Summary
Clinical characteristics.
Fibrodysplasia ossificans progressiva (FOP) is characterized by congenital bilateral hallux valgus malformations and early-onset heterotopic ossification, which may be spontaneous or precipitated by trauma including intramuscular vaccinations. Painful, recurrent soft-tissue swellings (flare-ups) may precede localized heterotopic ossification. Heterotopic ossification can occur at any location, but typically affects regions in close proximity to the axial skeleton in the early/mild stages, before progressing to the appendicular skeleton. This can lead to restriction of movement as a result of ossification impacting joint mobility. Problems with swallowing and speaking can occur with ossification affecting the jaw, head, and neck, and restriction of the airway and breathing may lead to thoracic insufficiency syndrome.
Diagnosis/testing.
The diagnosis of FOP is established in a proband with heterotopic ossification, hallux valgus malformations, and/or a heterozygous pathogenic variant in ACVR1 identified by molecular genetic testing.
Management.
Treatment of manifestations: Avoid intramuscular injections and arterial punctures. Fall prevention using household safety measures and ambulatory devices; use of protective headgear to reduce sequelae of falls; prompt medical attention after a fall with consideration of prophylactic corticosteroid use; management by a dietician for those with feeding difficulties; preventative dental care with precautions to avoid injury; orthodontic treatment with a practitioner with experience in FOP; consultation with an expert anesthetist with experience in FOP prior to elective anesthesia; use of singing, swimming, incentive spirometry; positive pressure ventilation when indicated for mechanical respiratory difficulties including thoracic insufficiency syndrome; anti-inflammatory medications for flare-ups; consider corticosteroids for flare-ups of the submandibular region or jaw, major joints, after significant soft-tissue trauma, and for prophylaxis prior to dental and surgical procedures. Conservative management for scoliosis. Consider bisphosphonates for corticosteroid-induced osteopenia; fractures should be managed by an expert in FOP; hearing aids and appliances for conductive hearing impairment; encourage hydration and avoidance of high protein and high salt intake to prevent renal stones; occupational therapy; warm water hydrotherapy for mobility difficulties; lower extremity elevation, DVT prophylaxis, and supportive stockings while avoiding traumatic compression for lymphedema. Psychological support.
Surveillance: Annual clinical evaluation including evaluation for scoliosis with orthopedist or geneticist familiar with FOP; annual nutrition evaluation and examination for jaw ankylosis; baseline pulmonary function assessment, sleep assessment, and echocardiogram before age ten years followed by annual clinical evaluation of respiratory status; annual evaluation for fracture risk; audiology assessment every 12 to 24 months; annual assessment for signs and symptoms of nephrocalcinosis, gastrointestinal complications, and skin integrity; dental examinations every six months; Doppler ultrasound if DVT is suspected.
Agents/circumstances to avoid: Avoid procedures that predispose to soft-tissue injury, including intramuscular injections such as vaccinations, arterial punctures, dental procedures, procedures related to anesthesia, biopsies, removal of heterotopic bone, and all nonemergent surgical procedures. Avoid contact sports, overstretching of soft tissues, muscle fatigue, and passive range of motion. Avoid falls. In individuals with thoracic insufficiency syndrome, avoid supplemental oxygen, which can suppress respiratory drive.
Genetic counseling.
FOP is inherited in an autosomal dominant manner. The majority of affected individuals represent simplex cases (i.e., a single occurrence in a family) resulting from a de novo ACVR1 pathogenic variant. Rarely, an individual diagnosed with FOP has an affected parent. If a parent of the proband is affected and/or is known to have the pathogenic variant identified in the proband, the risk to sibs is 50%. Once the ACVR1 pathogenic variant has been identified in an affected family member, prenatal testing for a pregnancy at increased risk and preimplantation genetic testing are possible.
Diagnosis
There are no formal diagnostic criteria for fibrodysplasia ossificans progressiva (FOP).
Suggestive Findings
FOP should be suspected in individuals with the following clinical and radiographic findings.
Clinical findings
- Congenital hallux valgus deformity that is most often bilateral
- Progressive heterotopic ossification (extraosseous bone formation) that may manifest as a palpable mass. Ossification is either spontaneous or in response to soft-tissue trauma, including iatrogenic trauma from vaccinations or surgical procedures.
- Painful, recurrent soft-tissue swellings (flare-ups) that may precede localized heterotopic ossification. This may occur in the form of scalp nodules in infancy, which may be an early or presenting feature.
- Limb reduction defects that may affect the fingers in atypical or nonclassic FOP and may be mistaken for a brachydactyly syndrome in individuals who have not yet developed heterotopic ossifications
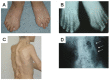
Imaging findings (See Figure 1.)
- Prenatal ultrasound may identify a hallux valgus deformity as early as 23 weeks' gestation [Maftei et al 2015].
- Radiographs of the halluces demonstrate short, malformed first metatarsals and a single dysplastic phalanx.
- Radiographs of affected areas demonstrate heterotopic ossification (extraosseous bone formation).
Note: Individuals with suspected FOP should avoid biopsy, elective surgery, and immunizations until diagnosis is confirmed [Kaplan et al 2019].
Establishing the Diagnosis
The diagnosis of FOP is established in a proband with hallux valgus malformations, heterotopic ossification, and/or a heterozygous pathogenic (or likely pathogenic) variant in ACVR1 identified by molecular genetic testing (see Table 1).
Note: (1) Per ACMG/AMP variant interpretation guidelines, the terms "pathogenic variants" and "likely pathogenic variants" are synonymous in a clinical setting, meaning that both are considered diagnostic and both can be used for clinical decision making [Richards et al 2015]. Reference to "pathogenic variants" in this section is understood to include any likely pathogenic variants. (2) Identification of a heterozygous ACVR1 variant of uncertain significance does not establish or rule out the diagnosis.
Molecular genetic testing approaches can include a combination of gene-targeted testing (single-gene testing, multigene panel) and comprehensive genomic testing (exome sequencing, genome sequencing) depending on the phenotype.
Gene-targeted testing requires that the clinician determine which gene(s) are likely involved, whereas genomic testing does not. Individuals with the distinctive features described in Suggestive Findings are likely to be diagnosed using gene-targeted testing (see Option 1), whereas individuals in whom the diagnosis of FOP has not been considered are more likely to be diagnosed using genomic testing (see Option 2).
Option 1
When the phenotypic and radiographic findings suggest the diagnosis of FOP, molecular genetic testing approaches can include single-gene testing or use of a multigene panel:
- Single-gene testing. Sequence analysis of ACVR1 to detect the most common pathogenic variant (c.617G>A; p.Arg206His) and other missense variants associated with FOP. Note: Since FOP occurs through a gain-of-function mechanism and large intragenic deletions or duplications have not been reported, testing for intragenic deletions or duplication is not indicated in individuals in whom a diagnosis of FOP is strongly suspected. Pathogenic loss of function variants in ACVR1 such as nonsense, frameshift, and splice-site variants have not been described.
- A skeletal dysplasia multigene panel that includes ACVR1 and other genes of interest (see Differential Diagnosis) may be considered to identify the genetic cause of the condition, compared to comprehensive genomic testing, while limiting identification of variants of uncertain significance and pathogenic variants in genes that do not explain the underlying phenotype. Note: (1) The genes included in the panel and the diagnostic sensitivity of the testing used for each gene vary by laboratory and are likely to change over time. (2) Some multigene panels may include genes not associated with the condition discussed in this GeneReview. (3) In some laboratories, panel options may include a custom laboratory-designed panel and/or custom phenotype-focused exome analysis that includes genes specified by the clinician. (4) Methods used in a panel may include sequence analysis, deletion/duplication analysis, and/or other non-sequencing-based tests.
Option 2
When the diagnosis of FOP has not been considered, including in individuals with atypical phenotypic features and/or the absence of congenital hallux malformation, comprehensive genomic testing (which does not require the clinician to determine which gene[s] are likely involved) may be the best option. Exome sequencing is most commonly used; genome sequencing is an increasingly used alternative.
For an introduction to comprehensive genomic testing click here. More detailed information for clinicians ordering genomic testing can be found here.
Table 1.
Molecular Genetic Testing Used in Fibrodysplasia Ossificans Progressiva
Clinical Characteristics
Clinical Description
Fibrodysplasia ossificans progressiva (FOP) is characterized by congenital bilateral hallux valgus malformations and early-onset heterotopic ossification, which may be spontaneous or precipitated by trauma, including intramuscular vaccinations [Pignolo et al 2016].
To date, more than 800 individuals with over 20 pathogenic variants in ACVR1 have been reported (overview in Kaplan et al [2019]). The following description of the phenotypic features associated with this condition is based on these reports and reports of classic FOP phenotype in individuals who did not have molecular genetic testing.
Table 2.
Select Features of Fibrodysplasia Ossificans Progressiva
Hallux valgus malformation. Hallux valgus malformations are present from birth and may be identifiable on prenatal imaging [Maftei et al 2015]. The first metatarsal is short with a hallux valgus malformation and/or monophalangism with a single dysplastic phalanx (see Figure 1) [Towler et al 2020]. Additional hallux malformations can include a delta-shaped, dysplastic proximal phalanx. Hallux valgus malformations are most often bilateral but can be unilateral or absent in a minority of individuals with atypical FOP.
Heterotopic ossification
- Extraosseous bone formation (abnormal bone formation in soft connective tissues outside of the normal skeleton) may manifest as a palpable hard lump or mass. Onset of ossification in individuals with the most common pathogenic variant (c.617G>A [p.Arg206His]) is age one to ten years, while onset of heterotopic ossification may be later in some individuals with atypical FOP.
- Heterotopic ossification can be spontaneous or in response to soft-tissue trauma, including iatrogenic trauma from intramuscular vaccinations, falls, and surgical procedures. Painful, recurrent soft-tissue swelling may precede localized heterotopic ossification.
- Heterotopic ossification can occur at any location, typically affecting regions in close proximity to the axial skeleton in the early/mild stages, before progressing to the appendicular skeleton. This can lead to restriction of movement as a result of ossification affecting joint mobility. Ossification of the jaw, head, and neck can affect swallowing and speaking.
- Heterotopic ossification occurring in the thoracic region, submandibular region, throat, or other locations near the airway may impact the airway or respiratory function. In addition, costovertebral involvement, ossification of intercostal muscles, paravertebral muscles, and aponeuroses, as well as progressive spinal deformity with kyphoscoliosis may lead to thoracic insufficiency syndrome, the predominant cause of mortality. Pneumonia, hypoxemia, hypercarbia, pulmonary hypertension, and right-sided heart failure may occur in individuals with thoracic insufficiency syndrome.
- Heterotopic ossification may be misdiagnosed as tumors or isolated osteochondromas such as those seen in hereditary multiple osteochondromas, especially if the hallux malformations are not recognized.
Soft-tissue swellings
- Soft-tissue swellings (flare-ups) may be spontaneous or follow an injury. They are characterized by painful swellings in soft connective tissue including skeletal muscles, tendons, ligaments, fascia, and aponeuroses. They may precede the development of localized heterotopic ossification.
- Scalp nodules occurring in neonates and infants have been described in 40% of individuals from a national disease registry [Piram et al 2011]. The nodules were large, firm, immobile, and tender, with rapid growth when they first appear. They generally regress spontaneously without treatment. The overlying skin was normal. Scalp nodules may be a localized manifestation of the soft-tissue swellings.
Additional skeletal malformations and manifestations variably seen:
- Variable thumb malformations may be present in some individuals, including hypoplasia and dysplastic phalanges.
- Limb reduction defects affecting the fingers may be seen in atypical FOP.
- Cervical spine fusions between C2 and C7 may be noted on cervical spine radiographs and may contribute to limitations in mobility as heterotopic ossification progresses. This occurs from intra-articular ankylosis of facet joints and early degenerative changes of the cervical spine.
- Scoliosis affects up to 65% of individuals, may be rapidly progressive as a result of paravertebral lesions, and may contribute to thoracic insufficiency syndrome.
- Pelvic radiographs may identify congenital short broad femoral necks, which rarely affect function.
- Developmental hip dysplasia is present in 60% of individuals with acute hip pain.
- Osteochondromas are reported in up to 90% of individuals, with the proximal medial tibia the most common location.
- Enchondromas, a benign tumor originating in cartilaginous tissue, have been described in several individuals [Tabas et al 1993, Rafati et al 2016]; the prevalence is unknown.
Fractures. Individuals with FOP are at increased risk for fractures of both normotropic and heterotopic bone because of the increased risk for falls, immobility, and corticosteroid-related osteopenia. Fractures in individuals with FOP usually heal with minimal heterotopic bone formation. Open reduction and internal fixation can lead to rapid onset of heterotopic ossification and is not recommended.
Hearing loss. Conductive hearing loss is present in 50% of individuals with FOP and may be slowly progressive. Onset is usually in childhood and may result from middle ear ossification. In some individuals, a sensorineural component may be present. Acute hearing loss is not usually associated with FOP and should prompt evaluation for other causes.
Renal stones. Individuals with FOP have a threefold increased risk of renal stones, which may be due to a combination of immobilization coupled with increased bone turnover. There has been no comprehensive study of stone composition in individuals with FOP.
Lymphedema may occur with flare-ups affecting the limbs. This may be acute, subacute, or chronic. In some individuals, underlying deep vein thrombosis may be present.
Genotype-Phenotype Correlations
The c.617G>A (p.Arg206His) pathogenic variant is associated with the classic FOP phenotype, including bilateral hallux valgus malformations and early-onset heterotopic ossification [Kaplan et al 2009].
Specific gain-of-function variants at amino acid residue 328 (p.Gly328Arg [c.982G>A and c.982G>C], p.Gly328Trp [c.982G>T], and p.Gly328Glu [c.983G>A]) have been associated with a characteristic phenotype that includes limb reduction defects, which may be misdiagnosed as an amniotic band defect or a brachydactyly syndrome, most commonly brachydactyly type B [Kaplan et al 2009].
Penetrance
The penetrance of gain-of-function variants in ACVR1 is estimated to be near complete; there are no reported individuals with nonpenetrance [Shore et al 2006b]. Among reported individuals with the most common pathogenic variant (c.617G>A [p.Arg206His]), none are unaffected.
Nomenclature
In the 2023 revision of the Nosology of Genetic Skeletal Disorders [Unger et al 2023], FOP is referred to as ACVR1-related fibrodysplasia ossificans progressiva and is included in the disorganized development of skeletal components group.
Prevalence
Based on studies in French [Baujat et al 2017] and British [Connor & Evans 1982] populations, the prevalence of FOP is estimated at one in one million (0.6-1.36:1,000,000). Individuals with FOP have been reported in diverse populations, and no racial, ethnic, sex, or geographic predisposition has been identified.
Genetically Related (Allelic) Disorders
No phenotypes other than those discussed in this GeneReview are known to be associated with germline pathogenic variants in ACVR1.
Differential Diagnosis
The diagnosis of fibrodysplasia ossificans progressiva (FOP) is often missed, due in part to the rarity of the condition. Nearly 90% of individuals with FOP initially receive a misdiagnosis, with two thirds undergoing unnecessary and potentially dangerous procedures that lead to permanent harm and lifelong disability in as many as 50% [Kitterman et al 2005].
Disorders that may present with clinical features similar to those of FOP are summarized in Table 3.
Table 3.
Genes of Interest in the Differential Diagnosis of Fibrodysplasia Ossificans Progressiva
Disorders to consider in individuals presenting with an isolated clinical feature characteristic of FOP [Kaplan et al 2008]:
- Hallux malformations may represent isolated congenital malformations (e.g., isolated brachydactyly) or juvenile bunions.
- Tumor-like swellings may be associated with sarcoma, desmoid tumor, aggressive juvenile fibromatosis, or lymphedema.
Management
Evaluations Following Initial Diagnosis
To establish the extent of disease and needs in an individual diagnosed with fibrodysplasia ossificans progressiva (FOP), the evaluations summarized in Table 4 (if not performed as part of the evaluation that led to the diagnosis) are recommended.
Table 4.
Recommended Evaluations Following Initial Diagnosis in Individuals with Fibrodysplasia Ossificans Progressiva
Table 5.
Clinical Staging of Fibrodysplasia Ossificans Progressiva
Treatment of Manifestations
There is currently no definitive medical treatment for FOP and management is supportive. Clinical trials investigating experimental treatments are in progress (see Therapies Under Investigation). Guidelines for the management of individuals with FOP have been developed by a multidisciplinary team of experts [Kaplan et al 2019].
A hallmark of FOP management is prevention of soft-tissue injury and muscle damage to prevent inflammatory soft-tissue swellings and heterotopic ossification [Kaplan et al 2019].
Table 6.
Treatment of Manifestations in Individuals with Fibrodysplasia Ossificans Progressiva
Note: Treatments for which no definitive evidence supports their use in FOP include chemotherapy, radiotherapy, bone marrow transplantation, and the chronic use of antiangiogenic agents, calcium binders, colchicine, fluoroquinolone antibiotics, propranolol, mineralization inhibitors, PPAR-gamma antagonists, and TNF-α inhibitors [Kaplan et al 2019].
Surveillance
Table 7.
Recommended Surveillance for Individuals with Fibrodysplasia Ossificans Progressiva
Agents/Circumstances to Avoid
It is imperative that iatrogenic harm is limited by avoiding procedures that predispose to soft-tissue injury, including intramuscular injections such as vaccinations, dental procedures, procedures related to anesthesia, biopsies, removal of heterotopic bone, and all nonemergent surgical procedures [Kaplan et al 2019].
Other activities to avoid include soft-tissue injuries, contact sports, overstretching of soft tissues, muscle fatigue, and passive range of motion (caution is required during treatment with physical therapists) [Kaplan et al 2019].
Falls should be actively avoided. Protective headwear should be considered for children who have upper limb involvement to prevent fall-induced head injury. Mobility aids may be effective in reducing falls in all age groups [Kaplan et al 2019].
In individuals with thoracic insufficiency syndrome, avoid supplemental oxygen, which can suppress respiratory drive.
Administration of vaccinations must be carefully managed in individuals with FOP. Detailed guidelines are available [Kaplan et al 2019] (see pdf). In brief, intramuscular vaccinations and all diphtheria-tetanus-pertussis (DTP) type vaccinations should be avoided. When the benefit outweighs the risk, subcutaneous vaccinations may be given at least six to eight weeks following recovery from soft-tissue flare-ups. Vaccinations to prevent respiratory disease (influenza, pneumococcal) are particularly important, and family members of individuals with FOP should receive influenza and pertussis vaccinations.
Evaluation of Relatives at Risk
It may be appropriate to clarify the genetic status of apparently asymptomatic young sibs of an affected individual in order to identify individuals at risk of iatrogenic harm (e.g., intramuscular injections) and other sources of trauma that may precipitate heterotopic ossification.
Note: For adult at-risk family members of a proband with classic FOP, molecular genetic testing in the absence of supportive physical examination findings (i.e., hallux deformity and signs of heterotopic ossification) is not usually required. However, for the evaluation of adult family members of a proband with atypical FOP, molecular genetic testing is recommended because manifestations of FOP may not be clinically apparent on physical examination.
See Genetic Counseling for issues related to testing of at-risk relatives for genetic counseling purposes.
Pregnancy Management
It is not known whether women with FOP have impaired fertility. Pregnancy in women with FOP is uncommon, as the disease manifestations at reproductive age limit reproductive potential. FOP poses major life-threatening risks to mother and fetus because of potential mechanical restrictions secondary to heterotopic ossification affecting the pelvis and surrounding regions, as well as breathing difficulties in later pregnancy secondary to restrictive chest wall disease. There is an increased risk of thromboembolism exacerbated by immobility. Ideally, pregnancy in a woman with FOP should be provided at a high-risk pregnancy center and follow established guidelines for the management of pregnancy in women with FOP (see Kaplan et al [2019]).
See MotherToBaby for further information on medication use during pregnancy.
Therapies Under Investigation
Research to develop treatments for FOP has focused on targeted inhibition of the ACVR1 receptor, ACVR1 ligands, BMP pathway signaling, pre-osseous chondrogenic heterotopic ossification, and inflammatory triggers of disease activity.
Palovarotene is a RAR-gamma agonist that reduces BMP signaling and may reduce the volume of heterotopic ossification.
REGN2477 is an antibody that binds to activin A and blocks its activity. By binding and blocking activin A, REN2477 may prevent the formation and stop the growth of heterotopic ossification in individuals with FOP. REGN2477 is currently in Phase II clinical trials in individuals older than age 18 years.
Sirolimus is an mTOR inhibitor that may reduce heterotopic ossification. Sirolimus is currently in Phase II clinical trials in individuals older than age six years.
Several other agents are currently undergoing safety and tolerability assessment in Phase I clinical trials. Further information on therapies under investigation is available in Kaplan et al [2019].
Search ClinicalTrials.gov in the US and EU Clinical Trials Register in Europe for further information on clinical studies for FOP.
Genetic Counseling
Genetic counseling is the process of providing individuals and families with information on the nature, mode(s) of inheritance, and implications of genetic disorders to help them make informed medical and personal decisions. The following section deals with genetic risk assessment and the use of family history and genetic testing to clarify genetic status for family members; it is not meant to address all personal, cultural, or ethical issues that may arise or to substitute for consultation with a genetics professional. —ED.
Mode of Inheritance
Fibrodysplasia ossificans progressiva (FOP) is inherited in an autosomal dominant manner.
Risk to Family Members
Parents of a proband
- Most individuals diagnosed with FOP have the disorder as the result of a de novo ACVR1 pathogenic variant.
- Rarely, an individual diagnosed with FOP has an affected parent.
- Molecular genetic testing is recommended for the parents of a proband with an apparent de novo pathogenic variant in order to confirm the genetic status of the parents and to allow reliable recurrence risk counseling. Note: Physical examination of both parents of a proband with the ACVR1 c.617G>A (p.Arg206His) pathogenic variant and classic FOP can be used to exclude a clinical diagnosis of FOP. In these families, confirmatory genetic testing of the parents is not required for reliable recurrence risk assessment.
- If the pathogenic variant found in the proband cannot be detected in the leukocyte DNA of either parent, the proband most likely has a de novo pathogenic variant. Another possible explanation is germline mosaicism in a parent.* Presumed germline mosaicism was reported in a family with sib recurrence [Janoff et al 1996].* Misattributed parentage can also be explored as an alternative explanation for an apparent de novo pathogenic variant.
- The family history of some individuals diagnosed with atypical or non-classic FOP may appear to be negative because of failure to recognize the disorder in other family members. Therefore, an apparently negative family history cannot be confirmed unless molecular genetic testing has been performed on the parents of the proband.
- Note: If the parent is the individual in whom the pathogenic variant first occurred, the parent may have somatic (and germline) mosaicism for the variant and may be mildly/minimally affected. Possible parental mosaicism was reported in the mildly affected father (who had minimal evidence of heterotopic ossification without hallux malformation) of a daughter with classic heterotropic ossification and hallux malformation. The daughter was heterozygous for the common c.617G>A (p.Arg206His) pathogenic variant, while the pathogenic variant was not detected in the father (the father could not be further tested for suspected somatic/germline mosaicism as he was deceased) [Shore et al 2006b].
Sibs of a proband. The risk to the sibs of the proband depends on the genetic status of the proband's parents:
- If a parent of the proband is affected and/or is known to have the pathogenic variant identified in the proband, the risk to the sibs is 50%. The penetrance of FOP is estimated to be near complete with intrafamilial clinical variability.
- If the proband has a known ACVR1 pathogenic variant that cannot be detected in the leukocyte DNA of either parent, the recurrence risk to sibs is slightly greater than that of the general population because of the possibility of parental germline mosaicism [Janoff et al 1996].
- If the parents have not been tested for the ACVR1 pathogenic variant but are clinically unaffected, the risk to the sibs of a proband appears to be low. However, sibs of a proband with clinically unaffected parents are still presumed to be at increased risk for FOP because of the possibility of parental germline mosaicism or, if the proband has atypical FOP, the absence of clinical manifestations in a heterozygous parent.
Offspring of a proband. Each child of an individual with FOP has a 50% chance of inheriting the ACVR1 pathogenic variant; as the penetrance is estimated to be near complete, a child who has inherited a gain-of-function ACVR1 variant is expected to develop features of FOP.
Other family members. The risk to other family members depends on the genetic status of the proband's parents: if a parent has the ACVR1 pathogenic variant, the parent's family members are at risk.
Related Genetic Counseling Issues
See Management, Evaluation of Relatives at Risk for information on evaluating at-risk relatives for the purpose of early diagnosis and treatment.
Family planning
- The optimal time for determination of genetic risk and discussion of the availability of prenatal/preimplantation genetic testing is before pregnancy.
- It is appropriate to offer genetic counseling (including discussion of potential risks to offspring and reproductive options) to young adults who are affected or at risk.
Prenatal Testing and Preimplantation Genetic Testing
Once the ACVR1 pathogenic variant has been identified in an affected family member, prenatal and preimplantation genetic testing are possible.
Differences in perspective may exist among medical professionals and within families regarding the use of prenatal testing. While most centers would consider use of prenatal testing to be a personal decision, discussion of these issues may be helpful.
Resources
GeneReviews staff has selected the following disease-specific and/or umbrella support organizations and/or registries for the benefit of individuals with this disorder and their families. GeneReviews is not responsible for the information provided by other organizations. For information on selection criteria, click here.
- International Clinical Council on Fibrodysplasia Ossificans Progressiva
- International Fibrodysplasia Ossificans Progressiva Association (IFOPA)PO Box 196217Winter Springs FL 32719-6217Phone: 407-365-4194Fax: 407-365-3213Email: together@ifopa.org
- National Library of Medicine Genetics Home Reference
- FOP Patient RegistryPhone: 866-761-0145Email: info@fopregistry.org
Molecular Genetics
Information in the Molecular Genetics and OMIM tables may differ from that elsewhere in the GeneReview: tables may contain more recent information. —ED.
Table A.
Fibrodysplasia Ossificans Progressiva: Genes and Databases
Table B.
OMIM Entries for Fibrodysplasia Ossificans Progressiva (View All in OMIM)
Molecular Pathogenesis
ACVR1 encodes ACVR1, a transmembrane serine/threonine kinase. All individuals with FOP have overactive BMP signaling mediated by ACVR1. Disease-causing variants cause a conformational change of the receptor that alters its sensitivity and activity, resulting in induced BMP signaling in a BMP-independent and BMP-responsive manner to activate downstream signaling. In contrast to mouse models of BMP-pathway overactivation, which are embryonic-lethal, disease-causing variants in ACVR1 are more mildly activating, which may explain its compatibility with life [Shore & Kaplan 2010].
Mechanism of disease causation. Gain of function. To date, only missense variants in the GS-rich and protein kinase functional domains have been described in individuals with FOP, suggesting a mechanism of action involving altered protein function and BMP pathway activation, rather than a loss-of-function mechanism [Kaplan et al 2009, Shore & Kaplan 2010].
Table 8.
Notable ACVR1 Pathogenic Variants
Cancer and Benign Tumors
Somatic gain-of-function variants in ACVR1 have been identified in 20%-25% of diffuse intrinsic pontine gliomas (DIPG), with a wider variant spectrum than that seen in FOP. There is no reported increased incidence of DIPG in individuals with FOP [Han et al 2018].
Chapter Notes
Revision History
- 11 May 2023 (sw) Revision: "ACVR1-Related Fibrodysplasia Ossificans Progressiva" added as a synonym; Nosology of Genetic Skeletal Disorders: 2023 Revision [Unger et al 2023] added to Nomenclature
- 11 June 2020 (me) Review posted live
- 19 February 2020 (la) Original submission
References
Published Guidelines / Consensus Statements
- Kaplan FS, Al Mukaddam M, Baujat G, Brown M, Cali A, Cho T-J, Crowe C, De Cunto C, Delai P, Diecidue R, Di Rocco M, Eekhoff EMW, Friedman C, Grunwald Z, Haga N, Hsiao E, Keen R, Kitterman J, Levy C, Morhart R, Netelenbos C, Scott C, Shore EM, Zasloff M, Zhang K, Pignolo RJ. The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Proc Intl Clin Council FOP. 2019;1:1-111. Available online. Accessed 5-2-23.
Literature Cited
- Baujat G, Choquet R, Bouee S, Jeanbat V, Courouve L, Ruel A, Michot C, Le Quan Sang KH, Lapidus D, Messiaen C, Landais P, Cormier-Daire V. Prevalence of fibrodysplasia ossificans progressiva (FOP) in France: an estimate based on a record linkage of two national databases. Orphanet J Rare Dis. 2017;12:123. [PMC free article: PMC5493013] [PubMed: 28666455]
- Connor JM, Evans DA. Genetic aspects of fibrodysplasia ossificans progressiva. J Med Genet. 1982;19:35–9. [PMC free article: PMC1048816] [PubMed: 7069743]
- Han HJ, Jain P, Resnick AC. Shared ACVR1 mutations in FOP and DIPG: opportunities and challenges in extending biological and clinical implications across rare diseases. Bone. 2018;109:91–100. [PMC free article: PMC7888549] [PubMed: 28780023]
- Janoff HB, Muenke M, Johnson LO, Rosenberg A, Shore EM, Okereke E, Zasloff M, Kaplan FS. Fibrodysplasia ossificans progressiva in two half-sisters: evidence for maternal mosaicism. Am J Med Genet. 1996;61:320–4. [PubMed: 8834042]
- Kaplan FS, Al Mukaddam M, Baujat G, Brown M, Cali A, Cho T-J, Crowe C, De Cunto C, Delai P, Diecidue R, Di Rocco M, Eekhoff EMW, Friedman C, Grunwald Z, Haga N, Hsiao E, Keen R, Kitterman J, Levy C, Morhart R, Netelenbos C, Scott C, Shore EM, Zasloff M, Zhang K, Pignolo RJ. The medical management of fibrodysplasia ossificans progressiva: current treatment considerations. Proc Intl Clin Council FOP. 2019;1:1-111. Available online. Accessed 5-4-23.
- Kaplan FS, Al Mukaddam M, Pignolo RJ. A cumulative analogue joint involvement scale (CAJIS) for fibrodysplasia ossificans progressiva (FOP). Bone. 2017;101:123–8. [PubMed: 28465250]
- Kaplan FS, Xu M, Glaser DL, Collins F, Connor M, Kitterman J, Sillence D, Zackai E, Ravitsky V, Zasloff M, Ganguly A, Shore EM. Early diagnosis of fibrodysplasia ossificans progressiva. Pediatrics. 2008;121:e1295–300. [PMC free article: PMC3502043] [PubMed: 18450872]
- Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Koster B, Pauli RM, Reardon W, Zaidi SA, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30:379–90. [PMC free article: PMC2921861] [PubMed: 19085907]
- Kitterman JA, Kantanie S, Rocke DM, Kaplan FS. Iatrogenic harm caused by diagnostic errors in fibrodysplasia ossificans progressiva. Pediatrics. 2005;116:e654–61. [PubMed: 16230464]
- Maftei C, Rypens F, Thiffault I, Dube J, Laberge AM, Lemyre E. Fibrodysplasia ossificans progressiva: bilateral hallux valgus on ultrasound a clue for the first prenatal diagnosis for this condition-clinical report and review of the literature. Prenat Diagn. 2015;35:305–7. [PubMed: 25346098]
- Pignolo RJ, Bedford-Gay C, Liljesthrom M, Durbin-Johnson BP, Shore EM, Rocke DM, Kaplan FS. The natural history of flare-ups in fibrodysplasia ossificans progressiva (FOP): a comprehensive global assessment. J Bone Miner Res. 2016;31:650–6. [PMC free article: PMC4829946] [PubMed: 27025942]
- Pignolo RJ, Kaplan FS. Clinical staging of fibrodysplasia ossificans progressiva (FOP). Bone. 2018;109:111–4. [PubMed: 28943457]
- Piram M, Le Merrer M, Bughin V, De Prost Y, Fraitag S, Bodemer C. Scalp nodules as a presenting sign of fibrodysplasia ossificans progressiva: a register-based study. J Am Acad Dermatol. 2011;64:97–101. [PubMed: 21055844]
- Rafati M, Mohamadhashem F, Hoseini A, Hoseininasab F, Ghaffari SR. A novel ACVR1 mutation detected by whole exome sequencing in a family with an unusual skeletal dysplasia. Eur J Med Genet. 2016;59:330–6. [PubMed: 27182040]
- Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. [PMC free article: PMC4544753] [PubMed: 25741868]
- Shore E, Xu M, Connor JM, Kaplan FS. Mutations in the BMP type I receptor ACVR1 in patients with fibrodysplasia ossificans progressiva (FOP). J Bone Miner Res. 2006a;21:S75. [PubMed: 16753021]
- Shore EM, Kaplan FS. Insights from a rare genetic disorder of extra-skeletal bone formation, fibrodysplasia ossificans progressiva (FOP). Bone. 2008;43:427–33. [PMC free article: PMC2601573] [PubMed: 18590993]
- Shore EM, Kaplan FS. Inherited human diseases of heterotopic bone formation. Nat Rev Rheumatol. 2010;6:518–27. [PMC free article: PMC3551620] [PubMed: 20703219]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006b;38:525–7. [PubMed: 16642017]
- Tabas JA, Zasloff M, Fallon MD, Gannon FH, Cohen RB, Kaplan FS. Enchondroma in a patient with fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1993;(294):277–80. [PubMed: 8358928]
- Towler OW, Shore EM, Kaplan FS. Skeletal malformations and developmental arthropathy in individuals who have fibrodysplasia ossificans progressiva. Bone. 2020;130:115116. [PubMed: 31655222]
- Unger S, Ferreira CR, Mortier GR, Ali H, Bertola DR, Calder A, Cohn DH, Cormier-Daire V, Girisha KM, Hall C, Krakow D, Makitie O, Mundlos S, Nishimura G, Robertson SP, Savarirayan R, Sillence D, Simon M, Sutton VR, Warman ML, Superti-Furga A. Nosology of genetic skeletal disorders: 2023 revision. Am J Med Genet A. 2023;191:1164–209. [PMC free article: PMC10081954] [PubMed: 36779427]
- Zhang W, Zhang K, Song L, Pang J, Ma H, Shore EM, Kaplan FS, Wang P. The phenotype and genotype of fibrodysplasia ossificans progressiva in China: a report of 72 cases. Bone. 2013;57:386–91. [PMC free article: PMC3975922] [PubMed: 24051199]
Publication Details
Author Information and Affiliations
Royal Melbourne Hospital;
University of Melbourne
Melbourne, Victoria, Australia
Murdoch Children's Research Institute;
University of Melbourne
Parkville, Victoria, Australia
Publication History
Initial Posting: June 11, 2020; Last Revision: May 11, 2023.
Copyright
GeneReviews® chapters are owned by the University of Washington. Permission is hereby granted to reproduce, distribute, and translate copies of content materials for noncommercial research purposes only, provided that (i) credit for source (http://www.genereviews.org/) and copyright (© 1993-2024 University of Washington) are included with each copy; (ii) a link to the original material is provided whenever the material is published elsewhere on the Web; and (iii) reproducers, distributors, and/or translators comply with the GeneReviews® Copyright Notice and Usage Disclaimer. No further modifications are allowed. For clarity, excerpts of GeneReviews chapters for use in lab reports and clinic notes are a permitted use.
For more information, see the GeneReviews® Copyright Notice and Usage Disclaimer.
For questions regarding permissions or whether a specified use is allowed, contact: ude.wu@tssamda.
Publisher
University of Washington, Seattle, Seattle (WA)
NLM Citation
Akesson LS, Savarirayan R. Fibrodysplasia Ossificans Progressiva. 2020 Jun 11 [Updated 2023 May 11]. In: Adam MP, Feldman J, Mirzaa GM, et al., editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993-2024.