NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline. Washington (DC): National Academies Press (US); 1998.

Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline.
Show detailsSUMMARY
Vitamin B12 (cobalamin) functions as a coenzyme for a critical methyl transfer reaction that converts homocysteine to methionine and for a separate reaction that converts l-methylmalonyl-coenzyme A (CoA) to succinyl-CoA. The Recommended Dietary Allowance (RDA) for vitamin B12 is based on the amount needed for the maintenance of hematological status and normal serum vitamin B12 values. An assumed absorption of 50 percent is included in the recommended intake. The RDA for adults is 2.4 μg/day of vitamin B12. Because 10 to 30 percent of older people may be unable to absorb naturally occurring vitamin B12, it is advisable for those older than 50 years to meet their RDA mainly by consuming foods fortified with vitamin B12 or a vitamin B12-containing supplement. Individuals with vitamin B12 deficiency caused by a lack of intrinsic factor require medical treatment. The median intake of vitamin B12 from food in the United States was estimated to be approximately 5 μg/day for men and 3.5 μg/day for women. The ninety-fifth percentile of vitamin B12 intake from both food and supplements was approximately 27 μg/day. In one Canadian province the mean dietary intake was estimated to be approximately 7 μg/day for men and 4 μg/day for women. There is not sufficient scientific evidence to set a Tolerable Upper Intake Level (UL) for vitamin B12 at this time.
BACKGROUND INFORMATION
Cobalamin is the general term used to describe a group of cobalt-containing compounds (corrinoids) that have a particular structure that contains the sugar ribose, phosphate, and a base (5, 6-dimethyl benzimidazole) attached to the corrin ring. Vitamin B12 can be converted to either of the two cobalamin coenzymes that are active in human metabolism: methylcobalamin and 5-deoxyadenosylcobalamin. Although the preferred scientific use of the term vitamin B12 is usually restricted to cyanocobalamin, in this report, B12 will refer to all potentially biologically active cobalamins.
In the United States, cyanocobalamin is the only commercially available B12 preparation used in supplements and pharmaceuticals. It is also the principal form used in Canada (B. A. Cooper, Department of Hematology, Stanford University, personal communication, 1997). Another form, hydroxocobalamin, has been used in some studies of B12. Compared with hydroxocobalamin, cyanocobalamin binds to serum proteins less well and is excreted more rapidly (Tudhope et al., 1967).
Function
B12 is a cofactor for two enzymes: methionine synthase and l-methylmalonyl-CoA mutase. Methionine synthase requires methylcobalamin as a cofactor for the methyl transfer from methyltetrahydrofolate to homocysteine to form methionine and tetrahydrofolate. l-Methymalonyl-CoA mutase requires adenosylcobalamin to convert l-methymalonyl-CoA to succinyl-CoA in an isomerization reaction. In B12 deficiency, folate may accumulate in the serum as a result of slowing of the B12-dependent methyltransferase. An adequate supply of B12 is essential for normal blood formation and neurological function.
Physiology of Absorption, Metabolism, Storage, and Excretion
Small amounts of B12 are absorbed via an active process that requires an intact stomach, intrinsic factor (a glycoprotein that the parietal cells of the stomach secrete after being stimulated by food), pancreatic sufficiency, and a normally functioning terminal ileum. In the stomach, food-bound B12 is dissociated from proteins in the presence of acid and pepsin. The released B12 then binds to R proteins (haptocorrins) secreted by the salivary glands and the gastric mucosa. In the small intestine, pancreatic proteases partially degrade the R proteins, releasing B12 to bind with intrinsic factor. The resulting complex of intrinsic factor and B12 attaches to specific receptors in the ileal mucosa; after internalization of the complex, B12 enters the enterocyte. Approximately 3 to 4 hours later, B12 enters the circulation. All circulating B12 is bound to the plasma binding proteins—transcobalamin I, II, or III (TCI, TCII, or TCIII). Although TCI binds approximately 80 percent of the B12 carried in the blood, TCII is the form that delivers B12 to the tissues through specific receptors for TCII (Hall and Finkler, 1966; Seetharam and Alpers, 1982). The liver takes up approximately 50 percent of the B12 and the remainder is transported to other tissues.
If there is a lack of intrinsic factor (as is the case in the condition called pernicious anemia), malabsorption of B12 results; if this is untreated, potentially irreversible neurological damage and life-threatening anemia develop.
The average B12 content of liver tissue is approximately 1.0 μg/g of tissue in healthy adults (Kato et al., 1959; Stahlberg et al., 1967). Estimates of the average total-body B12 pool in adults range from 0.6 (Adams et al., 1972) to 3.9 mg (Grasbeck et al., 1958), but most estimates are between 2 and 3 mg (Adams, 1962; Adams et al., 1970; Heinrich, 1964; Reizenstein et al., 1966). The highest estimate found for an individual's total body B12 store was 11.1 mg (Grasbeck et al., 1958). Excretion of B12 is proportional to stores (see “Excretion”).
Absorption
Studies to measure the actual absorption of B12 involve whole-body counting of radiolabeled B12, counting of radiolabeled B12 in the stool, or both. No data are available on whether B12 absorption varies with B12 status, but fractional absorption decreases as the oral dose is increased (Chanarin, 1979). Total absorption increases with increasing intake. Adams and colleagues (1971) measured fractional absorption of radiolabeled cyanocobalamin and reported that nearly 50 percent was retained at a 1-μg dose, 20 percent at a 5-μg dose, and just over 5 percent at a 25-μg dose. The second of two doses of B12 given 4 to 6 hours apart is absorbed as well as the first (Heyssel et al., 1966). When large doses of crystalline B12 are ingested, up to approximately 1 percent of the dose may be absorbed by mass action even in the absence of intrinsic factor (Berlin et al., 1968; Doscherholmen and Hagen, 1957).
Absorption from Food. The approximate percentage absorption of B12 from a few foods is presented in Table 9-1. These values apply to normal, healthy adults. No studies were found on the absorption of B12 from dairy foods or from red meat other than mutton and liver. The absorption efficiency of B12 from liver reportedly was low because of its high B12 content. Although evidence indicates that a B12 content of 1.5 to 2.5 μg/meal saturates ileal receptors and thus limits further absorption (Scott, 1997), absorption of as much as 7 μg in one subject (18 percent) was reported from a serving of liver paste that contained 38 μg of B12 (average absorption was 4.1 μg or 11 percent) (Heyssel et al., 1966).
TABLE 9-1
Percentage Absorption of Vitamin B12 from Foods by Healthy Adults.
Assumptions Used in this Report. Because of the lack of data on dairy foods and most forms of red meat and fish, a conservative adjustment for the bioavailability of naturally occurring B12 is used in this report. In particular, it is assumed that 50 percent of dietary B12 is absorbed by healthy adults with normal gastric function. A smaller fractional absorption would apply, however, if a person consumed a large portion of foods rich in B12. Different levels of absorption are assumed under various conditions, as shown in Table 9-2. Crystalline B12 appears in the diet only in foods that have been fortified with B12, such as breakfast cereals and liquid meal replacements.
TABLE 9-2
Assumed Vitamin B12 Absorption under Different Conditions.
Enterohepatic Circulation
B12 is continually secreted in the bile. In healthy individuals most of this B12 is reabsorbed and available for metabolic functions. El Kholty et al. (1991) demonstrated that the secretion of B12 into the bile averaged 1.0 ± 0.44 nmol/day (1.4 μg/day) in eight cholecystectomized patients, and this represented 55 percent of total corrinoids. If approximately 50 percent of this B12 is assumed to be reabsorbed, the average loss of biliary B12 in the stool would be 0.5 nmol/day (0.7 μg/day). Research with baboons (Green et al., 1982) suggests that the form of B12 present in bile may be absorbed more readily than is cyanocobalamin, but the absorption of both forms was enhanced by intrinsic factor. Both Green and colleagues (1982) and Teo and coworkers (1980) reported data suggesting that bile enhances B12 absorption. However, in the absence of intrinsic factor, essentially all the B12 from the bile is excreted in the stool rather than recirculated. Thus, B12 deficiency develops more rapidly in individuals who have no intrinsic factor or who malabsorb B12 for other reasons than it does in those who become complete vegetarians and thus ingest no B12.
Excretion
If the circulating B12 exceeds the B12 binding capacity of the blood, the excess is excreted in the urine. This typically occurs only after injection of B12. The highest losses of B12 ordinarily occur through the feces. Sources of fecal B12 include unabsorbed B12 from food or bile, desquamated cells, gastric and intestinal secretions, and B12 synthesized by bacteria in the colon. Other losses occur through the skin and metabolic reactions. Fecal (Reizenstein, 1959) and urinary losses (Adams, 1970; Heinrich, 1964; Mollin and Ross, 1952) decrease when B12 stores decrease. Various studies have indicated losses of 0.1 to 0.2 percent of the B12 pool per day (Amin et al., 1980; Boddy and Adams, 1972; Bozian et al., 1963; Heinrich, 1964; Heyssel et al., 1966; Reizenstein et al., 1966) regardless of the size of the store, with the 0.2 percent value generally applicable to those with pernicious anemia.
Clinical Effects of Inadequate Intake
Hematological Effects of Deficiency
The major cause of clinically observable B12 deficiency is pernicious anemia (see “Pernicious Anemia”). The hematological effects of B12 deficiency are indistinguishable from those of folate deficiency (see Chapter 8). These include pallor of the skin associated with a gradual onset of the common symptoms of anemia, such as diminished energy and exercise tolerance, fatigue, shortness of breath, and palpitations. As in folate deficiency, the underlying mechanism of anemia is an interference with normal deoxyribonucleic acid (DNA) synthesis. This results in megaloblastic change, which causes production of larger-than-normal erythrocytes (macrocytosis). This leads first to an increase in the erythrocyte distribution width index and ultimately to an elevated mean cell volume. Oval macrocytes and other abnormally shaped erythrocytes are present in the blood. Typically, as with folate deficiency, the appearance of hypersegmentation of polymorphonuclear leukocytes precedes the development of macrocytosis. However, the sensitivity of this finding has recently been questioned (Carmel et al., 1996). By the time anemia has become established, there is usually also some degree of neutropenia and thrombocytopenia because the megaloblastic process affects all rapidly dividing bone marrow elements. The hematological complications are completely reversed by treatment with B12.
Neurological Effects of Deficiency
Neurological complications are present in 75 to 90 percent of individuals with clinically observable B12 deficiency and may, in about 25 percent of cases, be the only clinical manifestation of B12 deficiency. Evidence is mounting that the occurrence of neurological complications of B12 deficiency is inversely correlated with the degree of anemia; patients who are less anemic show more prominent neurological complications and vice versa (Healton et al., 1991; Savage et al., 1994a). Neurological manifestations include sensory disturbances in the extremities (tingling and numbness), which are worse in the lower limbs. Vibratory and position sense are particularly affected. Motor disturbances, including abnormalities of gait, also occur. Cognitive changes may occur, ranging from loss of concentration to memory loss, disorientation, and frank dementia, with or without mood changes. In addition, visual disturbances, insomnia, impotency, and impaired bowel and bladder control may develop. The progression of neurological manifestations is variable but generally gradual. Whether neurological complications are reversible after treatment depends on their duration. The neurological complications of B12 deficiency occur at a later stage of depletion than do the indicators considered below and were, therefore, not used for estimating the requirement for B12. Moreover, neurological complications are not currently amenable to easy quantitation nor are they specific to B12 deficiency.
Gastrointestinal Effects of Deficiency
B12 deficiency is also frequently associated with various gastrointestinal complaints, including sore tongue, appetite loss, flatulence, and constipation. Some of these complaints may be related to the underlying gastric disorder in pernicious anemia.
SELECTION OF INDICATORS FOR ESTIMATING THE REQUIREMENT FOR VITAMIN B12
Search of the literature revealed numerous indicators that could be considered as the basis for deriving an Estimated Average Requirement (EAR) for vitamin B12 for adults. These include but are not limited to hematological values such as erythrocyte count, hemoglobin concentration or hematocrit, and mean cell volume (MCV), blood values such as plasma B12, and the metabolite methylmalonic acid (MMA).
Indicators of Hematological Response
Measurements used to indicate a hematological response that could be considered as indicative of B12 sufficiency have consisted of either a minimal but significant increase in hemoglobin, hematocrit, and erythrocyte count; a decrease in MCV; or an optimal rise in reticulocyte number.
In the earliest studies, MCV was a calculated value that was derived from relatively imprecise erythrocyte counts. Although MCV is now directly measured and precise, the response time of this measurement to changes in dietary intake is slow because of the 120-day longevity of erythrocytes. Consequently, the MCV is of limited usefulness. The erythrocyte count, hemoglobin, and hematocrit values are all robust measurements of response. Again, however, the response time is slow before an improvement in B12 status leads to a return to normal values. Partial responses are of limited value because they do not predict the ultimate completeness or maintenance of response.
The reticulocyte count is a useful measure of hematological response because an increase is apparent within 48 hours of B12 administration and reaches a peak at 5 to 8 days.
Serum or Plasma Vitamin B12
The concentration of B12 in the serum or plasma reflects both the B12 intake and stores. The lower limit is considered to be approximately 120 to 180 pmol/L (170 to 250 pg/mL) for adults but varies with the method used and the laboratory conducting the analysis. As deficiency develops, serum values may be maintained at the expense of B12 in the tissues. Thus, a serum B12 value above the cutoff point does not necessarily indicate adequate B12 status (see the section “Vitamin B12 Deficiency”) but a low value may represent a long-term abnormality (Beck, 1991) or prolonged low intake.
Methylmalonic Acid
The range that represents expected variability (2 standard deviations) for serum MMA is 73 to 271 nmol/L (Pennypacker et al., 1992). The concentration of MMA in the serum rises when the supply of B12 is low. Elevation of MMA may also be caused by renal failure or intravascular volume depletion (Stabler et al., 1988), but Lindenbaum and coworkers (1994) reported that moderate renal dysfunction in the absence of renal failure does not affect MMA values as strongly as does inadequate B12 status. MMA values tend to rise in the elderly (Joosten et al., 1996); in most cases this appears to reflect inadequate B12 intake or absorption. Lindenbaum and coworkers (1988) reported that elevated serum MMA concentrations are present in many patients with neuropsychiatric disorders caused by B12 deficiency. Pennypacker and colleagues (1992) found that intramuscular injections of B12 reduced the elevated MMA values in their elderly subjects. The reduction of elevated MMA values with B12 therapy has also been reported in other studies (Joosten et al., 1993; Naurath et al., 1995; Norman and Morrison, 1993). Increased activity of anaerobic flora in the intestinal tract may increase serum MMA values; treatment with antibiotics decreases the serum MMA concentration in this situation (Lindenbaum et al., 1990). Because the presence of elevated concentrations of MMA in serum represents a metabolic change that is highly specific to B12 deficiency, the serum MMA concentration is a preferred indicator of B12 status. However, data were not sufficient to use MMA as the criterion on which to base the EAR in this report. Serum MMA values from older studies may not be comparable with those obtained recently because of improvements of methods over time (Beck, 1991; Green and Kinsella, 1995). More importantly, no studies were found that examined directly the relationship of B12 intake and MMA concentrations.
Homocysteine
Serum total homocysteine concentration is commonly elevated in elderly persons whose folate status is normal but who have a clinical response to treatment with B12 (Stabler et al., 1996). Because a lack of folate, vitamin B6, or both also results in an elevated serum and plasma homocysteine concentration, this indicator has poor specificity and does not provide a useful basis for deriving an EAR.
Formiminoglutamic Acid, Propionate, and Methylcitrate
Although most patients with untreated B12 deficiency excrete an increased amount of formiminoglutamic acid (FIGLU) in the urine after an oral loading dose of histidine, FIGLU excretion is also almost invariably increased in folate deficiency as well. The test, therefore, lacks specificity for the diagnosis of either vitamin deficiency. Concentrations of propionate, the metabolic precursor of methylmalonate, also rise with B12 deficiency. Propionate may be converted to 2-methylcitrate, serum and cerebrospinal fluid concentrations of which also rise in B12 deficiency (Allen et al., 1993). However, the measurement of either propionate or methyl citrate offers no advantages over serum MMA for the detection of B12 deficiency.
Holotranscobalamin II
Among the three plasma B12 binding proteins, transcobalamin II (TCII) is responsible for receptor-mediated uptake of B12 into cells. However, only a small fraction of the plasma B12 (10 to 20 percent) is present as the TCII-B12 complex. This fraction, termed holoTCII, may provide a good indication of B12 status, and methods have been described to measure this fraction (Herzlich and Herbert, 1988; Vu et al., 1993). These methods are currently considered to be insufficiently robust for routine clinical use.
METHODOLOGICAL ISSUES
Vitamin B12 Content
The two primary microbial organisms used to determine the vitamin B12 content of serum, urine, and stool are Euglena gracilis and Lactobacillus leichmannii. Although either organism will yield essentially similar results, L. leichmannii is the preferred method for reasons of convenience (Chanarin, 1969). Microbiological assays have been largely supplanted by radioligand binding assays. Until 1978 radioligand binding assays frequently gave higher results; the binding protein for B12 used in these assays would also bind analogues of B12 (Beck, 1991; Russell, 1992). Since 1978 the use of purified intrinsic factor as the binder in commercial radioisotope dilution assay kits has resulted in serum concentrations of B12 comparable with those obtained from microbiological assays. More recently, nonisotopic serum B12 assays have been introduced, which has resulted in cutoff levels for B12 deficiency again rising. Care must be taken in comparing studies because much variation has been noted across laboratories, and different cutoff points have been used to identify deficiency (Beck, 1991; Green and Kinsella, 1995; Miller et al., 1991; Rauma et al., 1995; WHO, 1970; Winawer et al., 1967).
The serum B12 value may be misleading as an indicator because it includes all the B12 regardless of the protein to which it is bound. Transcobalamin II (TCII) is the key transport protein, and it has been proposed that only the TCII-bound fraction of the serum B12 (holoTCII) is important in relation to B12 nutritional and metabolic status (Herzlich and Herbert, 1988; Vu et al., 1993). However, at this time, there is no reliable method to determine holoTCII.
Retention
Studies of the retention of parenterally administered B12 indicate that percentage retention depends on the dose and the route of administration (intramuscular [IM] or intravenous). The expected percentage retention of IM cyanocobalamin is shown in Table 9-3. These values, which vary from 15 to 100 percent, are useful when IM doses of B12 are used to estimate the B12 requirement.
TABLE 9-3
Change in Percentage Retention of Vitamin B12 with Increasing Intramuscular Dose.
DIAGNOSIS
Vitamin B12 Deficiency
Early detection of vitamin B12 deficiency depends on biochemical measurements. Lindenbaum and colleagues (1990) reported that metabolites that arise from B12 insufficiency are more sensitive indicators of B12 deficiency than is the serum B12 value. This was found in patients with pernicious anemia or previous gastrectomy who experienced early hematological relapse: serum methylmalonic acid (MMA), total homocysteine, or both were elevated in 95 percent of the instances of relapse whereas the serum B12 value was low (less than 150 pmol/L [200 pg/mL]) in 69 percent. Similarly, serum B12 was found to be an insensitive indicator in a review of records of patients with clinically significant B12 deficiency. Five deficient individuals had neurological disorders that were responsive to B12 and had elevated serum MMA and homocysteine values even though their serum B12 values were greater than 150 pmol/L (200 pg/mL) and anemia was absent or mild. In a recent series of 173 patients, 5.2 percent of those with recognized B12 deficiency had serum B12 values in the normal range. Similar findings were reported elsewhere (e.g., Carmel, 1988; Pennypacker et al., 1992; Stabler et al., 1996). At present, the techniques developed to measure serum MMA and homocysteine (capillary gas chromatography and mass spectrometry) are costly and may be beyond the scope of routine laboratories. Conditions that may warrant assessment of B12 status because they may result in B12 deficiency are summarized in Table 9-4.
TABLE 9-4
Conditions That May Result in Vitamin B12 Deficiency.
Pernicious Anemia
Pernicious anemia is the end stage of an autoimmune disorder in which parietal cell autoantibodies against H+K+-adenosine triphosphatase cause loss of gastric parietal cells. The loss of parietal cells reduces and then completely prevents production of intrinsic factor. In addition, blocking autoantibodies can bind to the B12 binding site for intrinsic factor and prevent the formation of the B12-intrinsic factor complex. Deficiency of intrinsic factor gradually results in B12 deficiency (see “Clinical Effects of Inadequate Intake”).
The prevalence of undiagnosed, untreated pernicious anemia was recently estimated to be approximately 2 percent in a nonrandom sample of free-living elderly aged 60 years or older in Southern California (Carmel, 1996). Rates were higher for white and black women than for Latin American or Asian women and for all men. These estimates are consistent with the 2.9 percent prevalence of intrinsic factor antibody in individuals older than 60 years (Krasinski et al., 1986). Earlier studies reported a higher prevalence of anti–intrinsic factor antibody in blacks with pernicious anemia than in whites with pernicious anemia (Carmel, 1992) and an earlier onset of pernicious anemia in blacks (Carmel et al., 1987; Houston et al., 1985) and Hispanics (Carmel et al., 1987). Approximately 20 percent of relatives of patients with pernicious anemia also have pernicious anemia (Toh et al., 1997). Pernicious anemia carries an excess risk of gastric carcinoma (1 to 3 percent) and of gastric carcinoid tumors (Hsing et al., 1993).
A flow sheet for the diagnosis of pernicious anemia appears in Figure 9-1. Autoantibodies to gastric parietal cells should be measured along with intrinsic factor. The demonstration of circulating intrinsic factor autoantibodies is almost diagnostic of type A gastritis and pernicious anemia (Toh et al., 1997).
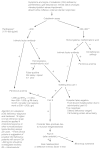
FIGURE 9-1
The diagnosis of pernicious anemia. Reprinted with permission from Green and Kinsella (1995). Copyright 1995 by Lippincott-Raven Publishers.
FACTORS AFFECTING THE VITAMIN B12 REQUIREMENT
Aging
Plasma vitamin B12 tends to decrease and serum methylmalonic acid (MMA) concentration tends to increase with age. These changes may represent a decline in B12 status. Factors that may contribute to these changes include a decrease in gastric acidity, the presence of atrophic gastritis and of bacterial overgrowth accompanied by food-bound B12 malabsorption, severity of atrophic gastritis, compromised functional and structural integrity of the B12 binding proteins, and a lack of liver B12 stores (van Asselt et al., 1996). Percentage absorption of crystalline B12 does not appear to decrease with age (McEvoy et al., 1982). In a study of 38 healthy subjects each 76 years old taken from a larger cohort study (Nilsson-Ehle et al., 1986), cyanocobalamin absorption was found to be comparable with that reported in eight other studies of healthy younger people.
Studies of absorption in the elderly have yielded somewhat contradictory results. van Asselt and coworkers (1996) found no significant difference in cobalamin absorption (either free or protein bound) between subjects younger than 64 years (median 57) and those 65 years and older (median 75 years). These investigators could not explain the high prevalence of low cobalamin values in the elderly by either the aging process or the occurrence of mild-to-moderate atrophic gastritis. In contrast Krasinski and coworkers (1986) demonstrated that although a small proportion of the elderly with atrophic gastritis have a low serum concentration of B12 (less than 88 pmol/L [120 pg/mL]), those with lowest serum B12 values tend to have severe atrophic gastritis. Scarlett and colleagues (1992) reported a reduction in dietary B12 absorption with age that was associated with elevated serum gastrin, which indicates reduced gastric acidity.
Prevalence of Atrophic Gastritis
Large differences in the prevalence of atrophic gastritis in the elderly, ranging from approximately 10 to 30 percent, have been reported in Australia (Andrews et al., 1967), Missouri (Hurwitz et al., 1997), Scandinavia (Johnsen et al., 1991), and Boston (Krasinski et al., 1986). In the general elderly population, many cases of atrophic gastritis may remain undiagnosed.
Food-Bound B12 Malabsorption
Testing of individuals who have low serum B12 values but who do not have pernicious anemia reveals a substantial proportion with malabsorption of protein-bound B12 (Carmel et al., 1987, 1988; Jones et al., 1987). More importantly, Carmel and coworkers (1988) found that 60 percent of those with neurological, cerebral, or psychological abnormalities malabsorbed food-bound B12. Food-bound malabsorption is found in persons with certain gastric dysfunctions (e.g., hypochlorhydria or achlorhydria with an intact stomach, post-gastric surgery such as Billroth I or II, and postvagotomy with pyloroplasty) and in some persons with initially unexplained low serum B12 (Carmel et al., 1988; Doscherholmen et al., 1983). Suter and colleagues (1991) reported that subjects with atrophic gastritis absorb significantly less B12 than do healthy control subjects but that the difference disappears after antibiotic therapy.
Miller and colleagues (1992) studied the absorption of radiolabeled B12 in patients who had not had gastric surgery but who had low B12 values. All patients with elevated serum gastrin levels absorbed food-bound B12 poorly compared with 21 percent of all those with normal serum gastrin values. In this study normal values were specified as greater than 12 percent absorption of food-bound B12 and greater than 33 percent absorption of free B12 as measured by direct body radioactivity measurements. Control subjects with normal serum B12 values (median 173 pmol/L [234 pg/mL], range 125 to 284 pmol/L [170 to 385 pg/mL]) absorbed 12 to 39 percent of food-bound B12 and 54 to 97 percent of free B12 (median 75 percent). The median age of this group was 61 years (range 49 to 69 years). Available evidence does not indicate that aging or atrophic gastritis increases the amount of B12 that must actually be absorbed to meet the body's needs.
Smoking
The high cyanide intake that occurs with cigarette smoking may disturb the metabolism of B12. In a study of healthy adults (Linnell et al., 1968), mean urinary B12 excretion was significantly higher in the 16 smokers than in the 16 nonsmokers (81.2 ± 8.7 [standard error] and 60.3 ± 7.9, respectively, p < 0.02), and urinary thiocyanate excretion (an index of the exogenous cyanide load) was inversely associated with serum B12. Similarly, in a study of pregnant women, the distribution of values of serum B12 was slightly lower for smokers than for nonsmokers. However, in a cross-sectional study, differences in B12 concentrations of smokers and nonsmokers were not significant in multivariate analyses. The effect of smoking on the B12 requirement thus appears to be negligible.
Gender
In a cross-sectional study of 77 young men and 82 young women (Fernandes-Costa et al., 1985), the women were found to have significantly higher serum B12 values and unsaturated cobalamin binding capacity than did the men (p < 0.001 and 0.05, respectively). Subjects were excluded if they were taking vitamin supplements, oral contraceptive agents, or other medications other than patent analgesics. Mean serum B12 values were 477 and 604 pmol/L (647 and 819 pg/mL) for men and women, respectively—well above the cutoff of adequacy. Other investigators have reported similar findings (Low-Beer et al., 1968; Metz et al., 1971). Studies that have found no difference in mean B12 values were smaller and less well-controlled for other factors that could influence B12 values (Rosner and Schreiber, 1972; Scott et al., 1974). Taken together, these studies do not provide sufficient evidence on which to quantitate a difference in B12 requirements by gender.
Nutrient-Nutrient Interactions
Folate with B12
Although adequate or high folate intake may mitigate the effects of a B12 deficiency on normal blood formation, there is no evidence that folate intake or status changes the requirement for B12.
Vitamin C with B12
Low serum B12 values reported in persons receiving megadoses of vitamin C are likely to be artifacts of the effect of ascorbate on the radioisotope assay for B12 (Herbert et al., 1978)—and thus not a true nutrient-nutrient interaction.
Other Food Components
Although it is clear that protein-bound B12 is less well absorbed than crystalline B12, the effect varies greatly with the specific protein and may be modified by gastric factors (see “Food-Bound B12 Malabsorption”). Data on absorption from different types of diets (e.g., high in dairy products or beef) are not sufficient to use as a basis for adjusting the estimated requirement for B12.
No evidence was found that a high-fiber diet increases the amount of B12 that should be consumed. A single study (Doi et al., 1983) was found that examined the effect of dietary fiber (specifically, konjac mannan, or glucomannan) on the absorption of B12. A 3.9-g dose of the fiber with a meal did not change the rate of B12 absorption in either normal subjects or those with diabetes mellitus.
Genetic Defects
Underutilization of B12 has been reported in individuals with genetic defects that involve deletions or defects of MMA-CoA mutase, transcobalamin II, or enzymes in the pathway of cobalamin adenosylation (Kano et al., 1985; Rosenberg and Fenton, 1989).
FINDINGS BY LIFE STAGE AND GENDER GROUP
Infants Ages 0 through 12 Months
Methods Used to Set the Adequate Intake
An Adequate Intake (AI) is set for the recommended intake for infants. The AI reflects the observed average vitamin B12 intake of infants fed principally with human milk.
Reported values for the concentration of the vitamin in human milk vary widely, partly because of differences in methods of analysis and partly because of differences in maternal B12 status and current intake. Despite high intraindividual diurnal variability within a group of lactating women, no consistent effect on B12 concentration of time of day, breast, or time within a feed has been demonstrated. Thus, casual samples of human milk can be used to represent concentrations for the group (Trugo and Sardinha, 1994). However, the wide intraindividual variability may lead to inaccuracies in reported mean values if the number of individuals sampled is small. Median values are substantially lower than average values (Casterline et al., 1997; Donangelo et al., 1989). Acceptable methods of analysis include Euglena gracilis after pretreatment with papain to release the vitamin from the R protein in milk and radioassays in which the vitamin is released by heating (Areekul et al., 1977; Trugo and Sardinha, 1994). Studies used for estimating the concentration of the vitamin in human milk are limited to those that used one of these two methods.
The single longitudinal study of the change in B12 concentration in human milk over time (Trugo and Sardinha, 1994) suggests somewhat higher concentrations in colostrum than in mature milk (≤ 21 days postpartum) but little change after the first month of lactation.
Ages 0 through 6 Months
The AI for infants ages 0 through 6 months is based on the B12 intake of infants fed human milk. B12 deficiency does not occur in infants fed milk from mothers with adequate B12 status. In samples collected from nine well-nourished Brazilian mothers who were not taking supplements and whose infants were receiving human milk exclusively, the average concentration of the vitamin was 0.42 μg/L at 2 months; this decreased to an average of 0.34 μg/L at 3 months (Trugo and Sardinha, 1994). Milk collected at least 2 months postpartum from 13 unsupplemented American mothers who were vegetarians was lower in B12 content, averaging 0.31 μg/L (Specker et al., 1990). The B12 content of milk in a large group of low-income Brazilian mothers (n = 83) who had received prenatal supplements containing B12 was much higher, averaging 0.91 μg/L after 1 month of lactation (Donangelo et al., 1989). Given that the average concentration at 2 months postpartum of well-nourished mothers whose infants received exclusively human milk was higher than those on vegetarian diets, the higher value of 0.42 μg/L is chosen in order to be sure adequate amounts are available. Using the average human milk volume of 0.78 L/day during the first 6 months and the higher average B12 content of 0.42 μg/L, the AI for B12 for the infant 0 through 6 months of age fed human milk would be 0.33 μg/day, rounded up to 0.4 μg.
Maintenance of Normal Methylmalonic Acid Concentrations. Data on methylmalonic acid (MMA) excretion is also available for infants. An infant may be born with low B12 stores and may consume human milk that is low in B12 if its mother is a vegan (a person who avoids all animal foods) or has untreated pernicious anemia. Such infants begin to show clinical signs of B12 deficiency at about 4 months postpartum. In the study of 13 vegan mothers and their infants, Specker and colleagues (1990) found increased urinary MMA in 2-to 14-month old (mean 7.3) infants predominantly fed human milk when the B12 concentration in human milk was below 0.49 μg/L. Assuming an average volume of human milk consumption of 0.78 L/day during the first 6 months, the infant of a vegan mother would be receiving an average of 0.24 μg/day of B12 (0.31 μg/L × 0.78 L/day).
In these infants, urinary MMA concentrations were strongly correlated with those of their mothers and inversely related to maternal plasma B12 concentrations, supporting the assumption that the elevations in infant urinary MMA were caused by poor maternal B12 status (Specker et al., 1988). Although these infants were probably born with depleted stores of the vitamin, the data suggest that a mean intake of 0.24 μg/day is inadequate to maintain B12 balance in infants.
Clinical signs of B12 deficiency are usually seen if the mother has been a strict vegetarian for at least 3 years. The B12 status of the infant is clearly abnormal by about 4 to 6 months of age. In case studies of infants born to strict vegetarians who were identified because of clinical signs of B12 deficiency, human milk concentrations have been reported to be 0.02 (Hoey et al., 1982), 0.037 (Gambon et al., 1986), 0.032 and 0.042 (Jadhav et al., 1962), 0.051 (Johnson and Roloff, 1982), and 0.085 (Kuhne et al., 1991) μg/L. If clinical signs appear within 9 months in infants consuming milk containing 0.085 μg/L of B12, this intake (approximately 0.07 μg/day) cannot support B12 requirements of the infant during the first year. However, from these data it cannot be determined how far this estimate falls below the average requirement for these infants.
Rate of Depletion of Stores. The liver of a well-nourished newborn infant contains 18 to 22 pmol (25 to 30 μg) of B12 (Baker et al., 1962; Loria et al., 1977; Vaz Pinto et al., 1975). There are no data on liver B12 content at birth in full-term infants born to depleted mothers, only data for two infants who died prematurely (Baker et al., 1962); thus, the utilization of B12 by infants remains speculative.
Summary. The AI for infants ages 0 through 6 months is 0.33 μg/day based on the average concentration of B12 in the milk of mothers with adequate B12 status. This value is rounded up to 0.4 μg/day. The adequacy of this intake is supported by evidence that it is above the intake level that has been associated with increased urinary MMA excretion.
Ages 7 through 12 Months
If the reference body weight ratio method described in Chapter 2 to extrapolate from the AI for B12 for infants ages 0 through 6 months is used, the AI for B12 for the older infants would be 0.5 μg/day after rounding up. This is a somewhat lower value than that obtained from the second method (see Chapter 2) by extrapolating down from the Estimated Average Requirement (EAR) for adults and adjusting for the expected variance to estimate a recommended intake, which results in an AI for B12 of 0.6 μg/day.
In one study of three infants exclusively fed human milk who had clinically observable B12 deficiency caused by low maternal consumption of animal products, one infant was treated parenterally with B12 whereas two infants were treated with small oral B12 doses (Jadhav et al., 1962). At 9 months of age, 0.1 μg/day of oral B12 normalized bone marrow within 5 days in one of the two infants given oral doses and produced profound improvements in behavior by 18 days (after a total of 1.8 μg of B12 had been given). The mother's milk contained 0.032 μg/L. In the second infant, who was 7 months old, 0.1 μg/day of B12 caused abnormal pigmentation to disappear and “an adequate hematologic response.” His mother's milk contained 0.042 μg/L. Although evidence of sustained recovery was not provided, it appears from these limited data that 0.1 μg/day may be adequate to improve clinical and hematological signs of deficiency in infants at this age. However, it is not known whether this level of intake is adequate to sustain normal plasma B12 and MMA concentrations or hematological response.
In the study of infants of vegan mothers (Specker et al., 1990) the mean age of infants was 7.3 months (ranged 2 to 14 months). As discussed, a mean intake of 0.23 μg/day was not adequate to maintain B12 balance in this group as determined by urinary MMA excretion.
B12 AI Summary, Ages 0 through 12 Months
| AI for Infants | ||
| 0–6 months | 0.4 μg/day of vitamin B12 | ≈0.05 μg/kg |
| 7–12 months | 0.5 μg/day of vitamin B12 | ≈0.05 μg/kg |
Special Considerations
Infants of vegan mothers should be supplemented with B12 at the AI from birth on the basis of evidence that their stores at birth are low and their mother's milk may supply very small amounts of the vitamin.
Children and Adolescents Ages 1 through 18 Years
Method Used to Estimate the Average Requirement
Only one study is available to provide data regarding B12 status and intake in young children. Plasma MMA was elevated in 11- to 22-month-old (mean 16.8 months) infants of Dutch vegan mothers. The sensitivity of plasma MMA to distinguish the group of infants born to macrobiotic mothers from those born to omnivorous mothers was 85 percent (Schneede et al., 1994). The average intake of B12 by these infants, who were exclusively fed human milk for a mean of 4.8 months and then at least partially fed human milk for 13.6 ± 6.6 (standard deviation) months and fed macrobiotic foods, was 0.3 ± 0.2 μg/day for the first 6 to 16 months compared with 2.9 ± 1.2 μg/day for well-nourished control infants (Dagnelie et al., 1991). These data suggest that an intake of 0.3 μg/day of B12 between 6 and 16 months of age was inadequate to prevent elevated plasma MMA concentrations of infants born to vegan mothers.
No other direct data were found on which to base an Estimated Average Requirement (EAR) for B12 for children or adolescents. In the absence of additional information, EARs and RDAs for children and adolescents have been estimated by using the method described in Chapter 2, which extrapolates down from adult values, and rounded up.
B12 EAR and RDA Summary, Ages 1 through 18 Years
The RDA for B12 is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for B12; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for B12 the RDA is 120 percent of the EAR).
Adults Ages 19 through 50 Years
Method Used to Estimate the Average Requirement
No single indicator was judged to be a sufficient basis for deriving an EAR for adults. It was not deemed appropriate to base the EAR on an examination limited to studies that provided data on mean cell volume (MCV) or serum B12 or any other single laboratory value. Data on men and women were examined together because of small numbers. Three general approaches were considered to derive the EAR for adults: determination of the amount of B12 needed to maintain adequate hematological status (as measured by stable hemoglobin value, normal MCV, and normal reticulocyte response) and serum B12 values in persons with pernicious anemia or with known intakes that were very low in dietary B12; use of daily B12 turnover to estimate the amount of B12 needed to maintain body stores at a specified level; and estimation of the dietary B12 intake by healthy adults that corresponds to adequate serum values of B12 and of MMA.
The first approach was chosen as the primary method for deriving an EAR because it is the only approach for which there are sufficient and reliable data for estimating need. A low serum B12 value in persons with pernicious anemia was assumed to indicate incomplete response to treatment.
Primary Criterion: Maintenance of Hematological Status and Serum B12 Values. The primary method used to derive the EAR for adults estimates the amount of B12 needed for the maintenance of hematological status and serum B12 values, primarily by using data derived from patients with pernicious anemia in remission. Data from studies of vegetarians were also examined to determine whether they provided information on levels of B12 intake needed to maintain hematological status. In some cases, neurological manifestations may be the earliest clinical sign of low B12 values (Beck, 1991; Karnaze and Carmel, 1990; Lindenbaum et al., 1988; Martin et al., 1992). Assumptions that were integral to the application of this method are shown in Box 9-1.
In brief, this method involves estimating the amount of B12 required daily to maintain hematological and serum B12 status of individuals with pernicious anemia in remission; subtracting the amount of endogenous B12 lost from the bile in excess of that lost by a healthy individual; and, because the value is to be used for individuals with normal ability to absorb B12 from food, correcting for bioavailability. The result is shown in Box 9-2.
BOX 9-2
Steps Used to Estimate the Vitamin B12 Requirement by Using Data Obtained from Subjects with Pernicious Anemia.
The following studies provide the basis for the estimate used in Step 1. These studies do not provide ideal data on which to base an EAR, but they bracket the requirement by providing values that are obviously too low or too high to meet the needs of 50 percent of the individuals in an age group.
Studies of Patients with Pernicious Anemia. Darby and coworkers (1958) studied the effects of various intramuscular (IM) doses of B12 in 20 subjects with pernicious anemia who had not previously been treated or who were in relapse. The diagnosis of pernicious anemia had been based on the clinical history and on the findings of macrocytic anemia, megaloblastic hyperplasia of bone marrow, histamine-fast achlorhydria, and a negative radiological examination of the gastrointestinal tract. These diagnoses were not made based on results of the Schilling test, first published as a method in 1953 (Schilling, 1953). The extent of the disease differed among the subjects; 14 had neurological manifestations. Of the 18 subjects who received doses of 1 μg/day of B12 or less for 2 weeks, 5 or fewer responded satisfactorily according to the standards used for erythrocytes (Isaacs et al., 1938) and reticulocytes (Isaacs and Friedman, 1938). At B12 dosages of less than 0.5 μg/day, no patient met those standards. Dosages used for maintenance were increased to 1 to 4 μg/day for a period of months to years. MCVs greater than 100 were considered macrocytic. No reticulocyte counts or serum B12 values were reported. According to the authors' interpretation, the data indicated that subjects achieved and maintained maximum erythropoiesis as indicated in Table 9-5. Approximately half (4 of 7) did so at a B12 intake of 1.4 μg/day IM.
TABLE 9-5
Effectiveness of Intramuscular Vitamin B12 Doses for Maintenance of Maximum Erythropoiesis.
Results of other studies of patients with pernicious anemia are presented in Table 9-6. The short-term study by Hansen and Weinfeld (1962) used relatively high B12 doses to restore normal status but did not assess maintenance requirement. The long-term studies by Bastrup-Madsen et al. (1983) and Lindenbaum et al. (1990) used different dosages and methods of reporting that make it impossible to draw precise conclusions. Nonetheless, the results indicate that 0.8 to 1.0 μg/day of B12 IM will maintain normal hematological, serum B12, and serum metabolite status in nearly half of the individuals over time and that 1.7 μg will maintain it in all individuals. The study conducted by Best and colleagues (1956) was designed to determine the effective dosage of intrinsic factor concentrates, not to estimate the B12 requirement, but it suggests that 1.4 μg of B12 exceeds the requirement for absorbed B12 in most of the subjects tested. The often-cited study of Sullivan and Herbert (1965) was interpreted as providing evidence that 0.1 μg/day of B12 was not sufficient for treating pernicious anemia and maintaining adequate B12 status. Similarly, the 0.6 to 0.7 μg/day of B12 supplied IM in the study by Will and coworkers (1959) was also judged too low to maintain a normal serum B12 concentration.
TABLE 9-6
Other Studies of Subjects with Pernicious Anemia Considered in Setting the Estimated Average Requirement for Vitamin B12 for Adults.
The study by Darby and colleagues (1958), which indicates an average requirement in such patients of approximately 1.5 μg, is supported by the supplementary data from the other studies described in Table 9-6. These studies provide support for a physiological average requirement of 1.0 μg/day of B12 after adjustment for the extra loss of B12 by subjects with pernicious anemia (0.5 μg/day) (Step 2 in Box 9-2). Adjusting for incomplete absorption of B12 from food of 50 percent (Step 3) converts this value to an EAR for B12 of 2.0 μg/day.
Studies of Individuals with Low B12 Intake. Studies of individuals with low B12 intake were examined to determine whether these reports (Table 9-7) supported the findings for subjects with pernicious anemia. Because B12 is not a component of plant foods, diets containing little or no animal food may lead to B12 deficiency. Deficiency develops slowly because of efficient reabsorption of biliary B12. It is also possible but not certain that vegans consume some B12 from animal products that contaminate plant food or from bacterial action. Studies of vegetarians generally have not analyzed the B12 content of the food, and accurate data are not available for some of the foods (e.g., certain algae) consumed by vegetarians. Without actual analyses it is not clear what B12 content should be assumed for vegans.
TABLE 9-7
Studies of Individuals with Low Vitamin B12 Intake Considered in Setting the Estimated Average Requirement for B12 for Adults.
The studies covered by Table 9-7 suggest that the B12 requirement is higher than the amounts reported to be consumed by the subjects and more than that provided by the treatments that were described. In three studies (Baker and Mathan, 1981; Jathar et al., 1975; Winawer et al., 1967), all adults required more than 1 μg/day of B12 by mouth. Two studies (Narayanan et al., 1991; Stewart et al., 1970) give evidence that 1.5 μg/day of dietary B12 is not sufficient to maintain hematological status and serum B12 in half of the subjects studied. The meager data provided by the studies of vegetarians indicate that the B12 average requirement should probably be at least 1.5 μg/day, but a higher average requirement is not ruled out.
Supportive Data: Maintenance of B12 Body Stores
Various studies have indicated losses of 0.1 to 0.2 percent/day of the B12 pool (e.g., Amin et al., 1980; Boddy and Adams, 1972; Heyssel et al., 1966; Reizenstein et al., 1966) regardless of the size of the pool. A loss of 0.2 percent appears to be typical for individuals who do not reabsorb biliary B12 because of pernicious anemia (Boddy and Adams, 1972). A person with a B12 pool of 1,000 μg and a loss of 0.1 percent would excrete 1 μg of B12 daily, and a person with a 3,000-μg pool would excrete 3 μg daily. If only 50 percent of dietary B12 is absorbed, the amounts required daily to replenish the pools are 2 and 6 μg of B12, respectively. The higher value would lead to less efficient use of B12, but the larger store of B12 would cover a longer period of inadequate B12 intake or absorption.
With a 0.1 percent loss, the period of protection afforded by the B12 pool can be estimated if the lowest pool size consistent with health is also known. If it is assumed that this value is 300 μg (derived from Bozian and coworkers [1963]), there is no absorption of B12 from food or supplements, and the enterohepatic circulation is intact, then stores of 1 mg would be expected to meet the body's needs for 3 years, 2 mg for about 5 years, and 3 mg for about 6 years. A 1.5 percent loss would reduce these estimates to 2, 3.6, and 4 years (see Appendix N for the method used to obtain these values).
The extent of the supply of reserve B12 may be an important consideration when persons approach the age of 50 and the risk increases for food-bound B12 malabsorption secondary to atrophic gastritis (see “Factors Affecting the Vitamin B12 Requirement” and section “Adults Ages 51 Years and Older”). Because the absorption of B12 from fortified foods, oral supplements, or the bile does not appear to be impaired, the combination of stores and absorbed crystalline B12 may cover needs for an extended period.
The estimates above for the period of protection afforded by body stores are consistent with the periods required to develop overt signs of B12 deficiency after a total gastrectomy; for example, megaloblastic anemia has been typically diagnosed 2 to 5 years after a total gastrectomy (Chanarin, 1990).
Possible Ancillary Method: Maintenance of a Serum B12 Concentration That Is Consistent with a Normal Circulating MMA Value
Several investigators have urged the use of the serum MMA concentration as the most sensitive indicator of B12 status (Lindenbaum et al., 1990; Moelby et al., 1990; Savage et al., 1994b; Stabler et al., 1996). This indicator could not be used as the criterion for setting the EAR for B12 because of a lack of direct data. At least one study (Lindenbaum et al., 1994) relates serum B12 to circulating MMA values. None link MMA with B12 intake. Moreover, although MMA is a metabolite that accumulates abnormally when the B12 supply is low, studies have not yet convincingly demonstrated that elevated MMA caused by insufficient B12 intake has adverse health consequences. However, because MMA values hold promise as a criterion for estimating the B12 requirement in the future, an indirect approach was used to estimate a requirement for B12 as a means of confirming or refining the EAR value derived by using the primary approach. For example, because the serum B12 value of 150 pmol/L (200 pg/mL) appears to be the level at which half the population would have an elevated MMA value (Lindenbaum et al., 1994), one could select the dietary intake that would maintain this value in healthy individuals in that population.
In a study of 548 surviving members of the original Framingham Heart Study cohort, aged 67 to 96 years, and 117 healthy control subjects younger than 65 years, Lindenbaum and colleagues (1994) reported on serum B12, MMA, and homocysteine values (Table 9-8). These investigators used a cutoff value equal to or greater than 260 pmol/L (350 pg/mL) of B12 as adequate; more than 15 percent of subjects below the cutoff value had elevated MMA concentrations whereas fewer than 10 percent of subjects above the cutoff did. Serum creatinine was elevated in 10 of those with both increased MMA and low B12 values, which would indicate confounding abnormal renal function. Slightly more than 40 percent of the 70 elderly subjects with serum B12 less than 150 pmol/L (200 pg/mL) had an elevated MMA concentration.
TABLE 9-8
Vitamin B12 Status: Occurrence of Low Serum Values for Two Age Groups.
Studies of B12 intake and serum B12 concentration provide very limited information on the relationship of the two. In Finland, vegans consuming an uncooked (“living food”) diet were estimated to consume a mean of 1.8 μg/day of B12 (range 0 to 12.8 μg) (Rauma et al., 1995), but the accuracy of the dietary intake data is uncertain. The 16 vegans who ate seaweed (the main source of B12 reported) had B12 concentrations twice as high as those not eating seaweed (mean of 220 pmol/L [300 pg/mL] compared with 105 pmol/L [142 pg/mL]). On this diet 57 percent of the vegans had serum B12 concentrations less than 200 pmol/L (270 pg/mL). A study by Draper and colleagues (1993) provided dietary data on vegans that were not sufficient for drawing conclusions about diet-B12 relationships. Neither Garry and coworkers (1984) nor Sahyoun and colleagues (1988) separated data with regard to supplement use, so their data are not interpretable for setting EARs. A study of a macrobiotic population (Miller et al., 1991) revealed that more than half of the adults had low serum B12 concentrations and nearly one-third were excreting high amounts of MMA, but dietary information from the study was not sufficient for drawing conclusions. Moreover, studies need to be conducted in younger persons in whom B12 absorption is more likely to be normal.
B12 EAR and RDA Summary, Ages 19 through 50 Years
On the basis of hematological evidence and serum B12 values, the EAR for B12 is estimated to be 2 μg/day for men and women ages 19 through 50 years. Sufficient data were not available to enable differences in requirements to be discerned for men and women in these age groups.
The RDA for B12 is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for B12; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for B12 the RDA is 120 percent of the EAR).
Adults Ages 51 Years and Older
Evidence Considered in Estimating the Average Requirement
Because 10 to 30 percent of people older than 50 years are estimated to have atrophic gastritis with low stomach acid secretion (Andrews et al., 1967; Hurwitz et al, 1997; Johnsen et al., 1991; Krasinski et al., 1986), they may have decreased bioavailability of B12 from food. Therefore, because of the high prevalence of this condition, 50 percent bioavailability of dietary B12 (see Box 9-2) cannot be assumed for this age group, and the EAR would be higher than 2.0 μg. Similarly, 2.4 μg of B12, which is the RDA for younger adults, might not meet the needs of 97 percent of this large age group. There is not sufficient information on which to base a bioavailability correction factor for persons with atrophic gastritis who obtain their B12 from animal foods. However, because the bioavailability of crystalline B12 is not altered in people with atrophic gastritis, the same EAR and RDA would apply if the dietary sources of B12 were foods fortified with B12, supplements, or a combination of both.
B12 EAR and RDA Summary, Ages 51 Years and Older
The EAR and RDA for B12 for adults ages 51 years and older are the same as for younger adults but with the recommendation that B12-fortified foods (such as fortified ready-to-eat cereals) or B12-containing supplements be used to meet much of the requirement.
The RDA for B12 is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for B12; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for B12 the RDA is 120 percent of the EAR).
Pregnancy
Evidence Considered in Estimating the Average Requirement
Absorption and Utilization of B12. There is some evidence that the absorption of B12 may increase during pregnancy. An increase in the number of intrinsic factor–B12 receptors was observed in pregnant mice and found to be regulated by placental lactogen (Robertson and Gallagher, 1983). A greater absorption of oral B12 was reported from the single study of pregnant women (Hellegers et al., 1957), but the methods used do not permit quantification of the increase.
Serum total B12 concentrations begin to decline early in the first trimester. In a longitudinal Dutch study of 23 subjects, serum B12 fell significantly by the end of the first trimester, more than could be accounted for by hemodilution (Fernandes-Costa and Metz, 1982). There were further decreases through the sixth month to about half of nonpregnancy concentrations. Some of the later decrease was due to hemodilution. However, transcobalamin I and III increase during the second and third trimesters, and transcobalamin II increases sharply in the third trimester to about one-third more than in nonpregnant, nonlactating control subjects (Fernandes-Costa and Metz, 1982).
Transfer to the Fetus. The serum B12 concentration of the newborn is twice that of the mother, decreasing to adult concentrations at about 6 to 7 months postpartum (Luhby et al., 1958). The placenta concentrates B12, which is then transferred to the fetus down a concentration gradient. Fetal and maternal B12 serum concentrations are quite strongly correlated (Fréry et al., 1992). It appears that only newly absorbed B12 is readily transported across the placenta and that maternal liver stores are a less important source of the vitamin for the fetus (Luhby et al., 1958). This implies that current maternal intake and absorption of the vitamin during pregnancy have a more important influence on the B12 status of the infant than do maternal B12 stores. The importance of adequate maternal intake during pregnancy is supported by the appearance of B12 deficiency in infants at 4 to 6 months when their mothers have been strict vegetarians for only 3 years (Specker et al., 1990).
Fetal Accumulation. The human fetus accumulates an average of 0.07 to 0.14 nmol/day (0.1 to 0.2 μg/day) of B12, a range based on three studies of the liver content of infants born to women who were adequate in B12 (Baker et al., 1962; Loria et al., 1977; Vaz Pinto et al., 1975) and an assumption that the liver contains half the total body B12 content. Placental B12 is negligible (0.01 nmol/L [14 ng/L]) (Muir and Landon, 1985). The low body content of B12 in the newborn implies that pregnancy is unlikely to deplete maternal stores.
B12 EAR and RDA Summary, Pregnancy
On the basis of a fetal deposition of 0.1 to 0.2 μg/day throughout pregnancy and evidence that maternal absorption of the vitamin becomes more efficient during pregnancy, the EAR is increased by 0.2 μg/day during pregnancy. No distinction is made for the age of the mother.
| EAR for Pregnancy | 14–18 years | 2.2 μg/day of vitamin B12 |
| 19–30 years | 2.2 μg/day of vitamin B12 | |
| 31–50 years | 2.2 μg/day of vitamin B12 |
The RDA for B12 is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for B12; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for B12 the RDA is 120 percent of the EAR).
| RDA for Pregnancy | 14–18 years | 2.6 μg/day of vitamin B12 |
| 19–30 years | 2.6 μg/day of vitamin B12 | |
| 31–50 years | 2.6 μg/day of vitamin B12 |
Lactation
Evidence Considered in Estimating the Average Requirement
As described earlier, the average amount of B12 secreted in the milk of mothers with adequate B12 status is approximately 0.33 μg/day during the first 6 months of lactation. During the second 6 months, the average amount of B12 secreted is slightly less: 0.25 μg/day.
The concentration of B12 in milk is usually similar to that in maternal plasma. In some studies, human milk and maternal plasma concentrations are strongly correlated (Srikantia and Reddy, 1967) but in others they are not (Casterline et al., 1997; Donangelo et al., 1989). The correlation appears to be stronger when maternal B12 status is marginal (Fréry et al., 1992).
Current maternal intake of the vitamin may have an important influence on secretion of the vitamin in milk. In several studies of infants with clinical signs of B12 deficiency caused by low maternal intake or absorption of the vitamin, maternal plasma concentrations of the vitamin were found to be normal or low normal, suggesting that maternal B12 stores are less important than current maternal intake (Hoey et al., 1982; Johnson and Roloff, 1982; Kuhne et al., 1991; Sklar, 1986). This is also indicated by the observation that the length of time that mothers had been strict vegetarians was not correlated with the urinary MMA concentrations of their infants (Specker et al., 1988).
Low B12 concentrations in human milk occur commonly in two situations involving inadequate intake: when the mother is a strict vegetarian and in developing countries where the usual consumption of animal products is low. When the B12 status of the mother is marginal, further maternal depletion may occur as reflected in decreasing concentrations of maternal plasma B12 (Black et al., 1994; Shapiro et al., 1965).
B12 EAR and RDA Summary, Lactation
To estimate the EAR for lactation, 0.33 μg/day of B12 is added to the EAR of 2 μg/day for adolescent girls and adult women; the result is rounded up.
| EAR for Lactation | 14–18 years | 2.4 μg/day of vitamin B12 |
| 19–30 years | 2.4 μg/day of vitamin B12 | |
| 31–50 years | 2.4 μg/day of vitamin B12 |
The RDA for B12 is set by assuming a coefficient of variation (CV) of 10 percent (see Chapter 1) because information is not available on the standard deviation of the requirement for B12; the RDA is defined as equal to the EAR plus twice the CV to cover the needs of 97 to 98 percent of the individuals in the group (therefore, for B12 the RDA is 120 percent of the EAR).
| RDA for Lactation | 14–18 years | 2.8 μg/day of vitamin B12 |
| 19–30 years | 2.8 μg/day of vitamin B12 | |
| 31–50 years | 2.8 μg/day of vitamin B12 |
Special Considerations
Persons with any malabsorption syndrome will likely require increased amounts of B12. Patients with pernicious anemia or Crohn's disease involving the terminal ileum and patients who have had a gastrectomy, gastric bypass surgery, or ileal resection will require B12 under a physician's direction. Persons who are positive for human immunodeficiency virus with chronic diarrhea may also require either increased oral or parenteral B12.
Patients with atrophic gastritis, pancreatic insufficiency, or prolonged omeprazole treatment (Bellou et al., 1996; Gueant et al., 1990; Suter et al., 1991; Termanini et al., 1998) will have decreased bioavailability of food-bound B12 and will require normal amounts of crystalline B12 (either in foods fortified with B12 or as a supplement).
INTAKE OF VITAMIN B12
Food Sources
Ordinarily, humans obtain vitamin B12 from animal foods. Unlike other B vitamins, B12 is not a normal constituent of plant foods except for certain algae (Ford and Hutner, 1955). B12 is not supplied by commonly eaten plant foods unless they have been exposed to bacterial action that has produced the vitamin; contaminated with soil, insects, or other substances that contain B12; or fortified with B12 (e.g., fortified ready-to-eat breakfast cereals and meal replacement formulas).
Data obtained from the 1995 Continuing Survey of Food Intakes by Individuals (CSFII) indicate that the greatest contribution to B12 intake of the U.S. adult population comes from the category of mixed foods (including sandwiches) with meat, fish, or poultry as the main ingredient (Table 9-9). For women, the second category contributing the most B12 is milk and milk drinks, whereas beef is the second category of B12 for men. Fortified ready-to-eat cereals contribute a greater proportion of dietary B12 for women than for men. The foods that are the richest sources of B12—shellfish, organ meats such as liver, some game meat, and a few kinds of fish (see Table 9-9)—are not a regular part of many people's diets.
TABLE 9-9
Food Groups Providing Vitamin B12 in the Diets of U.S. Men or Women Aged 10 Years and Older, CSFII, 1995.
Analyses of CSFII 1994 to 1995 intake data for food fortified with B12 for adults aged 51 through 70 years and older than 70 years were provided by the U.S. Department of Agriculture (A. Moshfegh, Agricultural Research Service, U.S. Department of Agriculture, personal communication, 1997). Because of the higher bioavailability of synthetic B12 than of protein-bound B12 for a substantial proportion of older adults, these results were examined to determine whether fortified foods contributed differently to the B12 content of the diet for different age groups (Table 9-10). These cross-sectional data suggest that fortified foods provide a larger proportion of the B12 consumed by older than by younger adults, especially men.
TABLE 9-10
Contribution of Fortified Foods to the Vitamin B12 Intake of U.S. Men and Women by Age Group, CSFII, 1995.
Few studies report cooking losses. However, Stewart and coworkers (1970) tested one sample and found that boiling milk for 10 minutes reduced its B12 content by about 50 percent. Reconstituted evaporated milk contains only about 25 percent of the B12 content of fluid whole milk (USDA, 1997). Such cooking losses may seriously limit B12 intake by vegetarians. Boiling milk, for example, was described as a common cooking practice among Hindu women in the United Kingdom (Stewart et al., 1970). With a B12 content of 0.4 mg/100 mL (0.9 mg/8 oz), fresh pasteurized fluid milk may be an important source of B12 for vegetarians.
Dietary Intake
Because a generous intake of animal foods is common in the United States and Canada, median B12 intake from food is well above the EAR. For example, in the United States the median daily intake from food by young adult men has been reported to be approximately 4 to 5 μg and by young adult women, 3 μg (Appendixes G and H). In one Canadian province, the mean dietary intake was reported as approximately 7 μg/day for men and 4 μg/day for women (Appendix I).
Intake by the elderly continues to be high relative to the EAR and RDA (Appendix F); however, quantitative data are not available on the amount of B12 provided by fortified foods. In a study of Boston elderly aged 60 to more than 90 years (Russell, 1992), median B12 intake by males who were not taking supplements was 3.4 μg/day. The median plasma B12 concentration for this unsupplemented group was 286 pmol/L (388 pg/mL). For females not taking supplements, the median B12 intake was 2.6 μg/day and the median plasma B12 concentration was 272 pmol/L (369 pg/mL). B12 intake was correlated with serum levels, but the actual correspondence of intake with plasma values was not determined.
Quinn and Basu (1996) reported on the dietary B12 intake estimated from 3-day (nonconsecutive) food records of 156 elderly males and females aged 65 to 77 years residing in Northern Alberta, Canada. Supplement users were excluded from the sample. The mean daily B12 intake by males was 3.7 ± 0.3 (standard error of the mean) μg and by females was 4.3 ± 1.0 μg. Mean plasma B12 was 286 ± 24 pmol/L (388 ± 33 pg/mL) for males and 335 ± 37 pmol/L (454 ± 50 pg/mL) for females, which is consistent with the difference in reported dietary intake. None of the males and 7 percent of the females had estimated intakes of less than 1.3 μg/day.
Intake from Supplements
Information from the Boston Nutritional Status Survey on supplement use of B12 by a free-living elderly population is given in Appendix F. For those taking supplements, the fiftieth percentile of supplemental B12 intake was 5.0 μg for men and 6.0 μg for women. Approximately 26 percent of all adults reported taking a B12-containing supplement in 1986 (Moss et al., 1989).
TOLERABLE UPPER INTAKE LEVELS
Hazard Identification
Adverse Effects
No adverse effects have been associated with excess B12 intake from food or supplements in healthy individuals. There is very weak evidence from animal studies suggesting that B12 intake enhances the carcinogenesis of certain chemicals (Day et al., 1950; Georgadze, 1960; Kalnev et al., 1977; Ostryanina, 1971). These findings are contradicted by evidence that increased B12 intake inhibits tumor induction in the human liver, colon, and esophagus (Rogers, 1975). Some studies suggest a possible association between high-dose, parenterally administered B12 (0.5 to 5 mg) and acne formation (Berlin et al., 1969; Dugois et al., 1969; Dupre et al., 1979; Puissant et al., 1967; Sherertz, 1991). However, the acne lesions were primarily associated with hydroxocobalamin rather than cyanocobalamin, the form used in the United States and Canada. Furthermore, iodine particles in commercial B12 preparations may have been responsible for the acne. In conclusion, the evidence from these data was considered not sufficient for deriving a Tolerable Upper Intake Level (UL).
Studies involving periodic parenteral administration of B12 (1 to 5 mg) to patients with pernicious anemia provide supportive evidence for the lack of adverse effects at high doses (Boddy and Adams, 1968; Mangiarotti et al., 1986; Martin et al., 1992). Periodic doses of 1 mg are used in standard clinical practice to treat patients with pernicious anemia. As indicated earlier, when high doses are given orally (see “Absorption”) only a small percentage of B12 can be absorbed from the gastrointestinal tract, which may explain the apparent low toxicity.
Special Considerations
B12-deficient individuals who are at risk for Leber's optic atrophy should not be given cyanocobalamin to treat the B12 deficiency. Leber's optic atrophy is a genetic disorder caused by chronic cyanide intoxication (present in tobacco smoke, alcohol, and some plants). Reduced serum B12 concentrations have been associated with a reduced ability to detoxify the cyanide in exposed individuals (Foulds, 1968, 1969a, b, 1970; Wilson and Matthews, 1966). Cyanocobalamin may increase the risk of irreversible neurological damage (from the optic atrophy). Hydroxocobalamin is a cyanide antagonist and therefore not associated with adverse effects when given to these individuals.
Dose-Response Assessment
The data on adverse effects of B12 intake were considered not sufficient for a dose-response assessment and derivation of a UL.
Intake Assessment
In 1986 approximately 26 percent of adults in the United States took a supplement containing B12 (Moss et al., 1989). Although no UL can be set for B12, an exposure assessment is provided here for possible future use. Based on data from the Third National Health and Nutrition Examination Survey (see Appendix H), the highest median intake of B12 from diet and supplements for any life stage and gender group was for males aged 31 through 50 years: 17 μg/day. The highest reported intake at the ninety-fifth percentile was 37 μg/day for pregnant females aged 14 through 55 years.
Risk Characterization
On the basis of the review of data involving high-dose intakes of B12, there appear to be essentially no risks of adverse effects to the general population even at the current ninety-fifth percentile of intake noted above. Furthermore, there appear to be no risks associated with intakes of supplemental B12 that are more than two orders of magnitude higher than the ninety-fifth percentile of intake. Although there are extensive data showing no adverse effects associated with high intakes of supplemental B12, the studies in which such intakes were reported were not designed to assess adverse effects.
RESEARCH RECOMMENDATIONS FOR VITAMIN B12
High-Priority Recommendations
Priority should be given to three topics of research related to vitamin B12:
- The prevalence of B12 deficiency as diagnosed by biochemical, neurological, or hematological abnormalities (e.g., methylmalonic acid and holotranscobalamin II).
- Improved, economical, and sensitive methods to detect B12 malabsorption and deficiency before adverse neurological and hematological changes occur.
- Effective methods to reduce the risk of suboptimal B12 status resulting from B12 malabsorption or vegetarian diets. For elderly persons with food-bound malabsorption, research is needed on the form and amount of B12 that can normalize and maintain B12 stores. For vegetarians, information is needed about the absorption of B12 from dairy products, algae, and fortified food products.
Other Research Areas
Two additional topics also merit attention:
- The feasibility and potential benefits and adverse effects of fortification of cereal grain foods with B12, considering stability, identity of any degradation products, and bioavailability for normal individuals and those who malabsorb protein-bound B12.
- The contribution of bacterial overgrowth to elevated serum methylmalonic acid.
REFERENCES
- Adams JF. The measurement of the total assayable vitamin B12 in the body. In: Heinrich HC, editor. Vitamin B12 und Intrinsic Faktor. Stuttgart, Germany: Ferdinand Enke; 1962. pp. 397–403.
- Adams JF. Correlation of serum and urine vitamin B12. Br Med J. 1970;1:138–139. [PMC free article: PMC1699041] [PubMed: 5413949]
- Adams JF, Tankel HI, MacEwan F. Estimation of the total body vitamin B12 in the live subject. Clin Sci. 1970;39:107–113. [PubMed: 4915181]
- Adams JF, Ross SK, Mervyn RL, Boddy K, King P. Absorption of cyanocobalamin, coenzyme B12, methylcobalamin, and hydroxocobalamin at different dose levels. Scand J Gastroenterol. 1971;6:249–252. [PubMed: 5560708]
- Adams JF, Boddy K, Douglas AS. Interrelation of serum vitamin B12, total body vitamin B12, peripheral blood morphology and the nature of erythropoiesis. Br J Haematol. 1972;23:297–305. [PubMed: 4628061]
- Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism. 1993;42:1448–1460. [PubMed: 7694037]
- Amin S, Spinks T, Ranicar A, Short MD, Hoffbrand AV. Long-term clearance of [57Co]cyanocobalamin in vegans and pernicious anaemia. Clin Sci. 1980;58:101–103. [PubMed: 6766367]
- Andrews GR, Haneman B, Arnold BJ, Booth JC, Taylor K. Atrophic gastritis in the aged. Australas Ann Med. 1967;16:230–235. [PubMed: 4861884]
- Areekul S, Oumarum K, Dougbarn J. Determination of vitamin B12 and vitamin B12-binding protein in human and cow's milk. Mod Med Asia. 1977;13:17–23.
- Baker SJ, Mathan VI. Evidence regarding the minimal daily requirement of dietary vitamin B12. Am J Clin Nutr. 1981;34:2423–2433. [PubMed: 7304484]
- Baker SJ, Jacob E, Rajan KT, Swaminathan SP. Vitamin B12 deficiency in pregnancy and the puerperium. Br Med J. 1962;1:1658–1661. [PMC free article: PMC1958841] [PubMed: 13864166]
- Bastrup-Madsen P, Helleberg-Rasmussen I, Norregaard S, Halver B, Hansen T. Long term therapy of pernicious anaemia with the depot cobalamin preparation Betolvex® Scand J Haematol. 1983;31:57–62. [PubMed: 6867609]
- Beck WS. Neuropsychiatric consequences of cobalamin deficiency. Adv Intern Med. 1991;36:33–56. [PubMed: 2024586]
- Bellou A, Aimone-Gastin I, De Korwin JD, Bronowicki JP, Moneret-Vautrin A, Nicolas JP, Bigard MA, Gueant JL. Cobalamin deficiency with megaloblastic anaemia in one patient under long-term omeprazole therapy. J Intern Med. 1996;240:161–164. [PubMed: 8862126]
- Berlin H, Berlin R, Brante G. Oral treatment of pernicious anemia with high doses of vitamin B12 without intrinsic factor. Acta Med Scand. 1968;184:247–258. [PubMed: 5751528]
- Berlin H, Berlin R, Brante G. Treatment with high oral doses of vitamin B12 Five years experience. Lakartidningen. 1969;66:153–158. [PubMed: 5779939]
- Best WR, White WF, Robbins KC, Landmann WA, Steelman SL. Studies on urinary excretion of vitamin B12Co60 in pernicious anemia for determining effective dosage of intrinsic factor concentrates. Blood. 1956;11:338–351. [PubMed: 13304122]
- Black AK, Allen LH, Pelto GH, de Mata M, Chávez A. Iron, vitamin B-12 and folate status in Mexico: Associated factors in men and women and during pregnancy and lactation. J Nutr. 1994;124:1179–1188. [PubMed: 8064368]
- Boddy K, Adams JF. Excretion of cobalamins and coenzyme B12 following massive parenteral doses. Am J Clin Nutr. 1968;21:657–664. [PubMed: 5666644]
- Boddy K, Adams JF. The long-term relationship between serum vitamin B12 and total body vitamin B12. Am J Clin Nutr. 1972;25:395–400. [PubMed: 4622019]
- Bozian RC, Ferguson JL, Heyssel RM, Meneely GR, Darby WJ. Evidence concerning the human requirement for vitamin B12. Use of the whole body counter for determination of absorption of vitamin B12. Am J Clin Nutr. 1963;12:117–129. [PubMed: 14014759]
- Carmel R. Pernicious anemia. The expected findings of very low serum cobalamin levels, anemia, and macrocytosis are often lacking. Arch Intern Med. 1988;148:1712–1714. [PubMed: 3401092]
- Carmel R. Reassessment of the relative prevalences of antibodies to gastric parietal cell and to intrinsic factor in patients with pernicious anaemia: Influence of patient age and race. Clin Exp Immunol. 1992;89:74–77. [PMC free article: PMC1554402] [PubMed: 1628426]
- Carmel R. Prevalence of undiagnosed pernicious anemia in the elderly. Arch Intern Med. 1996;156:1097–1100. [PubMed: 8638997]
- Carmel R, Sinow RM, Karnaze DS. Atypical cobalamin deficiency. Subtle biochemical evidence of deficiency is commonly demonstrable in patients without megaloblastic anemia and is often associated with protein-bound cobalamin malabsorption. J Lab Clin Med. 1987;109:454–463. [PubMed: 3819580]
- Carmel R, Sinow RM, Siegel ME, Samloff IM. Food cobalamin malabsorption occurs frequently in patients with unexplained low serum cobalamin levels. Arch Intern Med. 1988;148:1715–1719. [PubMed: 3401093]
- Carmel R, Green R, Jacobsen DW, Qian GD. Neutrophil nuclear segmentation in mild cobalamin deficiency: Relation to metabolic tests of cobalamin status and observations on ethnic differences in neutrophil segmentation. Am J Clin Pathol. 1996;106:57–63. [PubMed: 8701933]
- Casterline JE, Allen LH, Ruel MT. Vitamin B-12 deficiency is very prevalent in lactating Guatemalan women and their infants at three months postpartum. J Nutr. 1997;127:1966–1972. [PubMed: 9311952]
- Chanarin I. The Megaloblastic Anaemias. 1st ed. Oxford: Blackwell Scientific; 1969.
- Chanarin I. The Megaloblastic Anaemias. 2nd ed. Oxford: Blackwell Scientific; 1979.
- Chanarin I. The Megaloblastic Anaemias. 3rd ed. Boston: Blackwell Scientific; 1990.
- Dagnelie PC, van Staveren WA, Hautvast JG. Stunting and nutrient deficiences in children on alternative diets. Acta Pediatr Scand Suppl. 1991;374:111–118. [PubMed: 1957614]
- Darby WJ, Bridgforth EB, Le Brocquy J, Clark SL, De Oliviera JD, Kevany J, McGanity WJ, Perez C. Vitamin B12 requirement of adult man. Am J Med. 1958;25:726–732. [PubMed: 13582982]
- Day PL, Payne LD, Dinning JS. Procarcinogenic effect of vitamin B12 on p-dimethylaminoazobenzene-fed rats. Proc Soc Exp Biol Med. 1950;74:854–857. [PubMed: 14781201]
- Doi K, Matsuura M, Kawara A, Tanaka T, Baba S. Influence of dietary fiber (konjac mannan) on absorption of vitamin B12 and vitamin E. Tohoku J Exp Med. 1983;141:677–681. [PubMed: 6096987]
- Donangelo CM, Trugo NM, Koury JC, Barreto Silva MI, Freitas LA, Feldheim W, Barth C. Iron, zinc, folate and vitamin B12 nutritional status and milk composition of low-income Brazilian mothers. Eur J Clin Nutr. 1989;43:253–266. [PubMed: 2661218]
- Doscherholmen A, Hagen PS. A dual mechanism of vitamin B12 plasma absorption. J Clin Invest. 1957;36:1551–1557. [PMC free article: PMC1072759] [PubMed: 13475493]
- Doscherholmen A, McMahon J, Ripley D. Vitamin B12 absorption from eggs. Proc Soc Exp Biol Med. 1975;149:987–990. [PubMed: 1172618]
- Doscherholmen A, McMahon J, Ripley D. Vitamin B12 assimilation from chicken meat. Am J Clin Nutr. 1978;31:825–830. [PubMed: 645629]
- Doscherholmen A, McMahon J, Economon P. Vitamin B12 absorption from fish. Proc Soc Exp Biol Med. 1981;167:480–484. [PubMed: 7279926]
- Doscherholmen A, Silvis S, McMahon J. Dual isotope Schilling test for measuring absorption of food-bound and free vitamin B12 simultaneously. Am J Clin Pathol. 1983;80:490–495. [PubMed: 6684880]
- Draper A, Lewis J, Malhotra N, Wheeler E. The energy and nutrient intakes of different types of vegetarian: A case for supplements? Br J Nutr. 1993;69:3–19. [PubMed: 8457537]
- Dugois P, Amblard P, Imbert R, Bignicourt B. Acne caused by vitamin B12. Lyon Med. 1969;221:1165–1167. [PubMed: 4249537]
- Dupre A, Albarel N, Bonafe JL, Christol B, Lassere J. Vitamin B12-induced acnes. Cutis. 1979;24:210–211. [PubMed: 157854]
- El Kholty S, Gueant JL, Bressler L, Djalali M, Boissel P, Gerard P, Nicolas JP. Portal and biliary phases of enterohepatic circulation of corrinoids in humans. Gastroenterology. 1991;101:1399–1408. [PubMed: 1936810]
- Fernandes-Costa F, Metz J. Levels of transcobalamins I, II, and III during pregnancy and in cord blood. Am J Clin Nutr. 1982;35:87–94. [PubMed: 7064881]
- Fernandes-Costa F, van Tonder S, Metz J. A sex difference in serum cobalamin and transcobalamin levels. Am J Clin Nutr. 1985;41:784–786. [PubMed: 3984931]
- Ford JE, Hutner SH. Role of vitamin B12 in the metabolism of micro-organisms. Vitam Horm. 1955;13:101–136. [PubMed: 13291771]
- Foulds WS. Hydroxocobalamin in the treatment of Leber's hereditary optic atrophy. Lancet. 1968;1:896–897. [PubMed: 4171494]
- Foulds WS. Cyanide induced optic neuropathy. Ophthalmologica. 1969;158:350–358. [PubMed: 5359735]
- Foulds WS. The optic neuropathy of pernicious anemia. Arch Ophthalmol. 1969;82:427–432. [PubMed: 5344936]
- Foulds WS. The investigation and therapy of the toxic amblyopias. Trans Ophthalmol Soc UK. 1970;90:739–763. [PubMed: 5314698]
- Fréry N, Huel G, Leroy M, Moreau T, Savard R, Blot P, Lellouch J. Vitamin B12 among parturients and their newborns and its relationship with birth-weight. Eur J Obstet Gynecol Reprod Biol. 1992;45:155–163. [PubMed: 1511760]
- Gambon RC, Lentze MJ, Rossi E. Megaloblastic anaemia in one of monozygous twins breast fed by their vegetarian mother. Eur J Pediatr. 1986;145:570–571. [PubMed: 3816865]
- Garry PJ, Goodwin JS, Hunt WC. Folate and vitamin B12 status in a healthy elderly population. J Am Geriatr Soc. 1984;32:719–726. [PubMed: 6481051]
- Georgadze GE. Effect of vitamin B1 and B12 on induction of malignant growths in hamsters. Vopr Onkol. 1960;6:54–58. [PubMed: 13704446]
- Grasbeck T, Nyberg W, Reizenstein P. Biliary and fecal vitamin B12 excretion in man. An isotope study. Proc Soc Exp Biol Med. 1958;97:780–784. [PubMed: 13554481]
- Green R, Kinsella LJ. Current concepts in the diagnosis of cobalamin deficiency. Neurology. 1995;45:1435–1440. [PubMed: 7644036]
- Green R, Jacobsen DW, Van Tonder SV, Kew MC, Metz J. Absorption of biliary cobalamin in baboons following total gastrectomy. J Lab Clin Med. 1982;100:771–777. [PubMed: 7130832]
- Gueant JL, Champigneulle B, Gaucher P, Nicolas JP. Malabsorption of vitamin B12 in pancreatic insufficiency of the adult and of the child. Pancreas. 1990;5:559–567. [PubMed: 2235967]
- Hall CA, Finkler AE. Function of transcobalamin II: A B12 binding protein in human plasma. Proc Soc Exp Biol Med. 1966;123:55–58. [PubMed: 5924456]
- Hansen HA, Weinfeld A. Metabolic effects and diagnostic value of small doses of folic acid and B12 in megaloblastic anemias. Acta Med Scand. 1962;172:427–443. [PubMed: 13952598]
- Healton EB, Savage DG, Brust JC, Garrett TJ, Lindenbaum J. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore). 1991;70:229–245. [PubMed: 1648656]
- Heinrich HC. Metabolic basis of the diagnosis and therapy of vitamin B12 deficiency. Semin Hematol. 1964;1:199–249. [PubMed: 14184274]
- Hellegers A, Okuda K, Nesbitt RE Jr, Smith DW, Chow BF. Vitamin B12 absorption in pregnancy and in the newborn. Am J Clin Nutr. 1957;5:327–331. [PubMed: 13424485]
- Herbert V, Jacob E, Wong KT, Scott J, Pfeffer RD. Low serum vitamin B12 levels in patients receiving ascorbic acid in megadoses: Studies concerning the effect of ascorbate on radioisotope vitamin B12 assay. Am J Clin Nutr. 1978;31:253–258. [PubMed: 23674]
- Herzlich B, Herbert V. Depletion of serum holotranscobalamin II. An early sign of negative vitamin B12 balance. Lab Invest. 1988;58:332–337. [PubMed: 3347009]
- Heyssel RM, Bozian RC, Darby WJ, Bell MC. Vitamin B12 turnover in man. The assimilation of vitamin B12 from natural foodstuff by man and estimates of minimal daily requirements. Am J Clin Nutr. 1966;18:176–184. [PubMed: 5948742]
- Hoey H, Linnell JC, Oberholzer VG, Laurance BM. Vitamin B12 deficiency in a breastfed infant of a mother with pernicious anaemia. J R Soc Med. 1982;75:656–658. [PMC free article: PMC1438012] [PubMed: 7108883]
- Houston GA, Files JC, Morrison FS. Race, age, and pernicious anemia. South Med J. 1985;78:69–70. [PubMed: 3966173]
- Hsing AW, Hansson LE, McLaughlin JK, Nyren O, Blot WJ, Ekbom A, Faumeni JF. Pernicious anemia and subsequent cancer: A population-based cohort study. Cancer. 1993;71:745–750. [PubMed: 8431855]
- Hurwitz A, Brady DA, Schaal SE, Samloff IM, Dedon J, Ruhl CE. Gastric acidity in older adults. J Am Med Assoc. 1997;278:659–662. [PubMed: 9272898]
- Isaacs R, Friedman A. Standards for maximum reticulocyte percentage after intramuscular liver therapy in pernicious anemia. Am J Med Sci. 1938;196:718–719.
- Isaacs R, Bethell FH, Riddle MC, Friedman A. Standards for red blood cell increase after liver and stomach therapy in pernicious anemia. JAMA. 1938;111:2291.
- Jadhav M, Webb JK, Vaishnava S, Baker SJ. Vitamin B12 deficiency in Indian infants. Lancet. 1962;1962:903–907. [PubMed: 13964414]
- Jathar VS, Inamdar-Deshmukh AB, Rege DV, Satoskar RS. Vitamin B12 and vegetarianism in India. Acta Haematol. 1975;53:90–97. [PubMed: 804798]
- Johnsen R, Bernersen B, Straume B, Forde OH, Bostad L, Burhol PG. Prevalences of endoscopic and histological findings in subjects with and without dyspepsia. Br Med J. 1991;302:749–752. [PMC free article: PMC1669538] [PubMed: 2021764]
- Johnson PR Jr, Roloff JS. Vitamin B12 deficiency in an infant strictly breastfed by a mother with latent pernicious anemia. J Pediatr. 1982;100:917–919. [PubMed: 7086591]
- Jones BP, Broomhead AF, Kwan YL, Grace CS. Incidence and clinical significance of protein-bound vitamin B12 malabsorption. Eur J Haematol. 1987;38:131–136. [PubMed: 3595808]
- Joosten E, Pelemans W, Devos P, Lesaffre E, Goossens W, Criel A, Verhaeghe R. Cobalamin absorption and serum homocysteine and methylmalonic acid in elderly subjects with low serum cobalamin. Eur J Haematol. 1993;51:25–30. [PubMed: 8348941]
- Joosten E, Lesaffre E, Riezler R. Are different reference intervals for methylmalonic acid and total homocysteine necessary in elderly people? Eur J Haematol. 1996;57:222–226. [PubMed: 8898926]
- Kalnev VR, Rachkus I, Kanopkaite SI. Influence of methylcobalamin and cyanocobalamin on the neoplastic process in rats. Prikl Biochim Mikrobiol. 1977;13:677. [PubMed: 199903]
- Kano Y, Sakamoto S, Miura Y, Takaku F. Disorders of cobalamin metabolism. Crit Rev Oncol Hematol. 1985;3:1–34. [PubMed: 2861915]
- Karnaze DS, Carmel R. Neurologic and evoked potential abnormalities in subtle cobalamin deficiency states, including deficiency without anemia and with normal absorption of free cobalamin. Arch Neurol. 1990;47:1008–1012. [PubMed: 2396929]
- Kato N, Narita Y, Kamohara S. Liver vitamin B12 levels in chronic liver diseases. J Vitam. 1959;5:134–140. [PubMed: 14404737]
- Krasinski SD, Russell RM, Samloff IM, Jacob RA, Dallal GE, McGandy RB, Hartz SC. Fundic atrophic gastritis in an elderly population: Effect on hemoglobin and several serum nutritional indicators. J Am Geriatr Soc. 1986;34:800–806. [PubMed: 3771980]
- Kuhne T, Bubl R, Baumgartner R. Maternal vegan diet causing a serious infantile neurological disorder due to vitamin B12 deficiency. Eur J Pediatr. 1991;150:205–208. [PubMed: 2044594]
- Lindenbaum J, Healton EB, Savage DG, Brust JC, Garrett TJ, Podell ER, Marcell PD, Stabler SP, Allen RH. Neuropsychiatric disorders caused by cobalamin deficiency in the absence of anemia or macrocytosis. N Engl J Med. 1988;318:1720–1728. [PubMed: 3374544]
- Lindenbaum J, Savage DG, Stabler SP, Allen RH. Diagnosis of cobalamin deficiency: 2. Relative sensitivities of serum cobalamin, methylmalonic acid, and total homocysteine concentrations. Am J Hematol. 1990;34:99–107. [PubMed: 2339684]
- Lindenbaum J, Rosenberg IH, Wilson PW, Stabler SP, Allen RH. Prevalence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr. 1994;60:2–11. [PubMed: 8017332]
- Linnell JC, Smith AD, Smith CL, Wilson J, Matthews DM. Effects of smoking on metabolism and excretion of vitamin B12. Br Med J. 1968;2:215–216. [PMC free article: PMC1985886] [PubMed: 5653029]
- Loria A, Vaz-Pinto A, Arroyo P, Ramirez-Mateos C, Sanchez-Medal L. Nutritional anemia. 6. Fetal hepatic storage of metabolites in the second half of pregnancy. J Pediatr. 1977;91:569–573. [PubMed: 908975]
- Low-Beer TS, McCarthy CF, Austad WI, Brzechwa-Ajdukiewicz A, Read AE. Serum vitamin B12 levels and vitamin B12 binding capacity in pregnant and non-pregnant Europeans and West Indians. Br Med J. 1968;4:160–161. [PMC free article: PMC1911963] [PubMed: 5681054]
- Luhby AL, Cooperman JM, Donnenfeld AM, Herrero JM, Teller DN, Wenig JB. Observations on transfer of vitamin B12 from mother to fetus and newborn. Am J Dis Child. 1958;96:532–533.
- Mangiarotti G, Canavese C, Salomone M, Thea A, Pacitti A, Gaido M, Calitri V, Pelizza D, Canavero W, Vercellone A. Hypervitaminosis B12 in maintenance hemodialysis patients receiving massive supplementation of vitamin B12. Int J Artif Organs. 1986;9:417–420. [PubMed: 3818116]
- Martin DC, Francis J, Protetch J, Huff J. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168–172. [PubMed: 1740602]
- McEvoy AW, Fenwick JD, Boddy K, James OF. Vitamin B12 absorption from the gut does not decline with age in normal elderly humans. Age Ageing. 1982;11:180–183. [PubMed: 7124557]
- Metz J, Hart D, Harpending HC. Iron, folate, and vitamin B12 nutrition in a hunter-gatherer people: A study of the Kung Bushmen. Am J Clin Nutr. 1971;24:229–242. [PubMed: 5545850]
- Miller DR, Specker BL, Ho L, Norman EJ. Vitamin B-12 status in a macrobiotic community. Am J Clin Nutr. 1991;53:524–529. [PubMed: 1989421]
- Miller A, Furlong D, Burrows BA, Slingerland DW. Bound vitamin B12 absorption in patients with low serum B12 levels. Am J Hematol. 1992;40:63–166. [PubMed: 1609768]
- Moelby L, Rasmussen K, Jensen MK, Pedersen KO. The relationship between clinically confirmed cobalamin deficiency and serum methylmalonic acid. J Intern Med. 1990;228:373–378. [PubMed: 2266346]
- Mollin DL, Ross GI. The vitamin B12 concentrations of serum and urine of normals and of patients with megaloblastic anaemias and other diseases. J Clin Pathol. 1952;5:129–139. [PMC free article: PMC1023543] [PubMed: 14938452]
- Moss AJ, Levy AS, Kim I, Park YK. Use of Vitamin and Mineral Supplements in the United States: Current Users, Types of Products, and Nutrients. Hyattsville, MD: National Center for Health Statistics; 1989. Advance Data, Vital and Health Statistics of the National Center for Health Statistics No. 174.
- Muir M, Landon M. Endogenous origin of microbiologically-inactive cobalamins (cobalamin analogues) in the human fetus. Br J Haematol. 1985;61:303–306. [PubMed: 4041374]
- Narayanan MN, Dawson DW, Lewis MJ. Dietary deficiency of vitamin B12 is associated with low serum cobalamin levels in non-vegetarians. Eur J Haematol. 1991;47:115–118. [PubMed: 1889479]
- Naurath HJ, Joosten E, Riezler R, Stabler SP, Allen RH, Lindenbaum J. Effects of vitamin B12, folate, and vitamin B6 supplements in elderly people with normal serum vitamin concentrations. Lancet. 1995;346:85–89. [PubMed: 7603218]
- Nilsson-Ehle H, Jagenburg R, Landahl S, Lindstedt G, Swolin B, Westin J. Cyanocobalamin absorption in the elderly: Results for healthy subjects and for subjects with low serum cobalamin concentration. Clin Chem. 1986;32:1368–1371. [PubMed: 3719947]
- Norman EJ, Morrison JA. Screening elderly populations for cobalamin (vitamin B12) deficiency using the urinary methylmalonic acid assay by gas chromatography mass spectrometry. Am J Med. 1993;94:589–594. [PubMed: 8506883]
- Ostryanina AD. Effect of vitamin B12 on the induction of tumors in mouse skin. Patol Fiziol Eksperim Terapiya. 1971;15:48–53. [PubMed: 5141928]
- Pennypacker LC, Allen RH, Kelly JP, Matthews LM, Grigsby J, Kaye K, Lindenbaum J, Stabler SP. High prevalence of cobalamin deficiency in elderly outpatients. J Am Geriatr Soc. 1992;40:1197–1204. [PubMed: 1447433]
- Puissant A, Vanbremeersch F, Monfort J, Lamberton JN. A new iatrogenic dermatosis: Acne caused by vitamin B12. Bull Soc Fr Dermatol Syphiligr. 1967;74:813–815. [PubMed: 4232220]
- Quinn K, Basu TK. Folate and vitamin B12 status of the elderly. Eur J Clin Nutr. 1996;50:340–342. [PubMed: 8793412]
- Rauma AL, Torronen R, Hanninen O, Mykkanen H. Vitamin B-12 status of long-term adherents of a strict uncooked vegan diet (“living food diet”) is compromised. J Nutr. 1995;125:2511–2515. [PubMed: 7562085]
- Reizenstein P. Excretion of non-labeled vitamin B12 in man. Acta Med Scand. 1959;165:313–320. [PubMed: 13854492]
- Reizenstein P, Ek G, Matthews CM. Vitamin B12 kinetics in man. Implications on total-body B12 determinations, human requirements, and normal and pathological cellular B12 uptake. Phys Med Biol. 1966;11:295–306. [PubMed: 5954616]
- Robertson JA, Gallagher ND. Increased intestinal uptake of cobalamin in pregnancy does not require synthesis of new receptors. Biochim Biophys Acta. 1983;757:145–150. [PubMed: 6303438]
- Rogers AE. Variable effects of a lipotrobe-deficient, high-fat diet on chemical carcinogens in rats. Cancer Res. 1975;35:2469–2474. [PubMed: 50131]
- Rosenberg LE, Fenton WA. Disorders of propionate and methylmalonate metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic Basis of Inherited Disease. 6th ed. New York: McGraw-Hill; 1989. pp. 821–844.
- Rosner F, Schreiber ZA. Serum vitamin B12 and vitamin B12 binding capacity in chronic myelogenous leukemia and other disorders. Am J Med Sci. 1972;263:473–480. [PubMed: 4505888]
- Russell RM. Vitamin B12. In: Hartz SC, Russell RM, Rosenberg IH, editors. Nutrition in the Elderly The Boston Nutritional Status Survey. London: Smith-Gordon; 1992. pp. 141–145.
- Sahyoun NR, Otradovec CL, Hartz SC, Jacob RA, Peters H, Russell RM, McGandy RB. Dietary intakes and biochemical indicators of nutritional status in an elderly, institutionalized population. Am J Clin Nutr. 1988;47:524–533. [PubMed: 3348164]
- Savage D, Gangaidzo I, Lindenbaum J. Vitamin B12 deficiency is the primary cause of megaloblastic anemia in Zimbabwe. Br J Haematol. 1994;86:844–850. [PubMed: 7880241]
- Savage DG, Lindenbaum J, Stabler SP, Allen RH. Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med. 1994;96:239–246. [PubMed: 8154512]
- Scarlett JD, Read H, O'Dea K. Protein-bound cobalamin absorption declines in the elderly. Am J Hematol. 1992;39:79–83. [PubMed: 1550110]
- Schilling RF. Intrinsic factor studies II. The effect of gastric juice on the urinary excretion of radioactivity after the oral administration of radioactive vitamin B12. J Lab Clin Med. 1953;42:860–866. [PubMed: 13109347]
- Schneede J, Dagnelie PC, van Staveren WA, Vollset SE, Refsum H, Ueland PM. Methylmalonic acid and homocysteine in plasma as indicators of functional cobalamin deficiency in infants on macrobiotic diets. Pediatr Res. 1994;36:194–201. [PubMed: 7970934]
- Scott JM. Bioavailability of vitamin B12. Eur J Clin Nutr. 1997;51 Suppl 1:S49–S53. [PubMed: 9023481]
- Scott JM, Bloomfield FJ, Stebbins R, Herbert V. Studies on derivation of transcobalamin 3 from granulocytes. Enhancement by lithium and elimination by fluoride of in vitro increments in vitamin B12-binding capacity. J Clin Invest. 1974;53:228–239. [PMC free article: PMC301458] [PubMed: 4202670]
- Seetharam B, Alpers DH. Absorption and transport of cobalamin (vitamin B12). Annu Rev Nutr. 1982;2:343–369. [PubMed: 6313022]
- Shapiro J, Alberts HW, Welch P, Metz J. Folate and vitamin B12 deficiency associated with lactation. Br J Haematol. 1965;11:498–504. [PubMed: 14317444]
- Sherertz EF. Acneiform eruption due to “megadose” vitamins B6 and B12. Cutis. 1991;48:119–120. [PubMed: 1834437]
- Sklar R. Nutritional vitamin B12 deficiency in a breast-fed infant of a vegan-diet mother. Clin Pediatr. 1986;25:219–221. [PubMed: 3948463]
- Specker BL, Miller D, Norman EJ, Greene H, Hayes KC. Increased urinary methylmalonic acid excretion in breast-fed infants of vegetarian mothers and identification of an acceptable dietary source of vitamin B12. Am J Clin Nutr. 1988;47:89–92. [PubMed: 3337042]
- Specker BL, Black A, Allen L, Morrow F. Vitamin B-12: Low milk concentrations are related to low serum concentrations in vegetarian women and to methylmalonic aciduria in their infants. Am J Clin Nutr. 1990;52:1073–1076. [PubMed: 2239784]
- Srikantia SG, Reddy V. Megaloblastic anaemia of infancy and vitamin B12. Br J Haematol. 1967;13:949–953. [PubMed: 6075449]
- Stabler SP, Marcell PD, Podell ER, Allen RH, Savage DG, Lindenbaum J. Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J Clin Invest. 1988;81:466–474. [PMC free article: PMC329593] [PubMed: 3339129]
- Stabler SP, Lindenbaum J, Allen RH. The use of homocysteine and other metabolites in the specific diagnosis of vitamin B-12 deficiency. J Nutr. 1996;126:1266S–1272S. [PubMed: 8642468]
- Stahlberg KG, Radner S, Norden A. Liver B12 in subjects with and without vitamin B12 deficiency. A quantitative and qualitative study. Scand J Haematol. 1967;4:312–330. [PubMed: 6078062]
- Stewart JS, Roberts PD, Hoffbrand AV. Response of dietary vitamin B12 deficiency to physiological oral doses of cyanocobalamin. Lancet. 1970;2:542–545. [PubMed: 4195205]
- Sullivan LW, Herbert V. Studies on the minimum daily requirement for vitamin B12. Hematopoietic responses to 0.1 microgram of cyanocobalamin or coenzyme B12 and comparison of their relative potency. N Engl J Med. 1965;272:340–346. [PubMed: 14239115]
- Suter PM, Golner BB, Goldin BR, Morrow FD, Russell RM. Reversal of protein-bound vitamin B12 malabsorption with antibiotics in atrophic gastritis. Gastroenterology. 1991;101:1039–1045. [PubMed: 1889697]
- Teo NH, Scott JM, Neale G, Weir DG. Effect of bile on vitamin B12 absorption. Br Med J. 1980;281:831–833. [PMC free article: PMC1714280] [PubMed: 7427470]
- Termanini B, Gibril F, Sutliff VE, Yu F, Venzon DJ, Jensen RT. Effect of long-term gastric acid suppressive therapy on serum vitamin B12 levels in patients with Zollinger-Ellison syndrome. Am J Med. 1998;104:422–430. [PubMed: 9626024]
- Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441–1448. [PubMed: 9358143]
- Trugo NM, Sardinha F. Cobalamin and cobalamin-binding capacity in human milk. Nutr Res. 1994;14:22–33.
- Tudhope GR, Swan HT, Spray GH. Patient variation in pernicious anaemia, as shown in a clinical trial of cyanocobalamin, hydroxocobalamin and cyanocobalamin-zinc tannate. Br J Haematol. 1967;13:216–228. [PubMed: 6019807]
- USDA (U.S. Department of Agriculture). USDA, ARS Nutrient Data Laboratory. 1997. [WWW document]. URL http://www
.nal.usda.gov/fnic/foodcomp/ - van Asselt DZ, van den Broek WJ, Lamers CB, Corstens FH, Hoefnagels WH. Free and protein-bound cobalamin absorption in healthy middle-aged and older subjects. J Am Geriatr Soc. 1996;44:949–953. [PubMed: 8708306]
- Vaz Pinto A, Torras V, Sandoval JF, Dillman E, Mateos CR, Cordova MS. Folic acid and vitamin B12 determination in fetal liver. Am J Clin Nutr. 1975;28:1085–1086. [PubMed: 1180242]
- Vu T, Amin J, Ramos M, Flener V, Vanyo L, Tisman G. New assay for the rapid determination of plasma holotranscobalamin II levels: Preliminary evaluation in cancer patients. Am J Hematol. 1993;42:202–211. [PubMed: 8438881]
- WHO (World Health Organization). Requirements of Ascorbic Acid, Vitamin D, Vitamin B12, Folate, and Iron. Geneva: WHO; 1970. Report of a Joint FAO/WHO Expert Group. Technical Report Series No. 452. [PubMed: 4991708]
- Will JJ, Mueller JF, Brodine C, Kiely CE, Friedman B, Hawkins VR, Dutra J, Vilter RN. Folic acid and vitamin B12 in pernicious anemia. Studies on patients treated with these substances over a ten-year period. J Lab Clin Med. 1959;53:22–38. [PubMed: 13621020]
- Wilson J, Matthews DM. Metabolic inter-relationships between cyanide, thiocyanate and vitamin B12 in smokers and non-smokers. Clin Sci. 1966;31:1–7. [PubMed: 5912703]
- Winawer SJ, Streiff RR, Zamcheck N. Gastric and hematological abnormalities in a vegan with nutritional vitamin B12 deficiency: Effect of oral vitamin B12. Gastroenterology. 1967;53:130–135. [PubMed: 6027227]
Footnotes
- *
It is advisable for most of this amount to be obtained by consuming foods fortified with B12 or a B12-containing supplement.
- SUMMARY
- BACKGROUND INFORMATION
- SELECTION OF INDICATORS FOR ESTIMATING THE REQUIREMENT FOR VITAMIN B12
- METHODOLOGICAL ISSUES
- DIAGNOSIS
- FACTORS AFFECTING THE VITAMIN B12 REQUIREMENT
- FINDINGS BY LIFE STAGE AND GENDER GROUP
- INTAKE OF VITAMIN B12
- TOLERABLE UPPER INTAKE LEVELS
- RESEARCH RECOMMENDATIONS FOR VITAMIN B12
- REFERENCES
- Vitamin B12 - Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin...Vitamin B12 - Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline
Your browsing activity is empty.
Activity recording is turned off.
See more...