Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
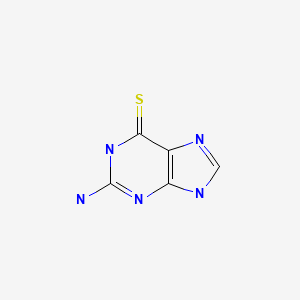
CASRN: 154-42-7
Drug Levels and Effects
Summary of Use during Lactation
Thioguanine nucleosides are active intracellular metabolites of azathioprine and have been measured in breastmilk and in infant serum following maternal use of azathioprine as an immunosuppressant. Thioguanine levels in milk are low after immunosuppressant doses of the drug or of azathioprine. It might be desirable to monitor exclusively breastfed infants with a complete blood count with differential, and liver function tests if thioguanine is used during lactation, although some authors feel that monitoring is unnecessary.[1] Most sources consider the thiopurines acceptable to use in low, immunosuppressant doses.[2-5]
Most sources consider breastfeeding to be contraindicated during maternal antineoplastic drug therapy, although antimetabolites such as thioguanine appear to pose the least risk to breastfed infants.[6] After high-dose thioguanine use in chemotherapy, it might be possible to breastfeed safely during intermittent therapy with an appropriate period of breastfeeding abstinence. Although no data are available to determine an appropriate period to withhold breastfeeding, the drug's terminal half-life suggests that withholding breastfeeding for 4 days may be sufficient. Chemotherapy may adversely affect the normal microbiome and chemical makeup of breastmilk.[7]
Drug Levels
Maternal Levels. Four women receiving an immunomodulator to treat inflammatory bowel disease had metabolite levels measured in milk during the first 6 weeks postpartum. The abstract does not mention the specific drug and dose being taken, but the azathioprine metabolite 6-thioguanine nucleosides (6-TGNs) were measured. Although therapeutic levels were found in maternal serum, 6-TGNs was undetectable (<123 mcg/L) in milk (time of collection not stated).[8]
One woman received thioguanine for inflammatory bowel disease during pregnancy and lactation. She was receiving thioguanine 0.7 mg/kg daily in divided doses to treat colitis. Her breastmilk level at delivery was 48 nanomoles/L (8.03 mcg/L).[9]
Infant Levels. Four infants were breastfed (3 exclusively, 1 rarely received formula) during maternal use of azathioprine orally in dosages of 1.2 to 2.1 mg/kg daily. All of the mothers and infants had the wild type TPMT *1/*1 genotype and all of the mothers had normal enzyme activity. At 3 to 3.5 months of age, all of the infants had undetectable blood levels of 6-TGNs.[10]
Three infants whose mothers were taking azathioprine for inflammatory bowel disease (n = 2) or systemic lupus erythematosus (n = 1) were breastfed during maternal use of azathioprine. Azathioprine doses were 100 mg (plus prednisolone), 150 mg (plus infliximab) and 175 mg daily. In 1 infant, thioguanine was low, but detectable in blood at 3 days of age; at 3 weeks of age, thioguanine was not detectable. In another infant, thioguanine was undetectable at 3 weeks of age. Neither assay limits nor specific maternal doses were stated in the published abstract.[11]
A woman began taking azathioprine 100 mg (1.4 mg/kg) daily for Crohn's disease while breastfeeding (extent not stated) her 3-month-old infant. After 8 days and 3 months of maternal therapy, 6-TGNs were measured, although breastfeeding had been tapered to zero by 3 months. On both occasions, 6-TGNs were not detectable in the blood of the infant. The assay limit was not stated.[1]
Effects in Breastfed Infants
Thioguanine is an active metabolite of azathioprine. Numerous infants breastfed during maternal azathioprine in dosages up to 250 mg daily have been reported with no adverse effects noted. See the Azathioprine record for details.
Ninety-nine women, mostly with Crohn’s disease, who were taking thioguanine were followed during pregnancy and postpartum. In 98 pregnancies, thioguanine was continued during breastfeeding (extent not stated) in 38% of infants. No reported complications were reported in the 37 breastfed infants.[3]
Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
References
- 1.
- Christensen LA, Dahlerup JF, Nielsen MJ, et al. Azathioprine treatment during lactation: Authors' reply. Aliment Pharmacol Ther 2009;30:91. doi:.10.1111/j.1365-2036.2009.03996.x [PubMed: 18761704] [CrossRef]
- 2.
- Sammaritano LR, Bermas BL, Chakravarty EE, et al. 2020 American College of Rheumatology Guideline for the Management of Reproductive Health in Rheumatic and Musculoskeletal Diseases. Arthritis Rheumatol 2020;72:529-56. [PubMed: 32090480]
- 3.
- Crouwel F, Simsek M, de Boer MA, et al. Exposure to thioguanine during 117 pregnancies in women with inflammatory bowel disease. J Crohns Colitis 2023;17:738-45. [PMC free article: PMC10155742] [PubMed: 36521000]
- 4.
- Torres J, Chaparro M, Julsgaard M, et al. European Crohn's and Colitis Guidelines on Sexuality, Fertility, Pregnancy, and Lactation. J Crohns Colitis 2023;17:1-27. [PubMed: 36005814]
- 5.
- Laube R, Selinger CP, Seow CH, et al. Australian inflammatory bowel disease consensus statements for preconception, pregnancy and breast feeding. Gut 2023;72:1040-53. [PubMed: 36944479]
- 6.
- Pistilli B, Bellettini G, Giovannetti E, et al. Chemotherapy, targeted agents, antiemetics and growth-factors in human milk: How should we counsel cancer patients about breastfeeding? Cancer Treat Rev 2013;39:207-11. doi:10.1016/j.ctrv.2012.10.002 [PubMed: 23199900] [CrossRef]
- 7.
- Urbaniak C, McMillan A, Angelini M, et al. Effect of chemotherapy on the microbiota and metabolome of human milk, a case report. Microbiome 2014;2:24. [PMC free article: PMC4109383] [PubMed: 25061513]
- 8.
- Kane SV, Present DH. Metabolites to immunomodulators are not detected in breast milk. Am J Gastroenterol 2004;99 (10 Suppl. S):S246-7. doi:10.1111/j.1572-0241.2004.001_1.x [CrossRef]
- 9.
- Pavlidis P, Ansari A, Duley J, et al. Splitting a therapeutic dose of thioguanine may avoid liver toxicity and be an efficacious treatment for severe inflammatory bowel disease: A 2-center observational cohort study. Inflamm Bowel Dis 2014;20:2239-46. [PubMed: 25230165]
- 10.
- Gardiner SJ, Gearry RB, Roberts RL, et al. Exposure to thiopurine drugs through breast milk is low based on metabolite concentrations in mother-infant pairs. Br J Clin Pharmacol 2006;62:453-6. [PMC free article: PMC1885151] [PubMed: 16995866]
- 11.
- Bernard N, Garayt C, Chol F, et al. Prospective clinical and biological follow-up of three breastfed babies from azathioprine-treated mothers. Fundam Clin Pharmacol 2007;21 (Suppl. 1):62-3. doi:10.1111/j.1472-8206.2007.00481.x [CrossRef]
Substance Identification
Substance Name
Thioguanine
CAS Registry Number
154-42-7
Drug Class
Breast Feeding
Lactation
Milk, Human
Antimetabolites
Antineoplastic Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.