By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999.

Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition.
Show detailsMany neuropeptides were originally identified as pituitary or gastrointestinal hormones
Probably the first neuropeptide to be identified was vasopressin, a nine-amino-acid peptide secreted by the nerve endings in the neural lobe of the pituitary. The source of the vasopressin is the magnocellular neurons of the hypothalamus, which send axons to the neurohypophysis, which is the site of release into the blood, in classic neurosecretory fashion. Like vasopressin, a number of gastrointestinal peptides, such as cholecystokinin (CCK), are also found at high concentrations in the nervous system. In the gastrointestinal (GI) system, CCK is secreted by the duodenum and governs the delivery of digestive enzymes and bile acids into the intestine. In contrast to vasopressin and CCK, the hypothalamic releasing factors are peptides released into a special portal blood system that bathes the anterior pituitary, controlling the secretion of pituitary hormones. In this system, “portal” means two successive capillary beds, one in the hypothalamus and one in the anterior pituitary. Substance P was first purified as a “sialogogic peptide,” causing salivation in a bioassay. Now substance P is recognized as a major bioactive peptide in many neuronal pathways, including pain signaling. Since there are so many peptides, this chapter focuses on the principles of how neuropeptides are synthesized, stored and released and how they act on the cells they regulate. Comparisons among peptides and smaller, “conventional” neurotransmitters will be emphasized. It is significant to note that the number of known neuropeptides far exceeds the number of classical neurotransmitters.
Peptides can be grouped by structural and functional similarity
Like GABA and glutamate, which differ by only a single carboxyl group yet have very different functions, many neuropeptides with similar structures have very different functions. Vasopressin and oxytocin are the two major neurohypophyseal peptides, and each consists of nine amino acids. These two peptides are identical at seven of those residues and are thought to be the result of gene duplication early in evolution. The actions of the two peptides are distinct: oxytocin causes milk letdown and uterine contraction, while vasopressin causes water retention in the kidney and blood vessel contraction. Likewise, the opiate peptides share a common Tyr-Gly-Gly-Phe-Met/Leu sequence at the NH2 terminus, and all are potent endogenous opiates but with distinct patterns of selectivity at the various classes of opiate receptor. The three glycoprotein hormones from the anterior pituitary, thyroid stimulating hormone (TSH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH), share a common α subunit but have distinct β subunits, and only the αβ dimer is biologically active. The tachykinin group includes substance P and various frog skin peptides, all with similar core sequences and -Phe-X-Gly-Leu-Met-NH2 at the COOH terminus. The GI peptides CCK and gastrin share a common COOH-terminal sequence (Trp-Met-Asp-Phe-NH2) and are among the few peptides which undergo tyrosine sulfation. The sites of action of CCK and gastrin are distinct: gastrin stimulates gastric acid secretion, while CCK stimulates enzyme and bile acid delivery to the small intestine. Interestingly, the common COOH-terminal tetrapeptide, while inactive in the GI tract, is abundant in the cerebral cortex and has important behavioral actions.
The function of peptides as first messengers is evolutionarily very old
In phylogenetic terms, neuropeptides were established very early as molecules effecting intercellular communication. In coelenterates, such as Hydra, there are many peptides used in neurotransmission, but many of the “conventional” neurotransmitter systems, such as acetylcholine (ACh), catecholamines and serotonin, covered in previous chapters, are not found [1,2]. The nerve net is strongly peptidergic in the lowest animal group with a nervous system, the cnidarians, which includes sea anemones, corals, jellyfishes and Hydra. Yeast use bioactive peptides such as a- and α-mating factors to communicate.
Various techniques are used to identify additional neuropeptides
Although the list of neuropeptides is already quite long, as seen in the partial listing in Figure 18-1, additional neuropeptides are still being identified. A number of experimental approaches are in use.

Figure 18-1
Selected bioactive peptides are grouped by structural similarity or by tissue source.
Bioassays are the oldest and surest way to identify biologically active peptides: a skin-darkening assay led to the discovery of α-melanotropin, increased salivation was used to identify substance P and CCK was identified as the factor causing bile acid secretion. More recently, with the advent of radioreceptor assays, peptides have been identified by their ability to bind to a known receptor and thus to displace a ligand or to produce a biological response. This was one of the methods used to search for opiate peptides, assaying for peptides that could displace [3H]-etorphine or other opiate ligands. Similarly, several of the peptides in Figure 18-1 are routinely assayed by their ability to increase adenylyl cyclase activity in membrane preparations due to their stimulatory interaction with peptide receptors. Use of a relatively homogeneous tissue source, such as adrenal chromaffin granules, enabled identification of peptides derived from chromogranin A. Mass spectrometry is being used to characterize peptides in certain large neurons of invertebrates. As noted above, peptides important in the nervous system were often identified first in some peripheral source, such as CCK in the gut, or even in unusual places, such as frog skin. Since α-amidation of the COOH-terminus of peptides has proven to be a signature of bioactive peptides, assays specific for COOH-terminal α-amides were developed and used to discover neuropeptide Y and several other peptides.
Molecular biological approaches have been used to discover many new peptides. Neuropeptide genes have been cloned from a single identified neuron, such as the R3-14 and L5-67 propeptides of Aplysia. Subtractive hybridization and differential display have been used to screen for unique transcripts, allowing identification of both the cocaine and amphetamine-regulated transcript (CART), and RESP18, a dopamine-regulated transcript [3,4]. Finally, screens using orphan receptors, which have no known ligand, were used to find natural ligands, such as orphanin FQ or prepronociceptin [5].
The neuropeptides exhibit a few key differences from the classical neurotransmitters
First, neuropeptides are present in tissues at much lower concentrations than classical neurotransmitters but are also active at receptors at correspondingly lower concentrations. For example, the concentration of ACh in synaptic vesicles is in the 100 mM range (see Chap. 11), while the concentration of neuropeptide in a large dense core vesicle is 3 to 10 mM at most. Correspondingly, the affinity of ACh for its receptors is in the 100 μM to 1 mM range, while peptides typically bind to their receptors with nanomolar to micromolar affinities.
Probably the most striking difference between neuropeptides and conventional neurotransmitters is in their biosynthesis (Fig. 18-2). Neuropeptides are derived from larger, inactive precursors that are generally at least 90 amino acid residues in length [6–8]. The simplest example is prolactin, a pituitary product. The signal sequence for prolactin must be removed and disulfide linkages must form, but no further cleavages are necessary. The next simplest case is somatostatin, in which a single cleavage after signal peptide removal produces the bioactive peptide. Neuropeptide Y (NPY) comes from proneuropeptide Y after signal peptide removal, cleavage between NPY and the C-terminal flanking peptide of NPY (CPON) and additional modifications which are discussed below. The pro-opiomelanocortin (POMC) precursor includes several different bioactive peptides, as does the egg-laying hormone (ELH) precursor [8]. One interesting attribute common to peptide precursors in evolutionarily older species is the existence of multiple copies of the same bioactive peptide in one precursor; this is exemplified by the FMRF-NH2 (Phe-Met-Arg-Phe-amide) precursor, with 29 copies of the active peptide [1]. Even in yeast, a similar process is used, so that four copies of α-mating factor are produced from the α-mating factor precursor. Precursors with multiple copies of bioactive peptide are much less common in evolutionarily more advanced species, although the rat TRH precursor contains five copies of the TRH tripeptide.

Figure 18-2
Structures of selected bioactive peptide precursors are diagrammed. The structures of prolactin (PRL), somatostatin, neuropeptide Y (NPY), pro-opiomelanocortin (POMC), egg-laying hormone (ELH), yeast α-mating factor (αMF) and FMRF-amide (more...)
The supply of conventional neurotransmitters in small synaptic vesicles is replenished in nerve terminals by local synthesis, and many conventional neurotransmitters are recaptured after secretion. In striking contrast, neuropeptides are initially synthesized in the cell soma, sequestered within the lumen of the secretory pathway and transported down the axon while undergoing cleavages and other processing events, after which the peptide-containing, large dense core vesicle (LDCV) is used once. After exocytosis, the membrane components of the LDCV must be reinternalized and either destroyed or reutilized after transport to the cell body. Thus, no synaptic re-use occurs of either the neuropeptides or their immediate precursors.
Release is another area of difference: conventional neurotransmitters are secreted from small secretory vesicles (SSVs) after cytosolic [Ca2+] transiently reaches concentrations of 50 to 100 μM, while peptides are released from LDCVs at lower concentrations of cytosolic [Ca2+]. Conventional neurotransmitter release is thought to occur very close to the site of Ca2+ entry (see Chaps. 9 and 10), while neuropeptides are typically released at a distance from the site of Ca2+ entry. Furthermore the Ca2+ that stimulates exocytosis from LDCVs may come from either internal stores or the transmembrane current (Fig. 18-3). Thus, the location of LDCVs relative to the site of Ca2+ influx can determine the amount of Ca2+ necessary for secretion to occur.

Figure 18-3
Intracellular pathway of bioactive peptide biosynthesis, processing and storage. Neuropeptide precursors are synthesized on ribosomes at the endoplasmic reticulum and processed through the Golgi. Axonal transport of the large dense core vesicle to the (more...)
Neuropeptides are often found in neurons with conventional neurotransmitters
As diagrammed in Figure 18-3, both conventional neurotransmitters and neuropeptides are found at a majority of the synapses in the nervous system. Neuropeptide expression is extremely plastic, even in the adult. For example, the hypothalamic neurons which express vasopressin and those which synthesize corticotropin-releasing hormone (CRH) are situated close to each other but constitute separate and virtually nonoverlapping populations of neurons in the normal animal. However, after glucocorticoid concentrations are lowered by blockade of adrenal cortical function or removal of the adrenal glands, vasopressin neurons begin to express CRH and CRH neurons begin to synthesize vasopressin. This adaptive response can be understood from a teleological point of view by knowing that CRH normally stimulates the adrenocorticotropic hormone (ACTH)-producing cells of the anterior pituitary to secrete ACTH and that ACTH stimulates glucocorticoid production in the adrenal cortex. Vasopressin acts synergistically to increase ACTH secretion in times of need, such as adrenalectomy. In addition to neuropeptides, many LDCVs contain ATP, just as many conventional neurotransmitter vesicles do, so that ATP is released along with neuropeptides. ATP and adenosine can have potent synaptic actions in their own right (see Chap. 17).
The biosynthesis of neuropeptides is fundamentally different from that of conventional neurotransmitters
To add to the complexity discussed above, the processing of neuropeptide precursors is tissue-specific, with a general rule that most precursors are expressed in more than one tissue and that the processing is not identical in different tissues (Fig. 18-4) [6–10]. For example, anterior pituitary corticotropes cleave POMC to ACTH(1–39), a molecule that stimulates adrenal glucocorticoid production. Neurons in the arcuate nucleus cleave ACTH and α-amidate the smaller peptide to create ACTH(1–13)NH2, which cannot stimulate the adrenal cortex but does have potent behavioral effects in the CNS. Intermediate pituitary melanotropes go one step further and α-N-acetylate this molecule to produce α-melanocyte-stimulating hormone (MSH), which has skin-darkening activity, especially in lower vertebrates for which background color adaptation is protective. Similarly, corticotropes produce β-lipotropin (βLPH), which has no activity as an opiate peptide, while melanotropes and CNS neurons cleave βLPH to produce the potent opiate peptide β-endorphin(1–31). In some tissues, the β-endorphin may be shortened at the COOH-terminus, which decreases its opiate activity, or α-N-acetylated at the NH2-terminus, which abolishes opiate activity. The cellular control of these different patterns of processing is beginning to be understood, with the identification of some of the enzymes that mediate these steps (see below).

Figure 18-4
Tissue-specific processing of the pro-opiomelanocortin (POMC) precursor yields a wide array of bioactive peptide products. Processing of the POMC precursor varies in various tissues. In anterior pituitary, adrenocorticotropic hormone [ACTH (1–39) (more...)
Other examples of tissue-specific processing include proenkephalin, proglucagon, procholecystokinin and prosomatostatin. Somatostatin neurons in the hypothalamus primarily produce a 14-residue form of the peptide, while somatostatin endocrine cells of the pancreas and intestine produce a 28-residue form derived from the same precursor [11]. Proenkephalin is processed in the adrenal medulla to a set of opiate peptides of 15 to 35 residues, while proenkephalin in the brain is cleaved primarily to the pentapeptides met-enkephalin and leuenkephalin. Procholecystokinin in the gut is processed to peptides of approximately 30 residues, which act on the pancreas and gallbladder, while smaller CCK-related peptides with behavioral effects are found in the brain. These smaller CCK-related peptides have no effects when applied to the pancreas or gallbladder [9].
Many of the enzymes involved in peptide biogenesis have been identified
The most common steps in precursor processing and the enzymes involved are shown in Figure 18-5. The endoproteases involved are prohormone convertases 1 and 2 (PC1 and PC2), the exopeptidase is carboxypeptidase E (CPE, also called CPH and enkephalin convertase) and the α-amidating enzyme is peptidylglycine α-amidating mono-oxygenase (PAM). Many steps in their biosynthesis are not unique to neuropeptides, such as signal peptide cleavage, disulfide bond formation, the addition and subsequent modification of N-linked and O-linked oligosaccharides, phosphorylation and sulfation. As diagrammed in Figure 18-3, many of the post-translational steps occur as the maturing neuropeptides travel down the axon toward the synapse in LDCVs. The later steps in neuropeptide biosynthesis (Fig. 18-5) are unique to neurons and endocrine cells.

Figure 18-5
Sequential enzymatic steps lead from the peptide precursor to bioactive peptides. The neuropeptide Y (NPY) precursor shown at the left is processed sequentially by the enzymes of the large dense-core vesicles (LDCV) shown at right. ER, endoplasmic reticulum; (more...)
Key enzymes in neuropeptide biosynthesis include endoproteases, exoproteases and enzymes modifying the ends of the peptides. The discovery and characterization of Kex2p, the endoprotease that cleaves yeast pro-α-mating factor to produce four copies of the pheromone α-mating factor (Fig. 18-2), were key to the discovery of the mammalian prohormone convertases, including furin, PC1/3, PC2, PC4, PC5/6, PC7/8/LPC, and PACE4 [6,7]. The prohormone convertases share homology with bacterial subtilisins and have an Asp-His-Ser catalytic triad, which consists of three key amino acids involved in catalysis (denoted D, H, and S in Fig. 18-5). The proregion of each (Fig. 18-5) must be present during biosynthesis for the protease to fold correctly but must be removed to yield an activated protease. For PC1 and furin, removal of the proregion occurs within a few minutes of biosynthesis while the enzyme is in the endoplasmic reticulum and is most likely an autocatalytic event. For the other prohormone convertases removal of the proregion is much slower. Expression of active PC2 requires coexpression of the peptide 7B2 (Fig. 18-5), which appears to perform a chaperone function and may also prevent expression of PC2 endoproteolytic activity until PC2 has been deposited into secretory granules. No corresponding chaperone/inhibitor peptide has been identified for the other prohormone convertases.
The mammalian endoproteases most clearly involved in neuropeptide processing are PC1 and PC2, Ca2+-dependent proteases found in secretory granules whose expression is limited to neurons and endocrine cells (Fig. 18-5). Several other members of this endoprotease family are more widely expressed, while still others are expressed in restricted locations distinct from neurons and endocrine cells. For example, furin is found in virtually all cells and is localized primarily to the trans-Golgi network; furin catalyzes cleavages important in peptide function, such as the initial cleavage of the ELH, nerve growth factor and parathyroid hormone precursors, as well as cleavage within the insulin receptor precursor to produce the active αβ dimer form of the receptor. Furin may also be instrumental in the activation of some of the other processing enzymes, such as PC2 and CPE.
PC1 and PC2 cleave at selected pairs of basic amino acids in peptide precursors: Lys-Arg, Arg-Arg, Lys-Lys and Arg-Lys. PC1 may also catalyze cleavages at the selected single Arg sites present in some precursors, such as prosomatostatin and procholecystokinin. Cleavages in LDCVs by PCs are tightly controlled, often occurring in a very orderly fashion (Fig. 18-6). The initial cleavages of POMC occur in less than 1 hr (Fig. 18-6, steps 1 and 2), while other cleavages occur only after several hours (Fig. 18-6, steps 6 and 7). The endoproteolytic cleavage of propeptides is often the rate-limiting reaction in peptide biosynthetic processing.
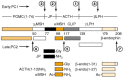
Figure 18-6
Processing of the pro-opiomelanocortin (POMC) precursor proceeds in an ordered, stepwise fashion. Cleavage of the POMC precursor occurs at seven sites, with some of the reactions being tissue-specific. The circled numbers indicate the temporal order of (more...)
The pattern of cleavages catalyzed by PC1, PC2 and furin when expressed in neurons and endocrine cells is much more selective than the pattern of cleavages seen in test tube assays with purified enzymes. For example, although prohormone convertases usually cleave at the COOH-terminus of a pair of basic residues in model peptide substrates, in cells the cleavages can be in the middle of the pairs of basic residues, as in the case of POMC cleavage (Fig. 18-6), where the basic residues are separated and remain with the two resulting mature peptides [8]. It is likely that the Ca2+ concentration and internal pH of LDCVs are two variables used by neurons and endocrine cells to regulate endoproteolytic activity in LDCVs.
Additional endoproteases may be shown to play a role in neuropeptide biosynthesis. Leading candidates are the mammalian homolog of the yeast aspartyl protease-3 (YAP-3) and the N-arginine dibasic (NRD) convertase [12,13]. An additional twist in peptide biosynthesis is seen in the heart, where proatrial natriuretic factor (proANF) is stored in LDCVs and yet mature ANF is released from atrial cells into the circulation. The processing of proANF, which involves cleavage after a single Arg residue in proANF, cannot involve PC1 or PC2 since there are negligible amounts of those PCs in the heart.
CPE is a soluble protein found in virtually all LDCVs in neurons and endocrine cells (Fig. 18-5) [14]. It removes basic residues, Lys or Arg, from the COOH termini of peptide intermediates produced by the prohormone convertases. It was originally identified by its tissue distribution and substrate specificity, along with its specific inhibition by guanidinoethylmercaptosuccinic acid (GEMSA). CPE is a Co2+- and Zn2+-activated enzyme with a short proregion that is normally removed during maturation of the enzyme; unlike the prohormone convertases, CPE is active with the proregion attached. The carboxypeptidase function of peptide processing is not normally rate-limiting since peptide intermediates with COOH-terminal basic residues are detected only at extremely low concentrations in tissue or LDCV extracts. Recently, additional carboxypeptidases have been identified, notably CPD, an integral membrane form of the enzyme with 3 carboxypeptidase domains. The relative importance of CPE and these additional carboxypeptidases to neuropeptide processing in vivo is unclear. Given that cleavage at a pair of basic residues can be in the middle of the pair, there is good reason to think that an aminopeptidase will be found in LDCVs.
PAM is a bifunctional enzyme found in nearly all LDCVs (Fig. 18-5) [15]. PAM acts on peptide substrates after endoproteolytic cleavage and exopeptidase action, when a COOH-terminal Gly residue is exposed, and converts the peptidyl-Gly into the corresponding peptide-NH2. About half of the known bioactive peptides are α-amidated, and α-amidation is generally crucial to biological potency. The peptidyl-Gly and peptide-COOH forms are usually inactive at physiological concentrations. The first step of the α-amidation reaction is performed by peptidylglycine α-hydroxylating mono-oxygenase (PHM), which is the NH2-terminal portion of the bifunctional PAM protein. PHM binds two Cu2+ atoms that participate in catalysis by undergoing cycles of reduction and oxidation. PHM uses ascorbic acid as the reductant, with one atom of oxygen from O2 incorporated into the peptide during the hydroxylation step. Thus, PHM is enzymatically very similar to dopamine β-mono-oxygenase (DBM), which converts dopamine to norepinephrine (see Chap. 12). The second step of the α-amidation reaction is performed by a second enzymatic domain of PAM, peptidyl-α-hydroxyglycine α-amidating lyase (PAL). The PAL domain constitutes a novel, divalent metal ion-dependent enzyme. Neurons primarily express an integral membrane form of the bifunctional PAM protein (Fig. 18-5), while an additional mRNA-splicing event enables some endocrine cells to express soluble versions of the protein, lacking the transmembrane domain. In the integral membrane forms of PAM, the short COOH-terminal domain extends into the cytoplasm and participates in the routing of PAM between LDCVs and the cell surface. The supply of reduced ascorbate in LDCVs is maintained by cytochrome B561, a protein that has five transmembrane domains and shuttles electrons from cytosolic ascorbate to ascorbate in the lumen of the LDCVs [16]. Cytochrome B561 is also found in catecholamine-containing vesicles, where it performs a similar function for DBM (see Chap. 12). Nervous and endocrine tissues maintain concentrations of reduced ascorbate about 100-fold above the blood concentration of ascorbate, while most other tissues do not concentrate ascorbate.
Several peptides have NH2-terminal pyroglutamic acid residues, also termed cyclic glutamic acid (<Glu), which are essential to bioactivity, for example, thyrotropin-releasing hormone (TRH) and gonadotropin-releasing hormone (GnRH). The enzyme responsible for this step is glutaminyl cyclase, which converts the original NH2-terminal Gln into <Glu. The regulation and function of glutaminyl cyclase has not yet been extensively studied. Another important but infrequent modification of peptides is α-N-acetylation (Figs. 18-6 and 18-7). During POMC processing, α-N-acetylation greatly increases the skin-darkening potency of ACTH(1–13)NH2 while abolishing both the adrenal steroidogenic potency of ACTH and the opiate activity of β-endorphin [8]. The enzyme(s) responsible for this modification has not yet been purified or cloned.

Figure 18-7
Cell-specific packaging of peptides into large dense core vesicles can lead to very different patterns of peptide secretion. Sorting of neuropeptides into distinct mature secretory granules (MSG) is shown for bag cell neurons but does not occur for endocrine (more...)
As an example, Figure 18-6 shows the pattern of processing steps in the POMC system [8]. The initial endoproteolytic steps (Fig. 18-6, steps 1–4) are mediated by PC1 and occur in all POMC-producing neurons and endocrine cells, usually in the numerical order shown. It is clear that steps 1 and 2 are initiated in the trans-Golgi network and continue in LDCVs, while step 4 occurs only in LDCVs. Steps 5–7 occur only in LDCVs and seem to require PC2. In the adult anterior pituitary, corticotropes contain PC1 but not PC2 and perform only cleavages 1–4. However, during early postnatal development, corticotropes also express PC2 and cleavages 5–7 are transiently seen in corticotropes. In the rat, expression of PC2 and cleavage within ACTH (cleavage 5) decline simultaneously a few weeks after birth, at about the time that the adult pattern of ACTH control over adrenal steroidogenesis appears.
Melanotropes and CNS neurons making POMC express both PC1 and PC2 and, thus, the smaller peptide products are seen in these cells. PAM is expressed in all POMC-producing cells, so the α-amidation of joining peptide (JP), a small peptide with no clear biological function, occurs rapidly in all POMC cells (Fig. 18-6). In the melanotropes of the intermediate pituitary and the POMC neurons of the nucleus of the solitary tract, α-N-acetylation of ACTH(1–13)NH2 and β-endorphin occurs. In melanotropes, α-N-acetylation of ACTH can occur before cleavage 5. As indicated in Figure 18-4, the particular cleavages made and the modifications made to the NH2- and COOH-termini of the peptide products determine the mixture of bioactive peptides released.
Neuropeptides are packaged into large dense core vesicles
In many cases, the peptide products from the processing of a propeptide are packaged together in an equimolar fashion in LDCVs and the peptides and the soluble processing enzymes (PC1, PHM, CPE) are all released together in response to stimuli (Fig. 18-7) [6–8]. By comparison, there are also examples where the products of propeptide processing are sorted into different LDCVs or are subject to degradation. In Aplysia bag cell neurons, ELH is formed from the COOH terminus of the pro-ELH precursor (Fig. 18-2), while α, β and γ bag cell peptides (BCPs) are formed from the NH2-terminal portion (Fig. 18-7). The initial cleavage of the pro-ELH precursor occurs in the trans-Golgi network and the peptides are then separated into two distinct types of LDCV, which are sent to different parts of the cell (Fig. 18-7). These two sets of peptides mediate a coordinated set of behaviors involved in egg laying. Similarly, in TRH neurons, the NH2 and COOH-terminal domains of the pro-TRH precursor are separated from each other and stored in distinct LDCVs.
Diversity is generated by families of propeptides, alternative splicing, proteolytic processing and post-translational modifications
The huge number of biologically active peptides is the result of many factors. First, there are several families of genes which clearly evolved from a common ancestor (Fig. 18-1): examples include the three precursors to β-endorphin, dynorphin and the enkephalins; the precursors to gastrin and CCK; and the precursors to oxytocin and vasopressin. Second, there are several peptide precursors which yield multiple copies of bioactive peptide: examples include the pro-ELH precursor, the α-mating factor precursor, the FMRF-NH2 precursor and the enkephalin precursor. Likewise, several distinct biological activities are found within the POMC and ELH precursors. Third, there is alternative splicing of mRNAs encoding preprohormones, first discovered in the calcitonin and calcitonin generelated peptide precursors but also seen in the case of the preprotachykinin precursor, which yields substance P, substance K and several other peptides, depending on the splicing pattern (Fig. 18-8) [17]. Finally, RNA editing can be involved, as in the case of the amphibian bombesin-like peptides, where nucleotides in the mRNA are changed and the final protein is not a direct reflection of the sequence encoded in the gene. RNA editing is also seen in the glutamate and serotonin receptors (Chaps. 13 and 15) and probably will be found elsewhere as detection methods become more sophisticated.
![Figure 18-8. Several mechanisms through which the substance P gene gives rise to different bioactive peptides in different neurons (adapted from [17]).](/books/NBK28247/bin/ch18f8.gif)
Figure 18-8
Several mechanisms through which the substance P gene gives rise to different bioactive peptides in different neurons (adapted from [17]). Alternative splicing of mRNA leads to translation of distinct precursors, and subsequent processing leads to unique (more...)
- Many neuropeptides were originally identified as pituitary or gastrointestinal hormones
- Peptides can be grouped by structural and functional similarity
- The function of peptides as first messengers is evolutionarily very old
- Various techniques are used to identify additional neuropeptides
- The neuropeptides exhibit a few key differences from the classical neurotransmitters
- Neuropeptides are often found in neurons with conventional neurotransmitters
- The biosynthesis of neuropeptides is fundamentally different from that of conventional neurotransmitters
- Many of the enzymes involved in peptide biogenesis have been identified
- Neuropeptides are packaged into large dense core vesicles
- Diversity is generated by families of propeptides, alternative splicing, proteolytic processing and post-translational modifications
- The Neuropeptides - Basic NeurochemistryThe Neuropeptides - Basic Neurochemistry
Your browsing activity is empty.
Activity recording is turned off.
See more...