Attribution Statement: LactMed is a registered trademark of the U.S. Department of Health and Human Services.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Drugs and Lactation Database (LactMed®) [Internet]. Bethesda (MD): National Institute of Child Health and Human Development; 2006-.
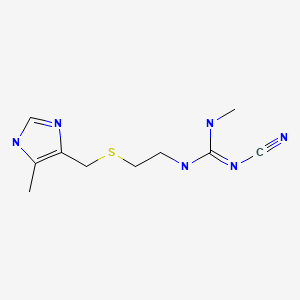
CASRN: 51481-61-9
Drug Levels and Effects
Summary of Use during Lactation
Maternal cimetidine doses of 1000 to 1200 mg daily result in infant dosages that are much less than reported neonatal dosages of 5 to 10 mg/kg daily. Cimetidine would not be expected to cause any adverse effects in breastfed infants, especially if the infant is older than 2 months. However, because of its potential for causing hepatic enzyme inhibition, other drugs might be preferred. Cimetidine can increase serum prolactin and it has been used, but not validated, as a galactogogue.
Drug Levels
Maternal Levels. After a single oral dose of 400 mg of cimetidine, the peak milk level of 5 mg/L occurred about 3 hours after the dose and was about equal to the peak serum level in a woman who had been breastfeeding for 6 months. Milk levels remained higher than serum levels for the duration of the dosing interval. With a multiple-dose regimen of 200 mg 3 times daily and 400 mg at bedtime, milk levels just prior to the doses were relatively constant between 4.9 and 6 mg/L.[1] These levels indicate that a fully breastfed infant would receive between 0.74 and 0.9 mg/kg daily.
Twelve healthy, lactating human volunteers who were 6 to 45 weeks postpartum were given single doses of cimetidine 100 mg, 600 mg and 1200 mg on 3 different days within a 15-day period. Blood and milk samples were donated before each dose and 11 times over the next 24 hours. Average peak cimetidine levels in milk occurred at 3.3 hours after the dose. The dose-normalized peak milk level was 2.5 to 3.1 mg/L for each 100 mg of dosage. The half-life in milk was 2.5 hours, which was longer than in serum (1.4 hours).[2] Using data from the paper, a fully breastfed infant whose mother was receiving 1200 mg daily would receive an average dose of 1.4 mg/kg daily, which is in the neonatal dosage range. Average milk values equated to a steady-state weight-adjusted relative infant dose of 1.1% of the maternal weight-adjusted dose
Infant Levels. Relevant published information was not found as of the revision date.
Effects in Breastfed Infants
Relevant published information was not found as of the revision date.
Effects on Lactation and Breastmilk
Histamine H2-receptor blockade is known to stimulate prolactin secretion. In addition, cimetidine may have additional, nonspecific actions that stimulate prolactin secretion.[3] Oral cimetidine doses of 400 mg 4 times daily increased serum prolactin by 50 to 112% in 6 patients. Cimetidine has caused dose-related gynecomastia and galactorrhea in men and nonnursing women.[4-6] The prolactin level in a mother with established lactation may not affect her ability to breastfeed.
One clinician reported that she used cimetidine 200 or 300 mg 4 times daily to nursing mothers with a marginal or low milk supply. Subjective reports indicated an increase in milk supply.[7]
Alternate Drugs to Consider
References
- 1.
- Somogyi A, Gugler R. Cimetidine excretion into breast milk. Br J Clin Pharmacol 1979;7:627-9. [PMC free article: PMC1429661] [PubMed: 465286]
- 2.
- Oo CY, Kuhn RJ, Desai N, et al. Active transport of cimetidine into human milk. Clin Pharmacol Ther 1995;58:548-55. [PubMed: 7586949]
- 3.
- Knigge UP. Histaminergic regulation of prolactin secretion. Dan Med Bull 1990;37:109-24. [PubMed: 2188799]
- 4.
- Delle Fave FG, Tamburrano G, De Magistris L, et al. Gynaecomastia with cimetidine. Lancet 1977;309:1319. [PubMed: 68422]
- 5.
- Bateson MC, Browning MCK, Maconnachie A. Galactorrhoea with cimetidine. Lancet 1977;310:247-8. [PubMed: 69853]
- 6.
- García Rodríguez, LA, Jick H. Risk of gynaecomastia associated with cimetidine, omeprazole, and other antiulcer drugs. BMJ 1994;308:503-6. [PMC free article: PMC2542783] [PubMed: 8136667]
- 7.
- Mouzoon ME. Broader options for galactagogue therapy - Consideration of H2 receptor blockers. Breastfeed Med 2023;18:A10-A1. doi:10.1089/bfm.2023.29253.abstracts [CrossRef]
Substance Identification
Substance Name
Cimetidine
CAS Registry Number
51481-61-9
Drug Class
Breast Feeding
Lactation
Milk, Human
Anti-Ulcer Agents
Histamine H2 Antagonists
Gastrointestinal Agents
Disclaimer: Information presented in this database is not meant as a substitute for professional judgment. You should consult your healthcare provider for breastfeeding advice related to your particular situation. The U.S. government does not warrant or assume any liability or responsibility for the accuracy or completeness of the information on this Site.
- User and Medical Advice Disclaimer
- Drugs and Lactation Database (LactMed) - Record Format
- LactMed - Database Creation and Peer Review Process
- Fact Sheet. Drugs and Lactation Database (LactMed)
- Drugs and Lactation Database (LactMed) - Glossary
- LactMed Selected References
- Drugs and Lactation Database (LactMed) - About Dietary Supplements
- Breastfeeding Links
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Verapamil.[Drugs and Lactation Database (...]Review Verapamil.. Drugs and Lactation Database (LactMed®). 2006
- Review Alcohol.[Drugs and Lactation Database (...]Review Alcohol.. Drugs and Lactation Database (LactMed®). 2006
- Review Metoclopramide.[Drugs and Lactation Database (...]Review Metoclopramide.. Drugs and Lactation Database (LactMed®). 2006
- Review Pilocarpine.[Drugs and Lactation Database (...]Review Pilocarpine.. Drugs and Lactation Database (LactMed®). 2006
- Review Clonidine.[Drugs and Lactation Database (...]Review Clonidine.. Drugs and Lactation Database (LactMed®). 2006
- Cimetidine - Drugs and Lactation Database (LactMed®)Cimetidine - Drugs and Lactation Database (LactMed®)
Your browsing activity is empty.
Activity recording is turned off.
See more...
