NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
The thyroid, like any other structure, may be the seat of an acute or chronic suppurative or non-suppurative inflammation. Various systemic infiltrative disorders may leave their mark on the thyroid gland as well as elsewhere. Infectious thyroiditis is a rare condition, usually the result of bacterial invasion of the gland. Its signs are the classic ones of inflammation: heat, pain, redness, and swelling, and special ones conditioned by local relationships, such as dysphagia and a desire to keep the head flexed on the chest in order to relax the paratracheal muscles. The treatment is that for any febrile disease, including specific antibiotic drugs if the invading organism has been identified and its sensitivity to the drug established. Otherwise, a broad-spectrum antibiotic may be used. Surgical drainage may be necessary and a search for a pyriform sinus fistula should be made, particularly in children with thyroiditis involving the left lobe. Important to differentiate from the acute bacterial infection of acute suppurative thyroiditis (AST), is subacute (granulomatous) thyroiditis (SAT) which is far more common than AST and is characterized by a more protracted course, usually involving the thyroid symmetrically. The gland is also swollen and tender, and the systemic reaction may be severe, with fever and an elevated erythrocyte sedimentation rate. During the acute phase of the disorder, tests of thyroid function often disclose a suppression of TSH, increased serum concentrations of T4, T3, and thyroglobulin while a diminished thyroidal RAIU is observed. The cause of SAT has been established in only a few instances in which a viral infection has been the initiating factor. There may be repeated recurrences of diminishing severity. Usually, but not always, the function of the thyroid is normal after the disease has subsided. Subacute thyroiditis may be treated with rest, non-steroidal anti-inflammatory drugs or aspirin, and thyroid hormone. If the disease is severe and protracted, it is usually necessary to resort to administration of glucocorticoids, but recurrence may follow their withdrawal. It is precisely the observational nature of SAT therapy combined with the use of glucocorticoids that make it so critical to rule out the bacterial etiology of AST in the patient presenting with a painful thyroid. Riedel's thyroiditis is a chronic sclerosing replacement of the gland that is exceedingly rare. The process extends to adjacent structures, making any surgical intervention very difficult and potentially harmful. The exact cause of Riedel’s thyroiditis remains unknown, and no specific treatment is available beyond limited resection of the thyroid gland to relieve the symptoms of tracheal or esophageal compression. The use of anti-inflammatory medical treatments has been demonstrated to have significant benefits to outcome. Sarcoidosis may involve the thyroid, and amyloid may be deposited in the gland in quantities sufficient to cause goiter. In all of these diseases, it may be necessary to give the patient levothyroxine replacement therapy if the function of the gland has been impaired. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
CLASSIFICATION
The diagnostic term thyroiditis includes a group of inflammatory or inflammatory-like conditions. The terminology that has been employed is confusing, and no classification is ideal. We prefer the following nomenclature, which takes into account the cause when known.
1, Infectious thyroiditis, also referred to as either acute or chronic, and which in fact may be either, along with the qualifying term suppurative (AST), nonsuppurative, or septic thyroiditis. It includes all forms of infection, other than viral, and is caused by invasion of the thyroid by bacteria, mycobacteria, fungi, protozoa, or flatworms. The disorder is rare.
2. De Quervain's thyroiditis, commonly known as (painful) subacute thyroiditis (SAT) but also termed subacute nonsuppurative thyroiditis, granulomatous, pseudotuberculous, pseudo-giant cell or giant cell thyroiditis, migratory or creeping thyroiditis, and struma granulomatosa. This condition, most likely of post viral origin, lasts for a week to a few months, with a tendency to recur.
3. Autoimmune thyroiditis, commonly referred to as chronic, Hashimoto's, or lymphocytic thyroiditis and also known as lymphadenoid goiter and struma lymphomatosa. This indolent disease usually persists for years and in the Western world is the principal cause of non-iatrogenic primary hypothyroidism. Nonspecific focal thyroiditis, characterized by local lymphoid cell infiltration without parenchymal changes, may be a variant of the autoimmune disease. The condition is covered in detail in the Endotext chapter on Hashimoto’s Thyroiditis.
Another form of thyroiditis, also believed to be of autoimmune cause, has been described. It has been variably referred to as painless, silent, occult, subacute, subacute nonsuppurative, and atypical (silent) subacute thyroiditis, as well as “hyperthyroiditis”, transient thyrotoxicosis with low thyroidal RAIU and lymphocytic thyroiditis with spontaneously resolving hyperthyroidism. There is no agreement on an inclusive name. The features of this disease entity overlap with de Quervain's thyroiditis and Hashimoto's thyroiditis. The clinical course, with the exception of a very high erythrocyte sedimentation rate and pain in the thyroid are indistinguishable from de Quervain's thyroiditis. Yet, histologically, the condition cannot be differentiated from a milder form of Hashimoto's disease. This condition often occurs in the postpartum period and is also termed postpartum thyroiditis. All forms of autoimmune thyroiditis are considered in other Endotext chapters.
4. Riedel's thyroiditis, another disorder of unknown etiology. Synonyms include Riedel's struma, ligneous thyroiditis and invasive fibrous or chronic sclerosing thyroiditis. This condition is characterized by overgrowth of connective tissue that often extends into neighboring structures.
5. Miscellaneous varieties of thyroid inflammation or infiltration including local manifestations of a generalized disease processes. Among these are sarcoid and amyloid involvement of the thyroid. Radiation and direct trauma to the thyroid gland may also cause thyroiditis. Rarely, acute thyroiditis has been reported after parathyroid surgery (1).
INFECTIOUS THYROIDITIS
The thyroid gland is remarkably resistant to infection. This has been attributed to its high vascularity, lymphatic drainage, the presence of large amounts of iodine in the tissue, the fact that hydrogen peroxide is generated within the gland as a requirement for the synthesis of thyroid hormone, and its normal encapsulated position away from external structures. Acute suppurative thyroiditis (AST) is a rare condition, reported to account for 0.1-0.7% of thyroid disease (2,3) which may result in up to 12% or higher mortality if left untreated (2,4,5). In the pre-antimicrobial era, the case fatality rate of AST was as high as 22% (6) which makes early recognition of AST crucial in order to prevent life-threatening complications.
Predisposing Factors
Acute thyroiditis may involve a normal gland, arise in a multinodular goiter (7) or even Hashimoto’s thyroiditis. Presence of certain predisposing factors (Table 1) makes the gland susceptible to infections. A persistent fistula from the pyriform sinus may make the left lobe of the thyroid particularly susceptible to abscess formation, particularly in children (8-18). In one study, 7 out of 48 (15%) of children undergoing piriform sinus fistula surgery presented with a thyroid abscess (19). The possibility of a persistent thyroglossal duct should be considered for patients with midline infections (20). The infection of the thyroid gland is a result of direct extension from an internal fistula from the pyriform sinus (11,13,14,21-24). This tract is thought to represent the course of migration of the ultimobranchial body from the site of its embryonic origin in the fifth pharyngeal pouch (15). Careful histopathological studies of these fistulae have demonstrated that they are lined by squamous columnar or ciliated epithelium and occasionally form branches in the thyroid lobe (11,14). In addition, occasional cells positive for calcitonin have been found in the fistulae and increased numbers of C-cells were noted in the thyroid lobe at the point of termination of the tract. The predominance of acute thyroiditis in the left lobe of the thyroid gland, particularly in infants and children, is explained by the fact that the right ultimobranchial body is often atrophic and does not develop in the human (as well as in other species such as reptiles). Ninety-two percent of cases involve the left thyroid lobe, 6% the right lobe, and 2% are bilateral (25). The left-sided predominance may be due to embryological asymmetry of the transformation of the fourth branchial arch to form the aortic and innominate arteries (26) or to poor development of the ultimobrachial body on the right side of the embryo (27).
Recurrent left-sided thyroid abscess has also been reported due to a fourth branchial arch sinus fistula (28). A review of 526 cases of congenital fourth branchial arch anomalies (29) noted that they presented with acute suppurative thyroiditis in 45% of cases. Acute thyroiditis from a periapical abscess of an inferior molar has been reported (30). Acute suppurative thyroiditis associated with thyroid metastasis from esophageal cancer has also been reported (31). Acute thyroiditis can occur in an immuno-compromised state, predisposing them to unusual bacteria such as nocardia (32,33), salmonella(34) and fungi like candida (35-38), coccidioides immitis (39) and aspergillus (40). Among patients > 20 years old in the study by Yu et al. 32/66 (49%) were immunocompromised (5). Occasionally, acute bacterial suppurative thyroiditis occurs in children receiving cancer chemotherapy (41). Rarely, infection will occur in a cystic or degenerated nodule (42,43) or presumed hematogenous spread in the setting of endocarditis (44). Acute thyroiditis has arisen as the initial presentation of juvenile systemic lupus erythematosus (45) and has also occurred due to septic emboli derived from infective endocarditis (44,46,47). As will be discussed, the principal differential diagnosis is generally between acute (AST), infectious, and subacute (SAT), meaning post-viral (non-infectious) inflammation of the gland.
Table 1.
Predisposing Factors for Acute Thyroiditis
| Pyriform sinus fistula |
| Third and fourth arch abnormalities |
| Immunocompromised states |
| Rarely: endocarditis, tooth abscess, fine needle aspiration |
Etiology
Virtually any bacterium can infect the thyroid (Table 2), but at times no causative organism can be demonstrated. Streptococcus, staphylococcus, pneumococcus, salmonella (34,48-51), Klebsiella (52), Bacteroides, Treponema pallidum, Pasteurella spp (53,54), porphyromonas (55), Eikenella (51,56-58), and Mycobacterium tuberculosis (59-63) have all been described. Rare cases of disseminated nocardia infections with thyroiditis along with subcutaneous nodules have been reported (32,64-66). This subject has been extensively reviewed (21,36,67). In addition, certain fungi, including Coccidioides immitis (39), Aspergillus (40,68), Actinomyces (69-71), Blastomyces (72,73), Candida albicans (35-38), Actinobacter baumanii (5), Cryptococcus (74), and Pneumocystis (75) have also been associated with thyroiditis. In a recent meta-analysis, 94% of the patients with fungal AST were immunocompromised (76). Most of these patients who were immunocompromised either had malignancy or AIDS (33,34,77,78). Rarely acute suppurative thyroiditis is due to thyroid abscess with deep neck infection (79) and fistulous connection (80). Coccidioides immitis from infected donor tissue in an immunocompromised host has also been reported (39). Thyroid abscess due to clostridium perfringens has been reported (81) and clostridium septicum is almost always associated with carcinoma of the colon (82). Metastatic breast cancer has been described as presenting clinically with acute thyroiditis (83). Hashimoto’s disease (84,85), large goiters (86), or thyroid cancer could predispose individuals (87), but AST could also arise by hematogenous or lymphatic spread or by iatrogenic infections after fine needle aspiration biopsy (FNA). Recently, the role of diagnostic fine needle thyroid aspiration has been emphasized as a factor in the cause of acute suppurative thyroiditis (81,88-92). Care should be taken when performing FNA in patients who may be susceptible to tracking of infection into the thyroid.
Table 2.
Microbiology of Acute Suppurative Thyroiditis
| Usual Organisms Aerobic: Staphylococcus aureus, Streptococcus pyogenes, Streptococcus epidermidis, Streptococcus pneumoniae, Escherichia coli (111) |
| Anaerobic: Clostridium septicum (82), gram-negative bacilli, Peptostreptococcus spp. |
| Rare Organisms |
| Bacterial: Atypical mycobacteria, Clostridium perfringens (81), Eikenella corrodens, Enterobacteriaceae, Haemophilus influenza, Klebsiella spp., Mycobacterium tuberculosis, Porphyromonas (55), Salmonella spp., Streptococcus viridans, Treponema pallidum, Brucella. (112), Lactococcus (113), Citrobacter freundii (114), Nocardia |
| Fungal: Aspergillus spp., Blastomyces, Candida spp., Coccidioides immitis, Pneumocystis jiroveci |
| Parasitic: Trypanosoma (21), Echinococcus spp., |
Pathology
Pathological examination reveals characteristic changes of acute inflammation. With bacterial infections, heavy polymorphonuclear and lymphocytic cellular infiltrate is found in the initial phase, often with necrosis and abscess formation. Fibrosis is prominent as healing occurs. In material obtained by fine needle aspiration, the infectious agent may be seen on a gram, acid fast or appropriate fungal stain (13), and grown out in culture for antibiotic sensitivity assessment.
Clinical Manifestations
Although acute thyroiditis is quite rare (about two patients per year in a large tertiary care hospital), cases of suppurative thyroiditis are increasing due to the higher incidence of immune-compromised patients. A recent meta-analysis of about 200 cases of AST published in 148 articles between 2000-2020 noted that the median duration of symptoms prior to presentation was 6 days [IQR 3-12 days] in bacterial AST and longer symptom duration in fungal (21 days [IQR 12-26]) and tuberculous AST (30 days [18-60]) (76).
Recently, another case series of six otherwise healthy adult patients without anatomic anomalies with AST was published (93). Of the 6 patients, 5 were female and the median age at presentation was 51 years (28-73 years). None had third or fourth left branchial cleft anomalies or an immunosuppressed state. All patients were successfully treated with antibiotics for an average of 13.5 days (10–41 days), drainage occurred in three, and surgery was performed twice in the acute phase in one and at a later state in another. The length of hospital stay was 7.5 days (4–79 days). AST has been estimated to be much more common in the pediatric age group because of its relationship with pyriform sinus fistulae, where 90% of lesions develop in the left lobe of the thyroid (44) although it is still quite unusual. It has been estimated that about 8% of cases occur in adulthood (25,44,94-99). The dominant clinical symptom is pain in the region of the thyroid gland that may subsequently enlarge and become palpably hot and tender. The patient is unable to extend the neck and often sits with the neck flexed in order to avoid pressure on the thyroid gland. Swallowing is painful. There are usually signs of infection in structures adjacent to the thyroid, local lymphadenopathy as well as temperature elevation and, if bacteremia occurs, chills. Gas formation with suppurative thyroiditis has been noted (100-103). Symptoms are generally more obvious in children than in adults. Adults may present with a vague slightly painful mass in the thyroid region without fever, which may raise the possibility of a malignancy. Suppurative thyroiditis may even spread to the chest producing necrotizing mediastinitis and pericarditis in the absence of a pyriform sinus fistula (79,104-106). It may occur more commonly in the fall and winter following upper respiratory tract infections.
White cell counts are elevated in 80% of bacterial AST but in only 40% and 26% of fungal and tuberculous AST respectively.
Previous reviews have found that thyrotoxicosis was not common in AST (5). The recent meta-analysis by Lafontaine et al. found that 42% of bacterial and 40% of fungal AST cases were thyrotoxic at presentation and at least 36% of bacterial AST cases had significant thyrotoxicosis with fT4 more than twice the upper limit of normal (76). Tuberculous AST was least likely to be associated with hyperthyroidism (12%). Thyrotoxicosis due to AST is plausible, given the pathogenesis of AST and the release of pre-formed thyroid hormone secondary to the destruction of thyroid follicles. It is therefore important to consider AST in patients with apparent hyperthyroidism and a painful neck, making the differentiation with SAT difficult (76).
In general, there are no signs or symptoms of hyper- or hypothyroidism. However, exceptions to both have been reported particularly if the thyroiditis is generalized, such as occurs with fungal processes (74) or mycobacterial infections. At times, even in patients with bacterial thyroiditis, destruction of the thyroid gland is extensive enough to release thyroid hormone in amounts sufficient to cause symptomatic thyrotoxicosis (54,59). Associated thyrotoxicosis has also been reported in children and adults (17,54,88,107); in one series, 12% presented with thyrotoxicosis, and 17% were said to be hypothyroid (5). This variety of thyroid function findings clearly increases the difficulty of differentiating AST from SAT as both present with thyroidal pain. Unique presentations of AST have been reported where initial thyrotoxicosis has been followed by hypothyroidism and spontaneous normalization of thyroid function after treatment of the AST (55,108). Complications described in various cases included internal jugular vein thrombophlebitis, mediastinitis and pericarditis, esophageal perforation, fistula and obstruction, laryngeal edema requiring tracheostomy, obstructive symptoms, Horner’s syndrome and multisystem organ failure (76).
Diagnosis
Pain in the anterior neck will usually lead to a consideration of thyroiditis. The meta-analysis by Lafontaine et al. showed that the most common symptoms in bacterial AST were neck pain (89%) and fever (82%), followed by dysphagia (46%). Neck pain and fever were the most common symptoms in all cases, occurring in 78% and 63% of fungal AST, and 40% and 48% of tuberculous AST cases respectively (76). Since the differential diagnosis will lie between acute suppurative thyroiditis and subacute thyroiditis, it is critical to compare the history, physical, and particularly laboratory data in these two conditions (see Table 4). In general, the patient with acute thyroiditis appears septic, has greater and more localized pain in the thyroid gland, may have an associated upper respiratory infection, has lymphadenopathy and may be immuno-compromised. Localization of tenderness to the left lobe should suggest the possibility of an infection as should any erythema or apparent abscess formation. The presence of an elevated white blood count with a shift to the left would argue for infection, however, elevations in sedimentation rate are common in both acute and subacute thyroiditis. As mentioned above, patients with bacterial thyroiditis are usually euthyroid but a thyrotoxic presentation has been noted in 8-12% (5,109) and hypothyroidism was noted in 17% of one series (109). Thyrotoxicosis is clearly more common with longer duration, 52% at 7 days and 65% by 30 days of neck pain in patients with subacute thyroiditis (110). The thyrotoxic presentation therefore poses a difficult differential diagnostic problem to separate AST from SAT, which may have significant impact in the selection of initial therapy.
Depending on the patient’s age and clinical circumstances, one may wish to proceed with invasive or non-invasive studies. Discriminating tests differentiating AST from SAT have been considered a radio-nuclide uptake (RAIU) and/or scanning usually showing a very low uptake value in subacute thyroiditis with a normal value found in the patient with localized mild bacterial thyroiditis (21). More frequently however both conditions are associated with a low 123-I uptake at initial presentation (33,108,115,116) limiting the power of iodine based nuclear studies to effectively differentiated these two conditions.
In the early inflammatory phase of AST, when obvious abscess formation is not evident, an ultrasound may show a localized hypoechoic process with an obscure border and effacement between the thyroid and surrounding perithyroidal tissues(117). During the acute inflammatory stage of AST, clear cut abscess formation is noted in the affected thyroidal tissue (117). Perithyroidal unifocal hypoechoic space and effacement of the plane between the thyroid and perithyroidal tissues have been noted to be specific signs of AST (117). Alternatively, the application of sonoelastography may reveal very stiff lesions corresponding to the areas of the thyroid which are especially painful (118) during acute phases of the AST episode which soften significantly as the patient responds to treatment (118). As AST resolves with appropriate treatment, ultrasound images may demonstrate deformity of the gland characterized by atrophy of the affected lobe, air/fluid levels in the thyroidal tissue and scarring of the perithyroidal tissues (117).
A CT scan may be useful in identifying the location of the abscess, but is required only in unusual situations (119). The CT findings also vary with the stage of AST. In the early inflammatory stage, nonspecific low density areas in the swollen thyroid along with potential tracheal displacement may be seen (117). In the acute inflammatory stage, a CT can also demonstrate edema of the ipsilateral hypopharynx, and abscess formation. In the late inflammatory stage, deformity of the thyroid, atrophy of the affected lobe and scarring of the perithyroidal tissues may be observed (117). Recent reviews indicate a significant role for CT in the initial evaluation of those with AST (2,117). As outlined above, during the earliest stages of AST both CT and ultrasound findings may fail to effectively differentiate between AST and SAT. In this circumstance, the use of a fine needle aspiration (FNA) has been demonstrated to be very useful as outlined below. Localization of gallium to the thyroid gland in the course of an evaluation for a fever of unknown origin is very useful finding confirming thyroid inflammation as the source of the problem but the differential of gallium positive thyroid tissue will also include the presence of Riedel’s thyroiditis (120).
If an infectious process is identified, particularly of the left lobe of a younger individual, then a barium swallow should be performed with attention to the possibility of a fistulous tract located on the left side between the pyriform sinus and the thyroid gland. The barium swallow has very good sensitivity in detecting the presence of the fistula tracts as 89-97% of those examined in early and acute stages of AST have been confirmed with this technique (117). Other methods of documenting the presence of a fistula are also utilized. On follow up ultrasound an ‘emerging echogenic tract sign’ suggests an associated pyriform sinus thyroid fistula (121). During a CT scan procedure the patient can be asked to blow into a syringe, the so called “trumpet maneuver”, which may help to identify a piriform sinus fistula (122), a reported series suggests that timing may influence the ability of this maneuver to demonstrate the presence of a fistula as only 20% of those examined in the acute inflammatory phase revealed a fistula while 54% of those evaluated in the late inflammatory phase had a fistula documented (117) with the “trumpet maneuver”. A ‘light guided procedure’ to visualize the tract may also help (123). Transnasal flexible fiberoptic laryngoscopy has become increasingly utilized to identify the presence of fistular tracts (2). This approach has been estimated to have similar sensitivity of documenting the tracts as barium swallow and CT methods (124-126) and can also be utilized for the instillation of chemo-cauterizing agents at an appropriate time after the resolution of the acute infection (109,124,126,127).
Occasionally, pain from an infectious process elsewhere in the neck will present as anterior neck tenderness. For example, a retropharyngeal abscess may present with typical symptoms of acute thyroiditis. The thyroid gland, however, will have a normal ultrasound appearance, be normal on scanning, and only on CT scan will the retropharyngeal abscess be recognized. The tendency for the pain of thyroid inflammation to be referred to the throat or ears should be kept in mind, although recognition of the anatomic source of the problem is usually not difficult in patients with acute thyroiditis due to their localized symptoms. While patients with tuberculosis or parasitic infections tend to have a more indolent course, these infections can present with acute symptoms and this possibility should be considered if the epidemiology is consistent. For example, thyroidal echinococcosis occurs in countries in which this parasite is endemic (128). Trypanosomiasis of the thyroid has also been reported (21).
A fine needle aspiration (FNA) performed in the acute phase of AST is important as an aspirate has a superior ability to differentiate the patient with AST from those with subacute thyroiditis not only by cytological criteria and also provides appropriate bacteriologic specificity allowing smears and cultures providing a more accurate antibiotic selection (2) for the patient documented to have AST. In addition, transcutaneous drainage of the infectious material can be performed to relieve pressure on a displaced trachea in patients with a compromised airway (2). Finally FNA may be seen as the most accurate means of differential diagnosis (129) when a thyrotoxic presentation is encountered. Establishing a firm diagnosis of AST allows timely antibiotic therapy to be prescribed when a trial of glucocorticoids for empirically assumed SAT might result in both delay in diagnosis as well as initiation of a potentially wrong therapy (55).
Prompt treatment is necessary as the infection may cause destruction of the thyroid and the parathyroid glands, spread to other organs, or cause abscess rupture, vocal cord palsy and fistulae to the trachea or esophagus (130,131).
Treatment
There has been a trend toward less invasive management during active inflammation and infection (2). A recent study observed that 32% of the cases with bacterial AST were managed with antibiotics and a single needle aspiration, 3% required multiple needle aspirations, and 13% had a needle aspiration and antibiotics but subsequently required surgery. In both the immediate surgery group and those with needle aspiration and antibiotics, incision and drainage was the most common procedure (57% and 53% respectively), followed by partial thyroidectomy (30% and 40%) with or without excision of a fistula tract, and total thyroidectomy (13% and 7%). The median duration of antibiotics was 17 days (IQR 14- 30) (76).
In contrast, only 22% of cases of fungal AST went directly to surgery (11% for incision and drainage, 11% underwent a partial thyroidectomy), 56% had a single needle aspiration and antifungals, and 22% failed needle aspiration and antifungals and subsequently required surgery. The mean duration of antifungal therapy was 42 days. Of the patients with tuberculous AST, 41% had needle aspiration and antibiotics; only 3% failed needle aspiration and antibiotics and subsequently required partial thyroidectomy (76).
Despite a lack of randomized controlled trials, algorithms for acute and long-term management have been suggested by several authors. Miyauchi (115), who has extensive experience with the condition, has cautioned that consideration of the basic anomaly predisposing the patient to thyroid gland infection must be duly considered. Microscopic examination and appropriate staining of a fine needle aspirate often aid the diagnosis and choice of antibiotic therapy. The procedure is best done under ultrasound guidance so that the source of the specimen is identified. It may also serve as a mechanism for decompression of an abscess and can be repeated to facilitate healing. Some abscesses will require surgical exploration and drainage. The choice of therapy will also depend on the immune status of the patient. Systemic antibiotics are required for severe infections. Candida albicans thyroiditis may be treated with appropriate doses of amphotericin B and fluconazole. Successful antifungal combination therapy and a surgical approach for Aspergillus spp associated AST has been reported (132). The proper treatment of an acute thyroiditis in children generally requires the surgical removal of the fistula (11,13,14), although surgical treatment should be delayed until the inflammatory process is resolved (133,134). Combining this with partial thyroidectomy may further decrease the recurrence rate (12,29). In addition, a lobectomy may be the safer option as it provides an adequate identification of the recurrent laryngeal nerve in the re-operative field (135). Alternatively, fistula tract ablation can be achieved either by surgical resection which has been associated with recurrence free survival (117), or less invasively obliterated with the instillation of a chemo-cauterizing agent which has also been demonstrated to result is satisfactory outcomes (117,124,126,127). Newer, minimally invasive transoral video-laryngoscopic surgery (TOVS) (136) and endoscopy assisted surgery (137) have been reported to be safe and reliable methods of pyriform sinus fistula treatment. Ultrasound-guided aspiration with or without lavage had a good treatment effect and without adverse events for the management of AST secondary to pyriform sinus fistula (138).
Prognosis
The disease may occasionally prove fatal (106). In some patients with thyroiditis, the destruction may be sufficiently severe that permanent hypothyroidism results (7). Thus, patients with a particularly diffuse thyroiditis should have follow-up thyroid function studies performed to determine the need for thyroid hormone replacement. Surgical removal of a fistula or branchial pouch sinus (133,134) is required to prevent recurrence.
SUBACUTE THYROIDITIS
Case Illustration
J.G., a 56-year-old woman, presented to her primary care physician in January, with 4 weeks of low anterior neck pain and 2 days of fatigue, chills and shivers. She was prescribed a course of antibiotics with no relief. A non-contrast CT scan of the neck was done which showed mild diffuse thyroid enlargement, multiple nodules and area of hypo-attenuation in the right lobe with no evidence of abscess formation. She was referred to Endocrinology for further evaluation. Upon further questioning, she reported having intermittent fever, nervousness, and slight difficulty during swallowing, nearly 5-pound weight loss but no changes in her appetite or bowel habits. A family history of thyroid disease was not elicited. She has been taking Naproxen 200 mg four times a day and a full dose aspirin with minimal relief.
On physical examination she appeared to be in pain, BP was 144/88, and pulse 108/min and regular. Clinically, she appeared euthyroid. The thyroid gland was estimated to be 40 grams in weight and was tender, firm, and slightly irregular. The remainder of the examination was non-contributory.
Laboratory data included an erythrocyte sedimentation rate of 58 mm/min, FT4 of 2.7 ng/dl (reference range 0.76 to 1.46 ng/dl), FT3 5.8 pg/ml (2.3 to 4.2 pg/mL) and a negative thyroid stimulating immunoglobulin. CRP was 31.3 mg/L (reference range 0.0-8.0 mg/L). RAI uptake was 1%.
Subacute thyroiditis (SAT) sometimes referred to as granulomatous or De Quervain's thyroiditis is a spontaneously remitting inflammatory condition of the thyroid gland that may last for weeks to several months (21,139,140). It has a tendency to recur. The gland is typically involved as a whole, and thyroidal RAIU is much depressed. Transient hyperthyroxinemia, elevation of the serum thyroglobulin concentration and the erythrocyte sedimentation rate, and sometimes the WBC, during the early acute phase are characteristic if not pathognomonic.
Etiology
An infections cause can rarely be established. A tendency for the disease to follow upper respiratory tract infections or sore throats has suggested initiation by a viral infection. Earlier suggestions that the disease may represent a bacterial infection have been disproven. An autoimmune reaction is also unlikely. The development during the illness of cell-mediated immunity against various thyroid cell particulate fractions or crude antigens appears to be related to the release of these materials during tissue destruction (141,142).
Although the search for a viral cause has usually been unrewarding, a few cases have been associated with the virus that causes mumps (139,143). The disease has occurred in epidemic form. High titers of mumps antibodies have been found in some patients with subacute thyroiditis, and occasionally parotitis or orchitis are associated with the thyroiditis. The mumps virus has been cultured directly from thyroid tissue involved by subacute thyroiditis. Although the mumps virus may be one discrete etiologic factor, the disease has also been reported in association with other viral conditions including measles, influenza, H1N1 influenza (144) adenovirus infection, infectious mononucleosis (145), myocarditis, HIV (146), cat scratch fever, and coxsackie virus (147). SAT has been reported following hand-foot-mouth disease due to coxsackie B4 (148), cytomegalovirus (149), hepatitis E virus (150,151) and scrub typhus infection (116). Case reports suggesting SAT as a rare facet of Dengue expanded syndrome have been published (152-154).
Most recently, SAT has been associated with SARS-COV-2/COVID 19 infection (155). Two comprehensive studies (156,157) failed to find evidence of enteroviruses in 27 patients and Epstein-Barr (EB) virus or cytomegalovirus in 10 patients, respectively, but a single case report has implicated EB virus in a case of subacute thyroiditis with typical clinical features (158) and cytomegalovirus has been reported in an infant (159).
Numerous attempts to culture viruses from cases not associated with mumps have failed. Virus-like particles have been demonstrated in the follicular epithelium of a single patient suffering from subacute thyroiditis (147). However, viral antibody titers to common respiratory tract viruses are often elevated in these patients. Since the titers fall promptly, and multiple viral antibodies may appear in the same patient, the titer elevation may represent an anamnestic response to the inflammatory condition. It is likely that the thyroid gland could respond with thyroiditis after invasion by a variety of different viruses but no single agent is likely to be causative (160).
Histocompatibility studies show that 72% of patients with subacute thyroiditis manifest HLA-BW35 (161). Familial occurrence of subacute thyroiditis associated with HLA-B35 has been reported (162-165). The correlation between the SAT occurrence and the presence of HLA-B*18:01 and DRB1*01, as well as HLA-C*04:01 has been demonstrated, with the latter one being in linkage disequilibrium with a well-known SAT risk haplotype HLA-B*35 (166). These new three antigens, together with the known HLA-B*35, allow confirmation of a genetic predisposition in almost all patients with SAT. The haplotypes HLA-B*18:01, -DRB1*01 and HLA-B*35 are all independent SAT risk factors. Recent studies demonstrated for the first time that the risk of SAT recurrence is indeed HLA-dependent, and the high-risk group includes patients with co-occurrence of HLA-B*18:01 and -B*35 (166). It seems that the presence of HLA B18:01 significantly changes the course of SAT. The risk of recurrence was significantly influenced by the presence of HLA-B*18:01, but only with the concurrent presence of HLA-B*35. Although demonstration that the co-occurrence of HLA-B*18:01 and -B*35 carries the risk of SAT recurrence should be confirmed in further studies.
Thus, a susceptibility to subacute thyroiditis seems genetically influenced and it has been suggested that subacute thyroiditis might occur by transmission of viral infection in genetically predisposed individuals (159). A reported association between subacute thyroiditis and acute febrile neutrophilic dermatosis (Sweet's syndrome) (167,168), may imply a common role for cytokines in both these conditions.
New treatments, particularly those in which there is manipulation of the immune system, have led to the development of a subacute thyroiditis like clinical course (169). Infusion of interleukin 2 caused hyperthyroxinemia with a low radioiodine uptake in six patients who received this in combination with tumor necrosis factor (TNF) α or γ interferon (170). The patients proceeded through the pattern of hyperthyroidism followed by transient hypothyroidism, with a re-establishment of normal thyroid function typical of patients with autoimmune painless thyroiditis. However, none of the patients had detectable antithyroid antibodies. This condition is thus intermediate between subacute lymphocytic (painless) thyroiditis and subacute thyroiditis, which is typically painful.
The advent of immunotherapy has revolutionized cancer therapy. Immune checkpoint inhibitors (ICI) are a group of monoclonal antibodies that target the receptors cytotoxic T-lymphocyte antigen 4 (CTLA-4) and programmed cell death protein 1 (PD-1) or its associated ligand (PD-L-1). The thyroid gland is the endocrine gland most frequently affected in association with immune checkpoint inhibitors (ICIs). With the increase in use of immunotherapy for various malignancies, thyroid immune related adverse events (irAE) are on the rise (171). Thyroid dysfunction has been more frequently associated with PD-1 inhibitors rather than CTLA-4 inhibitors (172). About 20% of the patients receiving PD-1 inhibitors present with thyroid dysfunction, occurring early in the course of treatment (median onset 6 weeks after first infusion) (173,174) The exact underlying pathophysiologic mechanisms for thyroid irAEs are still unclear. It has been thought to be secondary to destructive, immune mediated thyroiditis and may include T cell, NK cell, and/or monocyte-mediated pathways (175). However, the onset and clinical manifestations are highly variable and not all patients develop the classic thyroiditis like presentation(176). Based on the limited data available, the PD-1 inhibitor induced thyroiditis may histologically present as a granulomatous inflammation with active destruction of thyroid follicles(177).
In one of the studies, in which thyroid function was prospectively monitored in patients with melanoma receiving PD-1 inhibitor therapy, most patients presenting with thyrotoxicosis developed hypothyroidism within 1-3 months (173). A recent study that looked at thyroid dysfunction in patients with melanoma undergoing CTLA-4 or PD-1 based treatment reported many distinct phenotypes (178). Of the 1246 patients studied, 42% developed thyroid irAEs. The most common presentation was subclinical hyperthyroidism followed by overt hyperthyroidism, subclinical hypothyroidism, and overt hypothyroidism.
The most common thyroid dysfunction is thyrotoxicosis followed by hypothyroidism. Incidences of hypothyroidism were lower with the anti-CTLA-4 antibody (2.5%-5.2%) than with anti-PD-1/anti-PD-L1 (3.9%-8.5%), while combination therapy was associated with the highest estimated incidence (10.2%-16.4%). Similarly, for thyrotoxicosis differences according to the class of ICIs had been reported, with ipilimumab having low frequencies (0.2%–1.7%), anti-PD-1/anti-PD-L1 drugs having higher frequencies (0.6%–3.7%), and combination therapy having the highest frequency (8.0%–11.1%) (179). Moreover the risk of thyrotoxicosis was significantly greater with anti-PD-1 antibodies than with anti-PD-L1 antibodies and differences among anti-PD-1 drugs were also observed, with nivolumab having lower risk for hyperthyroidism than pembrolizumab (179).
In the majority of the patients who develop thyroid dysfunction, ICI therapy can be continued, unless they experience symptoms of severe thyrotoxicosis or there is concern for thyroid storm (180). Current guidelines recommend initiation of beta-blockers for symptomatic relief and if there is persistence of hypothyroidism, levothyroxine should be initiated after ruling out adrenal insufficiency, which can also occur with ICI therapy.
Patients have developed subacute thyroiditis after influenza vaccination (181-183) suggesting immune alteration as a contributory factor. In patients with chronic hepatitis C, studies following interferon therapy (IFN) have shown that a minority (15%) developed a destructive thyroiditis while others had a mild elevation of TSH (170,184). IFN can exacerbate previous thyroid autoimmunity and cause destructive thyroidal changes de novo. Subacute thyroiditis has also been noted in patients treated with combination therapy of IFN plus ribavirin for this disease (185,186) as well as during treatment of hepatitis B with interferon-a (187). Peginterferon alpha-2a has been reported to cause subacute thyroiditis (188) and the condition has been seen in Takayasu's arteritis suggesting an immune abnormality (189). On the other hand, SAT has also been reported in patients receiving long-term immunosuppressive therapy suggesting a minimal role for activating autoimmunity in the condition (190,191). A phase 2 trial conducted with alemtuzumab, a monoclonal anti-CD52 antibody for relapsing and remitting type of multiple sclerosis found that 34% of the subjects developed thyroid dysfunction and 4% had subacute thyroiditis (192). Use of TNF inhibitor therapy has been associated with thyroid dysfunction that closely resembles subacute thyroditis (193,194). A recent report of SAT associated with the use of the kinase inihibitor, dasatinib has been published (195). Other reports of the occurrence of a SAT-like picture with renal cell carcinoma (196), following the administration of cardiac catheterization dye (197), after gastric bypass(198), or after ginger ingestion (199) do not clearly contribute to an enhanced understanding of its etiology.
SARS-COV-2 Infection and Subacute Thyroiditis
Severe acute respiratory syndrome coronavirus 2 (SARSCoV-2) has infected more than 190 million people worldwide and the pandemic is still spreading. The first case of SAT after a SARS-CoV-2 infection was published from Italy in 2020 (155). Although additional cases were soon reported, this entity is likely underrecognized (200-202).
A case series of 4 patients with SAT after SARS-COV2 infection was published (155). In this case series, all 4 patients were female (age, 29-46 years). SAT developed 16 to 36 days after the resolution of coronavirus disease 2019 (COVID-19). Neck pain radiated to the jaw and palpitations were the main presenting symptoms and were associated with fever and asthenia. One patient was hospitalized because of atrial fibrillation. Laboratory exams during the acute phase of SAT, available for 3 patients, were typical of destructive thyroiditis: thyroid hormones, and particularly free thyroxine, were increased, TSH was low to undetectable, serum thyroglobulin was high, and TSH receptor antibodies were undetectable.
At neck ultrasound (performed in all patients) the thyroid was enlarged, with diffuse and bilateral hypoechoic areas. At color Doppler ultrasonography (performed in 3 patients) thyroid vascularization was absent. One patient had a thyroid scintiscan with 99mtechnetium, which showed absent uptake, as typical of the destructive phase of SAT. Symptoms subsided in all patients a few days after they commenced treatment (prednisone 25 mg/day in 3 patients and nonsteroidal anti-inflammatory drug in 1 patient). Six weeks after the onset of SAT symptoms, inflammatory markers had returned to normal range in all patients. Two patients were euthyroid and 2 were diagnosed with subclinical hypothyroidism. No patient experienced a relapse of COVID-19.
Expression of the mRNA encoding for the ACE-2 receptor has been documented in thyroid follicular cells, making them a potential target for SARS-COV-2 entry (203). The expression of ACE-2 mRNA in follicular cells was confirmed by analyzing primary cultures of thyroid cells, which expressed the ACE-2 mRNA at levels similar to thyroid tissues. It is important to note that, as recently demonstrated, SARS-CoV-2 infection requires the ACE-2 receptor to coexist with type II serine protease trans-membranes (TMPRSS2) (204).
More recently, three cases of SAT were reported from Turkey after inactivated SARS-CoV-2 vaccination (CoronaVac®) was administered. Three female healthcare workers presented with anterior neck pain and fatigue 4 to 7 days after SARS-CoV-2 vaccination and were diagnosed to have SAT, as a part of an autoimmune/inflammatory syndrome induced by adjuvants (ASIA syndrome). This can be seen as a postvaccination phenomenon that occurs after exposure to adjuvants in vaccines that increase the immune responses. However, two of these patients were in the postpartum period, which may have facilitated the development of ASIA syndrome after the SARS-CoV-2 vaccination (205).
Pathology
The thyroid gland may be adherent to its capsule or to the strap muscles, but it can usually be dissected free, a feature distinguishing subacute thyroiditis from Riedel's thyroiditis. The involved tissue appears yellowish or white and is firmer than normal. The gland is enlarged, usually bilaterally and uniform, but may be asymmetrical, with predominant involvement of one lobe. Although the lesion may extend to the capsular surface, it can also be confined to the thyroid parenchyma or merely be palpable as a suspiciously hard area.
Macroscopically, yellow-white, solidified foci of different sizes are visible, which occur focally, asymmetrically or less often, bilaterally. Clinically and macroscopically, malignancy can be suspected due to the ill-defined delimitation of these foci. There is a characteristic picture of a granulomatous inflammatory reaction with focal destruction of the follicular epithelial cells histologically. Due to the destruction of the follicles in the early stages of the disease, colloid emerges; neutrophils dominate, which can form granulomas with central micro-abscesses (206).
In the florid phase, lymphocytes, histiocytes and plasma cells predominate in the inflammatory infiltrate. The typical granulomas of this phase consist of cell necrosis, macrophages, multinucleated colloid phagocytic giant cells and lymphocytes (207).
The regeneration phase is characterized by focal fibrosis of the affected thyroid area with regenerative cell and nuclear changes in the immediately adjacent unaffected thyroid tissue. The characteristic juxtaposition of the different histological stages of inflammation indicates that the disease is evolving in parallel zones (206).
The macroscopic pathologic picture of subacute thyroiditis frequently bears a striking resemblance to a thyroid malignancy. The lesion is firm to dense in consistency, pale white in color, and has poorly defined margins that encroach irregularly on the adjacent normal thyroid. Microscopically, one sees a mixture of subacute, chronic, and granulomatous inflammatory changes associated with zones of parenchymal destruction and scar tissue. Early infiltration with polymorphonuclear leukocytes is replaced by lymphocytes and macrophages. The normal follicles may be largely replaced by an inflammatory reaction, but a few small follicles containing colloid remain (Fig. 1, below). Three dimensional cytomorphological analysis of fine needle aspiration biopsy samples from patients with subacute thyroiditis examined with scanning and transmission electron microscopy has shown a loss of a uniform, honeycomb cellular arrangement; variation in size and a decrease or shortening of microvilli in follicular cells together with the appearance of round or ovoid giant cells (208). The most distinctive feature is the granuloma, consisting of giant cells clustered about foci of degenerating thyroid follicles (Fig. 1). Mast cells play an important part in the repair process of thyroid tissue affected by the disease via production of growth factors and biomolecules which modulate thyroid folliculogenesis and angiogenesis (209).The early literature contains accounts of tuberculous thyroiditis, a diagnosis largely based on the granulomatous tissue reaction, from which the descriptive but unfortunate term pseudotuberculous thyroiditis arose (210). Data on the mechanism of inflammation and the pathogenesis of subacute thyroiditis at the cellular level are sparse. However, a study of apoptosis and expression of Bcl 1-2 family proteins in 11 patients with SAT suggests that apoptotic mechanisms may be involved in the development of SAT (211). Growth factor rich monocytes/macrophages (containing VEGF, beta FGF, PDGF and TGF beta 1) are involved in the granulomatous stage (212). EGF is important in the regenerative stage as it has mitogenic effects on the thyrocyte. VEGF and beta FGF contribute to the angiogenesis at both these stages of the disease. Factors influencing the severity of the acute phase response during the course of SAT include serum interleukin -1 receptor antagonist, which may have a significant anti-inflammatory role (213); also, a decrease in TNF alpha results in earlier resolution of experimentally induced granulomatous thyroiditis (214). TNF- related apoptosis-inducing ligand (TRAIL) has been shown to promote resolution of granulomatous autoimmune thyroiditis in animal models (215).
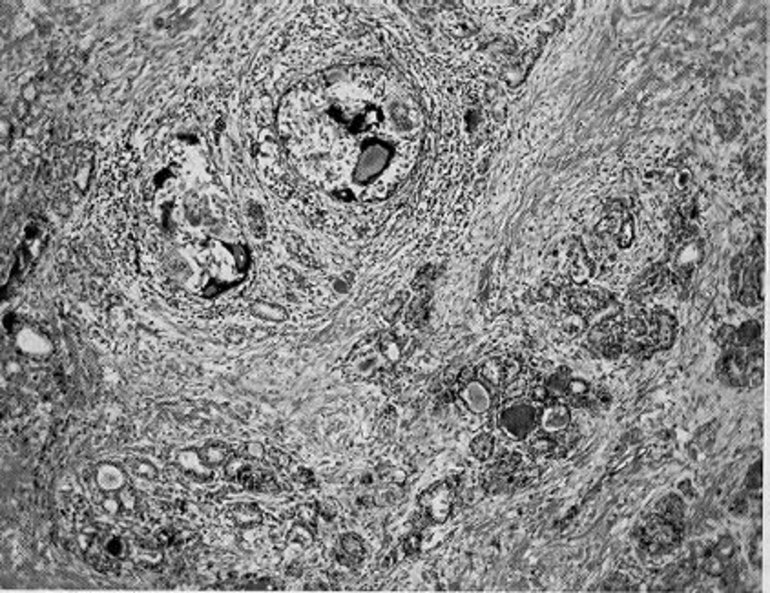
Figure 1.
Subacute thyroiditis. Note the discrete granulomas, with giant cells, and the diffuse fibrosis (85 X).
Incidence and Prevalence
Subacute thyroiditis is encountered in up to 5% of patients with thyroid illness (216). Woolner et al. (210) collected 162 cases diagnosed on clinical grounds at the Mayo Clinic over a 5-year period; during the same time, 1,250 patients with Graves' disease were seen. Thus, the disease had approximately one-eighth the incidence of Graves' disease in this clinical population. Between 1970 and 1997, in the Epidemiology Project in Olmsted county, Minnesota 94 patients with subacute thyroiditis were identified (217). They report an incidence of 12.1 cases per 100,000/year with a higher incidence in females than in males (19.1 and 4.1 per 100,000/year, respectively). It is most common in young adulthood (24 per 100,000/year) and middle age (35 per 100,000/year), and it decreases in frequency with increasing age.
During an evaluation of subtypes of hypothyroidism over a 4-year period in Denmark, an incidence of subacute thyroiditis of 1.8% was found in a cohort of 685 patients with hypothyroidism (218). Although the disease has been described at all ages, it is rare in children (24,140). Female patients have outnumbered male patients in a ratio of 1.9-6:1, with a preponderance of cases in the third to fifth decades (67,139,210,219,220) and it has been noted as a rare cause of hyperthyroidism in pregnancy (221,222).
Clinical Manifestations
Characteristically, the patient has severe pain and extreme tenderness in the thyroid region. A small number of patients have been noted to present with painless or minimally painful subacute thyroiditis following viral symptomatology (223). These may be regarded as atypical subacute thyroiditis patients but the natural history of their disease is not known. Subacute thyroiditis has been reported to occur during the first trimester of pregnancy (221). When the symptom is difficulty in swallowing, the disorder may be initially mistaken for pharyngitis. Transient vocal cord paresis may occur (224). At times, the pain begins in one pole and then spreads rapidly to involve the rest of the gland ("creeping thyroiditis"). Pain may radiate to the jaw or the ears. Malaise, fatigue, myalgia and arthralgia are common. A mild to moderate fever is expected, and at times is high, swinging fever with temperatures above 104°F (40.0°C). The disease may reach its peak within 3 to 4 days, subside, and disappear within a week, but more typically, a gradual onset extends over 1 to 2 weeks and continues with fluctuating intensity for 3 to 6 weeks. Several recurrences of diminishing intensity extending over many months have also been reported.
The thyroid gland is typically enlarged two or three times the normal size or larger and is tender to palpation, sometimes exquisitely so. It is smooth and firm. Occasionally the condition may be confined to one lobe (225,226). Approximately one-half of the patients present during the first weeks of the illness, with symptoms of thyrotoxicosis, including nervousness, heat intolerance, palpitations - including ventricular tachycardia (227), tremulousness, and increased sweating. These symptoms are caused by excessive release of preformed thyroid hormone from the thyroid gland during the acute phase of the inflammatory process. At least 3 cases of thyroid storm due to subacute thyroiditis have been described (228,229) and adverse cardiac outcomes have been reported even in individuals without preexisting cardiac history or lesions (230). As the disease process subsides, transient hypothyroidism occurs in about one-quarter of the patients. Ultimately thyroid function returns to normal and permanent hypothyroidism occurs in less than 10 percent of the cases (21,67,139). Occasionally the condition may be painless and present as fever of unknown origin (231-233) or associated with other findings and mimicking conditions such as temporal arteritis (234). Some clinical and laboratory features recorded in 2 series of SAT are shown in Table 3 (110,235). Liver function test abnormalities are found in half the patients and return to normal in a few months (236).
Table 3.
Clinical Features of Subacute Thyroiditis
| Japan | Israel | |
|---|---|---|
| Number | 852 | 56 |
| Females (%) | 87 | 70 |
| Season | summer-autumn | no effect |
| Recurrence | 1.6% | 9% |
| Temp >380 | 28% | -- |
| Thyrotoxic symptoms | 60% | -- |
| Hypothyroid phase | -- | 55% |
| Laboratory - peak levels | 1 week | -- |
| Antithyroid antibodies | -- | 25% |
| Ultra Sound | ||
| Bilateral hypoechogenicity | 50% | 70% |
| Nodules | -- | 70% |
| Disease duration (days) | -- | 77 |
Diagnosis
Table 4 provides a comparison between the clinical and laboratory findings of patients with subacute and acute thyroiditis (21,237-242). Laboratory examination may disclose a moderate leukocytosis. A striking elevation of the erythrocyte sedimentation rate, at times above 100 mm/hr, or an elevated level of serum C-reactive protein (243) are useful diagnostic clues. The identification of CRP in salivary samples can also provide a convenient source for documenting the presence of abnormal levels in patients with SAT (244). Short of a tissue diagnosis, the characteristic combination of elevated erythrocyte sedimentation rate, high serum T4, T3, (T3:T4 <20) and hyroglobulin concentrations in the presence of low thyroidal RAIU, TSH, and an absent or low titer of circulating TPO and TG antibodies are the most helpful parameters. While the estimation of thyrotropin receptor antibodies (TRAb) in a thyrotoxic patient may be clinically useful in identifying Graves' disease, there have been reports of positive TRAb in patients with subacute thyroiditis although the frequency of this finding is low (245-249). Mild anemia and hyperglobulinemia may be present.
The value of a 99m-Tc-pertechnetate scintigraphy as a marker of disease activity and severity has been described (250). Pertechnetate scanning, which is inexpensive and convenient, typically reveals little to no uptake, and thus no thyroid tissue visualization during the SAT process (250,251), a finding consistently reported in the literature (144,148,230,252-254). Further imaging studies have shown diffuse increased uptake of Tc-99m sestamibi (251) and Tc-99m tetrofosmin (250) in the thyroid region of patients in the acute phase (thyrotoxic) of subacute thyroiditis suggesting an ability of both agents to detect the inflammatory process associated with the disease (250,251). In the same patients Doppler flow assessment ultrasonography has shown an absence of vascular flow in the acute phase and the utility of this finding in the differential diagnosis of unclear cases has been emphasized (255,256). Standard ultrasonographic images are characterized by a hypoechoic appearance of the affected tissue, the volume of which correlates with the severity of clinical discomfort (257,258). Cervical adenopathy may be observed (259). The application of newer technologies such as sonoelastography has the capacity to demonstrate markedly decreased elasticity (enhanced stiffness) in SAT lesions (118). Subacute thyroiditis may obscure the coexistence of papillary carcinoma in cases presenting with ultrasonographically diffuse hypoechoic areas (260). Subacute thyroiditis with thyrotoxicosis may also be distinguished from Graves' hyperthyroidism by using T1- and T2- diffusion weighted magnetic resonance imaging (261) and as an intense area of uptake on (18) F-FDG PET/CT (254,262), although these investigation may not be necessary. Altered F-18 FDG uptake in skeletal muscle and reduced hepatic uptake has been observed during the hyperthyroid phase (263,264). Rarely, a sensor-navigated (124) iodine PET/ultrasound (I-124-PET/US) fusion has been implemented to establish this diagnosis (265). Fine needle aspiration biopsy is often diagnostic although patients are often alarmed at the prospect of this test due to the pain in the thyroid. However FNA may be helpful in ruling out malignancy (266) and the infection associated with localized, painful lesions of AST (see above).
Table 4.
Features Useful in Differentiating Acute Suppurative Thyroiditis and Subacute Thyroiditis
| Characteristic | Acute Thyroiditis | Subacute Thyroiditis | |
|---|---|---|---|
| History | Preceding upper respiratory infection | 88% | 17% |
| Fever | 100% | 54% | |
| Symptoms of thyrotoxicosis | Uncommon | 47% | |
| Sore throat | 90% | 36% | |
| Physical examination of the thyroid | Painful thyroid swelling | 100% | 77% |
| Left side affected | 85+% | Not specific | |
| Migrating thyroid tenderness | Possible | 27% | |
| Erythema of overlying skin | 83% | Not usually | |
| Laboratory | Elevated white blood cell count | 57% | 25-50% |
| Erythrocyte sedimentation rate (>30 mm/hr) | 100% | 85% | |
| Abnormal thyroid hormone levels (elevated or depressed) | 5-10% | 60% | |
| Alkaline phosphatase, transaminases increased | Rare | Common | |
| Needle Aspiration | Purulent, bacteria or fungi present | ~100% | 0 |
| Lymphocytes, macrophages, some polys, giant cells | 0 | ~100% | |
| Radiological | 123I uptake low | Common | ~100% |
| Abnormal thyroid scan | 92% | Non-visualized | |
| Thyroid scan or ultrasound helpful in diagnosis | 75% | Non-specific | |
| Gallium scan positive | ~100% | ~100% | |
| 18F-FDG-PET | Positive | Positive | |
| Barium swallow showing fistula | Common | 0 | |
| CT scan useful | Varies | Not indicated | |
| Clinical Course | Clinical response to glucocorticoid treatment | Transient | 100% |
| Incision and drainage required | 85% | No | |
| Recurrence following operative drainage | 16% | No | |
| Pyriform sinus fistula discovered | 96% | No |
If subacute thyroiditis affects only one part of the thyroid gland, the serum T4 concentration and overall thyroidal RAIU may be entirely normal. A thyroid scan done with either radioactive iodine or 99m-Tc-pertchnetate will demonstrate failure of the involved areas of the gland to concentrate the tracer. When the thyroid is diffusely involved, which is more typical, a dramatic disturbance in iodine metabolism is observed.
During the initial phase of the disease, the RAIU is depressed or entirely absent and the concentrations of serum T4 and T3 are often elevated but the ratio of T3 to T4 is typically less than 20 (compared to > 20 in typical Graves’ disease). Due to the concomitant release of non-hydrolyzed iodoproteins from the inflamed tissue, the serum thyroglobulin level is also high. During this phase, the serum TSH level is low. Analysis of the TSH suppression reported over 20 years ago with a sensitive assay, measured in thyrotoxic patients, indicated that patients with SAT may demonstrate suppressed but detectable levels of TSH while those with Graves’ disease or silent thyroiditis typically have undetectable TSH values (267). It has been postulated that those with SAT are evaluated sooner in the course of thyrotoxicosis due to the pain of the condition, and thus the duration of the thyrotoxicosis is less, leading to proportionally less TSH suppression. This finding has been proposed to be useful in the differential diagnosis of these thyrotoxic states (267). The TSH response to TRH stimulation is also typically suppressed (238) due to the high levels of circulating thyroid hormone. Iodide that is collected and metabolized by the gland is rapidly secreted because of the decreased ability to store colloid (240). At this time, the involved tissue shows decreased but not necessarily depleted stores of iodine, as determined by x-ray fluorescence (237,240), a study which is not readily available in most clinical settings in the USA. Administration of TSH will fail to produce a normal increase in RAIU. Evidently, thyroid cell damage reduces the ability of the gland to respond to TSH. As the process subsides, the serum T4, T3, and TG levels decline, but the serum TSH level remains suppressed. The normal concentrations of SHBG sometimes observed in the thyrotoxic phase probably reflects the short duration of exposure to increased thyroid hormone (268). Later, during the recovery phase, the RAIU becomes elevated with the resumption of the ability of the thyroid gland to take up and concentrate iodide in response to TSH. The serum T4 concentration may fall below normal; the TSH level may become elevated. Usually after several weeks or months, all the parameters of thyroid function return to normal (Fig. 2). Restoration of iodine stores appears to be much slower and may take more than a year after the complete clinical remission (237,240). In about 2% of patients subacute thyroiditis may trigger auto-reactive B cells to produce TSH receptor antibodies, resulting in TSH antibody associated thyroid dysfunction in some patients (246).This finding may be a potential explanation of the apparent occurrence of Graves’ disease following an episode of SAT (249,269,270).
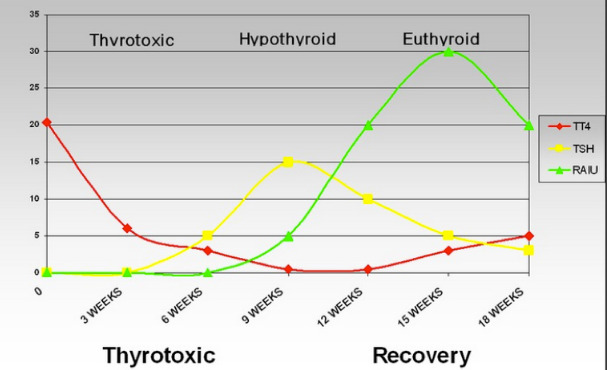
Figure 2.
Thyroid function in a patient during the course of de Quervain’s (subacute) thyroiditis. During the thyrotoxic phase (days 10 to 20), the serum TG concentration was greatly elevated, the FTI was high, TSH was suppressed; the erythrocyte sedimentation rate was 86 mm/hr, and the thyroidal RAIU was 2 percent. The thyroglobulin level and FTI declined in parallel. During the phase of hypothyroidism (days 30 to 63), when the FTI was below normal, a modest transient increase in the serum thyroglobulin level occurred in parallel with the increase in serum TSH. All parameters of thyroid function were normal by day 150, 5 months after the onset of symptoms.
Differential Diagnosis
The patient presenting with painful neck symptoms is frequently empirically treated with antibiotics with minimal evaluation in general practice only later to be found to have thyroid related disease (253). With an acutely enlarged, tender thyroid, an RAIU near zero, and elevated serum T4, T3, (T3:T4 <20), thyroglobulin concentrations, and ESR, the diagnosis is almost certain. Circulating thyroid autoantibodies are absent or the titer is low. Among the diagnostic alternatives, the uncommon presentation of thyrotoxicosis in infectious thyroiditis must be considered (55) and the possibility of invading bacteria excluded (see Table 2 and 4). Rarely a fever of unknown origin may suggest temporal arteritis but is actually due to subacute thyroiditis (234). Additionally, because of the radiation of painful thyroid into the jaw area the presence of dental pain may be confused with SAT (271). The thyroid in Hashimoto's thyroiditis may be slightly tender and painful, but this event is rare, and the typical disturbances in iodine metabolism and erythrocyte sedimentation rate are rarely found. Markers of inflammation such as CRP as measured in the saliva are normal in Hashimoto’s thyroiditis when compared to controls but are grossly elevated in the patient with SAT (244).
Standard thyroid ultrasonography may appear similar with hypoechoic tissue in both Hashimoto’s thyroiditis and SAT. Doppler measured blood flow is usually robust in Hashimoto’s thyroiditis and Graves’ disease but minimal in SAT while assessment by sono-elastography reveals that the SAT gland is profoundly stiffer than Hashimoto’s thyroiditis tissue which is itself somewhat stiffer than normal controls (118). The radio nuclide thyroid uptake and scanning in Hashimoto’s thyroiditis is variable with elevated, depressed or normal results reported. 18F-FDG-PET in Hashimoto’s on the other hand is similar to that seen in SAT with usually very positive uptake reported (254,272,273). Magnetic resonance imaging does not differentiate between Hashimoto’s thyroiditis and SAT (261) and is therefore, like 131/123-I and PET scanning, of little value in separating the patient with painful Hashimoto’s from the SAT patient.
Hemorrhage into a cyst in a nodular thyroid gland may be acutely painful and therefore confused with subacute thyroiditis although the condition may be associated with an initially autonomously functioning nodule (274). The clinical presentation of a nodule hemorrhage is usually sudden and transient, a fluctuant mass may be found in the involved region, which may be confirmed as fluid filled and avascular ultrasonographically, and further differentiated as the erythrocyte sedimentation rate is normal. Occasionally, subacute thyroiditis mimics endogenous hyperthyroidism (Graves’ or toxic nodular goiter) in a patient whose RAIU is suppressed by the administration of exogenous iodine. This event occurs particularly in thyrotoxicosis induced by iodine (Jod-Basedow phenomenon) (241). The sudden onset of subacute thyroiditis, the presence of toxic symptoms without the typical signs of long-term hyperthyroidism, the tender gland, the constitutional symptoms, and the high erythrocyte sedimentation rate are helpful in making the differentiation. In some instances, measurement of antibodies and thyroid-stimulating immunoglobulins, and observation of the course of the illness may be required to confirm the diagnosis.
The single disease entity that is probably most difficult to differentiate from SAT is a variant of lymphocytic thyroiditis (242). This condition is unrelated to iodine ingestion and most likely is a variant of autoimmune thyroiditis. The patient presents with goiter, thyrotoxicosis, and a low RAIU. The biochemical course of the disease is indistinguishable from that of subacute thyroiditis and proceeds from a thyrotoxic phase through a hypothyroid phase to spontaneous remission with normalization of thyroid function. The goiter is however, typically painless and there are no associated systemic symptoms. This condition has been formerly confused with subacute (de Quervain's) thyroiditis, which likely has led to the descriptive terms of silent, painless, or atypical subacute thyroiditis to refer to this entity. The most helpful distinguishing features, short of histologic examination of biopsy material, are the absence of pain, the positivity of anti-thyroid antibodies and a normal erythrocyte sedimentation rate.
Localized subacute thyroiditis, with induration, mild tenderness, and depressed iodine uptake visualized on scan, can clearly be very suggestive of acute suppurative thyroiditis or even thyroid cancer. One series indicated a surprisingly high frequency of focal involvement observed among those with SAT (256). Indeed, this differential is quite difficult when incidentally discovered lesions are evaluated. Focal thyroid lesions incidentally identified by 18F-FDG-PET/CT are said to have malignant potential in up to 14-63% of cases (275,276). Among the other diagnostic findings reported to account for such FDG-PET incidentalomas is focal SAT (262). Usually, the degree of pain and tenderness, elevated erythrocyte sedimentation rate, leukocytosis, and remission or spread to other parts of the gland make clinical differentiation possible. Traditional ultrasonography may reveal localized hypoechoic area in the thyroid and gray-scale and Doppler sonography may be helpful in this situation (255,277). Sonoelastography of these nodular lesions yields abnormally inelastic results in both SAT as well as thyroid cancer (278). Occasionally, magnetic resonance imaging (261), where the image of SAT is characterized by low intensity, may assist the clinician in differential of these nodular lesions. The hypoechoic area on ultrasound can reflect the degree of inflammation and thyroid hormone levels (257). However, a fine needle aspiration may be necessary for a definitive differentiation between these two processes (274), as well as the other entities noted above (129).
Therapy
In some patients with SAT, no treatment is required. However, for many, some form of analgesic therapy is warranted to treat the symptoms of the disease until it resolves. At times, this relief of symptoms can be achieved with non-steroidal anti-inflammatory agents or aspirin. However, if this fails, as it often does when the symptoms are severe, and after acute suppurative thyroiditis had been definitively ruled out as outlined above, prednisone administration should be employed (67,139).Compared to the use of NSAIDs, use of steroids has been shown to reduce time to resolution of symptoms (279). Large doses promptly relieve the symptoms through non-specific anti-inflammatory effects. Treatment is generally begun with a single daily dose of 40 mg prednisone. After one week of this treatment, the dosage is tapered over a period of 6 weeks or so. The relief of the tenderness in the neck is so dramatic as to be virtually diagnostic of subacute thyroiditis. As the dose is tapered, most patients have no recrudescence of symptoms, but occasionally this does occur, and the dose must be increased again. A dose as low as 15 mg of prednisolone has been shown to be as effective (280) and further studies should be conducted to determine the lowest effective doses. A newer therapeutic approach with local injection of lidocaine and dexamethasone through an insulin syringe has been reported to alleviate symptoms earlier than standard treatment with systemically administered prednisone and needs further evaluation in larger studies (281). The recurrence rate of subacute thyroiditis after cessation of prednisolone therapy is about 20% but no predictive factors have been found in routine laboratory data between recurrent and non-recurrent groups of patients (282). A recent study that evaluated the results of the steroid and NSAID treatments in SAT in relation to persistent hypothyroidism and recurrence, concluded that NSAIDs fail to provide clinical remission in more than half of SAT patients, and symptomatic response to NSAIDs is lower in patients with higher ESR and CRP levels. Despite the high recurrence rate observed in steroid-treated SAT patients, steroid treatment appears to be protective against permanent hypothyroidism. Steroid therapy should therefore be considered, especially in anti-TPO positive SAT patients and patients with high-level ESR and CRP (283). In this study, initial laboratory data, treatment response, and long-term results of 295 SAT patients treated with ibuprofen or methylprednisolone were evaluated. After the exclusion of 78 patients, evaluation was made of 126 patients treated with 1800 mg ibuprofen and 91 patients treated with 48 mg methylprednisolone. In 59.5% of 126 patients treated with ibuprofen, there was no adequate clinical response at the first control visit. In 54% of patients, the treatment was changed to steroids after a mean of 9.5 days. Symptomatic remission was achieved within two weeks in all patients treated with methylprednisolone. The total recurrence rate was 19.8%, and recurrences were observed more frequently in patients receiving only steroid therapy than in patients treated with NSAID only (23% vs. 10.5% p:0.04). Persistent hypothyroidism developed in 22.8% of patients treated only with ibuprofen and in 6.6% of patients treated with methylprednisolone only. Treatment with only ibuprofen (p:0.039) and positive thyroid peroxidase antibody (anti-TPO) (p:0.029) were determined as the main risk factors for permanent hypothyroidism.
During the recovery process, there may be a marked but transient increase in the 24-hour radioactive iodine uptake which can reach levels typically seen in Graves' disease but of course thyrotoxicosis is not simultaneously present. This elevation of iodine uptake occurs prior to re-establishment of normal thyroid function and should not be confused (taken out of context) with hyperthyroidism due to Graves ‘disease. Surgical intervention is not the primary treatment for subacute thyroiditis but rarely this has been performed due to presence of indeterminate cytology on FNA (284-286) or pain (287). Experience from the Mayo clinic (284) has shown, however, that if surgery is performed for a clinically indeterminate thyroid nodule, resection is safe and with low morbidity. Because of the possibility of associated papillary cancer further cytological examination should be performed in patients presenting with a persistent hypoechoic area larger than 1 cm by ultrasonography (260).
Prognosis
In most patients, there is a complete and spontaneous recovery and a return to normal thyroid function. However, the thyroid glands of patients with SAT may exhibit irregular scarring between islands of residual functioning parenchyma, although the patient has no symptoms. A recent study that followed 61 patients for 2 years following diagnosis of SAT explored the early indicators of hypothyroidism and the final changes in thyroid volume in SAT patients (288). They noted that the thyroid gland volumes of SAT patients, especially those with hypothyroidism, were smaller than those of healthy controls after the acute stage of the disease. They also suggested that the higher early maximum TSH value within 3 months after SAT onset may be the risk factor for the incidence of hypothyroidism 2 years later.
SAT may recur in up to 2.8 to 4% of patients (219,289). Up to 10% of the patients may become hypothyroid and require permanent replacement with levothyroxine. The choice of treatment, use of steroid, NSAID or both may not predict the development of permanent hypothyroidism.(290) In a retrospective study of 252 patients with SAT, permanent hypothyroidism occurred in 5.9%. All of these had bilateral hypoechoic areas on thyroid ultrasound at initial presentation suggesting that this may be a useful prognostic marker for the potential development of thyroid dysfunction after SAT (291). However, permanent hypothyroidism was significantly less common in SAT compared to the outcome noted in amiodarone induced thyrotoxicosis type 2 (destructive thyroiditis) (292). It is of interest that elevated levels of serum thyroglobulin may persist well over a year after the initial diagnosis, indicating that disordered follicular architecture and/or low grade inflammation can persist for a relatively long period (293).
A minority (< 1%) of those presenting with clinical SAT in Japan have been reported to return (n= 7) a mean 4.7 months later with findings consistent with Graves’ disease (GD) (269). Review of the other 26 cases summarized in the report of Nakano et al. indicates a similar interval between the diagnosis of SAT and subsequent GD presentation, a clearly elevated RAIU in the GD phase of all the reports where an uptake is reported (14/26 [54%]) and a change in thyroid antibody positivity in 50% of those evaluated in both (6/26 [23%]) the SAT and GD presentation(269). Combining the case series by Nakano et al. with their review of the literature, 21/31 [68%] of cases labeled as SAT were diagnosed clinically without a radioactive iodine uptake assessment, and a further 4/12 [33%] of those diagnosed as SAT with a RAIU available had an uptakes greater than 10% at the time of diagnosis(269). This brings into question the true incidence of this reported transition from presumably non-autoimmune SAT to clearly immune mediated GD.
RIEDEL'S THYROIDITIS
Riedel’s thyroiditis is a chronic sclerosing thyroiditis, occurring especially in women, that tends to progress inexorably to complete destruction of the thyroid gland and frequently causes pressure symptoms in the neck (294-296). Initially described by Semple in 1864 and Bolby in 1888 (297), it was later reported in 1896 by Riedel as an “eisenharte Struma” (iron hard goiter) fixed and usually painless enlargement of the thyroid (294,298,299). It is exceedingly rare with estimated incidence of 1.06 cases per 100,000 population and 37/57,000 (0.06%) of thyroid surgical outcomes over a 64 year period (300). In the Mayo Clinic series (300), it occurred approximately one-fiftieth as frequently as Hashimoto's thyroiditis. It is more frequent in women (F:M 3.1:1) (67,163,294,301,302) who were recently reported to represent 81% of those with confirmed Riedel’s in a Mayo clinic series and further confirmed in a meta-analysis (302,303). Riedel’s thyroiditis is principally reported to occur in the 30- to 50 year age group and has a reported median age of 47 years (67,301-303).
Pathology
The thyroid gland is normal in size or enlarged, focally or symmetrically involved, and extremely (woody) hard. The gland is replaced by the inflammatory process which may extend into adjacent structures including parathyroid, skeletal muscle, nerves, blood vessels as well as the trachea (304). Gross observation of the mass reveals a pale gray appearance similar to a malignant lesion (305). There are no tissues planes visible and the cut surface of the mass is stark white due to the hypovascularity of the tissue (306). Histologically, normal tissue is replaced by inflammatory cells, predominantly lymphocytes, plasma cells, eosinophils (301,307), and small amounts of colloid (308-310) in a dense matrix of hyalinized connective tissue. Characteristically, an inflammatory reaction of the venous vascular structures has been described (305). An oft-stated criterion useful in assuring the pathologic diagnosis is to note the absence of granulomatous tissue and malignancy (301,305,306). A potentially difficult differential diagnostic decision may be encountered with diffuse sclerosing variant of papillary thyroid cancer and the nodular sclerosing variant of Hodgkin’s disease (302), rare sarcomas of the thyroid region (311), or with the pauci-cellular variant of anaplastic thyroid cancer, both of which, although similar in gross appearance, will have distinctive histopathologic immunohistochemical findings (312).
Etiology
Although the etiology remains unclear, Riedel’s thyroiditis has been characterized in various ways including as the cervical manifestation of a systemic fibrosing disorder with identical histopathological appearance (313). Further, Riedel’s thyroiditis has been called a variant of Hashimoto’s, a primary infiltrative disease of the thyroid and even a manifestation of end stage de Quervain’s thyroiditis (295,308,314,315). Riedel’s thyroiditis has been reported following subacute thyroiditis (315) and a case of concurrent Riedel’s, Hashimoto’s and acute thyroiditis has also been reported (316). The report of a case of Graves’ disease following Riedel’s thyroiditis (317) and the observation that the B cell proliferation observed in the course of these diseases has been shown to be polyclonal (318) supports the notion of autoimmune mechanisms in the etiology of the Riedel’s condition. The occurrence of marked tissue eosinophilia and the extracellular deposition of eosinophil granule major basic protein suggests a role for eosinophils and their products in the development of fibrosis in Riedel's thyroiditis (307). Fibrosis may also be related to the action of TGF beta 1, as seen in murine thyroiditis (319). Most recently links between Hashimoto’s, IgG4-related systemic disease (IgG4-RSD) and Riedel’s thyroiditis have been reported (320-322). Supporting evidence showing the presence of IgG4-bearing plasma cells in thyroidectomy specimens and other affected organs (302,322,323). A comprehensive review of potential etiology has been published (304).
IgG4-related disease (IgG4-RD) was first described in 1961 as a distinctive presentation of pancreatitis which was observed to be associated with hypergammaglobulinemia (324). A specific association with IgG4 was published in 2001 (325) and eventually an international consensus was established to define the criteria for recognizing IgG4-RD (326,327). Through this understanding of pathophysiology, the term IgG4-RD has been adopted to describe a common underlying pathology found in a variety of fibrosing disorders that over the years have been designated in various ways primarily based on the organ of involvement and the names of the initial authors of reports describing their occurrence. Under the umbrella of IgG4-RD, newer nomenclature captures entities such as Mikulicz syndrome as IgG4 related dacryoadentis and sialadenitis, and Riedel’s thyroiditis as IgG4 related thyroid disease (IgG4RTD) (328,329). More recently, IgG4 related thyroid disease has included Riedel’s thyroiditis, fibrosing variant of Hashimoto’s thyroiditis and few patients of Graves’ orbitopathy to represent IgG4-related thyroid disease (330). One or several organs may be involved at the time of diagnosis or subsequent to the identification of IgG4-RD in a particular organ. The most frequently involved organs include the pancreas, bile ducts, salivary glands, lachrymal glands and kidneys (328). The majority of cases are identified by the presence of characteristic fibrosing pathology and it is expected that serum IgG4 levels be elevated in most cases. The diagnosis if IgG4-RD is based on the identification of a (1) mass in one or more organs associated with an (2) elevated serum IgG4 level (greater than 1.35 g/L) and (3) histopathology demonstrating marked lymphocytic and plasma cell infiltration, more than 10 IgG4 positive plasma cells per high powered field (greater than a 40% IgG4/IgG ratio) and storiform fibrosis(326,331). Definitive diagnosis is assured when all 3 criteria are present, the diagnosis is probable when the first and 3rd criteria are met and possible when only the 1st and second criteria are present (328).
Based on these criteria, the position of Riedel’s thyroiditis among the IgG4-RDs would be considered probable as the vast majority of cases reported thus far are not associated with elevated serum IgG4 levels although the typical histopathologic criteria are met when applied. A recent review of 10 cases studied in Japan confirmed the IgG4-RD connection in histopathological data and note a paucity of serum IgG4 levels in their summary of the published literature (332). Most recent cases specifically noting this association and including the association with IgG4-RD in their titles document the first and third diagnostic criteria (322,332-335), while only a few have documented an elevated serum IgG4 level (336,337). A recent series of cases from Sweden, where serum IgG4 levels were measured in 66% of the subjects, indicated that none of the 4 evaluated had elevated serum IgG4 levels (338). The most recent meta-analysis does not detail any serum IgG4 data among the 212 subjects reviewed in published reports from 1925-2019 (302).
Clinical Features
Riedel’s thyroiditis usually presents as a hard thyroid mass, frequently associated with compressive symptoms including dyspnea, stridor, hoarse voice, dysphagia for months before diagnosis (6,67,294,295,300-303) and historically has been diagnosed by a surgeon faced with an inflammatory mass of fibrosclerosing tissue (339,340) when expecting a thyroid tumor (309). Intraoperative diagnostic confusion with anaplastic thyroid cancer (312), sarcoma of the thyroid (311), thyroid lymphoma (341), or fibrosing Hashimoto’s thyroiditis (342) have been reported. A case of asymptomatic Riedel’s associated with a benign follicular adenoma has also been reported (343). Riedel’s thyroiditis may occur in a multinodular goiter or as a rapidly growing hard neck mass in a previously normal gland mimicking thyroid cancer (340,344,345). As the extent of the fibrosis increases, or concomitant Hashimoto’s is present, involvement of a critical mass of the thyroid tissue results in primary hypothyroidism in 25-80% of cases (295,303,309,310,346). Antithyroid antibodies may be present in 36-90% of reported cases (296,302,303). A detailed breakdown of antibodies encountered has recently been reported in the context of systemic review of documented Riedel’s thyroiditis reports where TPO was positive in 43% of those tested, thyroglobulin antibodies were detected in 27% of those evaluated and thyrotropin receptor antibodies were considered present in 20% when they were drawn (302). Extension of the inflammatory process into underlying parathyroid glands may result in non-surgical hypoparathyroidism (346-351) in up to 14% cases encountered (303). The fibrosis may remain relatively stable or progress resulting in local complications by compressing the trachea or esophagus and resulting in symptoms of local pressure, dyspnea, dysphagia as well as stridor out of proportion of the size of the mass (352,353), with subsequent hoarseness, and aphonia, with involvement of the recurrent laryngeal nerves (342,349). Further extension of the inflammatory process involving other neck structures can result in Tolosa-Hunt syndrome (354), Horner’s syndrome (349), or occlusive phlebitis of cervical vessels (355-357). The occurrence of cerebral sinus thrombosis suggests that Riedel's thyroiditis may cause venous stasis, vascular damage, and possibly hypercoagulability (358). Estimates as high as 38% associate Riedel's thyroiditis with similar fibro-sclerotic processes in other areas (303). Subcutaneous fibrosclerosis has also been noted but it is very rare (359). The lesions appear in the lacrimal glands, orbits (360), parotid glands (361), mediastinum (300,303,305,309), coronary arteries (303), retroperitoneal tissues (295,300,346,362,363), bile ducts (301,364) and pancreas (364) in varying combinations in the syndrome of multifocal fibro-sclerositis (365,366).
Clinical Evaluation
Initially the patient with a thyroid mass will need an assessment of thyroid function, and may benefit from screening thyroid antibodies (367). A complete blood count reveals normal to elevated white blood cell counts. The erythrocyte sedimentation rate is usually moderately elevated (308,309). Due to the potential of hypoparathyroidism an assessment of calcium status is prudent (304). Ultrasonography of the thyroid typically reveals a diffuse, hypoechoic, hypovascular appearance due to the extensive fibrosing process (317,340,346,368,369). Unique to the findings in Riedel’s thyroiditis is an encasement of the carotid arteries, not typically seen in other forms of multinodular or Hashimoto’s goiter (303,370). Sonographic elastography demonstrates significant stiffness of the tissue compared to normal thyroid (370). At this point in the evaluation, a fine needle aspiration (FNA) of the thyroid mass is usually obtained. FNA results are typically non-diagnostic due the lack of thyroid follicular cells (67,301,303,306) but may contain evidence of the inflammatory process (303), fibrous tissue and myofibroblasts (371), or even cytopathology findings consistent with follicular neoplasm (348). A novel case illustrates a potential use of FNA to obtain protein used in proteomic analysis which was successful in differentiating the tissue of a patient with Riedel’s thyroiditis from the tissue profile of anaplastic thyroid cancer (372).
In patients with significant obstructive symptomatology, a neck computed tomography (CT) study may be ordered to assess tracheal integrity. CT images characteristically demonstrate hypodense tissue which does not enhance with iodinated contrast in the affected area (368). CT images readily reveal extrathyroidal extension of the inflammatory process (368,373), and have been reported to document arterial encasement in about half of subjects and jugular involvement in about one third of cases (303). Magnetic resonance imaging (MRI) can be expected to show hypointense images on both T1 and T2 weighted images (368) and variable enhancement patterns after gadolinium enhancement (368,369,373-375). Unlike the hypointense images produced by CT and MRI, 18FDG-PET images have shown metabolic activity not only in extrathyroidal masses associated with the systemic inflammatory process but also increased glucose metabolism in the Riedel’s thyroid, likely as a result of active inflammation involving lymphocytes, plasma cells and fibroblast proliferation (370,376,377). FDG metabolic activity can also be used to assess a patient's response to therapy (376,377), but not all reports of this phenomenon have documented the usefulness of this effect (370).
Although not typically indicated in the evaluation of a eu- or hypothyroid individual with a thyroid mass, 99mTc-pertechnetate or 123/131-I scanning in Riedel’s thyroiditis is typically compromised due to low uptake and patchy images typical of other forms of chronic thyroiditis (301,308,309). An exception to the utility of radionuclide scanning is found in the thyrotoxic patient presenting with a thyroid mass. In those with Graves’ disease or a toxic thyroid nodule, the hyperfunctioning portion of the thyroid is indeed well visualized while the portion involved with Riedel’s thyroiditis typically demonstrates no uptake (317). Finally, it has been documented that gallium scanning may, as expected, also demonstrate significant uptake in the Riedel’s lesion (120).
Establishing the diagnosis of Riedel’s thyroiditis requires histopathologic confirmation at the present time. Biopsy material may be obtained by Tru-cut needle biopsy (378), open biopsy (67), or at the time of decompressive thyroidectomy. Histopathologic findings required to establish this diagnosis include: 1) the presence of an inflammatory process in the thyroid with extension into surrounding tissue; 2) the inflammatory infiltrate should contain no giant cells, lymphoid follicles, oncocytes or granulomas; 3) there should be evidence of occlusive phlebitis; and 4) there should be no evidence of thyroid malignancy (379). More recent work has suggested that the IgG4+ plasma cells per high powered field (HPF) and an IgG4+/IgG+ > 40% criteria for IgG4-Related Disease is seldom met in the thyroid and more modest finding of 10 IgG4+ plasma cells/HPF and a IgG4+/IgG ratio of 20% would be a more appropriate diagnostic threshold (380). In light of recent work defining Riedel’s thyroiditis as a potential manifestation of the IgG4-related systemic sclerosing disease, the role of incorporating immunohistochemical assessment of tissue lymphocytes and the measurement of serum IgG4 levels into working diagnostic criteria is very supportive but remains to be defined.
Management of Riedel's Thyroiditis
Although there is no specific therapy for Riedel's thyroiditis, several management strategies are available dependent on the clinical features of the disease in the individual patient. Patients commonly undergo surgery for relief of obstructive symptoms. Histopathology then allows for the definitive establishment of the diagnosis. Most are then treated medically for associated hormone deficiencies with levothyroxine and /or calcium along with calcitriol, but with exception of one case where reduction of the size of the inflammatory mass was observed (345), this supplementation is not thought to influence the course of the disease. Finally, anti-inflammatory treatment aimed at diminishing the inflammatory tissue mass is applied and may even result in resolution of limited biochemical findings such as primary hypoparathyroidism (351).
Surgical therapy for debulking and symptom relief should usually be limited to isthmusectomy (6,67,301,348) when total thyroidectomy is not possible. Due to the obliteration of tissue planes there is an enhanced danger of hypoparathyroidism and recurrent laryngeal nerve injury even when limited surgery is performed by experienced surgical specialists as documented in a series from the Mayo clinic where 39% of patients with Riedel’s thyroiditis suffered surgical complications (303). Previous and contemporary experience therefore recommends that extensive surgical procedures be considered inappropriate (67,303,306,348). Microscopic surgery has been attempted to minimize complications (381).
Medical therapy to arrest progression of symptomatic disease should be pursued after establishment of a firm diagnosis. Corticosteroid therapy has been found to be effective in some cases (296,302,347,351,358,365,374,378,382-387), probably most in those with active inflammation (322,386). Initial doses of up to 100 mg per day of prednisone have been used (301) but sustained improvement has been reported with lower doses of 15-60 mg per day for about 3 months (302,341,349,365,382,385,387). There are no controlled trials of steroid therapy in Riedel's and a variety of medications have been used including most frequently prednisone, prednisolone, dexamethasone methylprednisolone, and betamethasone (302). Although some patients obtain long-term benefit after steroid withdrawal (313,365,386) others may relapse, usually leading to reintroduction of glucocorticoids or the addition of alternative anti-inflammatory therapy (349,388,389). The reasons for this variation are unclear but inflammatory activity and duration of disease may be relevant factors. More recently, the observation that smoking history may play a role in the responsiveness of Riedel’s pathology to glucocorticoid therapy has been published (303).
In those who fail to respond to glucocorticoid therapy, or relapse after withdrawal, tamoxifen therapy is the next most common therapy reported to have been tried. Twenty eight reports have described an encouraging response with this agent when administered in doses of 20 mg daily for an average of 8 months, admittedly in only a small number of patients (302,349,351,388,390-395). It is possible that tamoxifen acts in Riedel's by inhibition of fibroblast proliferation through the stimulation of TGF beta (396-398). Tamoxifen in combination with prednisone or tamoxifen as monotherapy have both been reported to be effective (349,388,393,395). There appears to be a persistent benefit of tamoxifen therapy during continued application in most but not all cases (303,389). Limited data on effective therapy with other immunosuppressive agents indicates that responses to azathioprine in doses ranging from 40 to 150 mg daily have been reported in 4 patients (302). Also a combination of mycophenolate mofetil and prednisone has been observed to have successfully treated an individual who failed a prednisone and tamoxifen combination (389) and 3 cases of rituximab use have also been reported to be useful (334,399,400). The potential usefulness and relative effectiveness of these interventions awaits confirmation.
Summary of Riedel’s Thyroiditis
Riedel’s thyroiditis should be suspected in patients presenting with a thyroid mass and unique clinical features. Findings increasing the likelihood of Riedel’s thyroiditis include local restrictive or infiltrative symptoms out of proportion to the size or extent of the mass or simultaneous hypocalcemia. Surgical intervention should be limited to rule out the presence of malignancy and obtain the histopathologic confirmation. Once the diagnosis of Riedel’s thyroiditis is established, a search for related fibrotic conditions and medical treatment should be pursued. Replacement with levothyroxine and, when appropriate, calcium and active vitamin D metabolites should be begun when indicated along with anti-inflammatory medications.
RARE INFLAMMATORY OR INFILTRATIVE DISEASES
In addition to the varieties of thyroiditis already mentioned, which are diseases specifically of the thyroid gland, generalized or systemic diseases may also involve the thyroid gland (67). The lesions of sarcoid may appear in the thyroid gland of 1-4% of patients with systemic sarcoidosis (401). Thyroid dysfunction has been reported very infrequently (1-3%)(402) in systemic sarcoidosis but a recent series of patients with cutaneous sarcoidosis noted abnormal TSH values in 26% compared to the US population expectation of about 10% (402). Most thyroid dysfunction was mild, a male to female ratio of abnormal thyroid function of 1:1 was noted, Caucasians were more frequently affected than African Americans and 20% of those with abnormal TSH were eventually classified as hypothyroid (402). Infiltration of the thyroid with sarcoidosis is reported to occur in about 5% of patients with sarcoidosis (403). Multinodular goiter has been described as an initial presenting manifestation in a woman eventually diagnosed with systemic sarcoidosis (401). This case illustrates the difficulty in diagnosing the cause of supine dyspnea in patients with sarcoidosis, illustrating the potential of a thyroid contribution to the overall clinical picture (401).
Deposits of amyloid are quite common in systemic amyloidosis (404) but this uncommonly causes goiter (404-407). Although transthyretin amyloidosis is primarily associated with amyloid deposits in the heart and the nervous tissue, familial forms of amyloidosis due to transthyretin gene mutations are associated with deposits of amyloid in multiple tissues (408). AL amyloidosis (primary), linked to plasma cell dyscrasias can be local or systemic. Localized primary amyloidosis presenting with isolated amyloid goiter are rare (405,409,410). Secondary amyloidosis is associated with chronic inflammatory conditions such as Familial Mediterranean Fever (FMF) (411), inflammatory bowel disease (412), rheumatoid arthritis (407,413), end-stage renal disease (414), tuberculosis (415) and bronchiectasis (416). Clinically, an amyloid goiter may be progressive, diffuse and rapidly lead to compressive symptoms (404,405,411). Thyroid function in association with an amyloid goiter is normal in 2/3 of cases, 1/7 present with hypothyroidism and fewer demonstrate other abnormalities of thyroid function (404). In addition to the focal deposition of amyloid in thyroid tissues associated with most cases of medullary thyroid cancer (417,418), several cases of papillary thyroid cancer have been reported in association of amyloid goiter (404,419-421). Amyloid goiter may be readily diagnosed by fine needle aspiration biopsy (422) and has been reported in conjunction with infiltration of other endocrine organs such as the pituitary (406). It has been suggested that the thyroid FNA is relatively safe and sensitive to confirm the presence of systemic amyloidosis(404,423). Some may require surgery to relieve the compressive symptoms or for a confirmatory diagnosis. Using a calcitonin immunostaining technique may help delineate between amyloidosis and medullary thyroid cancer (424). Once the diagnosis of amyloid goiter is established, the patient should be screened for predisposing causes and the extent of the disease.
Painless thyroiditis has been noted in a woman with rheumatoid arthritis and secondary amyloidosis infiltrating the thyroid gland (425). Radiotherapy for tonsillar carcinoma has been reported to result in thyroiditis (426). Irradiation to the thyroid during therapy for breast cancer or lymphoma can also induce hypothyroidism. Following 131-I therapy for Graves’ disease or toxic multinodular goiter, thyroiditis, which is occasionally symptomatic, may develop. This situation is discussed in other Endotext chapters. Therapy should be directed toward the primary disease rather than the thyroid, but administration of thyroid hormone may be necessary if destruction of thyroid tissue is sufficient to produce hypothyroidism. Finally, surgery to the neck, associated with mechanical manipulation of the thyroid during laryngectomy or parathyroid surgery can result in a painless subacute thyroiditis like picture (427-429).
ACKNOWLEDGEMENT
The authors are grateful to the extensive groundwork performed by Dr. John Lazarus, the founding author of this chapter. Additionally, we are privileged to update this summary with the most recent developments in the field while maintaining the historical perspective of those who have preceded us.
REFERENCES
- 1.
- Chan GC, Lee PC, Kwan LP, Yip TP, Tang SC. Acute thyroiditis: An under-recognized complication of parathyroidectomy in end-stage renal failure patients with secondary hyperparathyroidism. Nephrology (Carlton). 2017;22(7):572. [PubMed: 28621009]
- 2.
- Paes JE, Burman KD, Cohen J, Franklyn J, McHenry CR, Shoham S, Kloos RT. Acute bacterial suppurative thyroiditis: a clinical review and expert opinion. Thyroid. 2010;20(3):247–255. [PubMed: 20144025]
- 3.
- Al-Dajani N, Wootton SH. Cervical lymphadenitis, suppurative parotitis, thyroiditis, and infected cysts. Infect Dis Clin North Am. 2007;21(2):523–541. viii. [PubMed: 17561081]
- 4.
- Hendrick JW. Diagnosis and management of thyroiditis. J Am Med Assoc. 1957;164(2):127–133. [PubMed: 13415949]
- 5.
- Yu EH, Ko WC, Chuang YC, Wu TJ. Suppurative Acinetobacter baumanii thyroiditis with bacteremic pneumonia: case report and review. Clin Infect Dis. 1998;27(5):1286–1290. [PubMed: 9827283]
- 6.
- Pearce EN, Farwell AP, Braverman LE. Thyroiditis. N Engl J Med. 2003;348(26):2646–2655. [PubMed: 12826640]
- 7.
- Dugar M, da Graca Bandeira A, Bruns J Jr, Som PM. Unilateral hypopharyngitis, cellulitis, and a multinodular goiter: a triad of findings suggestive of acute suppurative thyroiditis. AJNR Am J Neuroradiol. 2009;30(10):1944–1946. [PMC free article: PMC7051277] [PubMed: 19461065]
- 8.
- Chi H, Lee YJ, Chiu NC, Huang FY, Huang CY, Lee KS, Shih SL, Shih BF. Acute suppurative thyroiditis in children. Pediatr Infect Dis J. 2002;21(5):384–387. [PubMed: 12150173]
- 9.
- Chang P, Tsai WY, Lee PI, Hsiao PH, Huang LM, Lee JS, Peng SF, Li YW. Clinical characteristics and management of acute suppurative thyroiditis in children. J Formos Med Assoc. 2002;101(7):468–471. [PubMed: 12353338]
- 10.
- Brook I. Microbiology and management of acute suppurative thyroiditis in children. Int J Pediatr Otorhinolaryngol. 2003;67(5):447–451. [PubMed: 12697345]
- 11.
- Hatabu H, Kasagi K, Yamamoto K, Iida Y, Misaki T, Hidaka A, Endo K, Konishi J. Acute suppurative thyroiditis associated with piriform sinus fistula: sonographic findings. AJR Am J Roentgenol. 1990;155(4):845–847. [PubMed: 2119120]
- 12.
- Parida PK, Gopalakrishnan S, Saxena SK. Pediatric recurrent acute suppurative thyroiditis of third branchial arch origin--our experience in 17 cases. Int J Pediatr Otorhinolaryngol. 2014;78(11):1953–1957. [PubMed: 25219934]
- 13.
- Lucaya J, Berdon WE, Enriquez G, Regas J, Carreno JC. Congenital pyriform sinus fistula: a cause of acute left-sided suppurative thyroiditis and neck abscess in children. Pediatr Radiol. 1990;21(1):27–29. [PubMed: 2152547]
- 14.
- Miyauchi A, Yokozawa T, Matsuzuka F, Kuma K. Acute suppurative thyroiditis; infection in thyroid nodules or infection through a piriform sinus fistula. Thyroidol Clin Exp. 1998;10:75–79.
- 15.
- Miyauchi A, Matsuzuka F, Kuma K, Katayama S. Piriform sinus fistula and the ultimobranchial body. Histopathology. 1992;20(3):221–227. [PubMed: 1563708]
- 16.
- Shah SS, Baum SG. Diagnosis and Management of Infectious Thyroiditis. Curr Infect Dis Rep. 2000;2(2):147–153. [PubMed: 11095850]
- 17.
- Fukata S, Miyauchi A, Kuma K, Sugawara M. Acute suppurative thyroiditis caused by an infected piriform sinus fistula with thyrotoxicosis. Thyroid. 2002;12(2):175–178. [PubMed: 11916288]
- 18.
- Gan YU, Lam SL. Imaging findings in acute neck infection due to pyriform sinus fistula. Ann Acad Med Singapore. 2004;33(5):636–640. [PubMed: 15531961]
- 19.
- Sheng Q, Lv Z, Xiao X, Zheng S, Huang Y, Huang X, Li H, Wu Y, Dong K, Liu J. Diagnosis and management of pyriform sinus fistula: experience in 48 cases. J Pediatr Surg. 2014;49(3):455–459. [PubMed: 24650477]
- 20.
- Mohan PS, Chokshi RA, Moser RL, Razvi SA. Thyroglossal duct cysts: a consideration in adults. Am Surg. 2005;71(6):508–511. [PubMed: 16044932]
- 21.
- Szabo SM, Allen DB. Thyroiditis. Differentiation of acute suppurative and subacute. Case report and review of the literature. Clin Pediatr (Phila). 1989;28(4):171–174. [PubMed: 2649297]
- 22.
- Hopwood NJ, Kelch RP. Thyroid masses: approach to diagnosis and management in childhood and adolescence. Pediatr Rev. 1993;14(12):481–487. [PubMed: 8115286]
- 23.
- Sai Prasad TR, Chong CL, Mani A, Chui CH, Tan CE, Tee WS, Jacobsen AS. Acute suppurative thyroiditis in children secondary to pyriform sinus fistula. Pediatr Surg Int. 2007;23(8):779–783. [PubMed: 17534632]
- 24.
- Wasniewska M, Vigone MC, Cappa M, Cassio A, Scognamillo R, Aversa T, Rubino M, De Luca F. Acute suppurative thyroiditis in childhood: spontaneous closure of sinus pyriform fistula may occur even very early. J Pediatr Endocrinol Metab. 2007;20(1):75–77. [PubMed: 17315532]
- 25.
- Cases JA, Wenig BM, Silver CE, Surks MI. Recurrent acute suppurative thyroiditis in an adult due to a fourth branchial pouch fistula. J Clin Endocrinol Metab. 2000;85(3):953–956. [PubMed: 10720022]
- 26.
- Miyauchi A, Matsuzuka F, Kuma K, Takai S. Piriform sinus fistula: an underlying abnormality common in patients with acute suppurative thyroiditis. World J Surg. 1990;14(3):400–405. [PubMed: 2368443]
- 27.
- Kingsbury BF. On the fate of the ultimobrachial body within the hyman thyroid gland. Anat Rec. 1935;61:155–167.
- 28.
- Minhas SS, Watkinson JC, Franklyn J. Fourth branchial arch fistula and suppurative thyroiditis: a life-threatening infection. J Laryngol Otol. 2001;115(12):1029–1031. [PubMed: 11779342]
- 29.
- Nicoucar K, Giger R, Pope HG Jr, Jaecklin T, Dulguerov P. Management of congenital fourth branchial arch anomalies: a review and analysis of published cases. J Pediatr Surg. 2009;44(7):1432–1439. [PubMed: 19573674]
- 30.
- Acocelia A, Nardi P. Sacco, Agostini T. Acute thyroiditis of odontogenic origin. Minerva Stomatol. 2007;56:461–467. [PubMed: 17938625]
- 31.
- Dai L, Lin S, Liu D, Wang Q. Acute suppurative thyroiditis with thyroid metastasis from oesophageal cancer. Endokrynol Pol. 2020;71(1):106–107. [PubMed: 31681975]
- 32.
- Vandjme A, Pageaux GP, Bismuth M, Fabre JM, Domergue J, Perez C, Makeieff M, Mourad G, Larrey D. Nocardiosis revealed by thyroid abscess in a liver--kidney transplant recipient. Transpl Int. 2001;14(3):202–204. [PubMed: 11499912]
- 33.
- Teckie G, Bhana SA, Tsitsi JM, Shires R. Thyrotoxicosis followed by Hypothyroidism due to Suppurative Thyroiditis Caused by in a Patient with Advanced Acquired Immunodeficiency Syndrome. Eur Thyroid J. 2014;3(1):65–68. [PMC free article: PMC4005252] [PubMed: 24847469]
- 34.
- Kazi S, Liu H, Jiang N, Glick J, Teng M, LaBombardi V, Szporn AH, Chen H. Salmonella thyroid abscess in human immunodeficiency virus-positive man: a diagnostic pitfall in fine-needle aspiration biopsy of thyroid lesions. Diagn Cytopathol. 2015;43(1):36–39. [PubMed: 24550178]
- 35.
- Massolt ET, Rijneveld AW, Vernooij MW, Kevenaar ME, van Kemenade FJ, Peeters RP. Acute Candida thyroiditis complicated by abscess formation in a severely immunocompromised patient. J Clin Endocrinol Metab. 2014;99(11):3952–3953. [PubMed: 25033071]
- 36.
- Berger SA, Zonszein J, Villamena P, Mittman N. Infectious diseases of the thyroid gland. Rev Infect Dis. 1983;5(1):108–122. [PubMed: 6338569]
- 37.
- Fernandez JF, Anaissie EJ, Vassilopoulou-Sellin R, Samaan NA. Acute fungal thyroiditis in a patient with acute myelogenous leukaemia. J Intern Med. 1991;230(6):539–541. [PubMed: 1748861]
- 38.
- Gandhi RT, Tollin SR, Seely EW. Diagnosis of Candida thyroiditis by fine needle aspiration. J Infect. 1994;28(1):77–81. [PubMed: 8163838]
- 39.
- McAninch EA, Xu C, Lagari VS, Kim BW. Coccidiomycosis thyroiditis in an immunocompromised host post-transplant: case report and literature review. J Clin Endocrinol Metab. 2014;99(5):1537–1542. [PubMed: 24606101]
- 40.
- Alvi MM, Meyer DS, Hardin NJ, Dekay JG, Marney AM, Gilbert MP. Aspergillus thyroiditis: a complication of respiratory tract infection in an immunocompromised patient. Case Rep Endocrinol. 2013;2013:741041. [PMC free article: PMC3878395] [PubMed: 24455333]
- 41.
- Imai C, Kakihara T, Watanabe A, Ikarashi Y, Hotta H, Tanaka A, Uchiyama M. Acute suppurative thyroiditis as a rare complication of aggressive chemotherapy in children with acute myelogeneous leukemia. Pediatr Hematol Oncol. 2002;19(4):247–253. [PubMed: 12051591]
- 42.
- Yildar M, Demirpolat G, Aydin M. Acute suppurative thyroiditis accompanied by thyrotoxicosis after fine-needle aspiration: treatment with catheter drainage. J Clin Diagn Res. 2014;8(11):ND12–14. [PMC free article: PMC4290286] [PubMed: 25584265]
- 43.
- Unluturk U, Ceyhan K, Corapcioglu D. Acute suppurative thyroiditis following fine-needle aspiration biopsy in an immunocompetent patient. J Clin Ultrasound. 2014;42(4):215–218. [PubMed: 23893617]
- 44.
- Inoue K, Kozawa J, Funahashi T, Nakata Y, Mitsui E, Kitamura T, Maeda N, Kishida K, Otsuki M, Okita K, Iwahashi H, Imagawa A, Shimomura I. Right-sided acute suppurative thyroiditis caused by infectious endocarditis. Intern Med. 2011;50(23):2893–2897. [PubMed: 22129504]
- 45.
- Robazzi TC, Alves C, Mendonca M. Acute suppurative thyroiditis as the initial presentation of juvenile systemic lupus erythematosus. J Pediatr Endocrinol Metab. 2009;22(4):379–383. [PubMed: 19554814]
- 46.
- Cabizuca CA, Bulzico DA, de Almeida MH, Conceicao FL, Vaisman M. Acute thyroiditis due to septic emboli derived from infective endocarditis. Postgrad Med J. 2008;84(994):445–446. [PubMed: 18832408]
- 47.
- Yegya-Raman N, Copeland T, Parikh P. Acute Suppurative Thyroiditis in an Intravenous Drug User with a Preexisting Goiter. Case Reports in Medicine. 2018 [PMC free article: PMC5892251] [PubMed: 29780423]
- 48.
- Chiovato L, Canale G, Maccherini D, Falcone V, Pacini F, Pinchera A. Salmonella brandenburg: a novel cause of acute suppurative thyroiditis. Acta Endocrinol (Copenh). 1993;128(5):439–442. [PubMed: 8317191]
- 49.
- Dai MS, Chang H, Peng MY, Ho CL, Chao TY. Suppurative salmonella thyroiditis in a patient with chronic lymphocytic leukemia. Ann Hematol. 2003;82(10):646–648. [PubMed: 12879283]
- 50.
- Su DH, Huang TS. Acute suppurative thyroiditis caused by Salmonella typhimurium: a case report and review of the literature. Thyroid. 2002;12(11):1023–1027. [PubMed: 12490081]
- 51.
- Akhanli P, Bayir O, Bayram SM, Hepsen S, Badirshaev M, Cakal E, Saylam G, Korkmaz MH. Acute spontaneous suppurative thyroiditis caused by Eikenella corrodens presented with thyrotoxicosis. Einstein (Sao Paulo). 2020;18:eRC5273. [PMC free article: PMC7087456] [PubMed: 32215470]
- 52.
- Bukvic B, Diklic A, Zivaljevic V. Acute suppurative klebsiella thyroiditis: a case report. Acta Chir Belg. 2009;109(2):253–255. [PubMed: 19499695]
- 53.
- Fernandez Pena C, Morales Gorria MJ, Morano Amado LE, Lopez Miragalla MI, Pena Gonzalez E. Pasteurella spp: a newmicroorganism to the cause of acute suppurative thyroiditis. An Med Interna. 1999;16:637–638. [PubMed: 10686718]
- 54.
- McLaughlin SA, Smith SL, Meek SE. Acute suppurative thyroiditis caused by Pasteurella multocida and associated with thyrotoxicosis. Thyroid. 2006;16(3):307–310. [PubMed: 16571095]
- 55.
- Spitzer M, Alexanian S, Farwell AP. Thyrotoxicosis with Post-Treatment Hypothyroidism in a Patient with Acute Suppurative Thyroiditis Due to Porphyromonas. Thyroid. 2011 [PubMed: 22136210]
- 56.
- Iniguez JL, Duyckaerts V, Badoual J. Arch Fr Pediatr. 1989;46(10):745–747. [Acute thyroiditis caused by Eikenella corrodens and abnormality of the left pyriform sinus] [PubMed: 2697197]
- 57.
- Queen JS, Clegg HW, Council JC, Morton D. Acute suppurative thyroiditis caused by Eikenella corrodens. J Pediatr Surg. 1988;23(4):359–361. [PubMed: 3290424]
- 58.
- Yoshino Y, Inamo Y, Fuchigami T, Hashimoto K, Ishikawa T, Abe O, Tahara D, Hayashi K. A pediatric patient with acute suppurative thyroiditis caused by Eikenella corrodens. J Infect Chemother. 2010;16(5):353–355. [PubMed: 20424880]
- 59.
- Nieuwland Y, Tan KY, Elte JW. Miliary tuberculosis presenting with thyrotoxicosis. Postgrad Med J. 1992;68(802):677–679. [PMC free article: PMC2399548] [PubMed: 1448412]
- 60.
- Das DK, Pant CS, Chachra KL, Gupta AK. Fine needle aspiration cytology diagnosis of tuberculous thyroiditis. A report of eight cases. Acta Cytol. 1992;36(4):517–522. [PubMed: 1636345]
- 61.
- Orlandi F, Fiorini S, Gonzatto I, Saggiorato E, Pivano G, Angeli A, Pasquali R. Tubercular involvement of the thyroid gland: a report of two cases. Horm Res. 1999;52(6):291–294. [PubMed: 10965210]
- 62.
- Terzidis K, Tourli P, Kiapekou E, Alevizaki M. Thyroid tuberculosis. Hormones (Athens). 2007;6(1):75–79. [PubMed: 17324921]
- 63.
- Kataria SP, Tanwar P, Singh S, Kumar S. Primary tuberculosis of the thyroid gland: a case report. Asian Pac J Trop Biomed. 2012;2(10):839–840. [PMC free article: PMC3609221] [PubMed: 23569858]
- 64.
- Karabinis A, Douzinas E, Clouva P, Papanicolaou M, Kakaviatos N, Bilalis D. Presse Med. 1993;22(1):34. [Acute necrotic thyroiditis caused by Candida albicans immediately after acute hemorrhagic rectocolitis] [PubMed: 8469659]
- 65.
- Carriere C, Marchandin H, Andrieu JM, Vandome A, Perez C. Nocardia thyroiditis: unusual location of infection. J Clin Microbiol. 1999;37(7):2323–2325. [PMC free article: PMC85148] [PubMed: 10364605]
- 66.
- Lewin SR, Street AC, Snider J. Suppurative thyroiditis due to Nocardia asteroides. J Infect. 1993;26(3):339–340. [PubMed: 8505575]
- 67.
- Singer PA. Thyroiditis. Acute, subacute, and chronic. Med Clin North Am. 1991;75(1):61–77. [PubMed: 1987447]
- 68.
- Tan J, Shen J, Fang Y, Zhu L, Liu Y, Gong Y, Zhu H, Hu Z, Wu G. A suppurative thyroiditis and perineal subcutaneous abscess related with aspergillus fumigatus: a case report and literature review. BMC Infect Dis. 2018;18(1):702. [PMC free article: PMC6307113] [PubMed: 30587135]
- 69.
- Karatoprak N, Atay Z, Erol N, Goksugur SB, Ceran O. Actinomycotic suppurative thyroiditis in a child. J Trop Pediatr. 2005;51(6):383–385. [PubMed: 15947015]
- 70.
- Park YH, Baik JH, Yoo J. Acute thyroiditis of actinomycosis. Thyroid. 2005;15(12):1395–1396. [PubMed: 16405415]
- 71.
- Trites J, Evans M. Actinomycotic thyroiditis in a child. J Pediatr Surg. 1998;33(5):781–782. [PubMed: 9607501]
- 72.
- Moinuddin S, Barazi H, Moinuddin M. Acute blastomycosis thyroiditis. Thyroid. 2008;18(6):659–661. [PubMed: 18578618]
- 73.
- Rao N, Mann SJ. Fine needle aspiration cytology of acute blastomycosis thyroiditis. Diagnostic Cytopathology. 2017;45(12):1119–1121. [PubMed: 28792123]
- 74.
- Avram AM, Sturm CA, Michael CW, Sisson JC, Jaffe CA. Cryptococcal thyroiditis and hyperthyroidism. Thyroid. 2004;14(6):471–474. [PubMed: 15242578]
- 75.
- Zavascki AP, Maia AL, Goldani LZ. Pneumocystis jiroveci thyroiditis: report of 15 cases in the literature. Mycoses. 2007;50(6):443–446. [PubMed: 17944703]
- 76.
- Lafontaine N, Learoyd D, Farrel S, Wong R. Suppurative thyroiditis: Systematic review and clinical guidance. Clin Endocrinol (Oxf). 2021;95(2):253–264. [PubMed: 33559162]
- 77.
- Orkar KS, Dakum NK, Kidmas AT, Awani KU. Pyogenic thyroiditis and HIV infection. West Afr J Med. 2001;20(2):173–175. [PubMed: 11768021]
- 78.
- Tien KJ, Chen TC, Hsieh MC, Hsu SC, Hsiao JY, Shin SJ, Hsin SC. Acute suppurative thyroiditis with deep neck infection: a case report. Thyroid. 2007;17(5):467–469. [PubMed: 17542677]
- 79.
- Iwama S, Kato Y, Nakayama S. Acute suppurative thyroiditis extending to descending necrotizing mediastinitis and pericarditis. Thyroid. 2007;17(3):281–282. [PubMed: 17381365]
- 80.
- Premawardhana LD, Vora JP, Scanlon MF. Suppurative thyroiditis with oesophageal carcinoma. Postgrad Med J. 1992;68(801):592–593. [PMC free article: PMC2399380] [PubMed: 1437962]
- 81.
- Valina S, Lotter O, Schaller HE, Rahmanian-Schwarz A. Zentralbl Chir. 2011 [Abscess Formation after Puncture of a Thyroid Cyst - A Case Report.] [PubMed: 21667445]
- 82.
- Kale SU, Kumar A, David VC. Thyroid abscess--an acute emergency. Eur Arch Otorhinolaryngol. 2004;261(8):456–458. [PubMed: 14652771]
- 83.
- Jimenez-Heffernan JA, Perez F, Hornedo J, Perna C, Lapuente F. Massive thyroid tumoral embolism from a breast carcinoma presenting as acute thyroiditis. Arch Pathol Lab Med. 2004;128(7):804–806. [PubMed: 15214816]
- 84.
- Robillon JF, Sadoul JL, Guerin P, Iafrate-Lacoste C, Talbodec A, Santini J, Canivet B, Freychet P. Mycobacterium avium intracellulare suppurative thyroiditis in a patient with Hashimoto's thyroiditis. J Endocrinol Invest. 1994;17(2):133–134. [PubMed: 8006334]
- 85.
- Visser R, de Mast Q, Netea-Maier RT, van der Ven AJ. Hashimoto's thyroiditis presenting as acute painful thyroiditis and as a manifestation of an immune reconstitution inflammatory syndrome in a human immunodeficiency virus-seropositive patient. Thyroid. 2012;22(8):853–855. [PubMed: 22784301]
- 86.
- Kale SU, Kumar A, David VC. Thyroid abscess: an acute emergency. Eur Arch Otorhinolarngol. 2004;261(8):456–481. [PubMed: 14652771]
- 87.
- Kalladi Puthanpurayil S, Francis GL, Kraft AO, Prasad U, Petersson RS. Papillary thyroid carcinoma presenting as acute suppurative thyroiditis: A case report and review of the literature. Int J Pediatr Otorhinolaryngol. 2018;105:12–15. [PubMed: 29447798]
- 88.
- Nishihara E, Miyauchi A, Matsuzuka F, Sasaki I, Ohye H, Kubota S, Fukata S, Amino N, Kuma K. Acute suppurative thyroiditis after fine-needle aspiration causing thyrotoxicosis. Thyroid. 2005;15(10):1183–1187. [PubMed: 16279853]
- 89.
- Chen HW, Tseng FY, Su DH, Chang YL, Chang TC. Secondary infection and ischemic necrosis after fine needle aspiration for a painful papillary thyroid carcinoma: a case report. Acta Cytol. 2006;50(2):217–220. [PubMed: 16610694]
- 90.
- Puthanpurayil SK, Francis GL, Kraft AO, Prasad U, Petersson RS. Papillary thyroid carcinoma presenting as acute suppurative thyroiditis: A case report and review of the literature. International Journal of Pediatric Otorhinolaryngology. 2018;105:12–15. [PubMed: 29447798]
- 91.
- George MM, Goswamy J, Penney SE. Embolic suppurative thyroiditis with concurrent carcinoma in pregnancy: lessons in management through a case report. Thyroid Research. 2015:8. [PMC free article: PMC4349691] [PubMed: 25741383]
- 92.
- Sicilia V, Mezitis S. A case of acute suppurative thyroiditis complicated by thyrotoxicosis. J Endocrinol Invest. 2006;29(11):997–1000. [PubMed: 17259797]
- 93.
- Falhammar H, Wallin G, Calissendorff J. Acute suppurative thyroiditis with thyroid abscess in adults: clinical presentation, treatment and outcomes. BMC Endocr Disord. 2019;19(1):130. [PMC free article: PMC6889346] [PubMed: 31791298]
- 94.
- Takai SI, Miyauchi A, Matsuzuka F, Kuma K, Kosaki G. Internal fistula as a route of infection in acute suppurative thyroiditis. Lancet. 1979;1(8119):751–752. [PubMed: 85992]
- 95.
- Yamashita J, Ogawa M, Yamashita S, Saishoji T, Nomura K, Tsuruta J. Acute suppurative thyroiditis in an asymptomatic woman: an atypical presentation simulating thyroid carcinoma. Clin Endocrinol (Oxf). 1994;40(1):145–149. [PubMed: 8306474]
- 96.
- Miyauchi A, Matsuzuka F, Takai S, Kuma K, Kosaki G. Piriform sinus fistula. A route of infection in acute suppurative thyroiditis. Arch Surg. 1981;116(1):66–69. [PubMed: 7469735]
- 97.
- Nonomura N, Ikarashi F, Fujisaki T, Nakano Y. Surgical approach to pyriform sinus fistula. Am J Otolaryngol. 1993;14(2):111–115. [PubMed: 8484475]
- 98.
- Himi T, Kataura A. Distribution of C cells in the thyroid gland with pyriform sinus fistula. Otolaryngol Head Neck Surg. 1995;112(2):268–273. [PubMed: 7838551]
- 99.
- Bar-Ziv J, Slasky BS, Sichel JY, Lieberman A, Katz R. Branchial pouch sinus tract from the piriform fossa causing acute suppurative thyroiditis, neck abscess, or both: CT appearance and the use of air as a contrast agent. AJR Am J Roentgenol. 1996;167(6):1569–1572. [PubMed: 8956599]
- 100.
- Gaafar H, El-Garem F. Acute thyroiditis with gas formation. J Laryngol Otol. 1975;89(3):323–327. [PubMed: 1127327]
- 101.
- Bussman YC, Wong ML, Bell MJ, Santiago JV. Suppurative thyroiditis with gas formation due to mixed anaerobic infection. J Pediatr. 1977;90(2):321–322. [PubMed: 830929]
- 102.
- Reksoprawiro S. Suppurative thyroiditis with gas formation. Asian J Surg. 2003;26(3):180–182. [PubMed: 12925295]
- 103.
- Al-Kordi RS, Alenizi E, Elgazzar AH. Acute suppurative thyroiditis with abscess, gas formation, and thyrotoxic crisis. Nuklearmedizin. 2008;47(4):N44–46. [PubMed: 18763371]
- 104.
- Lemariey Hamelin. Muler. Ann Otolaryngol. 1955;72(7):571–573. [Acute thyroiditis complicating mediastinitis] [PubMed: 13302921]
- 105.
- Dordain ML, Coutant G, Algayres JP, Jancovici R, Pats B, Daly JP. Presse Med. 1997;26(7):319–320. [Suppurative mediastinitis secondary to acute thyroiditis in a patient under corticotherapy] [PubMed: 9122140]
- 106.
- Pereira O, Prasad DS, Bal AM, McAteer D, Abraham P. Fatal descending necrotizing mediastinitis secondary to acute suppurative thyroiditis developing in an apparently healthy woman. Thyroid. 2010;20(5):571–572. [PubMed: 20384489]
- 107.
- Yung BC, Loke TK, Fan WC, Chan JC. Acute suppurative thyroiditis due to foreign body-induced retropharyngeal abscess presented as thyrotoxicosis. Clin Nucl Med. 2000;25(4):249–252. [PubMed: 10750960]
- 108.
- Wu C, Zhang Y, Gong Y, Hou Y, Li S, Zou Y, Ge J. Two cases of bacterial suppurative thyroiditis caused by Streptococcus anginosus. Endocr Pathol. 2013;24(1):49–53. [PubMed: 23435638]
- 109.
- Miyauchi A, Inoue H, Tomoda C, Amino N. Evaluation of chemocauterization treatment for obliteration of pyriform sinus fistula as a route of infection causing acute suppurative thyroiditis. Thyroid. 2009;19(7):789–793. [PubMed: 19508119]
- 110.
- Nishihara E, Ohye H, Amino N, Takata K, Arishima T, Kudo T, Ito M, Kubota S, Fukata S, Miyauchi A. Clinical characteristics of 852 patients with subacute thyroiditis before treatment. Intern Med. 2008;47(8):725–729. [PubMed: 18421188]
- 111.
- Hong JT, Lee JH, Kim SH, Hong SB, Nam M, Kim YS, Chu YC. Case of concurrent Riedel's thyroiditis, acute suppurative thyroiditis, and micropapillary carcinoma. Korean J Intern Med. 2013;28(2):236–241. [PMC free article: PMC3604615] [PubMed: 23526581]
- 112.
- Akdemir Z, Karaman E, Akdeniz H, Alptekin C, Arslan H. Giant Thyroid Abscess Related to Postpartum Brucella Infection. Case Reports in Infectious Diseases. 2015 [PMC free article: PMC4378610] [PubMed: 25861492]
- 113.
- Campos R, Perez B, Armengod L, Munez E, Ramos A. Lactococcus lactis thyroid abscess in an immunocompetent patient. Endocrinologia y Nutricion. 2015;62(4):204–206. [PubMed: 25601537]
- 114.
- Mohi GK, Datta P, Chander J, Das A. Citrobacter freundii as a cause of acute suppurative thyroiditis in an immunocompetent adult female. Indian Journal of Pathology and Microbiology. 2017;60(2):282–284. [PubMed: 28631657]
- 115.
- Miyauchi A. Thyroid gland: A new management algorithm for acute suppurative thyroiditis? Nat Rev Endocrinol. 2010;6(8):424–426. [PubMed: 20657545]
- 116.
- Kim S, Park TS, Baek HS, Jin HY. Subacute painful thyroiditis accompanied by scrub typhus infection. Endocrine. 2013;44(2):546–548. [PubMed: 23564597]
- 117.
- Masuoka H, Miyauchi A, Tomoda C, Inoue H, Takamura Y, Ito Y, Kobayashi K, Miya A. Imaging studies in sixty patients with acute suppurative thyroiditis. Thyroid. 2011;21(10):1075–1080. [PubMed: 21875365]
- 118.
- Ruchala M, Szczepanek-Parulska E, Zybek A, Moczko J, Czarnywojtek A, Kaminski G, Sowinski J. The role of sonoelastography in acute, subacute and chronic thyroiditis - a novel application of the method. Eur J Endocrinol. 2011 [PubMed: 22143319]
- 119.
- Bernard PJ, Som PM, Urken ML, Lawson W, Biller HF. The CT findings of acute thyroiditis and acute suppurative thyroiditis. Otolaryngol Head Neck Surg. 1988;99(5):489–493. [PubMed: 3147442]
- 120.
- Yung G, Kannangara K, Bui C, Mansberg R, Champion B. Riedel thyroiditis demonstrated on gallium scintigraphy. Clin Nucl Med. 2010;35(8):614–617. [PubMed: 20631515]
- 121.
- Park NH, Park HJ, Park CS, Kim MS, Park SI. The emerging echogenic tract sign of pyriform sinus fistula: an early indicator in the recovery stage of acute suppurative thyroiditis. AJNR Am J Neuroradiol. 2011;32(3):E44–46. [PMC free article: PMC8013085] [PubMed: 20133389]
- 122.
- Miyauchi A, Tomoda C, Uruno T, Takamura Y, Ito Y, Miya A, Kobayashi K, Matsuzuka F, Fukata S, Amino N, Kuma K. Computed tomography scan under a trumpet maneuver to demonstrate piriform sinus fistulae in patients with acute suppurative thyroiditis. Thyroid. 2005;15(12):1409–1413. [PubMed: 16405421]
- 123.
- Ukiyama E, Endo M, Yoshida F, Watanabe T. Light guided procedure for congenital pyriform sinus fistula; new and simple procedure for impalpable fistula. Pediatr Surg Int. 2007;23(12):1241–1243. [PubMed: 17968563]
- 124.
- Kim KH, Sung MW, Koh TY, Oh SH, Kim IS. Pyriform sinus fistula: management with chemocauterization of the internal opening. Ann Otol Rhinol Laryngol. 2000;109(5):452–456. [PubMed: 10823473]
- 125.
- Smith SL, Pereira KD. Suppurative thyroiditis in children: a management algorithm. Pediatr Emerg Care. 2008;24(11):764–767. [PubMed: 18955909]
- 126.
- Pereira KD, Smith SL. Endoscopic chemical cautery of piriform sinus tracts: a safe new technique. Int J Pediatr Otorhinolaryngol. 2008;72(2):185–188. [PubMed: 18031833]
- 127.
- Jordan JA, Graves JE, Manning SC, McClay JE, Biavati MJ. Endoscopic cauterization for treatment of fourth branchial cleft sinuses. Arch Otolaryngol Head Neck Surg. 1998;124(9):1021–1024. [PubMed: 9738814]
- 128.
- Rauhofer U, Prager G, Hormann M, Auer H, Kaserer K, Niederle B. Cystic echinococcosis of the thyroid gland in children and adults. Thyroid. 2003;13(5):497–502. [PubMed: 12855018]
- 129.
- Mordes DA, Brachtel EF. Cytopathology of subacute thyroiditis. Diagn Cytopathol. 2011 [PubMed: 22045514]
- 130.
- Adler ME, Jordan G, Walter RM Jr. Acute suppurative thyroiditis: diagnostic, metabolic and therapeutic observations. West J Med. 1978;128(2):165–168. [PMC free article: PMC1238033] [PubMed: 629054]
- 131.
- Schweitzer VG, Olson NR. Thyroid abscess. Otolaryngol Head Neck Surg. 1981;89(2):226–229. [PubMed: 6787517]
- 132.
- Nicole S, Lanzafame M, Cazzadori A, Vincenzi M, Mangani F, Colato C, El Dalati G, Brazzarola P, Concia E. Successful Antifungal Combination Therapy and Surgical Approach for Aspergillus fumigatus Suppurative Thyroiditis Associated with Thyrotoxicosis and Review of Published Reports. Mycopathologia. 2017;182(9-10):839–845. [PubMed: 28555254]
- 133.
- Pereira KD, Losh GG, Oliver D, Poole MD. Management of anomalies of the third and fourth branchial pouches. Int J Pediatr Otorhinolaryngol. 2004;68(1):43–50. [PubMed: 14687686]
- 134.
- Nicoucar K, Giger R, Jaecklin T, Pope HG Jr, Dulguerov P. Management of congenital third branchial arch anomalies: a systematic review. Otolaryngol Head Neck Surg. 2010;142(1):21–28 e22. [PubMed: 20096218]
- 135.
- Kruijff S, Sywak MS, Sidhu SB, Shun A, Novakovic D, Lee JC, Delbridge LW. Thyroidal abscesses in third and fourth branchial anomalies: not only a paediatric diagnosis. ANZ J Surg. 2014 [PubMed: 24674380]
- 136.
- Kamide D, Tomifuji M, Maeda M, Utsunomiya K, Yamashita T, Araki K, Shiotani A. Minimally invasive surgery for pyriform sinus fistula by transoral videolaryngoscopic surgery. Am J Otolaryngol. 2015;36(4):601–605. [PubMed: 25748690]
- 137.
- Xiao X, Zheng S, Zheng J, Zhu L, Dong K, Shen C, Li K. Endoscopic-assisted surgery for pyriform sinus fistula in children: experience of 165 cases from a single institution. J Pediatr Surg. 2014;49(4):618–621. [PubMed: 24726124]
- 138.
- Yang H, Li Ye X, Cheng J, Jia Z, Huang X, Wang X, Xu Y. Aspiration with or without lavage in the treatment of acute suppurative thyroiditis secondary to pyriform sinus fistula. Arch Endocrinol Metab. 2020;64(2):128–137. [PMC free article: PMC10118946] [PubMed: 32236305]
- 139.
- Volpe R. The management of subacute (DeQuervain's) thyroiditis. Thyroid. 1993;3(3):253–255. [PubMed: 8257868]
- 140.
- Ogawa E, Katsushima Y, Fujiwara I, Iinuma K. Subacute thyroiditis in children: patient report and review of the literature. J Pediatr Endocrinol Metab. 2003;16(6):897–900. [PubMed: 12948304]
- 141.
- Tamai H, Nozaki T, Mukuta T, Morita T, Matsubayashi S, Kuma K, Kumagai LF, Nagataki S. The incidence of thyroid stimulating blocking antibodies during the hypothyroid phase in patients with subacute thyroiditis. J Clin Endocrinol Metab. 1991;73(2):245–250. [PubMed: 1856259]
- 142.
- Galluzzo A, Giordano C, Andronico F, Filardo C, Andronico G, Bompiani G. Leukocyte migration test in subacute thyroiditis: hypothetical role of cell-mediated immunity. J Clin Endocrinol Metab. 1980;50(6):1038–1041. [PubMed: 6989848]
- 143.
- Parmar RC, Bavdekar SB, Sahu DR, Warke S, Kamat JR. Thyroiditis as a presenting feature of mumps. Pediatr Infect Dis J. 2001;20(6):637–638. [PubMed: 11419514]
- 144.
- Dimos G, Pappas G, Akritidis N. Subacute thyroiditis in the course of novel H1N1 influenza infection. Endocrine. 2010;37(3):440–441. [PubMed: 20960165]
- 145.
- Volta C, Carano N, Street ME, Bernasconi S. Atypical subacute thyroiditis caused by Epstein-Barr virus infection in a three-year-old girl. Thyroid. 2005;15(10):1189–1191. [PubMed: 16279854]
- 146.
- Bouillet B, Petit JM, Piroth L, Duong M, Bourg JB. A case of subacute thyroiditis associated with primary HIV infection. Am J Med. 2009;122(4):e5–6. [PubMed: 19332218]
- 147.
- Satoh M. Virus-like particles in the follicular epithelium of the thyroid from a patient with subacute thyroiditis (deQuervain's). Acta Pathol Jpn. 1975;25:499–501. [PubMed: 1180050]
- 148.
- Engkakul P, Mahachoklertwattana P, Poomthavorn P. de Quervain thyroiditis in a young boy following hand-foot-mouth disease. Eur J Pediatr. 2011;170(4):527–529. [PubMed: 20886354]
- 149.
- Andre R, Opris A, Costantino F, Hayem G, Breban M. Cytomegalovirus subacute thyroiditis in a patient treated by infliximab for psoriatic arthritis. Joint Bone Spine. 2016;83(1):109–110. [PubMed: 26184533]
- 150.
- Martinez-Artola Y, Poncino D, Garcia ML, Munne MS, Gonzalez J, Garcia DS. Acute hepatitis E virus infection and association with a subacute thyroiditis. Annals of Hepatology. 2015;14(1):141–142. [PubMed: 25536654]
- 151.
- Mo ZM, Dong YX, Chen XL, Yao HY, Zhang B. Acute transverse myelitis and subacute thyroiditis associated with dengue viral infection: A case report and literature review. Experimental and Therapeutic Medicine. 2016;12(4):2331–2335. [PMC free article: PMC5038889] [PubMed: 27703498]
- 152.
- Mangaraj S. Subacute thyroiditis complicating dengue fever - Case report and brief review of literature. Trop Doct. 2021;51(2):254–256. [PubMed: 33302815]
- 153.
- Sheraz F, Tahir H, Saqi J, Daruwalla V. Dengue Fever Presenting Atypically with Viral Conjunctivitis and Subacute Thyroiditis. J Coll Physicians Surg Pak. 2016;26(6) Suppl:S33–34. [PubMed: 27376214]
- 154.
- Assir MZ, Jawa A, Ahmed HI. Expanded dengue syndrome: subacute thyroiditis and intracerebral hemorrhage. BMC Infect Dis. 2012;12:240. [PMC free article: PMC3482561] [PubMed: 23033818]
- 155.
- Brancatella A, Ricci D, Viola N, Sgro D, Santini F, Latrofa F. Subacute Thyroiditis After Sars-COV-2 Infection. J Clin Endocrinol Metab. 2020;105(7) [PMC free article: PMC7314004] [PubMed: 32436948]
- 156.
- Luotola K, Hyoty H, Salmi J, Miettinen A, Helin H, Pasternack A. Evaluation of infectious etiology in subacute thyroiditis--lack of association with coxsackievirus infection. APMIS. 1998;106(4):500–504. [PubMed: 9637274]
- 157.
- Mori K, Yoshida K, Funato T, Ishii T, Nomura T, Fukuzawa H, Sayama N, Hori H, Ito S, Sasaki T. Failure in detection of Epstein-Barr virus and cytomegalovirus in specimen obtained by fine needle aspiration biopsy of thyroid in patients with subacute thyroiditis. Tohoku J Exp Med. 1998;186(1):13–17. [PubMed: 9915102]
- 158.
- Espino Montoro A, Medina Perez M, Gonzalez Martin MC, Asencio Marchante R, Lopez Chozas JM. An Med Interna. 2000;17(10):546–548. [Subacute thyroiditis associated with positive antibodies to the Epstein-Barr virus] [PubMed: 11109652]
- 159.
- Al Maawali A, Al Yaarubi S, Al Futaisi A. An infant with cytomegalovirus-induced subacute thyroiditis. J Pediatr Endocrinol Metab. 2008;21(2):191–193. [PubMed: 18422033]
- 160.
- Desailloud R, Hober D. Viruses and thyroiditis: an update. Virol J. 2009;6:5. [PMC free article: PMC2654877] [PubMed: 19138419]
- 161.
- Buc M, Nyulassy S, Hnilica P, Stefanovic J. HLA-BW35 and subacute de Quervain's thyroiditis. Diabete Metab. 1976;2(3):163. [proceedings] [PubMed: 1010141]
- 162.
- Hamaguchi E, Nishimura Y, Kaneko S, Takamura T. Subacute thyroiditis developed in identical twins two years apart. Endocr J. 2005;52(5):559–562. [PubMed: 16284433]
- 163.
- Kabalak T, Ozgen AG. Familial occurrence of subacute thyroiditis. Endocr J. 2002;49(2):207–209. [PubMed: 12081240]
- 164.
- Zein EF, Karaa SE, Megarbane A. Familial occurrence of painful subacute thyroiditis associated with human leukocyte antigen-B35. Presse Med. 2007;36(5 Pt 1):808–809. [PubMed: 17383147]
- 165.
- Kramer AB, Roozendaal C, Dullaart RP. Familial occurrence of subacute thyroiditis associated with human leukocyte antigen-B35. Thyroid. 2004;14(7):544–547. [PubMed: 15307945]
- 166.
- Stasiak M, Tymoniuk B, Stasiak B, Lewinski A. The Risk of Recurrence of Subacute Thyroiditis Is HLA-Dependent. Int J Mol Sci. 2019;20(5) [PMC free article: PMC6429176] [PubMed: 30832406]
- 167.
- Kalmus Y, Kovatz S, Shilo L, Ganem G, Shenkman L. Sweet's syndrome and subacute thyroiditis. Postgrad Med J. 2000;76(894):229–230. [PMC free article: PMC1741542] [PubMed: 10727568]
- 168.
- Richard J, Lazarte S, Calame A, Lingvay I. Sweet's syndrome and subacute thyroiditis: an unrecognized association? Thyroid. 2010;20(12):1425–1426. [PubMed: 21114383]
- 169.
- Vassilopoulou-Sellin R, Sella A, Dexeus FH, Theriault RL, Pololoff DA. Acute thyroid dysfunction (thyroiditis) after therapy with interleukin-2. Horm Metab Res. 1992;24(9):434–438. [PubMed: 1427615]
- 170.
- Amenomori M, Mori T, Fukuda Y, Sugawa H, Nishida N, Furukawa M, Kita R, Sando T, Komeda T, Nakao K. Incidence and characteristics of thyroid dysfunction following interferon therapy in patients with chronic hepatitis C. Intern Med. 1998;37(3):246–252. [PubMed: 9617858]
- 171.
- Martins F, Sofiya L, Sykiotis GP, Lamine F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A, Guex-Crosier Y, Kuntzer T, Michielin O, Peters S, Coukos G, Spertini F, Thompson JA, Obeid M. Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol. 2019;16(9):563–580. [PubMed: 31092901]
- 172.
- Gonzalez-Rodriguez E, Rodriguez-Abreu D. Spanish Group for Cancer I-B. Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist. 2016;21(7):804–816. [PMC free article: PMC4943391] [PubMed: 27306911]
- 173.
- de Filette J, Jansen Y, Schreuer M, Everaert H, Velkeniers B, Neyns B, Bravenboer B. Incidence of Thyroid-Related Adverse Events in Melanoma Patients Treated With Pembrolizumab. J Clin Endocrinol Metab. 2016;101(11):4431–4439. [PMC free article: PMC5095250] [PubMed: 27571185]
- 174.
- Lee H, Hodi FS, Giobbie-Hurder A, Ott PA, Buchbinder EI, Haq R, Tolaney S, Barroso-Sousa R, Zhang K, Donahue H, Davis M, Gargano ME, Kelley KM, Carroll RS, Kaiser UB, Min L. Characterization of Thyroid Disorders in Patients Receiving Immune Checkpoint Inhibition Therapy. Cancer Immunol Res. 2017;5(12):1133–1140. [PMC free article: PMC5748517] [PubMed: 29079654]
- 175.
- Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, Dietz AB, Ryder M. Pembrolizumab-Induced Thyroiditis: Comprehensive Clinical Review and Insights Into Underlying Involved Mechanisms. J Clin Endocrinol Metab. 2017;102(8):2770–2780. [PMC free article: PMC5546861] [PubMed: 28609832]
- 176.
- Muir CA, Menzies AM, Clifton-Bligh R, Tsang VHM. Thyroid Toxicity Following Immune Checkpoint Inhibitor Treatment in Advanced Cancer. Thyroid. 2020;30(10):1458–1469. [PubMed: 32264785]
- 177.
- Neppl C, Kaderli RM, Trepp R, Schmitt AM, Berger MD, Wehrli M, Seiler CA, Langer R. Histology of Nivolumab-Induced Thyroiditis. Thyroid. 2018;28(12):1727–1728. [PubMed: 30319070]
- 178.
- Muir CA, Clifton-Bligh RJ, Long GV, Scolyer RA, Lo SN, Carlino MS, Tsang VHM, Menzies AM. Thyroid Immune-related Adverse Events Following Immune Checkpoint Inhibitor Treatment. J Clin Endocrinol Metab. 2021;106(9):e3704–e3713. [PubMed: 33878162]
- 179.
- Stelmachowska-Banas M, Czajka I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: an updated review. Endocr Connect. 2020;9(10):R207–R228. [PMC free article: PMC7576644] [PubMed: 33064663]
- 180.
- Deligiorgi MV, Panayiotidis MI, Trafalis DT. Endocrine adverse events related with immune checkpoint inhibitors: an update for clinicians. Immunotherapy. 2020;12(7):481–510. [PubMed: 32345074]
- 181.
- Hernan Martinez J, Corder E, Uzcategui M, Garcia M, Sostre S, Garcia A. Subacute thyroiditis and dyserythropoesis after influenza vaccination suggesting immune dysregulation. Bol Asoc Med P R. 2011;103(2):48–52. [PubMed: 22111471]
- 182.
- Hsiao JY, Hsin SC, Hsieh MC, Hsia PJ, Shin SJ. Subacute thyroiditis following influenza vaccine (Vaxigrip) in a young female. Kaohsiung J Med Sci. 2006;22(6):297–300. [PubMed: 16793568]
- 183.
- Momani MS, Zayed AA, Bakri FG. Subacute thyroiditis following influenza vaccine: a case report and literature review. Italian Journal of Medicine. 2015;9(4):384–386.
- 184.
- Shen L, Bui C, Mansberg R, Nguyen D, Alam-Fotias S. Thyroid dysfunction during interferon alpha therapy for chronic hepatitis C. Clin Nucl Med. 2005;30(8):546–547. [PubMed: 16024950]
- 185.
- Kryczka W, Brojer E, Kowalska A, Zarebska-Michaluk D. Thyroid gland dysfunctions during antiviral therapy of chronic hepatitis C. Med Sci Monit. 2001;7 Suppl 1:221–225. [PubMed: 12211724]
- 186.
- Parana R, Cruz M, Lyra L, Cruz T. Subacute thyroiditis during treatment with combination therapy (interferon plus ribavirin) for hepatitis C virus. J Viral Hepat. 2000;7(5):393–395. [PubMed: 10971829]
- 187.
- Omur O, Daglyoz G, Akarca U, Ozcan Z. Subacute thyroiditis during interferon therapy for chronic hepatitis B infection. Clin Nucl Med. 2003;28(10):864–865. [PubMed: 14508288]
- 188.
- Moser C, Furrer J, Ruggieri F. [Neck pain and fever after peginterferon alpha-2a]. Praxis (Bern 1994). 2007;96(6):205-207. [PubMed: 17330412]
- 189.
- Ohta Y, Ohya Y, Fujii K, Tsuchihashi T, Sato K, Abe I, Iida M. Inflammatory diseases associated with Takayasu's arteritis. Angiology. 2003;54(3):339–344. [PubMed: 12785027]
- 190.
- Obuobie K, Al-Sabah A, Lazarus JH. Subacute thyroiditis in an immunosuppressed patient. J Endocrinol Invest. 2002;25(2):169–171. [PubMed: 11929089]
- 191.
- Ozdogu H, Boga C, Bolat F, Ertorer ME. Wegener's granulomatosis with a possible thyroidal involvement. J Natl Med Assoc. 2006;98(6):956–958. [PMC free article: PMC2569360] [PubMed: 16775921]
- 192.
- Daniels GH, Vladic A, Brinar V, Zavalishin I, Valente W, Oyuela P, Palmer J, Margolin DH, Hollenstein J. Alemtuzumab-related thyroid dysfunction in a phase 2 trial of patients with relapsing-remitting multiple sclerosis. J Clin Endocrinol Metab. 2014;99(1):80–89. [PubMed: 24170099]
- 193.
- Kawashima J, Naoe H, Sasaki Y, Araki E. A rare case showing subacute thyroiditis-like symptoms with amyloid goiter after anti-tumor necrosis factor therapy. Endocrinology Diabetes and Metabolism Case Reports. 2015 [PMC free article: PMC4424874] [PubMed: 25969738]
- 194.
- Senlis M, Ottaviani S, Gardette A, Palazzo E, Coustet B, Dieude P. Subacute thyroiditis in psoriatic arthritis treated by adalimumab. Joint Bone Spine. 2017;84(6):745–746. [PubMed: 27825573]
- 195.
- Vazquez Friol MDC, Bravo Blazquez I, Tejera Perez C. Subacute thyroiditis by dasatinib. Med Clin (Barc). 2020;155(6):270–271. [PubMed: 31351661]
- 196.
- Algun E, Alici S, Topal C, Ugras S, Erkoc R, Sakarya ME, Ozbey N. Coexistence of subacute thyroiditis and renal cell carcinoma: a paraneoplastic syndrome. CMAJ. 2003;168(8):985–986. [PMC free article: PMC152681] [PubMed: 12695381]
- 197.
- Calvi L, Daniels GH. Acute thyrotoxicosis secondary to destructive thyroiditis associated with cardiac catheterization contrast dye. Thyroid. 2011;21(4):443–449. [PubMed: 21385076]
- 198.
- Carneiro JR, Macedo RG, Da Silveira VG. Thyrotoxicosis after gastric bypass. Obes Surg. 2004;14(5):699–701. [PubMed: 15186642]
- 199.
- Sanavi S, Afshar R. Subacute thyroiditis following ginger (Zingiber officinale) consumption. Int J Ayurveda Res. 2010;1(1):47–48. [PMC free article: PMC2876930] [PubMed: 20532098]
- 200.
- Ippolito S, Dentali F, Tanda ML. SARS-CoV-2: a potential trigger for subacute thyroiditis? Insights from a case report. J Endocrinol Invest. 2020;43(8):1171–1172. [PMC free article: PMC7266411] [PubMed: 32488726]
- 201.
- Asfuroglu Kalkan E, Ates I. A case of subacute thyroiditis associated with Covid-19 infection. J Endocrinol Invest. 2020;43(8):1173–1174. [PMC free article: PMC7273820] [PubMed: 32504458]
- 202.
- Ruggeri RM, Campenni A, Siracusa M, Frazzetto G, Gullo D. Subacute thyroiditis in a patient infected with SARS-COV-2: an endocrine complication linked to the COVID-19 pandemic. Hormones (Athens). 2021;20(1):219–221. [PMC free article: PMC7365600] [PubMed: 32676935]
- 203.
- Rotondi M, Coperchini F, Ricci G, Denegri M, Croce L, Ngnitejeu ST, Villani L, Magri F, Latrofa F, Chiovato L. Detection of SARS-COV-2 receptor ACE-2 mRNA in thyroid cells: a clue for COVID-19-related subacute thyroiditis. J Endocrinol Invest. 2021;44(5):1085–1090. [PMC free article: PMC7538193] [PubMed: 33025553]
- 204.
- Ma D, Chen CB, Jhanji V, Xu C, Yuan XL, Liang JJ, Huang Y, Cen LP, Ng TK. Expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in human primary conjunctival and pterygium cell lines and in mouse cornea. Eye (Lond). 2020;34(7):1212–1219. [PMC free article: PMC7205026] [PubMed: 32382146]
- 205.
- Iremli BG, Sendur SN, Unluturk U. Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Postvaccination ASIA Syndrome. J Clin Endocrinol Metab. 2021;106(9):2600–2605. [PMC free article: PMC8194612] [PubMed: 34043800]
- 206.
- Synoracki S, Ting S, Schmid KW. Pathologe. 2016;37(3):215–223. [Inflammatory diseases of the thyroid gland] [PubMed: 27100868]
- 207.
- Harach HR, Williams ED. The pathology of granulomatous diseases of the thyroid gland. Sarcoidosis. 1990;7(1):19–27. [PubMed: 2189175]
- 208.
- Chang TC, Lai SM, Wen CY, Hsiao YL. Three-dimensional cytomorphology in fine needle aspiration biopsy of subacute thyroiditis. Acta Cytol. 2004;48(2):155–160. [PubMed: 15085746]
- 209.
- Toda S, Tokuda Y, Koike N, Yonemitsu N, Watanabe K, Koike K, Fujitani N, Hiromatsu Y, Sugihara H. Growth factor-expressing mast cells accumulate at the thyroid tissue-regenerative site of subacute thyroiditis. Thyroid. 2000;10(5):381–386. [PubMed: 10884184]
- 210.
- Woolner LB, Mc CW, Beahrs OH. Granulomatous thyroiditis (De Quervain's thyroiditis). J Clin Endocrinol Metab. 1957;17(10):1202–1221. [PubMed: 13475461]
- 211.
- Koga M, Hiromatsu Y, Jimi A, Toda S, Koike N, Nonaka K. Immunohistochemical analysis of Bcl-2, Bax, and Bak expression in thyroid glands from patients with subacute thyroiditis. J Clin Endocrinol Metab. 1999;84(6):2221–2225. [PubMed: 10372734]
- 212.
- Toda S, Nishimura T, Yamada S, Koike N, Yonemitsu N, Watanabe K, Matsumura S, Gartner R, Sugihara H. Immunohistochemical expression of growth factors in subacute thyroiditis and their effects on thyroid folliculogenesis and angiogenesis in collagen gel matrix culture. J Pathol. 1999;188(4):415–422. [PubMed: 10440753]
- 213.
- Luotola K, Mantula P, Salmi J, Haapala AM, Laippala P, Hurme M. Allele 2 of interleukin-1 receptor antagonist gene increases the risk of thyroid peroxidase antibodies in subacute thyroiditis. APMIS. 2001;109(6):454–460. [PubMed: 11506478]
- 214.
- Chen K, Wei Y, Sharp GC, Braley-Mullen H. Decreasing TNF-alpha results in less fibrosis and earlier resolution of granulomatous experimental autoimmune thyroiditis. J Leukoc Biol. 2007;81(1):306–314. [PMC free article: PMC1748426] [PubMed: 17046971]
- 215.
- Fang Y, Sharp GC, Yagita H, Braley-Mullen H. A critical role for TRAIL in resolution of granulomatous experimental autoimmune thyroiditis. J Pathol. 2008;216(4):505–513. [PMC free article: PMC2637621] [PubMed: 18810759]
- 216.
- Greene JN. Subacute thyroiditis. Am J Med. 1971;51(1):97–108. [PubMed: 4936649]
- 217.
- Golden SH, Robinson KA, Saldanha I, Anton B, Ladenson PW. Clinical review: Prevalence and incidence of endocrine and metabolic disorders in the United States: a comprehensive review. J Clin Endocrinol Metab. 2009;94(6):1853–1878. [PMC free article: PMC5393375] [PubMed: 19494161]
- 218.
- Carle A, Laurberg P, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Jorgensen T. Epidemiology of subtypes of hypothyroidism in Denmark. Eur J Endocrinol. 2006;154(1):21–28. [PubMed: 16381987]
- 219.
- Fatourechi V, Aniszewski JP, Fatourechi GZ, Atkinson EJ, Jacobsen SJ. Clinical features and outcome of subacute thyroiditis in an incidence cohort: Olmsted County, Minnesota, study. J Clin Endocrinol Metab. 2003;88(5):2100–2105. [PubMed: 12727961]
- 220.
- Qari FA, Maimani AA. Subacute thyroiditis in Western Saudi Arabia. Saudi Med J. 2005;26(4):630–633. [PubMed: 15900374]
- 221.
- Anastasilakis AD, Karanicola V, Kourtis A, Makras P, Kampas L, Gerou S, Giomisi A. A case report of subacute thyroiditis during pregnancy: difficulties in differential diagnosis and changes in cytokine levels. Gynecol Endocrinol. 2011;27(6):384–390. [PubMed: 20528573]
- 222.
- Hiraiwa T, Kubota S, Imagawa A, Sasaki I, Ito M, Miyauchi A, Hanafusa T. Two cases of subacute thyroiditis presenting in pregnancy. J Endocrinol Invest. 2006;29(10):924–927. [PubMed: 17185903]
- 223.
- Daniels GH. Atypical subacute thyroiditis: preliminary observations. Thyroid. 2001;11(7):691–695. [PubMed: 11484899]
- 224.
- Dedivitis RA, Coelho LS. Vocal fold paralysis in subacute thyroiditis. Braz J Otorhinolaryngol. 2007;73(1):138. [PMC free article: PMC9443580] [PubMed: 17505618]
- 225.
- Nakamura S, Saio Y, Ishimori M. Recurrent hemithyroiditis: a case report. Endocr J. 1998;45(4):595–600. [PubMed: 9881913]
- 226.
- Sari O, Erbas B, Erbas T. Subacute thyroiditis in a single lobe. Clin Nucl Med. 2001;26(5):400–401. [PubMed: 11317018]
- 227.
- Alper AT, Hasdemir H, Akyol A, Cakmak N. Incessant ventricular tachycardia due to subacute thyroiditis. Int J Cardiol. 2007;116(1):e22–24. [PubMed: 17134771]
- 228.
- Sherman SI, Simonson L, Ladenson PW. Clinical and socioeconomic predispositions to complicated thyrotoxicosis: a predictable and preventable syndrome? Am J Med. 1996;101(2):192–198. [PubMed: 8757360]
- 229.
- Swinburne JL, Kreisman SH. A rare case of subacute thyroiditis causing thyroid storm. Thyroid. 2007;17(1):73–76. [PubMed: 17274754]
- 230.
- Kim HJ, Jung TS, Hahm JR, Hwang SJ, Lee SM, Jung JH, Kim SK, Chung SI. Thyrotoxicosis-induced acute myocardial infarction due to painless thyroiditis. Thyroid. 2011;21(10):1149–1151. [PubMed: 21875344]
- 231.
- Mizokami T, Okamura K, Sato K, Hirata T, Yamasaki K, Fujishima M. Localized painful giant-cell thyroiditis without inflammatory signs in a euthyroid patient followed by serial sonography. J Clin Ultrasound. 1998;26(6):329–332. [PubMed: 9641397]
- 232.
- Muqtadir F, Ahmed A, Gufran K, Bin Hamza MO. CASE OF SUBACUTE THYROIDITIS PRESENTING AS THE CAUSE OF PYREXIA OF UNKNOWN ORIGIN. Journal of Evolution of Medical and Dental Sciences-Jemds. 2015;4(88):15373–15375.
- 233.
- Popovska-Jovicic B, Canovic P, Gajovic O, Rakovic I, Mijailovic Z. Fever of unknown origin: Most frequent causes in adults patients. Vojnosanitetski Pregled. 2016;73(1):21–25. [PubMed: 26964380]
- 234.
- Cunha BA, Chak A, Strollo S. Fever of unknown origin (FUO): de Quervain's subacute thyroiditis with highly elevated ferritin levels mimicking temporal arteritis (TA). Heart Lung. 2010;39(1):73–77. [PubMed: 20109988]
- 235.
- Benbassat CA, Olchovsky D, Tsvetov G, Shimon I. Subacute thyroiditis: clinical characteristics and treatment outcome in fifty-six consecutive patients diagnosed between 1999 and 2005. J Endocrinol Invest. 2007;30(8):631–635. [PubMed: 17923793]
- 236.
- Matsumoto Y, Amino N, Kubota S, Ikeda N, Morita S, Nishihara E, Ohye H, Kudo T, Ito M, Fukata S, Miyauchi A. Serial changes in liver function tests in patients with subacute thyroiditis. Thyroid. 2008;18(7):815–816. [PubMed: 18631018]
- 237.
- Fragu P, Rougier P, Schlumberger M, Tubiana M. Evolution of thyroid 127I stores measured by X-ray fluorescence in subacute thyroiditis. J Clin Endocrinol Metab. 1982;54(1):162–166. [PubMed: 7054213]
- 238.
- Gordin A, Lamberg BA. Serum thyrotrophin response to thyrotrophin releasing hormone and the concentration of free thyroxine in subacute thyroiditis. Acta Endocrinol (Copenh). 1973;74(1):111–121. [PubMed: 4128225]
- 239.
- Intenzo CM, Park CH, Kim SM, Capuzzi DM, Cohen SN, Green P. Clinical, laboratory, and scintigraphic manifestations of subacute and chronic thyroiditis. Clin Nucl Med. 1993;18(4):302–306. [PubMed: 8386991]
- 240.
- Rapoport B, Block MB, Hoffer PB, DeGroot LJ. Depletion of thyroid iodine during subacute thyroiditis. J Clin Endocrinol Metab. 1973;36(3):610–611. [PubMed: 4685396]
- 241.
- Savoie JC, Massin JP, Thomopoulos P, Leger F. Iodine-induced thyrotoxicosis in apparently normal thyroid glands. J Clin Endocrinol Metab. 1975;41(4):685–691. [PubMed: 1176580]
- 242.
- Woolf PD. Transient painless thyroiditis with hyperthyroidism: a variant of lymphocytic thyroiditis? Endocr Rev. 1980;1(4):411–420. [PubMed: 7018893]
- 243.
- Pearce EN, Bogazzi F, Martino E, Brogioni S, Pardini E, Pellegrini G, Parkes AB, Lazarus JH, Pinchera A, Braverman LE. The prevalence of elevated serum C-reactive protein levels in inflammatory and noninflammatory thyroid disease. Thyroid. 2003;13(7):643–648. [PubMed: 12964969]
- 244.
- Rao NL, Shetty S, Upadhyaya K. R MP, Lobo EC, Kedilaya HP, Prasad G. Salivary C-Reactive Protein in Hashimoto's Thyroiditis and Subacute Thyroiditis. Int J Inflam. 2010;2010:514659. [PMC free article: PMC2989710] [PubMed: 21151525]
- 245.
- Fujii S, Miwa U, Seta T, Ohoka T, Mizukami Y. Subacute thyroiditis with highly positive thyrotropin receptor antibodies and high thyroidal radioactive iodine uptake. Intern Med. 2003;42(8):704–709. [PubMed: 12924496]
- 246.
- Iitaka M, Momotani N, Hisaoka T, Noh JY, Ishikawa N, Ishii J, Katayama S, Ito K. TSH receptor antibody-associated thyroid dysfunction following subacute thyroiditis. Clin Endocrinol (Oxf). 1998;48(4):445–453. [PubMed: 9640411]
- 247.
- Kamijo K. TSH-receptor antibody measurement in patients with various thyrotoxicosis and Hashimoto's thyroiditis: a comparison of two two-step assays, coated plate ELISA using porcine TSH-receptor and coated tube radioassay using human recombinant TSH-receptor. Endocr J. 2003;50(1):113–116. [PubMed: 12733717]
- 248.
- Takasu N, Kamijo K, Sato Y, Yoshimura H, Nagata A, Ochi Y. Sensitive thyroid-stimulating antibody assay with high concentrations of polyethylene glycol for the diagnosis of Graves' disease. Clin Exp Pharmacol Physiol. 2004;31(5-6):314–319. [PubMed: 15191404]
- 249.
- Fang F, Yan S, Zhao L, Jin YB, Wang YF. Concurrent Onset of Subacute Thyroiditis and Graves' Disease. American Journal of the Medical Sciences. 2016;352(2):224–226. [PubMed: 27524225]
- 250.
- Hiromatsu Y, Ishibashi M, Miyake I, Nonaka K. Technetium-99m tetrofosmin imaging in patients with subacute thyroiditis. Eur J Nucl Med. 1998;25(10):1448–1452. [PubMed: 9818287]
- 251.
- Hiromatsu Y, Ishibashi M, Nishida H, Kawamura S, Kaku H, Baba K, Kaida H, Miyake I. Technetium-99 m sestamibi imaging in patients with subacute thyroiditis. Endocr J. 2003;50(3):239–244. [PubMed: 12940451]
- 252.
- Alonso O, Mut F, Lago G, Aznarez A, Nunez M, Canepa J, Touya E. 99Tc(m)-MIBI scanning of the thyroid gland in patients with markedly decreased pertechnetate uptake. Nucl Med Commun. 1998;19(3):257–261. [PubMed: 9625501]
- 253.
- Janssen OE. Dtsch Med Wochenschr. 2011;136(11):519–522. [Atypical presentation of subacute thyroiditis] [PubMed: 21387209]
- 254.
- Song YS, Jang SJ, Chung JK, Lee DS. F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) and Tc-99m pertechnate scan findings of a patient with unilateral subacute thyroiditis. Clin Nucl Med. 2009;34(7):456–458. [PubMed: 19542956]
- 255.
- Kunz A, Blank W, Braun B. De Quervain's subacute thyroiditis -- colour Doppler sonography findings. Ultraschall Med. 2005;26(2):102–106. [PubMed: 15852172]
- 256.
- Park SY, Kim EK, Kim MJ, Kim BM, Oh KK, Hong SW, Park CS. Ultrasonographic characteristics of subacute granulomatous thyroiditis. Korean J Radiol. 2006;7(4):229–234. [PMC free article: PMC2667608] [PubMed: 17143025]
- 257.
- Omori N, Omori K, Takano K. Association of the ultrasonographic findings of subacute thyroiditis with thyroid pain and laboratory findings. Endocr J. 2008;55(3):583–588. [PubMed: 18490832]
- 258.
- Cappelli C, Pirola I, Gandossi E, Formenti AM, Agosti B, Castellano M. Ultrasound findings of subacute thyroiditis: a single institution retrospective review. Acta Radiol. 2014;55(4):429–433. [PubMed: 23969266]
- 259.
- Ohta T, Nishioka M, Nakata N, Fukuda K, Shirakawa T. Significance of perithyroidal lymph nodes in benign thyroid diseases. Journal of Medical Ultrasonics. 2018;45(1):81–87. [PubMed: 28656512]
- 260.
- Nishihara E, Hirokawa M, Ohye H, Ito M, Kubota S, Fukata S, Amino N, Miyauchi A. Papillary carcinoma obscured by complication with subacute thyroiditis: sequential ultrasonographic and histopathological findings in five cases. Thyroid. 2008;18(11):1221–1225. [PubMed: 18925839]
- 261.
- Tezuka M, Murata Y, Ishida R, Ohashi I, Hirata Y, Shibuya H. MR imaging of the thyroid: correlation between apparent diffusion coefficient and thyroid gland scintigraphy. J Magn Reson Imaging. 2003;17(2):163–169. [PubMed: 12541222]
- 262.
- Yeo SH, Lee SK, Hwang I, Ahn EJ. Subacute thyroiditis presenting as a focal lesion on [18F] fluorodeoxyglucose whole-body positron-emission tomography/CT. AJNR Am J Neuroradiol. 2011;32(4):E58–60. [PMC free article: PMC7965895] [PubMed: 20299440]
- 263.
- Kim MH, Kim DW, Park SA, Kim CG. Transiently Altered Distribution of F-18 FDG in a Patient with Subacute Thyroiditis. Nuclear Medicine and Molecular Imaging. 2018;52(1):82–84. [PMC free article: PMC5777952] [PubMed: 29391918]
- 264.
- Yoshida K, Yokoh H, Toriihara A, Fujii H, Harata N, Isogai J, Tateishi U. F-18-FDG PET/CT imaging of atypical subacute thyroiditis in thyrotoxicosis. A case report. Medicine. 2017;96(30) [PMC free article: PMC5627825] [PubMed: 28746199]
- 265.
- Freesmeyer M, Opfermann T. Diagnosis of de quervain's subacute thyroiditis via sensor-navigated (124)Iodine PET/ultrasound (I-124-PET/US) fusion. Endocrine. 2015;49(1):293–295. [PubMed: 25060209]
- 266.
- Shabb NS, Salti I. Subacute thyroiditis: fine-needle aspiration cytology of 14 cases presenting with thyroid nodules. Diagn Cytopathol. 2006;34(1):18–23. [PubMed: 16355394]
- 267.
- Ito M, Takamatsu J, Yoshida S, Murakami Y, Sakane S, Kuma K, Ohsawa N. Incomplete thyrotroph suppression determined by third generation thyrotropin assay in subacute thyroiditis compared to silent thyroiditis or hyperthyroid Graves' disease. J Clin Endocrinol Metab. 1997;82(2):616–619. [PubMed: 9024264]
- 268.
- Vierhapper H, Bieglmayer C, Nowotny P, Waldhausl W. Normal serum concentrations of sex hormone binding-globulin in patients with hyperthyroidism due to subacute thyroiditis. Thyroid. 1998;8(12):1107–1111. [PubMed: 9920365]
- 269.
- Nakano Y, Kurihara H, Sasaki J. Graves' disease following subacute thyroiditis. Tohoku J Exp Med. 2011;225(4):301–309. [PubMed: 22112923]
- 270.
- Hallengren B, Planck T, Asman P, Lantz M. Presence of Thyroid-Stimulating Hormone Receptor Antibodies in a Patient with Subacute Thyroiditis followed by Hypothyroidism and Later Graves' Disease with Ophthalmopathy: A Case Report. European Thyroid Journal. 2015;4(3):197–200. [PMC free article: PMC4637508] [PubMed: 26558237]
- 271.
- Tesfaye H, Cimermanova R, Cholt M, Sykorova P, Pechova M, Prusa R. Subacute thyroiditis confused with dental problem. Cas Lek Cesk. 2009;148(9):438–441. [PubMed: 19899734]
- 272.
- Meller J, Sahlmann CO, Scheel AK. 18F-FDG PET and PET/CT in fever of unknown origin. J Nucl Med. 2007;48(1):35–45. [PubMed: 17204697]
- 273.
- Yasuda S, Shohtsu A, Ide M, Takagi S, Takahashi W, Suzuki Y, Horiuchi M. Chronic thyroiditis: diffuse uptake of FDG at PET. Radiology. 1998;207(3):775–778. [PubMed: 9609903]
- 274.
- Liel Y. The survivor: association of an autonomously functioning thyroid nodule and subacute thyroiditis. Thyroid. 2007;17(2):183–184. [PubMed: 17316124]
- 275.
- King DL, Stack BC Jr, Spring PM, Walker R, Bodenner DL. Incidence of thyroid carcinoma in fluorodeoxyglucose positron emission tomography-positive thyroid incidentalomas. Otolaryngol Head Neck Surg. 2007;137(3):400–404. [PubMed: 17765765]
- 276.
- Van den Bruel A, Maes A, De Potter T, Mortelmans L, Drijkoningen M, Van Damme B, Delaere P, Bouillon R. Clinical relevance of thyroid fluorodeoxyglucose-whole body positron emission tomography incidentaloma. J Clin Endocrinol Metab. 2002;87(4):1517–1520. [PubMed: 11932274]
- 277.
- Zacharia TT, Perumpallichira JJ, Sindhwani V, Chavhan G. Gray-scale and color Doppler sonographic findings in a case of subacute granulomatous thyroiditis mimicking thyroid carcinoma. J Clin Ultrasound. 2002;30(7):442–444. [PubMed: 12210464]
- 278.
- Xie P, Xiao Y, Liu F. Real-time ultrasound elastography in the diagnosis and differential diagnosis of subacute thyroiditis. J Clin Ultrasound. 2011;39(8):435–440. [PubMed: 21674511]
- 279.
- Sato J, Uchida T, Komiya K, Goto H, Takeno K, Suzuki R, Honda A, Himuro M, Watada H. Comparison of the therapeutic effects of prednisolone and nonsteroidal anti-inflammatory drugs in patients with subacute thyroiditis. Endocrine. 2017;55(1):218–223. [PubMed: 27688010]
- 280.
- Kubota S, Nishihara E, Kudo T, Ito M, Amino N, Miyauchi A. Initial treatment with 15 mg of prednisolone daily is sufficient for most patients with subacute thyroiditis in Japan. Thyroid. 2013;23(3):269–272. [PubMed: 23227861]
- 281.
- Ma SG, Bai F, Cheng L. A novel treatment for subacute thyroiditis: administration of a mixture of lidocaine and dexamethasone using an insulin pen. Mayo Clin Proc. 2014;89(6):861–862. [PubMed: 24943704]
- 282.
- Mizukoshi T, Noguchi S, Murakami T, Futata T, Yamashita H. Evaluation of recurrence in 36 subacute thyroiditis patients managed with prednisolone. Intern Med. 2001;40(4):292–295. [PubMed: 11334386]
- 283.
- Sencar ME, Calapkulu M, Sakiz D, Hepsen S, Kus A, Akhanli P, Unsal IO, Kizilgul M, Ucan B, Ozbek M, Cakal E. An Evaluation of the Results of the Steroid and Non-steroidal Anti-inflammatory Drug Treatments in Subacute Thyroiditis in relation to Persistent Hypothyroidism and Recurrence. Sci Rep. 2019;9(1):16899. [PMC free article: PMC6858328] [PubMed: 31729433]
- 284.
- Duininck TM, van Heerden JA, Fatourechi V, Curlee KJ, Farley DR, Thompson GB, Grant CS, Lloyd RV. de Quervain's thyroiditis: surgical experience. Endocr Pract. 2002;8(4):255–258. [PubMed: 12173910]
- 285.
- Ranganath R, Shaha MA, Xu B, Migliacci J, Ghossein R, Shaha AR. de Quervain's thyroiditis: A review of experience with surgery. American Journal of Otolaryngology. 2016;37(6):534–537. [PMC free article: PMC5574176] [PubMed: 27686396]
- 286.
- Park HK, Kim DW, Lee YJ, Ha TK, Kim DH, Bae SK, Jung SJ. Suspicious Sonographic and Cytological Findings in Patients With Subacute Thyroiditis Two Case Reports. Diagnostic Cytopathology. 2015;43(5):399–402. [PubMed: 25350937]
- 287.
- Mazza E, Quaglino F, Suriani A, Palestini N, Gottero C, Leli R, Taraglio S. Thyroidectomy for Painful Thyroiditis Resistant to Steroid Treatment: Three New Cases with Review of the Literature. Case Reports in Endocrinology. 2015 [PMC free article: PMC4468277] [PubMed: 26137327]
- 288.
- Zhao N, Wang S, Cui XJ, Huang MS, Wang SW, Li YG, Zhao L, Wan WN, Li YS, Shan ZY, Teng WP. Two-Years Prospective Follow-Up Study of Subacute Thyroiditis. Front Endocrinol (Lausanne). 2020;11:47. [PMC free article: PMC7058985] [PubMed: 32184756]
- 289.
- Iitaka M, Momotani N, Ishii J, Ito K. Incidence of subacute thyroiditis recurrences after a prolonged latency: 24-year survey. J Clin Endocrinol Metab. 1996;81(2):466–469. [PubMed: 8636251]
- 290.
- Saklamaz A. IS. THERE A DRUG EFFECT ON THE DEVELOPMENT OF PERMANENT HYPOTHYROIDISM IN SUBACUTE THYROIDITIS? Acta Endocrinologica-Bucharest. 2017;13(1):119–123. [PMC free article: PMC6525754] [PubMed: 31149159]
- 291.
- Nishihara E, Amino N, Ohye H, Ota H, Ito M, Kubota S, Fukata S, Miyauchi A. Extent of hypoechogenic area in the thyroid is related with thyroid dysfunction after subacute thyroiditis. J Endocrinol Invest. 2009;32(1):33–36. [PubMed: 19337012]
- 292.
- Bogazzi F, Dell'Unto E, Tanda ML, Tomisti L, Cosci C, Aghini-Lombardi F, Sardella C, Pinchera A, Bartalena L, Martino E. Long-term outcome of thyroid function after amiodarone-induced thyrotoxicosis, as compared to subacute thyroiditis. J Endocrinol Invest. 2006;29(8):694–699. [PubMed: 17033257]
- 293.
- Izumi M, Larsen PR. Correlation of sequential changes in serum thyroglobulin, triiodothyronine, and thyroxine in patients with Graves' disease and subacute thyroiditis. Metabolism. 1978;27(4):449–460. [PubMed: 580305]
- 294.
- Riedel BM. Die chronische zur Bildung eisenharter Tumoren fuehrende Entzuendung der Shilddruese. Verh Ges Chir. 1896;25:101–105.
- 295.
- de Lange WE, Freling NJ, Molenaar WM, Doorenbos H. Invasive fibrous thyroiditis (Riedel's struma): a manifestation of multifocal fibrosclerosis? A case report with review of the literature. Q J Med. 1989;72(268):709–717. [PubMed: 2690181]
- 296.
- Zimmermann-Belsing T, Feldt-Rasmussen U. Riedel's thyroiditis: an autoimmune or primary fibrotic disease? J Intern Med. 1994;235(3):271–274. [PubMed: 8120524]
- 297.
- Goodman HI. Riedel's Thyroiditis: a review and report of two cases. American Journal of Surgery. 1941;54(2):472–478.
- 298.
- Riedel BM. Vorstellung eines Kranken mit chronischer Strumitis. Verh Ges Chir. 1896;26:127–129.
- 299.
- Riedel BM. Ueber Verlauf und Ausgang der chronischer Strumitis. Munch Med Wochenschr. 1910;57:1946–1947.
- 300.
- Hay ID. Thyroiditis: a clinical update. Mayo Clin Proc. 1985;60(12):836–843. [PubMed: 3906289]
- 301.
- Guimaraes VC. Subacute and Reidel's Thyroiditis. In: Jameson JL, De Groot LJ, eds. Endocrinology: Adult and Pediatric. Vol 2. 6th ed. Philadelphia: Elsevier; 2010:1600-1603.
- 302.
- Zala A, Berhane T, Juhlin CC, Calissendorff J, Falhammar H. Riedel Thyroiditis. J Clin Endocrinol Metab. 2020;105(9) [PubMed: 32687163]
- 303.
- Fatourechi MM, Hay ID, McIver B, Sebo TJ, Fatourechi V. Invasive fibrous thyroiditis (riedel thyroiditis): the mayo clinic experience, 1976-2008. Thyroid. 2011;21(7):765–772. [PubMed: 21568724]
- 304.
- Hennessey JV. Clinical review: Riedel's thyroiditis: a clinical review. J Clin Endocrinol Metab. 2011;96(10):3031–3041. [PubMed: 21832114]
- 305.
- Balach ZW, LiVolsi VA. Pathology. In: Braverman LE, Utiger RE, eds. Werner & Ingbar's The Thyroid; A Fundamental and Clinical Text. Ninth ed. Philadelphia: Lippincott Williams & Wilkins; 2005:427.
- 306.
- Lee SL, Ananthakrishnan S. Infiltartive thyroid disease. In: Rose BD, Mulder JE, eds. UpToDate. Wellesley, MA: BDR, Inc.; 2011:1-21.
- 307.
- Heufelder AE, Goellner JR, Bahn RS, Gleich GJ, Hay ID. Tissue eosinophilia and eosinophil degranulation in Riedel's invasive fibrous thyroiditis. J Clin Endocrinol Metab. 1996;81(3):977–984. [PubMed: 8772560]
- 308.
- Volpe R. Subacute and Sclerosing Thyroiditis. In: De Groot LJ, ed. Endocrinology. 3rd ed. Philadelphia: WB Saunders; 1995:742-751.
- 309.
- Schwaegerle SM, Bauer TW, Esselstyn CB Jr. Riedel's thyroiditis. Am J Clin Pathol. 1988;90(6):715–722. [PubMed: 3057862]
- 310.
- Beahrs OH, McConahey WM, Woolner LB. Invasive fibrous thyroiditis (Riedel's struma). J Clin Endocrinol Metab. 1957;17(2):201–220. [PubMed: 13398460]
- 311.
- Torres-Montaner A, Beltran M, Romero de la Osa A, Oliva H. Sarcoma of the thyroid region mimicking Riedel's thyroiditis. J Clin Pathol. 2001;54(7):570–572. [PMC free article: PMC1731483] [PubMed: 11429435]
- 312.
- Wan SK, Chan JK, Tang SK. Paucicellular variant of anaplastic thyroid carcinoma. A mimic of Reidel's thyroiditis. Am J Clin Pathol. 1996;105(4):388–393. [PubMed: 8604680]
- 313.
- Katsikas D, Shorthouse AJ, Taylor S. Riedel's thyroiditis. Br J Surg. 1976;63(12):929–931. [PubMed: 1009341]
- 314.
- LiVolsi VA, LoGerfo P, eds. Thyroiditis. Boca Raton: CRC Press; 1981.
- 315.
- Cho MH, Kim CS, Park JS, Kang ES, Ahn CW, Cha BS, Lim SK, Kim KR, Lee HC. Riedel's thyroiditis in a patient with recurrent subacute thyroiditis: a case report and review of the literature. Endocr J. 2007;54(4):559–562. [PubMed: 17603227]
- 316.
- Pirola I, Morassi ML, Braga M, De Martino E, Gandossi E, Cappelli C. A Case of Concurrent Riedel's, Hashimoto's and Acute Suppurative Thyroiditis. Case Report Med. 2009;2009:535974. [PMC free article: PMC2728608] [PubMed: 19724638]
- 317.
- McIver B, Fatourechi MM, Hay ID, Fatourechi V. Graves' disease after unilateral Riedel's thyroiditis. J Clin Endocrinol Metab. 2010;95(6):2525–2526. [PubMed: 20525902]
- 318.
- Kojima M, Nakamura S, Yamane Y, Shimizu K, Sugiharal S, Masawa N. Riedel's thyroiditis containing cytologically atypically appearing B-cells: a case report. Pathol Res Pract. 2003;199(7):497–501. [PubMed: 14521268]
- 319.
- Chen K, Wei Y, Sharp GC, Braley-Mullen H. Characterization of thyroid fibrosis in a murine model of granulomatous experimental autoimmune thyroiditis. J Leukoc Biol. 2000;68(6):828–835. [PubMed: 11129650]
- 320.
- Li Y, Bai Y, Liu Z, Ozaki T, Taniguchi E, Mori I, Nagayama K, Nakamura H, Kakudo K. Immunohistochemistry of IgG4 can help subclassify Hashimoto's autoimmune thyroiditis. Pathol Int. 2009;59(9):636–641. [PubMed: 19712131]
- 321.
- Neild GH, Rodriguez-Justo M, Wall C, Connolly JO. Hyper-IgG4 disease: report and characterisation of a new disease. BMC Med. 2006;4:23. [PMC free article: PMC1618394] [PubMed: 17026742]
- 322.
- Dahlgren M, Khosroshahi A, Nielsen GP, Deshpande V, Stone JH. Riedel's thyroiditis and multifocal fibrosclerosis are part of the IgG4-related systemic disease spectrum. Arthritis Care Res (Hoboken). 2010;62(9):1312–1318. [PubMed: 20506114]
- 323.
- Yamamoto M, Takahashi H, Shinomura Y. Rinsho Byori. 2010;58(5):454–465. [IgG4-related systemic disease/systemic IgG4-related disease] [PubMed: 20560455]
- 324.
- Sarles H, Sarles JC, Muratore R, Guien C. Chronic inflammatory sclerosis of the pancreas--an autonomous pancreatic disease? Am J Dig Dis. 1961;6:688–698. [PubMed: 13746542]
- 325.
- Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N, Kiyosawa K. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344(10):732–738. [PubMed: 11236777]
- 326.
- Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S, Hamano H, Kamisawa T, Shimosegawa T, Shimatsu A, Ito T, Notohara K, Sumida T, Tanaka Y, Mimori T, Chiba T, Mishima M, Hibi T, Tsubouchi H, Inui K, Ohara H. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22(1):21–30. [PubMed: 22218969]
- 327.
- Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Sumida T, Mimori T, Tanaka Y, Tsubota K, Yoshino T, Kawa S, Suzuki R, Takegami T, Tomosugi N, Kurose N, Ishigaki Y, Azumi A, Kojima M, Nakamura S, Inoue D. A novel clinical entity, IgG4-related disease (IgG4RD): general concept and details. Mod Rheumatol. 2012;22(1):1–14. [PMC free article: PMC3278618] [PubMed: 21881964]
- 328.
- Palazzo E, Palazzo C, Palazzo M. IgG4-related disease. Joint Bone Spine. 2014;81(1):27–31. [PubMed: 23849464]
- 329.
- Dutta D, Ahuja A, Selvan C. Immunoglobulin G4 related thyroid disorders: Diagnostic challenges and clinical outcomes. Endokrynologia Polska. 2016;67(5):520–524. [PubMed: 27828690]
- 330.
- Stan MN, Sonawane V, Sebo TJ, Thapa P, Bahn RS. Riedel's thyroiditis association with IgG4-related disease. Clinical endocrinology. 2017;86(3):425–430. [PubMed: 27647429]
- 331.
- Deshpande V, Zen Y, Chan JK, Yi EE, Sato Y, Yoshino T, Kloppel G, Heathcote JG, Khosroshahi A, Ferry JA, Aalberse RC, Bloch DB, Brugge WR, Bateman AC, Carruthers MN, Chari ST, Cheuk W, Cornell LD, Fernandez-Del Castillo C, Forcione DG, Hamilos DL, Kamisawa T, Kasashima S, Kawa S, Kawano M, Lauwers GY, Masaki Y, Nakanuma Y, Notohara K, Okazaki K, Ryu JK, Saeki T, Sahani DV, Smyrk TC, Stone JR, Takahira M, Webster GJ, Yamamoto M, Zamboni G, Umehara H, Stone JH. Consensus statement on the pathology of IgG4-related disease. Mod Pathol. 2012;25(9):1181–1192. [PubMed: 22596100]
- 332.
- Takeshima K, Inaba H, Ariyasu H, Furukawa Y, Doi A, Nishi M, Hirokawa M, Yoshida A, Imai R, Akamizu T. Clinicopathological features of Riedel's thyroiditis associated with IgG4-related disease in Japan. Endocr J. 2015 [PubMed: 26052139]
- 333.
- Pusztaszeri M, Triponez F, Pache JC, Bongiovanni M. Riedel's thyroiditis with increased IgG4 plasma cells: evidence for an underlying IgG4-related sclerosing disease? Thyroid. 2012;22(9):964–968. [PubMed: 22827716]
- 334.
- Soh SB, Pham A, O'Hehir RE, Cherk M, Topliss DJ. Novel use of rituximab in a case of Riedel's thyroiditis refractory to glucocorticoids and tamoxifen. J Clin Endocrinol Metab. 2013;98(9):3543–3549. [PubMed: 23824414]
- 335.
- Sakai Y, Imamura Y. Case report: IgG4-related mass-forming thyroiditis accompanied by regional lymphadenopathy. Diagnostic pathology. 2018;13(1):3. [PMC free article: PMC6389070] [PubMed: 29378608]
- 336.
- Oriot P, Amraoui A, Rousseau E, Malvaux P, Dechambre S, Delcourt A. Fibrosis of the thyroid gland caused by an IgG4-related sclerosing disease: three years of follow-up. Acta Clin Belg. 2014;69(6):446–450. [PubMed: 25224542]
- 337.
- Ghys C, Depierreux M, Ozalp E, Velkeniers B. Cervical lymph nodes, thyroiditis and ophthalmopathy: the pleomorphic face of an immunoglobulin g4-related disease. Eur Thyroid J. 2014;3(4):252–257. [PMC free article: PMC4311305] [PubMed: 25759802]
- 338.
- Falhammar H, Juhlin CC, Barner C, Catrina SB, Karefylakis C, Calissendorff J. Riedel's thyroiditis: clinical presentation, treatment and outcomes. Endocrine. 2018;60(1):185–192. [PMC free article: PMC5845586] [PubMed: 29380231]
- 339.
- Lu L, Gu F, Dai WX, Li WY, Chen J, Xiao Y, Zeng ZP. Clinical and pathological features of Riedel's thyroiditis. Chin Med Sci J. 2010;25(3):129–134. [PubMed: 21180272]
- 340.
- Annaert M, Thijs M, Sciot R, Decallonne B. Riedel's thyroiditis occurring in a multinodular goiter, mimicking thyroid cancer. J Clin Endocrinol Metab. 2007;92(6):2005–2006. [PubMed: 17554052]
- 341.
- Vigouroux C, Escourolle H, Mosnier-Pudar H, Thomopoulos P, Louvel A, Chapuis Y, Varet B, Luton JP. Presse Med. 1996;25(1):28–30. [Riedel's thyroiditis and lymphoma. Diagnostic difficulties] [PubMed: 8728889]
- 342.
- Sheu SY, Schmid KW. Pathologe. 2003;24(5):339–347. [Inflammatory diseases of the thyroid gland. Epidemiology, symptoms and morphology] [PubMed: 12961022]
- 343.
- Ozgur T, Gokce H, Ustun I, Yaldiz M, Akin MM, Gokce C. A case of asymptomatic riedel thyroiditis with follicular adenoma in a patient with a multinodular goiter: an unusual association. Eur Thyroid J. 2012;1(3):204–207. [PMC free article: PMC3821478] [PubMed: 24783021]
- 344.
- Shahi N, Abdelhamid MF, Jindall M, Awad RW. Riedel's thyroiditis masquerading as anaplastic thyroid carcinoma: a case report. J Med Case Reports. 2010;4:15. [PMC free article: PMC2821392] [PubMed: 20157436]
- 345.
- Kumar SS, Fraser S, Scarsbrook A, Maclennan K, Lansdown M, Murray RD. Atypical Presentation of Riedel's Thyroiditis: Multifocal Nodular Fibrosis and Resolution with Levothyroxine. Eur Thyroid J. 2012;1(4):259–263. [PMC free article: PMC3821490] [PubMed: 24783028]
- 346.
- Best TB, Munro RE, Burwell S, Volpe R. Riedel's thyroiditis associated with Hashimoto's thyroiditis, hypoparathyroidism, and retroperitoneal fibrosis. J Endocrinol Invest. 1991;14(9):767–772. [PubMed: 1761813]
- 347.
- Chopra D, Wool MS, Crosson A, Sawin CT. Riedel's struma associated with subacute thyroiditis, hypothyroidism, and hypoparathyroidism. J Clin Endocrinol Metab. 1978;46(6):869–871. [PubMed: 263470]
- 348.
- Marin F, Araujo R, Paramo C, Lucas T, Salto L. Riedel's thyroiditis associated with hypothyroidism and hypoparathyroidism. Postgrad Med J. 1989;65(764):381–383. [PMC free article: PMC2429333] [PubMed: 2608578]
- 349.
- Yasmeen T, Khan S, Patel SG, Reeves WA, Gonsch FA, de Bustros A, Kaplan EL. Clinical case seminar: Riedel's thyroiditis: report of a case complicated by spontaneous hypoparathyroidism, recurrent laryngeal nerve injury, and Horner's syndrome. J Clin Endocrinol Metab. 2002;87(8):3543–3547. [PubMed: 12161472]
- 350.
- Nazal EM, Belmatoug N, de Roquancourt A, Lefort A, Fantin B. Hypoparathyroidism preceding Riedel's thyroiditis. Eur J Intern Med. 2003;14(3):202–204. [PubMed: 12798222]
- 351.
- Stan MN, Haglind EG, Drake MT. Early Hypoparathyroidism Reversibility with Treatment of Riedel's Thyroiditis. Thyroid. 2015;25(9):1055–1059. [PubMed: 26200816]
- 352.
- Heufelder AE, Hay ID. Further evidence for autoimmune mechanisms in the pathogenesis of Riedel's invasive fibrous thyroiditis. J Intern Med. 1995;238(1):85–86. [PubMed: 7608652]
- 353.
- Heufelder AE, Bahn RS. Modulation of Graves' orbital fibroblast proliferation by cytokines and glucocorticoid receptor agonists. Invest Ophthalmol Vis Sci. 1994;35(1):120–127. [PubMed: 8300338]
- 354.
- Khan MA, Hashmi SM, Prinsley PR, Premachandra DJ. Reidel's thyroiditis and Tolosa-Hunt syndrome, a rare association. J Laryngol Otol. 2004;118(2):159–161. [PubMed: 14979959]
- 355.
- Meijer S, Hoitsma HF, Scholtmeijer R. Idiopathic retroperitoneal fibrosis in multifocal fibrosclerosis. Eur Urol. 1976;2(5):258–260. [PubMed: 1009988]
- 356.
- Meyer S, Hausman R. Occlusive phlebitis in multifocal fibrosclerosis. Am J Clin Pathol. 1976;65(3):274–283. [PubMed: 943929]
- 357.
- Geissler B, Wagner T, Dorn R, Lindemann F. Extensive sterile abscess in an invasive fibrous thyroiditis (Riedel's thyroiditis) caused by an occlusive vasculitis. J Endocrinol Invest. 2001;24(2):111–115. [PubMed: 11263468]
- 358.
- Vaidya B, Coulthard A, Goonetilleke A, Burn DJ, James RA, Kendall-Taylor P. Cerebral venous sinus thrombosis: a late sequel of invasive fibrous thyroiditis. Thyroid. 1998;8(9):787–790. [PubMed: 9777750]
- 359.
- Natt N, Heufelder AE, Hay ID, Grant CS, Goellner JR. Extracervical fibrosclerosis causing obstruction of a ventriculo-peritoneal shunt in a patient with hydrocephalus and invasive fibrous thyroiditis (Riedel's struma). Clin Endocrinol (Oxf). 1997;47(1):107–111. [PubMed: 9302380]
- 360.
- Egsgaard Nielsen V, Hecht P, Krogdahl AS, Andersen PB, Hegedus L. A rare case of orbital involvement in Riedel's thyroiditis. J Endocrinol Invest. 2003;26(10):1032–1036. [PubMed: 14759078]
- 361.
- Hines RC, Scheuermann HA, Royster HP, Rose E. Invasive fibrous (Riedel's) thyroiditis with bilateral fibrous parotitis. JAMA. 1970;213(5):869–871. [PubMed: 5468639]
- 362.
- Rao CR, Ferguson GC, Kyle VN. Retroperitoneal fibrosis associated with Riedel's struma. Can Med Assoc J. 1973;108(8):1019–1021. [PMC free article: PMC1941367] [PubMed: 4699273]
- 363.
- Julie C, Vieillefond A, Desligneres S, Schaison G, Grunfeld JP, Franc B. Hashimoto's thyroiditis associated with Riedel's thyroiditis and retroperitoneal fibrosis. Pathol Res Pract. 1997;193(8):573–577. [PubMed: 9406251]
- 364.
- Brihaye B, Lidove O, Sacre K, Laissy JP, Escoubet B, Valla D, Papo T. Diffuse periarterial involvement in systemic fibrosclerosis with Riedel's thyroiditis, sclerosing cholangitis, and retroperitoneal fibrosis. Scand J Rheumatol. 2008;37(6):490–492. [PubMed: 18728935]
- 365.
- Owen K, Lane H, Jones MK. Multifocal fibrosclerosis: a case of thyroiditis and bilateral lacrimal gland involvement. Thyroid. 2001;11(12):1187–1190. [PubMed: 12186507]
- 366.
- Hamed G, Tsushima K, Yasuo M, Kubo K, Yamazaki S, Kawa S, Hamano H, Yamamoto H. Inflammatory lesions of the lung, submandibular gland, bile duct and prostate in a patient with IgG4-associated multifocal systemic fibrosclerosis. Respirology. 2007;12(3):455–457. [PubMed: 17539856]
- 367.
- Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, Schlumberger M, Sherman SI, Steward DL, Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167–1214. [PubMed: 19860577]
- 368.
- Ozgen A, Cila A. Riedel's thyroiditis in multifocal fibrosclerosis: CT and MR imaging findings. AJNR Am J Neuroradiol. 2000;21(2):320–321. [PMC free article: PMC7975359] [PubMed: 10696016]
- 369.
- Papi G, Corrado S, Cesinaro AM, Novelli L, Smerieri A, Carapezzi C. Riedel's thyroiditis: clinical, pathological and imaging features. Int J Clin Pract. 2002;56(1):65–67. [PubMed: 11831840]
- 370.
- Slman R, Monpeyssen H, Desarnaud S, Haroche J, Fediaevsky LD, Fabrice M, Seret-Begue D, Amoura Z, Aurengo A, Leenhardt L. Ultrasound, Elastography, and Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography Imaging in Riedel's Thyroiditis: Report of Two Cases. Thyroid. 2011 [PubMed: 21615310]
- 371.
- Harigopal M, Sahoo S, Recant WM, DeMay RM. Fine-needle aspiration of Riedel's disease: report of a case and review of the literature. Diagn Cytopathol. 2004;30(3):193–197. [PubMed: 14986301]
- 372.
- Iacconi P, Giusti L, Da Valle Y, Ciregia F, Giannaccini G, Torregrossa L, Proietti A, Donatini G, Mazzeo S, Basolo F, Lucacchini A. Proteomic approach used in the diagnosis of Riedel's thyroiditis: a case report. J Med Case Rep. 2012;6:103. [PMC free article: PMC3349472] [PubMed: 22480342]
- 373.
- Perez Fontan PJ, Cordido Carbillido F, Pompo Felipe F, Mosquera Oses J, Villalba Martin C. Riedel thyroiditis: US, CT, and MR evaluation. J Comput Assist Tomogr. 1993;17(2):324–325. [PubMed: 8454764]
- 374.
- Lo JC, Loh KC, Rubin AL, Cha I, Greenspan FS. Riedel's thyroiditis presenting with hypothyroidism and hypoparathyroidism: dramatic response to glucocorticoid and thyroxine therapy. Clin Endocrinol (Oxf). 1998;48(6):815–818. [PubMed: 9713573]
- 375.
- Takahashi N, Okamoto K, Sakai K, Kawana M, Shimada-Hiratsuka M. MR findings with dynamic evaluation in Riedel's thyroiditis. Clin Imaging. 2002;26(2):89–91. [PubMed: 11852213]
- 376.
- Drieskens O, Blockmans D, Van den Bruel A, Mortelmans L. Riedel's thyroiditis and retroperitoneal fibrosis in multifocal fibrosclerosis: positron emission tomographic findings. Clin Nucl Med. 2002;27(6):413–415. [PubMed: 12045432]
- 377.
- Kotilainen P, Airas L, Kojo T, Kurki T, Kataja K, Minn H, Nuutila P. Positron emission tomography as an aid in the diagnosis and follow-up of Riedel's thyroiditis. Eur J Intern Med. 2004;15(3):186–189. [PubMed: 15245724]
- 378.
- Moulik PK, Al-Jafari MS, Khaleeli AA. Steroid responsiveness in a case of Riedel's thyroiditis and retroperitoneal fibrosis. Int J Clin Pract. 2004;58(3):312–315. [PubMed: 15117103]
- 379.
- Papi G. LiVolsi VA. Current concepts on Riedel thyroiditis. Am J Clin Pathol. 2004;121 Suppl:S50–63. [PubMed: 15298150]
- 380.
- Yu Y, Liu J, Yu N, Zhang Y, Zhang S, Li T, Gao Y, Lu G, Zhang J, Guo X. IgG4 immunohistochemistry in Riedel's thyroiditis and the recommended criteria for diagnosis: A case series and literature review. Clin Endocrinol (Oxf). 2021;94(5):851–857. [PubMed: 33301600]
- 381.
- Jung K-Y. Surgical Treatment for Riedel’s Thyroiditis: a Case Report. International Journal of Thyroidology. 2017;10(1):66–69.
- 382.
- Vaidya B, Harris PE, Barrett P, Kendall-Taylor P. Corticosteroid therapy in Riedel's thyroiditis. Postgrad Med J. 1997;73(866):817–819. [PMC free article: PMC2431527] [PubMed: 9497955]
- 383.
- Tutuncu NB, Erbas T, Bayraktar M, Gedik O. Multifocal idiopathic fibrosclerosis manifesting with Riedel's thyroiditis. Endocr Pract. 2000;6(6):447–449. [PubMed: 11155216]
- 384.
- Hostalet F, Hellin D, Ruiz JA. Tumefactive fibroinflammatory lesion of the head and neck treated with steroids: a case report. Eur Arch Otorhinolaryngol. 2003;260(4):229–231. [PubMed: 12709810]
- 385.
- Bagnasco M, Passalacqua G, Pronzato C, Albano M, Torre G, Scordamaglia A. Fibrous invasive (Riedel's) thyroiditis with critical response to steroid treatment. J Endocrinol Invest. 1995;18(4):305–307. [PubMed: 7560814]
- 386.
- Thomson JA, Jackson IM, Duguid WP. The effect of steroid therapy on Riedel's thyroiditis. Scott Med J. 1968;13(1):13–16. [PubMed: 5694137]
- 387.
- Rodriguez I, Ayala E, Caballero C, De Miguel C, Matias-Guiu X, Cubilla AL, Rosai J. Solitary fibrous tumor of the thyroid gland: report of seven cases. Am J Surg Pathol. 2001;25(11):1424–1428. [PubMed: 11684960]
- 388.
- Few J, Thompson NW, Angelos P, Simeone D, Giordano T, Reeve T. Riedel's thyroiditis: treatment with tamoxifen. Surgery. 1996;120(6):993–998. [PubMed: 8957485]
- 389.
- Levy JM, Hasney CP, Friedlander PL, Kandil E, Occhipinti EA, Kahn MJ. Combined mycophenolate mofetil and prednisone therapy in tamoxifen- and prednisone-resistant Reidel's thyroiditis. Thyroid. 2010;20(1):105–107. [PubMed: 20067381]
- 390.
- De M, Jaap A, Dempster J. Tamoxifen therapy in steroid-resistant Riedels disease. Scott Med J. 2002;47(1):12–13. [PubMed: 11980291]
- 391.
- Dabelic N, Jukic T, Labar Z, Novosel SA, Matesa N, Kusic Z. Riedel's thyroiditis treated with tamoxifen. Croat Med J. 2003;44(2):239–241. [PubMed: 12698518]
- 392.
- Erdogan MF, Anil C, Turkcapar N, Ozkaramanli D, Sak SD, Erdogan G. A case of Riedel's thyroiditis with pleural and pericardial effusions. Endocrine. 2009;35(3):297–301. [PubMed: 19381890]
- 393.
- Jung YJ, Schaub CR, Rhodes R, Rich FA, Muehlenbein SJ. A case of Riedel's thyroiditis treated with tamoxifen: another successful outcome. Endocr Pract. 2004;10(6):483–486. [PubMed: 16033720]
- 394.
- Clark CP, Vanderpool D, Preskitt JT. The response of retroperitoneal fibrosis to tamoxifen. Surgery. 1991;109(4):502–506. [PubMed: 2008655]
- 395.
- Pritchyk K, Newkirk K, Garlich P, Deeb Z. Tamoxifen therapy for Riedel's thyroiditis. Laryngoscope. 2004;114(10):1758–1760. [PubMed: 15454767]
- 396.
- Butta A, MacLennan K, Flanders KC, Sacks NP, Smith I, McKinna A, Dowsett M, Wakefield LM, Sporn MB, Baum M, et al. Induction of transforming growth factor beta 1 in human breast cancer in vivo following tamoxifen treatment. Cancer Res. 1992;52(15):4261–4264. [PubMed: 1322240]
- 397.
- Colletta AA, Wakefield LM, Howell FV, van Roozendaal KE, Danielpour D, Ebbs SR, Sporn MB, Baum M. Anti-oestrogens induce the secretion of active transforming growth factor beta from human fetal fibroblasts. Br J Cancer. 1990;62(3):405–409. [PMC free article: PMC1971440] [PubMed: 1698443]
- 398.
- Arteaga CL, Tandon AK, Von Hoff DD, Osborne CK. Transforming growth factor beta: potential autocrine growth inhibitor of estrogen receptor-negative human breast cancer cells. Cancer Res. 1988;48(14):3898–3904. [PubMed: 3164252]
- 399.
- Falhammar H, Juhlin C, Barner C, Catrina S, Karefylakis C, Calissendorff J. Riedel's thyroiditis: clinical presentation, treatment and outcomes. Endocrine. 2018;60(1):185–192. [PMC free article: PMC5845586] [PubMed: 29380231]
- 400.
- Hunt L, Harrison B, Bull M, Stephenson T, Allahabadia A. Rituximab: a novel treatment for refractory Riedel's thyroiditis. Endocrinology, diabetes & metabolism case reports. 2018;2018. [PMC free article: PMC5811771] [PubMed: 29472985]
- 401.
- Hoang TD, Mai VQ, Clyde PW, Glister BC, Shakir MK. Multinodular goiter as the initial presentation of systemic sarcoidosis: limitation of fine-needle biopsy. Respir Care. 2011;56(7):1029–1032. [PubMed: 21352663]
- 402.
- Anolik RB, Schaffer A, Kim EJ, Rosenbach M. Thyroid dysfunction and cutaneous sarcoidosis. J Am Acad Dermatol. 2012;66(1):167–168. [PubMed: 22177644]
- 403.
- Vailati A, Marena C, Aristia L, Sozze E, Barosi G, Inglese V, Luisetti M, Bossolo PA. Sarcoidosis of the thyroid: report of a case and a review of the literature. Sarcoidosis. 1993;10(1):66–68. [PubMed: 8134720]
- 404.
- Ozdemir D, Dagdelen S, Erbas T. Endocrine involvement in systemic amyloidosis. Endocr Pract. 2010;16(6):1056–1063. [PubMed: 20570812]
- 405.
- Sethi Y, Gulati A, Singh I, Rao S, Singh N. Amyloid goiter: a case of primary thyroid amyloid disease. Laryngoscope. 2011;121(5):961–964. [PubMed: 21520108]
- 406.
- Ozdemir D, Dagdelen S, Erbas T, Sokmensuer C, Erbas B, Cila A. Amyloid goiter and hypopituitarism in a patient with systemic amyloidosis. Amyloid. 2011;18(1):32–34. [PubMed: 21231858]
- 407.
- Kazdaghli Lagha E, M'Sakni I, Bougrine F, Laabidi B, Ben Ghachem D, Bouziani A. Amyloid goiter: first manifestation of systemic amyloidosis. Eur Ann Otorhinolaryngol Head Neck Dis. 2010;127(3):108–110. [PubMed: 20822765]
- 408.
- Vanguri VK, Nose V. Transthyretin amyloid goiter in a renal allograft recipient. Endocr Pathol. 2008;19(1):66–73. [PubMed: 18369743]
- 409.
- Lari E, Burhamah W, Lari A, Alsafran S, Ismail A. Amyloid goiter - A rare case report and literature review. Ann Med Surg (Lond). 2020;57:295–298. [PMC free article: PMC7452006] [PubMed: 32874558]
- 410.
- Joung KH, Park JY, Kim KS, Koo BS. Primary amyloid goiter mimicking rapid growing thyroid malignancy. Eur Arch Otorhinolaryngol. 2014;271(2):417–420. [PubMed: 24150545]
- 411.
- Vergneault H, Terre A, Buob D, Buffet C, Dumont A, Ardois S, Savey L, Pardon A, Michel PA, Boffa JJ, Grateau G, Georgin-Lavialle S. Amyloid Goiter in Familial Mediterranean Fever: Description of 42 Cases from a French Cohort and from Literature Review. J Clin Med. 2021;10(9) [PMC free article: PMC8125620] [PubMed: 34063105]
- 412.
- Aydin B, Koca YS, Koca T, Yildiz I, Gerek Celikden S, Ciris M. Amyloid Goiter Secondary to Ulcerative Colitis. Case Rep Endocrinol. 2016;2016:3240585. [PMC free article: PMC4802030] [PubMed: 27051538]
- 413.
- Seker A, Erkinuresin T, Demirci H. Amyloid Goiter in a Patient with Rheumatoid Arthritis and End-Stage Renal Disease. Indian J Nephrol. 2020;30(2):125–128. [PMC free article: PMC7132848] [PubMed: 32269439]
- 414.
- Jakubovic-Cickusic A, Hasukic B, Sulejmanovic M, Cickusic A, Hasukic S. Amyloid Goiter: A Case Report and Review of the Literature. Saudi J Med Med Sci. 2020;8(2):151–155. [PMC free article: PMC7305682] [PubMed: 32587498]
- 415.
- Villa F, Dionigi G, Tanda ML, Rovera F, Boni L. Amyloid goiter. Int J Surg. 2008;6 Suppl 1:S16–18. [PubMed: 19168408]
- 416.
- Goldsmith JD, Lai ML, Daniele GM, Tomaszewski JE. LiVolsi VA. Amyloid goiter: report of two cases and review of the literature. Endocr Pract. 2000;6(4):318–323. [PubMed: 11242609]
- 417.
- Hamed G, Heffess CS, Shmookler BM, Wenig BM. Amyloid goiter. A clinicopathologic study of 14 cases and review of the literature. Am J Clin Pathol. 1995;104(3):306–312. [PubMed: 7677120]
- 418.
- Pinto A, Nose V. Localized amyloid in thyroid: are we missing it? Adv Anat Pathol. 2013;20(1):61–67. [PubMed: 23232573]
- 419.
- Coca-Pelaz A, Vivanco-Allende B, Alvarez-Marcos C, Suarez-Nieto C. Multifocal papillary thyroid carcinoma associated with primary amyloid goiter. Auris Nasus Larynx. 2011 [PubMed: 22075140]
- 420.
- Nessim S, Tamilia M. Papillary thyroid carcinoma associated with amyloid goiter. Thyroid. 2005;15(4):382–385. [PubMed: 15876164]
- 421.
- Coli A, Bigotti G, Zucchetti F, Negro F, Massi G. Papillary carcinoma in amyloid goitre. J Exp Clin Cancer Res. 2000;19(3):391–394. [PubMed: 11144534]
- 422.
- Ozdemir BH, Akman B, Ozdemir FN. Amyloid goiter in Familial Mediterranean Fever (FMF): a clinicopathologic study of 10 cases. Ren Fail. 2001;23(5):659–667. [PubMed: 11725912]
- 423.
- Ozdemir BH, Uyar P, Ozdemir FN. Diagnosing amyloid goitre with thyroid aspiration biopsy. Cytopathology. 2006;17(5):262–266. [PubMed: 16961655]
- 424.
- Hill K, Diaz J, Hagemann IS, Chernock RD. Multiple Myeloma Presenting as Massive Amyloid Deposition in a Parathyroid Gland Associated with Amyloid Goiter: A Medullary Thyroid Carcinoma Mimic on Intra-operative Frozen Section. Head Neck Pathol. 2018;12(2):269–273. [PMC free article: PMC5953875] [PubMed: 28879586]
- 425.
- Bando Y, Ushiogi Y, Toya D, Tanaka N, Fujisawa M. Painless thyroiditis associated with severe inflammatory reactions in amyloid goiter: a case report. Endocr J. 2001;48(3):323–329. [PubMed: 11523903]
- 426.
- Bryer-Ash M, Lodhi W, Robbins K, Morrison R. Early thyrotoxic thyroiditis after radiotherapy for tonsillar carcinoma. Arch Otolaryngol Head Neck Surg. 2001;127(2):209–211. [PubMed: 11177042]
- 427.
- Espiritu RP, Dean DS. Parathyroidectomy-induced thyroiditis. Endocr Pract. 2010;16(4):656–659. [PubMed: 20350919]
- 428.
- McDermott A, Onyeaka CV, Macnamara M. Surgery-induced thyroiditis: fact or fiction? Ear Nose Throat J. 2002;81(6):408–410. [PubMed: 12092285]
- 429.
- Blenke EJ, Vernham GA, Ellis G. Surgery-induced thyroiditis following laryngectomy. J Laryngol Otol. 2004;118(4):313–314. [PubMed: 15117475]
- Review Clinical review: Riedel's thyroiditis: a clinical review.[J Clin Endocrinol Metab. 2011]Review Clinical review: Riedel's thyroiditis: a clinical review.Hennessey JV. J Clin Endocrinol Metab. 2011 Oct; 96(10):3031-41. Epub 2011 Aug 10.
- Review Case of concurrent Riedel's thyroiditis, acute suppurative thyroiditis, and micropapillary carcinoma.[Korean J Intern Med. 2013]Review Case of concurrent Riedel's thyroiditis, acute suppurative thyroiditis, and micropapillary carcinoma.Hong JT, Lee JH, Kim SH, Hong SB, Nam M, Kim YS, Chu YC. Korean J Intern Med. 2013 Mar; 28(2):236-41. Epub 2013 Feb 27.
- Review Riedel's thyroiditis in a patient with recurrent subacute thyroiditis: a case report and review of the literature.[Endocr J. 2007]Review Riedel's thyroiditis in a patient with recurrent subacute thyroiditis: a case report and review of the literature.Cho MH, Kim CS, Park JS, Kang ES, Ahn CW, Cha BS, Lim SK, Kim KR, Lee HC. Endocr J. 2007 Aug; 54(4):559-62. Epub 2007 Jul 2.
- Review Thyroiditis: differential diagnosis and management.[Am Fam Physician. 2000]Review Thyroiditis: differential diagnosis and management.Slatosky J, Shipton B, Wahba H. Am Fam Physician. 2000 Feb 15; 61(4):1047-52, 1054.
- [Acute suppurative thyroiditis in a patient with prior subacute thyroiditis].[Minerva Endocrinol. 1992][Acute suppurative thyroiditis in a patient with prior subacute thyroiditis].Pantaleoni M, Velardo A, Smerieri A, Toschi E, Zizzo G, Marrama P. Minerva Endocrinol. 1992 Jul-Sep; 17(3):133-6.
- Acute and Subacute, and Riedel’s Thyroiditis - EndotextAcute and Subacute, and Riedel’s Thyroiditis - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
