NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Hoshida Y, editor. Hepatocellular Carcinoma: Translational Precision Medicine Approaches [Internet]. Cham (CH): Humana Press; 2019. doi: 10.1007/978-3-030-21540-8_15

Hepatocellular Carcinoma: Translational Precision Medicine Approaches [Internet].
Show detailsIn the large majority of cases, hepatocellular carcinoma (HCC) develops on the background of chronic liver inflammation and fibrosis. Liver microenvironment plays a crucial role in hepatocarcinogenesis, HCC progression, response to treatment, and patients’ long-term prognosis. Chronic liver inflammation and hepatocyte damage recruit and activate immune and stromal cells that release cytokines stimulating cell proliferation and producing liver fibrosis, hepatocellular stress, DNA damage, and chromosomal alterations that finally drive hepatocyte degeneration. Moreover, immune and stromal cells, e.g., cancer-associated fibroblasts, promote HCC progression by reducing tumor immunosurveillance, stimulating angiogenesis, and recruiting cancer stem cells. Activation of stromal and immune cells leads finally to epithelial–mesenchymal transition that confers increased initiation and metastasis of cancer cells and a greater resistance to therapies. Tumor microenvironment is also a relevant target for HCC treatment. Indeed, compounds targeting exhausted immune cells infiltrating HCC (i.e., nivolumab and pembrolizumab) have recently been shown to increase survival of HCC patients after sorafenib failure and were FDA-approved as a second-line treatment for advanced HCC.
Keywords:
HCC microenvironment, Cancer-associated fibroblasts, Tumor-associated macrophages, Chronic inflammation, NF-ҡB, STAT3, Epithelial-to-mesenchymal transitionIntroduction
The liver is a multifunctional organ that plays a key role in metabolism and detoxification as well as in regulation of immune response and tolerance. The liver is physiologically exposed to many pathogens and toxic substances derived from the gut and has the largest population of resident macrophages (i.e., Kupffer cells, KCs) in the body and a high prevalence of natural killer cells (NK), natural killer T cells (NKT), and T cells. In normal conditions, the liver removes a large amount of microbes and pathogen-associated and damage-associated molecular patterns (PAMPs and DAMPs) and maintains an immunosuppressive environment [1].
Following chronic hepatocyte damage, immune and stromal cells modify a liver environment, which triggers chronic inflammation and ultimately promotes hepatocellular carcinoma (HCC) [2]. Indeed, independently from the etiology, chronic liver disease is characterized by a deregulation in the liver immune network that stimulates cellular stress and death favoring liver fibrosis, hepatocyte proliferation, and epithelial-to-mesenchymal transition (EMT) [2]. A combination of EMT, genetic mutations, and epigenetic alterations that accumulate during cell proliferation is the most important driver of hepatocarcinogenesis [3].
Once HCC has developed, liver microenvironment greatly affects tumor progression and response to therapy [4]. This is the reason why gene expression signatures in liver tissues adjacent to the HCC—and the not in tumor itself—highly correlate with long-term survival of patients with liver fibrosis [5]. Similarly, HCC infiltration by non-parenchymal cells (e.g., regulatory T cells, Treg) has been associated with tumor progression [5–8]. New therapies targeting liver microenvironment are recently developed or under clinical investigation for both chronic liver disease (e.g., nonalcoholic steatohepatitis, NASH) and HCC.
Hence, liver microenvironment plays an essential role in both hepatocarcinogenesis and tumor progression and it is an important therapeutic target for HCC prevention and treatment.
From Chronic Inflammation to Hepatocellular Carcinoma
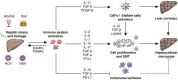
HCC almost universally evolves on the background of chronic liver inflammation and liver fibrosis [9]. Chronic hepatocyte cell injury induces activation of the immune system that initiates and supports chronic inflammation by generation of proinflammatory cytokines and chemokines and activation of hepatic stellate cells (HSCs), finally resulting in liver fibrosis, cirrhosis, and cancer [10] (Fig. 15.1).
During chronic infections (e.g., hepatitis B virus, HBV, or hepatitis C virus, HCV) as well as metabolic (e.g., NASH) or toxic diseases (e.g., alcoholic steatohepatitis, ASH), immune cells—first of all KCs—are activated by the release of PAMPs and DAMPs produced by hepatocyte apoptosis and death. Activated KCs present viral antigens to T cells and/or secrete cytokines and chemokines that recruit circulating monocytes, lymphocytes, and neutrophils [11]. Proinflammatory signals are mainly mediated by the accumulation of tumor necrosis factor alpha (TNF-α); interleukins (IL) such as IL-6, IL-1β, IL-2, IL-7, IL-15, IL-17; C-C motif chemokine ligand 2 (CCL2); and interferon gamma (IFN-ɣ).
Following activation by antigen-presenting cells, T cells and especially T-helper 17 (Th17) cells and the mucosal-associated invariant T (MAIT) cells are major promoters of liver inflammation primarily by secretion of IL-17 [12, 13]. IL-17 secreted by T cells as well as transforming growth factor beta 1 (TGF-β1) and platelet-derived growth factor subunit B (PDGF-B) secreted by KCs and monocyte-derived macrophages are able to activate and differentiate HSC into collagen-producing myofibroblasts [12, 13]. Finally, also DAMPs can directly activate HSC and participate in fibrosis [7, 14]. HSC-derived myofibroblasts account for abnormal production of collagen in the liver and are main components of the hepatic precancerous microenvironment [15].
The inflammatory microenvironment causes hepatocellular stress, accompanied by epigenetic modifications, mitochondrial alterations, DNA damage, and chromosomal alterations that determine cell transformations [7]. Inflammation has been shown to upregulate nuclear factor kappa B (NF-κB) and signal transducer and activator of transcription 3 (STAT3) thereby affecting cell proliferation, survival, angiogenesis, and chemotaxis [16–18]. STAT3 is further induced by several other cytokines and growth factors that are known to be upregulated under conditions of chronic liver inflammation [19]. Regarding chronic HBV and HCV infection, upregulation of the cytokines lymphotoxin beta and TNF-α in CD4+ and CD8+ T cells has been shown to promote hepatocarcinogenesis [20, 21].
Collectively, persistence of infection by hepatotropic viruses or toxic condition may cause a chronic inflammatory state, accompanied by continual cell death and promotion of compensatory tissue repair mechanisms, finally resulting in liver cirrhosis and cell transformation. Since chronic inflammation induces impaired immune surveillance due to exhausted T cells, chronic inflammatory liver status not only provokes cell transformation but also attenuates physiological antitumor defense mechanisms by the immune system. Thus, tumor cell attack by cytolytic T cells is weakened in chronic inflammatory liver tissue and HCC microenvironment [22–24].
Moreover, upregulation of immunosuppressive Treg cells has been related to chronic inflammation associated with attenuated immune surveillance contributing to risk of HCC development [25, 26]. The inducible type 1 T regulatory (Tr1) cells possess many immunosuppressive functions by secretion of the cytokines IL-10 and TGF-β, as well as by expression of the checkpoint inhibitors cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and programmed death 1 (PD1) on the cell surface [27–29]. Treg or KC-secreted IL-10 was reported to reduce immune surveillance by suppressing macrophage activation, T-cell proliferation, and IFN-ɣ production, hereby inhibiting antitumor response mediated by the immune system [30–32]. Moreover, TGF-β is known to inhibit IL-2-dependent T-cell proliferation as well as production of proinflammatory cytokines and performance of cytolytic functions by effector cells [33–35]. Suggesting its involvement in chronic inflammatory liver disease and contribution to hepatocarcinogenesis, levels of the immunoregulatory cytokine IL-10 and TGF-β have been reported to be elevated in patients with chronic liver disease and related to disease progression and patients’ survival [30, 36, 37].
Immune Cells in HCC Microenvironment
Leukocytes are one of the main drivers in chronic inflammation. They are highly enriched in both the precancerous state of liver cirrhosis and in malignant tissue of HCC. Indeed, liver carcinoma is characterized by an immunogenic microenvironment, consisting of high amounts of lymphocytes, including NK cells, NKT cells, B cells, and T cells [38]. T-cell exhaustion due to chronic inflammation hereby shapes an immunogenic microenvironment that is characterized by an enhanced immunotolerance. Thus, the endogenous antitumor function of cytotoxic lymphocytes can be restored by antigen-presenting cells, which are typically reduced in the HCC microenvironment [39]. Indeed, decreased activity of NK cells, one of the most important antigen-presenting cells, correlates with an increased incidence of HCC in patients with liver cirrhosis [40]. Moreover, infiltration and density of T cells in human HCCs correlate with better patient prognosis, whereas tumor-infiltrating B cells reduce tumor viability [41].
Macrophages perpetuate chronic inflammation following liver injury and promote fibrogenesis via HSC activation. This therefore represents a significant component of HCC microenvironment. Of note, tumor-associated macrophages (TAMs) are considered to promote tumor development and favor angiogenesis and tumor cell migration [42, 43]. Moreover, TAMs may stimulate tumor growth by suppression of the adaptive immune system. They express high levels of cell death-ligand 1 (PD-L1), thereby suppressing the antitumor cytotoxic T-cell responses [44]. TAMs provide cytokines and growth factors that enhance tumor cell proliferation and NF-κB-mediated protection from cancer cell apoptosis and angiogenesis [45]. Accordingly, TAM infiltration correlates with HCC progression and poor survival [46, 47].
Dendritic cells (DCs) are a heterogeneous cell population and one of the most powerful antigen-presenting cells which regulate the primary immune response and the immune homeostasis in the liver [48]. By forming a bridge between the innate and the adaptive immune system [49], DCs are regarded as key players in immune regulation [50, 51]. An impaired DC function has frequently been suggested as an important factor contributing to an immunosuppressive microenvironment in chronic liver disease, which is favoring tumor development. Accordingly, several studies report lower DC numbers in both the peripheral blood and liver tissue of patients with HCC [52, 53]. A reduced IL-12 secretion by DCs is hereby attributed to an attenuated stimulation of T cells [54]. Moreover, DC inhibition and its effects on downstream effector cells have further been identified as immune escape mechanisms of HCC [55, 56].
Stromal Cells Participate in HCC Development and Progression
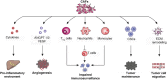
Liver cirrhosis is one of the main risk factors for hepatocarcinogenesis and therefore regarded as a precancerous liver state [57]. Thus, the lifetime risk of HCC development in patients with advanced liver cirrhosis is approximately 30%, and 80–90% of HCCs evolve in cirrhotic liver tissue [58, 59]. Considering HSCs as the most important progenitor cells of myofibroblasts that account for enhanced production of the extracellular matrix in liver fibrosis and liver cirrhosis, HSC-derived myofibroblasts are the main components of the hepatic precancerous microenvironment as well as the HCC tumor environment. Indeed, differentiation of HSCs from pericyte-like cells to collagen-producing myofibroblasts provides 85–95% of the myofibroblasts in liver fibrosis and liver cirrhosis, independent of the underlying trigger [15]. Hence, together with bone marrow (BM)-derived fibroblasts and portal fibroblasts (PF), HSC-derived myofibroblasts compose the stromal population of cancer-associated myofibroblasts (CAFs) that contribute actively to HCC development and progression [60]. Of note, CAFs show a markedly altered phenotype compared to normal fibroblasts [61, 62]. Normal fibroblasts may suppress tumor growth by contact inhibition [62], whereas CAFs promote an immune-tolerant tumor environment by interaction with monocytes and lymphocytes [63]. Indeed, CAFs inhibit lymphocyte tumor infiltration, increase the activity of immunosuppressive regulatory T cells, and induce apoptosis in monocytes [64, 65]. Furthermore, CAFs were reported to impair antitumor functions of T cells via activation of neutrophils [66]. CAFs may further promote hepatocarcinogenesis by downregulation of tumor-suppressive microRNAs [67, 68]. CAF activity has also been associated with tumor angiogenesis. CAFs have been shown to secrete vascular endothelial growth factor (VEGF) and angiopoietin 1 or 2 [69–71]. The cross talk between CAFs and cancer cells is crucial for HCC biology. The secretion of laminin 5 (LAMA5) [72] and IL-1β [73] by CAFs has been shown to promote HCC migration, and on the other hand, highly metastatic HCC cells were found to be able to convert normal fibroblasts to CAFs, which in turn promote cancer progression by secretion of proinflammatory cytokines [74]. Several studies further suggest an association of CAFs and CSCs that are thought to promote tumor development and to mediate therapeutic resistance. CAFs have been reported to recruit CSCs and to drive their self-renewal [75, 76]. Moreover, CAFs have been observed to increase expression of keratin 19 by paracrine interactions [77], a marker for hepatic stem cells that has been observed to be correlated with poor prognosis [78]. In summary, CAFs are key drivers in hepatic carcinogenesis by increasing angiogenesis, inflammation, and proliferation and attenuating immune surveillance [60] (Fig. 15.2). CAFs correlate with HCC tumor stage and progression, tumor recurrence after surgery, as well as overall prognosis [79–81].
Lymphatic vessels function as a tissue drainage and immunological control system. They are highly enriched in the liver, carrying approximately 25–50% of the thoracic duct’s lymph flow [82]. For a long time, lymphatic vessels were considered to affect carcinogenesis only by providing the structural pathway for metastatic spread of tumor cells. However, recent observations indicate a functional role of the lymphatic endothelium also in the hepatocytes’ immunogenic microenvironment, which is affecting the development of chronic liver disease and hepatocarcinogenesis [83]. Thus, lymphatic endothelial cells (LECs) guide immune cell migration by lining the inner surface of lymphatic capillaries and regulate the expression of adhesion molecules and cytokines [84, 85]. Moreover, by secretion of immunosuppressive cytokines (i.e., TGF-β) and the overexpression of co-inhibitory checkpoint proteins (i.e., PD-L1), LECs suppress a maturation and proliferation of circulating immune cells [84–86]. LECs further mediate CD4+ and CD8+ T-cell tolerance by expression of self-antigens in the presence of inhibitory ligands [87].
Lymphangiogenesis is increased in liver fibrosis and cirrhosis and positively correlate with portal venous pressure and disease severity [88–90]. The enhanced interstitial flow and increased number of LECs is accompanied by increased cytokine production and immune cell recruitment to the inflammatory environment present in almost all chronic liver diseases [91]. The primarily immunosuppressive functions of LECs hereby contribute to an immunotolerant microenvironment favoring HCC development [83, 92]. Moreover, expression of chemokines by LECs may facilitate lymphogenic metastatic tumor spread [84]. Vascular endothelial growth factor C (VEGF-C) is an important stimulator of LEC growth and lymphangiogenesis. VEGF-C is enhanced in liver cirrhosis and HCC, and its expression in HCCs correlates with metastasis and poor patients’ outcome [93, 94].
Epithelial-to-Mesenchymal Transition in HCC
Epithelial-to-mesenchymal transition (EMT) describes a reversible process, by which epithelial cell types gradually develop mesenchymal characteristics leading to higher motility and invasive properties that are essential in embryogenic development and wound healing but also implicated in hepatic fibrogenesis and carcinogenesis [95, 96]. Thus, while epithelial cells are characterized by polarity and stable morphology, mesenchymal cells lack polarity, show a loose arrangement, and exhibit the capacity of migration [97]. EMT can be divided in three different biological subtypes [98]. While type 1 EMT determines embryonal development and organogenesis, types 2 and 3 EMT affect liver disease progression and can be activated by several proinflammatory cytokines and growth factors present in the inflammatory state of the liver [99].
Type 2 EMT occurs in response to cell injury as a mechanism of tissue repair and may cause fibrosis due to generation of collagen-producing fibroblasts. TGF-β, a cytokine increased under condition of chronic inflammation, has been shown to be one of the strongest activators of type 2 EMT that can affect hepatocytes, cholangiocytes, and hepatic stellate cells (HSC) [100]. Quiescent HSCs, the most frequent progenitor cells of collagen-producing fibroblasts [15], are actually regarded as transitional cells that have undergone partial EMT from epithelial cells and may complete transition upon inflammatory signals [101]. Hence, EMT is regarded as one of the most important promoters of liver fibrogenesis in response to chronic inflammation [101].
Type 3 EMT may occur due to genetic and epigenetic changes during malignant transformation of epithelial cells and is implicated in HCC growth and progression [3]. Cells generated by type 3 EMT differ significantly from types 1 and 2 EMT cells and develop properties of invasion and migration as well as escape from apoptosis. Weakened or loss of E-cadherin expression, characteristic for development of the mesenchymal unpolarized phenotype, could be revealed in 58% of human HCC patients and correlated with the presence of metastases and patients’ survival [102]. Besides proinflammatory cytokines and growth factors, several studies further indicate induction of type 3 EMT by core proteins of HCV itself [103]. Given not only the correlation of EMT with tumor stage but also response to therapy [104], therapeutic targeting of molecular key players in EMT is highly clinically relevant.
Clinical Perspectives
Considering the implication of stromal and immunogenic cell compounds in HCC development and progression, medical treatments targeting these factors represent promising tools for future medical treatment of advanced HCC. Presently, sorafenib, an oral multikinase inhibitor targeting vascular endothelial growth factor receptor (VEGFR-2/VEGFR-3) and platelet-derived growth factor receptor (PDGFR), produced by the stromal HCC microenvironment already represents the standard of care treatment for patients with advanced HCC [105]. Lenvatinib, another tyrosine kinase inhibitor with multiple targets, has recently been revealed to be noninferior compared to sorafenib according to the REFLECT trial and has lately been approved by the FDA as first-line treatment for unresectable HCC [106]. Moreover, recently therapeutic strategies targeting the immunogenic tumor microenvironment have been demonstrated to be effective as systemic therapy for several cancer types. Consequently, drugs targeting exhausted lymphocytes expressing PD1 and infiltrating the tumor are able to activate T-cell-driven immune response against cancer cells and were approved for melanoma and non-small cell lung cancer treatment [107, 108]. Preliminary results from open-label trials of these drugs in HCC treatment are encouraging. Indeed, nivolumab and pembrolizumab, anti-PD1 monoclonal antibodies, have been demonstrated to be more effective than placebo in patients with advanced unresectable HCC previously treated with sorafenib [109, 110]. For that reason, these compounds were recently approved by FDA as a second-line treatment for advanced HCC. Moreover, currently several randomized controlled trials investigate the effects of other drugs targeting the HCC immunogenic and stromal microenvironment. Thus, aiming to activate tumor-targeting cytotoxic T lymphocytes, a growing number of studies recently worked on ex vivo tumor-antigen-loaded dendritic cells as an approach of cancer immunotherapy by DC vaccination [111–113]. Several other studies are focused on immunotherapy targeting TAMs, aiming to decrease TAM population present in the HCC by elimination, blocking recruitment, or functional reprogramming of TAM polarization [43]. The results of current ongoing clinical studies are expected in the next few years and may revolutionize future HCC medical treatment.
References
- 1.
- Jenne CN, Kubes P. Immune surveillance by the liver. Nat Immunol. 2013;14:996. https://doi
.org/10.1038/ni.2691. https://www .nature.com/articles/ni .2691#supplementary-information. [PubMed: 24048121] [CrossRef] - 2.
- Marrone G, Shah VH, Gracia-Sancho J. Sinusoidal communication in liver fibrosis and regeneration. J Hepatol. 2016;65(3):608–17. https://doi
.org/10.1016/j .jhep.2016.04.018. [PMC free article: PMC4992446] [PubMed: 27151183] [CrossRef] - 3.
- van Zijl F, Zulehner G, Petz M, Schneller D, Kornauth C, Hau M, et al. Epithelial-mesenchymal transition in hepatocellular carcinoma. Future Oncol. 2009;5(8):1169–79. https://doi
.org/10.2217/fon.09.91. [PMC free article: PMC2963061] [PubMed: 19852728] [CrossRef] - 4.
- Nishida N, Kudo M. Oncogenic signal and tumor microenvironment in hepatocellular carcinoma. Oncology. 2017;93(Suppl 1):160–4. https://doi
.org/10.1159/000481246. [PubMed: 29258072] [CrossRef] - 5.
- Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359(19):1995–2004. https://doi
.org/10.1056/NEJMoa0804525. [PMC free article: PMC2963075] [PubMed: 18923165] [CrossRef] - 6.
- Caja L, Dituri F, Mancarella S, Caballero-Diaz D, Moustakas A, Giannelli G, et al. TGF-beta and the tissue microenvironment: relevance in fibrosis and cancer. Int J Mol Sci. 2018;19(5) https://doi
.org/10.3390/ijms19051294. [PMC free article: PMC5983604] [PubMed: 29701666] [CrossRef] - 7.
- Ringelhan M, Pfister D, O'Connor T, Pikarsky E, Heikenwalder M. The immunology of hepatocellular carcinoma. Nat Immunol. 2018;19(3):222–32. https://doi
.org/10.1038 /s41590-018-0044-z. [PubMed: 29379119] [CrossRef] - 8.
- Robinson MW, Harmon C, O'Farrelly C. Liver immunology and its role in inflammation and homeostasis. Cell Mol Immunol. 2016;13(3):267–76. https://doi
.org/10.1038/cmi.2016.3. [PMC free article: PMC4856809] [PubMed: 27063467] [CrossRef] - 9.
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. https://doi
.org/10.1038/nrdp.2016.18. [PubMed: 27158749] [CrossRef] - 10.
- Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14(3):181–94. https://doi
.org/10.1038/nri3623. [PubMed: 24566915] [CrossRef] - 11.
- Mossanen JC, Krenkel O, Ergen C, Govaere O, Liepelt A, Puengel T, et al. Chemokine (C-C motif) receptor 2-positive monocytes aggravate the early phase of acetaminophen-induced acute liver injury. Hepatology. 2016;64(5):1667–82. https://doi
.org/10.1002/hep.28682. [PubMed: 27302828] [CrossRef] - 12.
- Lemmers A, Moreno C, Gustot T, Marechal R, Degre D, Demetter P, et al. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49(2):646–57. https://doi
.org/10.1002/hep.22680. [PubMed: 19177575] [CrossRef] - 13.
- Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, et al. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143(3):765–76 e3. https://doi
.org/10.1053/j .gastro.2012.05.049. [PMC free article: PMC3635475] [PubMed: 22687286] [CrossRef] - 14.
- Tu T, Calabro SR, Lee A, Maczurek AE, Budzinska MA, Warner FJ, et al. Hepatocytes in liver injury: victim, bystander, or accomplice in progressive fibrosis? J Gastroenterol Hepatol. 2015;30(12):1696–704. https://doi
.org/10.1111/jgh.13065. [PubMed: 26239824] [CrossRef] - 15.
- Mederacke I, Hsu CC, Troeger JS, Huebener P, Mu X, Dapito DH, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. https://doi
.org/10.1038/ncomms3823. [PMC free article: PMC4059406] [PubMed: 24264436] [CrossRef] - 16.
- Maeda S, Kamata H, Luo JL, Leffert H, Karin M. IKKbeta couples hepatocyte death to cytokine-driven compensatory proliferation that promotes chemical hepatocarcinogenesis. Cell. 2005;121(7):977–90. https://doi
.org/10.1016/j .cell.2005.04.014. [PubMed: 15989949] [CrossRef] - 17.
- Akira S, Nishio Y, Inoue M, Wang XJ, Wei S, Matsusaka T, et al. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77(1):63–71. [PubMed: 7512451] [CrossRef]
- 18.
- Mackey-Lawrence NM, Petri WA Jr. Leptin and mucosal immunity. Mucosal Immunol. 2012;5(5):472–9. https://doi
.org/10.1038/mi.2012.40. [PMC free article: PMC3425733] [PubMed: 22692456] [CrossRef] - 19.
- McCartney EM, Helbig KJ, Narayana SK, Eyre NS, Aloia AL, Beard MR. Signal transducer and activator of transcription 3 is a proviral host factor for hepatitis C virus. Hepatology. 2013;58(5):1558–68. https://doi
.org/10.1002/hep.26496. [PubMed: 23703790] [CrossRef] - 20.
- Haybaeck J, Zeller N, Wolf MJ, Weber A, Wagner U, Kurrer MO, et al. A lymphotoxin-driven pathway to hepatocellular carcinoma. Cancer Cell. 2009;16(4):295–308. https://doi
.org/10.1016/j .ccr.2009.08.021. [PMC free article: PMC4422166] [PubMed: 19800575] [CrossRef] - 21.
- Wolf MJ, Adili A, Piotrowitz K, Abdullah Z, Boege Y, Stemmer K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26(4):549–64. https://doi
.org/10.1016/j .ccell.2014.09.003. [PubMed: 25314080] [CrossRef] - 22.
- Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature. 2011;479(7374):547–51. https://doi
.org/10.1038/nature10599. [PubMed: 22080947] [CrossRef] - 23.
- Ma C, Kesarwala AH, Eggert T, Medina-Echeverz J, Kleiner DE, Jin P, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531(7593):253–7. https://doi
.org/10.1038/nature16969. [PMC free article: PMC4786464] [PubMed: 26934227] [CrossRef] - 24.
- van der Windt DJ, Sud V, Zhang H, Varley PR, Goswami J, Yazdani HO, et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology. 2018;68(4):1347–60. https://doi
.org/10.1002/hep.29914. [PMC free article: PMC6173613] [PubMed: 29631332] [CrossRef] - 25.
- Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie Y, et al. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun. 2017;8:15129. https://doi
.org/10.1038/ncomms15129. [PMC free article: PMC5529670] [PubMed: 28541302] [CrossRef] - 26.
- Li K, Liu H, Guo T. Th17/Treg imbalance is an indicator of liver cirrhosis process and a risk factor for HCC occurrence in HBV patients. Clin Res Hepatol Gastroenterol. 2017;41(4):399–407. https://doi
.org/10.1016/j .clinre.2016.12.004. [PubMed: 28169127] [CrossRef] - 27.
- Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192(2):295–302. [PMC free article: PMC2193261] [PubMed: 10899916] [CrossRef]
- 28.
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. [PubMed: 10485649] [CrossRef]
- 29.
- Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32(3):634–43. https://doi
.org/10.1002 /1521-4141(200203)32 :3<634::AID-IMMU634>3.0.CO;2-9. [PubMed: 11857337] [CrossRef] - 30.
- Knolle P, Schlaak J, Uhrig A, Kempf P, Meyer zum Buschenfelde KH, Gerken G. Human Kupffer cells secrete IL-10 in response to lipopolysaccharide (LPS) challenge. J Hepatol. 1995;22(2):226–9. [PubMed: 7790711] [CrossRef]
- 31.
- Hattori E, Okumoto K, Adachi T, Takeda T, Ito J, Sugahara K, et al. Possible contribution of circulating interleukin-10 (IL-10) to anti-tumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatol Res. 2003;27(4):309–14. [PubMed: 14662119] [CrossRef]
- 32.
- Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. https://doi
.org/10.1146/annurev .immunol.19.1.683. [PubMed: 11244051] [CrossRef] - 33.
- Kehrl JH, Wakefield LM, Roberts AB, Jakowlew S, Alvarez-Mon M, Derynck R, et al. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986;163(5):1037–50. [PMC free article: PMC2188095] [PubMed: 2871125] [CrossRef]
- 34.
- Espevik T, Waage A, Faxvaag A, Shalaby MR. Regulation of interleukin-2 and interleukin-6 production from T-cells: involvement of interleukin-1 beta and transforming growth factor-beta. Cell Immunol. 1990;126(1):47–56. [PubMed: 2302741] [CrossRef]
- 35.
- Smyth MJ, Strobl SL, Young HA, Ortaldo JR, Ochoa AC. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol. 1991;146(10):3289–97. [PubMed: 1827481]
- 36.
- Othman MS, Aref AM, Mohamed AA, Ibrahim WA. Serum levels of Interleukin-6 and Interleukin-10 as biomarkers for hepatocellular carcinoma in Egyptian patients. ISRN Hepatol. 2013;2013:412317. https://doi
.org/10.1155/2013/412317. [PMC free article: PMC4890868] [PubMed: 27335826] [CrossRef] - 37.
- El-Emshaty HM, Nasif WA, Mohamed IE. Serum cytokine of IL-10 and IL-12 in chronic liver disease: the immune and inflammatory response. Dis Markers. 2015;2015:707254. https://doi
.org/10.1155/2015/707254. [PMC free article: PMC4689924] [PubMed: 26783377] [CrossRef] - 38.
- Thomson AW, Knolle PA. Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol. 2010;10(11):753–66. https://doi
.org/10.1038/nri2858. [PubMed: 20972472] [CrossRef] - 39.
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39(1):1–10. https://doi
.org/10.1016/j .immuni.2013.07.012. [PubMed: 23890059] [CrossRef] - 40.
- Nakajima T, Mizushima N, Kanai K. Relationship between natural killer activity and development of hepatocellular carcinoma in patients with cirrhosis of the liver. Jpn J Clin Oncol. 1987;17(4):327–32. [PubMed: 2826847]
- 41.
- Garnelo M, Tan A, Her Z, Yeong J, Lim CJ, Chen J, et al. Interaction between tumour-infiltrating B cells and T cells controls the progression of hepatocellular carcinoma. Gut. 2017;66(2):342–51. https://doi
.org/10.1136 /gutjnl-2015-310814. [PMC free article: PMC5284473] [PubMed: 26669617] [CrossRef] - 42.
- Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124(2):263–6. https://doi
.org/10.1016/j .cell.2006.01.007. [PubMed: 16439202] [CrossRef] - 43.
- Degroote H, Van Dierendonck A, Geerts A, Van Vlierberghe H, Devisscher L. Preclinical and clinical therapeutic strategies affecting tumor-associated macrophages in hepatocellular carcinoma. J Immunol Res. 2018;2018:7819520. https://doi
.org/10.1155/2018/7819520. [PMC free article: PMC6206557] [PubMed: 30410942] [CrossRef] - 44.
- Wu K, Kryczek I, Chen L, Zou W, Welling TH. Kupffer cell suppression of CD8+ T cells in human hepatocellular carcinoma is mediated by B7-H1/programmed death-1 interactions. Cancer Res. 2009;69(20):8067–75. https://doi
.org/10.1158/0008-5472 .CAN-09-0901. [PMC free article: PMC4397483] [PubMed: 19826049] [CrossRef] - 45.
- Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66(6):1300–12. https://doi
.org/10.1016/j .jhep.2017.02.026. [PubMed: 28267621] [CrossRef] - 46.
- Yeung OW, Lo CM, Ling CC, Qi X, Geng W, Li CX, et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J Hepatol. 2015;62(3):607–16. https://doi
.org/10.1016/j .jhep.2014.10.029. [PubMed: 25450711] [CrossRef] - 47.
- Ding T, Xu J, Wang F, Shi M, Zhang Y, Li SP, et al. High tumor-infiltrating macrophage density predicts poor prognosis in patients with primary hepatocellular carcinoma after resection. Hum Pathol. 2009;40(3):381–9. https://doi
.org/10.1016/j .humpath.2008.08.011. [PubMed: 18992916] [CrossRef] - 48.
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392(6673):245–52. https://doi
.org/10.1038/32588. [PubMed: 9521319] [CrossRef] - 49.
- Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr Top Microbiol Immunol. 2006;311:17–58. [PubMed: 17048704]
- 50.
- Almand B, Resser JR, Lindman B, Nadaf S, Clark JI, Kwon ED, et al. Clinical significance of defective dendritic cell differentiation in cancer. Clin Cancer Res. 2000;6(5):1755–66. [PubMed: 10815894]
- 51.
- Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med. 2007;13(10):1155–9. https://doi
.org/10.1038/nm1643. [PubMed: 17917664] [CrossRef] - 52.
- Kakumu S, Ito S, Ishikawa T, Mita Y, Tagaya T, Fukuzawa Y, et al. Decreased function of peripheral blood dendritic cells in patients with hepatocellular carcinoma with hepatitis B and C virus infection. J Gastroenterol Hepatol. 2000;15(4):431–6. [PubMed: 10824889] [CrossRef]
- 53.
- Chen S, Akbar SM, Tanimoto K, Ninomiya T, Iuchi H, Michitaka K, et al. Absence of CD83-positive mature and activated dendritic cells at cancer nodules from patients with hepatocellular carcinoma: relevance to hepatocarcinogenesis. Cancer Lett. 2000;148(1):49–57. [PubMed: 10680592] [CrossRef]
- 54.
- Ormandy LA, Farber A, Cantz T, Petrykowska S, Wedemeyer H, Horning M, et al. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J Gastroenterol. 2006;12(20):3275–82. [PMC free article: PMC4087975] [PubMed: 16718852] [CrossRef]
- 55.
- Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–103. [PubMed: 8837607] [CrossRef]
- 56.
- Beckebaum S, Zhang X, Chen X, Yu Z, Frilling A, Dworacki G, et al. Increased levels of interleukin-10 in serum from patients with hepatocellular carcinoma correlate with profound numerical deficiencies and immature phenotype of circulating dendritic cell subsets. Clin Cancer Res. 2004;10(21):7260–9. https://doi
.org/10.1158/1078-0432 .CCR-04-0872. [PubMed: 15534100] [CrossRef] - 57.
- Maier KP. Cirrhosis of the liver as a precancerous condition. Praxis (Bern 1994). 1998;87(44):1462–5. [PubMed: 9847685]
- 58.
- Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol. 2015;13(12):2140–51. https://doi
.org/10.1016/j .cgh.2015.08.014. [PMC free article: PMC4618036] [PubMed: 26284591] [CrossRef] - 59.
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118–27. https://doi
.org/10.1056/NEJMra1001683. [PubMed: 21992124] [CrossRef] - 60.
- Affo S, Yu LX, Schwabe RF. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. 2017;12:153–86. https://doi
.org/10.1146 /annurev-pathol-052016-100322. [PMC free article: PMC5720358] [PubMed: 27959632] [CrossRef] - 61.
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. https://doi
.org/10.1016/j .cell.2011.02.013. [PubMed: 21376230] [CrossRef] - 62.
- Bissell MJ, Hines WC. Why don’t we get more cancer? A proposed role of the microenvironment in restraining cancer progression. Nat Med. 2011;17(3):320–9. https://doi
.org/10.1038/nm.2328. [PMC free article: PMC3569482] [PubMed: 21383745] [CrossRef] - 63.
- Ji J, Eggert T, Budhu A, Forgues M, Takai A, Dang H, et al. Hepatic stellate cell and monocyte interaction contributes to poor prognosis in hepatocellular carcinoma. Hepatology. 2015;62(2):481–95. https://doi
.org/10.1002/hep.27822. [PMC free article: PMC4515211] [PubMed: 25833323] [CrossRef] - 64.
- Zhao W, Su W, Kuang P, Zhang L, Liu J, Yin Z, et al. The role of hepatic stellate cells in the regulation of T-cell function and the promotion of hepatocellular carcinoma. Int J Oncol. 2012;41(2):457–64. https://doi
.org/10.3892/ijo.2012.1497. [PMC free article: PMC3582803] [PubMed: 22641338] [CrossRef] - 65.
- Zhao W, Zhang L, Yin Z, Su W, Ren G, Zhou C, et al. Activated hepatic stellate cells promote hepatocellular carcinoma development in immunocompetent mice. Int J Cancer. 2011;129(11):2651–61. https://doi
.org/10.1002/ijc.25920. [PubMed: 21213212] [CrossRef] - 66.
- Cheng Y, Li H, Deng Y, Tai Y, Zeng K, Zhang Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9(4):422. https://doi
.org/10.1038 /s41419-018-0458-4. [PMC free article: PMC5859264] [PubMed: 29556041] [CrossRef] - 67.
- Zhang Z, Li X, Sun W, Yue S, Yang J, Li J, et al. Loss of exosomal miR-320a from cancer-associated fibroblasts contributes to HCC proliferation and metastasis. Cancer Lett. 2017;397:33–42. https://doi
.org/10.1016/j .canlet.2017.03.004. [PubMed: 28288874] [CrossRef] - 68.
- Wang F, Li L, Piontek K, Sakaguchi M, Selaru FM. Exosome miR-335 as a novel therapeutic strategy in hepatocellular carcinoma. Hepatology. 2018;67(3):940–54. https://doi
.org/10.1002/hep.29586. [PMC free article: PMC5826829] [PubMed: 29023935] [CrossRef] - 69.
- Torimura T, Ueno T, Kin M, Harada R, Taniguchi E, Nakamura T, et al. Overexpression of angiopoietin-1 and angiopoietin-2 in hepatocellular carcinoma. J Hepatol. 2004;40(5):799–807. https://doi
.org/10.1016/j .jhep.2004.01.027. [PubMed: 15094228] [CrossRef] - 70.
- Taura K, De Minicis S, Seki E, Hatano E, Iwaisako K, Osterreicher CH, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology. 2008;135(5):1729–38. https://doi
.org/10.1053/j .gastro.2008.07.065. [PubMed: 18823985] [CrossRef] - 71.
- Lin N, Chen Z, Lu Y, Li Y, Hu K, Xu R. Role of activated hepatic stellate cells in proliferation and metastasis of hepatocellular carcinoma. Hepatol Res. 2015;45(3):326–36. https://doi
.org/10.1111/hepr.12356. [PubMed: 24827154] [CrossRef] - 72.
- Santamato A, Fransvea E, Dituri F, Caligiuri A, Quaranta M, Niimi T, et al. Hepatic stellate cells stimulate HCC cell migration via laminin-5 production. Clin Sci (Lond). 2011;121(4):159–68. https://doi
.org/10.1042/CS20110002. [PubMed: 21413933] [CrossRef] - 73.
- Okabe H, Beppu T, Ueda M, Hayashi H, Ishiko T, Masuda T, et al. Identification of CXCL5/ENA-78 as a factor involved in the interaction between cholangiocarcinoma cells and cancer-associated fibroblasts. Int J Cancer. 2012;131(10):2234–41. https://doi
.org/10.1002/ijc.27496. [PubMed: 22337081] [CrossRef] - 74.
- Fang T, Lv H, Lv G, Li T, Wang C, Han Q, et al. Tumor-derived exosomal miR-1247-3p induces cancer-associated fibroblast activation to foster lung metastasis of liver cancer. Nat Commun. 2018;9(1):191. https://doi
.org/10.1038 /s41467-017-02583-0. [PMC free article: PMC5768693] [PubMed: 29335551] [CrossRef] - 75.
- Jiang J, Ye F, Yang X, Zong C, Gao L, Yang Y, et al. Peri-tumor associated fibroblasts promote intrahepatic metastasis of hepatocellular carcinoma by recruiting cancer stem cells. Cancer Lett. 2017;404:19–28. https://doi
.org/10.1016/j .canlet.2017.07.006. [PubMed: 28716525] [CrossRef] - 76.
- Liu C, Liu L, Chen X, Cheng J, Zhang H, Zhang C, et al. LSD1 stimulates cancer-associated fibroblasts to drive Notch3-dependent self-renewal of liver cancer stem-like cells. Cancer Res. 2018;78(4):938–49. https://doi
.org/10.1158/0008-5472 .CAN-17-1236. [PubMed: 29259010] [CrossRef] - 77.
- Rhee H, Kim HY, Choi JH, Woo HG, Yoo JE, Nahm JH, et al. Keratin 19 expression in hepatocellular carcinoma is regulated by fibroblast-derived HGF via a MET-ERK1/2-AP1 and SP1 Axis. Cancer Res. 2018;78(7):1619–31. https://doi
.org/10.1158/0008-5472 .CAN-17-0988. [PubMed: 29363547] [CrossRef] - 78.
- Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, et al. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54(5):1707–17. https://doi
.org/10.1002/hep.24559. [PubMed: 22045674] [CrossRef] - 79.
- Coulouarn C, Corlu A, Glaise D, Guenon I, Thorgeirsson SS, Clement B. Hepatocyte-stellate cell cross-talk in the liver engenders a permissive inflammatory microenvironment that drives progression in hepatocellular carcinoma. Cancer Res. 2012;72(10):2533–42. https://doi
.org/10.1158/0008-5472 .CAN-11-3317. [PMC free article: PMC3498759] [PubMed: 22419664] [CrossRef] - 80.
- Ju MJ, Qiu SJ, Fan J, Xiao YS, Gao Q, Zhou J, et al. Peritumoral activated hepatic stellate cells predict poor clinical outcome in hepatocellular carcinoma after curative resection. Am J Clin Pathol. 2009;131(4):498–510. https://doi
.org/10.1309/AJCP86PPBNGOHNNL. [PubMed: 19289585] [CrossRef] - 81.
- Zhang DY, Goossens N, Guo J, Tsai MC, Chou HI, Altunkaynak C, et al. A hepatic stellate cell gene expression signature associated with outcomes in hepatitis C cirrhosis and hepatocellular carcinoma after curative resection. Gut. 2016;65(10):1754–64. https://doi
.org/10.1136 /gutjnl-2015-309655. [PMC free article: PMC4848165] [PubMed: 26045137] [CrossRef] - 82.
- Pupulim LF, Vilgrain V, Ronot M, Becker CD, Breguet R, Terraz S. Hepatic lymphatics: anatomy and related diseases. Abdom Imaging. 2015;40(6):1997–2011. https://doi
.org/10.1007 /s00261-015-0350-y. [PubMed: 25579171] [CrossRef] - 83.
- Lund AW, Wagner M, Fankhauser M, Steinskog ES, Broggi MA, Spranger S, et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. J Clin Invest. 2016;126(9):3389–402. https://doi
.org/10.1172/JCI79434. [PMC free article: PMC5004967] [PubMed: 27525437] [CrossRef] - 84.
- Swartz MA. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunol Res. 2014;2(8):701–7. https://doi
.org/10.1158/2326-6066 .CIR-14-0115. [PubMed: 25092811] [CrossRef] - 85.
- Lukacs-Kornek V, Malhotra D, Fletcher AL, Acton SE, Elpek KG, Tayalia P, et al. Regulated release of nitric oxide by nonhematopoietic stroma controls expansion of the activated T cell pool in lymph nodes. Nat Immunol. 2011;12(11):1096–104. https://doi
.org/10.1038/ni.2112. [PMC free article: PMC3457791] [PubMed: 21926986] [CrossRef] - 86.
- Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010;207(4):689–97. https://doi
.org/10.1084/jem.20092642. [PMC free article: PMC2856033] [PubMed: 20308362] [CrossRef] - 87.
- Rouhani SJ, Eccles JD, Riccardi P, Peske JD, Tewalt EF, Cohen JN, et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nat Commun. 2015;6:6771. https://doi
.org/10.1038/ncomms7771. [PMC free article: PMC4403767] [PubMed: 25857745] [CrossRef] - 88.
- Vollmar B, Wolf B, Siegmund S, Katsen AD, Menger MD. Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. Am J Pathol. 1997;151(1):169–75. [PMC free article: PMC1857941] [PubMed: 9212743]
- 89.
- Yamauchi Y, Michitaka K, Onji M. Morphometric analysis of lymphatic and blood vessels in human chronic viral liver diseases. Am J Pathol. 1998;153(4):1131–7. https://doi
.org/10.1016 /S0002-9440(10)65657-X. [PMC free article: PMC1853063] [PubMed: 9777944] [CrossRef] - 90.
- Yokomori H, Oda M, Kaneko F, Kawachi S, Tanabe M, Yoshimura K, et al. Lymphatic marker podoplanin/D2-40 in human advanced cirrhotic liver--re-evaluations of microlymphatic abnormalities. BMC Gastroenterol. 2010;10:131. https://doi
.org/10.1186/1471-230X-10-131. [PMC free article: PMC2995474] [PubMed: 21059220] [CrossRef] - 91.
- Limatola E, Filosa S. Exogenous vitellogenesis and micropinocytosis in the lizard, Podarcis sicula, treated with follicle-stimulating hormone. Gen Comp Endocrinol. 1989;75(2):165–76. [PubMed: 2509281] [CrossRef]
- 92.
- Shields JD, Kourtis IC, Tomei AA, Roberts JM, Swartz MA. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science. 2010;328(5979):749–52. https://doi
.org/10.1126/science.1185837. [PubMed: 20339029] [CrossRef] - 93.
- Xiang Z, Zeng Z, Tang Z, Fan J, Sun H, Wu W, et al. Increased expression of vascular endothelial growth factor-C and nuclear CXCR4 in hepatocellular carcinoma is correlated with lymph node metastasis and poor outcome. Cancer J. 2009;15(6):519–25. https://doi
.org/10.1097/PPO .0b013e3181c6aa6b. [PubMed: 20010172] [CrossRef] - 94.
- Yamaguchi R, Yano H, Nakashima O, Akiba J, Nishida N, Kurogi M, et al. Expression of vascular endothelial growth factor-C in human hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21(1 Pt 1):152–60. https://doi
.org/10.1111/j .1440-1746.2005.04217.x. [PubMed: 16706827] [CrossRef] - 95.
- Nieto MA, Huang RY, Jackson RA, Thiery JP. Emt: 2016. Cell. 2016;166(1):21–45. https://doi
.org/10.1016/j .cell.2016.06.028. [PubMed: 27368099] [CrossRef] - 96.
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2018; https://doi
.org/10.1038 /s41580-018-0080-4. [PubMed: 30459476] [CrossRef] - 97.
- Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–73. https://doi
.org/10.1038/nrc2620. [PubMed: 19262571] [CrossRef] - 98.
- Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119(6):1438–49. https://doi
.org/10.1172/JCI38019. [PMC free article: PMC2689100] [PubMed: 19487820] [CrossRef] - 99.
- Yan L, Xu F, Dai CL. Relationship between epithelial-to-mesenchymal transition and the inflammatory microenvironment of hepatocellular carcinoma. J Exp Clin Cancer Res. 2018;37(1):203. https://doi
.org/10.1186 /s13046-018-0887-z. [PMC free article: PMC6114477] [PubMed: 30157906] [CrossRef] - 100.
- Zavadil J, Bottinger EP. TGF-beta and epithelial-to-mesenchymal transitions. Oncogene. 2005;24(37):5764–74. https://doi
.org/10.1038/sj.onc.1208927. [PubMed: 16123809] [CrossRef] - 101.
- Choi SS, Diehl AM. Epithelial-to-mesenchymal transitions in the liver. Hepatology. 2009;50(6):2007–13. https://doi
.org/10.1002/hep.23196. [PMC free article: PMC2787916] [PubMed: 19824076] [CrossRef] - 102.
- Zhai B, Yan HX, Liu SQ, Chen L, Wu MC, Wang HY. Reduced expression of E-cadherin/catenin complex in hepatocellular carcinomas. World J Gastroenterol. 2008;14(37):5665–73. [PMC free article: PMC2748200] [PubMed: 18837082] [CrossRef]
- 103.
- Battaglia S, Benzoubir N, Nobilet S, Charneau P, Samuel D, Zignego AL, et al. Liver cancer-derived hepatitis C virus core proteins shift TGF-beta responses from tumor suppression to epithelial-mesenchymal transition. PLoS One. 2009;4(2):e4355. https://doi
.org/10.1371/journal .pone.0004355. [PMC free article: PMC2629560] [PubMed: 19190755] [CrossRef] - 104.
- Fuchs BC, Fujii T, Dorfman JD, Goodwin JM, Zhu AX, Lanuti M, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68(7):2391–9. https://doi
.org/10.1158/0008-5472 .CAN-07-2460. [PubMed: 18381447] [CrossRef] - 105.
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. https://doi
.org/10.1056/NEJMoa0708857. [PubMed: 18650514] [CrossRef] - 106.
- Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–73. https://doi
.org/10.1016 /S0140-6736(18)30207-1. [PubMed: 29433850] [CrossRef] - 107.
- Sui H, Ma N, Wang Y, Li H, Liu X, Su Y, et al. Anti-PD-1/PD-L1 therapy for non-small-cell lung cancer: toward personalized medicine and combination strategies. J Immunol Res. 2018;2018:6984948. https://doi
.org/10.1155/2018/6984948. [PMC free article: PMC6109480] [PubMed: 30159341] [CrossRef] - 108.
- Herzberg B, Fisher DE. Metastatic melanoma and immunotherapy. Clin Immunol. 2016;172:105–10. https://doi
.org/10.1016/j .clim.2016.07.006. [PMC free article: PMC5569887] [PubMed: 27430520] [CrossRef] - 109.
- Killock D. Immunotherapy: Nivolumab keeps HCC in check and opens avenues for checkmate. Nat Rev Clin Oncol. 2017;14(7):392. https://doi
.org/10.1038/nrclinonc .2017.70. [PubMed: 28485409] [CrossRef] - 110.
- Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940–52. https://doi
.org/10.1016 /S1470-2045(18)30351-6. [PubMed: 29875066] [CrossRef] - 111.
- Shang N, Figini M, Shangguan J, Wang B, Sun C, Pan L, et al. Dendritic cells based immunotherapy. Am J Cancer Res. 2017;7(10):2091–102. [PMC free article: PMC5665855] [PubMed: 29119057]
- 112.
- Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, et al. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49(1):124–32. https://doi
.org/10.1002/hep.22626. [PubMed: 18980227] [CrossRef] - 113.
- El Ansary M, Mogawer S, Elhamid SA, Alwakil S, Aboelkasem F, Sabaawy HE, et al. Immunotherapy by autologous dendritic cell vaccine in patients with advanced HCC. J Cancer Res Clin Oncol. 2013;139(1):39–48. https://doi
.org/10.1007 /s00432-012-1298-8. [PMC free article: PMC5223882] [PubMed: 22886490] [CrossRef]
- * Antonio Saviano and Natascha Roehlen are co-first authors of this chapter
- Review Stromal cell-derived factor-1 (SDF-1) as a target in liver diseases.[Am J Physiol Gastrointest Live...]Review Stromal cell-derived factor-1 (SDF-1) as a target in liver diseases.Liepelt A, Tacke F. Am J Physiol Gastrointest Liver Physiol. 2016 Aug 1; 311(2):G203-9. Epub 2016 Jun 16.
- Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib.[Gastroenterology. 2016]Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib.Zhou SL, Zhou ZJ, Hu ZQ, Huang XW, Wang Z, Chen EB, Fan J, Cao Y, Dai Z, Zhou J. Gastroenterology. 2016 Jun; 150(7):1646-1658.e17. Epub 2016 Feb 26.
- Review The current status of tumor microenvironment and cancer stem cells in sorafenib resistance of hepatocellular carcinoma.[Front Oncol. 2023]Review The current status of tumor microenvironment and cancer stem cells in sorafenib resistance of hepatocellular carcinoma.Chen S, Du Y, Guan XY, Yan Q. Front Oncol. 2023; 13:1204513. Epub 2023 Jul 27.
- CD8(+) T cells mediate the antitumor activity of frankincense and myrrh in hepatocellular carcinoma.[J Transl Med. 2018]CD8(+) T cells mediate the antitumor activity of frankincense and myrrh in hepatocellular carcinoma.Xu C, Lu X, Liu W, Chen A, Meng G, Zhang H, Li B, Zhang Y, Wu J, Wei J. J Transl Med. 2018 May 21; 16(1):132. Epub 2018 May 21.
- Increased expression of SLC46A3 to oppose the progression of hepatocellular carcinoma and its effect on sorafenib therapy.[Biomed Pharmacother. 2019]Increased expression of SLC46A3 to oppose the progression of hepatocellular carcinoma and its effect on sorafenib therapy.Zhao Q, Zheng B, Meng S, Xu Y, Guo J, Chen LJ, Xiao J, Zhang W, Tan ZR, Tang J, et al. Biomed Pharmacother. 2019 Jun; 114:108864. Epub 2019 Apr 10.
- Stromal and Immune Drivers of Hepatocarcinogenesis - Hepatocellular CarcinomaStromal and Immune Drivers of Hepatocarcinogenesis - Hepatocellular Carcinoma
Your browsing activity is empty.
Activity recording is turned off.
See more...
 1,2.
1,2.