NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Mobley HLT, Mendz GL, Hazell SL, editors. Helicobacter pylori: Physiology and Genetics. Washington (DC): ASM Press; 2001.
Helicobacter pylori must be able to modulate properties of its immediate environment to colonize effectively and to persist within the human stomach (12, 21, 34, 86). An important aspect of environmental remodeling is the bacterium's ability to control and facilitate movement of both small and large molecules from the intracellular compartment to the extracellular environment. Proteins destined for the extracellular milieu must be translocated through a differentiated cell envelope composed of inner and outer membranes that sandwich one or more peptidoglycan layer(s) and the periplasmic space (10, 13, 18, 26, 39, 40, 45–47, 63, 75, 80, 88, 90, 96). In gram-negative bacteria, most proteins are secreted by either type I or type II mechanisms (reviewed in another chapter of this volume). However, recent data suggest that in addition to these important and well-studied secretion pathways, some H. pylori strains use alternative strategies, including type IV secretion (16, 20), bacterial autolysis (60), and the generation of outer membrane vesicles (8, 62, 99), to release proteins from the bacterial cell. Each of these alternative mechanisms is fundamentally different from the others in terms of the cellular machinery involved, overall mechanisms, and fate of the transported proteins. At the same time, all three mechanisms represent potentially important approaches for facilitating adaptation of H. pylori to the unique environment of the human gastric mucosa.
The purpose of this chapter is to review recent results concerning alternative strategies that H. pylori strains employ to introduce proteins into the environment. The chapter is divided into three sections. The first section describes the cellular machinery comprising the type IV secretion system of H. pylori. The second section discusses evidence that H. pylori undergoes autolysis as a mechanism for releasing cytoplasmic proteins directly into the extracellular environment. Finally, the third section reviews recent data supporting the theory that cellular envelope proteins are released from H. pylori via the formation of outer membrane vesicles, which appear to have the ability to be taken up into mammalian cells.
Type IV Secretion: Adaptation of Conjugative Cellular Machinery
A number of pathogenic bacteria have adapted their conjugation machinery for protein secretion (16, 20). Bacterial conjugation machinery not only facilitates the transport of proteins to the extracellular matrix; in the case of H. pylori, effector molecules are injected directly into recipient eukaryotic cells (16, 20). This mechanism of bacterial conjugation machinery-mediated secretion is now widely known as type IV secretion. Most of our current knowledge of the conjugation apparatus is derived from the Agrobacterium tumefaciens T-DNA transfer machine, which delivers oncogenic nucleoprotein particles into plant cells (19, 43, 57, 58, 61, 78, 101, 102). Bacterial pathogens with known or putative type IV systems include Bordetella pertussis (20, 98), Legionella pneumophila (83, 85), Rickettsia prowazekii (4), and Brucella spp. (71).
The proteins comprising the type IV secretion systems are encoded by genes generally associated with pathogenicity islands, suggesting horizontal transfer of these conjugative systems (20). As discussed elsewhere in this volume, the genes associated with the H. pylori type IV apparatus are encoded within the cag (cytotoxin-associated gene) pathogenicity island (1, 17). The 145-kDa effector protein CagA is directly secreted from bacterial cells into epithelial cells via the cag-encoded type IV translocation apparatus. The strong correlation of the presence of the cag pathogenicity island with H. pylori strains associated with gastric disease, as well as the potential role of cag in bacterial pathogenesis, has been discussed in detail elsewhere in this volume. This chapter focuses on the cag genes linked to the H. pylori type IV secretion apparatus.
Components of the Type IV Secretion Apparatus
The conjugation machines of gram-negative bacteria are composed of two surface structures, the mating channel through which the cargo is transferred and the conjugative pilus for contacting recipient cells, along with membrane-associated ATPases (37, 98). High-resolution structural data are not available for any mating channel, although various conjugative pili have been visualized by lower resolution techniques such as electron microscopy (48). Most of the current information contributing to the model of bacterial conjugation machines derives from studies focused on interactions between specific protein subunits of the A. tumefaciens T-pilus transporter, as well as localization of individual transporter components in the assembled secretion machinery. The vir genes that encode the type IV secretion apparatus in A. tumefaciens can be divided into three functional groups that encode the following: (i) proteins comprising the mating channel, (ii) cytoplasmic membrane ATPases, and (iii) proteins that localize exocellularly to form the T-pilus or other adhesive structures (20, 58). Although these components are hypothesized to form a large supramolecular complex, there is not yet direct evidence for a physical association between the mating channel and the conjugative pilus.
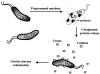
Unraveling the structure of the H. pylori secretion apparatus represents a rich area for future research. Information about the functions and properties of specific Cag proteins have been derived primarily from sequence homologies with the Vir proteins of the A. tumefaciens conjugation machine (1, 17). It is striking that of the 31 genes identified within the H. pylori pathogenicity island, only six cag gene products demonstrate substantial amino acid similarities to the vir gene products (Fig. 1A). On the basis of biochemical and genetic studies of the A. tumefaciens secretion apparatus (58), the locations of the six H. pylori Cag proteins are predicted via analogy to their Vir homologs (Fig. 1B) to be either part of the mating channel, or, alternatively, one of the putative ATPases (Table 1). There are no Cag homologs to Vir proteins, which are involved exocellularly in contact or adhesion to host cells. The lack of additional homology between Vir and Cag proteins suggests that the H. pylori cag secretion apparatus may be substantially different from the type IV apparatus of A. tumefaciens. Alternatively, it cannot be ruled out that other Cag proteins may be able to carry out similar or identical functions to the Vir proteins in the A. tumefaciens secretion apparatus.
Table 1
H. pylori type IV system.
Homologs of A. tumefaciens mating channel components
The VirB6-VirB10 gene products from A. tumefaciens, as well as one or more of the three ATPases, are believed to be channel subunits (19). VirB7, an outer membrane lipoprotein (35), is homologous to H. pylori CagT; and VirB9 is a homolog to H. pylori Cag-528 (1, 17). VirB7 forms homodimers with neighboring VirB7 and interacts with VirB9 via reactive cysteines present in each protein that are oxidized to form disulfide linkages (3, 87), The VirB7-VirB9 heterodimer has been localized to the outer membrane and appears to be essential for stabilizing other VirB proteins during assembly of the A. tumefaciens transfer apparatus (35). By analogy to VirB7 and VirB9, CagT and Cag-528 may also be localized to the H. pylori outer membrane, and serve a role for assembly of the type IV secretion machinery in H. pylori.
H. pylori Cag-527 is homologous to A. tumefaciens VirB10 (Fig. 1A) (1, 17). VirB10 is believed to interact directly with VirB9 based on yeast two-hybrid analysis (24). In support of this hypothesis, VirB9 is essential for chemically cross-linking VirB10 into higher ordered multimers predicted to be homotrimers (6). It is thus reasonable to hypothesize that Cag-527 and Cag-528 (the VirB9 homolog) may interact within the H. pylori "mating channel" and carry out the same or similar functions during H. pylori type IV secretion as their homologs do in A. tumefaciens (Fig. 1B). Although VirB10 is proposed to bridge the cytoplasmic and outer membrane VirB subcomplexes (6), no evidence yet exists to support such a role for Cag-527.
Interestingly, there is no clearly identified H. pylori homolog to VirB6, which is a highly hydrophobic protein thought to span the cytoplasmic membrane several times (23). VirB6 is potentially the best candidate for a channel-forming protein in A. tumefaciens. Presumably, if the H. pylori type IV transfer apparatus is similar to that of the conjugation machine of A. tumefaciens, such a putative channel protein must also exist in H. pylori but may be more specifically tailored to the translocation requirements of this gastric bacterium.
Homologs of A. tumefaciens membrane ATPases
Type IV secretion requires functional cytoplasmic membrane ATPases. H. pylori CagE and Cag-525 are required for type IV translocation of effector molecules into mammalian cells and have considerable homology to the putative ATPase proteins of A. tumefaciens VirB4 and VirB11 (1, 17, 54). These VirB proteins contain conserved Walker A nucleotide-binding motifs required for function (19, 91). Purified forms of VirB4 and VirB11 possess weak in vitro ATPase activities (19). Both proteins appear to assemble minimally in vivo as homodimers. Dimerization of VirB4 is mediated by a domain in the amino-terminal third of the protein. In contrast, dimerization of VirB11 is mediated by domains located in each half of the protein (22, 77, 100). VirB11 and the H. pylori homolog Cag-525 have been shown to form higher ordered homohexameric rings in solution, further supporting the view that they may share similar or identical properties and functions (53, 77).
Another putative A. tumefaciens ATPase, VirD4, is believed to be important for DNA processing and transfer reactions by discriminating for transferred DNA (42). The H. pylori homolog of VirD4 is HP524, which is required for transport of the H. pylori protein effector molecule CagA into mammalian cells (89), suggesting that this family of putative ATPases may not be specific for the type of molecule transported by the type IV secretion machinery.
Homologs of A. tumefaciens exocellular T-pilus components
The third component of the A. tumefaciens secretion apparatus is composed of the exocellular VirB proteins localized to form the adhesive and contact structure for direct interactions with host cells (20, 58). There are no cag gene products that share significant sequence homology to A. tumefaciens proteins comprising the scaffold of the T pilus (1, 17). The lack of clearly identifiable homologs may represent differences in adaptive requirements for H. pylori and A. tumefaciens, which interact with gastric epithelial cells and plant cells, respectively. The proteins that comprise the exocellular structure required for contact and adhesion of the H. pylori type IV machine remain undefined, and their identification and characterization represent important areas for future investigations.
Bacterial Autolysis: An "Altruistic" Mechanism for H. pylori Adaptation within a Hostile Environment?
Nascent proteins targeted for secretion by type I or type II mechanisms are transported rapidly subsequent to their expression in the cytoplasm (70, 74). Consequently, proteins destined for secretion by these mechanisms are not generally localized simultaneously within the cytoplasm and extracellular milieu. In contrast, typical cytoplasmic H. pylori proteins, such as urease and catalase, have been found in the cytoplasm, localized to the outer membrane, and/or in the extracellular medium (14, 33, 73). This phenomenon has been observed in vitro, but it is thought that extracellular transport of H. pylori cytoplasmic proteins also occurs in vivo. Supporting this hypothesis are the observations that administration of urease, catalase, and other proteins confers protection against H. pylori challenge in animal models (11, 28, 30, 41, 59, 64, 68, 76).
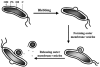
Recent studies have suggested that specific H. pylori cytoplasmic proteins are localized to the outer membrane by a mechanism of bacterial autolysis (33, 73). According to this model (Fig. 2), individual bacteria are lysed by a highly regulated and controlled process that results in the release of cytoplasmic proteins from the bacterial cytosol. Notably, a number of other pathogenic bacteria, including Streptococcus pneumoniae and Neisseria gonorrhoeae, convincingly have been demonstrated to undergo a regulated program of autolysis (60, 69, 79, 84, 85). In H. pylori, some of the released cytoplasmic proteins are then adsorbed to the surface of the outer membrane of neighboring bacteria. Such a model has been called "altruistic autolysis" since the lysis of a fraction of the bacterial population presumably benefits the remaining viable bacteria (5, 32, 33, 73). This type of mechanism has been suggested by a number of investigators to explain how vaccination with a cytoplasmic protein such as urease can confer protection against challenges with Helicobacter spp. in animal models, as described (11, 30, 41, 59, 68). Because H. pylori is noninvasive, autolysis might also facilitate presentation of virulence factors and immunogens to the host (5, 32, 33, 73).
Experimental Evidence for Autolysis
Evidence supporting a model of altruistic autolysis is based primarily on recent H. pylori urease localization studies (5, 32, 33, 73). All H. pylori isolates generate large quantities of a potent urease, which catalyzes the hydrolysis of urea in the stomach to produce ammonia and bicarbonate ions, as detailed elsewhere in this volume. H. pylori urease has been categorized as a virulence factor because the enzyme is required for bacterial colonization of the gastric mucosa (65). In addition, urease functions in the utilization of exogenous urea as a nitrogen source for amino acid synthesis (97). Although urease is localized in the cytoplasm of H. pylori and other bacterial species, cryoimmunoelectron microscopy showed the enzyme to be found both in vitro and in vivo on the cell surface under specific conditions (73). The release of urease and other cytoplasmic proteins from H. pylori has been demonstrated now in vitro by three independent groups (73, 81, 82), and an autolytic mechanism has been proposed to explain their location (73, 81).
How does cytoplasmic urease localize to the H. pylori outer membrane surface? Urease does not possess an amino-terminal signal peptide for targeting to the type II (Sec-dependent) secretion pathway. Recombinant H. pylori urease expressed in E. coli shows cytoplasmic activity, demonstrating the efficacy of expressing a functional protein in a heterologous system (55). However, no urease was detectable on the outer surface of E. coli, suggesting that the mechanism employed by H. pylori for surface localization of the enzyme is not ubiquitous to all gram-negative bacteria.
In H. pylori, autolysis is sensitive to the phase of bacterial growth. Urease, catalase, and the heat shock protein HspB are localized only in the cytoplasmic compartment during exponential growth phase (ca. 24 h) in H. pylori in vitro cultures (73). However, bacteria cultivated for 72 h demonstrated significant amounts of surface-bound and extracellular urease, suggesting that urease is localized on the outer surface only in older bacteria. Catalase and HspB are also localized outside the cytoplasm in older or subcultured preparations, suggesting that the extracellular release of cytoplasmic proteins with age is not a process that is exclusive to urease.
The presence of an autolytic mechanism for release of some H. pylori proteins remains controversial because of observations not properly explained by this mechanism. H. pylori yielded differential release patterns of proteins from the cytoplasm to the extracellular supernatant, and kinetic studies indicated that proteins were not released at the same rate (94). Collectively, these data were interpreted to support a role for specific secretion mechanism(s) rather than autolysis for the release of cytoplasmic proteins (94). Nonetheless, autolysis of some gram-negative bacteria by a regulated and controlled mechanism is gaining acceptance (60, 69, 79, 84, 85), and the current data supporting H. pylori autolysis warrants additional investigation of this problem.
Autolysis is a strategy for survival in a hostile environment
The presence of cytoplasmic proteins on the surface of the H. pylori outer membrane both in vitro and in vivo is consistent with a mechanism of bacterial autolysis. In addition, the dependence of cytoplasmic protein release on the phase of bacterial growth in vitro suggests that the process is regulated.
H. pylori autolysis has been termed "altruistic" because surface-bound urease is important for bacterial survival in acid. Using membrane-impermeable (flurofamide) and -permeable (acetohydroxamic acid) inhibitors, it was demonstrated that decreasing only surface-bound urease activity had almost as great an effect on H. pylori acid resistance as decreasing both surface and cytoplasmic urease activity (55). Moreover, H. pylori lacking surface-associated urease was unable to withstand exposure to acid (55). Specifically, H. pylori cultures grown for 24 h did not survive the acid environment in the presence of urea, whereas cultures, which had been grown and passaged every 72 h, demonstrated a marked ability to survive acid treatment (pH 2 in the presence of 5 mM urea). In support of the importance of surface-bound urease to bacterial acid resistance is the observation that E. coli strains expressing only cytoplasmic urease activity were not resistant to acid (55), although alternative explanations for sensitivity of these bacteria to acidic conditions are possible. Collectively, these data suggest that surface-bound urease performs an essential function in H. pylori acid resistance, leading to the conclusion that the release of this protein by some bacteria has beneficial effects on the overall population.
Machinery for autolysis
An autolytic mechanism influenced by environmental factors and regulated by genetic factors requires cellular components that contribute directly to autolysis. In other bacterial species, autolysis requires disruption of the cell wall peptidoglycan layers by specific proteins called autolysins. These proteins can be penicillin-binding proteins or non-penicillin-binding proteins. They have been identified for S. pneumoniae, which undergoes genetically regulated autolysis both in vivo and in vitro (72). S. pneumoniae autolysis occurs under conditions of nutrient starvation or antibiotic treatment. Direct genetic evidence for the role of autolysins comes from the inability of autolysin-deficient mutants to undergo autolysis during the stationary phase of growth (72) and from restoration of the autolytic phenotype of S. pneumoniae mutants by genetic complementation with a gene encoding the autolysin (7, 79). The importance of autolysis in bacterial pathogenesis is suggested from results showing that autolysin-negative S. pneumoniae strains are less virulent in animal models than wild-type strains (7, 38, 79). N. gonorrhoeae apparently undergoes autolysis as a mechanism of releasing DNA for uptake by other bacteria (27). The mechanism of N. gonorrhoeae autolysis also appears to be regulated. Significantly, autolysin-deficient mutants are not lysed under conditions that generally promote autolysis in this bacterium (27).
An autolytic mechanism in H. pylori would require the bacterium to produce factors that function as autolysins. Genome analyses of H. pylori predict open reading frames encoding a putative lytic transglycosylase and an amidase (2, 92). In addition, the genes HP597, HP1556, and HP1565 encode proteins with sequence similarities to penicillin-binding proteins from Escherichia coli, Haemophilus influenzae, and Bacillus subtilis (92), but in the H. pylori genome no sequence similarities are detected to lower molecular weight penicillin-binding proteins with carboxy-peptidase or endopeptidase activities that may be involved in peptidoglycan degradation. Recent work with biotin- and digoxigenin-labeled antibiotics served to identify numerous putative penicillin-binding proteins with an assortment of molecular weights (25, 29, 44). A novel penicillin-binding protein (PBP4) has been discovered in both the soluble and membrane fractions of H. pylori preparations (56), whose expression increases significantly during mid- to late-exponential phase of H. pylori. The sequence of PBP4 has highly conserved penicillin-binding motifs arranged in a novel fashion, and it is hypothesized that PBP4 has endopeptidase activity to degrade peptidoglycan (56). These features of PBP4 suggest a possible link to autolysis.
Although evidence is accumulating that H. pylori may release cytoplasmic proteins into the extracellular milieu in a regulated fashion and that the bacterium may possess putative autolysins, many aspects of the altruistic autolysis model remain untested. In particular, it will be important to establish the significance of an autolytic mechanism to colonization and survival of the bacterium in its unique environment. Notwithstanding that H. pylori apparently releases cytoplasmic proteins in vitro as a function of the growth phase, the signals that may trigger autolysis in vivo remain unidentified. Nonetheless, altruistic autolysis remains an intriguing hypothesis to explain bacterial adaptation to hostile conditions.
Outer Membrane Vesicles: Bundling Cell Envelope Proteins for Delivery?
Recently, it has been shown that H. pylori releases small vesicles from its outer membrane by a process that bears striking similarity to the release of membrane vesicles by a number of other bacterial pathogens (36), namely, Neisseria meningitidis, H. influenzae, Borrelia burgdorferi, Pseudomonas aeruginosa, and Campylobacter jejuni (9, 15, 49–52, 62, 66, 99). H. pylori strains examined from gastric biopsies of infected patients generate small trilayered membranous vesicles, and it is hypothesized that they derive from the bacterial outer membrane (Fig. 3). Significantly, these outer membrane vesicles frequently contain numerous antigens and virulence factors. The production of these vesicles potentially represents an important mechanism for bacterial pathogens to modulate their environment within the host.
Production of Outer Membrane Vesicles
To investigate whether outer membrane vesicles are produced by H. pylori cultured in vitro as well as in vivo, bacteria were examined by ultrastructural and immunochemical methods (36). These studies provided insights on the composition and production of H. pylori vesicles. Outer membrane vesicle formation appears to be a multistep process (Fig. 3). Vesicles originate as "blebs" that protrude from the body of the cell as outward expansions of the periplasmic space surrounded by the outer membrane. The observed protruding blebs are likely to represent the early stages of vesicle formation. The blebs continue to bud, with concomitant focal expansion of the periplasmic space, and finally, vesicles are released from the cell. The vesicles are much smaller than the bacteria from which they originated, but still of significant size, ranging from 50 to 300 nm in diameter. H. pylori-derived outer membrane vesicles accumulate in the culture supernatants over time.
The vesicles contain outer membrane proteins such as porins (36). Interestingly, outer membrane vesicles did not contain detectable urease activity or cross-reacting material, suggesting that their formation is a fundamentally different process than the proposed autolytic mechanism described above. Moreover, vesicle formation occurs during H. pylori exponential growth, while the release of cytoplasmic proteins by the putative autolytic mechanism described above occurs during the stationary phase.
Outer membrane vesicles and environmental adaptation
Outer membrane blebs have been found in every examined biopsy of H. pylori-infected patients, whether the outer membranes of bacteria were adjacent to the gastric epithelium or not (36). Besides being distributed around bacteria, the vesicles adhere to the luminal surface of gastric superficial-foveolar epithelium. The outer membrane blebs and vesicles contain vacuolating toxin (VacA), which is an important virulence factor in H. pylori-mediated disease pathogenesis; and vesicles containing VacA were detected inside cytoplasmic tubulovesicles or vacuoles of the epithelium. These vesicles were found also within dilated endosomes and the vacuoles formed by progressive dilation and fusion of these structures (31). These observations suggest an in vivo role for outer membrane vesicles (36).
The role of outer membrane-derived vesicles in H. pylori growth and survival is poorly understood. In common with other gram-negative bacteria, vesicle formation in H. pylori may represent an important mechanism for the delivery of various virulence factors and antigens to host tissues that otherwise would be insoluble and/or susceptible to degradation in the extracellular matrix. Antigens derived from outer membrane vesicles might traverse the gastric epithelial monolayer and ultimately reach the lamina propria, thus providing a mechanism for antigen presentation resulting in local and systemic immune responses (31).
Many interesting problems concerning H. pylori outer membrane vesicles remain unsolved, for example, ascertaining the way in which the release of outer membrane vesicles is regulated, determining the gene products that specifically facilitate membrane blebbing, and establishing the fate of the H. pylori cell from which the membrane vesicle is derived.
Conclusions
Bacterial secretion remains a fertile area of research. H. pylori has classical type I and type II gram-negative bacterial secretion pathways, but it is becoming apparent that this bacterium uses alternative mechanisms for exporting proteins to the outside environment. The ability of a noninvasive bacterium such as H. pylori to modulate its environment is an important strategy for colonization and survival within the human stomach. Each of the mechanisms described above has the potential of allowing H. pylori to carry out important functions within the host. The type IV secretion system facilitates direct transport of protein effectors from the bacterium into the host cell. This adaptation of the conjugal machinery of many gram-negative bacteria bypasses the need for active uptake by the host cell. Bacterial autolysis probably involves mechanisms of peptidoglycan degradation to release cytoplasmic contents into the extracellular medium, which can then be strategically relocalized onto the outer membrane of other bacteria. In the case of H. pylori, localization of urease to the outer membrane appears to assist its survival in the acidic environment of the gastric mucosa. Finally, the release of outer membrane vesicles may allow H. pylori to bundle up cellular envelope proteins and deliver them to gastric epithelial cells and potentially to other locations distal to the site of colonization. Clearly, these three mechanisms represent remarkable adaptation strategies of H. pylori.
Acknowledgments
Research funding from the National Institutes of Health (ROI AI45928), the American Heart Association (98B 6472), and the Robert A. Welch Foundation (E-1311) is gratefully acknowledged.
References
- 1.
- Akopyants N. S., Clifton S. W., Kersulyte D., Crabtree J. E., Youree B. E., Reece C. A., Bukanov N. O., Drazek E. S., Roe B. A., Berg D. E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol. Microbiol. 1998;28:37–53. [PubMed: 9593295]
- 2.
- Alm R. A., Ling L. S., Moir D. T., King B. L., Brown E. D., Doig P. C., Smith D. R., Noonan B., Guild B. C., deJonge B. L., Carmel G., Tummino P. J., Caruso A., Uria-Nickelsen M., Mills D. M., Ives C., Gibson R., Merberg D., Mills S. D., Jiang Q., Taylor D. E., Vovis G. F., Trust T. J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. [PubMed: 9923682]
- 3.
- Anderson L. B., Hertzel A. V., Das A. Agrobacterium tumefaciens VirB7 and VirB9 form a disulfide-linked protein complex. Proc. Natl. Acad. Sci. USA. 1996;93:8889–8894. [PMC free article: PMC38564] [PubMed: 8799123]
- 4.
- Andersson S. G., Zomorodipour A., Andersson J. O., Sicheritz-Ponten T., Alsmark U. C., Podowski R. M., Naslund A. K., Eriksson A. S., Winkler H. H., Kurland C. G. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. [PubMed: 9823893]
- 5.
- Bauerfeind P., Garner R., Dunn B. E., Mobley H. L. Synthesis and activity of Helicobacter pylori urease and catalase at low pH. Gut. 1997;40:25–30. [PMC free article: PMC1027003] [PubMed: 9155571]
- 6.
- Beaupre C. E., Bohne J., Dale E. M., Binns A. N. Interactions between VirB9 and VirB10 membrane proteins involved in movement of DNA from Agrobacterium tumefaciens into plant cells. J. Bacteriol. 1997;179:78–89. [PMC free article: PMC178664] [PubMed: 8981983]
- 7.
- Berry A. M., Paton J. C., Hansman D. Effect of insertional inactivation of the genes encoding pneumolysin and autolysin on the virulence of Streptococcus pneumoniae type 3. Microb. Pathog. 1992;12:87–93. [PubMed: 1350046]
- 8.
- Beveridge T. J. Structures of gram-negative cell walls and their derived membrane vesicles. J. Bacteriol. 1999;181:4725–4733. [PMC free article: PMC93954] [PubMed: 10438737]
- 9.
- Beveridge T. J., Kadurugamuwa J. L. Periplasm, periplasmic spaces, and their relation to bacterial wall structure: novel secretion of selected periplasmic proteins from Pseudomonas aeruginosa. Microb. Drug Resist. 1996;2:1–8. [PubMed: 9158716]
- 10.
- Binet R., Letoffe S., Ghigo J. M., Delepelaire P., Wandersman C. Protein secretion by gram-negative bacterial ABC exporters—a review. Gene. 1997;192:7–11. [PubMed: 9224868]
- 11.
- Blanchard T. G., Czinn S. J., Maurer R., Thomas W. D., Soman G., Nedrud J. G. Urease-specific monoclonal antibodies prevent Helicobacter felis infection in mice. Infect. Immun. 1995;63:1394–1399. [PMC free article: PMC173165] [PubMed: 7890401]
- 12.
- Blaser M. Helicobacter pylori and gastric diseases. Br. Med. J. 1998;316:1507–1510. [PMC free article: PMC1113159] [PubMed: 9582144]
- 13.
- Blight M. A., Chervaux C., Holland I. B. Protein secretion pathway in Escherichia coli. Curr. Opin. Biotechnol. 1994;5:468–474. [PubMed: 7765458]
- 14.
- Bode G., Malfertheiner P., Lehnhardt G., Nilius M., Ditschuneit H. Ultrastructural localization of urease of Helicobacter pylori. Med. Microbiol. Immunol. (Berlin). 1993;182:233–242. [PubMed: 8283959]
- 15.
- Buommino E., Morelli F., Metafora S., Rossano F., Perfetto B., Baroni A., Tufano M. A. Porin from Pseudomonas aeruginosa induces apoptosis in an epithelial cell line derived from rat seminal vesicles. Infect. Immun. 1999;67:4794–4800. [PMC free article: PMC96811] [PubMed: 10456933]
- 16.
- Burns D. L. Biochemistry of type IV secretion. Curr. Opin. Microbiol. 1999;2:25–29. [PubMed: 10047550]
- 17.
- Censini S., Lange C., Xiang Z., Crabtree J. E., Ghiara P., Borodovsky M., Rappuoli R., Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA. 1996;93:14648–14653. [PMC free article: PMC26189] [PubMed: 8962108]
- 18.
- China B., Goffaux F. Secretion of virulence factors by Escherichia coli. Vet. Res. 1999;30:181–202. [PubMed: 10367354]
- 19.
- Christie P. J. Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multi-functional transporters in eubacteria. J. Bacteriol. 1997;179:3085–3094. [PMC free article: PMC179082] [PubMed: 9150199]
- 20.
- Christie P. J., Vogel J. P. Bacterial type IV secretion: conjugation systems adapted to deliver effector molecules to host cells. Trends Microbiol. 2000;8:354–360. [PMC free article: PMC4847720] [PubMed: 10920394]
- 21.
- Cover T. Helicobacter pylori factors associated with disease. Gastroenterology. 1999;117:257–261. [PubMed: 10381935]
- 22.
- Dang T. A., Zhou X. R., Graf B., Christie P. J. Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on the assembly and function of the T-DNA transporter. Mol. Microbiol. 1999;32:1239–1253. [PMC free article: PMC3918219] [PubMed: 10383764]
- 23.
- Das A., Xie Y. H. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol. Microbiol. 1998;27:405–414. [PubMed: 9484895]
- 24.
- Das A., Xie Y. H. The Agrobacterium T-DNA transport pore proteins VirB8, VirB9, and VirB10 interact with one another. J. Bacteriol. 2000;182:758–763. [PMC free article: PMC94340] [PubMed: 10633111]
- 25.
- DeLoney C. R., Schiller N. L. Competition of various beta-lactam antibiotics for the major penicillin-binding proteins of Helicobacter pylori: antibacterial activity and effects on bacterial morphology. Antimicrob. Agents Chemother. 1999;43:2702–2709. [PMC free article: PMC89546] [PubMed: 10543750]
- 26.
- d'Enfert C., Reyss I., Wandersman C., Pugsley A. P. Protein secretion by gram-negative bacteria. Characterization of two membrane proteins required for pullulanase secretion by Escherichia coli K-12. J. Biol. Chem. 1989;264:17462–17468. [PubMed: 2677007]
- 27.
- Dillard J. P., Selfert H. S. A peptidoglycan hydrolase similar to bacteriophage endolysins acts as an autolysin in Neisseria gonorrhoeae. Mol. Microbiol. 1997;25:893–901. [PubMed: 9364915]
- 28.
- Doidge C., Crust I., Lee A., Buck F., Hazell S., Manne U. 1994Therapeutic immunisation against Helicobacter infection Lancet 343914–915.. (Letter.) [PubMed: 7908372]
- 29.
- Dore M. P., Graham D. Y., Sepulveda A. R. Different penicillin-binding protein profiles in amoxicillin-resistant Helicobacter pylori. Helicobacter. 1999;4:154–161. [PubMed: 10469189]
- 30.
- Dubois A., Lee C. K., Fiala N., Kleanthous H., Mehlman P. T., Monath T. Immunization against natural Helicobacter pylori infection in nonhuman primates. Infect. Immun. 1998;66:4340–4346. [PMC free article: PMC108524] [PubMed: 9712786]
- 31.
- Dunn B. E., Cohen H., Blaser M. J. Helicobacter pylori. Clin. Microbiol. Rev. 1997;10:720–741. [PMC free article: PMC172942] [PubMed: 9336670]
- 32.
- Dunn B. E., Phadnis S. H. Structure, function and localization of Helicobacter pylori urease. Yale J. Biol. Med. 1998;71:63–73. [PMC free article: PMC2578883] [PubMed: 10378351]
- 33.
- Dunn B. E., Vakil N. B., Schneider B. G., Miller M. M., Zitzer J. B., Peutz T., Phadnis S. H. Localization of Helicobacter pylori urease and heat shock protein in human gastric biopsies. Infect. Immun. 1997;65:1181–1188. [PMC free article: PMC175115] [PubMed: 9119449]
- 34.
- Falk P. Theoretical and experimental approaches for studying factors defining the Helicobacter pylori-host relationship. Trends Microbiol. 2000;8:321–329. [PubMed: 10878767]
- 35.
- Fernandez D., Spudich G. M., Zhou X. R., Christie P. J. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J. Bacteriol. 1996;178:3168–3176. [PMC free article: PMC178067] [PubMed: 8655495]
- 36.
- Fiocca R., Necchi V., Sommi P., Ricci V., Telford J., Cover T. L., Solcia E. Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium. J. Pathol. 1999;188:220–226. [PubMed: 10398168]
- 37.
- Frost L. S., Ippen-Ihler K., Skurray R. A. Analysis of the sequence and gene products of the transfer region of the F sex factor. Microbiol Rev. 1994;58:162–210. [PMC free article: PMC372961] [PubMed: 7915817]
- 38.
- Garcia J. L., Sanchez-Puelles J. M., Garcia P., Lopez R., Ronda C., Garcia E. Molecular characterization of an autolysin-defective mutant of Streptococcus pneumoniae. Biochem. Biophys. Res. Commun. 1986;137:614–619. [PubMed: 2873814]
- 39.
- Genin S., Boucher C. A. A superfamily of proteins involved in different secretion pathways in gram-negative bacteria: modular structure and specificity of the N-terminal domain. Mol. Gen. Genet. 1994;243:112–118. [PubMed: 8190064]
- 40.
- Gennity J. M., Inouye M. Protein secretion in bacteria. Curr. Opin. Biotechnol. 1991;2:661–667. [PubMed: 1369460]
- 41.
- Guy B., Hessler C., Fourage S., Haensler J., Vialon-Lafay E., Rokbi B., Millet M. J. Systemic immunization with urease protects mice against Helicobacter pylori infection. Vaccine. 1998;16:850–856. [PubMed: 9627943]
- 42.
- Hamilton C. M., Lee H., Li P. L., Cook D. M., Piper K. R., von Bodman S. B., Lanka E., Ream W., Farrand S. K. TraG from RP4 and TraG and VirD4 from Ti plasmids confer relaxosome specificity to the conjugal transfer system of pTiC58. J. Bacteriol. 2000;182:1541–1548. [PMC free article: PMC94450] [PubMed: 10692358]
- 43.
- Hansen G., Chilton M. D. Lessons in gene transfer to plants by a gifted microbe. Curr. Top. Microbiol. Immunol. 1999;240:21–57. [PubMed: 10394714]
- 44.
- Harris A. G., Hazell S. L., Netting A. G. Use of digoxigenin-labelled ampicillin in the identification of penicillin-binding proteins in Helicobacter pylori. J. Antimicrob. Chemother. 2000;45:591–598. [PubMed: 10797079]
- 45.
- Hirst T. R., Hillary J. B., Ruddock L. W., Yu J. Translocation of folded proteins across bacterial outer membranes: a novel secretory phenomenon. Biochem. Soc. Trans. 1995;23:985–991. [PubMed: 8654880]
- 46.
- Hirst T. R., Welch R. A. Mechanisms for secretion of extracellular proteins by gram-negative bacteria. Trends Biochem. Sci. 1988;13:265–269. [PubMed: 3076724]
- 47.
- Jacob-Dubuisson F., Kuehn M., Hultgren S. J. A novel secretion apparatus for the assembly of adhesive bacterial pili. Trends Microbiol. 1993;1:50–55. [PubMed: 7913856]
- 48.
- Kado, C. 2000. On the mechanism of horizontal gene transfer by Agrobacterium tumefaciens, p. 68–76. In Plant Genetic Engineering: towards the Third Millennium.
- 49.
- Kadurugamuwa J. L., Beveridge T. J. Bacteriolytic effect of membrane vesicles from Pseudomonas aeruginosa on other bacteria including pathogens: conceptually new antibiotics. J. Bacteriol. 1996;178:2767–2774. [PMC free article: PMC178010] [PubMed: 8631663]
- 50.
- Kadurugamuwa J. L., Beveridge T. J. Natural release of virulence factors in membrane vesicles by Pseudomonas aeruginosa and the effect of aminoglycoside antibiotics on their release. J. Antimicrob. Chemother. 1997;40:615–621. [PubMed: 9421308]
- 51.
- Kadurugamuwa J. L., Beveridge T. J. Membrane vesicles derived from Pseudomonas aeruginosa and Shigella flexneri can be integrated into the surfaces of other gram-negative bacteria. Microbiology. 1999;145:2051–2060. [PubMed: 10463171]
- 52.
- Kolling G. L., Matthews K. R. Export of virulence genes and Shiga toxin by membrane vesicles of Escherichia coli O157:H7. Appl. Environ. Microbiol. 1999;65:1843–1848. [PMC free article: PMC91264] [PubMed: 10223967]
- 53.
- Krause S., Barcena M., Pansegrau W., Lurz R., Carazo J. M., Lanka E. Sequence-related protein export NTPases encoded by the conjugative transfer region of RP4 and by the cag pathogenicity island of Helicobacter pylori share similar hexameric ring structures. Proc. Natl. Acad. Sci. USA. 2000;97:3067–3072. [PMC free article: PMC16193] [PubMed: 10716714]
- 54.
- Krause S., Pansegrau W., Lurz R., de la Cruz F., Lanka E. Enzymology of type IV macromolecule secretion systems: the conjugative transfer regions of plasmids RP4 and R388 and the cag pathogenicity island of Helicobacter pylori encode structurally and functionally related nucleoside triphosphate hydrolases. J. Bacteriol. 2000;182:2761–2770. [PMC free article: PMC101984] [PubMed: 10781544]
- 55.
- Krishnamurthy P., Parlow M., Zitzer J. B., Vakil N. B., Mobley H. L., Levy M., Phadnis S. H., Dunn B. E. Helicobacter pylori containing only cytoplasmic urease is susceptible to acid. Infect. Immun. 1998;66:5060–5066. [PMC free article: PMC108630] [PubMed: 9784504]
- 56.
- Krishnamurthy P., Parlow M. H., Schneider J., Burroughs S., Wickland C., Vakil N. B., Dunn B. E., Phadnis S. H. Identification of a novel penicillin-binding protein from Helicobacter pylori. J. Bacteriol. 1999;181:5107–5110. [PMC free article: PMC94005] [PubMed: 10438788]
- 57.
- Lai E. M., Chesnokova O., Banta L. M., Kado C. I. Genetic and environmental factors affecting T-pilin export and T-pilus biogenesis in relation to flagellation of Agrobacterium tumefaciens. J. Bacteriol. 2000;182:3705–3716. [PMC free article: PMC94541] [PubMed: 10850985]
- 58.
- Lai E. M., Kado C. I. The T-pilus of Agrobacterium tumefaciens. Trends Microbiol. 2000;8:361–369. [PubMed: 10920395]
- 59.
- Lee C. K., Soike K., Hill J., Georgakopoulos K., Tibbitts T., Ingrassia J., Gray H., Boden J., Kleanthous H., Giannasca P., Ermak T., Weltzin R., Blanchard J., Monath T. P. Immunization with recombinant Helicobacter pylori urease decreases colonization levels following experimental infection of rhesus monkeys. Vaccine. 1999;17:1493–1505. [PubMed: 10195786]
- 60.
- Lewis K. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 2000;64:503–514. [PMC free article: PMC99002] [PubMed: 10974124]
- 61.
- Li P. L., Hwang I., Miyagi H., True H., Farrand S. K. Essential components of the Ti plasmid trb system, a type IV macromolecular transporter. J. Bacteriol. 1999;181:5033–5041. [PMC free article: PMC93993] [PubMed: 10438776]
- 62.
- Li Z., Clarke A. J., Beveridge T. J. Gram-negative bacteria produce membrane vesicles which are capable of killing other bacteria. J. Bacteriol. 1998;180:5478–5483. [PMC free article: PMC107602] [PubMed: 9765585]
- 63.
- Lory S. Determinants of extracellular protein secretion in gram-negative bacteria. J. Bacteriol. 1992;174:3423–3428. [PMC free article: PMC206022] [PubMed: 1592799]
- 64.
- Marchetti M., Arico B., Burroni D., Figura N., Rappuoli R., Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. [PubMed: 7886456]
- 65.
- Marshall B. J., Barrett L. J., Prakash C., McCallum R. W., Guerrant R. L. Urea protects Helicobacter (Campylobacter) pylori from the bactericidal effect of acid. Gastroenterology. 1990;99:697–702. [PubMed: 2379775]
- 66.
- Mayrand D., Grenier D. Biological activities of outer membrane vesicles. Can. J. Microbiol. 1989;35:607–613. [PubMed: 2670152]
- 67.
- Munzenmaier A., Lange C., Glocker E., Covacci A., Moran A., Bereswill S., Baeuerle P. A., Kist M., Pahl H. L. A secreted/shed product of Helicobacter pylori activates transcription factor nuclear factor-kappa B. J. Immunol. 1997;159:6140–6147. [PubMed: 9550415]
- 68.
- Myers G. A., Ermak T. H., Georgakopoulos K., Tibbits T., Ingrassia J., Gray H., Kleanthous H., Lee C. K., Monath T. P. Oral immunization with recombinant Helicobacter pylori urease confers long-lasting immunity against Helicobacter felis infection. Vaccine. 1999;17:1394–1403. [PubMed: 10195775]
- 69.
- Novak R., Tuomanen E. Pathogenesis of pneumococcal pneumonia. Semin. Respir. Infect. 1999;14:209–217. [PubMed: 10501308]
- 70.
- Nunn D. Bacterial type II protein export and pilus biogenesis: more than just homologies? Trends Cell Biol. 1999;9:402–408. [PubMed: 10481178]
- 71.
- O'Callaghan D., Cazevieille C., Allardet-Servent A., Boschiroli M. L., Bourg G., Foulongne V., Frutos P., Kulakov Y., Ramuz M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 1999;33:1210–1220. [PubMed: 10510235]
- 72.
- Paton J. C., Andrew P. W., Boulnois G. J., Mitchell T. J. Molecular analysis of the pathogenicity of Streptococcus pneumoniae: the role of pneumococcal proteins. Annu. Rev. Microbiol. 1993;47:89–115. [PubMed: 7903033]
- 73.
- Phadnis S. H., Parlow M. H., Levy M., Ilver D., Caulkins C. M., Connors J. B., Dunn B. E. Surface localization of Helicobacter pylori urease and a heat shock protein homolog requires bacterial autolysis. Infect. Immun. 1996;64:905–912. [PMC free article: PMC173855] [PubMed: 8641799]
- 74.
- Pugsley A. P. The complete general secretory pathway in gram-negative bacteria. Microbiol. Rev. 1993;57:50–108. [PMC free article: PMC372901] [PubMed: 8096622]
- 75.
- Pugsley A. P., d'Enfert C., Reyss I., Kornacker M. G. Genetics of extracellular protein secretion by gram-negative bacteria. Annu. Rev. Genet. 1990;24:67–90. [PubMed: 2088181]
- 76.
- Radcliff F. J., Hazell S. L., Kolesnikow T., Doidge C., Lee A. Catalase, a novel antigen for Helicobacter pylori vaccination. Infect. Immun. 1997;65:4668–4674. [PMC free article: PMC175669] [PubMed: 9353048]
- 77.
- Rashkova S., Zhou X. R., Chen J., Christie P. J. Self-assembly of the Agrobacterium tumefaciens VirB11 traffic ATPase. J. Bacteriol. 2000;182:4137–4145. [PMC free article: PMC101883] [PubMed: 10894719]
- 78.
- Ream W. Import of Agrobacterium tumefaciens virulence proteins and transferred DNA into plant cell nuclei. Subcell. Biochem. 1998;29:365–384. [PubMed: 9594654]
- 79.
- Ronda C., Garcia J. L., Garcia E., Sanchez-Puelles J. M., Lopez R. Biological role of the pneumococcal amidase. Cloning of the lytA gene in Streptococcus pneumoniae. Eur. J. Biochem. 1987;164:621–624. [PubMed: 3569279]
- 80.
- Sandkvist M., Bagdasarian M. Secretion of recombinant proteins by gram-negative bacteria. Curr. Opin. Biotechnol. 1996;7:505–511. [PubMed: 8939628]
- 81.
- Schraw W., McClain M. S., Cover T. L. Kinetics and mechanisms of extracellular protein release by Helicobacter pylori. Infect. Immun. 1999;67:5247–5252. [PMC free article: PMC96877] [PubMed: 10496902]
- 82.
- Scott D. R., Weeks D., Hong C., Postius S., Melchers K., Sachs G. The role of internal urease in acid resistance of Helicobacter pylori. Gastroenterology. 1998;114:58–70. [PubMed: 9428219]
- 83.
- Segal G., Russo J. J., Shuman H. A. Relationships between a new type IV secretion system and the icm/dot virulence system of Legionella pneumophila. Mol. Microbiol. 1999;34:799–809. [PubMed: 10564519]
- 84.
- Shockman G. D. The autolytic (`suicidase') system of Enterococcus hirae: from lysine depletion autolysis to biochemical and molecular studies of the two muramidases of Enterococcus hirae ATCC 9790. FEMS Microbiol Lett. 1992;79:261–267. [PubMed: 1362171]
- 85.
- Shockman G. D., Daneo-Moore L., Kariyama R., Massidda O. Bacterial walls, peptidoglycan hydrolases, autolysins, and autolysis. Microb. Drug Resist. 1996;2:95–98. [PubMed: 9158729]
- 86.
- Sipponen P. Pathogenesis of the transformation from gastritis to malignancy. Aliment. Pharmacol. Ther. 1998;12(Suppl.):61–71. [PubMed: 9701004]
- 87.
- Spudich G. M., Fernandez D., Zhou X. R., Christie P. J. Intermolecular disulfide bonds stabilize VirB7 homodimers and VirB7/VirB9 heterodimers during biogenesis of the Agrobacterium tumefaciens T-complex transport apparatus. Proc. Natl. Acad. Sci. USA. 1996;93:7512–7517. [PMC free article: PMC38776] [PubMed: 8755505]
- 88.
- Stathopoulos C., Hendrixson D. R., Thanassi D. G., Hultgren S. J., Geme 3rd J. W. St., Curtiss 3rd R. Secretion of virulence determinants by the general secretory pathway in gram-negative pathogens: an evolving story. Microb. Infect. 2000;2:1061–1072. [PubMed: 10967286]
- 89.
- Stein M., Rappuoli R., Covacci A. Tyrosine phosphorylation of the Helicobacter pylori CagA antigen after cag-driven host cell translocation. Proc. Natl. Acad. Sci. USA. 2000;97:1263–1268. [PMC free article: PMC15590] [PubMed: 10655519]
- 90.
- Stephens C. Protein secretion: getting folded proteins across membranes. Curr. Biol. 1998;8:R578–R581. [PubMed: 9707393]
- 91.
- Stephens K. M., Roush C., Nester E. Agrobacterium tumefaciens VirB11 protein requires a consensus nucleotide-binding site for function in virulence. J. Bacteriol. 1995;177:27–36. [PMC free article: PMC176552] [PubMed: 7798144]
- 92.
- Tomb J.-F., White O., Kerlavage A. R., Clayton R. A., Sutton G. G., Fleishmann R. D., Ketchum K. A., Klenk H. P., Gill S., Dougherty B. A., Nelson K., Quackenbush J., Zhou L., Kirkness E. F., Peterson S., Loftus B., Richardson D., Dodson R., Khalak H. G., Glodek A., McKenney K., Fitzegerald L. M., Lee N., Adams M. D., Hickey E., Berg D. E., Gocayne J. D., Utterback T. R., Peterson J. D., Kelley J. M., Cotton M. D., Weidman J. M., Fujii C., Bowman C., Watthey L., Wallin E., Hayes W. S., Borodovsky M., Karp P. D., Smith H. O., Fraser C. M., Venter J. C. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. [PubMed: 9252185]
- 93.
- Tummuru M. K., Sharma S. A., Blaser M. J. Helicobacter pylori picB, a homologue of the Bordetella pertussis toxin secretion protein, is required for induction of IL-8 in gastric epithelial cells. Mol. Microbiol. 1995;18:867–876. [PubMed: 8825091]
- 94.
- Vanet A., Labigne A. Evidence for specific secretion rather than autolysis in the release of some Helicobacter pylori proteins. Infect. Immun. 1998;66:1023–1027. [PMC free article: PMC108011] [PubMed: 9488391]
- 95.
- Vogel J. P., Andrews H. L., Wong S. K., Isberg R. R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. [PubMed: 9452389]
- 96.
- Wandersman C. Secretion across the bacterial outer membrane. Trends Genet. 1992;8:317–322. [PubMed: 1365398]
- 97.
- Williams C. L., Preston T., Hossack M., Slater C., McColl K. E. Helicobacter pylori utilises urea for amino acid synthesis. FEMS Immunol. Med. Microbiol. 1996;13:87–94. [PubMed: 8821403]
- 98.
- Winans S. C., Burns D. L., Christie P. J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. [PMC free article: PMC4848025] [PubMed: 8820569]
- 99.
- Zhou L., Srisatjaluk R., Justus D. E., Doyle R. J. On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol Lett. 1998;163:223–228. [PubMed: 9673026]
- 100.
- Zhou X. R., Christie P. J. Suppression of mutant phenotypes of the Agrobacterium tumefaciens VirB11 ATPase by overproduction of VirB proteins. J. Bacteriol. 1997;179:5835–5842. [PMC free article: PMC179474] [PubMed: 9294442]
- 101.
- Zupan J., Muth T. R., Draper O., Zambryski P. The transfer of DNA from Agrobacterium tumefaciens into plants: a feast of fundamental insights. Plant J. 2000;23:11–28. [PubMed: 10929098]
- 102.
- Zupan J. R., Ward D., Zambryski P. Assembly of the VirB transport complex for DNA transfer from Agrobacterium tumefaciens to plant cells. Curr. Opin. Microbiol. 1998;1:649–655. [PubMed: 10066547]
- Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium.[J Pathol. 1999]Release of Helicobacter pylori vacuolating cytotoxin by both a specific secretion pathway and budding of outer membrane vesicles. Uptake of released toxin and vesicles by gastric epithelium.Fiocca R, Necchi V, Sommi P, Ricci V, Telford J, Cover TL, Solcia E. J Pathol. 1999 Jun; 188(2):220-6.
- Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery.[Mol Microbiol. 2007]Novel nickel transport mechanism across the bacterial outer membrane energized by the TonB/ExbB/ExbD machinery.Schauer K, Gouget B, Carrière M, Labigne A, de Reuse H. Mol Microbiol. 2007 Feb; 63(4):1054-68.
- [UreI: a Helicobacter pylori protein essential for resistance to acidity and for the early steps of murine gastric mucosa infection].[Gastroenterol Clin Biol. 2001][UreI: a Helicobacter pylori protein essential for resistance to acidity and for the early steps of murine gastric mucosa infection].Bury-Moné S, Skouloubris S, Labigne A, De Reuse H. Gastroenterol Clin Biol. 2001 Jun-Jul; 25(6-7):659-63.
- Review Structure, function and localization of Helicobacter pylori urease.[Yale J Biol Med. 1998]Review Structure, function and localization of Helicobacter pylori urease.Dunn BE, Phadnis SH. Yale J Biol Med. 1998 Mar-Apr; 71(2):63-73.
- Review The gastric biology of Helicobacter pylori.[Annu Rev Physiol. 2003]Review The gastric biology of Helicobacter pylori.Sachs G, Weeks DL, Melchers K, Scott DR. Annu Rev Physiol. 2003; 65:349-69. Epub 2002 May 1.
- Alternative Mechanisms of Protein Release - Helicobacter pyloriAlternative Mechanisms of Protein Release - Helicobacter pylori
Your browsing activity is empty.
Activity recording is turned off.
See more...