By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Siegel GJ, Agranoff BW, Albers RW, et al., editors. Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition. Philadelphia: Lippincott-Raven; 1999.

Basic Neurochemistry: Molecular, Cellular and Medical Aspects. 6th edition.
Show detailsThe basal ganglia consist of several large, anatomically distinct masses of gray matter situated in the core of the cerebral hemispheres among ascending and descending tracts of white matter and astride the brainstem. These constitute the striatum, comprised of the caudate and putamen, and the pallidum, comprised of the internal and external portions of the globus pallidus. The striatopallidal system is a complex, integrated unit that has many afferent and efferent connections to other parts of the brain and, with several other subcortical structures, constitutes an extrapyramidal system regulating sensorimotor activity, including tone and posture. It is also a part of a frontal cortical—subcortical system implicated in schizophrenia (Chap. 51). The neurotransmitters present in various regions of the brain have been identified, the enzymes involved in the biosynthesis and degradation of many of these transmitters have been described and the presence of uptake sites for inactivation of transmitters as well as receptors mediating their effects on neuronal activity has been demonstrated. To better understand the functional role of the relevant neurotransmitters and neuromodulators, it is important to consider also the neuronal circuits and connections of the basal ganglia.
Neurotransmitter systems involving catecholamines, serotonin and GABA have been identified in the basal ganglia
A number of important techniques for precise cellular localization of neurotransmitters, enzymes involved in their synthesis or degradation, receptors and their subtypes and transporters have been applied to studies of the various neuronal pathways projecting to or arising from the basal ganglia. Catecholamines (CAs), including dopamine (DA), norepinephrine (NE) and epinephrine, as well as serotonin (5-HT), can be converted to fluorescent derivatives, which can then be used to delineate their distribution in neurons and their processes. Antibodies to neurotransmitters such as GABA and peptides, as well as against specific proteins, are useful for their immunohistological demonstration in brain. Radiolabeled neurotransmitters and ligands or blockers of transporters and receptors have been used for autoradiographic localization of uptake sites and receptor subtypes. The application of in situ hybridization using cDNA probes for mRNAs that encode peptide precursors, receptors, transporters, biosynthetic enzymes and specific neuronal proteins has provided a wealth of information regarding neuronal pathways throughout the brain, including the basal ganglia. These techniques provide quantitative as well as anatomical information, and together with biochemical assays, neurophysiologically monitored responses and behavioral studies have enormously enhanced our understanding of the basal ganglia, associated structures and the involved neurotransmitters (Fig. 45-1).
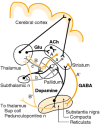
Figure 45-1
The direct pathway contains two inhibitory GABAergic synapses: A or A' (dark orange) is between the striatum and the medial pallidum, or the substantia nigra pars reticulata (SNr); B or B' (light orange) is between the medial pallidum or SNr and the ventroanterior (more...)
The basal ganglia are heterogeneous structures
In the striatum, differences in the intensity of staining with several neuronal markers show that the populations of cells in various regions are not uniform. Compartmentalization was first suggested by differences in the intensity of staining for acetylcholinesterase (AChE). Whereas most of the striatum stains heavily for this enzyme, AChE-poor islands, termed striasomes or patches, constitute 10 to 20% of the striatum. The striasomes constitute a labyrinthine mesh embedded in a matrix of AChE-rich striatal tissue. In addition to differences in AChE content, these areas have been found to differ quantitatively from the remainder of the striatum with respect to the concentration of several other substances (see below); furthermore, the neurons in the striasomes appear to receive distinct cortical input and to project differently from those in the matrix [1,2].
Excitatory amino acids provide afferent input to the basal ganglia
Glutamate and aspartate are excitatory amino acid neurotransmitters present in many neurons throughout the brain (Chap. 15). They are involved in basal ganglia afferent, connecting and efferent pathways, which together with thalamic and subthalamic nuclei constitute feedback circuits that modulate cerebral cortical function. The major afferent neuronal pathways to the striatum are from the sensorimotor and other cortical areas and from the thalamus; these fibers release excitatory amino acids, mostly glutamate, in synapses with dendrites on striatal neurons. Glutamate is also the excitatory neurotransmitter released from terminals of the subthalamic neurons projecting to the pallidum, particularly to the internal layer of the globus pallidus, and from the axon terminals of thalamic neurons that send fibers mainly to the cortex.
In the striatum, release of the excitatory transmitters is modulated by presynaptic receptors for acetylcholine (ACh), DA, GABA, opioids and adenosine. Also present are many peptides and other neuromodulators (Table 45-1), which may have modulatory effects similar to those of the cotransmitters. Nitric oxide (NO) is a prime example of a neuromodulator (see Chap. 10). It is a small, gaseous, lipophilic molecular mediator, which readily diffuses across cellular membranes. Thus, it can elicit both anterograde and retrograde signaling at the synapses. In addition, it plays a vital role in N-methyl-d-aspartate (NMDA)-mediated transmission. NO is synthesized from l-arginine by a family of NO synthases (NOS). NOS is found in both glia and neurons, particularly in the medium aspiny neurons of the striatum and other brain regions.
Table 45-1
Peptides and Neuromodulators Found in the Basal Ganglia.
Ionotropic and metabotropic excitatory amino acid receptors are localized on striatal neurons
The excitatory amino acids glutamate and aspartate are unevenly distributed in the basal ganglia thalamocortical circuits (Fig. 45-2A). The receptors that respond to excitatory amino acids, particularly to glutamate, may be ionotropic or metabotropic (see Chap. 15). There are at least three types of ionotropic glutamate receptors that can be distinguished in the striatum by their responses to selective agonists. These are NMDA, kainate (KA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA). It is believed that NMDA receptors reside mainly on the GABAergic efferent neurons of the striatonigral pathway. NMDA receptors are associated with ligand-gated Ca2+ channels, whereas KA and AMPA receptors are associated with ligand-gated K+/Na+ channels. Since decortication of rats does not diminish significantly the binding of radiolabeled NMDA, KA or AMPA in the striatum whereas destruction of striatal neurons with the excitotoxin quinolinic acid does strikingly decrease binding of these ligands, it appears that most of these receptors are postjunctional. Four subtypes of excitatory amino acid metabotropic glutamate receptors have also been identified in the striatum, mGluR1—mGluR4.
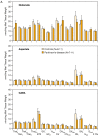
Figure 45-2
Concentrations in the basal ganglia of control and Parkinson's disease human brains. A: Glutamate, aspartate and GABA. Values are presented as mean ± SEM. Thala, anterior thalamus; Thalvl, ventral lateral thalamus; Thalva, ventral anterior thalamus; (more...)
GABA is the neurotransmitter of most striatal efferent neurons
High concentrations of GABA are found in the substantia nigra, globus pallidus and subthalamic nucleus (Fig. 45-2). The striatum is reputed to contain a population of GABAergic aspiny interneurons. In addition, a vast number of medium spiny neurons release GABA as a neurotransmitter. This was first demonstrated by use of an antibody specific for the enzyme responsible for GABA formation, glutamic acid decarboxylase (GAD) and, later, with antibodies to GABA (Chap. 16). Antibodies to peptide neurotransmitters or probes for mRNA encoding the peptides or their precursors have been used to demonstrate colocalization of these peptides in subpopulations of GABAergic neurons. Some peptides that have been found in the striatum are listed in Table 45-1. GABAergic efferent neurons of the striatum project to the external and internal layers of the pallidum and to the substantia nigra; the latter constitute the striatonigral path (Fig. 45-1). Those that project to the external layer of the globus pallidus often contain enkephalin as a cotransmitter. They synapse on GABAergic neurons which project from the external layer of the globus pallidus to the glutamatergic neurons in the subthalamic nucleus; these form the indirect pathway (Fig. 45-1). The GABAergic striatal neurons that project to the internal layer of the globus pallidus and substantia nigra pars reticulata often contain substance P and/or dynorphin; these form the direct pathway (Fig. 45-1). GABAergic neurons in the striasomes appear to project to the substantia nigra pars compacta. Many of these neurons contain neurotensin; tachykinins, including substance P or K; and/or dynorphin.
GABA receptors are abundant in the basal ganglia, hypothalamus, hippocampus and dorsal horn of the spinal cord. Two GABA receptor subtypes have been characterized, GABAA and GABAB (see Chap. 16). The postsynaptic GABAA receptor executes the inhibitory effects of the neurotransmitter, primarily by altering the membrane permeability to chloride ions. In contrast, the presynaptic GABAB receptor modulates the release of GABA.
Acetylcholine is the neurotransmitter of striatal interneurons
ACh is the neurotransmitter contained in many of the large spiny neurons that make up about 5% of the striatal neurons. These neurons, which are distinguished by immunoreactivity to choline acetyltransferase (ChAT), the enzyme responsible for synthesis of ACh, receive several types of synaptic input: (i) glutamatergic fibers from the cerebral cortex; (ii) tyrosine hydroxylase (TH)-immunoreactive, presumably dopaminergic, fibers which form small, symmetrical synapses on the soma and proximal dendrites; and (iii) substance P/GAD-immunopositive boutons, believed to be axon collaterals from medium spiny neurons. These cholinergic interneurons have relatively short axons that are confined to the striatum and that innervate predominantly the medium spiny neurons.
Both muscarinic and nicotinic cholinergic receptors are found in the striatum (see Chap. 11). Muscarinic receptors on glutamatergic terminals are thought to inhibit release of the excitatory transmitter, acting as a modulator of glutamatergic stimulation of striatal neurons, whereas nicotinic receptor activation enhances transmitter release.
Dopamine is the neurotransmitter of the nigrostriatal pathway
DA is widely distributed throughout the brain, but, as shown in Figure 45-2, particularly high concentrations are found in the striatal areas [3]. Indeed, although DA accounts for about half of the total CAs in the brain, over 80% of DA in the brain is in the basal ganglia and its concentration in the striatum is much greater than that of NE. Such differences in distribution were the first indication of its role as a neurotransmitter. Histofluorescence techniques for visualizing DA and immunohistological localization of TH, the rate-limiting enzyme for its biosynthesis, have been used to map DA-containing neurons and the pathways of their axons to the terminal arborizations at their target regions (Chap. 12).
The principal dopaminergic fiber systems in brain are the nigrostriatal, from the substantia nigra pars compacta to the caudate nucleus and putamen; the mesolimbic, from the ventral tegmental area to the nucleus accumbens; the mesocortical, from the ventral tegmental area to the cerebral cortex; and the tuberoinfundibular, from the arcuate nucleus to the median eminence. There are also DA-containing neurons and terminals in other brain regions, in the retina and in the spinal cord. These neurons store DA in vesicles within varicosities of the nerve terminals. Intravesicular concentrations of DA are maintained at high concentrations, 1 to 20 mg/g fresh weight.
DA in the striatum is contained in a dense aborization of the fine terminals derived from the DA-containing neurons of the substantia nigra pars compacta. These structures contain TH so that DA can be synthesized directly at the terminal varicosities. TH requires iron and tetrahydropteridine in order to oxidize tyrosine to 3,4-dihydroxyphenylalanine (levodopa, l-DOPA) (Chap. 12). Only small amounts of l-DOPA are found in the tissue; however, it is readily decarboxylated by aromatic amino acid decarboxylase (AADC). This enzyme is present in many tissues, including serotonergic neurons, where it decarboxylates 5-hydroxytryptophan (5-HTP) to form serotonin (see Chap. 13). Like other amino acid decarboxylases, AADC requires pyridoxyl phosphate as its coenzyme. AADC is inhibited by many compounds, a few of which have been used therapeutically. The chemical structures of some decarboxylase inhibitors are shown in Figure 45-3. DA synthesis in the nerve terminals is accelerated during depolarization-induced release of the neurotransmitter. This is the result of rapid activation of TH. Enhanced release of DA results in elevated levels of its metabolites in the tissues and in the surrounding fluids.
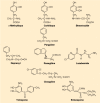
Figure 45-3
Inhibitors of enzymes involved in the metabolism of levodopa and of dopamine. Aromatic amino acid decarboxylase (AADC) is inhibited by α-methyldopa, carbidopa and benserazide. Pargyline inhibits both monoamine oxidase (MAO) subtypes, whereas deprenyl, (more...)
Both D1 and D2 receptors have been defined pharmacologically; thus, there are specific ligands for these receptors (see Chap. 12 for receptor classification). D1 receptors contain D1 and D5 receptor gene products and D2 receptors are comprised of D2, D3 and D4 gene products. However, the more recent identification of D3, D4 and D5 is chiefly based on findings from molecular genetic studies; therefore, the classical pharmacological nomenclature is still often adopted. D1 receptors are present on dynorphin/substance P-containing medium spiny neurons, and both D1 and D2 receptors are present on a subpopulation of striatal neurons. There are D2 receptors on the terminals of corticostriatal projections, on enkephalin-containing medium spiny neurons and cholinergic interneurons and, as autoreceptors, on dopaminergic neurons on the substantia nigra pars compacta.
There is considerable evidence that there are important functional distinctions and interactions between the D1 and D2 families of receptors. D1 and D5 receptors are excitatory; they stimulate adenylyl cyclase activity and, possibly, phosphoinositide hydrolysis. D2, D3 and D4 receptors are inhibitory, and they reduce adenylyl cyclase activity (see Chaps. 12 and 22). D1 and D2 receptors, which are both abundant in the striatum, are the principal DA receptors involved in the pathophysiology of Parkinson's disease. D1 is the main type of DA receptor on the striatal neurons of the direct pathway, while D2 is the main type in the indirect pathway (Fig. 45-1). Combined administration of D1 and D2 agonists appears to have synergistic biochemical and behavioral effects. Functional studies related to the basal ganglia demonstrate that, for the implementation of motor behavior, the synergistic action of both D1 and, mainly, D2 receptors is required.
Dopamine is primarily metabolized by O-methylation and oxidative deamination
The major metabolites of DA are products of O-methylation and/or oxidative deamination. Major products include 3-methoxytyramine (3-MT), 3,4-dihydroxyphenylacetic acid (DOPAC) and 4-hydroxy-3-methoxyphenylacetic acid, or homovanillic acid (HVA) (Fig. 45-2). These are depicted in Figure 45-4, which also shows the alternative pathways of metabolism of DA. Some of the compounds shown in Figure 45-4 are conjugated with sulfate or glucuronate before excretion in the urine or bile.
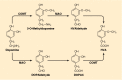
Figure 45-4
Dopamine and its major metabolites. Dopamine is sequentially deaminated and O-methylated or vice versa, depending on the site of metabolism (see text). MAO, monoamine oxidase; COMT, catechol-O-methyltransferase; HVA, homovanillic acid; DOPAC, 3,4-dihydroxyphenylacetic (more...)
The amine and its O-methyl derivative are both subject to the action of monoamine oxidase (MAO), a flavoprotein present in the outer membrane of the mitochondria. MAO exists in two forms: MAO type A (MAO-A) is especially sensitive to inhibition by clorgyline and is present in catecholaminergic neurons and their axons, whereas MAO type B (MAO-B) is inhibited selectively by deprenyl and predominates in serotonin-containing neurons and in astrocytes. Pargyline irreversibly inhibits both MAO-A and MAO-B. The chemical structures of these three propargylamine inhibitors are shown in Figure 45-3.
Products of the MAO reaction include the aldehyde corresponding to the amine substrate, hydrogen peroxide and ammonia. Most of the aldehyde undergoes further dehydrogenation to form, in the case of DA, DOPAC, which is the substrate for catechol-O-methyltransferase (COMT), an enzyme that catalyzes the transfer of the methyl group from S-adenosylmethionine to the m-hydroxyl group. This process appears to be very efficient in human brain because normally there are only very small quantities of DOPAC in the striatum but substantial amounts of HVA. A portion of the aldehyde undergoes reduction catalyzed by alcohol dehydrogenase or a similar enzyme, with the participation of nicotinamide coenzyme. The alcoholic products that are formed are far less abundant than the acidic metabolites of DA but are major metabolites of NE.
The black pigment neuromelanin, which accumulates in the neurons of the substantia nigra pars compacta with aging (see Chap. 30), is derived from polymerization of quinones formed from catechols, mostly l-DOPA and DA. The function, if any, of neuromelanin is unknown. It may be related to the vulnerability of these neurons to toxic damage or degenerative processes, including the release of iron from ferritin. These factors may relate to Parkinson's disease (see below).
The circuits of the basal ganglia are regulated by feedback systems and modulatory peptide cotransmitters
The two principal intrinsic basal ganglia circuits presently recognized consist of the direct and indirect pathways between the striatum and the thalamus, as depicted in Figure 45-1. The direct path is composed of GABAergic inhibitory fibers from the striatum to the internal or medial globus pallidus (GPm) and to the substantia nigra pars reticulata (SNr), the main efferent nuclei of the basal ganglia. From these nuclei, another set of GABAergic inhibitory fibers extends to the ventroanterior (VA) and ventrolateral (VL) nuclei of the thalamus. The thalamic VA and VL nuclei project excitatory glutamatergic fibers to the cortex, where these fibers modulate cortical outflows back to the striatum, to the spinal cord and elsewhere. DA fibers from the substantia nigra pars compacta (SNc) stimulate the direct path through D1 receptors on the GABAergic striatal neurons, which results in disinhibition of the thalamus and net activation of the cortex. The indirect path is formed of GABAergic inhibitory fibers from the striatum to the external or lateral globus pallidus (GPl), which extends GABAergic inhibitory fibers to the subthalamic nucleus (STN); the latter, in turn, projects excitatory glutamatergic fibers to the efferent stage, GPm and SNr. Thus, excitation of the indirect path would lead to inhibition of the thalamus. The interposition in the indirect circuit of additional excitatory and inhibitory synapses has the dual effect of delaying an impulse in the indirect relative to the direct circuit and, when activated, of producing net inhibition rather than stimulation of cortical neurons. However, there is a critical difference in the DA effects on these two routes. The indirect circuit is inhibited by SNc DA fibers through inhibitory D2 receptors, leading to disinhibition by these DA fibers as in the direct path. The striatal neurons are modulated normally by both excitatory and inhibitory transmitters and receptors. Loss of the nigrostriatal DA input from the SNc, as in Parkinson's disease (see below), results in complicated patterns of imbalance in these circuits and generally a greatly increased inhibitory striatal outflow through the GPm and SNr with net reductions in thalamocortical activation.
Cholinergic and substance P axons terminate on the proximal portions of the dendrites of the medium spiny neurons in the striatum. The extensive axon collaterals of these spiny neurons release GABA, and probably their peptide cotransmitters, into synapses near or on the cell bodies of other spiny neurons. Interneurons that release ACh also contain somatostatin and neuropeptide Y (NPY), which may modulate striatal neuron activity. Best known and perhaps most important, dopaminergic fibers from the SNc that synapse on the medium spiny neurons, as well as fibers that synapse on the more distal parts of their dendritic tree, modulate striatal efferent GABAergic output.
As indicated above, dopaminergic neurons project mainly from the SNc to innervate the striatum, and 80% of the DA content in the brain is found in the basal ganglia. DA plays a multifunctional role in the basal ganglia. It regulates cortically initiated basal ganglia—thalamic conduction. DA released in the striatum can act at any of several receptor sites to modulate outflow of GABAergic neuronal activity to the globus pallidus or substantia nigra. The complexity of interconnections via neuronal networks, feedback circuits and D1 and D2 receptors on the same cell defies simple analysis, but it is evident that the net effects of DA deficiency are responsible for the striking motor abnormalities seen in parkinsonian syndromes (Table 45-2). Conversely, conditions that cause overactivity of DA or the systems normally activated by DA result in an array of involuntary dyskinesias. These sometimes appear in association with the hypokinetic features of parkinsonism and are generally attributed to disorders of the basal ganglia and its output pathways. Thus, basal ganglia disorders are associated with parkinsonian hypokinetic, as well as a variety of hyperkinetic, conditions.
Table 45-2
Striatal Dopamine Concentration in Parkinson's Disease, Parkinsonian Syndromes and Other Neurodegenerative Disorders.
- Neurotransmitter systems involving catecholamines, serotonin and GABA have been identified in the basal ganglia
- The basal ganglia are heterogeneous structures
- Excitatory amino acids provide afferent input to the basal ganglia
- Ionotropic and metabotropic excitatory amino acid receptors are localized on striatal neurons
- GABA is the neurotransmitter of most striatal efferent neurons
- Acetylcholine is the neurotransmitter of striatal interneurons
- Dopamine is the neurotransmitter of the nigrostriatal pathway
- Dopamine is primarily metabolized by O-methylation and oxidative deamination
- The circuits of the basal ganglia are regulated by feedback systems and modulatory peptide cotransmitters
- Biochemical Anatomy of the Basal Ganglia and Associated Neural Systems - Basic N...Biochemical Anatomy of the Basal Ganglia and Associated Neural Systems - Basic Neurochemistry
Your browsing activity is empty.
Activity recording is turned off.
See more...