorrect study design. Incorrect interventions. Inappropriate comparison |
| Kim 2012160 | Incorrect interventions |
| Kim 2013159 | No relevant outcomes |
| Kjeldsen 2002161 | Inappropriate comparison |
| Kjeldsen 2006164 | Incorrect interventions |
| Kjeldsen 2008163 | Incorrect population |
| Kjeldsen 2016162 | Incorrect interventions |
| Ko 2001165 | Not review population |
| Kohlmann 2009166 | Inappropriate comparison |
| Kostis 2005167 | Inappropriate comparison |
| Kuwajima 2001168 | Not review population |
| Lacourciere 2000169 | Incorrect study design |
| Lane 2007170 | Less than minimum duration, incorrect population |
| Laufer 1998171 | Incorrect interventions. No relevant outcomes |
| Laurent 2014172 | Inappropriate comparison |
| Lavenius 1982173 | Less than minimum duration |
| Leonetti 2002174 | Inappropriate comparison |
| Levine 2001175 | Incorrect study design |
| Licata 1994176 | Less than minimum duration |
| Lim 2000177 | Less than minimum duration |
| Lin 1991178 | Incorrect interventions |
| Lin 1993179 | Less than minimum duration |
| Lin 1995180 | Incorrect interventions |
| Lind 1994181 | No relevant outcomes |
| Lindholm 1996183 | Incorrect interventions |
| Lindholm 2000184 | Incorrect interventions |
| Lindholm 2001182 | Not review population |
| Lindholm 2002186 | Inappropriate comparison |
| Lindholm 2002185 | Incorrect interventions. Incorrect study design |
| Lindner 1984187 | Article not in English |
| Lindroos 1984188 | Less than minimum duration |
| Littlejohn 2009189 | Less than minimum duration |
| Liu 1999191 | Inappropriate comparison |
| Liu 2000190 | Not in English |
| Lombardo 1997192 | Inappropriate comparison |
| López 1997193 | Article not in English |
| Lu 2017194 | Systematic review, references checked |
| Ludwig 2002195 | Inappropriate comparison |
| Luno 2017196 | Not review population |
| Lynch 2008197 | Inappropriate comparison |
| Lynch 2012198 | Inappropriate comparison |
| Maclean 1986200 | Not review population |
| Maclean 1986201 | Less than minimum duration |
| Mahmud 2005202 | Incorrect study population, incorrect intervention, less than minimum duration |
| Malacco 2003203 | Incorrect interventions. Incorrect study design |
| Malminiemi 2000204 | Inappropriate comparison. No relevant outcomes |
| Mancia 2007205 | Incorrect study design. Incorrect interventions |
| Mann 1998206 | Incorrect study design |
| Marfatia 2012207 | Less than minimum duration |
| Marre 2004208 | Incorrect interventions |
| Martinez-martin 2011209 | Inappropriate comparison |
| Mason 2005210 | Systematic review - references checked |
| Matsuno 2011211 | Not review population. No relevant outcomes |
| Matsushita 2010212 | Incorrect study design. Inappropriate comparison |
| Matsuzaki 2011213 | Inappropriate comparison |
| Mazza 2016214 | No relevant outcomes |
| M’Buyamba-Kabangu 1987199 | Less than minimum duration |
| Mcareavey 1983215 | No relevant outcomes |
| Mende 2017216 | Less than minimum duration |
| Metelitsa 1991217 | Incorrect interventions |
| Metelitsa 1991218 | Article not in English |
| Middeke 1990219 | No relevant outcomes |
| Middeke 1997220 | Inappropriate comparison |
| Misson 1984221 | No relevant outcomes |
| Mizuno 2017222 | Less than minimum duration |
| Morgan 1989223 | Less than minimum duration |
| Mroczek 1984224 | Inappropriate comparison |
| Muller 1986225 | no relevant outcomes |
| Nakae 2006226 | Article not in English |
| NCT228 | Citation only |
| Neutel 1999230 | Incorrect study design. Incorrect interventions |
| Neutel 2017229 | Not review population |
| Oberman 1983231 | Less than minimum duration |
| Ocón 1985232 | Not in English |
| Ogawa 2012233 | Incorrect interventions |
| Ogihara 2000234 | Inappropriate comparison |
| Ogihara 2012235 | Inappropriate comparison |
| Ogihara 2014236 | Inappropriate comparison |
| Ogihara 2015237 | Inappropriate comparison |
| Ohnishi 2001238 | No relevant outcomes |
| Okin 2012239 | Incorrect study design. Inappropriate comparison |
| Oshikawa 2014240 | Not review population |
| Ostergren 2008241 | Not review population |
| Park 2017242 | No relevant outcomes |
| Patay 2010243 | Incorrect study design |
| Persson 1986244 | Incorrect study design |
| Philip 1987245 | Less than minimum duration |
| Pierini 2013246 | Less than minimum duration. Inappropriate comparison |
| Piller 2006247 | Inappropriate comparison. No relevant outcomes |
| Remonti 2016248 | NMA, references checked |
| Ritter 2013249 | Incorrect study design |
| Rodilla, 2009250 | Less than minimum duration |
| Roush 2018251 | Inappropriate comparison |
| Ruoff 1986252 | Inappropriate comparison |
| Russell 1985253 | Inappropriate comparison |
| Safar 1994254 | Incorrect study design |
| Saha 2005255 | Less than minimum duration. Not review population |
| Saini 1998256 | Inappropriate comparison |
| Saku 1996257 | Inappropriate comparison |
| Sano 1994258 | Inappropriate comparison |
| Saruta 2015259 | Inappropriate comparison |
| Sato 2002260 | Inappropriate comparison. No relevant outcomes |
| Sato 2009261 | Citation only |
| Sato 2012262 | Incorrect study design |
| Sato 2013263 | Not review population |
| Seedat 1992264 | Less than minimum duration |
| Seedat 1998265 | Incorrect interventions |
| Soucek 2007266 | Article not in English |
| Spoelstra-de Man 2006267 | Inappropriate comparison |
| Stamler 1986268 | Incorrect interventions |
| Swales 1982269 | Incorrect study design |
| Thomopoulos 2017270 | Incorrect study design |
| Trimarco 2015271 | Incorrect study design |
| Umemoto 2017272 | Inappropriate comparison |
| Wallin 1983273 | Inappropriate comparison |
| White 2008274 | Inappropriate comparison |
I.2. Excluded health economic studies
No health economic studies were found.
Tables
Table 1PICO characteristics of review question
| Population | Adults (aged 18 years and older) with primary hypertension who are taking the maximally tolerated doses of at least 3 drugs (including a diuretic) and their blood pressure is still not controlled. |
|---|---|
| Intervention | Step 4 antihypertensive pharmacological treatment received for a minimum of 1 year. Examples include:
|
| Comparison | Compared against each other (class comparisons) Compared to placebo (class compared to placebo) |
| Outcomes | All outcomes to be measured at a minimum of 12 months. Where multiple time points are reported within each study, the longest time point only will be extracted. Critical
Important
|
| Study design | Randomised controlled trials (RCT) and systematic reviews (SR) |
Table 2UK costs of step 4 drugs
| Drug | Detail | Daily dose | Cost/month (£) | Cost/year (£) |
|---|---|---|---|---|
| Alpha blockers | ||||
| Doxazosin |
4 mg tablets, 28 pack = £1 | 4 mg per day | £1.13 | £13.56 |
| Beta blockers | ||||
| Bisoprolol fumarate |
5 mg tablets, 28 pack = £0.59 | 5 mg per day | £0.64 | £7.69 |
| Further diuretics | ||||
| Amiloride |
5 mg tablets, 28 pack = £2.60 | 5 mg per day | £2.82 | £33.89 |
| Spironolactone |
25 mg tablets, 28 pack = £1 | 25 mg per day | £1.09 | £13.04 |
| Direct renin inhibitors | ||||
| Aliskiren |
150 mg tablets, 28 pack = £28.51 | 150 mg per day | £30.97 | £371.65 |
| Centrally acting anti-hypertensives | ||||
| Moxonidine |
0.2 mg tablets, 28 pack = £1.02 | 0.4 mg per day | £2.22 | £26.59 |
- (a)
Costs are from the BNF drug tariff price. Accessed in May 2019.49
Final
Intervention evidence review underpinning recommendations 1.4.44 to 1.4.50 in the guideline
This evidence review was developed by the National Guideline Centre
Disclaimer: The recommendations in this guideline represent the view of NICE, arrived at after careful consideration of the evidence available. When exercising their judgement, professionals are expected to take this guideline fully into account, alongside the individual needs, preferences and values of their patients or service users. The recommendations in this guideline are not mandatory and the guideline does not override the responsibility of healthcare professionals to make decisions appropriate to the circumstances of the individual patient, in consultation with the patient and/or their carer or guardian.
Local commissioners and/or providers have a responsibility to enable the guideline to be applied when individual health professionals and their patients or service users wish to use it. They should do so in the context of local and national priorities for funding and developing services, and in light of their duties to have due regard to the need to eliminate unlawful discrimination, to advance equality of opportunity and to reduce health inequalities. Nothing in this guideline should be interpreted in a way that would be inconsistent with compliance with those duties.
NICE guidelines cover health and care in England. Decisions on how they apply in other UK countries are made by ministers in the Welsh Government, Scottish Government, and Northern Ireland Executive. All NICE guidance is subject to regular review and may be updated or withdrawn.