NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Obesity prevalence continues to increase globally, leading to ill-health and reduced life expectancy in those affected and an urgent need for effective preventative and therapeutic strategies. Until recently obesity was viewed simplistically as an imbalance between energy expenditure and consumption that could be easily corrected by lifestyle changes. However, obesity is now recognized to be a chronic progressive disease, with bodyweight controlled by a complex interplay between the central nervous system, peripheral signals of energy balance from adipose tissue and the gastrointestinal tract, environmental food cues, and a powerful biological drive to defend the highest weight achieved. Currently, bariatric surgery represents the most effective treatment for people with severe obesity, leading to marked sustained weight loss as a consequence of altered eating behavior with improved health and life expectancy. Bariatric surgical procedures were initially envisaged to limit calorie intake by physically restricting food passage and inducing malabsorption. However, it is now clear that the success of bariatric surgery lies rather in the impact of these procedures on the biological regulation of energy homeostasis. In this review we summarize the complex bi-directional communication system known as the gut-brain axis with special focus on gut hormones, bile acids and gut microbiota. We discuss the impact of obesity, lifestyle interventions and bariatric surgery upon the gut-brain axis. Finally, we discuss the progress being made to pharmacologically mimic the beneficial hormonal milieu of bariatric surgery. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Obesity, defined as the accumulation of excess adipose tissue that impairs health, is now recognized as a chronic progressive disease. Its prevalence continues to increase unabated (1). Globally in 2016, approximately 39% of the adult population were overweight (1.9 billion) and 13% had obesity (> 650 million) (1). Increased fat deposition is the result of an imbalance between energy expenditure and consumption, which in turn is due to an alteration of the homeostatic and/or hedonic systems that regulate energy homeostasis (2). This simplistic definition does not consider how complex obesity is, being the consequence of interactions between genetic, environmental, dietary, psychological and socio-economic factors (3, 4). Eating behavior is governed by specific brain areas that integrate peripheral signals regarding nutrient intake and energy stores (5).
The obese state is a very difficult condition to treat because of the coexistence of low-grade inflammation, dysbiosis, hormonal and neurogenic imbalances (3, 4, 6) (Figure 1). These factors also make major contributions to obesity-related diseases, such as type 2 diabetes (T2D), cardiovascular disease, and some types of cancer, impacting adversely upon health, socio-economic factors and life expectancy (1, 7, 8). Weight loss can improve these co-morbidities and increase life expectancy. However, current treatments that emphasize dietary (especially low-calorie diets) or lifestyle approaches for obesity lack long-term efficacy. A meta-analysis of weight loss clinical trials mediated by lifestyle interventions showed an average weight loss of 5% to 9% in the first 6 months, which back-tracked to 3% to 6% in those studies where 48-month data were available (9). Another review assessing the long-term outcomes of calorie-restricted diets showed that up to two-thirds of dieters regain more weight than they lost during their weight loss programs (10). The data for the impact of anti-obesity medication (AOM) on total weight loss percentage after 1 year are highly variable, ranging from 3% with lorcaserin to 9.4% with phentermine/topiramate (11).
Figure 1.
Schematic diagram comparing the simplistic definition of obesity, thought to be the result of an imbalance between energy expenditure and consumption (1), with the very complex physiopathology of the obese state (2). This is the result of genetic, inflammatory, microbiota, endocrine, neurogenic and other factors. This pathophysiological complexity underlies the difficulty in finding effective treatments to combat obesity.
Bariatric/metabolic procedures are currently the most effective treatments for people with severe obesity both in terms of weight loss amount and sustainability and the resolution of complications (12). The mechanisms behind the success of bariatric/metabolic surgeries remain to be fully elucidated but post-surgical changes in gut-derived hormonal peptides, bile acids (BA), gut microbiota, and vagal tone are suggested to be involved (13, 14). Importantly, research studies undertaken in animal models and patients with obesity undergoing bariatric surgery have significantly advanced our understanding of the important interplay between the central nervous system (CNS) and the gastrointestinal (GI) tract in regulating energy and glucose homeostasis. Several brain regions integrate continuous information provided by chemical messengers and neural networks arising from the periphery that reflect nutrient availability, the body's energy status, and play a key role in regulating energy homeostasis. The GI tract is responsible for generating the majority of inputs communicated to the CNS regarding both the quality and quantity of a meal. This complex bi-directional communication system between the GI tract and the CNS has come to be known as the gut-brain axis (4, 15) (Figure 2).

Figure 2.
Schematic diagram illustrating the gut-brain axis. The entire gastrointestinal tract (GIT) is responsible for generating multiple signals that inform the central nervous system (CNS) regarding quality and quantity of a meal. Key components include neural signals, gut hormones, bile acids, and gut microbiota.
In this review we will explore the gut-brain axis in detail, focusing on the role of gut hormones, Bas, and gut microbiota. We will concentrate our attention on the perturbations of the gut-brain axis in the obese state, and the compensatory response to weight loss induced by lifestyle interventions. Finally, we will discuss the impact of bariatric surgery upon gut hormones, Bas, and gut microbiota and the evidence supporting a role for these factors in mediating the beneficial weight and metabolic effects of bariatric surgeries.
THE PHYSIOLOGY OF BODY WEIGHT REGULATION AND THE GUT—BRAIN AXIS
During the majority of human evolution food has been scarce. It is therefore not a surprise that endogenous systems have evolved to prioritize food-seeking behaviours when necessary to ensure adequate nutrition for reproduction and survival. Neuronal circuits within the brain control energy homeostasis, integrating peripheral signals of energy availability originating from the GI tract, adipose tissue mass, muscle mass, and bone density, together with information from higher cognitive centers and external environmental food cues (16). Upon food consumption, sensory information reflecting nutrient availability is transferred from the GI vagal and/or somatosensory (spinal) afferent fibers to the nucleus tractus solitarius (NTS) that, in turn, are integrated and transferred to several other brain centers, including the hypothalamus (17).
Figure 3.
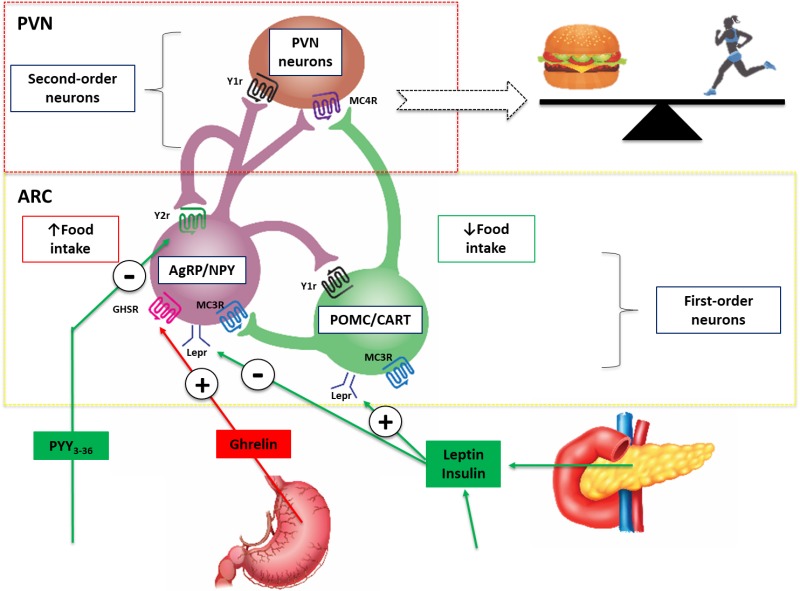
Schematic diagram illustrating the central effects of hormones that control eating. Leptin and insulin are secreted in proportions to body fat mass and decrease appetite by inhibiting neurons that produce the NPY and AgRP, while stimulating melanocortin-producing neurons in the ARC region of the hypothalamus, near the third ventricle of the brain. NPY and AgRP stimulate eating, and melanocortins inhibit eating, via higher-order neurons. Activation of NPY/AgRP-expressing neurons inhibits melanocortin-producing neurons. The gastric hormone acyl-ghrelin stimulates appetite by activating the NPY/AgRP-expressing neurons. Gut hormones released from the GI tract in response to eating, including PYY, inhibit these neurons and thereby suppress appetite and decrease energy intake. Abbreviations: AgRP, agouti-related peptide; ARC, arcuate nucleus; CART, cocaine and amphetamine-regulated transcript; NPY, neuropeptide Y, PVN, paraventricular nucleus; PYY, peptide tyrosine-tyrosine 3-36; POMC, pro-opiomelanocortin: Lepr, Leptin receptor; GHSR, Ghrelin receptor, MC3R, Melanocortin 3 receptor, MC4R, Melanocortin 4 receptor, Y1r, NPY receptor; Y2r, NPY/PYY3-36 receptor.
The hypothalamus is the key integrative brain site that governs reciprocal orexigenic and anorexigenic behavioral responses, as well as adaptive metabolic changes in response to alterations in food availability and activity levels (18) (Figure 3). The arcuate (ARC), paraventricular (PVN), ventromedial and dorsomedial nuclei, as well as the lateral hypothalamus, are the most important hypothalamic areas involved in energy homeostasis (19). The ARC responds to peripheral and central signals reflecting nutrient availability and energy expenditure by releasing neurotransmitters from two separate and reciprocally connected neuronal populations: pro-opiomelanocortin (POMC)/cocaine-and-amphetamine-regulated transcript (CART) and neuropeptide Y (NPY)/agouti-related protein (AgRP) neurons. NPY/AgRP neurons are situated in the medial ARC and release AgRP and NPY, which stimulate hunger, appetite, and decrease energy expenditure (17, 19-21). Neighboring POMC and CART-containing neurons located in the lateral ARC release α-melanocortin-stimulating hormone (α-MSH) and CART respectively (17). These neurotransmitters are antagonistic of AgRP and NPY and act via the melanocortin-4 receptor (MC4R) to decreased hunger and increased energy expenditure (22) (Figure 3). In addition to vagal signaling, gut hormones can also directly influence these hypothalamic circuits. For example, injected peptide YY (PYY) can inhibit food intake by binding to Y2 receptors localized to the ARC (23) (Figure 3).
Figure 4.
Schematic diagram illustrating the mechanisms involved in regulating feeding behavior. Nutrient entry into the GI tract causes gastric and intestinal distension, secretion of pancreatic enzymes and BA, altered enteric and vagal nerve signaling and exposure of EECs to nutrients with altered circulating gut hormone levels (e.g. decrease in orexigenic hormone acyl-ghrelin and increase in anorectic hormones PYY3-36 and GLP-1). Gut-derived signals (nutrients, hormones, and neural) and adipokines (e.g. leptin and others) act directly and indirectly upon the brainstem and hypothalamic areas (see Figure 3 for a detailed description of hypothalamic nuclei controlling energy homeostasis). All of these factors are involved in the regulation of homeostatic hunger. Social factors, emotion, reward, pleasure, increased food availability and sensory cues can influence brain reward and higher cognitive brain regions leading to altered feeding behavior (hedonic influences on hunger and appetite control). Taste and olfactory signals can also influence energy intake acting on both homeostatic and brain reward systems. Insulin leptin, GLP-1, PYY and ghrelin are present in saliva with cognate receptors on taste buds and olfactory neurons. Abbreviations: AgRP, agouti-related peptide; ARC, arcuate nucleus; CART, cocaine and amphetamine-regulated transcript; EEC’s, enteroendocrine cells; FGF-19, fibroblast growth factor-19; GLP-1, glucagon-like peptide 1; LHA, lateral hypothalamic area; NPY, neuropeptide Y, peripheral nervous system, PNS; PVN, paraventricular nucleus; PYY, peptide tyrosine-tyrosine 3-36; POMC, pro-opiomelanocortin, sympathetic nervous system, SNS.
Several other brain regions have key roles in energy homeostasis. `The area postrema (AP) is proximally located to NTS and, along with the ARC, are unique in that they have incomplete blood-brain-barriers, thus allowing them to be directly accessed and influenced by blood-borne gut hormones and other circulating factors (4). In animal models, AP lesions have been shown to result in diminished central effect of several gut hormones (24). Signals from the GI tract also interact with the brain reward systems that constitute dopaminergic neurons located in the ventral tegmental area, nucleus accumbens, and other sites. These neuronal pathways are thought to mediate effects of exposure to hedonic food cues present in obesogenic environments of Western societies, possibly contributing to the creation of the desire to eat in the absence of an energy requirement in what has been termed “hedonic obesity” (18) (Figure 4).
More broadly, the gut-brain axis includes bidirectional communication between the CNS and the enteric nervous system (ENS), autonomic nervous system (ANS) (with sympathetic and parasympathetic branches), neuroendocrine system, gut immune system, BAs, and gut microbiota (25, 26). Both acute and chronic alterations in these systems can arise in response in changes in energy expenditure and consumption (6). Peripheral energy-regulating signals are traditionally classified as long-term signals of energy balance, such as leptin and insulin levels reflective of body adipose stores (“adiposity signals”), and short-term signals, which convey information regarding nutrient and meal-derived energy availability (5) (“satiety signals”), whereas the role of the immune system of the GI tract is still under investigation (Figure 4).
Figure 5.
Schematic diagram illustrating the regulation of an L-cell, one of the several EECs present in the GI tract. Nutrients and their interaction with gut microbiota and BAs in the intestinal lumen activate luminal receptors located on the apical cell membrane, which then activate intracellular metabolism leading to calcium influx to induce the synthesis and release of gut hormones into the sub-epithelial space. Luminal receptors includes receptors for short-chain fatty acids (SCFAs) (e.g., GPR41, GPR43), long chain fatty acids (e.g., GPR40, GPR120), proteolytic products (e.g., CaSR) and BAs (e.g., TGR5). Various gut-derived hormones glucagon-like peptide 1 (GLP-1), peptide YY (PYY), and oxyntomodulin (OXM) are synthesized, secreted and released from L-cells systemically to induce an effect on various tissues throughout the body such as the brain. These hormones can act systemically or cooperate with the enterocytes via local paracrine action. Their systemic effects could be endocrine but also neural through the activation of afferent neurons located in the GI wall. The secretion of gut hormones can be stimulated, in turn, by circulating hormones and glucose or by stimulation from enteric nerves.
Most of these communications related to energy homeostasis involve hormonal and neural signals, which are quite substantial given that the GI tract releases more than 100 different hormonally active peptides (27) and contains approximately 500 million neurons (28). In response to nutrient ingestion, stretching of stomach mechanoreceptors generates the first ENS feedback signals to the CNS mediating meal cessation (4, 29). Subsequently, digested luminal nutrients come in contact with the microvilli of apical cell membranes of enteroendocrine cells (EECs) located in the epithelium of the GI tract to stimulate gut hormone release. Therefore, the majority of signaling and communication within the gut-brain axis is initiated in response to pre-absorptive nutrients (15) (Figure 5 illustrates in detail the biology of an L-cell as a model of an EEC). Following gut hormone receptor activation, nutrient-derived signals exert local control over intestinal function and are conveyed directly and indirectly to the brain via vagal and spinal afferents (30-32) (Figure 5).
Digestion and absorption take place predominantly in the stomach and small intestine where dense innervation originating from splanchnic and vagal nerves transmit neurological signals generated during the process of nutrient sensing (29). Additional roles of the ENS include regulation of gastric emptying by vagal activation (17, 33) and mediation of GI endocrine signaling via vagal nerve afferents that project into the lamina propria of the gut ending at the basolateral cell membrane of EECs. It is through these nutrient-specific sensory signals that the GI tract informs the CNS about a meal’s energy and macronutrient content (34).
LINKING THE GUT AND BRAIN: GUT HORMONES, BILE ACIDS AND THE GUT MICROBIOTA
Gut Hormones
As previously described, the gut-brain neuronal-signaling axis is initiated by nutrient-induced gut hormone secretion (32). EECs distributed throughout the entire GI tract length respond to luminal nutrients and release a panoply of gut hormones that act as endocrine, autocrine and paracrine regulators of energy and glucose homeostasis (35) (Figure 5). Although nutrient ingestion triggers the secretion of numerous gut hormones, in this review we will focus on glucagon-like peptide 1 (GLP-1), oxyntomodulin (OXM), and PYY (all of which are released from L-cells and have central appetite-suppressing effects (35, 36)), pancreatic polypeptide (PP), cholecystokinin (CCK), ghrelin, and anandamide.
The enteroendocrine L-cells responsible for secreting PYY, GLP-1 and OXM reside throughout the GI tract with the highest concentrations in the ileum and colon. In response to nutrient intake, circulating PYY, GLP-1 and OXM levels show a biphasic increase with an initial early peak within 15 minutes and then a later peak around 90 minutes post-ingestion (4, 37, 38). The early increase is thought to be mediated by neural (vagal) and/or hormonal mechanisms whilst the later peak, which is in proportion to the energy intake, is thought to result from the direct contact of nutrients with L-cells located in the distal small intestine and large intestine (39).
Glucagon-like peptide 1
Glucagon-like peptide 1 results from post-translational processing of the preproglucagon gene (37, 40). Glucagon-like peptide 1 exerts its metabolic effects by activating the GLP-1 receptor, which is widely expressed in the GI tract, pancreas, and CNS (41, 42). Actions mediated by the GLP-1 receptor include enhancing glucose-dependent insulin release (incretin effect) (43), inhibition of glucagon secretion (44), and stimulation of satiety centers in the ARC, NTS and AP leading to decreased hunger and increased satiation (45). In addition, GLP-1 limits energy intake by reducing the rate of gastric emptying, which in turn increases gastric distension (46). As a result of these functions, GLP-1 receptor agonists are currently used for the treatment of both T2D and obesity (47).
Glucagon-like peptide 1 is rapidly inactivated by the enzyme dipeptidyl peptidase-IV (DPP-IV) with only about 10% of GLP-1 reaching the systemic circulation (48-50). Thus, GLP-1 is thought to mainly act in a paracrine fashion. The vagus nerve is particularly important for the action of GLP-1 as demonstrated by vagotomy studies altering the effects of this hormone (51). Glucagon-like peptide 1 also stimulates the brainstem to enhance motor output and/or thermogenesis (52).
Oxyntomodulin
Like GLP-1, OXM is synthesized by post-translational processing of the preproglucagon gene (53, 54). Other similarities of OXM to GLP-1 include binding to GLP-1 receptors within the GI tract, the pancreas, and the ARC; subsequent reductions in gastric acid secretion, blood glucose concentrations and food intake (53, 55, 56); and degradation by DPP-IV (57). In addition, OXM administration enhances satiety and increases energy expenditure in both animal models and humans (53, 54).
Peptide YY
Circulating levels of PYY are low in the fasted state (58, 59) and increase during nutrient ingestion in proportion to the caloric content (60), exhibit differential responses according to the specific macronutrient composition of the meal (36, 59), and remain elevated for several hours after a meal with sustained endocrine effects (61).
Peptide YY circulates in two native forms: PYY1−36 (minor form) and PYY3−36 (major form) (36, 59). Peptide YY3−36 results from the N-terminal cleavage of PYY1−36 by the enzyme DPP-IV (59). Interestingly, PYY1−36 and PYY3−36 have divergent actions on appetite, glucose homeostasis and differential binding affinities of each form for the five neuropeptide Y receptor (YR) subtypes (59). Y2 receptors are located on the vagus nerve, in the NTS and in the ARC (23, 36, 60). Peptide YY1−36 has equivalent affinities for Y1R and Y2R, whereas PYY3−36 is a high-affinity ligand for Y2R (59). By binding to the Y2 receptor, PYY3-36 decreases energy intake by inhibiting the orexigenic effects of NPY neurons and activating the anorexigenic POMC neurons in the ARC (4, 20, 62) (Figure 3), physiological effects supported by studies showing that PYY knockout mice become hyperphagic and obese (36, 60). Increased PYY levels have been associated with prolonged appetite loss and food aversion during exogenous administration and following bariatric surgery (6, 63-65).
Pancreatic polypeptide
Pancreatic polypeptide is secreted by specialized F-cells located in the islets of Langerhans (66) during the pre-absorptive (cephalic) phase of nutrient metabolism and for up to 6 hours post-prandially in proportion to energy intake (67, 68). Pancreatic polypeptide acts centrally upon the Y4 receptor within the AP, NTS, and the ARC, reducing energy intake. Peripherally, it induces gallbladder relaxation, inhibits pancreatic secretion and delays gastric emptying thus inducing satiety (69, 70). Pancreatic polypeptide is a potent appetite suppressant (71) and studies have demonstrated a difference in PP concentrations in anorexic and obesogenic states, where it is increased and diminished respectively (61). Moreover, studies in people with Prader-Willi syndrome and obesity suggest that circulating post-prandial PP levels are reduced in comparison to healthy individuals. Furthermore, intravenous PP injection to these patients led to a significant decline in energy intake (72).
Cholecystokinin
Cholecystokinin is secreted from EECs located mainly in the duodenum and jejunum (73). Cholecystokinin release is stimulated by fat and protein ingestion and its circulating concentrations increase within 15 minutes after meal ingestion (6, 74). Cholecystokinin has a short half-life and it acts upon CCK-1 and CCK-2 receptors located throughout tissues of the GI tract and the CNS, including the vagal nerve, NTS and hypothalamus (6). Cholecystokinin increases gallbladder and GI motility and secretion but also has an active role in controlling food intake, energy expenditure and glucose utilization (17). Cholecystokinin reduces energy intake in a dose-dependent manner and it is a specific mediator of fat and protein satiation (6, 75). It has been suggested that it acts synergistically with leptin and amylin, a pancreatic hormone co-secreted with insulin (17). However, repeated doses can induce tolerance to CCK (6), potentially explaining why attempts to use CCK-derivatives as a medication to induce weight loss have failed (76).
Ghrelin
Ghrelin is a 28-amino-acid orexigenic hormone and is secreted by P/D1 cells located primarily in the gastric fundus in the absence of nutrient intake, leading to increased appetite and food intake (77). Ghrelin is also produced by the pituitary gland (77) and within the ARC and PVN area of the hypothalamus. Ghrelin is secreted as the inactive des-acyl-ghrelin. The active orexigenic form, acyl-ghrelin, is synthesized by the action of ghrelin O-acyltransferase enzyme (GOAT) (77) and can bind to growth hormone secretagogue receptor (GHS-R) to increase food intake in rodents (77) and humans (77, 78). The extremely complex ghrelin/GOAT/GHS-R system has a crucial role in the regulation of energy and metabolism as well as in the adaptation of energy homeostasis to environmental changes (77).
Acyl-ghrelin administration to humans has been used as an orexigenic agent in patients with anorexia that accompanies cachexia (79). A rising circulating ghrelin level precedes nutrient ingestion and decreases rapidly after a meal (78) which has led to the speculation that this is the first discovered “hunger hormone” (80). Plasma levels of ghrelin increase after diet induced weight loss, thought to be part of the body’s homeostatic adaptation response that restores lost weight, and are very high in patients with anorexia nervosa (81). Nutrient intake but not water ingestion is the main regulator of ghrelin leading to a decrease of its plasma levels (82). Peripheral ghrelin exerts its orexigenic actions through the stimulation of NPY/AgRP co-expressing neurons (83).
Anandamide
Anandamide and other bioactive lipids belonging to the endocannabinoid system contribute to the gut-brain axis. These molecules are secreted in the GI tract and activate endocannabinoid receptors 1 and 2 (CB1/CB2) (3, 4) which are expressed in the CNS, peripheral nervous system, liver, pancreas, adipose tissue, and immune cells (84). Exogenous cannabinoids convey orexigenic effects and so it is not a surprise that the endocannabinoid regulates gut motility and appetite (3, 84). Endocannabinoid receptor 1 antagonists were used to induce weight-loss in subjects with obesity before being withdrawn for their severe psychological side-effects (3, 85), including increased suicidality (86).
Bile Acids
Bile acids (BAs) are endogenous steroid molecules synthesized from cholesterol in the liver, stored in the gallbladder and secreted into the duodenum upon nutrient ingestion. These amphipathic molecules facilitate micelle formation, promoting the digestion of dietary fat and fat-soluble vitamins. More recently, BAs have also been shown to play a role in regulating glucose and lipid metabolism and energy expenditure via the activation of BA receptors in the liver, gut, and peripheral tissues (87, 88). Interactions between BAs, their receptors, and the gut microbiota determine synthesis, metabolism, and distribution of bile acids in the body (88).
There is complex cross talk between BAs, gut hormones and the microbiome (Figure 6). Bile acids stimulate GLP-1 secretion via activating G protein‐coupled receptors (TGR5) on L-cells and fasting total circulating BAs levels are positively correlated with post-prandial GLP-1 levels (89). TGR5 receptors are also located on skeletal muscle and in brown adipose tissue where they increase energy expenditure by promoting the conversion of inactive thyroxine (T4) into active thyroid hormones (T3) (90). Bile acids have been shown to act on farnesoid X receptors (FXR). During BA binding of FXR on pancreatic β cells, insulin release is increased (91). Bile acid activation of intestinal FXR-containing cells stimulates the secretion of fibroblast growth factor-19 (FGF-19), a protein that contributes to improved peripheral glucose disposal and lipid homeostasis, increased metabolic rate, and reduced weight (92, 93). In animal studies, BA supplementation has been shown to reduce weight gain (90). In humans, postprandial BA levels are also inversely related with body fat mass (94). Thus, the physiologic effects of BA likely extend beyond the gut and pancreas to include actions that improve body weight and glucolipid metabolism.
Figure 6.
Schematic diagram illustrating the complex cross talk between BAs, gut hormones, FGF-19, and the microbiome. BAs are important regulators of energy balance and glucose metabolism, primarily via the FXR and the TGR5. The trans-intestinal BAs flux activates intestinal FXR, inducing synthesis and secretion into the circulation of the ileal-derived enterokine FGF-19. FGF-19 can improve glucose tolerance by regulating insulin-independent glucose efflux and hepatic glucose production. FGF-19 can also increase energy expenditure with its central and peripheral effect in the adipose tissue. BAs acting via TGR5 stimulate L-cell secretion of GLP-1 (and PYY) then enhancing insulin secretion acting on β-cells. TGR5 activation in muscle and brown adipose tissue promotes the conversion of inactive thyroxine into active thyroid hormone inducing thermogenesis. BAs can reduce food intake centrally through FGF-19 and anorectic gut hormones. BAs also regulate gut microbiota composition. BAs are actively reabsorbed from the terminal ileum and returned via the portal circulation to the liver. A small percentage of BAs are deconjugated by gut bacteria, forming secondary BAs, which are reabsorbed or excreted in feces. Red dotted lines: FGF-19 effects. Blue dotted lines: IGF-1 effects. Green lines: BAs circulation.
The Gut Microbiota
The healthy human gut hosts trillions of microorganisms with a ratio of bacterial-to-human cells of 1.3:1, comprising a complex ecosystem referred to as the gut microbiota (95, 96). These microbes exist within a symbiotic relationship with their human host, who provides a nutrient-rich environment. The microbiota, in turn, provides metabolic processing of these nutrients that the host's genome does not possess (97, 98). With more than a thousand different bacterial species, the diversity and function of the microbiota is dynamic depending on the host’s diet, antibiotic exposure and other environmental factors (98, 99).
The extent of the symbiotic relationship between the host and the microbiota is highlighted by studies showing that mice lacking a microbiota (germ-free) have reduced adiposity, energy intake, and energy extraction from a standard rodent diet compared to conventionally-raised mice (100). More recent studies including germ-free rats transplanted with the microbiota of either obesity-prone or obesity-resistant rats confirmed the importance of the microbiome for the production of enzymes involved in energy harvesting from indigested carbohydrates (75). Both the Westernized-diet and obesity fecal transplant models are associated with an increased ratio of bacteria belonging to the Firmicutes phylum compared to the Bacteroidetes phylum, which is reversed upon surgical and dietary interventions (29, 101, 102).
Gut microbiota have been demonstrated to affect adiposity and weight-gain through several pathways. A typical Western diet contains indigestible carbohydrates, such as resistant starches and plant cell wall polysaccharides that are hydrolyzed by gut microbiota generating small-chain fatty acids (SCFA) (3). Short chain fatty acids in the form of butyrate, acetate, and propionate provide approximately 10% of the host's daily energy requirements (103). In the obesogenic state, feces contain an increased quantity of SCFA, especially propionate (103). Short-chain fatty acids are not always correlated with increased weight-gain as some may possess beneficial properties (4, 29). In animal models, prebiotics (indigestible compounds that can modulate the composition and activity of the gut microbiota) and oral or intestinal SCFA infusions lead to a reduction in food consumption and a decrease in body weight. This occurs when prebiotics and supplements promote the growth of favorable microbial species or when SCFA activates signaling pathways that ultimately increase gut hormone synthesis (104-106). For example, SCFA’s have also been shown to activate free fatty-acid receptors 2 and 3 (FFAR2/FFAR3) in the GI tract, immune cells, liver and adipose tissue (107). Intestinal FFAR2/FFAR3 receptors are expressed by EECs, in particular, L-cells and, when activated, can facilitate release of gut hormones, such as GLP-1 and PYY (107). Moreover, FFAR3 expressed within the ENS and ANS can stimulate the sympathetic tone in the adipose tissue regulating fat storage as well as glucose utilization in muscle and liver tissues (3, 108). The gut microbiota may also moderate the endocannabinoid tone affecting colonic CB1 expression and anandamide concentrations (3, 109). Finally, gut microbiota can enhance energy expenditure by intracellular thyroid hormone activation via FXR signaling (3, 90, 99), which may help to explain the observation that germ-free mice are resistance to adiposity despite an increased food intake (110).
Obesity is characterized by the presence of chronic low-grade inflammation. In another pathogenic pathway involving the microbiota, high-energy dense diets can lead to obesity and obesity-related diseases through changes in bacterial species composition that favors an increase in systemic lipopolysaccharide (LPS) concentrations (111, 112). Lipopolysaccharide is the pro-inflammatory component located in the cell wall of gram-negative bacteria that can enter the circulation when the permeability of intestinal epithelium is altered in a process called metabolic endotoxemia (111, 112). Leakage of LPS into the systemic circulation is a proposed trigger of a cascade of pro-inflammatory events in adipose tissue and throughout the whole body mediated by LPS stimulation of the toll-like receptor 4 (TLR4), which enhances the synthesis of inflammatory cytokines linked with reduced host insulin sensitivity (113, 114). Lipopolysaccharide can also inhibit the interstitial cells of Cajal, which are responsible for smooth muscle contraction in the gut and regulation of the ENS. This inhibition has been associated with disorders of both GI motility and gut hormones (4, 115).
The gut microbiota may also directly influence CNS-mediated stress and anxiety behaviours and the regulation of energy homeostasis (4). For example, germ-free mice have been found to have a resistance to adiposity despite an increased food intake (127). Germ-free mice have been found to have higher gene expression of food intake-regulating peptides like GLP1 and OXM within the brainstem and hypothalamus, when compared to normally-reared mice (116). On the other hand, a reduced leptin-mediated suppression of orexigenic peptides NPY and AgRP in the conventionally-raised mice has been noted, suggesting how the gut-microbiota could directly affect energy homeostasis leading to an increase in adiposity (116, 117). Further studies are needed, however, to understand if specific manipulations of the gut microbiota phenotype could be used as obesity treatments (Figure 7).

Figure 7:
Schematic diagram illustrating the possible causative links between an altered gut microbiota and obesity. The hydroxylation of indigestible carbohydrates and the altered intestinal permeability could lead to increased energy harvest and weight gain. The production of certain types of SCFA can reduce the sympathetic tone favoring fat accumulation in the adipose tissue and dysregulation of glucose utilization in muscles and the liver. Gut microbiota can induce a leakage of LPS into the systemic circulation (endotoxemia) and chronic low-grade inflammation. This is in turn responsible of insulin resistance and weight gain. The increased endocannabinoid tone could induce food intake. SCFAs, inflammation and BAs perturbations may all lead to a reduced activation of anorectic pathways. All these mechanisms could be responsible for weight gain, inflammation and obesity-related comorbidities. Abbreviations: FGF-19, fibroblast growth factor-19; GLP-1, glucagon-like peptide 1; PYY3-36, peptide tyrosine-tyrosine 3-36; SCFA; short chain fatty acid; LPS, Lipopolysaccharide; FFAR2 and FFAR3, free fatty-acid receptors 2 and 3; T2D, type 2 diabetes.
THE COMPLEXITIES OF ENERGY HOMEOSTASIS
The Physiology of Weight Regulation
As mentioned above (Figure 3), the adipokine leptin acts as a signal of long-term energy availability (fat mass), promoting satiety via its inhibitory action on orexigenic neurons located in the ARC of the hypothalamus (118). A recent report of a patient with leptin deficiency highlights key interactions between leptin and gut hormones. Leptin supplementation resulted in significant rises in meal-stimulated insulin, GLP-1, and PYY levels (61). In the same study, ghrelin levels were decreased, highlighting the regulatory effect of leptin on ghrelin secretion and the interplay between leptin, GLP-1 and PYY.
An important physiologic insight that has implications for pharmacological weight management is that gut hormones act synergistically. For example, GLP-1 and PYY in combination are more potent in reducing energy intake compared to either of the two hormones alone (119, 120). Oxyntomodulin, CCK and other gut hormones also act synergistically with GLP-1 to enhance its effects on appetite behaviours (120-123). An additional layer of complexity is added when considering that MC4Rs have been localized on L- and P/D1-cells and could, in turn, regulate GLP-1, PYY and ghrelin secretion (124). Furthermore, gut hormones influence energy homeostasis through interactions with the microbiome and BAs.
Hedonic factors can generate meaningful physiological responses that interact with homeostatic signals of energy availability in the regulation of body weight. This could lead to excess energy intake with possible weight gain. In humans, brain functional imaging studies, have shown that several gut hormones modulate neural activity in brain reward regions altering the reward value of food (2, 58, 125) by food cues, memory and social factors, and strongly influencing eating behavior (18). Exposure to food-related stimuli can induce changes in circulating gut hormone levels. Those in turn act upon brain reward pathways, either decreasing in the case of PYY or, increasing in the case of ghrelin, the reward value of food (23, 126). Those hormones are also present in saliva and their cognate receptors are present on taste buds and the olfactory bulb (127, 128). The taste and smell of food are key influencers of food selection with impacts on energy intake (127).
Adding to the complexity of the gut-brain regulation of body weight, studies have demonstrated that energy expenditure can increase without changes in activity but through the action of gut-derived neurohumoral signals that increase thermogenesis and basal metabolic rate (4). The existence of the gut-brain-brown adipose tissue axis has been hypothesized after studies showing how intestinal lipid-sensing activates vagal afferent fibers to enhance brown adipose tissue thermogenesis through a CCK-dependent pathway (129). Table 1 summarizes key gut hormone actions including their perturbations in the obese state and the changes induced by bariatric surgery procedures.
Table 1.
GI Tract Hormones Involved in the Control of Energy Balance and Changes in their Circulating Levels Induced by Obesity and Bariatric Surgery
| Hormone | EEC (Type) Location | Receptor | Food Intake | Other Effects | Obese State | Bariatric Surgery |
|---|---|---|---|---|---|---|
| PYY | Ileum (L cell) | Y2-R | ↓ | ↓ Gastric acid secretion and emptying ↓ Pancreatic and intestinal secretion ↓ Gastrointestinal motility ↑ Insulin secretion and vagus stimulation | ↓ | ↑ |
| GLP-1 | Ileum (L cell) | GLP-1R | ↓ | ↑ Insulin secretion ↑ β-cell proliferation and gene expression ↓ β-cell Apoptosis ↓ Gastric acid secretion and emptying | ↓ | ↑ |
| Ghrelin | Stomach (P/D1cell) | GHS-R | ↑ | ↑ Growth-hormone secretion ↑ Gastric acid secretion and emptying ↑ Vasodilatation ↓ Insulin secretion | ↓ | ↓ |
| CCK | Duodenum, jejunum and pancreas (I/L cell) | CCK 1, 2 | ↓ | ↓ Gastric emptying ↑ Pancreatic secretion ↑ Gallbladder contraction | ? | ↑ |
| PP | Pancreas (F-cell) | Y4, Y5 | ↓ | ↓ Gastric emptying ↓ Leptin levels ↑ Insulin secretion ↓ β-cell Apoptosis | ↓ | ↔ |
| GIP | Duodenum and jejunum (K-cell) | GIP-R | ? | ↑ Insulin secretion and β-cell proliferation ↓ β-cell Apoptosis ↑ Lipoprotein lipase activity and fat deposition ↑ Fatty acid synthesis | ↑ | ↓? |
| OXM | Ileum (L cell) | GLP-1R | ↓ | ↓ Gastric emptying and acid secretion ↓ Blood glucose ↑ Insulin secretion ↑ Energy expenditure | ↓ | ↑? |
| Glucagon | Pancreas (α-cell) | GCGR | ↓ | ↑ Energy expenditure ↑ Blood glucose | ? | ↑ |
| Amylin | Pancreas (β-cell) | AMY1-3 | ↓ | ↓ Gastric emptying and acid secretion ↓ Postprandial glucagon secretion ↓ Glucose elevation | ↑ | ↓ |
| Insulin | Pancreas (β-cell) | Insulin receptor | ↓ | ↑ Absorption ↑ Glycogen synthesis ↓ Blood glucose ↑ Lipid synthesis ↓ Lipolysis and proteolysis | ↑ | ↓ |
| Leptin | Adipose Tissue and gastric EECs | Leptin (Ob-R) | ↓ | ↓Glucose production and steatosis in the liver ↑ Glucose uptake and fatty acid oxidation in muscles ↓ Insulin and glucagon secretion ↑ Sympathetic nervous system tone ↑Thyroid hormones Modulates immunity and fertility | ↑ | ↓ |
| FGF-19 | Ileum (FXR activation from BA) | FGFR 1, 2, 3, 4 | ↓ | Regulates glucose and lipid metabolism, ↑Hepatic protein and glycogen synthesis ↑ energy expenditure | ↓ | ↑ |
| NT | Jejunum (L-cell) | NTR1, NTR2, NTR3 | ↓ | ↓ Reduces GI motility and gastric secretion, ↑ Pancreatic and biliary secretion | ? | ↑? |
Abbreviations: FGF-19, fibroblast growth factor-19; GLP-1, glucagon-like peptide 1; PYY3-36, peptide tyrosine-tyrosine 3-36; PP, pancreatic polypeptide, GIP, gastric inhibitory polypeptide; OXM, Oxyntomodulin; BA, Bile Acids: NT, Neurotensin. ? = effect not certain or not valid for every bariatric procedure.
The Obese State: Pathophysiologic Changes
Obesity is the result of a period of uncompensated chronic positive energy balance (130) when energy intake exceeds energy requirements. Dysregulation of the metabolic mechanisms controlling energy homeostasis includes an impaired gut hormone secretion response to nutrient ingestion (131). People with obesity have reduced circulating baseline and meal-stimulated levels of PYY and GLP-1 levels compared to lean subjects (131-133). Lower circulating ghrelin levels and a reduced suppression of circulation ghrelin levels after nutrient intake have been demonstrated in people with obesity, suggesting that dynamic changes more than absolute values are important in appetite regulation (7, 40). Animal models with diet-induced obesity show reduced circulating concentrations together with impaired circadian secretion profiles of PYY and GLP-1 (134), in addition to an increase in ghrelin-producing cells (135). However, reduced diurnal variability in circulating ghrelin is thought to contribute to the lack of a regular meal pattern and the frequent snacking behavior often observed in individuals with obesity (77, 136).
The directionality of the association between obesity and altered gut hormone profile remains to be fully elucidated. For example, high energy intake per se may affect gut hormone responsiveness to ingested nutrients. Moreover, intestinal EEC population differentiation and responsivity is reduced in people with obesity, which may underlie their blunted gut hormone secretion (137). Obesity has also been shown to blunt the rise in circulating post-prandial BAs levels (33). Paradoxically, most studies have found increased total BAs levels in subjects with obesity suggesting that BAs composition could shift unfavorably with detrimental metabolic effects (138). Interestingly, while obesity is thought to be due to resistance to the effects of insulin and leptin within key weight regulatory centers in the hypothalamus, sensitivity to the effects of PYY, GLP-1 and OXM during exogenous administration is preserved, suggesting these hormones and their receptor systems offer a viable therapeutic target for the treatment of obesity (139).
As detailed in the previous section, a dysbiotic relationship between host and gut microbiota has been suggested to contribute to the development of obesity (140), with profound differences found between the composition of the gut microbiota of obese and lean individuals (141). Obesity is associated with the relative increase or reduction of certain bacterial species and the importance of the relative proportions of those species remains an area of active investigation. Transplantation of gut bacteria from obese mice to normal-weight germ-free mice results in weight gain in the recipients (142). Conversely, fecal transplantation from lean human donors to recipient patients with metabolic syndrome led to improvements in insulin sensitivity (143). A dysbiotic relationship may affect host energy and nutrient metabolism by altering intestinal mucosal permeability, and promoting increased fat storage in adipose tissue (110). The mechanism for this could be by enhancing the absorption of SCFA derived by otherwise indigestible luminal polysaccharides and by triggering inflammatory responses through a process referred to as “metabolic endotoxemia” (144, 145) (Figure 7). Altered neural responses to food cues in people with obesity compared to people with normal weight have been confirmed by brain-imaging studies showing an increased stimulation of central reward pathways in response to eating or food cues (2). In addition, there is evidence that eating behavior in people with obesity becomes dissociated from perceptions of satiety and hunger (146, 147). Furthermore, in the obese state, enhanced endocannabinoid tone, CB1 expression, and plasma and adipose tissue endocannabinoid concentrations coexist (3). All these complex pathophysiological changes create an internal environment conducive to expression of unwanted weight gain, maintenance of the obese state, and resistance to diet-induced weight loss, providing an explanation as to why treating people with obesity can be challenging (Figure 7).
BARIATRIC/METABOLIC SURGERY
Bariatric/metabolic surgery is recognized as the most effective weight loss treatment for people with severe obesity (148). Procedures with the best outcomes involve surgical modifications of the anatomy of the GI tract that alter nutrient flow, thus affecting GI tract biology (83). Many clinical trials have demonstrated the superiority of bariatric surgery in terms of sustainability of weight loss and resolution of obesity-related comorbidities, especially diabetes, when compared with intensive medical interventions (12, 149, 150). Mechanisms other than restriction and/or malabsorption are responsible for this superiority and this has resulted in a marked increase in the number of procedures undertaken worldwide (151). Currently, the most commonly performed bariatric/metabolic procedures globally are sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB), whereas purely restrictive procedures, like gastric banding, are now performed less frequently (151) (Figure 8). However, post-operative weight loss can be highly variable (152), an important consideration given that total amount of weight loss plays a major role in determining post-operative remission of comorbidities (153).

Figure 8.
Schematic diagram illustrating the normal upper GI anatomy (a) and the two most commonly performed bariatric surgical procedures. The metabolic procedures: (b) RYGB and (c) SG (Refer to the main text for a detailed description of surgical techniques). Abbreviations: RYGB, Roux-en-Y gastric bypass, SG, Sleeve gastrectomy.
Roux-en-Y gastric bypass involves division of the stomach into two parts, generating a small gastric pouch (20-30 mL), which is then anastomosed with the mid-jejunum, creating the alimentary limb or Roux limb. Nutrients bypass most of the stomach, duodenum, and the proximal jejunum. In the common limb, after the anastomosis of the biliopancreatic limb with the jejunum, nutrients, BAs and pancreatic secretions mix and the absorption of nutrients occurs (154). In a SG, a transection along the greater curvature is performed, removing the fundus and body and creating a tube-like stomach (155). The transit of gastric contents into the duodenum is rapid. The SG was initially performed as a first-stage procedure followed by a second more invasive malabsorptive step (biliopancreatic diversion), but the significant weight loss results observed with this procedure led to its adoption as a standalone approach. Because it is a simpler operation compared with RYGB and has fewer complications with similar short-term weight-loss, SG have become the most common bariatric procedure worldwide (151).
Biological Changes Favoring Sustained Weight Loss and Metabolic Improvement Following Bariatric/Metabolic Surgery
A negative energy balance is a key component of many lifestyle interventions. Unfortunately, weight regain is very common after initial weight loss. Multiple powerful adaptive biological changes occur in response to weight loss from lifestyle alone that lead to increased hunger, enhanced neural responses to food cues and heightened drive to consume energy-dense foods. These include decreased total energy expenditure secondary to reduced lean muscle mass, sympathetic activity (156), circulating leptin, GLP-1 and PYY levels, along with increased ghrelin levels (147). Other changes following lifestyle-induced weight loss that have been described and may contribute to weight recidivism include impaired circulating BAs levels, an altered gut microbiome, and decreased vagal signal transmission (10, 157).

Figure 9.
Schematic diagram illustrating the different biological changes induced by weight loss achieved through dieting (upper part) compared to bariatric/metabolic surgery (lower part). Powerful compensatory biological changes contribute to the high rate of weight recidivism observed following lifestyle intervention weight management. Many homeostatic mechanisms act to restore a higher body weight and these includes hormonal alterations and a decreased energy expenditure leading to increased hunger and energy consumption. By contrast, bariatric surgery leads to a favorable biology that includes increased satiety hormones, reduced ghrelin, enhanced BA secretion and a “lean” microbiota. Together these mechanisms lead to reduced hunger and a shift towards healthier food options with a resetting of body weight “set point” to a lower level facilitating meaningful and sustained weight loss. References for this figure: (149, 158, 159). Abbreviations: GLP-1, glucagon-like peptide 1; PYY3-36; peptide tyrosine-tyrosine 3-36. *Suggestion that leptin sensitivity may improve
Weight loss following RYGB and SG are the result of multifactorial mechanisms and not from malabsorption or restricted stomach size alone (160, 161) (Figures 9 and 10). Reduced energy intake, as a result of altered eating behavior, is recognized as the main driver for weight loss following these procedures, and increased exposure of EECs to ingested nutrients is thought to play a key mediating role in the expression of these appetitive behaviours (83) (Table 1 and Figure 10). In contrast to lifestyle approaches to weight loss, favorable changes in these behaviours following bariatric/metabolic procedures include reduced hunger and neural responsiveness to food cues. Multiple studies have shown that bariatric surgery causes marked elevations in nutrient-stimulated levels of several anorectic hormones including PYY and GLP-1, along with decreased ghrelin levels, which have been reported post-RYGB but are more pronounced post-SG (162, 163). Following RYGB, increased nutrient-stimulated circulating levels of PYY and GLP-1 are most likely as a result of increased nutrient stimulation of L-cells as a consequence of anatomical rearrangement. Sleeve gastrectomy leads to rapid gastric emptying and enhanced exposure of L-cells to nutrients with increased nutrient-stimulated PYY and GLP-1 levels, but to a lesser extent than following RYGB. Sleeve gastrectomy leads to sustained and greater reduction in circulating acyl-ghrelin levels than RYGB because of the removal of the fundus of the stomach where most ghrelin-producing cells are located (164). These changes are present immediately after surgery and sustained up to 10 years post-operatively (165, 166). Oxyntomodulin levels are increased after RYGB (167) and a rise in CCK levels has been demonstrated following both RYGB and SG (163). Emerging evidence also suggest that the number of EECs changes after bariatric surgery. The total numbers of EECs in the stomach and duodenum of people with obesity are reduced when compared to lean individuals (31) and this has been found to normalize 3 months post-SG (158).

Figure 10.
Schematic diagram illustrating RYGB and SG and the mechanisms leading to weight loss and resolution of comorbidities. For every mechanism the effect of the procedure is represented with a “↑” when stimulating or “↓” when suppressing. A “+” means that the proposed mechanism is present only after surgery when compared to the pre-operative period. When the effect is stronger for one of the two procedures there is a double arrow compared with a single one. When the effect is missing for one procedure it means that the mechanism is procedure specific. Abbreviations: RYGB, Roux-en-Y gastric bypass; SG, Sleeve gastrectomy; GLP-1, glucagon-like peptide 1; PYY3-36; peptide tyrosine-tyrosine 3-36; GIP, gastric inhibitory polypeptide; FGF-19, fibroblast growth factor-19, CCK; cholecystokinin.
Variability in EEC secretion response may underlie differences in weight loss responses to bariatric/metabolic procedures. Profound anorexia and excessive weight loss post-SG have been associated with markedly elevated circulating fasted and post-meal PYY levels (65). Patients with poor weight loss after surgery have been found to have increased appetite coupled with lower meal-stimulated GLP-1 and PYY and higher ghrelin levels when compared with good responders (168). Additional support for the importance of EEC in weight loss responsiveness in the post-op period comes from data showing that administration of octreotide (a general inhibitor of EEC secretion), or selectively blocking GLP-1 and PYY, promotes appetite and weight gain (14, 65, 169).
Following SG and RYGB, food becomes less rewarding and there is a shift in preference from energy dense food rich in fat and sugar to healthier options enabling patients to adopt more favorable eating behaviours (158). These changes in eating behavior are the result of multiple mechanisms, some of which are common to both SG and RYGB and others that are procedure specific (Figure 10).
Studies of the physiological changes following bariatric/metabolic have also elucidated novel effectors of changes in weight and metabolism, many of which are gut-related and discussed above. For example, following RYGB and SG, changes in circulating BAs levels and composition are reported that may contribute to weight loss and improved glucose metabolism. Despite their anatomical differences, RYGB and SG exert similar effects on BA composition and circulating concentrations, although the changes observed following SG are more modest (87, 170). The exact mechanism responsible for elevated BAs following RYGB and SG is unclear, but animal studies suggests that an accelerated nutrient flow to the distal small intestine is a key mechanism (171). Indeed, in animal models, rerouting bile to the distal small bowel by transposing the common bile duct increases plasma BA levels similarly those seen after RYGB and results in weight loss, improved glucose metabolism, and reduced hepatic steatosis. The rise in circulating BAs appears even greater several months post-operation and may be due to intestinal cellular adaptations (172), increased hepatic synthesis, altered enterohepatic recirculation of bile, or a combination of these possibilities. Post-surgically increased BAs diversity might also impact on GLP-1 secretion and energy expenditure. Binding of BAs to TGR5 receptors in skeletal muscle and brown adipose tissue may contribute to enhanced action of thyroid hormones, thereby increasing energy expenditure (173). Therefore, BAs could contribute to weight loss and metabolic improvements after bariatric surgery through direct and indirect regulatory mechanisms.
Weight‐loss surgery can also affect the interplay between BAs and gut microbiota, which can have favorable metabolic effects in the post-operative period (174) (175). For example, in RYGB subjects, bacterial overgrowth in the biliopancreatic limb may generate secondary BAs species with altered affinity for FXR or TGR5 receptors (176). In rodent models, the importance of the FXR receptor in mediating weight loss and metabolic improvements after SG was demonstrated when FXR knockout mice regained lost weight following this procedure (173), although whether FXR signaling and/or FGF-19 contributes to the beneficial effects of bariatric surgery in humans is uncertain at present. Finally, a study that measured serum BAs levels before and after bariatric surgery showed that they were significantly increased only at one-year post‐surgery, whereas, the substantial increase in PYY and GLP-1 levels could be observed as soon as 1-week post-surgery. This finding suggest that increased plasma BAs may be less important in early metabolic improvements observed after bariatric surgery (170) but more so for long-term effects.
Alterations in intestinal microbiome following RYGB and SG have been described. Animal studies of fecal transplants from RYGB-treated to germ-free mice showed significantly greater weight loss in the germ-free mice, suggesting that the altered microbiome per se contributes to weight loss (177). RYGB can produce greater and more favorable changes in gut microbiota functional capacity and species than SG despite similar weight loss (178) (179, 180). Although the specific procedure-related mechanisms responsible for post-surgery gut microbiota changes remain to be delineated (181), potential explanations include differences in the physical manipulation of the GI tract and final anatomy, dietary changes, and weight loss differences between procedures. In rodents, these changes can be detected as early as 7 days after RYGB (175), with similar patterns observed in humans (102). Because of significant differences between the rodents and the humans, it is not possible to firmly conclude that gut bacteria are essential for the effects of metabolic procedures. However, it is evident in the rodent models that changes in gut microbiota induced by RYGB are sufficient to produce weight loss (174).
Other appetite-related post-surgery effects that may influence weight loss include changes in taste and smell that could, in turn, influence food preference (83). Interestingly, early data suggest that RYGB and SG may differently impact subjective changes in appetite, taste, olfaction and food aversion post-operatively (182). Finally, neurophysiological studies suggest that vagal nerve signaling also increases post-RYGB (157) and these changes may affect food intake in a procedure-specific fashion (183, 184).
Developing “Knifeless Surgery”
A multitude of compounds mimicking gut hormone actions are currently under development, opening a new era of pharmacotherapy for obesity. At present, GLP-1 analogues are broadly used in the management of people with T2DM and obesity (185). The longer acting GLP-1 analogue semaglutide has shown promising results for weight loss in early phase studies with both a weekly subcutaneous injection (186) and an oral compound form (187, 188). Intravenous administration of supra-physiological levels of native gut hormones like PYY, GLP-1 and others lead to reduced appetite and decreased energy intake (23, 189-191). Strategies aimed at reducing acyl-ghrelin and/or increasing des-acyl-ghrelin are also being developed and show promise. The inhibition of GOAT has been shown to reduce energy intake and bodyweight (192).
In order to mimic bariatric/metabolic procedures, the effects of combinations of hormones are under investigation with the aim of circumventing compensatory adaptive changes associated with energy restriction. For example, the co-infusion of GLP-1, PYY and OXM induced a 32% reduction in energy intake when compared to placebo (193). Animal models suggest a potential role of CCK as an adjunct to GLP-1 based therapies (194) or monomeric GLP-1/GIP/glucagon triagonism to reduce food intake and obesity (195).
Conclusion
Obesity is a complex disease where genetic, environmental, dietary, psychological and socio-economic factors interact complicating treatments for this life-threatening condition. Peripheral signals such as gut hormones, BAs and gut microbiota inform the CNS regarding the quality and the quantity of any ingested meal and are part of the complex bi-directional communication system known as the gut-brain axis. During the recent years many studies have identified perturbations of this system as a cause of weight gain. Current lifestyle approaches to weight loss lack efficacy because multiple powerful adaptive biological responses promote weight regain. Bariatric surgery, which reduces energy intake as a consequence of favorable gut-brain axis signaling, is currently the most successful treatment for people with severe obesity, leading to marked sustained weight loss and improved health. Understanding the hidden mechanisms responsible for this success is an exciting area of current research and holds promise to identify novel effective obesity pharmacotherapies.
Acknowledgements
The authors would like to thank Chiara Bullo for the illustrations, Janine Makaronidis and Cormac McGee for their critical review of the manuscript.
REFERENCES
- 1.
- Lauby-Secretan B., et al. Body fatness and cancer—viewpoint of the IARC Working Group. New England Journal of Medicine. 2016;375(8):794–798. [PMC free article: PMC6754861] [PubMed: 27557308]
- 2.
- Makaronidis J.M., Batterham R.L. Obesity, body weight regulation and the brain: insights from fMRI. Br J Radiol. 2018.:20170910. p. [PMC free article: PMC6223152] [PubMed: 29365284]
- 3.
- Moran C.P., Shanahan F. Gut microbiota and obesity: role in aetiology and potential therapeutic target. Best Pract Res Clin Gastroenterol. 2014;28(4):585–97. [PubMed: 25194177]
- 4.
- Bauer P.V., Hamr S.C., Duca F.A. Regulation of energy balance by a gut-brain axis and involvement of the gut microbiota. Cell Mol Life Sci. 2016;73(4):737–55. [PMC free article: PMC11108299] [PubMed: 26542800]
- 5.
- Miras A.D., le Roux C.W. Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol. 2013;10(10):575–84. [PubMed: 23835488]
- 6.
- Buhmann H., le Roux C.W., Bueter M. The gut-brain axis in obesity. Best Pract Res Clin Gastroenterol. 2014;28(4):559–71. [PubMed: 25194175]
- 7.
- Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. [PMC free article: PMC7615134] [PubMed: 27115820]
- 8.
- Afshin A., et al. Health effects of overweight and obesity in 195 countries over 25 years. The New England journal of medicine. 2017;377(1):13–27. [PMC free article: PMC5477817] [PubMed: 28604169]
- 9.
- Franz M.J., et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007;107(10):1755–67. [PubMed: 17904936]
- 10.
- Mann T., et al. Medicare's search for effective obesity treatments: diets are not the answer. Am Psychol. 2007;62(3):220–33. [PubMed: 17469900]
- 11.
- Pilitsi E., et al. Pharmacotherapy of obesity: Available medications and drugs under investigation. Metabolism. 2018 [PubMed: 30391259]
- 12.
- Schauer P.R., et al. Bariatric Surgery versus Intensive Medical Therapy for Diabetes - 5-Year Outcomes. N Engl J Med. 2017;376(7):641–651. [PMC free article: PMC5451258] [PubMed: 28199805]
- 13.
- Pucci A., Batterham R.L. Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different. J Endocrinol Invest. 2018 [PMC free article: PMC6394763] [PubMed: 29730732]
- 14.
- Santo M.A., et al. Weight Regain After Gastric Bypass: Influence of Gut Hormones. Obes Surg. 2016;26(5):919–25. [PubMed: 26450709]
- 15.
- Gribble F.M., Reimann F. Enteroendocrine Cells: Chemosensors in the Intestinal Epithelium. Annu Rev Physiol. 2016;78:277–99. [PubMed: 26442437]
- 16.
- Dokken B.B., Tsao T.-S. The Physiology of Body Weight Regulation: Are We Too Efficient for Our Own Good? Diabetes Spectrum. 2007;20(3):166–170.
- 17.
- Suzuki K., Jayasena C.N., Bloom S.R. Obesity and appetite control. Exp Diabetes Res. 2012;2012:824305. p. [PMC free article: PMC3415214] [PubMed: 22899902]
- 18.
- Berthoud H.R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol. 2011;21(6):888–96. [PMC free article: PMC3254791] [PubMed: 21981809]
- 19.
- Cone R.D., et al. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25 Suppl 5:S63–7. [PubMed: 11840218]
- 20.
- Dryden S., et al. Increased neuropeptide Y secretion in the hypothalamic paraventricular nucleus of obese (fa/fa) Zucker rats. Brain Res. 1995;690(2):185–8. [PubMed: 8535835]
- 21.
- Ollmann M.M., et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278(5335):135–8. [PubMed: 9311920]
- 22.
- Schwartz M.W., Morton G.J. Obesity: keeping hunger at bay. Nature. 2002;418(6898):595. [PubMed: 12167841]
- 23.
- Batterham R.L., et al. Gut hormone PYY 3-36 physiologically inhibits food intake. Nature. 2002;418(6898):650. [PubMed: 12167864]
- 24.
- van der Kooy D. Area postrema: site where cholecystokinin acts to decrease food intake. Brain Res. 1984;295(2):345–7. [PubMed: 6713193]
- 25.
- Grenham S., et al. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94. [PMC free article: PMC3232439] [PubMed: 22162969]
- 26.
- Mayer E.A., Tillisch K., Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125(3):926–38. [PMC free article: PMC4362231] [PubMed: 25689247]
- 27.
- Rehfeld J.F. A centenary of gastrointestinal endocrinology. Horm Metab Res. 2004;36(11-12):735–41. [PubMed: 15655701]
- 28.
- Monje M. Settling a Nervous Stomach: The Neural Regulation of Enteric Cancer. Cancer Cell. 2017;31(1):1–2. [PubMed: 28073000]
- 29.
- Bliss E.S., Whiteside E. The Gut-Brain Axis, the Human Gut Microbiota and Their Integration in the Development of Obesity. Front Physiol. 2018;9:900. [PMC free article: PMC6052131] [PubMed: 30050464]
- 30.
- Dockray G.J. Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol. 2013;13(6):954–8. [PubMed: 24064396]
- 31.
- Amato A., et al. Peripheral motor action of glucagon-like peptide-1 through enteric neuronal receptors. Neurogastroenterol Motil. 2010;22(6):664–e203. [PubMed: 20158614]
- 32.
- Costa M., Brookes S.J., Hennig G.W. Anatomy and physiology of the enteric nervous system. Gut. 2000;47 Suppl 4:iv15–9. [PMC free article: PMC1766806] [PubMed: 11076898]
- 33.
- Cooke A.R., Clark E.D. Effect of first part of duodenum on gastric emptying in dogs: response to acid, fat, glucose, and neural blockade. Gastroenterology. 1976;70(4):550–5. [PubMed: 1254139]
- 34.
- Hamr S.C., et al. Does nutrient sensing determine how we "see" food? Curr Diab Rep. 2015;15(6):604. [PubMed: 25956822]
- 35.
- Latorre R., et al. Enteroendocrine cells: a review of their role in brain–gut communication. Neurogastroenterology & Motility. 2016;28(5):620–630. [PMC free article: PMC4842178] [PubMed: 26691223]
- 36.
- Batterham R.L., et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4(3):223–33. [PubMed: 16950139]
- 37.
- Elliott R.M., et al. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138(1):159–66. [PubMed: 7852887]
- 38.
- Svendsen B., et al. An analysis of cosecretion and coexpression of gut hormones from male rat proximal and distal small intestine. Endocrinology. 2015;156(3):847–57. [PubMed: 25535831]
- 39.
- Oesch S., et al. Effect of gastric distension prior to eating on food intake and feelings of satiety in humans. Physiol Behav. 2006;87(5):903–10. [PubMed: 16549077]
- 40.
- Adam T.C., Westerterp-Plantenga M.S. Glucagon-like peptide-1 release and satiety after a nutrient challenge in normal-weight and obese subjects. Br J Nutr. 2005;93(6):845–51. [PubMed: 16022753]
- 41.
- Bliss E.S., Whiteside E. The Gut-Brain Axis, the Human Gut Microbiota and Their Integration in the Development of Obesity. Frontiers in Physiology. 2018;9(900) [PMC free article: PMC6052131] [PubMed: 30050464]
- 42.
- Yamato E., et al. Tissue-specific and glucose-dependent expression of receptor genes for glucagon and glucagon-like peptide-1 (GLP-1). Horm Metab Res. 1997;29(2):56–9. [PubMed: 9105899]
- 43.
- Holst J.J., Orskov C. The incretin approach for diabetes treatment: modulation of islet hormone release by GLP-1 agonism. Diabetes. 2004;53 Suppl 3:S197–204. [PubMed: 15561911]
- 44.
- Manning S., Pucci A., Batterham R.L. GLP-1: a mediator of the beneficial metabolic effects of bariatric surgery? Physiology (Bethesda). 2015;30(1):50–62. [PubMed: 25559155]
- 45.
- Larsen P.J., Tang-Christensen M., Jessop D.S. Central administration of glucagon-like peptide-1 activates hypothalamic neuroendocrine neurons in the rat. Endocrinology. 1997;138(10):4445–55. [PubMed: 9322962]
- 46.
- Edwards C.M., et al. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9-39. Diabetes. 1999;48(1):86–93. [PubMed: 9892226]
- 47.
- Pucci A., Finer N. New medications for treatment of obesity: metabolic and cardiovascular effects. Can J Cardiol. 2015;31(2):142–52. [PubMed: 25661549]
- 48.
- Holst J.J. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. [PubMed: 17928588]
- 49.
- Kuhre R.E., et al. Measurement of the incretin hormones: glucagon-like peptide-1 and glucose-dependent insulinotropic peptide. J Diabetes Complications. 2015;29(3):445–50. [PubMed: 25623632]
- 50.
- Vilsboll T., et al. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003;88(1):220–4. [PubMed: 12519856]
- 51.
- Ruttimann E.B., et al. Intrameal hepatic portal and intraperitoneal infusions of glucagon-like peptide-1 reduce spontaneous meal size in the rat via different mechanisms. Endocrinology. 2009;150(3):1174–81. [PMC free article: PMC2654737] [PubMed: 18948395]
- 52.
- Graaf C., et al. Glucagon-Like Peptide-1 and Its Class B G Protein-Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol Rev. 2016;68(4):954–1013. [PMC free article: PMC5050443] [PubMed: 27630114]
- 53.
- Baggio L.L., et al. Oxyntomodulin and glucagon-like peptide-1 differentially regulate murine food intake and energy expenditure. Gastroenterology. 2004;127(2):546–58. [PubMed: 15300587]
- 54.
- Cohen M.A., et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88(10):4696–701. [PubMed: 14557443]
- 55.
- Maida A., et al. The glucagon-like peptide-1 receptor agonist oxyntomodulin enhances beta-cell function but does not inhibit gastric emptying in mice. Endocrinology. 2008;149(11):5670–8. [PubMed: 18669601]
- 56.
- Pocai A. Action and therapeutic potential of oxyntomodulin. Mol Metab. 2014;3(3):241–51. [PMC free article: PMC3986661] [PubMed: 24749050]
- 57.
- Druce M.R., et al. Investigation of structure-activity relationships of Oxyntomodulin (Oxm) using Oxm analogs. Endocrinology. 2009;150(4):1712–22. [PubMed: 19074579]
- 58.
- Batterham R.L., et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–9. [PubMed: 17934448]
- 59.
- Manning S., Batterham R.L. The role of gut hormone peptide YY in energy and glucose homeostasis: twelve years on. Annu Rev Physiol. 2014;76:585–608. [PubMed: 24188711]
- 60.
- le Roux C.W., et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3–8. [PubMed: 16166213]
- 61.
- Batterham R.L., et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–8. [PubMed: 12954742]
- 62.
- Broberger C., et al. Subtypes Y1 and Y2 of the neuropeptide Y receptor are respectively expressed in pro-opiomelanocortin- and neuropeptide-Y-containing neurons of the rat hypothalamic arcuate nucleus. Neuroendocrinology. 1997;66(6):393–408. [PubMed: 9430445]
- 63.
- Di Francesco V., et al. Delayed postprandial gastric emptying and impaired gallbladder contraction together with elevated cholecystokinin and peptide YY serum levels sustain satiety and inhibit hunger in healthy elderly persons. J Gerontol A Biol Sci Med Sci. 2005;60(12):1581–5. [PubMed: 16424292]
- 64.
- El-Salhy M., et al. The role of peptide YY in gastrointestinal diseases and disorders. Int J Mol Med. 2013;31(2):275–82. (review) p. [PMC free article: PMC4042877] [PubMed: 23292145]
- 65.
- Pucci A., et al. A case of severe anorexia, excessive weight loss and high peptide YY levels after sleeve gastrectomy. Endocrinol Diabetes Metab Case Rep. 2015;2015:150020. p. [PMC free article: PMC4674657] [PubMed: 26664728]
- 66.
- Khandekar N., et al. The role of pancreatic polypeptide in the regulation of energy homeostasis. Mol Cell Endocrinol. 2015;418(Pt 1):33–41. [PubMed: 26123585]
- 67.
- Adrian T.E., et al. Distribution and release of human pancreatic polypeptide. Gut. 1976;17(12):940–44. [PMC free article: PMC1411244] [PubMed: 828120]
- 68.
- Teff K. Nutritional implications of the cephalic-phase reflexes: endocrine responses. Appetite. 2000;34(2):206–13. [PubMed: 10744911]
- 69.
- Lin S., et al. Critical role of arcuate Y4 receptors and the melanocortin system in pancreatic polypeptide-induced reduction in food intake in mice. PLoS One. 2009;4(12):e8488. p. [PMC free article: PMC2796177] [PubMed: 20041129]
- 70.
- Parker R.M., Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur J Neurosci. 1999;11(4):1431–48. [PubMed: 10103138]
- 71.
- Batterham R.L., et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab. 2003;88(8):3989–92. [PubMed: 12915697]
- 72.
- Berntson G.G., et al. Pancreatic polypeptide infusions reduce food intake in Prader-Willi syndrome. Peptides. 1993;14(3):497–503. [PubMed: 8332550]
- 73.
- Gibbs J., Young R.C., Smith G.P. Cholecystokinin elicits satiety in rats with open gastric fistulas. Nature. 1973;245(5424):323–5. [PubMed: 4586439]
- 74.
- Lieverse R.J., et al. Satiety effects of a physiological dose of cholecystokinin in humans. Gut. 1995;36(2):176–9. [PMC free article: PMC1382399] [PubMed: 7883212]
- 75.
- Duca F.A., Yue J.T. Fatty acid sensing in the gut and the hypothalamus: in vivo and in vitro perspectives. Mol Cell Endocrinol. 2014;397(1-2):23–33. [PubMed: 25261798]
- 76.
- Castillo E.J., et al. Effect of oral CCK-1 agonist GI181771X on fasting and postprandial gastric functions in healthy volunteers. Am J Physiol Gastrointest Liver Physiol. 2004;287(2):G363–9. [PubMed: 15246968]
- 77.
- Muller T.D., et al. Ghrelin. Mol Metab. 2015;4(6):437–60. [PMC free article: PMC4443295] [PubMed: 26042199]
- 78.
- Cummings, D.E., et al., A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. (0012-1797 (Print)). [PubMed: 11473029]
- 79.
- Neary N.M., et al. Ghrelin increases energy intake in cancer patients with impaired appetite: acute, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2004;89(6):2832–6. [PubMed: 15181065]
- 80.
- Cummings, D.E., et al., Plasma ghrelin levels and hunger scores in humans initiating meals voluntarily without time- and food-related cues. (0193-1849 (Print)). [PubMed: 15039149]
- 81.
- Cummings, D.E., et al., Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. (1533-4406 (Electronic)). [PubMed: 12023994]
- 82.
- Callahan H.S., et al. Postprandial suppression of plasma ghrelin level is proportional to ingested caloric load but does not predict intermeal interval in humans. J Clin Endocrinol Metab. 2004;89(3):1319–24. [PubMed: 15001628]
- 83.
- Makaronidis J.M., et al. Reported appetite, taste and smell changes following Roux-en-Y gastric bypass and sleeve gastrectomy: Effect of gender, type 2 diabetes and relationship to post-operative weight loss. Appetite. 2016;107:93–105. [PubMed: 27453553]
- 84.
- Mackie K. Cannabinoid receptors: where they are and what they do. J Neuroendocrinol. 2008;20 Suppl 1:10–4. [PubMed: 18426493]
- 85.
- Aronne L.J., et al. A clinical trial assessing the safety and efficacy of taranabant, a CB1R inverse agonist, in obese and overweight patients: a high-dose study. Int J Obes (Lond). 2010;34(5):919–35. [PubMed: 20157323]
- 86.
- Topol E.J., et al. Rimonabant for prevention of cardiovascular events (CRESCENDO): a randomised, multicentre, placebo-controlled trial. Lancet. 2010;376(9740):517–23. [PubMed: 20709233]
- 87.
- Seeley R.J., Chambers A.P., Sandoval D.A. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab. 2015;21(3):369–78. [PMC free article: PMC4351155] [PubMed: 25662404]
- 88.
- Schaap F.G., Trauner M., Jansen P.L. Bile acid receptors as targets for drug development. Nat Rev Gastroenterol Hepatol. 2014;11(1):55–67. [PubMed: 23982684]
- 89.
- Thomas C., et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10(3):167–77. [PMC free article: PMC2739652] [PubMed: 19723493]
- 90.
- Watanabe M., et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439(7075):484–9. [PubMed: 16400329]
- 91.
- Dufer M., et al. Bile acids acutely stimulate insulin secretion of mouse beta-cells via farnesoid X receptor activation and K(ATP) channel inhibition. Diabetes. 2012;61(6):1479–89. [PMC free article: PMC3357280] [PubMed: 22492528]
- 92.
- Lundasen T., et al. Circulating intestinal fibroblast growth factor 19 has a pronounced diurnal variation and modulates hepatic bile acid synthesis in man. J Intern Med. 2006;260(6):530–6. [PubMed: 17116003]
- 93.
- Fang S., et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21(2):159–65. [PMC free article: PMC4320010] [PubMed: 25559344]
- 94.
- Suzuki T., et al. Correlation between postprandial bile acids and body fat mass in healthy normal-weight subjects. Clin Biochem. 2014;47(12):1128–31. [PubMed: 24794786]
- 95.
- Turnbaugh P.J., et al. The human microbiome project. Nature. 2007;449(7164):804–10. [PMC free article: PMC3709439] [PubMed: 17943116]
- 96.
- Sender R., Fuchs S., Milo R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016;14(8):e1002533. p. [PMC free article: PMC4991899] [PubMed: 27541692]
- 97.
- Wang H.X., Wang Y.P. Gut Microbiota-brain Axis. Chin Med J (Engl). 2016;129(19):2373–80. [PMC free article: PMC5040025] [PubMed: 27647198]
- 98.
- Qin J., et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464(7285):59–65. [PMC free article: PMC3779803] [PubMed: 20203603]
- 99.
- Martinez K.B., Pierre J.F., Chang E.B. The Gut Microbiota: The Gateway to Improved Metabolism. Gastroenterol Clin North Am. 2016;45(4):601–614. [PMC free article: PMC5127273] [PubMed: 27837775]
- 100.
- Wostmann B.S., et al. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Lab Anim Sci. 1983;33(1):46–50. [PubMed: 6834773]
- 101.
- De Filippo C., et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107(33):14691–6. [PMC free article: PMC2930426] [PubMed: 20679230]
- 102.
- Furet J.P., et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes. 2010;59(12):3049–57. [PMC free article: PMC2992765] [PubMed: 20876719]
- 103.
- Schwiertz A., et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring). 2010;18(1):190–5. [PubMed: 19498350]
- 104.
- Bomhof M.R., et al. Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity (Silver Spring). 2014;22(3):763–71. [PubMed: 24124012]
- 105.
- Lin H.V., et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One. 2012;7(4):e35240. p. [PMC free article: PMC3323649] [PubMed: 22506074]
- 106.
- Pan X.D., et al. Prebiotic oligosaccharides change the concentrations of short-chain fatty acids and the microbial population of mouse bowel. J Zhejiang Univ Sci B. 2009;10(4):258–63. [PMC free article: PMC2666202] [PubMed: 19353743]
- 107.
- Kasubuchi M., et al. Dietary gut microbial metabolites, short-chain fatty acids, and host metabolic regulation. Nutrients. 2015;7(4):2839–49. [PMC free article: PMC4425176] [PubMed: 25875123]
- 108.
- Nohr M.K., et al. Expression of the short chain fatty acid receptor GPR41/FFAR3 in autonomic and somatic sensory ganglia. Neuroscience. 2015;290:126–37. [PubMed: 25637492]
- 109.
- Muccioli G.G., et al. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. [PMC free article: PMC2925525] [PubMed: 20664638]
- 110.
- Backhed F., et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A. 2004;101(44):15718–23. [PMC free article: PMC524219] [PubMed: 15505215]
- 111.
- Cani P.D., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–72. [PubMed: 17456850]
- 112.
- de La Serre C.B., et al. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G440–8. [PMC free article: PMC2928532] [PubMed: 20508158]
- 113.
- Caesar R., et al. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015;22(4):658–68. [PMC free article: PMC4598654] [PubMed: 26321659]
- 114.
- Vaure C., Liu Y. A comparative review of toll-like receptor 4 expression and functionality in different animal species. Front Immunol. 2014;5:316. [PMC free article: PMC4090903] [PubMed: 25071777]
- 115.
- Zuo D.C., et al. Inhibition of pacemaker activity in interstitial cells of Cajal by LPS via NF-kappaB and MAP kinase. World J Gastroenterol. 2013;19(8):1210–8. [PMC free article: PMC3587477] [PubMed: 23482668]
- 116.
- Schele E., et al. The gut microbiota reduces leptin sensitivity and the expression of the obesity-suppressing neuropeptides proglucagon (Gcg) and brain-derived neurotrophic factor (Bdnf) in the central nervous system. Endocrinology. 2013;154(10):3643–51. [PubMed: 23892476]
- 117.
- Schele E., et al. Regulation of body fat mass by the gut microbiota: Possible mediation by the brain. Peptides. 2016;77:54–9. [PubMed: 25934163]
- 118.
- Rosenbaum M., Leibel R.L. 20 years of leptin: role of leptin in energy homeostasis in humans. J Endocrinol. 2014;223(1):T83–96. [PMC free article: PMC4454393] [PubMed: 25063755]
- 119.
- De Silva A., et al. The gut hormones PYY3-36 and GLP-17-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell metabolism. 2011;14(5):700–706. [PMC free article: PMC3267038] [PubMed: 22000927]
- 120.
- Schmidt J.B., et al. Effects of PYY3–36 and GLP-1 on energy intake, energy expenditure, and appetite in overweight men. American Journal of Physiology-Endocrinology and Metabolism. 2014;306(11):E1248–E1256. [PubMed: 24735885]
- 121.
- De Silva A., et al. The gut hormones PYY 3-36 and GLP-1 7-36 amide reduce food intake and modulate brain activity in appetite centers in humans. Cell Metab. 2011;14(5):700–6. [PMC free article: PMC3267038] [PubMed: 22000927]
- 122.
- Neary N.M., et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology. 2005;146(12):5120–7. [PubMed: 16150917]
- 123.
- Field B.C., et al. PYY3-36 and oxyntomodulin can be additive in their effect on food intake in overweight and obese humans. Diabetes. 2010;59(7):1635–9. [PMC free article: PMC2889762] [PubMed: 20357366]
- 124.
- Panaro B.L., et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell metabolism. 2014;20(6):1018–1029. [PMC free article: PMC4255280] [PubMed: 25453189]
- 125.
- Hayes M.R., Schmidt H.D. GLP-1 influences food and drug reward. Current Opinion in Behavioral Sciences. 2016;9:66–70. [PMC free article: PMC4822543] [PubMed: 27066524]
- 126.
- Kroemer N.B., et al. Fasting levels of ghrelin covary with the brain response to food pictures. Addict Biol. 2013;18(5):855–62. [PubMed: 22974271]
- 127.
- Cummings D.E. Taste and the regulation of food intake: it's not just about flavor. Am J Clin Nutr. 2015;102(4):717–8. [PMC free article: PMC4588746] [PubMed: 26354541]
- 128.
- Zolotukhin S. Metabolic hormones in saliva: origins and functions. Oral diseases. 2013;19(3):219–229. [PMC free article: PMC3530011] [PubMed: 22994880]
- 129.
- Blouet C., Schwartz G.J. Duodenal lipid sensing activates vagal afferents to regulate non-shivering brown fat thermogenesis in rats. PLoS One. 2012;7(12):e51898. p. [PMC free article: PMC3522613] [PubMed: 23251649]
- 130.
- Piaggi P., et al. Energy expenditure in the etiology of human obesity: spendthrift and thrifty metabolic phenotypes and energy-sensing mechanisms. J Endocrinol Invest. 2018;41(1):83–89. [PMC free article: PMC5756119] [PubMed: 28741280]
- 131.
- Lean M.E., Malkova D. Altered gut and adipose tissue hormones in overweight and obese individuals: cause or consequence? Int J Obes (Lond). 2016;40(4):622–32. [PMC free article: PMC4827002] [PubMed: 26499438]
- 132.
- Steinert R.E., et al. Ghrelin, CCK, GLP-1, and PYY (3–36): Secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiological reviews. 2016;97(1):411–463. [PMC free article: PMC6151490] [PubMed: 28003328]
- 133.
- Auguet T., et al. Low Circulating Levels of Neurotensin in Women with Nonalcoholic Fatty Liver Disease Associated with Severe Obesity. Obesity (Silver Spring). 2018;26(2):274–278. [PubMed: 29276861]
- 134.
- Moghadam A.A., Moran T.H., Dailey M.J. Alterations in circadian and meal-induced gut peptide levels in lean and obese rats. Exp Biol Med (Maywood). 2017;242(18):1786–1794. [PMC free article: PMC5714147] [PubMed: 29191090]
- 135.
- Francois M., et al. High-fat diet increases ghrelin-expressing cells in stomach, contributing to obesity. Nutrition. 2016;32(6):709–15. [PubMed: 26856650]
- 136.
- Briggs D.I., et al. Diet-Induced Obesity Causes Ghrelin Resistance in Arcuate NPY/AgRP Neurons. Endocrinology. 2010;151(10):4745–4755. [PubMed: 20826561]
- 137.
- Wölnerhanssen B.K., et al. Deregulation of transcription factors controlling intestinal epithelial cell differentiation; a predisposing factor for reduced enteroendocrine cell number in morbidly obese individuals. Scientific reports. 2017;7(1):8174. [PMC free article: PMC5557953] [PubMed: 28811552]
- 138.
- Chavez-Talavera O., et al. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152(7):1679–1694.e3. [PubMed: 28214524]
- 139.
- Behary P., et al. Combined GLP-1, Oxyntomodulin, and Peptide YY Improves Body Weight and Glycemia in Obesity and Prediabetes/Type 2 Diabetes: A Randomized, Single-Blinded, Placebo-Controlled Study. Diabetes Care. 2019;42(8):1446–1453. [PubMed: 31177183]
- 140.
- Aron-Wisnewsky J., Clement K. The effects of gastrointestinal surgery on gut microbiota: potential contribution to improved insulin sensitivity. Curr Atheroscler Rep. 2014;16(11):454. [PubMed: 25214424]
- 141.
- Turnbaugh P.J., et al. A core gut microbiome in obese and lean twins. Nature. 2009;457(7228):480–4. [PMC free article: PMC2677729] [PubMed: 19043404]
- 142.
- Turnbaugh P.J., et al. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1(6):6ra14. [PMC free article: PMC2894525] [PubMed: 20368178]
- 143.
- Aron-Wisnewsky J., Clement K., Nieuwdorp M. Fecal Microbiota Transplantation: a Future Therapeutic Option for Obesity/Diabetes? Curr Diab Rep. 2019;19(8):51. [PubMed: 31250122]
- 144.
- Tambo A., Roshan M.H., Pace N.P. The Microbial Hypothesis: Contributions of Adenovirus Infection and Metabolic Endotoxaemia to the Pathogenesis of Obesity. Int J Chronic Dis. 2016;2016:7030795. p. [PMC free article: PMC5143720] [PubMed: 28004036]
- 145.
- Turnbaugh P.J., et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–31. [PubMed: 17183312]
- 146.
- Barkeling B., et al. Characterization of obese individuals who claim to detect no relationship between their eating pattern and sensations of hunger or fullness. Int J Obes (Lond). 2007;31(3):435–9. [PubMed: 16953260]
- 147.
- Sumithran P., et al. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(17):1597–604. [PubMed: 22029981]
- 148.
- Gloy V.L., et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. Bmj. 2013;347:f5934. [PMC free article: PMC3806364] [PubMed: 24149519]
- 149.
- Sjostrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273(3):219–34. [PubMed: 23163728]
- 150.
- Radaelli M.G., et al. NAFLD/NASH in patients with type 2 diabetes and related treatment options. J Endocrinol Invest. 2017 [PubMed: 29189999]
- 151.
- Angrisani L., et al. Bariatric Surgery and Endoluminal Procedures: IFSO Worldwide Survey 2014. Obes Surg. 2017;27(9):2279–2289. [PMC free article: PMC5562777] [PubMed: 28405878]
- 152.
- Manning S., et al. Early postoperative weight loss predicts maximal weight loss after sleeve gastrectomy and Roux-en-Y gastric bypass. Surg Endosc. 2015;29(6):1484–91. [PMC free article: PMC4422859] [PubMed: 25239175]
- 153.
- Pucci A., et al. Type 2 diabetes remission 2 years post Roux-en-Y gastric bypass and sleeve gastrectomy: the role of the weight loss and comparison of DiaRem and DiaBetter scores. Diabet Med. 2018;35(3):360–367. [PMC free article: PMC5836992] [PubMed: 29055156]
- 154.
- Olbers T., et al. Laparoscopic gastric bypass: development of technique, respiratory function, and long-term outcome. Obes Surg. 2003;13(3):364–70. [PubMed: 12841895]
- 155.
- Abu-Jaish W., Rosenthal R.J. Sleeve gastrectomy: a new surgical approach for morbid obesity. Expert Rev Gastroenterol Hepatol. 2010;4(1):101–19. [PubMed: 20136593]
- 156.
- Leibel R.L., Hirsch J. Diminished energy requirements in reduced-obese patients. Metabolism. 1984;33(2):164–70. [PubMed: 6694559]
- 157.
- Browning K.N., Fortna S.R., Hajnal A. Roux-en-Y gastric bypass reverses the effects of diet-induced obesity to inhibit the responsiveness of central vagal motoneurones. J Physiol. 2013;591(9):2357–72. [PMC free article: PMC3650700] [PubMed: 23459752]
- 158.
- Manning S., Pucci A., Batterham R.L. Roux-en-Y gastric bypass: effects on feeding behavior and underlying mechanisms. J Clin Invest. 2015;125(3):939–48. [PMC free article: PMC4362264] [PubMed: 25729850]
- 159.
- Leibel R.L., et al. Biologic Responses to Weight Loss and Weight Regain: Report From an American Diabetes Association Research Symposium. Diabetes. 2015;64(7):2299–309. [PubMed: 26106187]
- 160.
- Ionut V., Bergman R.N. Mechanisms responsible for excess weight loss after bariatric surgery. J Diabetes Sci Technol. 2011;5(5):1263–82. [PMC free article: PMC3208891] [PubMed: 22027328]
- 161.
- Odstrcil E.A., et al. The contribution of malabsorption to the reduction in net energy absorption after long-limb Roux-en-Y gastric bypass. Am J Clin Nutr. 2010;92(4):704–13. [PubMed: 20739420]
- 162.
- Yousseif A., et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obesity surgery. 2014;24(2):241–252. [PMC free article: PMC3890046] [PubMed: 23996294]
- 163.
- Peterli R., et al. Metabolic and hormonal changes after laparoscopic Roux-en-Y gastric bypass and sleeve gastrectomy: a randomized, prospective trial. Obes Surg. 2012;22(5):740–8. [PMC free article: PMC3319900] [PubMed: 22354457]
- 164.
- Yousseif A., et al. Differential effects of laparoscopic sleeve gastrectomy and laparoscopic gastric bypass on appetite, circulating acyl-ghrelin, peptide YY3-36 and active GLP-1 levels in non-diabetic humans. Obes Surg. 2014;24(2):241–52. [PMC free article: PMC3890046] [PubMed: 23996294]
- 165.
- le Roux C.W., et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Annals of surgery. 2007;246(5):780–785. [PubMed: 17968169]
- 166.
- Jirapinyo P., et al. A Meta-Analysis of GLP-1 After Roux-En-Y Gastric Bypass: Impact of Surgical Technique and Measurement Strategy. Obes Surg. 2017 [PubMed: 28871519]
- 167.
- Scott W.R., Batterham R.L. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy: understanding weight loss and improvements in type 2 diabetes after bariatric surgery. Am J Physiol Regul Integr Comp Physiol. 2011;301(1):R15–27. [PubMed: 21474429]
- 168.
- Dirksen C., et al. Gut hormones, early dumping and resting energy expenditure in patients with good and poor weight loss response after Roux-en-Y gastric bypass. International journal of obesity. 2013;37(11):1452–1460. [PubMed: 23419600]
- 169.
- Svane M.S., et al. Peptide YY and glucagon-like peptide-1 contribute to decreased food intake after Roux-en-Y gastric bypass surgery. Int J Obes (Lond). 2016;40(11):1699–1706. [PubMed: 27434221]
- 170.
- Steinert R.E., et al. Bile acids and gut peptide secretion after bariatric surgery: a 1-year prospective randomized pilot trial. Obesity (Silver Spring). 2013;21(12):E660–8. [PubMed: 23804517]
- 171.
- Pournaras D.J., et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology. 2012;153(8):3613–9. [PMC free article: PMC3404349] [PubMed: 22673227]
- 172.
- Ahmad N.N., Pfalzer A., Kaplan L.M. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond). 2013;37(12):1553–9. [PMC free article: PMC4157126] [PubMed: 23567924]
- 173.
- Ryan K.K., et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509(7499):183–8. [PMC free article: PMC4016120] [PubMed: 24670636]
- 174.
- Liu H., et al. Role of gut microbiota, bile acids and their cross-talk in the effects of bariatric surgery on obesity and type 2 diabetes. J Diabetes Investig. 2018;9(1):13–20. [PMC free article: PMC5754516] [PubMed: 28434196]
- 175.
- Liou A.P., et al. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med. 2013;5(178):178ra41. p. [PMC free article: PMC3652229] [PubMed: 23536013]
- 176.
- Midtvedt T., Norman A., Nygaard K. Bile acid transforming micro-organisms in rats with an intestinal blind segment. Acta Pathol Microbiol Scand. 1969;77(1):162–6. [PubMed: 4904667]
- 177.
- Tremaroli V., et al. Roux-en-Y Gastric Bypass and Vertical Banded Gastroplasty Induce Long-Term Changes on the Human Gut Microbiome Contributing to Fat Mass Regulation. Cell Metab. 2015;22(2):228–38. [PMC free article: PMC4537510] [PubMed: 26244932]
- 178.
- Murphy R., et al. Differential Changes in Gut Microbiota After Gastric Bypass and Sleeve Gastrectomy Bariatric Surgery Vary According to Diabetes Remission. Obes Surg. 2017;27(4):917–925. [PubMed: 27738970]
- 179.
- Medina D.A., et al. Distinct patterns in the gut microbiota after surgical or medical therapy in obese patients. PeerJ. 2017;5:e3443. p. [PMC free article: PMC5480389] [PubMed: 28649469]
- 180.
- Guo Y., et al. Gut microbiota after Roux-en-Y gastric bypass and sleeve gastrectomy in a diabetic rat model: Increased diversity and associations of discriminant genera with metabolic changes. Diabetes Metab Res Rev. 2017;33(3) [PubMed: 27572277]
- 181.
- Dethlefsen L., Relman D.A. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci U S A. 2011;108 Suppl 1:4554–61. [PMC free article: PMC3063582] [PubMed: 20847294]
- 182.
- Zakeri R., Batterham R.L. Potential mechanisms underlying the effect of bariatric surgery on eating behaviour. Curr Opin Endocrinol Diabetes Obes. 2018;25(1):3–11. [PubMed: 29120924]
- 183.
- Berthoud H.R. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20 Suppl 1:64–72. [PMC free article: PMC3617963] [PubMed: 18402643]
- 184.
- Berthoud H.R., Shin A.C., Zheng H. Obesity surgery and gut-brain communication. Physiol Behav. 2011;105(1):106–19. [PMC free article: PMC3118403] [PubMed: 21315095]
- 185.
- Pi-Sunyer X., et al. A Randomized, Controlled Trial of 3.0 mg of Liraglutide in Weight Management. New England Journal of Medicine. 2015;373(1):11–22. [PubMed: 26132939]
- 186.
- Marso S.P., et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2016;375(19):1834–1844. [PubMed: 27633186]
- 187.
- Husain M., et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2019;381(9):841–851. [PubMed: 31185157]
- 188.
- Aroda V.R., et al. PIONEER 1: Randomized Clinical Trial of the Efficacy and Safety of Oral Semaglutide Monotherapy in Comparison With Placebo in Patients With Type 2 Diabetes. Diabetes Care. 2019;42(9):1724–1732. [PubMed: 31186300]
- 189.
- Bagger J.I., et al. Effect of oxyntomodulin, glucagon, GLP-1, and combined glucagon+ GLP-1 infusion on food intake, appetite, and resting energy expenditure. The Journal of Clinical Endocrinology & Metabolism. 2015;100(12):4541–4552. [PubMed: 26445112]
- 190.
- Wynne K., et al. Oxyntomodulin increases energy expenditure in addition to decreasing energy intake in overweight and obese humans: a randomised controlled trial. International journal of obesity. 2006;30(12):1729. [PubMed: 16619056]
- 191.
- Reda T.K., Geliebter A., Pi‐Sunyer F.X. Amylin, food intake, and obesity. Obesity research. 2002;10(10):1087–1091. [PubMed: 12376591]
- 192.
- Allas S., et al. AZP-531, an unacylated ghrelin analog, improves food-related behavior in patients with Prader-Willi syndrome: A randomized placebo-controlled trial. PloS one. 2018;13(1):e0190849. p. [PMC free article: PMC5761957] [PubMed: 29320575]
- 193.
- Tan T., et al. The effect of a subcutaneous infusion of GLP-1, OXM, and PYY on energy intake and expenditure in obese volunteers. The Journal of Clinical Endocrinology & Metabolism. 2017;102(7):2364–2372. [PMC free article: PMC5505203] [PubMed: 28379519]
- 194.
- Pathak V., Flatt P.R., Irwin N. Cholecystokinin (CCK) and related adjunct peptide therapies for the treatment of obesity and type 2 diabetes. Peptides. 2018;100:229–235. [PubMed: 29412823]
- 195.
- Finan B., et al. A rationally designed monomeric peptide triagonist corrects obesity and diabetes in rodents. Nature medicine. 2015;21(1):27. [PubMed: 25485909]
- Review Neuroendocrine regulation of energy balance: Implications on the development and surgical treatment of obesity.[Nutr Health. 2017]Review Neuroendocrine regulation of energy balance: Implications on the development and surgical treatment of obesity.Farias G, Netto BDM, Bettini SC, Dâmaso AR, de Freitas ACT. Nutr Health. 2017 Sep; 23(3):131-146. Epub 2017 Aug 24.
- Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity.[Microbiome. 2022]Functional changes of the gastric bypass microbiota reactivate thermogenic adipose tissue and systemic glucose control via intestinal FXR-TGR5 crosstalk in diet-induced obesity.Münzker J, Haase N, Till A, Sucher R, Haange SB, Nemetschke L, Gnad T, Jäger E, Chen J, Riede SJ, et al. Microbiome. 2022 Jun 24; 10(1):96. Epub 2022 Jun 24.
- Review Neurohormonal Changes in the Gut-Brain Axis and Underlying Neuroendocrine Mechanisms following Bariatric Surgery.[Int J Mol Sci. 2022]Review Neurohormonal Changes in the Gut-Brain Axis and Underlying Neuroendocrine Mechanisms following Bariatric Surgery.Martinou E, Stefanova I, Iosif E, Angelidi AM. Int J Mol Sci. 2022 Mar 19; 23(6). Epub 2022 Mar 19.
- Review MECHANISMS IN ENDOCRINOLOGY: The gut-brain axis: regulating energy balance independent of food intake.[Eur J Endocrinol. 2021]Review MECHANISMS IN ENDOCRINOLOGY: The gut-brain axis: regulating energy balance independent of food intake.Nogueiras R. Eur J Endocrinol. 2021 Aug 3; 185(3):R75-R91. Epub 2021 Aug 3.
- Review Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different.[J Endocrinol Invest. 2019]Review Mechanisms underlying the weight loss effects of RYGB and SG: similar, yet different.Pucci A, Batterham RL. J Endocrinol Invest. 2019 Feb; 42(2):117-128. Epub 2018 May 5.
- Endocrinology of the Gut and the Regulation of Body Weight and Metabolism - Endo...Endocrinology of the Gut and the Regulation of Body Weight and Metabolism - Endotext
- Trichoblastoma and Trichoepithelioma - StatPearlsTrichoblastoma and Trichoepithelioma - StatPearls
- cell death regulator Aven [Homo sapiens]cell death regulator Aven [Homo sapiens]gi|9966841|ref|NP_065104.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...