By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Janeway CA Jr, Travers P, Walport M, et al. Immunobiology: The Immune System in Health and Disease. 5th edition. New York: Garland Science; 2001.

Immunobiology: The Immune System in Health and Disease. 5th edition.
Show detailsThe surface immunoglobulin that serves as the B-cell antigen receptor (BCR) has two roles in B-cell activation. First, like the antigen receptor on T cells, it transmits signals directly to the cell's interior when it binds antigen (see Section 6-1). Second, the B-cell antigen receptor delivers the antigen to intracellular sites where it is degraded and returned to the B-cell surface as peptides bound to MHC class II molecules (see Chapter 5). The peptide:MHC class II complex can be recognized by antigen-specific armed helper T cells, stimulating them to make proteins that, in turn, cause the B cell to proliferate and its progeny to differentiate into antibody-secreting cells. Some microbial antigens can activate B cells directly in the absence of T-cell help. The ability of B cells to respond directly to these antigens provides a rapid response to many important bacterial pathogens. However, somatic hypermutation and switching to certain immunoglobulin isotypes depend on the interaction of antigen-stimulated B cells with helper T cells and other cells in the peripheral lymphoid organs. Antibodies induced by microbial antigens alone are therefore less variable and less functionally versatile than those induced with T-cell help.
9-1. The humoral immune response is initiated when B cells that bind antigen are signaled by helper T cells or by certain microbial antigens alone
It is a general rule in adaptive immunity that naive antigen-specific lymphocytes are difficult to activate by antigen alone. Naive T cells require a co-stimulatory signal from professional antigen-presenting cells; naive B cells require accessory signals that can come either from an armed helper T cell or, in some cases, directly from microbial constituents.
Antibody responses to protein antigens require antigen-specific T-cell help. B cells can receive help from armed helper T cells when antigen bound by surface immunoglobulin is internalized and returned to the cell surface as peptides bound to MHC class II molecules. Armed helper T cells that recognize the peptide:MHC complex then deliver activating signals to the B cell. Thus, protein antigens binding to B cells both provide a specific signal to the B cell by cross-linking its antigen receptors and allow the B cell to attract antigenspecific T-cell help. These antigens are unable to induce antibody responses in animals or humans who lack T cells, and they are therefore known as thymus-dependent or TD antigens (Fig. 9.2, top two panels).
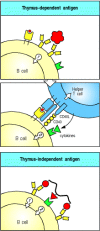
Figure 9.2
A second signal is required for B-cell activation by either thymus-dependent or thymus-independent antigens. The first signal required for B-cell activation is delivered through its antigen receptor (top panel). For thymus-dependent antigens, the second (more...)
The B-cell co-receptor complex of CD19:CD21:CD81 (see Section 6-8) can greatly enhance B-cell responsiveness to antigen. CD21 (also known as complement receptor 2, CR2) is a receptor for the complement fragment C3d (see Section 2-11). When mice are immunized with hen egg lysozyme coupled to three linked molecules of the complement fragment C3dg, the modified lysozyme induces antibody without added adjuvant at doses up to 10,000 times smaller than unmodified hen egg lysozyme. Whether binding of CD21 enhances B-cell responsiveness by increasing B-cell signaling, by inducing co-stimulatory molecules on the B cell, or by increasing the receptormediated uptake of antigen, is not yet known. As we will see later in this chapter, antibodies already bound to antigens can activate the complement system, thus coating the antigen with C3d and producing a more potent antigen, which in turn leads to more efficient B-cell activation and antibody production.
Although armed peptide-specific helper T cells are required for B-cell responses to protein antigens, many microbial constituents, such as bacterial polysaccharides, can induce antibody production in the absence of helper T cells. These microbial antigens are known as thymus-independent or TI antigens because they induce antibody responses in individuals who have no T lymphocytes. The second signal required to activate antibody production to TI antigens is either provided directly by recognition of a common microbial constituent (see Fig. 9.2, bottom panel) or by a nonthymus-derived accessory cell in conjunction with massive cross-linking of B-cell receptors, which would occur when a B cell binds repeating epitopes on the bacterial cell. Thymus-independent antibody responses provide some protection against extracellular bacteria, and we will return to them later.
9-2. Armed helper T cells activate B cells that recognize the same antigen
T-cell dependent antibody responses require the activation of B cells by helper T cells that respond to the same antigen; this is called linked recognition. This means that before B cells can be induced to make antibody to an infecting pathogen, a CD4 T cell specific for peptides from this pathogen must first be activated to produce the appropriate armed helper T cells. This presumably occurs by interaction with an antigen-presenting dendritic cell (see Section 8-1). Although the epitope recognized by the armed helper T cell must therefore be linked to that recognized by the B cell, the two cells need not recognize identical epitopes. Indeed, we saw in Chapter 5 that T cells can recognize internal peptides that are quite distinct from the surface epitopes on the same protein recognized by B cells. For more complex natural antigens, such as viruses, the T cell and the B cell might not even recognize the same protein. It is, however, crucial that the peptide recognized by the T cell be a physical part of the antigen recognized by the B cell, which can thus produce the appropriate peptide after internalization of the antigen bound to its B-cell receptors.
For example, by recognizing an epitope on a viral protein coat, a B cell can internalize a complete virus particle. After internalization, the virus particle is degraded and peptides from internal viral proteins as well as coat proteins can be displayed by MHC class II molecules on the B-cell surface. Helper T cells that have been primed earlier in an infection by macrophages or dendritic cells presenting these internal peptides can then activate the B cell to make antibodies that recognize the coat protein (Fig. 9.3).
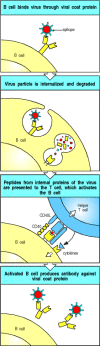
Figure 9.3
B cells and helper T cells must recognize epitopes of the same molecular complex in order to interact. An epitope on a viral coat protein is recognized by the surface immunoglobulin on a B cell and the virus is internalized and degraded. Peptides derived (more...)
The specific activation of the B cell by a T cell sensitized to the same antigen or pathogen depends on the ability of the antigen-specific B cell to concentrate the appropriate peptide on its surface MHC class II molecules. B cells that bind a particular antigen are up to 10,000 times more efficient at displaying peptide fragments of that antigen on their MHC class II molecules than are B cells that do not bind the antigen. Armed helper T cells will thus help only those B cells whose receptors bind an antigen containing the peptide they recognize.
The requirement for linked recognition has important consequences for the regulation and manipulation of the humoral immune response. One is that linked recognition helps ensure self tolerance, as will be described in Chapter 13. An important application of linked recognition is in the design of vaccines, such as that used to immunize infants against Haemophilus influenzae type B. This bacterial pathogen can infect the lining of the brain, called the meninges, causing meningitis and, in severe cases, neurological damage or death. Protective immunity to this pathogen is mediated by antibodies against its capsular polysaccharide. Although adults make very effective thymus-independent responses to these polysaccharide antigens, such responses are weak in the immature immune system of the infant. To make an effective vaccine for use in infants, therefore, the polysaccharide is linked chemically to tetanus toxoid, a foreign protein against which infants are routinely and successfully vaccinated (see Chapter 14). B cells that bind the polysaccharide component of the vaccine can be activated by helper T cells specific for peptides of the linked toxoid (Fig. 9.4).
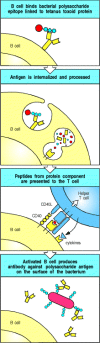
Figure 9.4
Protein antigens attached to polysaccharide antigens allow T cells to help polysaccharide-specific B cells. Haemophilus influenzae type B vaccine is a conjugate of bacterial polysaccharide and the tetanus toxoid protein. The B cell recognizes and binds (more...)
Linked recognition was originally discovered through studies of the production of antibodies to haptens (see Appendix I, Section A-1). Haptens are small chemical groups that cannot elicit antibody responses on their own because they cannot cross-link B-cell receptors and they cannot recruit T-cell help. When coupled at high density to a carrier protein, however, they become immunogenic, because the protein will carry multiple hapten groups that can now cross-link B-cell receptors. In addition, T-cell dependent responses are possible because T cells can be primed to peptides derived from the protein. Coupling of a hapten to a protein is responsible for the allergic responses shown by many people to the antibiotic penicillin, which reacts with host proteins to form a coupled hapten that can stimulate an antibody response, as we will learn in Chapter 12.
9-3. Antigenic peptides bound to self MHC class II molecules trigger armed helper T cells to make membrane-bound and secreted molecules that can activate a B cell
Armed helper T cells activate B cells when they recognize the appropriate peptide:MHC class II complex on the B-cell surface (Fig. 9.5). As with armed TH1 cells acting on macrophages, recognition of peptide:MHC class II complexes on B cells triggers armed helper T cells to synthesize both cellbound and secreted effector molecules that synergize in activating the B cell. One particularly important T-cell effector molecule is a membrane-bound molecule of the tumor necrosis factor (TNF) family known as CD40 ligand (CD40L, also known as CD154) because it binds to the B-cell surface molecule CD40. CD40 is a member of the TNF-receptor family of cytokine receptors (see Section 8-20) however, it does not contain a ‘death domain.’ It is involved in directing all phases of the B-cell response. Binding of CD40 by CD40L helps to drive the resting B cell into the cell cycle and is essential for B-cell responses to thymus-dependent antigens.
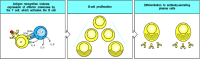
Figure 9.5
Armed helper T cells stimulate the proliferation and then the differentiation of antigen-binding B cells. The specific interaction of an antigen-binding B cell with an armed helper T cell leads to the expression of the B-cell stimulatory molecule CD40 (more...)
B cells are stimulated to proliferate in vitro when they are exposed to a mixture of artificially synthesized CD40L and the cytokine interleukin-4 (IL-4). IL-4 is also made by armed TH2 cells when they recognize their specific ligand on the B-cell surface, and IL-4 and CD40L are thought to synergize in driving the clonal expansion that precedes antibody production in vivo. IL-4 is secreted in a polar fashion by the TH2 cell and is directed at the site of contact with the B cell (Fig. 9.6) so that it acts selectively on the antigen-specific target B cell.The combination of B-cell receptor and CD40 ligation, along with IL-4 and other signals derived from direct T-cell contact, leads to B-cell proliferation. Some of these contact signals have recently been elucidated. They involve other TNF/TNF-receptor family members, including CD30 and CD30 ligand and BLyS (B lymphocyte stimulator) and its receptor on B cells, TACI. After several rounds of proliferation, B cells can further differentiate into antibody-secreting plasma cells. Two additional cytokines, IL-5 and IL-6, both secreted by helper T cells, contribute to these later stages of B-cell activation.

Figure 9.6
When an armed helper T cell encounters an antigen-binding B cell, it becomes polarized and secretes IL-4 and other cytokines at the point of cell-cell contact. On binding antigen on the B cell through its T-cell receptor, the helper T cell is induced (more...)
9-4. Isotype switching requires expression of CD40L by the helper T cell and is directed by cytokines
Antibodies are remarkable not only for the diversity of their antigen-binding sites but also for their versatility as effector molecules. The specificity of an antibody response is determined by the antigen-binding site, which consists of the two variable V domains, VH and VL; however, the effector action of the antibody is determined by the isotype of its heavy-chain C region (see Section 4-15). A given heavy-chain V domain can become associated with the C region of any isotype through the process of isotype switching (see Section 4-16). We will see later in this chapter how antibodies of each isotype contribute to the elimination of pathogens. The DNA rearrangements that underlie isotype switching and confer this functional diversity on the humoral immune response are directed by cytokines, especially those released by armed effector CD4 T cells.
All naive B cells express cell-surface IgM and IgD, yet IgM makes up less than 10% of the immunoglobulin found in plasma, where the most abundant isotype is IgG. Much of the antibody in plasma has therefore been produced by B cells that have undergone isotype switching. Little IgD antibody is produced at any time, so the early stages of the antibody response are dominated by IgM antibodies. Later, IgG and IgA are the predominant isotypes, with IgE contributing a small but biologically important part of the response. The overall predominance of IgG results, in part, from its longer lifetime in the plasma (see Fig. 4.16).
Isotype switching does not occur in individuals who lack functional CD40L, which
is necessary for productive interactions between B cells and helper T cells;
such individuals make only small amounts of IgM antibodies in response to
thymus-dependent antigens and have abnormally high levels of IgM ( Hyper IgM Immunodeficiency, in
Case Studies in Immunology, see Preface for details) in their
plasma. These IgM antibodies may be induced by thymus-independent antigens
expressed by the pathogens that chronically infect these patients, who suffer
from severe humoral immunodeficiency, as we will see in Chapter 11.
Hyper IgM Immunodeficiency, in
Case Studies in Immunology, see Preface for details) in their
plasma. These IgM antibodies may be induced by thymus-independent antigens
expressed by the pathogens that chronically infect these patients, who suffer
from severe humoral immunodeficiency, as we will see in Chapter 11.
Most of what is known about the regulation of isotype switching by helper T cells has come from experiments in which mouse B cells are stimulated with bacterial lipopolysaccharide (LPS) and purified cytokines in vitro. These experiments show that different cytokines preferentially induce switching to different isotypes. Some of these cytokines are the same as those that drive B-cell proliferation in the initiation of a B-cell response. In the mouse, IL-4 preferentially induces switching to IgG1 and IgE, whereas transforming growth factor (TGF)-β induces switching to IgG2b and IgA. TH2 cells make both of these cytokines as well as IL-5, which induces IgA secretion by cells that have already undergone switching. Although TH1 cells are relatively poor initiators of antibody responses, they participate in isotype switching by releasing interferon (IFN)-γ, which preferentially induces switching to IgG2a and IgG3. The role of cytokines in directing B cells to make the different antibody isotypes is summarized in Fig. 9.7.
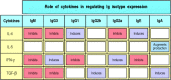
Figure 9.7
Different cytokines induce switching to different isotypes. The individual cytokines induce (violet) or inhibit (red) production of certain isotypes. Much of the inhibitory effect is probably the result of directed switching to a different isotype. These (more...)
Cytokines induce isotype switching by stimulating the formation and splicing of mRNA transcribed from the switch recombination sites that lie 5′ to each heavy-chain C gene (see Fig. 4.20). When activated B cells are exposed to IL-4, for example, transcription from a site upstream of the switch regions of Cγ1 and Cε can be detected a day or two before switching occurs (Fig. 9.8). Recent data suggest that the production of a spliced switch transcript has a role in directing switching, but the mechanism is not yet clear. Each of the cytokines that induces switching seems to induce transcription from the switch regions of two different heavy-chain C genes, promoting specific recombination to one or other of these genes only. Such a directed mechanism is supported by the observation that individual B cells frequently undergo switching to the same C gene on both chromosomes, even though the antibody heavy chain is only being expressed from one of the chromosomes. Thus, helper T cells regulate both the production of antibody by B cells and the isotype that determines the effector function of the antibody.
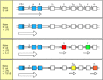
Figure 9.8
Isotype switching is preceded by transcriptional activation of heavy-chain C-region genes. Resting naive B cells transcribe the μ and δ genes at a low rate, giving rise to surface IgM and IgD. Bacterial lipopolysaccharide (LPS), which (more...)
9-5. Antigen-binding B cells are trapped in the T-cell zone of secondary lymphoid tissues and are activated by encounter with armed helper T cells
One of the most puzzling features of the antibody response is how an antigenspecific B cell manages to encounter a helper T cell with an appropriate antigen specificity. This question arises because the frequency of naive lymphocytes specific for any given antigen is estimated to be between 1 in 10,000 and 1 in 1,000,000. Thus, the chance of an encounter between a T lymphocyte and a B lymphocyte that recognize the same antigen should be between 1 in 108 and 1 in 1012. Achieving such an encounter is a far more difficult challenge than getting effector T cells activated, because, in the latter case, only one of the two cells involved has specific receptors. Moreover, T cells and B cells mostly occupy quite distinct zones in peripheral lymphoid tissue (see Fig. 1.8). As in naive T-cell activation (see Chapter 8), the answer seems to lie in the antigen-specific trapping of migrating lymphocytes.
When an antigen is introduced into an animal, it is captured and processed by professional antigen-presenting cells, especially the dendritic cells that migrate from the tissues into the T-cell zones of local lymph nodes. Recirculating naive T cells pass by such cells continuously and those rare T cells whose receptors bind peptides derived from the antigen are trapped very efficiently. This trapping clearly involves the specific antigen receptor on the T cell, although it is stabilized by the activation of adhesion molecules and chemokines as we learned in Sections 8-3 and 8-4. Ingenious experiments using mice transgenic for rearranged immunoglobulin genes show that, in the presence of the appropriate antigen, B cells with antigen-specific receptors are also trapped in the T-cell zones of lymphoid tissue by a similar mechanism. On encountering antigen, migrating antigen-binding B cells are arrested by the activation of adhesion molecules and the engagement of chemokine receptors such as CCR7, a receptor for MIP-3β and SLC.
Trapping of B cells in the T-cell zones provides an elegant solution to the problem posed at the beginning of this section. T cells are themselves trapped and activated to helper status in the T-cell zones, and when B cells migrate into lymphoid tissue through high endothelial venules they first enter these same T-cell zones. Most of the B cells move quickly through the T-cell zone into the B-cell zone (the primary follicle), but those B cells that have bound antigen are trapped. Thus, antigen-binding B cells are selectively trapped in precisely the correct location to maximize the chance of encountering a helper T cell that can activate them. Interaction with armed helper T cells activates the B cell to establish a primary focus of clonal expansion (Fig. 9.9). Here, at the border between T-cell and B-cell zones, both types of lymphocyte will proliferate for several days to constitute the first phase of the primary humoral immune response.
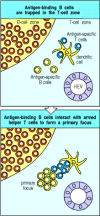
Figure 9.9
Antigen-binding cells are trapped in the T-cell zone. Upon entry into lymphoid tissues through a high endothelial venule (HEV), T cells and B cells home to different regions, as described in Chapter 7. Antigen-specific T cells remain in the T-cell zone (more...)
After several days, the primary focus of proliferation begins to involute. Many of the lymphocytes comprising the focus undergo apoptosis. However, some of the proliferating B cells differentiate into antibodysynthesizing plasma cells and migrate to the red pulp of the spleen or the medullary cords of the lymph node. The differentiation of a B cell into a plasma cell is accompanied by many morphological changes that reflect its commitment to the production of large amounts of secreted antibody. The properties of resting B cells and plasma cells are compared in Fig. 9.10. Plasma cells have abundant cytoplasm dominated by multiple layers of rough endoplasmic reticulum (see Fig. 1.19). The nucleus shows a characteristic pattern of peripheral chromatin condensation, a prominent perinuclear Golgi apparatus is visible, and the cisternae of the endoplasmic reticulum are rich in immunoglobulin, which makes up 10–20% of all the protein synthesized. MHC class II molecules are not expressed, so plasma cells can no longer present antigen to helper T cells, although these T cells may still provide important signals for plasma cell differentiation and survival, like IL-6 and CD40L. Surface immunoglobulin is still expressed on plasma cells at low levels, and recent evidence suggests that the survival of plasma cells may be determined in part by their ability to continue to bind antigen. Plasma cells have a range of life-spans. Some survive for only days to a few weeks after their final differentiation, whereas others are very long-lived and account for the persistence of antibody responses.

Figure 9.10
Plasma cells secrete antibody at a high rate but can no longer respond to antigen or helper T cells. Resting naive B cells carry surface immunoglobulin (usually IgM and IgD) and MHC class II molecules on their surface. Their V genes do not carry somatic (more...)
9-6. The second phase of the primary B-cell immune response occurs when activated B cells migrate to follicles and proliferate to form germinal centers
There is another fate for some of the B cells and T cells that proliferate in the primary focus. Some of these cells migrate into a primary lymphoid follicle (Fig. 9.11) where they continue to proliferate and ultimately form a germinal center (Fig. 9.12). Germinal centers are composed mainly of proliferating B cells, but antigen-specific T cells make up about 10% of germinal center lymphocytes and provide indispensable help to the B cells. The germinal center is essentially an island of cell division that sets up amidst a sea of resting B cells in the primary follicles; germinal center B cells displace the resting B cells toward the periphery of the follicle, forming a mantle zone of resting cells around the center. Primary follicles contain resting B cells clustered around a dense network of processes extending from a specialized cell type, the follicular dendritic cell (FDC). Follicular dendritic cells attract both naive and activated B cells into the follicles by secreting the chemokine BLC (see Section 7-30).

Figure 9.11
Activated B cells form germinal centers in lymphoid follicles. Some B cells activated in the primary focus migrate to form a germinal center within a primary follicle. Germinal centers are sites of rapid B-cell proliferation and differentiation. Follicles in (more...)

Figure 9.12
Germinal centers are formed when activated B cells enter lymphoid follicles. The germinal center is a specialized microenvironment in which B-cell proliferation, somatic hypermutation, and selection for antigen binding all occur. Rapidly proliferating (more...)
The early events in the primary focus lead to the prompt secretion of specific antibody that serves as immediate protection to the infected individual. The germinal center reaction, on the other hand, provides for a more effective later response, should the pathogen establish a chronic infection or the host become reinfected. To this end, B cells undergo a number of important modifications in the germinal center These include somatic hypermutation (see Chapter 4), which alters the V regions of B cells, affinity maturation, which selects for survival of B cells with high affinity for the antigen, and isotype switching (see Sections 9-4 and 4-16), which allows these selected B cells to express a variety of effector functions in the form of antibodies of different isotypes. The selected B cells will either differentiate into memory B cells, the function of which will be described in Chapter 10, or into plasma cells, which will begin to secrete higher-affinity and isotype-switched antibody during the latter part of the primary immune response.
The germinal center is a site of intense cell proliferation, with B cells dividing every 6 to 8 hours. Initially, these rapidly proliferating B cells dramatically reduce their expression of surface immunoglobulin, particularly of IgD. These B cells are termed centroblasts. As time goes on, some B cells reduce their rate of division and begin to express higher levels of surface immunoglobulin. These are termed centrocytes. The centroblasts at first proliferate in the dark zone of the germinal center (see Fig. 9.12), so called because the proliferating cells are densely packed. With further development, B cells begin to fill the light zone of the germinal center, an area of the follicle that is more richly supplied with follicular dendritic cells and less densely packed with cells. It was thought originally that only the centroblasts in the dark zone proliferated, whereas centrocytes in the light zone did not divide. Indeed, this may be the case in chronic germinal centers found in inflamed tonsils that have been surgically removed. However, in newly forming germinal centers in mice, it is now apparent that proliferation can occur in both light and dark zones, and that proliferative cells in the dark zone can express moderate amounts of immunoglobulin on their surface. So the distinction between dark and light zones as areas of B-cell proliferation or quiescence does not strictly apply to primary germinal centers, at least in mice. Follicular dendritic cells, which originally were most prominent in the light zone, appear to react to germinal center formation and begin to extend more prominently throughout the germinal center as it develops. The result is that a mature germinal center at day 15 after immunization more resembles a light zone, with few of the classic dark zone characteristics. This view of germinal center evolution may help to explain how B cells with high affinity for immunizing antigen are selected, as we now discuss.
9-7. Germinal center B cells undergo V-region somatic hypermutation and cells with mutations that improve affinity for antigen are selected
The process of somatic hypermutation, as one of the four mechanisms that create immunoglobulin diversity, was described in Chapter 4. Here we describe the signals that initiate hypermutation and the biological consequences of mutation for those cells. Somatic hypermutation is normally restricted to B cells that are proliferating in germinal centers. This was first shown by FACS sorting of germinal center B cells (see Appendix I, Section A-22) and sequencing of the V genes of cell lines derived from them; later, it was shown more directly by sequencing the V genes that were amplified by PCR of DNA isolated from germinal center B cells that had been micro-dissected from histologic sections. However, in vitro studies have shown that B cells can be induced to undergo hypermutation outside of germinal centers when their B-cell receptors are cross-linked and they receive help, including cytokines and CD40L stimulation, from activated T cells. In fact, mice that lack germinal centers owing to a mutation in the lymphotoxin-α gene (see Section 7-30) still support B-cell hypermutation, although where this takes place is unknown.
Unlike the other mechanisms of immunoglobulin diversification (see Section 4-6), which generate B cells with radically differing B-cell receptors, somatic hypermutation has the potential to create a series of related B cells that differ subtly in their specificity and affinity for antigen. This is because somatic hypermutation generally involves individual point mutations that change only a single amino acid. Immunoglobulin V-region genes accumulate mutations at a rate of about one base pair change per 103 base pairs per cell division. The mutation rates of all other somatic cell DNA are much lower: around one base pair change per 1010 base pairs per cell division. As each of the expressed heavy- and light-chain V-region genes is encoded by about 360 base pairs, and about three out of every four base changes results in an altered amino acid, every second B cell will acquire a mutation in its receptor at each division. These mutations also affect some DNA flanking the rearranged V gene but they generally do not extend into the C-region exons. Thus, random point mutations are somehow targeted to the rearranged V genes in a B cell.
The point mutations accumulate in a stepwise manner as B-cell clones expand in the germinal center. Generally, a B cell will not acquire more than one or two new mutations in each generation. Mutations can affect the ability of a B cell to bind antigen and thus will affect the fate of the B cell in the germinal center, as diagrammed in Fig. 9.13. Most mutations have a negative impact on the ability of the B-cell receptor to bind the original antigen. For example, some mutations will abolish receptor function altogether by introducing a stop codon that prevents proper translation; other deleterious mutations alter framework region amino acids that are essential for correct immunoglobulin folding; and still others alter amino acids in the complementarity-determining regions that are responsible for contacting antigen. These deleterious mutations are disastrous for the cells that harbor them; these cells are eliminated by apoptosis either because they can no longer make a B-cell receptor or because they cannot compete with sibling cells that bind antigen more strongly. Deleterious mutation is evidently a frequent event, as germinal centers are filled with apoptotic B cells that are quickly engulfed by macrophages, resulting in tingible body macrophages, which contain dark-staining nuclear debris in their cytoplasm and are a longrecognized histologic feature of germinal centers.
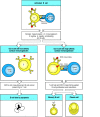
Figure 9.13
After T-cell-dependent activation, B cells undergo rounds of mutation and selection for higher-affinity mutants in the germinal center, ultimately resulting in high-affinity memory B cells and antibody secreted from plasma cells. B cells are first activated (more...)
More rarely, mutations will improve the affinity of a B-cell receptor for antigen. Cells that harbor these mutations are efficiently selected and expanded. Whether this is due to prevention of cell death and/or enhancement of cell division is still unclear. In either case, it is clear that selection is incremental. After each round of mutation, B cells begin to express the new receptor, and it determines the cell's fate, whether favorable or unfavorable. If favorable, the cell undergoes another round of division and mutation and the expression and selection process is repeated. In this way, the affinity and specificity of positively selected B cells is continually refined during the germinal center response. The fact that both centroblasts and centrocytes proliferate and can express immunoglobulin explains how mutation and positive selection can take place simultaneously throughout the germinal center without the need for migration back and forth between the dark and light zones. Evidence of positive and negative selection is seen in the pattern of somatic hyper-mutations in V regions of B cells that have survived passage through the germinal center (see Section 4-9). The existence of negative selection is shown by the relative scarcity of amino acid replacements in the framework regions, reflecting the loss of cells that had mutated any one of the many residues that are critical for immunoglobulin V-region folding. Negative selection is an important force in the germinal center, most likely eliminating about one in every two cells. Were it not for substantial negative selection, B cells dividing three to four times per day in a single germinal center would quickly create enough progeny to overwhelm the entire organism; more than a billion cells could be created in 10 days in a single germinal center. Instead, a germinal center actually contains a few thousand B cells at its peak.
The mark of positive selection, on the other hand, is an accumulation of numerous amino acid replacements in the complementarity-determining regions (see Fig. 4.9). The consequence of these cycles of proliferation, mutation, and selection, which all happen within the germinal center, is that the average affinity of the population of responding B cells for its antigen increases over time, largely explaining the observed phenomenon of affinity maturation of the antibody response. The selection process can be quite stringent: although 50 to 100 B cells may seed the germinal center, most of these leave no progeny, and by the time the germinal center reaches maximum size, it is typically composed of the descendants of only one or a few B cells.
9-8. Ligation of the B-cell receptor and CD40, together with direct contact with T cells, are all required to sustain germinal center B cells
Germinal center B cells are inherently prone to die and, in order to survive, they must receive specific signals. It was originally discovered in vitro that germinal center B cells could be kept alive by simultaneously cross-linking their B-cell receptors and ligating their cell-surface CD40. In vivo, these signals are delivered by antigen and T cells, respectively. Additional signals are also required for survival, which are delivered by direct contact with T cells. The nature of these signals is still obscure, but one signaling system involving the TNF-family member BLyS (the T-cell signal) and TACI (its receptor on B cells) has recently been found to be essential for the maintenance of germinal centers.
The source of antigen in the germinal center has been the matter of some controversy. Antigen can be trapped and stored for long periods of time in the form of immune complexes on follicular dendritic cells (Figs 9.14 and 9.15) and it was therefore assumed that this was the antigen that sustained germinal center B-cell proliferation. While this may be true under certain circumstances, there is now evidence that antigen on follicular dendritic cells is not required to sustain a normal germinal center response. Indeed, the role of the antigen depot on these cells is unknown, although it could be to maintain long-lived plasma cells. Where does the antigen that sustains the germinal center come from? Under normal circumstances, it is most likely that live pathogens carried to the lymphoid tissues and multiplying there will continue to provide antigens until they are eliminated by the immune response, after which the germinal center decays. Immunizations with protein antigens are usually given in a form that slowly releases the antigen over time, which mimics the situation with live pathogens. Indeed, it is difficult to stimulate germinal center formation by immunization without either a live replicating pathogen or a sustained release of antigen in adjuvant (see Appendix I, Section A-4).

Figure 9.14
Immune complexes bind to the surface of follicular dendritic cells. Radiolabeled antigen localizes to, and persists in, lymphoid follicles of draining lymph nodes (see light micrograph and the schematic representation below, showing a germinal center (more...)

Figure 9.15
Immune complexes bound to follicular dendritic cells form iccosomes, which are released and can be taken up by B cells in the germinal center. Follicular dendritic cells have a prominent cell body and many dendritic processes. Immune complexes, bound (more...)
How the various signals that maintain the germinal center exert their effects on B cells is not completely understood. The combined signals from the B-cell receptor and CD40 seem to upregulate a protein called Bcl-XL, a relative of Bcl-2, which promotes B-cell survival (see Chapter 6). There are doubtless many other signals yet to be discovered that promote B-cell differentiation.
9-9. Surviving germinal center B cells differentiate into either plasma cells or memory cells
The purpose of the germinal center reaction is to enhance the later part of the primary immune response. Some germinal center cells differentiate first into plasmablasts and then into plasma cells. Plasmablasts continue to divide rapidly but have begun to specialize to secrete antibody at a high rate; they are destined to become nondividing, terminally differentiated plasma cells and thus represent an intermediate stage of differentiation. These plasma cells will migrate to the bone marrow, where a subset of them will live for a long period of time. Plasma cells obtain signals from bone marrow stromal cells that are essential for their survival. These plasma cells provide a source of long-lasting high-affinity antibody.
Other germinal center cells differentiate into memory B cells. Memory B cells are long-lived descendents of cells that were once stimulated by antigen and had proliferated in the germinal center. These cells divide very slowly if at all; they express surface immunoglobulin, but do not secrete antibody at a high rate. Since the precursors of memory B cells once participated in a germinal center reaction, memory B cells inherit the genetic changes that occurred in germinal center cells, including somatic mutations and the gene rearrangements that result in isotype switch (see Sections 4-9 and 4-16). The signals that control which differentiation path a B cell takes, and even whether at any given point the B cell continues to divide instead of differentiating, are unclear.
It has been proposed that signals from follicular dendritic cells (FDCs) are important in stimulating a B cell to become a memory cell. However, memory cells can develop in mutant mice lacking FDCs, albeit with reduced efficiency, so there may be other sources of signals. Another possibility is that affinity for antigen controls B-cell differentiation, with high-affinity cells perhaps being preferentially stimulated to become memory cells while the lower-affinity cells are allowed to undergo further cycles of proliferation, mutation, and selection. This is just one of the mysteries of the germinal center that immunologists have yet to solve. Immunological memory is discussed in detail in Chapter 10.
9-10. B-cell responses to bacterial antigens with intrinsic ability to activate B cells do not require T-cell help
Although antibody responses to most protein antigens are dependent on helper T cells, humans and mice with T-cell deficiencies nevertheless make antibodies to many bacterial antigens. This is because the special properties of some bacterial polysaccharides, polymeric proteins, and lipopolysaccharides enable them to stimulate naive B cells in the absence of peptide-specific T-cell help. These antigens are known as thymus-independent antigens (TI antigens) because they stimulate strong antibody responses in athymic individuals. These nonprotein bacterial products cannot elicit classical T-cell responses, yet they induce antibody responses in normal individuals. However, B-cell responses to these TI antigens are influenced by the presence of T cells, perhaps indirectly through cytokines such as IL-5 since they are greatly diminished in animals that have no T cells at all.
Thymus-independent antigens fall into two classes that activate B cells by two different mechanisms. TI-1 antigens possess an intrinsic activity that can directly induce B-cell division. At high concentration, these molecules cause the proliferation and differentiation of most B cells regardless of their antigen specificity; this is known as polyclonal activation (Fig. 9.16, top two panels). TI-1 antigens are thus often called B-cell mitogens, a mitogen being a substance that induces cells to undergo mitosis. An example of a B-cell mitogen and TI-1 antigen is LPS, which binds to LPS-binding protein and CD14 (see Chapter 2), which then associate with the receptor TLR-4 on B cells. LPS activates B cells only at doses at least 100 times greater than those needed to activate dendritic cells. Thus, when B cells are exposed to concentrations of TI-1 antigens that are 103-105 times lower than those used for polyclonal activation, only those B cells whose B-cell receptors also specifically bind the TI-1 molecules become activated. At these low antigen concentrations, sufficient amounts of TI-1 for B-cell activation can only be concentrated on the B-cell surface with the aid of this specific binding (Fig. 9.16, bottom two panels). In the presence of large amounts of the TI-1 antigen, this concentrating effect is not required, and all B cells can be stimulated.

Figure 9.16
Thymus-independent type 1 antigens (TI-1 antigens) are polyclonal B-cell activators at high concentrations, whereas at low concentrations they induce an antigen-specific antibody response. At high concentrations, the signal delivered by the B-cell-activating moiety (more...)
It is likely that, as with any pathogen antigen, concentrations of TI-1 antigens are low during the early stages of infections in vivo; thus, only antigen-specific B cells are likely to be activated and these will produce antibodies specific for the TI-1 antigen. Such responses have an important role in defense against several extracellular pathogens, as they arise earlier than thymus-dependent responses since they do not require prior priming and clonal expansion of helper T cells. However, TI-1 antigens are inefficient inducers of isotype switching, affinity maturation, or memory B cells, all of which require specific T-cell help.
9-11. B-cell responses to bacterial polysaccharides do not require peptide-specific T-cell help
The second class of thymus-independent antigens consist of molecules such as bacterial capsular polysaccharides that have highly repetitive structures. These thymus-independent antigens, called TI-2 antigens, contain no intrinsic B-cell-stimulating activity. Whereas TI-1 antigens can activate both immature and mature B cells, TI-2 antigens can activate only mature B cells; immature B cells, as we saw in Chapter 7, are inactivated by repetitive epitopes. This might be why infants do not make antibodies to polysaccharide antigens efficiently; most of their B cells are immature. Responses to several TI-2 antigens are prominent among B-1 cells (also known as CD5 B cells), which comprise an autonomously replicating subpopulation of B cells, and among marginal zone B cells, another unique subset of nonrecirculating B cells that line the border of the splenic white pulp (see Chapter 7). Although B-1 cells arise early in development, young children do not make a fully effective response to carbohydrate antigens until about 5 years of age. On the other hand, marginal zone B cells are rare at birth and accumulate with age; they may thus be responsible for most physiological TI-2 responses, which also increase with age.
TI-2 antigens most probably act by extensively cross-linking the B-cell receptors of mature B cells specific for the antigen (Fig. 9.17, left panels). Excessive receptor cross-linking, however, renders mature B cells unresponsive or anergic, just as it does immature B cells. Thus, epitope density seems to be critical in the activation of B cells by TI-2 antigens: at too low a density, receptor cross-linking is insufficient to activate the cell; at too high a density, the B cell becomes anergic.
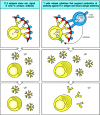
Figure 9.17
B-cell activation by thymus-independent type 2 antigens (TI-2 antigens) requires, or is greatly enhanced by, cytokines. Multiple cross-linking of the B-cell receptor by TI-2 antigens can lead to IgM antibody production (left panels), but there is evidence that (more...)
Although responses to TI-2 antigens can occur in nude mice (which lack a thymus), depletion of all T cells by knocking out the TCRβ and TCRδ loci eliminates responses to TI-2 antigens. Moreover, responses to TI-2 antigens can be augmented in vivo by transferring small numbers of T cells to these T-cell deficient mice. How T cells contribute to TI-2 responses is not clear. One possibility is that T cells can recognize and become activated by TI-2 antigens through cell-surface molecules shared by all T cells (Fig. 9.17, right panels). Alternatively, the help might come from γ:δ T cells or from CD4 CD8 double-negative α:β T cells. The T-cell receptors on these cells recognize certain polysaccharides bound to unconventional MHC class I or class I-like molecules such as CD1. Such T cells can develop outside the thymus, principally in the gut.
B-cell responses to TI-2 antigens provide a prompt and specific response to an
important class of pathogen. Many common extracellular bacterial pathogens are
surrounded by a polysaccharide capsule that enables them to resist ingestion by
phagocytes. The bacteria not only escape direct destruction by phagocytes but
also avoid stimulating T-cell responses through the presentation of bacterial
peptides by macrophages. Antibody that is produced rapidly in response to this
polysaccharide capsule without the help of peptide-specific T cells can coat
these bacteria, promoting their ingestion and destruction by phagocytes by
mechanisms we will describe later in this chapter. The common encapsulated
extracellular bacteria are often known as pyogenic bacteria, as they typically cause the formation of abundant
pus, which consists chiefly of dead and dying neutrophils that have been
recruited to the site of infection. Both IgM and IgG antibodies are induced by
TI-2 antigens and are likely to be an important part of the humoral immune response in many bacterial infections. We mentioned earlier the importance of
antibodies to the capsular polysaccharide of Haemophilus
influenzae type B, a TI-2 antigen, in protective immunity to this
bacterium. A further example of the importance of TI-2 responses can be seen in
patients with an immunodeficiency disease known as the Wiskott-Aldrich syndrome ( Wiskott-Aldrich Syndrome, in
Case Studies in Immunology, see Preface for
details). These patients can respond,
although poorly, to protein antigens but fail to make antibody against
polysaccharide antigens and are highly susceptible to infection with
encapsulated bacteria. Thus, the TI responses are important components of the
humoral immune response to nonprotein antigens that do not engage
peptide-specific T-cell help; the distinguishing features of thymus-dependent,
TI-1, and TI-2 antibody responses are summarized in Fig. 9.18.
Wiskott-Aldrich Syndrome, in
Case Studies in Immunology, see Preface for
details). These patients can respond,
although poorly, to protein antigens but fail to make antibody against
polysaccharide antigens and are highly susceptible to infection with
encapsulated bacteria. Thus, the TI responses are important components of the
humoral immune response to nonprotein antigens that do not engage
peptide-specific T-cell help; the distinguishing features of thymus-dependent,
TI-1, and TI-2 antibody responses are summarized in Fig. 9.18.

Figure 9.18
Properties of different classes of antigen that elicit antibody responses.
Summary
B-cell activation by many antigens, especially monomeric proteins, requires both binding of the antigen by the B-cell surface immunoglobulin—the B-cell receptor—and interaction of the B cell with antigen-specific helper T cells. Helper T cells recognize peptide fragments derived from the antigen internalized by the B cell and displayed by the B cells as peptide:MHC class II complexes. Helper T cells stimulate the B cell through the binding of CD40L on the T cell to CD40 on the B cell, through interaction of other TNF-TNF-receptor family ligand pairs, and by the directed release of cytokines. The initial interaction occurs in the T-cell area of secondary lymphoid tissue, where both antigen-specific and helper T cells and antigen-specific B cells are trapped as a consequence of binding antigen; further interactions between T cells and B cells occur after migration into the B-cell zone or follicle, and formation of a germinal center. Helper T cells induce a phase of vigorous B-cell proliferation, and direct the differentiation of the clonally expanded progeny of the naive B cells into either antibody-secreting plasma cells or memory B cells. During the differentiation of activated B cells, the antibody isotype can change in response to cytokines released by helper T cells, and the antigen-binding properties of the antibody can change by somatic hypermutation of V-region genes. Somatic hypermutation and selection for high-affinity binding occur in the germinal centers. Helper T cells control these processes by selectively activating cells that have retained their specificity for the antigen and by inducing proliferation and differentiation into plasma cells and memory B cells. Some nonprotein antigens stimulate B cells in the absence of linked recognition by peptide-specific helper T cells. These thymus-independent antigens induce only limited isotype switching and do not induce memory B cells. However, responses to these antigens have a critical role in host defense against pathogens whose surface antigens cannot elicit peptide-specific T-cell responses.
- The humoral immune response is initiated when B cells that bind antigen are signaled by helper T cells or by certain microbial antigens alone
- Armed helper T cells activate B cells that recognize the same antigen
- Antigenic peptides bound to self MHC class II molecules trigger armed helper T cells to make membrane-bound and secreted molecules that can activate a B cell
- Isotype switching requires expression of CD40L by the helper T cell and is directed by cytokines
- Antigen-binding B cells are trapped in the T-cell zone of secondary lymphoid tissues and are activated by encounter with armed helper T cells
- The second phase of the primary B-cell immune response occurs when activated B cells migrate to follicles and proliferate to form germinal centers
- Germinal center B cells undergo V-region somatic hypermutation and cells with mutations that improve affinity for antigen are selected
- Ligation of the B-cell receptor and CD40, together with direct contact with T cells, are all required to sustain germinal center B cells
- Surviving germinal center B cells differentiate into either plasma cells or memory cells
- B-cell responses to bacterial antigens with intrinsic ability to activate B cells do not require T-cell help
- B-cell responses to bacterial polysaccharides do not require peptide-specific T-cell help
- Summary
- B-cell activation by armed helper T cells - ImmunobiologyB-cell activation by armed helper T cells - Immunobiology
Your browsing activity is empty.
Activity recording is turned off.
See more...