NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Viswanathan M, Kahwati LC, Golin CE, et al. Medication Therapy Management Interventions in Outpatient Settings [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2014 Nov. (Comparative Effectiveness Reviews, No. 138.)
Background
Used appropriately, medications can alleviate distressing symptoms that compromise physical and psychological well-being, help prevent the onset of many acute and chronic illnesses, and improve patient health outcomes. Too often, however, medications are not used appropriately.1-3 In the United States in 2001, adverse drug events led to an estimated 4.3 million ambulatory visits.4 In addition to problems involving adverse drug events, many patients do not receive optimal pharmaceutical prescriptions. Even when optimal therapy is prescribed, patient inability to adhere closely to medication regimens may lead to poor health outcomes.5
Medication-related problems are especially pronounced among older adults.6 Individuals 65 years or older constitute 13 percent of the U.S. population, but they consume more than 30 percent of all prescription medications.6,7 A 2006 report found that nearly 60 percent of people in this age group were taking 5 or more medications and that nearly 20 percent were taking 10 or more medications,8 placing them at increased risk for experiencing adverse drug events.
Medication therapy management (MTM) services are intended to address issues of polypharmacy, preventable adverse drug events, medication adherence, and medication misuse.9 MTM is the current term that represents a suite of health care services that have evolved out of the philosophy and processes described in the early 1990s as “pharmaceutical care.”9 The Medicare Prescription Drug, Improvement, and Modernization Act of 2003 (Public Law 108-173)10 expanded patient access to MTM services and established the requirements that Medicare Part D Prescription Drug Benefit Plan sponsors have to meet with respect to cost and quality and the requirements for MTM programs sponsored by Part D drug benefit plans. The Centers for Medicare & Medicaid Services (CMS) requirements for Part D MTM programs have evolved since their implementation in 2006.
Within a year of the passage of Medicare Part D, 11 national pharmacy organizations established a consensus definition of MTM,11 and in 2008 a subset of national pharmacy organizations established five core elements for an MTM service model.12 These elements include a medication therapy review, a personal medication record, a medication action plan, intervention and/or referral, and documentation and followup.9 Also in 2008, Current Procedural Terminology (CPT®) for MTM services became available and further defined MTM and service-level expectations.13-15
The evolution from isolated research interventions studying the impact of pharmaceutical care interventions to large-scale commercial MTM programs or collaborative medication management within primary care represents a journey along a continuum of practice settings, patient populations, and intervention components and features. Over time, the practice and standards for these services have evolved, as have standards for describing and conducting research studies involving these interventions. A broadly defined scope for this review risks including studies that may be too different from each other to allow for meaningful comparison and synthesis. A narrowly defined scope for this review risks the omission of studies that met the definition of MTM but that predated the Part D era, were conducted in other countries, or used patient eligibility criteria that are less restrictive than Part D.
Scope and Key Questions
MTM is a complex intervention that could have different components depending on the goals and scope of the MTM program. This review seeks to catalog outpatient-based MTM intervention components, assess the overall effectiveness of outpatient-based MTM in comparison with usual care, examine the factors under which outpatient-based MTM is effective and optimally delivered, assess what types of patients are likely to benefit from outpatient-based MTM services, and clarify what types of patients may be at risk of harms from such programs. This review does not address (1) MTM services provided within inpatient settings or shortly after hospital discharge, (2) disease management services provided by pharmacists, or (3) interventions designed as a single episode of contact. The rationale for limiting the scope to exclude some types of MTM interventions is to ensure that included studies are reasonably comparable with respect to intended purpose and design of the MTM intervention.
The Key Questions (KQs) addressed in this review are—
KQ 1: What are the components and implementation features of MTM interventions?
KQ 2: In adults with one or more chronic diseases who are taking prescription medications, is MTM effective in improving the following:
- Intermediate outcomes, including biometric and laboratory measures, drug therapy problems identified, drug therapy problems resolved, medication adherence, goals of therapy met, and patient engagement in medication management?
- Patient-centered outcomes, such as disease-specific morbidity, disease-specific or all-cause mortality, adverse drug events, health-related quality of life, activities of daily living, patient satisfaction with health care, work or school absenteeism, and patient and caregiver participation in medical care and decisionmaking?
- Resource utilization, such as prescription drug costs, other health care costs, and health care utilization?
KQ 3: Does the effectiveness of MTM differ by MTM components and implementation features?
KQ 4; Does the effectiveness of MTM differ by patient characteristics, including but not limited to patient demographics and numbers and types of conditions and medications?
KQ 5: Are there harms of MTM, and if so, what are they?
Analytic Framework
The KQs are placed in relation to one another and the populations, interventions, comparators, outcomes, timing, and setting (PICOTS) in the analytic framework (Figure A). Specific details regarding patient population, intervention components, and outcomes are provided in the next section.
Populations, Interventions, Comparators, Outcomes, Timing, and Setting
Table A lays out the PICOTS for this review. For this review, we took a broad perspective on the population and interventions evaluated; we did not require CMS Part D MTM eligibility criteria. Specifically, we did not require multiple chronic conditions or a minimum number or level of expenditures on prescription drugs. We included randomized and controlled clinical trials, systematic reviews, and prospective and retrospective cohort studies. We included observational studies because we anticipated, from our topic refinement work, that a review limited to trials alone would fail to yield evidence on our wide range of prespecified benefits and harms for MTM interventions as a whole and for studies evaluating the modifying effects of specific intervention and patient characteristics on outcomes of MTM interventions.
Methods
Topic Refinement and Review Protocol
The topic of this report and preliminary KQs arose through a nomination from the Pharmacy Quality Alliance. Key Informants representing several clinical and scientific disciplines provided input on the initial KQs; we revised them as needed. An initial draft of the revised KQs was posted for public comment from March 6, 2013, through April 2, 2013, on the Agency for Healthcare Research and Quality (AHRQ) Effective Health Care Program Web site. We received comments from 23 professional organizations and individuals and further revised KQs as appropriate.
Literature Search and Identification Strategy
To identify articles relevant to each KQ, we began with a focused MEDLINE® search for MTM interventions using a combination of medical subject headings and title and abstract keywords, and limiting the search to English-language and human-only studies (inception through January 9, 2014). We also searched the Cochrane Library (inception through January 10, 2014) and the International Pharmaceutical Abstracts database (inception through January 10, 2014) using analogous search terms. We selected these databases based on preliminary searches and consultation with content experts. We conducted quality checks to ensure that the searches identified known studies (i.e., studies identified during topic nomination and refinement). Based on these quality checks, we revised and ran additional searches (specifically, using keywords such as “drug therapy management,” “drug therapy problem,” and “medications management”) to avoid missing articles that might prove eligible for this systematic review.
In addition, we searched the gray literature for unpublished studies relevant to this review and included studies that met all the inclusion criteria and contained enough methodological information to assess risk of bias. Specifically, sources of gray literature included ClinicalTrials.gov, the World Health Organization's International Clinical Trials Registry Platform, Health Services Research Projects in Progress (HSRProj), the National Institutes of Health Research Portfolio Online Reporting Tools, the Database of Promoting Health Effectiveness Reviews, the New York Academy of Medicine Grey Literature Report, and CMS.gov. In addition, we reviewed the yield from AHRQ's request for Scientific Information Packets in the Federal Register, posted for 30 days from September 16, 2013 onward.
We reviewed our search strategy with an independent information specialist and the Technical Expert Panel, and supplemented it according to their recommendations. In addition, to avoid retrieval bias, we manually searched the reference lists of landmark studies and background articles on this topic to identify any relevant citations that our electronic searches might have missed.
Two trained members of the research team independently reviewed each of the titles and abstracts against the inclusion/exclusion criteria listed in Table A. We applied the same criteria to systematic reviews and primary studies. Each article that either or both reviewers chose to include based on the abstract review underwent full-text review. Two reviewers reviewed the full text for eligibility against our inclusion/exclusion criteria. During full-text review, if both reviewers agreed that a study did not meet the eligibility criteria (including designation of high risk of bias), we excluded the study. Reviewers resolved conflicts by discussion and consensus or by consulting a third member of the review team.
For studies that met our inclusion criteria, a trained reviewer abstracted information into structured evidence tables; a second senior member of the team reviewed all data abstractions for completeness and accuracy. Reviewers resolved conflicts by discussion and consensus or by consulting a third member of the review team.
Assessment of Risk of Bias of Individual Studies
To assess the risk of bias of individual studies, we used predefined criteria developed by AHRQ.18 For randomized controlled trials (RCTs), we relied on the risk-of-bias tool developed by the Cochrane Collaboration.19 We assessed the risk of bias of observational studies using an item bank developed by RTI International.20
In general terms, results of a study with low risk of bias are considered valid. Studies marked low risk of bias did not have any major flaws in design or execution. A study with medium risk of bias is susceptible to some bias but probably not sufficient to invalidate its results. A study with high risk of bias has significant methodological flaws (e.g., stemming from serious errors in design or analysis) that may invalidate its results. Primary concerns for our review included selection bias, confounding, performance bias, detection bias, and attrition bias. Very high attrition rates, particularly when coupled with a failure to control for confounding or to conduct intention-to-treat analyses, resulted in a rating of high risk of bias for trials and prospective cohort studies. Likewise, we rated studies with an inherently high risk of confounding in design (e.g., observational studies comparing refusers vs. acceptors of MTM interventions) as high risk of bias if they failed to address confounding through design (e.g., matching) or analysis (e.g., regression). Specifically, we evaluated trials on the adequacy of randomization, allocation concealment, similarity of groups at baseline, masking, attrition, whether intention-to-treat analysis was used, method of handling dropouts and missing data, validity and reliability of outcome measures, and treatment fidelity. For observational studies, we did not assess adequacy of randomization or allocation concealment but assessed for confounding. We also evaluated trials for confounding due to randomization failure through biased selection or attrition. In other words, we evaluated trials with potential randomization failure for the same risks of bias as observational studies.
We excluded studies that we deemed at high risk of bias from our main data synthesis and main analyses. We included them for sensitivity analyses; in cases when we had no other available or credible evidence, we included in the report a brief synopsis of studies assessed as high risk of bias.
Data Synthesis
When we found three or more similar studies for a comparison of interest, we conducted meta-analysis of the data from those studies using Comprehensive Meta-Analysis software (Biostat, Inc, Englewood, NJ). For all analyses, we used random-effects models to estimate pooled or comparative effects. To determine whether quantitative analyses were appropriate, we assessed the clinical and methodological heterogeneity of the studies under consideration following established guidance;21 that is, we qualitatively assessed the PICOTS of the included studies, looking for similarities and differences. When we conducted quantitative syntheses (i.e., meta-analysis), we assessed statistical heterogeneity in effects between studies by calculating the chi-squared statistic and the I2 statistic (the proportion of variation in study estimates attributable to heterogeneity). The importance of the observed value of I2 depends on the magnitude and direction of effects and on the strength of evidence for heterogeneity (e.g., the p-value from the chi-squared test or a confidence interval for I2). Where relevant, we examined potential sources of heterogeneity using sensitivity analysis.
When quantitative analyses were not appropriate (e.g., because of heterogeneity, insufficient numbers of similar studies, or insufficiency or variation in outcome reporting), we synthesized the data qualitatively. Whenever possible, we computed confidence intervals for individual outcomes.
Numerous articles did not provide complete information about findings (e.g., 95% confidence intervals, statistical significance values, or between-group data). In many cases, therefore, we had to calculate odds ratios, mean differences or standardized mean differences, the relevant 95-percent confidence intervals, and p-values.
Grading Strength of Evidence for Individual Comparisons and Outcomes
We graded the strength of evidence based on the guidance established for the AHRQ Evidence-based Practice Center program.22 Developed to grade the overall strength of a body of evidence, this approach incorporates four key domains: study limitations (includes study design and aggregate quality), consistency (similar magnitude and direction of effect), directness (evidence links interventions directly to outcome of interest for the review), and precision of the evidence (degree of certainty surrounding an effect estimate based on sample size and number of events). In addition, the evidence may be rated as lower strength for bodies of evidence with suspected reporting bias from publication, selective outcome reporting, or selective analysis reporting. Regardless of the specific risk of bias of observational studies, this approach to grading the evidence assigns observational studies a grade of high for study limitations, which then leads to low strength of evidence. The strength of evidence from observational studies can be rated as higher for scenarios such as a strong dose-response association, plausible confounding that would decrease the observed effect, and a high strength of association (magnitude of effect). We evaluated optimal information size criteria to make judgments about precision based on guidance from Guyatt and colleagues23 and based our grades on RCTs with low or medium risk of bias or on observational studies unless none were available, based on guidance from the Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group24 and the AHRQ Evidence-based Practice Center program.22
Table B describes the grades of evidence that can be assigned.25 Grades reflect the strength of the body of evidence to answer the KQs on the overall effectiveness, comparative effectiveness, and harms of the interventions examined in this review. Two reviewers assessed each domain for each major outcome, and resolved any differences by consensus discussion or referral to a third, senior member of the team. We graded the strength of evidence for the outcomes deemed to be of greatest importance to decisionmakers and those commonly reported in the literature; we did not grade the strength of evidence for KQ 1 (on components and features of MTM services). The grades shown in Table B describe the state of evidence (which may demonstrate benefit, harm, or no effect) and the confidence in the stability of that state. An insufficient grade is not a statement about lack of efficacy or effectiveness; rather it is a statement about the lack of convincing evidence on benefit, harm, or lack of effect.
Assessing Applicability
We assessed applicability of the evidence following guidance from the “Methods Guide for Effectiveness and Comparative Effectiveness Reviews.”26 We used the PICOTS framework to explore factors that affect applicability. Some factors identified a priori that may limit the applicability of evidence include the following: age and health status of enrolled populations, health insurance coverage and access to health care, and complexity and intensity of the MTM intervention.
Results
We provide a summary of results by KQ below. Detailed descriptions of included studies, key points, detailed synthesis, summary tables, and expanded strength-of-evidence tables that include the magnitude of effect can be found in the full report. Our summary of results below presents the strength-of-evidence grades.
Results of Literature Searches
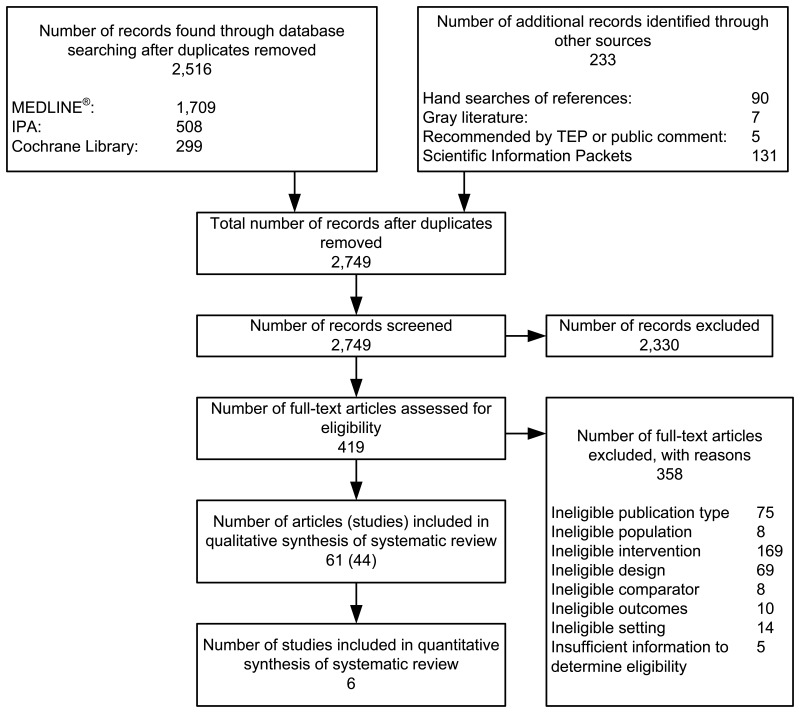
Figure B presents our literature search results through January 9, 2014. We identified 2,516 unduplicated citations. In addition, we identified 233 publications through gray literature searches, suggestions from Technical Experts or public comments received during topic refinement, hand searches of included studies, and Scientific Information Packets. After applying our eligibility and exclusion criteria to titles and abstracts of all 2,749 identified citations, we obtained full-text copies of 419 published articles. We reapplied our inclusion criteria and excluded 358 of these articles from further review before doing the risk-of-bias assessment. The 61 articles included after full-text review represent 44 studies.
This evidence base consisted of 44 studies (21 RCTs, 4 controlled clinical trials, and 19 cohort studies) reported in 61 articles. Most RCTs compared an MTM intervention with usual care rather than with a different active intervention; all observational studies were cohort studies. Numerous studies had methods problems that led us to rate them as having a medium or high risk of bias; only a few studies were of low risk of bias. When possible (enough studies similar in intervention, populations, and outcomes measured), we conducted meta-analyses of data from RCTs or cohort studies separately; when relevant, we did two sets of meta-analyses, one with and one without the trials that had high risk of bias.
Because of the wide variation in types of interventions classified as MTM, we first cataloged intervention components and implementation features of MTM interventions (KQ 1). We then evaluated the effect of MTM on intermediate, patient-centered, and resource utilization outcomes (KQ 2). We also reviewed the evidence to identify how these effects might vary by specific intervention components and features (KQ 3) and patient characteristics (KQ 4). Finally, we reviewed the evidence on harms associated with MTM (KQ 5).
Below, we summarize the main findings and strength of evidence, where applicable. We then discuss the findings in relationship to what is already known, applicability of the findings, implications for decisionmaking, limitations, research gaps, and conclusions.
Key Findings and Strength of Evidence
KQ 1. Intervention Components and Implementation Features
Of the 44 included studies, over three-quarters were broadly focused MTM interventions with patients who had a wide-ranging collection of conditions; the remaining studies were narrowly focused MTM interventions with patients who had a specific condition. All studies used a pharmacist as the interventionist. Services were provided face to face in half of included studies. Included studies provided interventions in a variety of clinical settings, including community pharmacies, centralized pharmacies or pharmacy call centers, and outpatient medical clinics, and some used home visits. Half of the narrowly focused interventions were delivered exclusively in an outpatient medical clinic.
Whether they used the term “pharmaceutical care” or “MTM,” studies did not describe intervention components and features in a consistent manner or in sufficient detail. These drawbacks were especially prevalent for intervention intensity and frequency of followup, method of patient enrollment for services, level of integration with usual care, and reimbursement characteristics for rendered MTM services. KQ 1 was descriptive in nature, so we did not grade strength of evidence.
KQ 2. Overall Effectiveness of MTM
Of the 44 studies included in this review, we rated 16 as high risk of bias overall; that is, concerns about randomization failure, confounding, or overall attrition increased the risk of bias for all outcomes. In addition, we rated some studies that were otherwise of low or medium risk of bias as high for individual outcomes, chiefly because of measurement or detection bias related to the specific outcome. These instances are specified in the relevant results section in the full report.
We rated the strength of evidence for each outcome from studies with low or medium risk of bias when available. MTM significantly improved objective measures of medication adherence, medication appropriateness assessed in general, and medication dosing (Table C). However, we did not find evidence of benefit for any other intermediate outcomes on which we had data. No studies addressed either goals of therapy or patient engagement.
Similarly, we did not have evidence of benefit for most patient-centered outcomes, including adverse drug events or mortality (Table D). MTM did not improve most measures of health-related quality of life (low strength of evidence for no benefit). We graded the “vitality” and “emotional role functioning” domains of the Medical Outcomes Study Short-Form (SF36) questionnaire as insufficient for this domain. For the SF-36, neither the other six domains nor the two component scores (physical health, mental health) showed significant benefit from MTM interventions. The various patient satisfaction items also showed no impact from MTM programs (low strength of evidence for no benefit). We found no evidence on activities of daily living, work or school absenteeism, and patient and caregiver participation in medical care and decisionmaking.
Outcomes related to using health resources were also not much influenced by MTM interventions (Table E). Two exceptions may merit attention: (1) health plan expenditures on medication costs and (2) the proportion and costs of hospitalization for patients with diabetes. MTM trials implemented in settings with a broad range of patients did not show a consistent signal of reduction in the number of hospitalizations, but a single cohort study that partially addressed confounding inherent in studies of refusers and acceptors found a lower mean number of inpatient visits for patients accepting MTM compared with patients refusing MTM. Overall, we judge the strength of evidence for this outcome to be insufficient owing to lack of consistency across studies.
Over all three categories of outcomes, each of which had a substantial number of individual measures, MTM improved outcomes in only a couple of instances. Study limitations, lack of consistency, and lack of precision of the estimates of effects limited the strength of evidence considerably. As discussed later, even the minimal findings of effectiveness are at best only narrowly applicable.
KQ 3. Effectiveness of MTM by Intervention Features
We found evidence from one study each on five intervention features: (1) access of pharmacists to patient records,27 (2) intensity of care coordination and followup after comprehensive medication review,28 (3) community pharmacy versus call center,29 (4) level of intensity of intervention,30 and (5) type of payer (private vs. Medicaid).31 With the exception of the study on pharmacists' access to patient records, we rated these studies as high risk of bias. Evidence was insufficient for most outcomes for the first two intervention features, with the following two exceptions. First, MTM delivered by community pharmacists increased the weighted generic dispensing ratio when compared with call-center pharmacists (low strength of evidence). Second, enhanced MTM with pharmacists' access to patient records reduced the mean number of adverse drug events; this finding suggested benefit when compared with basic MTM (low strength of evidence). We found insufficient evidence for all outcomes for intensity of intervention and type of payer.
KQ 4. Effectiveness of MTM by Patient Characteristics
We did not identify any studies that analyzed outcomes of MTM by patient characteristics.
KQ 5. Harms of MTM Interventions
Lack of precision and the limitations of a single study with high risk of bias meant that evidence was insufficient to judge whether MTM resulted in greater inconvenience32,33 than usual care. We found no evidence on other prespecified harms, specifically including care fragmentation, patient decisional conflict, patient anxiety, increased (actual) adverse drug events, prescriber confusion, and prescriber dissatisfaction.
Discussion
Key Findings
We included 44 eligible studies (21 randomized controlled trials, 4 controlled clinical trials, and 19 cohort studies) reported in 61 articles, described in detail in the full report (KQ 1). Evidence was insufficient on the effect of MTM on most outcomes (KQ 2). In a few instances, described below, the evidence led us to conclude with a low strength of evidence either a benefit or lack of benefit. Specifically, we found evidence that MTM results in improvement when compared with usual care for some measures of medication adherence and appropriateness; medication dosing; health plan expenditures on medication costs; and, for patients with diabetes, the proportion and costs of hospitalization. Similarly, we conclude based on a low strength of evidence that MTM conferred no benefit for patient satisfaction and most measures of health-related quality of life.
We found evidence on five intervention components and intervention features (KQ 3): one study provided information on each feature and yielded insufficient evidence for most outcomes, with the two following exceptions. MTM programs with pharmacist access to brief clinical summaries from the medical record reduced the mean number of adverse drug events when compared with basic MTM programs without such access (low strength of evidence). Community pharmacists increased the generic dispensing ratio more than pharmacists based in call centers (low strength of evidence). We found no relevant studies on patient characteristics moderating the effect of MTM interventions (KQ 4). Similarly, the evidence on harms associated with MTM was limited to one study on inconvenience and was rated as insufficient (KQ 5).
Findings in Relation to What Is Already Known
Our findings contrast with conclusions that Chisholm-Burns and colleagues reached in a recent systematic review.34 In that review, the authors concluded on page 923: “Pharmacist-provided direct patient care has favorable effects across various patient outcomes, health care settings, and disease states.”34 Several differences between the Chisholm-Burns review and the current review may account for the discrepant conclusions. First, the Chisholm-Burns review included all studies that cited evidence of pharmacist involvement in direct patient care. The interventions examined included chronic disease management and prospective and retrospective drug utilization review; we excluded these types of efforts because of the clinical heterogeneity those interventions would have introduced into the review. Notably, the Chisholm-Burns review did not use the term “medication therapy management” to categorize the interventions in the articles they reviewed. Second, approximately 30 percent of the studies in the Chisholm-Burns review were conducted entirely in institutional settings. In contrast, we did not identify any studies within institutional settings that met our MTM intervention definition criteria. Third, the Chisholm-Burns review included a total of 298 articles and did not omit from the analyses studies with a high risk of bias; in contrast, we based our strength-of-evidence grades in this review on only those studies with no more than medium risk of bias. Thus, a direct comparison of findings between these two reviews would be ill advised.
The striking differences between the conclusions reached in these two reviews emphasize two important needs for efforts to systematically review MTM programs. The first is for researchers to specify the MTM intervention based on existing definitions, taxonomies, or service models. The second is to develop consensus guidelines for describing intervention features and fidelity of intervention delivery in publications reporting findings from evaluation studies. Progress on these two steps would enable systematic reviews to differentiate better between different types of services and avoid the problem of overgeneralizing review results.
Applicability of the Findings
This body of evidence has significant clinical and methodological heterogeneity, which limits the ability to make any universal statements about effectiveness. However, the range of study designs, which includes RCTs, nonrandomized trials, and cohort studies, enhances the applicability of findings for real-world settings.26 Included studies ranged from relatively small interventions in single clinics provided by a single interventionist to evaluations of MTM services delivered on a large scale through integrated health systems or health plans as a Medicare Part D or other drug plan benefit. This diversity of studies enhanced the applicability of findings to a wide variety of settings, including outpatient clinics, community pharmacies, and centralized pharmacy call centers. A few studies conducted outside the United States included MTM as part of a home visits program; findings from this model may not be directly applicable within the United States.
The studies in this review are broadly applicable to a range of chronically ill adult patient populations. A majority of interventions were directed at populations with multiple and common chronic conditions, such as diabetes, chronic heart failure, and hypertension. Several specifically targeted adults age 65 years or older. Few studies reported sociodemographic characteristics beyond age and sex; thus, the applicability of findings to specific populations (e.g., rural, low socioeconomic status, cognitively impaired, uninsured) is unknown. The nature of the MTM intervention, which includes involving patients as active participants in the process, limits the extent to which findings can be generalized beyond patients who agree to participate in such interventions. Patients who agree to participate may be systematically different from those who decline to be in such a program. For that reason, the impact of such interventions at a population or health-plan level may be limited by the degree of uptake among interested patients.
The intervention used across most studies can be characterized as complex and moderately resource intensive. Components involve identifying applicable patients; initially assessing patients; providing counseling, education, and care coordination; and following patients over time. These services were provided per protocol in some studies and as needed or ad hoc in others. Most studies described intervention components in terms of “pharmaceutical care model” components or Medicare Part D MTM program criteria, but few provided detailed descriptions or measurement of implementation fidelity.
The comparator arm in all studies was usual medical or pharmacy care. This does not typically include distinct MTM services by health care providers other than prescribing providers (not common for the time period covered by most of the studies). Models of collaborative health care delivery are evolving, and the changing roles and training of pharmacists increase the potential applicability of MTM interventions in future models of health care.
The broad sets of outcomes evaluated across this body of evidence spanned a substantial range of both intermediate and health outcomes as well as outcomes related to resource use. Proximal and intermediate outcomes included number of drugs, identification of drug therapy problems, appropriateness of medication prescribing, and laboratory or biometric markers of disease control (e.g., hypertension, hemoglobin A1c, low-density lipoprotein cholesterol). Patient-centered outcomes focused on numerous measures of quality of life as well as adverse drug events. Many studies also reported outcomes involving health care resource use and expenditures (e.g., number and costs of hospitalizations, emergency department visits, outpatient visits).
Most studies did not, however, clearly indicate the expected, desired, or intended direction of effect on most resource use outcomes, making the applicability of using these interventions to reduce drug-related health care costs or expenditures difficult to assess. For example, it is not clear whether one should expect the number of medications prescribed for heart failure to increase or decrease under the careful scrutiny of an MTM intervention because the desired impact will be based on the goal of therapy for each individual.
The focus of outcome measurement in many studies was the short-term identification and characterization of drug therapy problems and their resolution; these endpoints are thought to be the outcomes most sensitive to change as a result of receiving MTM services. However, because identification of drug therapy problems is, by design, a part of the MTM intervention itself, differences between the nature of the intervention and that of the control programs mean that measuring these outcomes cannot be as rigorous in a usual-care comparison group as it is in the intervention group. In fact, many studies were able to measure changes in this outcome only in the intervention group. Hence, many studies failed to demonstrate a direct analytic link between the resolution of drug therapy problems as a result of MTM and impact on intermediate outcomes, patient-centered outcomes, and resource utilization. Thus, the applicability of studies that demonstrate an impact on the resolution of drug therapy problems is limited.
Implications for Clinical Practice and Policymakers
Although we found the evidence insufficient in general to draw definitive conclusions about the comparative effectiveness of MTM for most outcomes that we evaluated, our findings suggest some implications for practice and policy. MTM is already in widespread practice and is now shaped in the United States largely by Medicare Part D policy; this presents both challenges and opportunities. MTM programs sponsored and administered by Part D drug benefit plans are often centrally administered and delivered primarily by phone, and may be less integrated into routine health care than some of the interventions included in our review. MTM programs of the future have the potential to be more integrated into routine health care through participation in accountable care organizations or patient-centered medical home models. We were unable to answer definitively whether level of integration matters for effectiveness, but policymakers may need to consider expectations about the impact that MTM might have on patient-centered outcomes and resource use in the context of other health care delivery transformation activities or quality improvement initiatives that are also occurring. More integration of MTM services with other activities may be effective; however, the more integrated MTM becomes within routine medical care, the more difficult it becomes to isolate it as a discrete intervention for evaluation.
Policymakers could thus consider whether MTM services should be positioned as a contributor to overall improvement in processes of care, health status, and costs or positioned as an intervention to which effects can be discretely attributed. As noted earlier, improvements in medication appropriateness or drug therapy regimens may not always translate into improvements in health or costs, and even if they do, secular trends in related quality improvement (e.g., medication adherence interventions, regulatory requirements for medication reconciliation, meaningful use incentives for electronic health records) may make measuring outcomes attributable to MTM very challenging.
Future training of MTM providers would benefit from a better understanding of which MTM components really matter. At the moment, such information is lacking. Policymakers and funders who wish to understand the comparative effectiveness of different MTM components could encourage rigorous program evaluation designs that fit within the context of the real-world implementation of these programs. For example, positive deviance analyses35 with rigorous measurement of implementation features or stepped wedge trial designs36 may be useful approaches.
A typical approach for evaluating complex interventions is to identify the “core” components for standardization, while allowing for flexibility for peripheral components or variations in implementation. In complex practice-based innovations, such flexibility may reflect desirable (or unavoidable) adaptations to local circumstances. Policy governing MTM programs may warrant modifications to permit investigators to conduct rigorous and innovative evaluative designs to identify core components or effectiveness-enhancing modifications. As future research and evaluation elucidate these components or enhancements, policy will need to evolve to keep pace with best practices.
Finally, consideration of both patients' and prescribers' perspectives in future design and delivery of MTM services may be needed. In our current analytic framework, MTM interventions require a significant element of engagement by both patients and prescribers if the interventions are to have a reasonable likelihood of improving outcomes. Although “opt in” strategies may increase the reach of such interventions, keeping patients (and their prescribing providers) engaged in the intervention over a reasonable amount of time may be the key to translating the potential of MTM interventions into actual improvements. Further refinement of eligibility criteria based on evidence to provide interventions to those most at risk from drug-related problems, and therefore most likely to benefit, may also be warranted.
Limitations of the Comparative Effectiveness Review Process
The constraints for populations, interventions, and settings that we imposed on this systematic review may limit its applicability, as discussed above. During topic refinement and based on Technical Expert Panel inputs and public comment, we expanded the scope by removing an exclusion criterion that would have required MTM interventions to have been directed at a patient population with two or more chronic conditions. As a result, we included studies that focused on one chronic condition. Because of the prevalence of certain chronic conditions in the adult population, and particularly among Medicare beneficiaries, we think this decision was sensible and permitted us to examine a broader evidence base than would otherwise have been the case.
Although we tried to distinguish MTM from disease or case-management interventions, making this distinction was challenging. We created a threshold for the intervention components that were required to be present for this distinction. Specifically, we elected to emphasize whether the intervention entailed a comprehensive review of all medications; for that reason, we did not constrain studies of interest to those that targeted a single medication or drug regimen or that focused on a single condition such as diabetes or hypertension.
When we were unable to determine which medications the interventionist had reviewed, we wrote to the authors for additional information. We chose to pursue authors in an effort to permit us to use studies that had been designed as MTM but did not describe the comprehensive medication review component in detail.
Our approach may have been overly inclusive because it led us to include studies that addressed a single disease, as long as the pharmacist reviewed all medications. For example, 10 of the 44 studies were relatively narrowly focused; 2 of these addressed patients with chronic heart failure and 2 addressed patients with either hypertension or hypertension and diabetes. The remaining six studies focused on patients with diabetes, HIV, glucocorticoid-induced osteoporosis, or hemodialysis. The fact that we did not require patients to have more than one clinical condition resulted in an approach that was inclusive of these more narrowly focused (albeit often termed “MTM”) studies and may render our results less applicable to MTM interventions targeted to patients with a wide range of chronic conditions.
Also based on feedback during the process of setting out the scope of this review, we chose to include interventions that were broader than the Medicare Part D MTM-defined interventions. Put another way, we broadened our view of patient populations and intervention criteria, and we allowed studies not conducted in the United States into the evidence base. This decision led us to include interventions described as “pharmaceutical care,” which were generally based on the pharmaceutical care model principles;9 it also permitted us to examine investigations with elements of pharmaceutical care or MTM that did not specifically label the intervention as either MTM or pharmaceutical care. These studies were often described as “clinical pharmacist interventions.”
Furthermore, all the non-U.S. studies involved interventions within single-payer health systems. Hence, the interventions in this review constitute a more heterogeneous group than if we had allowed only those labeled as Medicare Part D MTM programs. This is both a limitation and a strength. Although our approach makes results more challenging to interpret, it enhances our ability not to miss interventions that include MTM components but lack the descriptor term “MTM.”
Studies did not often explicitly describe certain MTM components. In cases when we could not determine whether investigators had provided certain MTM components (such as patient education and counseling or coordination with other health care providers), we contacted the authors to gain additional information that would allow us to make an informed decision. We were fairly permissive in interpreting the presence of the MTM intervention components other than comprehensive medication review. The main reason is that we recognized that terms describing some components have evolved over time and may have been absent from the lexicon in earlier years or implicitly conveyed by authors by simply using the terms “MTM” or “pharmaceutical care” to describe their intervention.
Our approach to categorizing interventions for KQ 1 relied primarily on the short descriptions in published manuscripts and those we were able to obtain via email inquiries. Their similarities or differences substituted for any overarching taxonomy, because none that we considered seemed to fit our purpose. Thus, we have introduced intervention labels that, admittedly, do not fully describe or account for clinical heterogeneity among interventions. This approach limits our ability to make definitive statements about the effectiveness of various intervention components. We believe that the clusters and categorizations we used are useful heuristics, but some may regard them more as hypothesis generating than as reflecting settled principles of classification.
Finally, our search process was complicated by having to ensure coverage of all terms that could be used to describe MTM interventions over time. Adding to this challenge was our effort to examine the gray literature, where we thought we might find studies tilted toward effectiveness and real-world program evaluation. As it turned out, studies of these types of interventions were not indexed similarly; for that reason, we needed to rely heavily on hand searches of citation lists from key background articles to identify possibly relevant studies for inclusion. Thus, we may have missed some studies that might have qualified for inclusion. Given the considerable diversity in the evidence base we did have, however, we do not think that any potentially missed studies would have changed our conclusions in any material way. No meta-analyses included more than five studies; as a result, we did not examine included studies for publication bias quantitatively.
Limitations of the Evidence
As a body of evidence, the MTM literature evaluated in this review has measured numerous outcomes. As indicated in previous sections, very few outcomes, with the exception of harms, remain completely unexamined. Of the 44 studies in this review, we rated 28 as having medium, low, or mixed risk of bias. The 44 studies included 21 trials and 4 nonrandomized controlled studies. In other words, the literature on this topic is not marked by failure to consider important outcomes, universally high risk of bias, or pervasively weak designs.
Despite these advantages, we were unable to identify sufficient evidence on the majority of hypothesized outcomes of MTM. In several instances, our inability to rate evidence as higher than insufficient came from inconsistent and imprecise evidence or from a body of evidence with high study limitations. The choice of outcome measures in this body of evidence limited our ability to come to conclusions in some instances. For example, some studies did not focus on changes that proponents might expect MTM services to produce. Because effective MTM can either increase or decrease expenditures or use of services based on the needs of the patient, studies that did not prespecify the expected direction of change had no way to interpret their results as an appropriate change. Studies that demonstrated inconsistent results in direction of change (i.e., some showing an increase in resource use and others showing a decrease) may well have been consistent in terms of appropriate change, but because they generally failed to establish a priori the direction in which they expected to find an effect, we rated such evidence as indirect and inconsistent.
Similarly, studies often used nonstandardized or idiosyncratic measures for outcomes such as adverse events, adherence, and expenditures or costs; this tendency limited our ability to meta-analyze results. When studies focused on specific outcomes, they were often significantly underpowered to detect differences between groups (i.e., they did not meet optimal information size criteria). As a result, we rated several studies as imprecise.
MTM intervention studies are largely practice based and incorporate substantial heterogeneity in specific intervention elements and in patient populations targeted. Yet the evidence is sharply constrained in its ability to inform questions about the effectiveness of specific MTM components or intervention features (KQ 3 in our review) because study designs did not often capitalize on variants in MTM programs for a prospective evaluation of outcomes by those variants. Neither did they measure fidelity to intended MTM elements for post hoc evaluation. Similarly, the relatively untargeted nature of the MTM interventions meant that, in many studies, only small numbers of patients had any one specific condition, and most studies did not measure patient characteristics beyond age and sex, thus limiting our ability to address KQ 4 in our review. For this reason, the evidence we identified for this review was most relevant for KQ 2.
Research Gaps
In many bodies of research, questions regarding the comparative effectiveness of specific intervention components or implementation features are best answered after clear evidence of the overall effectiveness of the intervention relative to usual care has been established. Our review largely indicates insufficient evidence on the primary question of effectiveness relative to usual care. By definition, this limited what we could say about comparative effectiveness.
Nonetheless, the widespread implementation of MTM coexists with the urgent need for actionable information for policy, program policies, and training. This clinical and policy environment means that new research cannot afford to address causal claims relative to usual care first, followed by comparative effectiveness of the intervention elements in a relatively controlled environment, and finally, program evaluation of real-world implementation, all in sequential order.
In prioritizing among various research goals, therefore, funders may wish to consider the relative value of new evidence on overall effectiveness, effectiveness of implementation features, and program implementation and accountability. Trial research in narrow clinical settings can address questions of effectiveness but may lack applicability to real-world implementation. Likewise, evaluations of real-world programs with variable fidelity to interventions can answer questions about process and implementation, but they offer limited information on effectiveness. Research prioritization exercises will also need to account for already commissioned MTM intervention studies.
For new studies focusing on causal claims, a critical gap relates to the failure to specify the expected direction of effect. New research requires a strong theoretical foundation to help specify causal mechanisms and hypothesized effects. Without such an edifice, future research will continue to produce inconsistent and uninterpretable results.
Heightened attention to causal mechanisms will also help researchers convey their understanding of what outcomes these types of interventions are likely to influence. For instance, how should researchers wishing to establish direct causal links between MTM programs and outcomes evaluate distal outcomes such as patient-centered outcomes and resource utilization? This effort requires a better understanding of the relationship between proximal outcomes such as “drug therapy problems identified and resolved” and distal outcomes. For instance, MTM may reduce outpatient visits to address side effects. MTM may also result in the need for further testing and evaluation for some patients, which could, in turn, result in more rather than fewer outpatient visits. Unless the nature of change resulting from MTM is specified in relation to goals of drug therapy, studies cannot assert benefit or harm. Further, drug therapy problems are diverse and may not all have the same causal relationship to health, quality of life, patient satisfaction, or resource use outcomes. Furthermore, a causal model of these distal outcomes may need to take into account the competing or complementary contributions of MTM, new models of health care delivery (e.g., patient-centered medical homes), and other quality improvement interventions.
Investigators embarking on new studies focusing on causal links between MTM and outcomes may wish to consider the limitations of studies based on secondary data from existing MTM programs that use opt-in/opt-out patient enrollment mechanisms. Although these studies may provide invaluable information on process measures such as patient engagement, underlying issues of confounding severely limit the validity of causal claims from such studies.
Regardless of the goal of their future research, investigators should consider issues of sample size to ensure precision of their results. This issue is particularly relevant when evaluating outcomes likely to occur in smaller subgroups defined by patient risk, context, or highly adapted intervention features. Innovative designs (e.g., stepped wedge trials, statistical process control, time-series analysis, simulations, and factorial experiments) may permit both rigor and adequate sample size within the context of real-world implementation. With careful attention to fidelity, new studies may also inform questions about the effectiveness of intervention components and implementation features. Mixed-methods approaches may allow more information on variations in context and implementation. Such designs may also help inform our understanding of critical training elements for MTM service providers.
Regarding research gaps for specific outcomes such as patient satisfaction, measures specific to the types of services provided through MTM (e.g., patient education about medications) or to the proximal outcomes that MTM is intended to achieve (e.g., reduced medication side effects, improved disease control) may offer better insights into the effects of MTM. Similarly, a medication-related instrument may better measure patients' concerns that are directly related to medication use (e.g., experience of side effects, intrusiveness of the medication regimen) than generic tools do.
Conclusions
The evidence base offers low evidence of benefit for a limited number of intermediate and health utilization outcomes.We graded the evidence as insufficient for most outcomes because of inconsistency in direction, magnitude, and precision, rather than lack of evidence. Wide variations in populations and interventions, both within and across studies, likely explain these inconsistencies. Given the widespread implementation of MTM and urgent need for actionable information, optimal investments in new research require a process of research prioritization in which the value of information from each proposed study is carefully considered. Studies designed to identify causal relationships between MTM interventions and their outcomes require adequate controls for confounding but may offer limited information on which factors explain program success or failure. Studies designed to explore the reasons for program success or failure using qualitative or single-arm designs may offer hypothesis-generating rather than hypothesis-confirming insights on MTM effectiveness. New research, regardless of specific focus, will likely continue to find inconsistent results until underlying sources of heterogeneity are accounted for.
References
- 1.
- Adams K, Corrigan E. Priority areas for quality improvement. Washington, DC: Institute of Medicine of the National Academies, The National Academies Press; 2003. [PubMed: 25057643]
- 2.
- Kohn LT, Corrigan J, Donaldson MS. To Err Is Human: Building A Safer Health System. Washington, DC: National Academy Press; 2000. [PubMed: 25077248]
- 3.
- McGlynn EA, Asch SM, Adams J, et al. The quality of health care delivered to adults in the United States. N Engl J Med. 2003 Jun 26;348(26):2635–45. Epub: 2003/06/27. [PubMed: 12826639]
- 4.
- Zhan C, Arispe I, Kelley E, et al. Ambulatory care visits for treating adverse drug effects in the United States, 1995-2001. Jt Comm J Qual Patient Saf. 2005 Jul;31(7):372–8. Epub: 2005/09/01. [PubMed: 16130980]
- 5.
- Warholak TL, Nau DP. Quality and Safety in Pharmacy Practice. New York, NY: McGraw-Hill Medical; 2010.
- 6.
- Gurwitz JH, Rochon P., Food and Drug Administration. Improving the quality of medication use in elderly patients: a not-so-simple prescription. Arch Intern Med. 2002 Aug 12-26;162(15):1670–2. Epub: 2002/08/03. [PubMed: 12153368]
- 7.
- Mahan D. Out-of-Bounds: Rising Prescription Drug Prices for Seniors. Families USA Foundation Publication No 03-106. Washington, DC: Jul, 2003.
- 8.
- Kaufman DW, Kelly JP, Rosenberg L, et al. Recent patterns of medication use in the ambulatory adult population of the United States: the Slone survey. JAMA. 2002 Jan 16;287(3):337–44. Epub: 2002/01/16. [PubMed: 11790213]
- 9.
- Pellegrino AN, Martin MT, Tilton JJ, et al. Medication therapy management services: definitions and outcomes. Drugs. 2009;69(4):393–406. Epub: 2009/03/28. [PubMed: 19323584]
- 10.
- 108th U.S. Congress. Medicare Prescription Drug, Improvement and Modernization Act of 2003. 2003.
- 11.
- Bluml BM. Definition of medication therapy management: development of professionwide consensus. J Am Pharm Assoc (2003). 2005 Sep-Oct;45(5):566–72. Epub: 2005/11/22. [PubMed: 16295641]
- 12.
- Association of Chain Drug Stores Foundation. Medication therapy management in pharmacy practice: core elements of an MTM service model (version 2.0). J Am Pharm Assoc (2003). 2008 May-Jun;48(3):341–53. [PubMed: 18595820]
- 13.
- Isetts BJ, Buffington DE. CPT code-change proposal: national data on pharmacists' medication therapy management services. Consult Pharm. 2007 Aug;22(8):684–9. Epub: 2008/01/22. [PubMed: 18203409]
- 14.
- American Medical Association. CPT changes 2006: An Insider's View. Chicago, IL: American Medical Association; 2005. pp. 309–12.
- 15.
- Pharmacist Services Technical Advisory Coalition. Medication Therapy Management Service Codes. 2010. [March 5, 2013]. www
.pstac.org/services/mtms-codes.html. - 16.
- Tudor CG. CY 2014 Medication Therapy Management Program Guidance and Submission Instructions. Baltimore, MD: Centers for Medicare & Medicaid Services; 2013. [May 2, 2014]. www
.cms.gov/Medicare /Prescription-Drug-Coverage /PrescriptionDrugCovContra /Downloads /Memo-Contract-Year-2014-Medication-Therapy-Management-MTM-Program-Submission-v040513.pdf. - 17.
- Bodenheimer T, Berry-Millett R. Care Management of Patients With Complex Health Care Needs. Robert Wood Johnson Research Synthesis Report No 19. San Francisco, CA: Dec, 2009. www
.rwjf.org/content /dam/farm/reports/issue_briefs /2009/rwjf49853 /subassets/rwjf49853_1. [PubMed: 22052205] - 18.
- Viswanathan M, Ansari MT, Berkman ND, et al. Methods Guide for Effectiveness and Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Mar, 2012. Assessing the Risk of Bias of Individual Studies in Systematic Reviews of Health Care Interventions. AHRQ Publication No 12-EHC047-EF. January 2014 Update. Chapters available at www
.effectivehealthcare.ahrq.gov/ [PubMed: 22479713] - 19.
- Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. Epub: 2011/10/20. [PMC free article: PMC3196245] [PubMed: 22008217]
- 20.
- Viswanathan M, Berkman ND. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol. 2012 Feb;65(2):163–78. Epub: 2011/10/01. [PubMed: 21959223]
- 21.
- West SL, Gartlehner G, Mansfield AJ, et al. Methods Research Paper. Rockville, MD: Agency for Healthcare Research and Quality; Sep, 2010. Comparative Effectiveness Review Methods: Clinical Heterogeneity. AHRQ Publication No. 10-EHC070-EF. www
.effectivehealthcare.ahrq.gov/ [PubMed: 21433337] - 22.
- Berkman ND, Lohr KN, Ansari M, et al. Methods Guide for Comparative Effectiveness Reviews. Rockville, MD: Agency for Healthcare Research and Quality; Nov, 2013. Grading the Strength of a Body of Evidence When Assessing Health Care Interventions for the Effective Health Care Program of the Agency for Healthcare Research and Quality: An Update. (Prepared by the RTI-UNC Evidence-based Practice Center under Contract No 290-2007-10056-I) Chapters available at: www
.effectivehealthcare .ahrq.gov/reports/final.cfm. - 23.
- Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines 6. Rating the quality of evidence--imprecision. J Clin Epidemiol. 2011 Dec;64(12):1283–93. Epub: 2011/08/16. [PubMed: 21839614]
- 24.
- Guyatt GH, Oxman AD, Vist G, et al. GRADE guidelines: 4. Rating the quality of evidence--study limitations (risk of bias). J Clin Epidemiol. 2011 Apr;64(4):407–15. Epub: 2011/01/21. [PubMed: 21247734]
- 25.
- Owens DK, Lohr KN, Atkins D, et al. AHRQ series paper 5: grading the strength of a body of evidence when comparing medical interventions--Agency for Healthcare Research and Quality and the Effective Health-Care Program. J Clin Epidemiol. 2010 May;63(5):513–23. Epub: 2009/07/15. [PubMed: 19595577]
- 26.
- Atkins D, Chang SM, Gartlehner G, et al. Assessing applicability when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. 2011 Nov;64(11):1198–207. Epub: 2011/04/06. [PubMed: 21463926]
- 27.
- Touchette DR, Masica AL, Dolor RJ, et al. Safety-focused medication therapy management: a randomized controlled trial. J Am Pharm Assoc (2003). 2012 Sep-Oct;52(5):603–12. Epub: 2012/10/02. [PubMed: 23023840]
- 28.
- Grymonpre RE, Williamson DA, Montgomery PR. Impact of a pharmaceutical care model for non-institutionalised elderly: results of a randomised, controlled trial. Int J Pharm Pract. 2001;4:235–41.
- 29.
- Chrischilles EA, Carter BL, Lund BC, et al. Evaluation of the Iowa Medicaid pharmaceutical case management program. J Am Pharm Assoc (2003). 2004 May-Jun;44(3):337–49. Epub: 2004/06/12. [PubMed: 15191244]
- 30.
- Winston S, Lin YS. Impact on drug cost and use of Medicare part D of medication therapy management services delivered in 2007. J Am Pharm Assoc (2003). 2009 Nov-Dec;49(6):813–20. Epub: 2009/10/28. [PubMed: 19858047]
- 31.
- Witry MJ, Doucette WR, Gainer KL. Evaluation of the pharmaceutical case management program implemented in a private sector health plan. J Am Pharm Assoc (2003). 2011 Sep-Oct;51(5):631–5. Epub: 2011/09/08. [PubMed: 21896463]
- 32.
- Carter BL, Barnette DJ, Chrischilles E, et al. Evaluation of hypertensive patients after care provided by community pharmacists in a rural setting. Pharmacotherapy. 1997 Nov-Dec;17(6):1274–85. Epub: 1997/12/17. [PubMed: 9399611]
- 33.
- Barnette DJ, Murphy CM, Carter BL. Clinical skill development for community pharmacists. J Am Pharm Assoc (Wash). 1996 Sep;NS36(9):573–80. Epub: 1996/09/01. [PubMed: 8824077]
- 34.
- Chisholm-Burns MA, Kim Lee J, Spivey CA, et al. US pharmacists' effect as team members on patient care: systematic review and meta-analyses. Med Care. 2010 Oct;48(10):923–33. Epub: 2010/08/20. [PubMed: 20720510]
- 35.
- Bradley EH, Curry LA, Ramanadhan S, et al. Research in action: using positive deviance to improve quality of health care. Implement Sci. 2009;4:25. Epub: 2009/05/12. [PMC free article: PMC2690576] [PubMed: 19426507]
- 36.
- Brown CA, Lilford RJ. The stepped wedge trial design: a systematic review. BMC Med Res Methodol. 2006;6:54. Epub: 2006/11/10. [PMC free article: PMC1636652] [PubMed: 17092344]
Figures
Tables
Table APopulations, interventions, comparators, outcomes, timing, and settings
| PICOTS | Inclusion and Exclusion Criteria and Relevant Factors for Study Abstraction |
|---|---|
| Populations |
Inclusion criteria
Exclusion criteria
Relevant factors
|
| Interventions |
Inclusion criteria
Exclusion criteria
Relevant factors
|
| Comparators |
Inclusion criteria
|
| Outcomes |
Inclusion criteria
|
| Timing |
Inclusion criteria
Exclusion criteria
|
| Setting |
Inclusion criteria
Exclusion criteria
Relevant factors
|
MTM = medication therapy management; PICOTS = populations, interventions, comparators, outcomes, timing, and setting; PHQ9 = Patient Health Questionnaire 9.
Table BDefinitions of the grades of overall strength of evidence
| Grade | Definition |
|---|---|
| High | High confidence that the evidence reflects the true effect. Further research is very unlikely to change our confidence in the estimate of effect. |
| Moderate | Moderate confidence that the evidence reflects the true effect. Further research may change our confidence in the estimate of the effect and may change the estimate. |
| Low | Low confidence that the evidence reflects the true effect. Further research is likely to change our confidence in the estimate of the effect and is likely to change the estimate. |
| Insufficient | Evidence either is unavailable or does not permit estimation of an effect. |
Table CSummary of findings and strength of evidence for intermediate outcomes of MTM interventions
| Intermediate Outcome | Study Design: No. Studies (N Patients Analyzed) | Strength of Evidence | Supporting Judgment | Findings and Direction of Effect |
|---|---|---|---|---|
| Anticoagulation | RCT: 1 (10) | Insufficient | Medium study limitations, consistency unknown (single study), direct, imprecise | Therapeutic INR achieved, 100% vs. 16.7%; p = 0.048. |
| HbA1c | RCT: 2 (102) | Insufficient | Medium study limitations, inconsistent, direct, imprecise | One trial with significantly greater percentage of patients with HbA1c <7.5% at 12 months. |
| Cohort: 2 (2,688) | Insufficient | High study limitations, inconsistent, direct, imprecise | One study: adjusted findings significant at 12 months for percentage with HbA1c <7%, but findings not maintained at 24 months. Other study: no change in mean HbA1c or percentage <7% at 6 months. | |
| Low-density lipoprotein cholesterol | RCT: 1 (38) | Insufficient | Medium study limitations, consistency unknown, direct, imprecise | Calculated OR, 56.00; 95% CI, 5.583 to 561.753. |
| Cohort: 2 (3,062) | Insufficient | High study limitations, inconsistent, direct, imprecise | One study: adjusted difference in difference coefficient,1.95; 95% CI, 0.81 to 4.84; p = 0.13. Other study: calculated OR for achieving LDL goal,1.392; 95% CI, 1.160 to 1.670; p <0.001. | |
| BP | RCT: 1 (53) | Insufficient | Medium study limitations, consistency unknown, direct, imprecise | MTM group 28 times more likely to achieve BP goals than controls. |
| Cohort: 2 (2,507) | Insufficient | High study limitations, consistent within design but inconsistent with RCT, direct, imprecise | MTM group less likely to achieve BP goals than controls. | |
| Drug therapy problems identified | Cohort: 1 (582) | Insufficient | High study limitations, consistency unknown, indirect, imprecise | Risk difference, 6.1%; calculated p = 0.062. |
| Number of drug therapy problems resolved | Cohort: 1 (120) | Insufficient | High study limitations, consistency unknown, indirect, imprecise | Calculated mean difference, -1.00; 95% CI, -1.967 to -0.033; p = 0.04. |
| Medication adherence measured as proportion adherent to a threshold | RCT: 1 (69) | Insufficient | Medium study limitations, consistency unknown, direct, precise | 100% of intervention patients and 88.9% of controls were adherent; p = 0.115. |
| Cohort: 2 (224 to 200,722) | Low for benefit | High study limitations, inconsistent, direct, precise | Two studies with findings in opposite direction; larger study showing range of ORs for medication-specific adherence depending on medication. For comparison of PDP vs. controls, ORs ranged from 0.99 to 1.43; 95% CIs ranged from (0.90, 1.08) to (1.26, 1.62). For comparison of MA-PD vs. controls ORs ranged from 1.10 to 1.40; 95% CIs ranged from (0.83, 1.24) to (1.29, 1.52). For clinic-based MTM vs. usual care for adherence to aspirin, odds of adherence ranged from 5.981 (95% CI, 0.284 to 126.030; p = 0.250) during the intervention to 1.17 1 year after the intervention (95% CI, 0.072 to 18.903; p = 0.912). | |
| Medication adherence measured as percentage of prescribed doses taken | Cohort: 2 (120 to 4,500) | Low for benefit for adherence to treatment for hypertension and dyslipidemia Insufficient for treatment of patients with diabetes, depression, and asthma | High study limitations, inconsistent, direct, imprecise | Calculated mean difference from small study, -0.040; 95% CI, -0.101 to 0.021; p = 0.201. Larger study found a small (difference in adherence ∼4.6%) but statistically significant effect of MTM on adherence to medications for some (2 of 5) conditions but no significant effect for the other conditions. |
| Medication adherence using self-report measures | RCT: 1 (292) | Insufficient | Medium study limitations, consistency unknown, direct, imprecise | Calculated mean difference, 0.090; 95% CI, -0.076 to 0.256; p = 0.289. |
| Medication adherence, miscellaneous measures | RCT: 2 (365) | Insufficient | Medium study limitations, inconsistent, direct, imprecise | Two studies with opposite direction of effect, both with nonsignificant differences between groups. |
| Medication Appropriateness General Index Scores | RCT: 1 (208) | Low for benefit | Low study limitations, consistency unknown, direct, precise | Improvement in MTM group from score of 17.7 to 13.4 at 3 months and 12.8 at 12 months. |
| Medication-specific appropriateness | RCT: 2 (261) | Insufficient | Medium study limitations, inconsistent, direct, imprecise | Significant improvement in appropriateness in the MTM group for some medications but not others. |
| Medication dosing | RCT: 1 (56) | Low for benefit | Medium study limitations, consistency unknown, indirect, precise | Mean difference, -2.2 doses; calculated 95% CI, -3.738 to-0.662. |
| Goals of therapy | 0 | NA | NA | NA |
| Patient engagement | 0 | NA | NA | NA |
BP = blood pressure; CI = confidence interval; HbA1c = hemoglobin A1c; INR = International Normalized Ratio; LDL = low-density lipoprotein; MA-PD = Medicare Advantage Part D Plan; MTM = medication therapy management; NA = not applicable; OR = odds ratio; PDP = Medicare Part D Plan; RCT = randomized controlled trial.
Table DSummary of findings and strength of evidence for patient-centered outcomes of MTM interventions
| Patient-Centered Outcome | Study Design: No. Studies (N Patients Analyzed) | Strength of Evidence | Supporting Judgment | Findings and Direction of Effect |
|---|---|---|---|---|
| Adverse drug events | RCT: 2 (806) | Insufficient | Medium study limitations, inconsistent, direct, imprecise | Direction and magnitude of effect differs between the 2 trials. |
| Cognitive and physical function | RCT: 1 (133) | Insufficient | Medium study limitations, consistency unknown, direct, imprecise | No significant differences between arms. |
| Affective function | RCT: 2 (181) | Insufficient | Medium study limitations, inconsistent, direct, imprecise | One study with no significant calculated mean differences in depression or anxiety scores; the other study with significant differences in mean depression and anxiety scores, but no significant difference in percentage achieving a depression remission. |
| Mortality | RCT: 1 (181) | Insufficient | Medium study limitations consistency unknown, direct, imprecise | OR, 0.59; 95% CI, 0.12 to 2.49; p = 0.48. |
| Cohort: 2 (173,329) | Insufficient | High study limitations, inconsistent (magnitude), direct, imprecise | One study: OR, 0.5; 95% CI, 0.3 to 0.9. Second study: adjusted HR, 0.92; 95% CI, 0.87 to 0.96; p < 0.001. | |
| Gastrointestinal bleeding events | Cohort: 1 (unclear) | Insufficient | High study limitations, consistency unknown, direct, imprecise | RRR, 60%; p = 0.001. |
| General health-related quality of life domains other than vitality and emotional role functioning | RCT: 3 (1,169) | Low for no benefit | Medium study limitations; consistent for physical role functioning, general health perceptions, and social functioning domains; inconsistent for physical functioning, bodily pain, and mental health domains; direct; precise | Variable mean difference with CIs consistently spanning the null effect. |
| General health-related quality of life for vitality and emotional role functioning domain | RCT: 3 (1,169) | Insufficient | Medium study limitations, consistent, direct, imprecise (not corrected for multiple comparisons or wide CIs) | Vitality: Mean difference of 2.797; 95% CI, 0.655 to 4.939; p = 0.010. Emotional role functioning: Mean difference of 5.386; 95% CI, -7.244 to 18.013. |
| Condition-specific health-related quality of life (diabetes) | RCT: 1 (73) | Insufficient | Medium study limitations, consistency unknown, direct, imprecise, | Nonsignificant improvement of 0.1 point on a 5-point scale in the intervention group compared with no change in the control group. |
| Patient satisfaction | RCT: 3 (1,463) | Low for no benefit | Medium study limitations, consistent, direct, precise | No differences on 17 of 21 items of patient satisfaction; 4 statistically significant differences ranged in magnitude from -0.15 to -0.36, favoring MTM. |
| Activities of daily living | 0 | NA | NA | NA |
| Work or school absenteeism | 0 | NA | NA | NA |
| Patient and caregiver participation in medical care and decisionmaking | 0 | NA | NA | NA |
CI = confidence interval; HR = hazard ratio; MTM = medication therapy management; NA = not applicable; OR = odds ratio; RCT = randomized controlled trial; RRR = relative risk ratio.
Table ESummary of findings and strength of evidence for resource-utilization outcomes of MTM interventions
| Resource-Utilization Outcome | Study Design: No. Studies (N Patients Analyzed) | Strength of Evidence | Supporting Judgment | Study Findings and Direction of Effect |
|---|---|---|---|---|
| Use of generics | Cohort: 1 (63,198 to 200,722) | Insufficient | High study limitations, consistency unknown, direct, imprecise | Odds range from -0.01 to 0.006. |
| Medication costs: patient copayments | RCT: 1 (NR) | Insufficient | Medium study limitations, consistency unknown, indirect, precision cannot be determined | Calculated mean difference, -64 USD; variance not calculable. |
| Cohort: 1 (1,606) | Insufficient | High study limitations, consistency unknown, indirect, precise | Calculated mean difference for MTM vs. same-country control, 80.40 USD; 95% CI, 10.43 to 150.37; p = 0.024. Calculated mean difference for MTM vs. different country control, 88.60 USD; 95% CI, 24.61 to 152.59; p = 0.007. | |
| Medication costs: health plan expenditures | RCT: 3 (965) | Low for benefit | Medium study limitations, consistent, indirect, imprecise | Mean difference varies from -34 CAD to -293 USD over 6 months. |
| NRCT and cohort: 5 (120 to 200,722) | Insufficient | High study limitations, inconsistent, indirect, imprecise | Mean difference varies from -800 USD over 1 year to 425 USD over 2 years. | |
| Medication costs: total outlays | RCT: 6 (2,636) | Insufficient | Medium study limitations, inconsistent, indirect, imprecise | Mean difference varies from -20.16 USD to +5.25 USD per month. |
| Cohort: 2 (177,565) | Insufficient | High study limitations, inconsistent, indirect, imprecise | Mean difference varies from -563 USD to +310 USD annually. | |
| Medication costs: medication costs plus other expenditures | RCT: 2 (996) | Insufficient | Medium study limitations, inconsistent, indirect, imprecise | Differences in mean costs range from -8.1 CAD to 1,947 USD. |
| NRCT and cohort: 3 (5,300) | Insufficient | High study limitations, inconsistent, indirect, imprecise | Differences in mean costs range from -1,039 to 1,100 USD. | |
| Number of outpatient visits | RCT: 3 (2,208) | Insufficient | Medium study limitations, inconsistent, indirect, precise | Standardized mean difference, 0.049; 95% CI, -0.034 to 0.133; p = 0.247; I2 = 0. |
| Cohort: 1 (4,500) | Insufficient | High study limitations, consistency unknown, indirect, imprecise | Calculated mean difference, 2.48; 95% CI, 1.674 to 3.286; p <0.001. | |
| Outpatient costs | RCT: 3 (2,050) | Insufficient | Medium study limitations, inconsistent, indirect, imprecise | Variable estimates. |
| Number of laboratory tests | RCT: 2 (1,842) | Insufficient | Medium study limitations, inconsistent, indirect, imprecise | Differences range from +0.15 to -1.6 tests. |
| Costs of laboratory tests | RCT: 3 (2,050) | Insufficient | Medium study limitations, inconsistent, indirect, imprecise | Differences range from +15 CAD to -140 USD. |
| Number of emergency department visits | RCT: 3 (1,552) | Insufficient | Medium study limitations, inconsistent, direct, imprecise | Mean difference ranges from -0.7 (p not significant) to -0.03 (95% CI, -0.113 to 0.053). |
| Observational: 3 (795 to 200,722) | Insufficient | High study limitations, inconsistent, direct, imprecise | Adjusted OR ranges from 0.89 (95% CI, 0.6 to 1.3) to 1.09; mean difference (1 study), 0.04; 95% CI, -0.043 to 0.123; p = 0.346. | |
| Costs of emergency department visits | RCT: 2 (996) | Insufficient | Medium study limitations, consistent, direct, imprecise | Mean difference ranges from -52 USD to -5.6 CAD. |
| Cohort: 1 (150,470 to 200,722) | Insufficient | High study limitations, consistency unknown, direct, imprecise | Difference ranges from -16 USD to +12.8 USD. | |
| Hospitalization: number | RCT: 3 (2,208) | Low for no benefit | Medium study limitations, consistent, direct, precise | Mean difference, 0.037; 95% CI, -0.004 to 0.080. |
| Cohort: 1 (4,500) | Low for benefit | High study limitations, consistency unknown, direct, precise | Mean difference, -0.21; 95% CI, -0.265 to -0.155; p <0.001. | |
| Hospitalization: risk | RCT: 1 (556) | Insufficient | Low study limitations, consistency unknown, direct, imprecise | OR for basic MTM vs. usual care, 2.069; 95% CI, 1.104 to 3.878; p = 0.02. OR for enhanced MTM vs. usual care, 1.345; 95% CI, -0. 693 to 2.609; p = 0.381. |
| Cohort—CHF, COPD, or unspecified: 3 (795 to 200,722) Diabetes: 1 (150,470) | CHF, COPD, or unspecified: insufficient Diabetes: low for benefit | High study limitations, inconsistent, direct, imprecise High study limitations, consistency unknown, direct, precise | Adjusted OR ranges from 0.90 to 1.4. OR ranges from 0.91 to 0.93. | |
| Hospitalization: rate (patients with heart failure and home medicine review) | Cohort: 1 (5,717) | Low for benefit | High study limitations, consistency unknown, direct, precise | Adjusted HR, 0.55; 95% CI, 0.39 to 0.77. |
| Costs of hospitalization | 3; 2,151 (2,050) | Insufficient | Medium study limitations, inconsistent, direct, imprecise | Inconsistent direction of effect but consistent in lack of significant effect. |
| CHF or COPD: 1 (169,099 to 200,722) Diabetes: 1 (150,470) | Insufficient for CHF or COPD Low for benefit for diabetes | High study limitations, consistency unknown, direct, imprecise High study limitations, consistency unknown, direct, precise | Differences range from -526 USD to 200 USD for CHF and COPD. Differences range from -363 USD to -399 USD for diabetes. | |
| Length of hospital stay | RCT: 1 (208) | Insufficient | Low study limitations, consistency unknown, direct, imprecise | MTM reduced length of stay 1.8 days. |
CAD = Canadian dollar; CHF = congestive heart failure; CI = confidence interval; COPD = chronic obstructive pulmonary disease; HR = hazard ratio; MTM = medication therapy management; NR = not reported; NRCT = nonrandomized controlled trial; OR = odds ratio; RCT = randomized controlled trial; USD = U.S. dollar.