Context and Policy Issues
The Canadian Cancer Society estimates that breast cancer will account for 25.5% of new cancer cases for women in 2017, making breast cancer the leading cancer in Canadian women.1 Various gene expression tests have been developed to aid treatment planning in early-stage breast cancer. Commercially available gene expression tests include EndoPredict (Myriad Genetics), Oncotype DX (Genomic Health), MammaPrint (Agendia), Mammostrat (Clarient Diagnostic Services) and Prosigna (NanoString). The assays are prognostic and aim to address similar outcomes pertaining to cancer recurrence. The gene expression tests help to differentiate patients that would likely benefit from chemotherapy thus, avoiding overtreatment.2-4 The tests differ greatly in terms of the platforms used for assessing gene expression as well as the type and quantity of genes tested.2-4
EndoPredict assesses 11 genes (including three control genes) and predicts the risk of distant recurrence by categorizing patients as low or high risk.2,4 MammaPrint determines the risk of recurrence through the assessment of 70 genes. Recurrence is categorized as low or high risk.4,5 Prosignia (formally known as the PAM50 test) assesses 50 genes (plus five controls) to detect breast cancer subtypes and calculate a 10-year risk of recurrence score.4 Mammostrat assess five genes to derive a risk score that is typically categorized as high, moderate, or low risk.5 Oncotype DX is the most studied and widely used breast cancer gene expression test.2-6 This assay examines 21 genes (including 5 controls) from breast cancer tumor samples and produces a recurrence score (RS) from zero to 100.4 Patients are classified into 3 categories based on their RS for low, intermediate, or high risk of recurrence. A low RS predicts little benefit from chemotherapy.4 A 2014 CADTH report7 examining Oncotype DX found that test results have an impact on approximately 30% of treatment plans for patients with ER-positive, HER2-negative, lymph node negative early stage breast cancer, primarily resulting in lower rates of adjuvant chemotherapy when a low risk score is observed. However changes in patient clinical outcomes resulting from changes in treatment plans were unclear.7
While each gene expression test has been independently clinically validated using prospective or retrospective evidence, currently, there is no gold-standard for breast cancer gene expression tests. This highlights the need for comparative assessments of clinical utility and cost-effectiveness.4,5
Research Questions
- 1.
What is the comparative clinical utility of gene expression tests to either predict adjunct chemotherapy or evaluate cancer recurrence risk in women with early stage breast cancer?
- 2.
What is the comparative cost-effectiveness of gene expression tests to either predict adjunct chemotherapy or evaluate cancer recurrence risk in women with early stage breast cancer?
Key Findings
One moderate quality comparative clinical study provided evidence to suggest that Oncotype DX and EndoPredict were both prognostic for the risk of distant recurrence in the years zero through five. In years five through 10 EndoPredict was better able to predict distant recurrence compared to Oncotype DX. In comparisons between the high/non-low and the low risk groups, the hazard ratio was higher for EndoPredict.
One moderate quality comparative cost-effectiveness study determined that the use of Mammostrat was more cost-effective and was associated with a cost savings of $2,268 per patient compared to Oncotype DX.
Methods
Literature Search Methods
A limited literature search was conducted on key resources including Ovid Medline, PubMed, The Cochrane Library, University of York Centre for Reviews and Dissemination (CRD) databases , Canadian and major international health technology agencies, as well as a focused Internet search. No methodological filters were applied to limit retrieval by publication type from January 1, 2014 to September 12, 2017. An additional search was done to retrieve economic studies published since January 1, 2012. The search was limited to English language documents.
Rapid Response reports are organized so that the evidence for each research question is presented separately.
Selection Criteria and Methods
One reviewer screened citations and selected studies. In the first level of screening, titles and abstracts were reviewed and potentially relevant articles were retrieved and assessed for inclusion. The final selection of full-text articles was based on the inclusion criteria presented in .
Exclusion Criteria
Articles were excluded if they did not meet the selection criteria outlined in , they were duplicate publications, or were published prior to January 1, 2014 for clinical studies, or January 1, 2012 for economic studies.
Critical Appraisal of Individual Studies
The included clinical, non-randomized study were critically appraised using the Downs and Black Checklist,8 and the economic study was assessed using the Drummond checklist.9 Summary scores were not calculated for the included studies; rather, a review of the strengths and limitations of each included study were described narratively.
Summary of Evidence
Quantity of Research Available
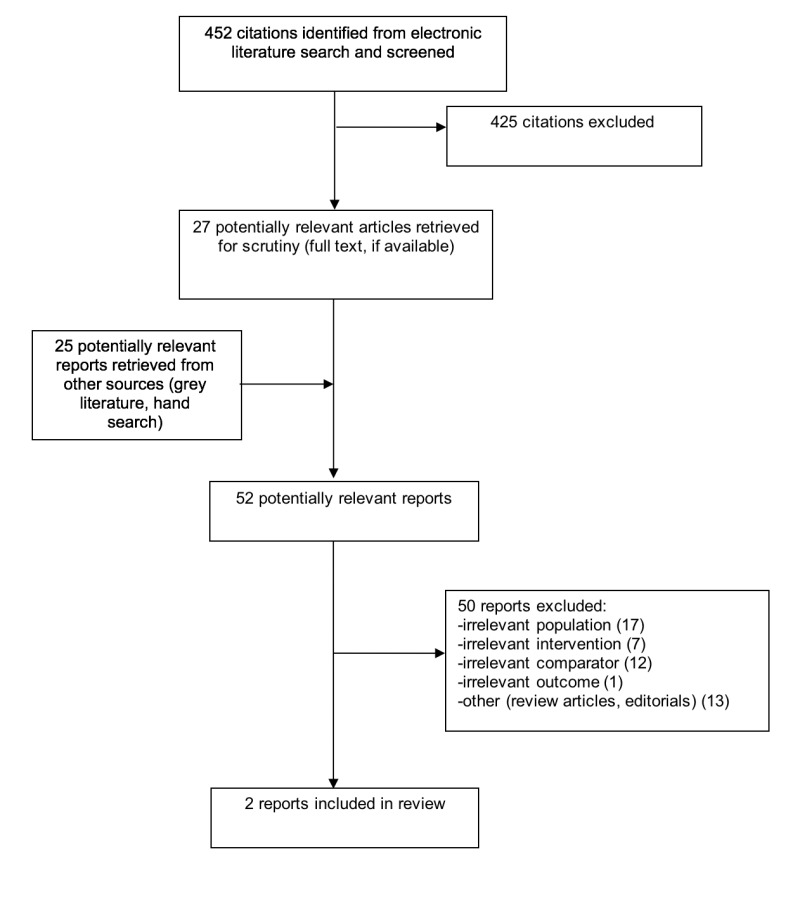
A total of 452 citations were identified in the
literature search. Following screening of titles
and abstracts, 425 citations were excluded and 27
potentially relevant reports from the electronic
search were retrieved for full-text review. Twenty-five potentially relevant publications were retrieved from the grey literature search. Of these potentially relevant articles, 50 publications were excluded for various reasons, while two publications met the inclusion criteria and were included in this report. Appendix 1 describes the PRISMA flowchart of the study selection.
Additional references of potential interest are
provided in Appendix 5. These publications did not meet the inclusion study for this Rapid Response.
Summary of Study Characteristics
The details of the individual study
characteristics for the included publications are provided in Appendix 2.
Study Design, Country of Origin, and Patient Population
Buus et al. published a clinical study based on data and RNA samples from the translational sub-study of the Arimidex, Tamoxifen, Alone or in combination trial (transATAC).2 This previously conducted translational RCT was designed to test the effectiveness of two endocrine therapies in post-menopausal women with hormone receptor-positive, HER2-negative, chemotherapy-naïve breast cancer. Patients were recruited from the United Kingdom (UK) and required to have an Oncotype DX recurrence score (RS) and at least 350 ng residual RNA available from the transATAC study to be used for the EndoPredict (EP) assay.
Mislick et al. published a cost-effectiveness study for the comparison of Mammostrat and Oncotype DX using a US third-party payer perspective.10 A 10-year Markov model was developed using model inputs derived from the National Surgical Adjuvant Breast and Bowel Project (NSABP). The NSABP collected data from women with node-negative, ER- positive, early stage breast cancer.10 The NSABP did not collect all types of data required for the cost-effectiveness study so assumptions were required for certain aspects of the model (i.e. proportion of women at each risk level who received chemotherapy). Mislick et al. used one set of values for the base-case analysis and conducted several sensitivity analyses to account for uncertainty.
Interventions and Comparators
Buus et al. compared the recurrence score (RS) from Oncotype DX with the EndoPredict (EP) and an alternative EndoPredict score (EPclin).2 EPclin combined the score from the original EndoPredict with clinical information on tumor size and nodal status.
The cost-effectiveness study by Mislick et al. compared the tumor subtyping assays Mammostrat and Oncotype DX.10 Model cost inputs included the cost of chemotherapy, the cost of Mammostrat and Oncotype DX, the cost of cancer recurrence, death due to chemotherapy cost, minor and severe chemotherapy adverse event cost, discount rate, and end of life costs.
Outcomes
The clinical study by Buus et al. assessed the prognostic value of the recurrence score from Oncotype DX (low/non-low), the EndoPredict (low/high risk) score, and the alternative EndoPredict (low/high) score for the assessment of distant recurrence risk.2 Distant recurrence was assessed in the period of zero to 10 years, as well as zero to five years, and five to 10 years.
The cost-effectiveness study by Mislick et al. aimed to assess the cost-effectiveness for the use of Mammostrat compared to Oncotype DX to inform the treatment of breast cancer using real and simulated data.10 The cost-effectiveness was reported as base-case costs and quality-adjusted life years.
Summary of Critical Appraisal
The details of the critical appraisal for the
included publications are provided in Appendix 3.
The clinical study by Buus et al. used data and RNA samples from transATAC.2 The aim, patient characteristics, main outcomes, and interventions of the Buus et al. study were clearly described. The results were reported for various sub-groups including a lymph node- negative group. Analysis was appropriately conducted and represented with 95% confidence intervals and actual probability values. The relevant sub-group (node-negative patients) accounted for 73.3% of the patient population. It is unclear if the main study or the node-negative sub-group analysis was sufficiently powered as the authors did not report power calculations. While the authors state that patients undergoing combination therapy were excluded from the analysis, it is unclear if the single therapy (arimidex or tamoxifen) examined in the original transATAC study is relevant to the main outcome (distant recurrence) as the authors did not include therapy-related sub-group analysis. The internal validity of the study was preserved through blinding of the analysts to clinical data. It was unclear if selection bias was present as the study required a minimum of 350 ng residual RNA for inclusion in the study. The authors did not provide an explanation for why some samples had insufficient RNA samples after the original transATAC study. The external validity was limited to the UK population and to women treated with arimidex or tamoxifen for five years. It is unclear if this treatment is comparable to treatment received in Canada for women with clinically similar cases.
The cost-effectiveness study by Mislick et al. used clear and relevant methodology and was generally well conducted.10 The research question, rationale and economic importance of the study were clearly stated. The model inputs and data sources were clearly described. The authors highlighted specific areas where real data was missing and simulated data based on assumptions were used. The data used in the study were derived from the NSABP. The clinical data from the NSABP was used in the development and validation of Mammostrat and Oncotype DX, and was thus determined to be an appropriate sample. The NSABP did not have data for the model parameters for the proportion of women at each risk level who received chemotherapy, thus the model relied on assumptions where the patients who were high, medium, and low risk were assumed to use chemotherapy at the following proportions: 0.8, 0.5 and 0.1, respectively. It was unclear if these assumptions about proportions were guided by the literature or if they would be applicable to a Canadian population. The base population for the analysis was not identical between the two test groups, as 20% to 30% of the samples were depleted in the initial assessment of Oncotype DX and were subsequently unavailable for the assessment with Mammostrat. To counter this limitation, the authors made statistical adjustments to make the study populations equivalent. Several relevant sensitivity analyses were conducted to assess the impact of changing model parameters. Finally, the model was based on a US third-party payer perspective, which inherently limits the external validity to the US population and reduces the applicability to the Canadian population.
Summary of Findings
What is the comparative clinical utility of gene expression tests to either predict adjunct chemotherapy or evaluate cancer recurrence risk in women with early stage breast cancer?
Oncotype DX RS and the two versions of Endopredict (EP and EPclin) were each similarly prognostic for the risk of distant recurrence in the years zero through five (likelihood ratio [LR] chi-square: EP=15.5; LR chi-square: EPclin= 17.0; LR chi-square: RS = 18.7).2 In the years five to 10, RS was less likely to predict distant recurrence compared to the EP and EPclin (LR chi-square: EP = 22.7; LR chi-square: EPclin = 15.5; LR chi-square: RS = 4.8). In this analysis the LR test was based on Cox proportional hazard models and indicates an increase in risk of distant recurrence associated with an increase in each assay’s score. In comparisons between the high/non-low and the low risk groups, the hazard ratio was similar for RS and EPclin at 3.72 (95% confidence interval [CI]: 2.17 to 6.39) and 3.90 (95% CI: 2.33 to 6.52), respectively. The hazard ratio was the highest for EP at 5.15 (95%CI: 2.44 to 10.85), indicating the greatest increase in the risk of recurrence with the high risk EP group versus low risk EP group compared to Oncotype DX RS and EPclin.
What is the comparative cost-effectiveness of gene expression tests to either predict adjunct chemotherapy or evaluate cancer recurrence risk in women with early stage breast cancer?
In a comparison of Mammostrat and Oncotype DX in a clinically similar population, Mammostrat was determined to be more cost-effective with a cost savings of $2,268 per patient.10 This study determined that both assays resulted in similar life years (9.880 and 9.882) and quality-adjusted life years (7.935 and 7.940) for Mammostrat and Oncotype DX, respectively. Sensitivity analyses based on alternative assumptions for chemotherapy cost, recurrence cost, mild and severe adverse event cost, and altering the recurrence-free proportion parameter showed a high degree of consistency with the main results with Mammostrat remaining cost-effective over Oncotype DX.
Limitations
The clinical study by Buus et al. was inherently limited as it used data from a previously conducted RCT that was designed to address a different research question.2 Thus, it was unclear if the sub-group analysis for node-negative patients was sufficiently powered. The external validity of this study was limited to the UK population; patients were treated with either treated with arimidex or tamoxifen for 5 years and it is unclear if this is comparable to the treatment Canadian women receive, or if use of the different treatments influenced the results.
The cost-effectiveness study was limited by assumptions on the proportion of women at each risk level who received chemotherapy in the 10-year model.10 It is unclear if these assumptions were based on evidence. Additionally, the model was based on a US third- party payer perspective, which inherently limits the external validity from the perspective of the Canadian population as costs associated with treatment were likely not equivalent.
Conclusions and Implications for Decision or Policy Making
The single comparative clinical utility study identified in this Rapid Response report evaluated the breast cancer assays Oncotype DX and EndoPredict.2 This study examined the prognostic value of both assays and determined that they provided a similar amount of prognostic information for distant recurrences occurring in the first five years. EndoPredict provided more prognostic information for distant recurrences occurring from five to ten years later. The hazard ratio for low versus high EndoPredict score was higher compared to low versus non-low Oncotype DX recurrence score. Overall, these results indicate that both Oncotype DX and EndoPredict are potentially useful assays in determining the risk of distant recurrence.
The single cost-effectiveness study identified in this Rapid Response evaluated the breast cancer assays Oncotype DX and MammaPrint.10 Using a combination of real and simulated data, this study determined that Mammostrat was more cost-effective with a cost savings of
$2,268 per patient using a US third-party payer perspective.10 In sensitivity analyses, varying the cost of chemotherapy, recurrence, and mild and severe adverse events still showed cost savings associated with Mammostrat. Varying the proportions of recurrence- free patients still showed cost savings with Mammostrat.
Overall, the findings for the comparative clinical utility and cost-effectiveness are limited by the quantity and scope of studies as one of each type of study was identified. The gene expression assays MammaPrint and Prosignia were not included in either of the studies evaluated in this Rapid Response. The US third-party payer perspective from the cost- effectiveness study limits its external validity to a Canadian population. Further comparative study is warranted.
References
- 1.
- 2.
- 3.
Zanotti L, Bottini A, Rossi C, Generali D, Cappelletti MR. Diagnostic tests based on gene expression profile in breast cancer: from background to clinical use.
Tumour Biol. 2014;35(9):8461–8470. [
PubMed: 25048969]
- 4.
Harbeck N, Sotlar K, Wuerstlein R, Doisneau-Sixou S. Molecular and protein markers for clinical decision making in breast cancer: today and tomorrow.
Cancer Treat Rev. 2014;40(3):434–444. [
PubMed: 24138841]
- 5.
Scope A, Essat M, Pandor A, Rafia R, Ward SE, Wyld L, et al. Gene expression profiling and expanded immunohistochemistry tests to guide selection of chemotherapy regimens in breast cancer management: a systematic review.
Int J Technol Assess Health Care. 2017;33(1):32–45. [
PubMed: 28486999]
- 6.
Gene-expression profiling to guide management of early-stage breast cancer [Internet]. Plymouth Meeting (PA): ECRI Institute; 2016 Jul. [cited 2017 Sep 29]. (ECRIgene). Available from:
www.ecri.org
Subscription required.
- 7.
- 8.
- 9.
Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions [Internet]. Version 5.1.0. London (England): The Cochrane Collaboration; 2011 Mar. Figure 15.5.a: Drummond checklist (Drummond 1996). Available from:
http://handbook-5- 1.cochrane.org/- 10.
Appendix 1. Selection of Included Studies
Appendix 2. Characteristics of Included Publications
Characteristics of Included Clinical Studies
Characteristics of Included Economic Study
Appendix 3. Critical Appraisal of Included Publications
Strengths and Limitations of Randomized Controlled Trials using the Downs and Black Checklist
Strengths and Limitations of Economic Studies using the Drummond Checklist
Appendix 4. Main Study Findings and Author’s Conclusions
Summary of Findings of Included Studies
Appendix 5. Additional References of Potential Interest
The following reference list includes publications that failed to meet the inclusion criteria for this Rapid Response. These publications included study populations that included both node-negative and node-positive breast cancer and did not include a sub-group analysis specific to the node-negative sub-group.
Bartlett JM, Bayani J, Marshall A, Dunn JA, Campbell A, Cunningham C, et al. Comparing breast cancer multiparameter tests in the OPTIMA prelim trial: no test is more equal than the others. J Natl Cancer Inst [Internet]. 2016 Sep [cited 2017 Sep 20];108(9). Available from: https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/djw050
Stein RC, Dunn JA, Bartlett JM, Campbell AF, Marshall A, Hall P, et al. OPTIMA prelim: a randomised feasibility study of personalised care in the treatment of women with early breast cancer. Health Technol Assess [Internet]. 2016 Feb [cited 2017 Sep 19];20(10):xxiii-
xxxix. Available from: https://www.journalslibrary.nihr.ac.uk/hta/hta20100/#/abstract
Marrone M, Stewart A, Dotson WD. Clinical utility of gene-expression profiling in women with early breast cancer: an overview of systematic reviews. Genet Med [Internet]. 2015 Jul [cited 2017 Sep 19];17(7):519-32. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4698966/pdf/nihms-744880.pdf
Meleth S, Reeder-Hayes K, Ashok M, Clark R, Funkhouser W, Wines R, et al. Technology assessment of molecular pathology testing for the estimation of prognosis for common cancers [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 May 29. [cited 2017 Sep 19]. (AHRQ Technology Assessments). Available from: https://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0073303/pdf/PubMedHealth_PMH007330
3.pdf
The following publication was lab-based. It included a relevant population and compared the risk classification for two of the relevant tests but did not include any clinical follow-up with patients.
Alvarado MD, Prasad C, Rothney M, Cherbavaz DB, Sing AP, Baehner FL, et al. A prospective comparison of the 21-gene recurrence score and the PAM50-based Prosigna in estrogen receptor-positive early-stage breast cancer. Adv Ther [Internet]. 2015 Dec [cited 2017 Sep 19];32(12):1237-47. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4679783/pdf/12325_2015_Article_269.pdf
About the Series
Rapid Response Report: Summary with Critical Appraisal
Suggested citation:
Gene expression tests for women with early stage breast cancer: a review of clinical utility and cost-effectiveness. Ottawa: CADTH; 2017 Oct. (CADTH rapid response report: summary with critical appraisal).
Disclaimer: The information in this document is intended to help Canadian health care decision-makers, health care professionals, health systems leaders, and policy-makers make well-informed decisions and thereby improve the quality of health care services. While patients and others may access this document, the document is made available for informational purposes only and no representations or warranties are made with respect to its fitness for any particular purpose. The information in this document should not be used as a substitute for professional medical advice or as a substitute for the application of clinical judgment in respect of the care of a particular patient or other professional judgment in any decision-making process. The Canadian Agency for Drugs and Technologies in Health (CADTH) does not endorse any information, drugs, therapies, treatments, products, processes, or services.
While care has been taken to ensure that the information prepared by CADTH in this document is accurate, complete, and up-to-date as at the applicable date the material was first published by CADTH, CADTH does not make any guarantees to that effect. CADTH does not guarantee and is not responsible for the quality, currency, propriety, accuracy, or reasonableness of any statements, information, or conclusions contained in any third-party materials used in preparing this document. The views and opinions of third parties published in this document do not necessarily state or reflect those of CADTH.
CADTH is not responsible for any errors, omissions, injury, loss, or damage arising from or relating to the use (or misuse) of any information, statements, or conclusions contained in or implied by the contents of this document or any of the source materials.
This document may contain links to third-party websites. CADTH does not have control over the content of such sites. Use of third-party sites is governed by the third-party website owners’ own terms and conditions set out for such sites. CADTH does not make any guarantee with respect to any information contained on such third-party sites and CADTH is not responsible for any injury, loss, or damage suffered as a result of using such third-party sites. CADTH has no responsibility for the collection, use, and disclosure of personal information by third-party sites.
Subject to the aforementioned limitations, the views expressed herein are those of CADTH and do not necessarily represent the views of Canada’s federal, provincial, or territorial governments or any third party supplier of information.
This document is prepared and intended for use in the context of the Canadian health care system. The use of this document outside of Canada is done so at the user’s own risk.
This disclaimer and any questions or matters of any nature arising from or relating to the content or use (or misuse) of this document will be governed by and interpreted in accordance with the laws of the Province of Ontario and the laws of Canada applicable therein, and all proceedings shall be subject to the exclusive jurisdiction of the courts of the Province of Ontario, Canada.