NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
PREFACE
The VA Evidence-based Synthesis Program (ESP) was established in 2007 to provide timely and accurate syntheses of targeted healthcare topics of particular importance to clinicians, managers, and policymakers as they work to improve the health and healthcare of Veterans. QUERI provides funding for four ESP Centers, and each Center has an active University affiliation. Center Directors are recognized leaders in the field of evidence synthesis with close ties to the AHRQ Evidence-based Practice Centers. The ESP is governed by a Steering Committee comprised of participants from VHA Policy, Program, and Operations Offices, VISN leadership, field-based investigators, and others as designated appropriate by QUERI/HSR&D.
The ESP Centers generate evidence syntheses on important clinical practice topics. These reports help:
- Develop clinical policies informed by evidence;
- Implement effective services to improve patient outcomes and to support VA clinical practice guidelines and performance measures; and
- Set the direction for future research to address gaps in clinical knowledge.
The ESP disseminates these reports throughout VA and in the published literature; some evidence syntheses have informed the clinical guidelines of large professional organizations.
The ESP Coordinating Center (ESP CC), located in Portland, Oregon, was created in 2009 to expand the capacity of QUERI/HSR&D and is charged with oversight of national ESP program operations, program development and evaluation, and dissemination efforts. The ESP CC establishes standard operating procedures for the production of evidence synthesis reports; facilitates a national topic nomination, prioritization, and selection process; manages the research portfolio of each Center; facilitates editorial review processes; ensures methodological consistency and quality of products; produces “rapid response evidence briefs” at the request of VHA senior leadership; collaborates with HSR&D Center for Information Dissemination and Education Resources (CIDER) to develop a national dissemination strategy for all ESP products; and interfaces with stakeholders to effectively engage the program.
Comments on this evidence report are welcome and can be sent to Nicole Floyd, ESP CC Program Manager, at vog.av@dyolF.elociN.
EXECUTIVE SUMMARY
Background
The ESP Coordinating Center is responding to a request from the Office of Community Engagement's Center for Compassionate Innovation for an evidence brief on use of near infrared spectroscopy (NIRS) to evaluate patients in nursing homes including VA Community Living Centers for the presence of brain hematomas after falls. Findings from this evidence brief will be used to inform Subject Matter Experts' consideration of NIRS' clinical use and research, as well as program prioritization, in the nursing home setting.
Methods
To identify studies, we searched MEDLINE®, Cochrane Database of Systematic reviews, Cochrane Central Register of Controlled Trials, and other sources up to June 2017. We used prespecified criteria for study selection, data abstraction, and rating internal validity and strength of the evidence. See our PROSPERO protocol for our full methods.
Falls are a common cause of injury among elderly populations, particularly those residing in nursing homes. Most falls in nursing homes occur at the ground level and are low impact. Injuries resulting from these types of falls are variable and range from serious orthopedic and head injury requiring transport to an emergency department (ED) or trauma center to mild injury that can be managed with closer short-term monitoring by nursing staff.1 The American Medical Directors Association (AMDA) Clinical Practice Guideline recommends that facilities have written policies to guide fall management including initial patient evaluation, monitoring for signs of delayed injuries, determination of the circumstances of the fall, and mitigation of risk factors for future falls.2
Initial patient evaluation after falls involves conducting a physical exam to evaluate the extent of injuries, including impaired consciousness as measured by the Glasgow Coma Scale (GCS), and considering baseline risk factors for intracranial hemorrhage, a potential complication of head injury. Identifying patients with moderate-severe injuries in need of transport and head imaging is usually straightforward. However, determining which patients with mild injuries need further evaluation can be more challenging as many elderly patients have pre-existing dementia or other cognitive disorders that impair the physical exam and GCS assessment, as well as higher baseline risk for intracranial hemorrhage due to use of anticoagulants. A further challenge in evaluating patients with possible head injury after falls is that a subset of patients who initially have a normal exam will develop delayed intracranial hemorrhage and could worsen quickly. For these reasons, guidelines on management of mild head injury including those by the American College of Emergency Physicians/Centers for Disease Control and Prevention commonly recommend head computed tomography (CT) for all patients ≥ 65 years old, even with a normal GCS.3
Despite these guidelines, excessive use of CT and implications for patient safety remains a concern due to radiation exposure.4 While the exact rate of normal CT scan findings in nursing home patients presenting after falls is unknown, most patients evaluated for head injury in the ED have normal CTs. A retrospective study using data from the National Hospital Ambulatory Medical Care Survey in the US found that 91% of the approximately 3.9 million head CTs obtained in ED patients to evaluate for head injury in 2009-2010 did not reveal a traumatic intracranial abnormality.5
Near infrared spectroscopy (NIRS) is a diagnostic tool that could be used to evaluate patients after falls and aid in decision-making to avoid unnecessary CTs. NIRS is an imaging technique that identifies intracranial hematomas by detecting asymmetry in light absorption over the right and left sides of the head. Handheld NIRS devices offer a portable, noninvasive, and quick means of evaluating patients for the presence of a brain hematoma. Infrascanner© 2000 is the only commercially available NIRS device in the US and is currently being used by clinical staff at the VA Pittsburgh's Community Living Center (CLC) to evaluate patients with mild injuries after falls when the clinical suspicion for head injury is low. The aim of this evidence brief was to evaluate the potential impact of NIRS as a diagnostic test for hematomas in nursing home patients after falls by synthesizing the evidence on the performance characteristics of NIRS for detecting brain hematoma, its impact on clinical decision-making, patient outcomes, potential harms, and cost-effectiveness.
Ideally, NIRS use would reduce unnecessary CTs and ED visits among nursing home patients who have mild injuries. This potential benefit must be weighed against the potential harm of a negative NIRS scan in a patient who truly has a brain hematoma in need of further intervention. In general, NIRS is not well-suited to identify bilateral hematomas, small hematomas, and deeply located hematomas with a greater distance from the scalp. Some hematomas that would not be detected by NIRS, but would be identified by CT, are unlikely to cause symptoms or a further change in patient's function. Other small hematomas could expand over time, causing symptoms and functional decline, and these hematomas would be important not to miss. In addition to the potential for missing a clinically important hematoma, there could be other unintended consequences of NIRS use. For example, fewer ED transfers could place additional strain on nursing home staff due to the need to monitor patients more frequently. Fewer ED transfers could also represent missed opportunities to identify reasons for patient falls if they do not undergo more extensive testing. The ideal study of NIRS would aim to capture not only the false positive rate, but these other potential harms.
Unfortunately, studies of NIRS to date have almost exclusively focused on demonstrating the technical feasibility of the device and its diagnostic accuracy in series of patients who are referred for a CT scan. These studies therefore provide little insight on how use of NIRS impacts clinical decision-making, patient outcomes, and healthcare utilization. Moreover, it is unknown how NIRS performs in older nursing home patients with mild injury after falls because this type of use has not been studied. Only one study of Infranscanner© 2000 has been conducted in patients with mostly mild injuries, and in that observational study of 85 patients presenting to a neurosurgical center with predominantly mild head injury (85% with GCS 12-15), Infrascanner© 2000 failed to identify 2 hematomas among 43 patients with hematomas on CT.6 Patients had a mean age of 48, high prevalence of hematomas (53%), and unreported baseline risk factors. The findings of this study are not necessarily predictive of how NIRS would perform in nursing homes with a different makeup of patients and overall lower prevalence of hematomas.
Given concerns about overuse of CT and the potential benefits of NIRS as a diagnostic tool in nursing home patients with mild injuries after falls, it would be reasonable to consider implementation of a NIRS protocol in a pilot study among VA CLCs. A pilot could provide reliable estimates of CTs and ED transfers averted. However, because positive CT scans are rare in this situation, a much larger study (or decision modeling) would be needed to assess the frequency and clinical consequences of false negative NIRS scans.
INTRODUCTION
PURPOSE
The ESP Coordinating Center (ESP CC) is responding to a request from the Office of Community Engagement's (OCE) Center for Compassionate Innovation (CCI) for an evidence brief on use of near infrared spectroscopy (NIRS) to evaluate patients in nursing homes including VA Community Living Centers (CLCs) for the presence of brain hematomas after falls. Findings from this evidence brief will be used to inform Subject Matter Experts' consideration of NIRS' clinical use and research, as well as program prioritization, in the nursing home setting.
BACKGROUND
Falls are a common cause of injury among elderly populations, particularly those residing in nursing homes. More than 60% of nursing home residents experience at least one fall per year, making a reduction in falls a focus of prevention efforts.1 Most falls in nursing homes occur at the ground level and are low impact. A prospective study of fall rates and characteristics among 528 nursing homes in Germany found that 41% of falls occurred during transfers and 36% occurred while walking.7 Injuries resulting from these types of falls are variable and range from serious orthopedic and head injury requiring transport to an emergency department (ED) or trauma center to mild injury that can be managed with closer short-term monitoring by nursing staff.1 The American Medical Directors Association (AMDA) Clinical Practice Guideline recommends that facilities have written policies to guide fall management including initial patient evaluation, monitoring for signs of delayed injuries, determination of the circumstances of the fall, and mitigation of risk factors for future falls.2
Management of moderate-severe injuries after falls is usually straightforward and most patients will be transported to an ED or trauma center. Management can be more challenging in patients who have mild injuries, particularly when the circumstances of the fall are unknown and patients cannot provide additional history or participate in the physical exam due to pre-existing dementia or other cognitive disorders. Clinicians may struggle with deciding which patients are appropriate to monitor and which patients are at risk of serious head injury and need to be transported to an ED to undergo head imaging with computed tomography (CT). A further challenge in evaluating patients with possible head injury after falls is that subset of patients who initially have a normal exam will develop delayed intracranial injuries and could worsen quickly. Intracranial hemorrhage, bleeding in the layers of tissue between the skull and the brain or within the brain itself, can occur at the time of injury or may be delayed by several hours or days.
Since CT came into widespread use, uncertainty has existed regarding which patients with mild head injury should undergo imaging, and this uncertainty led to efforts to define which clinical findings predict intracranial lesions.8 The Glasgow Coma Scale (GCS) is a widely used measure of impaired consciousness based on eye opening and verbal and motor responses that is used to assess the extent of traumatic brain injury (TBI) and is included in most decision-making tools regarding CT use in patients with head injury. Patients are classified has having mild injury (GCS ≥ 13), moderate injury (GCS 9-12), or severe injury (GCS 3-8). In an observational study of patients with minor head injury and normal GCS, Haydel et al8 found that patients with headache, vomiting, age ≥ 60 years, drug or alcohol intoxication, deficits in short-term memory, physical evidence of trauma above the clavicles, and seizure should undergo head CT, as these clinical findings predicted intracranial lesions. According to guidelines regarding CT use in patients with mild TBI, including guidelines by the American College of Emergency Physicians/Centers for Disease Control and Prevention, patients age ≥ 65 years should undergo CT even with a normal GCS.3
However, development of guidelines has not settled the controversy regarding which patients with mild head injury should undergo CT. Concern still exists regarding excessive use of CT and implications for patient safety due to radiation exposure.4 The radiation exposure associated with a head CT is equivalent to the radiation dose of 30 chest x-rays.9 While the exact rate of normal CT scan findings (and therefore unnecessary radiation exposure) in nursing home patients presenting after falls is unknown, the majority of patients evaluated for all types of TBI in the ED have normal CTs. A retrospective study using data from the National Hospital Ambulatory Medical Care Survey in the US found that 91% of the approximately 3.9 million head CTs obtained in ED patients to evaluate for TBI in 2009-2010 did not reveal a traumatic intracranial abnormality.5 A retrospective study of patients ≥ 75 years old with mostly normal GCS (95%) who underwent CTs in an ED in Poland found that 97.4% of 116 patients with low-energy accidents (mostly ground-level falls) had negative CT scans.10
Several new technologies are being developed to aid in decisions regarding which patients with mild head injuries should have CT scans. For example, the US Army has evaluated the BrainScope One, a handheld, non-invasive electroencephalogram-based device, which has been incorporated into a decision aid that pairs clinical risk factors with test results to predict the likelihood of intracranical injuries visible on CT.11 Developers of BrainScope One reported that use of this tool would have led to a 33% reduction in unnecessary CTs based on retrospective analysis of 564 patients with mild TBI.11 In this study, BrainScope One was negative in 7.7% of cases in which CT was positive, but Huff et al11 note that none of these patients developed worsening symptoms or required neurosurgical intervention.1 Other emerging diagnostic tools include protein S100B, thought to be a biomarker for neurologic injury,12 and artificial neural networks, mathematical models theorized to predict CT findings in older patients with mild TBI.13
Near infrared spectroscopy (NIRS) is another example of a tool that could be used to reduce unnecessary CTs. NIRS is an imaging technique that identifies intracranial hematomas based on the different light-absorbing properties of hemoglobin in extravascular compared to intravascular blood. When more extravascular blood is present, as in the case of a hematoma, the local concentration of hemoglobin is greater and leads to higher light absorption.14 NIRS works by comparing light absorption over the right and left sides of the head, with asymmetric findings suggesting the presence of a hematoma (measured as the change in optical density). Handheld NIRS devices provide results within minutes, require minimal staff training, and do not expose patients to radiation. Because they are portable, handheld NIRS devices can be used in multiple settings, including nursing homes, and can be used repeatedly to monitor patients after falls without harms associated with the scan itself. Proponents of NIRS suggest that it could be integrated into patient assessment after falls in the case of mild injuries when the clinical suspicion for a hematoma is low to provide reassurance to patients and clinicians that a hematoma is not present and thereby prevent unnecessary ED visits and CTs. NIRS has not been intended to be used as a substitute for clinical judgement or in nursing home patients with moderate-severe injuries who require transport for acute management.
Infrascanner© 2000 (developed by Infrascan Inc. with sponsorship from the US Office of Naval Research and the US Marine Corps) is the only commercially available handheld NIRS device in the US and obtained Food and Drug Administration (FDA) clearance as a Class II medical device in 2012.15 Infrascanner© 1000, an earlier model of Infrascanner© 2000, obtained FDA clearance in 2010. Infrascanner© 2000 is also marketed and sold in Europe. The cost of the device, as noted by Dr. Oldershaw (phone conversation, August 2017), may vary by health system, but is approximately $9500 (including the device itself and a set of disposable shields that form contact with the patient). A second handheld NIRS device, Crainscan© (developed by BYTech Inc.) is also commercially available in Europe. The devices work by the same principle with some differences in methods. Specifically, Infrascanner© 2000 and Crainscan© emit slightly different wavelengths of near infrared light (both are close to the ideal 805 nanometer wavelength to detect blood) and use different types of sensors placed at the scalp.16 Infrascanner© uses two 1.6mm optical fibers that form narrow contact with the scalp after maneuvering around hair follicles, while Crainscan© uses a wider detector that is placed directly over hair.16,17 No studies have compared the effectiveness of the 2 devices. Additional NIRS devices that have been developed for detection of brain hematomas include the Runman, which was an earlier prototype of Infrascanner©, Smartscan, and a research device developed by Francis et al.18
Currently available NIRS devices have inherent limitations in their ability to detect brain hematomas due to the nature of the technology itself. Because NIRS works by comparing light absorption of blood on the 2 sides of the head, results can be falsely negative in patients with bilateral hematomas. NIRS results may also be falsely negative in the cases of chronic subdural hematomas, because the absorption properties of blood change over time and can become less detectable by NIRS.14 The NIRS signal is also susceptible to confounding from structures that alter the path of light such as hair, scalp, and bone.16,19 Improving accuracy in this regard has been the focus of NIRS device development over time, and it is the rationale behind some of the differences in detection methods between Infrascanner© and Crainscan©. Specifically, Infrascan Inc. states that its design reduces the effect of hair color on scan results.16 The presence of scalp hematomas may lead to false positive results, although in many cases scalp hematomas may be detected by physical exam and avoided so that they do not interfere with NIRS measurements.20 In general, NIRS is best suited to detect larger hematomas and those closer to the brain surface. Infrascanner© can detect hematomas ≥ 3.5 ml and within 2.5 cm of the brain surface.14
Despite these limitations, Infrascanner© 2000 and Crainscan© are already being used in clinical practice in the US and Europe. In addition to use as an initial diagnostic test for brain hematomas after TBI, serial NIRS scans have been used to monitor for the development of delayed hematomas in hospitalized TBI patients. Other types of NIRS devices are used to monitor cerebral autoregulation and oxygenation in the hospital setting.19 Infrascanner© 2000 is currently being used by clinical staff at the VA Pittsburgh's CLC to evaluate patients with mild injuries after falls when the suspicion for head injury is low. In a comparison of patient fall rates and ED transfers in July 2014 and July 2015, 41% fewer patients were transferred after falls (19 out of 33 in July 2014 compared to 5 out of 30 in July 2015) with use of Infrascanner©, according to Dr. Oldershaw (phone conversation, August 2017). However, in interpreting these results, it is important to keep in mind that reduced ED use (and presumably reduced CT use) does not take into account the frequency or consequences of false negative NIRS test results. The false negative rate cannot be calculated from this experience because patients who had negative NIRS results did not get CT scans or receive long-term follow-up.2
An ideal study of NIRS would observe nursing home patients with mild injuries and evaluate how use of NIRS changes clinical decision-making. Specifically, it would measure the positive effects (fewer ED visits and CT scans) as well as the negative effects (missed hematomas and their consequences). Unfortunately, no study has evaluated the use of NIRS in this population and setting or examined its effect on decisions. For this reason, we posed broad key questions and included studies in other settings. While these studies may have some useful information, it is critical to keep in mind the limitations of generalizing the results of these studies to a different population and setting.
The aim of this evidence brief is to evaluate the potential impact of NIRS use among nursing home patients after falls by describing the technical feasibility of NIRS for detecting brain hematomas and synthesizing the evidence on the performance characteristics of NIRS, and its impact on diagnostic and therapeutic decision-making, patient outcomes, potential harms, and cost-effectiveness.
KEY QUESTIONS
KQ1: What are the performance characteristics of NIRS as a diagnostic test for patients with mild TBI suspected of having a brain hematoma or with risk factors for brain hematoma?
KQ2: What is the evidence that use of NIRS impacts diagnostic and/or therapeutic decision-making for patients suspected of having a brain hematoma or with risk factors for brain hematoma?
KQ3: What is the evidence that use of NIRS to detect brain hematomas impacts patient outcomes?
KQ4: What are potential adverse effects and unintended consequences of using NIRS for detection of brain hematoma?
KQ5: What is the cost-effectiveness of using NIRS for detecting brain hematoma?
KQ6: Do the effects of NIRS vary by patient population or setting?
ELIGIBILITY CRITERIA
The ESP included studies that met the following criteria:
- Population: Adults (≥ 18 years)
- Intervention: Near infrared spectroscopy (NIRS) device
- Comparator: Any (CT scan, MRI scan, etc)
- Outcomes:
- Diagnostic test performance: Sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV)
- Diagnostic impact: Earlier diagnosis or reduced need for additional diagnostic tests
- Therapeutic impact: Improved triage or earlier treatment
- Patient outcome impact: Morbidity, mortality, patient satisfaction, healthcare utilization, or quality of life
- Adverse effects or unintended consequences: Any
- Cost-effectiveness: Any
- Timing: Any
- Setting: Any
METHODS
To identify articles relevant to the key questions, our research associate searched MEDLINE®, Cochrane Database of Systematic reviews, Cochrane Central Register of Controlled Trials, PsycINFO, Health Technology Assessment, Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database, CINAHL, Military & Government Collection, and Scopus up to 6/30/2017, using terms for hematoma, hemorrhage, brain injury, near infrared, and spectroscopy; additional citations were identified from grey literature sources, hand-searching reference lists, and consultation with content experts (see Supplemental Materials Appendix A for complete search strategies). We limited the search to published and indexed articles involving human subjects available in the English language. We also contacted Infrascan, Inc. and BYTech Inc. with requests for scientific information. Study selection was based on eligibility criteria described above. Titles, abstracts, and full-text articles were sequentially reviewed by 2 investigators and checked by a third. All disagreements were resolved by consensus.
We used predefined criteria to rate the internal validity of all included studies. We used the Cochrane ROBIS tool to rate the internal validity of systematic reviews.21 We used QUADAS-2 tool to rate the internal validity of diagnostic accuracy studies.22 We abstracted data from all studies for prespecified study and patient characteristics of interest and results for each included outcome. For studies that did not report NIRS performance characteristics, we calculated sensitivity, specificity, PPV, and NPV when data on NIRS and CT results were available. For studies that reported NIRS performance characteristics for a subset of hematoma types, we performed additional calculations for all hematoma types when possible. All data abstraction and internal validity ratings were sequentially reviewed by 2 investigators and checked by a third. All disagreements were resolved by consensus.
We graded the strength of the evidence for Infrascanner© 2000 and Crainscan© (the 2 NIRS handheld devices that are currently commercially available) sensitivity and specificity based on the AHRQ Methods Guide for Comparative Effectiveness Reviews.23 This approach incorporates 4 key domains: risk of bias (includes study design and aggregate quality), consistency, directness, and precision of the evidence. It also considers other optional domains that may be relevant for some scenarios, such as a dose-response association, plausible confounding that would decrease the observed effect, strength of association (magnitude of effect), and publication bias. Strength of evidence is graded for each key outcome measure and ratings range from high to insufficient, reflecting our confidence that the evidence reflects the true effect.
Where studies were appropriately homogenous, we synthesized outcome data quantitatively Microsoft® Excel® for Windows (2016) to estimate pooled effects. Where meta-analysis was not suitable due to limited data or heterogeneity, we synthesized the evidence qualitatively.
A draft version of this report was reviewed by technical experts selected to represent relevant specialties including radiology, NIRS device development, and systematic review methodology. Their comments and our responses are available in Appendix F in the supplemental materials.
The complete description of our full methods can be found on the PROSPERO international prospective register of systematic reviews (http://www.crd.york.ac.uk/PROSPERO/; registration number CRD42017071444).
RESULTS
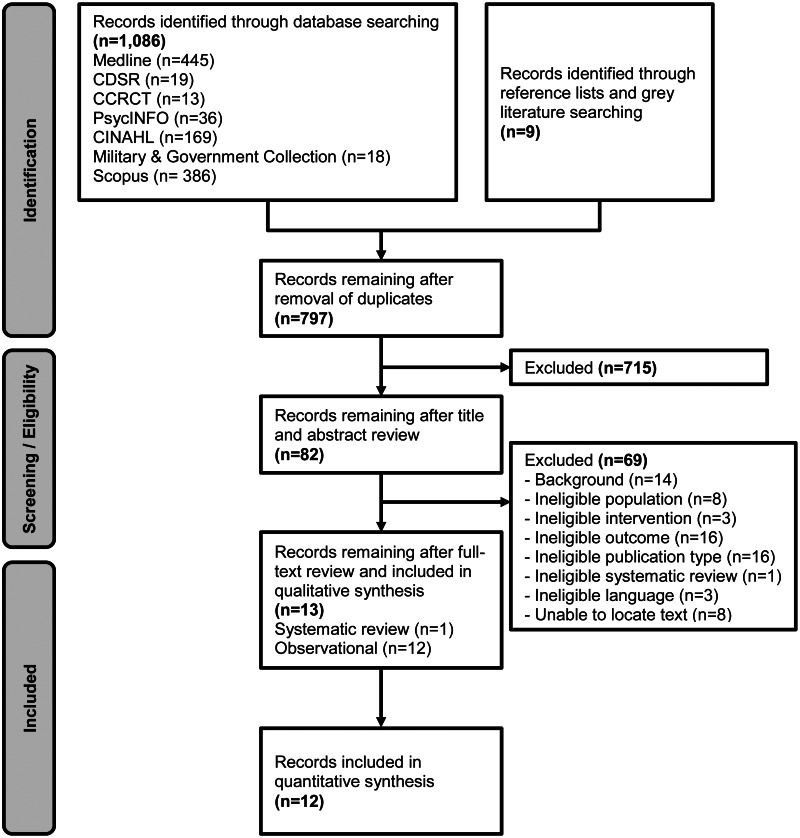
The literature flow diagram (Figure 1) summarizes the results of the search and study selection processes.
LITERATURE OVERVIEW
Searches resulted in 797 unique potentially relevant articles. We included 12 observational diagnostic accuracy studies (see Supplemental Materials Appendix B for list of excluded studies),6,14,18,20,24-31 with a median sample size of 89 (range 19-365) and a total population of 1,364 individuals. We identified a 2017 systematic review by Brogan et al16 that included 8 diagnostic accuracy studies on NIRS. Brogan et al16 included only 6 (50%) of the studies included in our review.14,18,20,26,29,30 Notably, the review by Brogan et. al.16 did not include the newest studies of Infrascanner© 20006,31 and Infrascanner© 100024 that were published after their search end date of 1/1/2015. Also, the review by Brogan et al16 included 2 additional studies in pediatric populations32,33 that were outside our scope. We received a scientific information packet from Infrascan, Inc. which did not include studies that we had not otherwise identified in our search.
Table 1 presents the characteristics of the 12 primary studies (see Supplemental Materials Appendix C for full evidence tables). Mean patient age for all studies was ≤ 60 years old. Most studies (67%) did not report on gender, but those that did included 51-83% male patients. Although skin and hair color are potential confounders of the NIRS signal, most studies (67%) did not report this information. Most patients had a history of TBI or suspected TBI and had been referred for CT scan. In Hennes et al,28 patients were already diagnosed with a subdural hematoma and in Kahraman et al,29 patients were already diagnosed with an epidural or subdural hematoma.28,29 2 studies included a control population of healthy individuals or those with minor injuries.6,29 Aside from Hennes et al,28 which only included patients with diagnosed hematomas, the prevalence of any type of hematoma ranged from 5-59%.
Table 1
Characteristics of Included Studies.
Severity of signs and symptoms at presentation was reported in 50% of studies. In 5 of the 6 studies that reported severity measures at presentation, most patients had a GCS score close to or ≥ 13, consistent with mild injury.6,14,20,24,30 In contrast, patients included in Peters et al31 had a mean GCS of 6.9, consistent with severe injury.
Studies varied in the type of handheld NIRS device used. Six studied used Infrascanner© 1000 or 2000 and 2 additional studies used the RunMan device. 6,14,20,24,25,27,28,31Three studies used Crainscan© or Smartscan.26,29,30 One study used a device developed by the study authors.18
No studies were conducted in elderly nursing homes patients with low clinical suspicion for hematoma after a fall. Only one study was conducted in the US (multicenter study conducted in the US and India by Robertson et al14). Most studies (83%) occurred in the emergency department or hospital setting. One study was conducted in the pre-hospital setting.31 Most studies (83%) were single-center.
Risk of bias was unclear in the majority of studies (67%) due to uncertainty regarding how patient selection was conducted and whether radiologists interpreting CTs were blinded to NIRS results. Risk of bias was low in the remainder of studies. Figure 2 shows the details of the risk of bias assessment.

Figure 2
Summary of QUADAS-2 Assessment.
KQ1. What are the performance characteristics of NIRS as a diagnostic test for patients suspected of having a brain hematoma or with risk factors for brain hematoma?
Performance characteristics are shown by study in Table 2 and by device in Figures 3 and 4 (see Supplemental Materials Appendices D and E for full details). 2 studies of Infrascanner© 2000,4,5 have the most practical application because Infrascanner© 2000 is the only commercially available NIRS handheld device in the US. In patients with predominantly mild TBI referred to a neurosurgery center, Infrascanner© 2000 correctly identified hematomas in 96% of patients (sensitivity) and correctly ruled out hematomas in 93% (specificity).6 Infrascanner© 2000 failed to identify 2 hematomas among 43 patients with hematomas on CT scans (false negatives).6 But, given that patients in this study had a mean age of 48, high prevalence of hematomas (53%), and unreported baseline risk factors, findings of this study are not necessarily predictive of how NIRS would perform in nursing homes with a different makeup of patients and overall lower prevalence of hematomas. When used in patients with predominantly mild TBI, CrainScan©30 and Infrascanner© 100014,20,24 correctly identified and ruled out hematomas at rates ranging from 68% to 89% (sensitivity) and 67% to 95% (specificity), respectively. NIRS' accuracy in correctly identifying and ruling out hematomas also ranged widely in patients with severe TBI31 and when used in patients already diagnosed with hematoma28. Collectively, these findings provide little insight on how NIRS would perform in elderly nursing home patients with mild injuries.
Table 2
Performance Characteristics.

Figure 3
NIRS Sensitivity by Device.

Figure 4
NIRS Specificity by Device.
Across studies, reasons cited for false negatives included bilateral hematomas, small hematomas below the NIRS detection threshold, deeply located hematomas with greater distance from the scalp, patient agitation or motion, and difficulty completing NIRS examination in transport.14,18,20,27-31
KQ2. What is the evidence that use of NIRS impacts diagnostic and/or therapeutic decision-making for patients suspected of having a brain hematoma or with risk factors for brain hematoma?
The majority of studies (92%) did not report the impact of NIRS on clinical decisions. In one study of patients with severe injuries (mean GCS 6.9) evaluated in the pre-hospital setting, one patient received 10% hypertonic saline as an addition to initial treatment based on the results of a NIRS scan.31 In this study, patients underwent NIRS prior to or during transport to the hospital and use of NIRS did not lead to decisions to transport any patient to a different location than was originally intended.
KQ3. What is the evidence that use of NIRS to detect brain hematomas impacts patient outcomes?
The majority of studies (92%) did not report the impact of NIRS on patient outcomes. One study of Infrascanner© 1000 use in ED patients with mild TBI (GCS ≥13) in Turkey found that in-hospital outcome was not significantly related to use of NIRS (p = 0.905).24
KQ4. What are potential adverse effects and unintended consequences of using NIRS for detection of brain hematoma?
No studies identified harms associated with the process of undergoing a NIRS scan. The scan itself is noninvasive and relatively quick to perform. Reported times to complete NIRS scans ranged from 2-4 minutes in studies of Infrascanner© 1000 and 2000 and did not interfere with delivery of usual care.14,24,31 Potential adverse effects of using NIRS are related to the possibility of false negative and false positive results as a function of the performance characteristics discussed above, as well as potential unintended consequences of transporting fewer patients to EDs.
KQ5. What is the cost-effectiveness of using NIRS for detecting brain hematoma?
No studies reported on the cost-effectiveness of NIRS. Several studies described NIRS as low cost, but did not include actual cost information.18,24,26
KQ6. Do the effects of NIRS vary by patient population or setting?
The available evidence does not demonstrate whether the effects of NIRS vary by patient population due to limited reporting of patient baseline characteristics and injury severity. Most studies took place in the ED or hospital setting, limiting comparisons of NIRS across settings.
SUMMARY AND CLINICAL IMPLICATIONS
We found no studies that evaluated the use of NIRS among nursing home patients after falls to avoid unnecessary CTs. Instead, studies of NIRS have almost exclusively focused on demonstrating the technical feasibility of the device and its performance characteristics, providing little insight on how the use of NIRS impacts clinical decision-making, patient outcomes, and healthcare utilization. Moreover, studies of NIRS have mostly been in patients ≤60 years old in the ED or hospital setting, with largely unreported disease severity and baseline risk factors for intracranial hemorrhage, thereby limiting applicability of findings to older patients in nursing homes.
Given that the nominator's highest priority was to evaluate the clinical impact of NIRS in nursing home patients after falls, we acknowledge that we excluded studies on the development of NIRS as a diagnostic test that were not designed to evaluate NIRS performance characteristics or other clinical outcomes. While the earliest studies of NIRS have value in terms of understanding the principles of NIRS and how NIRS measurements (change in optical density) compare to CT findings, studies that did not define when a NIRS scan was considered positive or negative (ie, state a cut-off point for the change in optical density that indicates a positive scan) or report performance characteristics have lower clinical relevance.
Early studies34-38 on RunMan are examples in which the authors did not state how they defined a positive NIRS scan, thereby limiting the conclusions that can be drawn from these studies about the performance characteristics of NIRS as a diagnostic test. Given more time, we could request and analyze raw individual patient data from these studies34-38 to derive additional sensitivity and specificity data based on published detection thresholds for Crainscan/Smartscan and Infrascanner. However, additional data on performance characteristics would still not address the most significant limitation of the current literature base – the lack of outcomes related to the impact of NIRS on clinical decision-making and healthcare utilization including use of CT.
We also acknowledge that we excluded studies35-38 evaluating the use of serial NIRS scans in hospitalized TBI patients in order to detect late-onset hematomas. However, using serial NIRS to monitor patients who are hospitalized for head injury and/or have already been diagnosed with a hematoma or undergone surgery is a distinct clinical scenario with different reference tests (such as intracranial pressure monitoring) that has limited applicability to the nominator's priority population.
Potential harms of NIRS as a test for hematomas in nursing home patients after falls are primarily related to the possibility of obtaining a negative NIRS scan in a patient who truly has a hematoma in need of further intervention. Across studies, reasons cited for false negative results reflect known limitations of NIRS as an imaging technique. In general, currently available NIRS devices are not well-suited to identify bilateral hematomas, small hematomas, and deeply located hematomas with a greater distance from the scalp. It is unknown how many patients could be harmed by a false negative NIRS scan if NIRS is used to evaluate nursing home patients with mild injury after falls. Some small hematomas that would not be detected by NIRS, but would be identified by CT, are unlikely to cause symptoms or a further change in patient's function. Other small hematomas could expand over time causing symptoms and functional decline, and these hematomas would be important not to miss. In addition, there could be unintended consequences of fewer ED transfers, including strain on nursing home staff due to more frequent monitoring of patients as well as missed opportunities to identify reasons for patient falls if they do not undergo more extensive testing in the ED.
LIMITATIONS
The evidence base included in this review has several important limitations. First, inconsistent reporting of patients' baseline characteristics and disease severity at presentation limits conclusions regarding NIRS performance among different patient populations. Second, mean patient age for all studies was ≤ 60 years old and findings could therefore have limited applicability for elderly populations. Third, most studies were conducted in the ED or hospital settings rather than in a nursing home or other pre-hospital settings. Fourth, while the use of serial NIRS scans has been evaluated in hospitalized TBI patients including those who have already been diagnosed with hematomas or undergone surgery, the use of serial NIRS scans has not been evaluated in patients with mild injuries in the pre-hospital setting. Finally, the most significant limitation of the current literature base is a lack of outcomes related to clinical decision-making and impact of NIRS on healthcare utilization including use of CT.
In terms of our review methods, limitations include our literature search with exclusion of non-English studies, our use of second-reviewer checking in lieu of dual independent review, and our scope. Given our focus on use of NIRS among nursing home patients after falls, our results may have limited applicability to the use of NIRS in other patient populations and clinical scenarios.
FUTURE RESEARCH
Given concerns about overuse of CT and the potential benefits of NIRS as a diagnostic tool in nursing home patients with mild injuries after falls, it would be reasonable to consider implementation of a NIRS protocol in a pilot study among VA CLCs. A pilot could provide reliable estimates of CTs and ED transfers averted. However, because positive CT scans are rare in this situation, a much larger study (or decision modeling) would be needed to assess the frequency and clinical consequences of false negative NIRS scans.3 In addition to evaluating the use of a single NIRS scan as diagnostic tool at the time of injury, future studies should evaluate the performance of serial NIRS scans for monitoring nursing home patients after falls who are not transferred to the ED. NIRS can be performed repeatedly on the same patient without exposure to radiation or harms associated with the scan itself and results from a series of scans may prove to be more clinically useful than a single scan. VA Pittsburgh's experience using Infrascanner© 2000 could provide guidance for other facilities to develop clinical policies regarding NIRS.
To avoid some of the methodologic limitations of existing studies of NIRS, future studies should: a) report patients' baseline characteristics including age, risk factors for intracranial hemorrhage including use of anticoagulants, and degree of injury after falls including GCS scores, b) ensure blinding of radiologists reading CTs to NIRS results, and c) include only completed NIRS scans in results (thereby limiting the potential for over-reporting of false negatives).
Another gap in the literature is better characterization of how many elderly patients with mild injuries after falls undergo CTs that are negative and therefore could have been avoided. Quantifying the rate of unnecessary CT use could strengthen the rationale for the use of NIRS as a tool to aid clinical decision-making for nursing home patients after falls.
CONCLUSIONS
Handheld NIRS devices offer a portable, noninvasive, and quick means of evaluating patients for the presence or absence of a brain hematoma with certain caveats, including the inability of NIRS to detect bilateral findings. NIRS has the potential to aid clinical decision-making in nursing home patients after falls when the need for head imaging is unclear. However, studies of NIRS to date have not been designed to evaluate the impact of NIRS in this clinical scenario.
REFERENCES
- 1.
- Gruneir A, Mor V. Nursing Home Safety: Current Issues and Barriers to Improvement. Annual Review of Public Health. 2008;29(1):369-382. [PubMed: 18173385]
- 2.
- Vance J. The clinical practice guideline for falls and fall risk. Translational Behavioral Medicine. 2012;2(2):241-243. [PMC free article: PMC3717891] [PubMed: 24073116]
- 3.
- Tavender EJ, Bosch M, Green S, et al. Quality and consistency of guidelines for the management of mild traumatic brain injury in the emergency department. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2011;18(8):880-889. [PubMed: 21843224]
- 4.
- Sierzenski PR, Linton OW, Amis ES, et al. Applications of justification and optimization in medical imaging: examples of clinical guidance for computed tomography use in emergency medicine. Journal of the American College of Radiology. 2014;11(1):36-44. [PubMed: 24135540]
- 5.
- Korley9 FK, Kelen GD, Jones CM, Diaz-Arrastia R. Emergency department evaluation of traumatic brain injury in the United States, 2009–2010. The Journal of Head Trauma Rehabilitation. 2016;31(6):379-387. [PMC free article: PMC4786477] [PubMed: 26360006]
- 6.
- Xu L, Tao X, Liu W, et al. Portable near-infrared rapid detection of intracranial hemorrhage in Chinese population. J Clin Neurosci. 2017;26. [PubMed: 28279553]
- 7.
- Rapp K, Becker C, Cameron ID, König H-H, Büchele G. Epidemiology of falls in residential aged care: analysis of more than 70,000 falls from residents of bavarian nursing homes. Journal of the American Medical Directors Association. 2012;13(2):187. e181-187. e186. [PubMed: 21816682]
- 8.
- Haydel MJ, Preston CA, Mills TJ, Luber S, Blaudeau E, DeBlieux PM. Indications for computed tomography in patients with minor head injury. New England Journal of Medicine. 2000;343(2):100-105. [PubMed: 10891517]
- 9.
- Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Archives of Internal Medicine. 2009;169(22):2078-2086. [PMC free article: PMC4635397] [PubMed: 20008690]
- 10.
- Timler D, Dworzynski MJ, Szarpak L, Gaszynska E, Dudek K, Galazkowski R. Head Trauma in Elderly Patients: Mechanisms of Injuries and CT Findings. Advances in clinical and experimental medicine : official organ Wroclaw Medical University. 2015;24(6):1045-1050. [PubMed: 26771978]
- 11.
- Huff JS, Naunheim R, Ghosh Dastidar S, Bazarian J, Michelson EA. Referrals for CT scans in mild TBI patients can be aided by the use of a brain electrical activity biomarker. Am J Emerg Med. 2017. [PubMed: 28579138]
- 12.
- Calcagnile O, Undén L, Undén J. Clinical validation of S100B use in management of mild head injury. BMC Emergency Medicine. 2012;12(1):13. [PMC free article: PMC3527238] [PubMed: 23102492]
- 13.
- Dusenberry MW, Brown CK, Brewer KL. Artificial neural networks: Predicting head CT findings in elderly patients presenting with minor head injury after a fall. The American Journal of Emergency Medicine. 2017;35(2):260-267. [PubMed: 27876174]
- 14.
- Robertson CS, Zager EL, Narayan RK, et al. Clinical evaluation of a portable near-infrared device for detection of traumatic intracranial hematomas. Journal of Neurotrauma. 2010;27(9):1597-1604. [PubMed: 20568959]
- 15.
- Food Drug Administration HHS. Medical devices; neurological devices; classification of the Near Infrared Brain Hematoma Detector. Final rule. Fed Regist. 2012;77(57):16925-16927. [PubMed: 22479734]
- 16.
- Brogan RJ, Kontojannis V, Garara B, Marcus HJ, Wilson MH. Near-infrared spectroscopy (NIRS) to detect traumatic intracranial haematoma: A systematic review and meta-analysis. Brain Inj. 2017;31(5):581-588. [PubMed: 28440675]
- 17.
- Infrascanner - White Paper: A Handheld Brain Hematoma Detector. In: Inc. I, ed2014.
- 18.
- Francis SV, Ravindran G, Visvanathan K, Ganapathy K. Screening for unilateral intracranial abnormalities using near infrared spectroscopy: a preliminary report. J Clin Neurosci. 2005;12(3):291-295. [PubMed: 15851084]
- 19.
- Sen AN, Gopinath SP, Robertson CS. Clinical application of near-infrared spectroscopy in patients with traumatic brain injury: a review of the progress of the field. Neurophotonics. 2016;3(3):031409. [PMC free article: PMC4874161] [PubMed: 27226973]
- 20.
- Leon-Carrion J, Dominguez-Roldan JM, Leon-Dominguez U, Murillo-Cabezas F. The Infrascanner, a handheld device for screening in situ for the presence of brain haematomas. Brain Inj. 2010;24(10):1193-1201. [PubMed: 20715889]
- 21.
- Whiting P, Savović J, Higgins JP, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. J Clin Epidemiol. 2016;69:225-234. [PMC free article: PMC4687950] [PubMed: 26092286]
- 22.
- Whiting PF, Rutjes AW, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155(8):529-536. [PubMed: 22007046]
- 23.
- Berkman ND, Lohr KN, Ansari M, et al. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville MD: Agency for Healthcare Research and Quality;2013. [PubMed: 24404627]
- 24.
- Akyol PY, Bayram B, Acerer A, et al. Comparison of near-infrared spectroscopy and head CT interpretations of the ED patients with minor head injury. Am J Emerg Med. 2016;34(8):1364-1368. [PubMed: 27133531]
- 25.
- Coskun F, Sezer EA, Karamercan MA, Akinci E, Vural K. An assessment on the use of infrascanner for the diagnosis of the brain hemotoma by using support vector machine. Scientific Research and Essays. 2010;5(14):1911-1915.
- 26.
- Ghalenoui H, Saidi H, Azar M, Yahyavi ST, Borghei Razavi H, Khalatbari M. Near-infrared laser spectroscopy as a screening tool for detecting hematoma in patients with head trauma. Prehospital Disaster Med. 2008;23(6):558-561. [PubMed: 19557974]
- 27.
- Hennes HJ, Lott C, Windirsch M, et al. Non invasive detection of intracerebral hemorrhage using near infrared spectroscopy (NIRS). SPIE. 1997;3194:42-54.
- 28.
- Hennes HJ, Richter B, Lott C, Dick W, Boor S, Hanley DF. Follow-up in patients with subdural haematomas using near infrared spectroscopy (NIRS). Proc SPIE Int Soc Opt Eng. 1999;3566:182-192.
- 29.
- Kahraman S, Kayali H, Atabey C, Acar F, Gocmen S. The accuracy of near-infrared spectroscopy in detection of subdural and epidural hematomas. J Trauma. 2006;61(6):1480-1483. [PubMed: 17159695]
- 30.
- Kessel B, Jeroukhimov I, Ashkenazi I, et al. Early detection of life-threatening intracranial haemorrhage using a portable near-infrared spectroscopy device. Injury. 2007;38(9):1065-1068. [PubMed: 17716603]
- 31.
- Peters J, Van Wageningen B, Hoogerwerf N, Tan E. Near-Infrared Spectroscopy: A Promising Prehospital Tool for Management of Traumatic Brain Injury. Prehospital Disaster Med. 2017:1-5. [PubMed: 28351447]
- 32.
- Bressan S, Daverio M, Martinolli F, et al. The use of handheld near-infrared device (Infrascanner)for detecting intracranial haemorrhages in children with minor head injury. Childs Nervous System. 2014;30(3):477-484. [PubMed: 24469947]
- 33.
- Salonia R, Bell MJ, Kochanek PM, Berger RP. The utility of near infrared spectroscopy in detecting intracranial hemorrhage in children. Journal of Neurotrauma. 2012;29(6):1047-1053. [PMC free article: PMC3325547] [PubMed: 22098538]
- 34.
- Gopinath S.P., Chance B., C.S. R. Near-infrared spectroscopy in head injury. In: Narayan RK, Wilberger J, J. P, eds. Neurotrauma. Vol 1. New York, NY McGraw-Hill; 1994:169-184.
- 35.
- Gopinath SP, Robertson CS, Contant CF, Narayan RK, Grossman RG, Chance B. Early detection of delayed traumatic intracranial hematomas using near-infrared spectroscopy. J Neurosurg. 1995;83(3):438-444. [PubMed: 7666220]
- 36.
- Gopinath SP, Robertson CS, Grossman RG, Chance B. Near-infrared spectroscopic localization of intracranial hematomas. J Neurosurg. 1993;79(1):43-47. [PubMed: 8315468]
- 37.
- Robertson CS, Gopinath SP, Chance B. A new application for near-infrared spectroscopy: detection of delayed intracranial hematomas after head injury. Journal of Neurotrauma. 1995;12(4):591-600. [PubMed: 8683610]
- 38.
- Robertson CS, Gopinath SP, Chance B. Use of near infrared spectroscopy to identify traumatic intracranial hematomas. J Biomed Opt. 1997;2(1):31-41. [PubMed: 23014820]
Footnotes
- 1
Note that Huff et al reported a false negative rate of 7.7% in the text, but Figure 2 supports a lower rate of 3.96%. We contacted the authors for clarification, but they did not respond by the publication date of this report.
- 2
Mathematically, when the chance of a positive CT scan is low, a very inaccurate test could appear to be useful unless false negatives are considered. For example, assume that 50 patients undergo a test and that 1 of them has a hematoma. If our “test” is a coin flip, about 25 patients would have a negative test (“tails”) and the number of CT scans would be reduced by 50%. If the one patient with a hematoma either had “heads” (and had a CT) or had “tails” but was not followed up, we would believe the coin toss was a very useful test.
- 3
For example, it would take a sample size of 811 to demonstrate 95% sensitivity and 95% specificity in a population with a hematoma prevalence of 9%, alpha =0.05, power= 0.80, and a margin of error of 5%.
Appendix A. Search Strategies
Appendix A. Search Strategies (PDF, 58K)
Appendix B. List of Excluded Studies
Appendix B. List of Excluded Studies (PDF, 49K)
Appendix C. Evidence Tables
Appendix C. Evidence Tables (PDF, 84K)
Appendix D. Forest Plots
Appendix D. Forest Plots (PDF, 72K)
Appendix E. Performance Characteristics
Appendix E. Performance Characteristics (PDF, 18K)
Appendix F. Peer Review
Appendix F. Peer Review (PDF, 36K)
REFERENCES Supplemental Materials
- 1.
- Akyol PY, Bayram B, Acerer A, et al. Comparison of near-infrared spectroscopy and head ct interpretations of the ed patients with minor head injury. Am J Emerg Med. 2016 Aug;34(8):1364–1368. [PubMed: 27133531]
- 2.
- Coskun F, Sezer EA, Karamercan MA, Akinci E, Vural K. An assessment on the use of infrascanner for the diagnosis of the brain hemotoma by using support vector machine. Scientific Research and Essays. 2010;5(14):1911–1915.
- 3.
- Francis SV, Ravindran G, Visvanathan K, Ganapathy K. Screening for unilateral intracranial abnormalities using near infrared spectroscopy: A preliminary report. J Clin Neurosci. 2005 Apr;12(3):291–295. [PubMed: 15851084]
- 4.
- Ghalenoui H, Saidi H, Azar M, Yahyavi ST, Borghei Razavi H, Khalatbari M. Near-infrared laser spectroscopy as a screening tool for detecting hematoma in patients with head trauma. Prehospital Disaster Med. 2008 Nov-Dec;23(6):558–561. [PubMed: 19557974]
- 5.
- Hennes HJ, Lott C, Windirsch M, et al. Non invasive detection of intracerebral hemorrhage using near infrared spectroscopy (nirs). SPIE. 1997;3194:42–54.
- 6.
- Hennes HJ, Richter B, Lott C, Dick W, Boor S, Hanley DF. Follow-up in patients with subdural haematomas using near infrared spectroscopy (nirs). Proc SPIE Int Soc Opt Eng. 1999;3566:182-192.
- 7.
- Kahraman S, Kayali H, Atabey C, Acar F, Gocmen S. The accuracy of near-infrared spectroscopy in detection of subdural and epidural hematomas. J Trauma. 2006 Dec;61(6):1480–1483. [PubMed: 17159695]
- 8.
- Kessel B, Jeroukhimov I, Ashkenazi I, et al. Early detection of life-threatening intracranial haemorrhage using a portable near-infrared spectroscopy device. Injury. 2007 Sep;38(9):1065–1068. [PubMed: 17716603]
- 9.
- Leon-Carrion J, Dominguez-Roldan JM, Leon-Dominguez U, Murillo-Cabezas F. The infrascanner, a handheld device for screening in situ for the presence of brain haematomas. Brain Inj. 2010;24(10):1193–1201. [PubMed: 20715889]
- 10.
- Peters J, Van Wageningen B, Hoogerwerf N, Tan E. Near-infrared spectroscopy: A promising prehospital tool for management of traumatic brain injury. Prehospital Disaster Med. 2017:1-5. [PubMed: 28351447]
- 11.
- Robertson CS, Zager EL, Narayan RK, et al. Clinical evaluation of a portable near-infrared device for detection of traumatic intracranial hematomas. Journal of Neurotrauma. 2010 Sep;27(9):1597–1604. [PubMed: 20568959]
- 12.
- Xu L, Tao X, Liu W, et al. Portable near-infrared rapid detection of intracranial hemorrhage in chinese population. J Clin Neurosci. 2017:26. [PubMed: 28279553]
- 13.
- Brogan RJ, Kontojannis V, Garara B, Marcus HJ, Wilson MH. Near-infrared spectroscopy (nirs) to detect traumatic intracranial haematoma: A systematic review and meta-analysis. Brain Inj. 2017;31(5):581–588. [PubMed: 28440675]
Prepared for: Department of Veterans Affairs, Veterans Health Administration, Quality Enhancement Research Initiative, Health Services Research & Development Service, Washington, DC 20420.
Prepared by: Evidence-based Synthesis Program (ESP), Coordinating Center, Portland VA Health Care System, Portland, OR, Mark Helfand, MD, MPH, MS, Director.
Recommended citation: Mackey K, Peterson K, Bourne D, Anderson J, Boundy E, Helfand M. Evidence Brief: Near Infrared Spectroscopy for Detecting Brain Hematoma. VA ESP Project #09-199; 2017.
This report is based on research conducted by the Evidence-based Synthesis Program (ESP) Coordinating Center located at the Portland VA Health Care System, Portland, OR, funded by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Quality Enhancement Research Initiative. The findings and conclusions in this document are those of the author(s) who are responsible for its contents; the findings and conclusions do not necessarily represent the views of the Department of Veterans Affairs or the United States government. Therefore, no statement in this article should be construed as an official position of the Department of Veterans Affairs. No investigators have any affiliations or financial involvement (eg, employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties) that conflict with material presented in the report.
Created: October 2017.
- NLM CatalogRelated NLM Catalog Entries
- PMCPubMed Central citations
- PubMedLinks to PubMed
- Evidence Brief: Near Infrared Spectroscopy for Detecting Brain HematomaEvidence Brief: Near Infrared Spectroscopy for Detecting Brain Hematoma
- UBE2Q1 ubiquitin conjugating enzyme E2 Q1 [Homo sapiens]UBE2Q1 ubiquitin conjugating enzyme E2 Q1 [Homo sapiens]Gene ID:55585Gene
- Gene Links for GEO Profiles (Select 114985465) (1)Gene
- Homologene neighbors for GEO Profiles (Select 114985462) (0)GEO Profiles
- Homologene neighbors for GEO Profiles (Select 101945743) (0)GEO Profiles
Your browsing activity is empty.
Activity recording is turned off.
See more...