Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
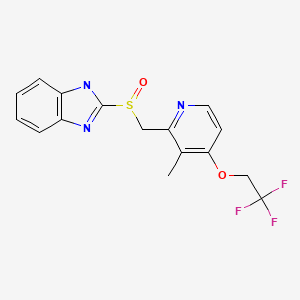
CASRN: 103577-45-3
Drug Levels and Effects
Summary of Use during Lactation
No information is available on the use of lansoprazole during breastfeeding. However, lansoprazole has been used safely in newborn infants, so it is unlikely that the amount in breastmilk would be harmful.
Drug Levels
Maternal Levels. Relevant published information was not found as of the revision date.
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
The Spanish pharmacovigilance system found 3 cases of gynecomastia associated with lansoprazole reported during the time period of 1982 to 2006.[1] A retrospective claims database study in the United States found that users of proton pump inhibitors had an increased risk of gynecomastia.[2]
A review article reported that a search of database from the European Pharmacovigilance Centre found 45 cases of gynecomastia, 11 cases of galactorrhea, 3 cases of breast pain and 5 cases of breast enlargement associated with lansoprazole. A search of the WHO global pharmacovigilance database found 123 cases of gynecomastia, 30 cases of galactorrhea, 36 cases of breast pain and 18 cases of breast enlargement associated with lansoprazole.[3]
In a retrospective study, out of total of 127 cases of gynecomastia in of 175 male patients, 11 patients were treated had been treated with lansoprazole.[4]
One case of elevated serum prolactin and galactorrhea was reported in a 21-year-old man. When omeprazole was substituted for lansoprazole, the serum prolactin decreased to the normal range and galactorrhea ceased. Although this case occurred in Spain, it was not included in the report above.[5]
A 13-year-old girl with a recent history of bilateral galactorrhea and hyperprolactinemia from omeprazole and domperidone on separate occasions was given lansoprazole to prevent gastrointestinal irritation following intravenous diclofenac for a severe headache. After 3 days of lansoprazole therapy, she again developed galactorrhea and an elevated serum prolactin that returned to normal a week after discontinuing lansoprazole.[6]
A 17-year-old woman using a progestin-containing IUD for 1 year began lansoprazole 15 mg daily and presented after 1 week with bilateral galactorrhea and hyperprolactinemia of 92 mcg/L. Seventy-two hours after discontinuation of lansoprazole, galactorrhea ceased. Four months later, serum prolactin was normal at 24.1 mcg/L with no recurrence of galactorrhea. The authors judged the adverse reaction likely to be caused by lansoprazole.[7]
The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
Alternate Drugs to Consider
Antacids, Cimetidine, Famotidine, Omeprazole, Pantoprazole, Sucralfate
References
- 1.
- Carvajal A, Macias D, Gutierrez A, et al. Gynaecomastia associated with proton pump inhibitors: A case series from the Spanish Pharmacovigilance System. Drug Saf. 2007;30:527–31. [PubMed: 17536878]
- 2.
- He B, Carleton B, Etminan M. Risk of gynecomastia with users of proton pump inhibitors. Pharmacotherapy. 2019;39:614–8. [PubMed: 30865318]
- 3.
- Ashfaq M, Haroon MZ, Alkahraman YM. Proton pump inhibitors therapy and risk of hyperprolactinemia with associated sexual disorders. Endocr Regul. 2022;56:134–47. [PubMed: 35489049]
- 4.
- Daniels IR, Layer GT. How should gynaecomastia be managed? ANZ J Surg. 2003;73:213–6. [PubMed: 12662229] [CrossRef]
- 5.
- Izquierdo Prieto OM, Moreno Alia E, Rosillo González A. Aten Primaria. 2004;34:325–6. [Galactorrhea induced by lansoprazole] [PMC free article: PMC7668912] [PubMed: 15491529]
- 6.
- Jabbar A, Khan R, Farrukh SN. Hyperprolactinaemia induced by proton pump inhibitor. J Pak Med Assoc. 2010;60:689–90. [PubMed: 20726208]
- 7.
- Duwicquet F, Gras-Champel V, Masmoudi K. Therapie. 2017;72:691–3. [Hyperprolactinemia with galactorrhea induced by lansoprazole: A case report] [PubMed: 29061292]
Substance Identification
Substance Name
Lansoprazole
CAS Registry Number
103577-45-3
Drug Class
Breast Feeding
Lactation
Milk, Human
Anti-Ulcer Agents
Gastrointestinal Agents
Proton Pump Inhibitors
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Dexlansoprazole.[Drugs and Lactation Database (...]Review Dexlansoprazole.. Drugs and Lactation Database (LactMed®). 2006
- Review Omeprazole.[Drugs and Lactation Database (...]Review Omeprazole.. Drugs and Lactation Database (LactMed®). 2006
- Review Metyrapone.[Drugs and Lactation Database (...]Review Metyrapone.. Drugs and Lactation Database (LactMed®). 2006
- Review Rabeprazole.[Drugs and Lactation Database (...]Review Rabeprazole.. Drugs and Lactation Database (LactMed®). 2006
- Synthesis of 2-[[(4-fluoroalkoxy-2-pyridyl)methyl]sulfinyl]-1H- benzimidazoles as antiulcer agents.[Chem Pharm Bull (Tokyo). 1990]Synthesis of 2-[[(4-fluoroalkoxy-2-pyridyl)methyl]sulfinyl]-1H- benzimidazoles as antiulcer agents.Kubo K, Oda K, Kaneko T, Satoh H, Nohara A. Chem Pharm Bull (Tokyo). 1990 Oct; 38(10):2853-8.
- Lansoprazole - Drugs and Lactation Database (LactMed®)Lansoprazole - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
