ABSTRACT
The pineal gland was described as the “Seat of the Soul” by Renee Descartes and it is located in the center of the brain. The main function of the pineal gland is to receive information about the state of the light-dark cycle from the environment and convey this information by the production and secretion of the hormone melatonin. Changing photoperiod is indicated by the duration of melatonin secretion and is used by photoperiodic species to time their seasonal physiology. The rhythmic production of melatonin, normally secreted only during the dark period of the day, is extensively used as a marker of the phase of the internal circadian clock. Melatonin itself is used as a therapy for certain sleep disorders related to circadian rhythm abnormalities such as delayed sleep phase syndrome, non-24h sleep wake disorder and jet lag. It might have more extensive therapeutic applications in the future, since multiple physiological roles have been attributed to melatonin. It exerts physiologic immediate effects during night or darkness and when suitably administered has prospective effects during daytime when melatonin levels are undetectable. In addition to its role in regulating seasonal physiology and influencing the circadian system and sleep patterns, melatonin is involved in cell protection, neuroprotection, and the reproductive system, among other possible functions. Pineal gland function and melatonin secretion can be impaired due to accidental and developmental conditions, such as pineal tumors, craniopharyngiomas, injuries affecting the sympathetic innervation of the pineal gland, and rare congenital disorders that alter melatonin secretion. This chapter summarizes the physiology and pathophysiology of the pineal gland and melatonin. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
PINEAL PHYSIOLOGY
Pineal Anatomy and Structure
The pineal gland in humans is a small (100-150 mg), highly vascularized, and a secretory neuroendocrine organ (1). It is located in the mid-line of the brain, outside the blood-brain barrier and attached to the roof of the third ventricle by a short stalk. In humans, the pineal gland usually shows a degree of calcification with age providing a good imaging marker (for a speculative discussion of pineal calcification see reference(2)). The principal innervation is sympathetic, arising from the superior cervical ganglia (3). Arterial vascularization of the pineal gland is supplied by both the anterior and posterior circulation, being the main artery supplying the lateral pineal artery, which originates from the posterior circulation (4). In mammals, the main cell types are pinealocytes (95%) followed by scattered glial cells (astrocytic and phagocytic subtypes) (5). Pinealocytes are responsible for the synthesis and secretion of melatonin.
Main Function of the Pineal Gland
The main function of the pineal gland is to receive and convey information about the current light-dark cycle from the environment via the production and secretion of melatonin cyclically at night (dark period) (6, 7). Although in cold-blooded vertebrates (lower-vertebrate species), the pineal gland is photosensitive, this property is lost in higher vertebrates. In higher vertebrates, light is sensed by the inner retina (retinal ganglion cells) that send neural signals to the visual areas of the brain. However, a few retinal ganglion cells contain melanopsin and have an intrinsic photoreceptor capability that sends neural signals to non-image forming areas of the brain, including the pineal gland, through complex neuronal connections. The photic information from the retina is sent to the suprachiasmatic nucleus (SCN), the major rhythm-generating system or “clock” in mammals, and from there to other hypothalamic areas. When the light signal is positive, the SCN secretes gamma-amino butyric acid, responsible for the inhibition of the neurons that synapse in the paraventricular nucleus (PVN) of the hypothalamus, consequently the signal to the pineal gland is interrupted and melatonin is not synthesized. On the contrary, when there is no light (darkness), the SCN secretes glutamate, responsible for the PVN transmission of the signal along the pathway to the pineal gland. However, it is important to note that in continuous darkness the SCN continues to generate rhythmic output without light suppression since it functions as an endogenous oscillator (master pacemaker or clock). The rhythm deviates from 24h and ‘free-runs’ in the absence of the important light time cue. Light-dark cycles serve to synchronize the rhythm to 24h.
The PVN nucleus communicates with higher thoracic segments of the spinal column, conveying information to the superior cervical ganglion that transmits the final signal to the pineal gland through sympathetic postsynaptic fibers by releasing norepinephrine (NE). NE is the trigger for the pinealocytes to produce melatonin by activating the transcription of the mRNA encoding the enzyme arylalkylamine N-acetyltransferase (AA-NAT), the first molecular step for melatonin synthesis (8) (Figure 1).

Figure 1.
Melatonin synthesis in the pineal gland.
MELATONIN SYNTHESIS AND METABOLISM
Melatonin Synthesis
Melatonin (N-acetyl-5-methoxytryptamine) is synthesized within the pinealocytes from tryptophan, mostly occurring during the dark phase of the day, when there is a major increase in the activity of serotonin-N-acetyltransferase (arylalkylamine N-acetyltransferase, AA-NAT), responsible for the transformation of 5-hydroxytryptamine (5HT, serotonin) to N-acetylserotonin (NAS) (Figure 1). Finally, N-acetylserotonin is converted to melatonin by acetylserotonin O-methyltransferase. The rapid decline in the synthesis with light treatment at night appears to depend on proteasomal proteolysis (9). Both AA-NAT and serotonin availability play a role limiting melatonin production. AA-NAT mRNA is expressed mainly in the pineal gland, retina, and to a lesser extent in some other brain areas, pituitary, and testis. Melatonin synthesis is also described in many other sites. AA-NAT activation is triggered by the activation of β1 and α1b adrenergic receptors by NE (9). NE is the major transmitter via β-1 adrenoceptors with potentiation by α-1 stimulation. NE levels are higher at night, approximately 180 degrees out of phase with the serotonin rhythm. Both availability of NE and serotonin are stimulatory for melatonin synthesis. Pathological, surgical or traumatic sympathetic denervation of the pineal gland or administration of β-adrenergic antagonists abolishes the rhythmic synthesis of melatonin and the light-dark control of its production.
There is evidence that melatonin can be synthesized in other sites of the body (skin, gastrointestinal tract, retina, bone marrow, placenta and others) acting in an autocrine or paracrine manner (10). Very recently it has been demonstrated that in the mouse brain melatonin is exclusively synthesized in the mitochondrial matrix. It is released to the cytoplasm, thereby activating a mitochondrial MT1 signal-transduction pathway which inhibits stress-mediated cytochrome c release and caspase activation: these are preludes to cell death and inflammation. This is a new mechanism whereby locally synthesized melatonin may protect against neurodegeneration. It is referred to as automitocrine signaling (11). Except for the pineal gland, other structures contribute little to circulating concentrations in mammals, since after pinealectomy, melatonin levels are mostly undetectable (12). Importantly several other factors, summarized in Table 1, have been related to the secretion and production of melatonin (13, 14).
Table 1.
Factors Influencing Human Melatonin Secretion and Production (11),(12)
Control of Melatonin Synthesis: A Darkness Hormone
The rhythm of melatonin production is internally generated and controlled by interacting networks of clock genes in the bilateral SCN (15). Damage to the SCN leads to a loss of the majority of circadian rhythms. The SCN rhythm is synchronized to 24 hours mainly by the light-dark cycle acting via the retina and the retinohypothalamic projection to the SCN; the longer the night the longer the duration of secretion is, and the ocular light serves to synchronize the rhythm to 24h and to suppress secretion at the end of the dark phase, as explained above. Light exposure is the most important factor related to pineal gland function and melatonin secretion. A single daily light pulse of suitable intensity and duration in otherwise constant darkness is enough to phase shift and to synchronize the melatonin rhythm to 24h (16). The amount of light required at night to suppress melatonin secretion varies across species. In humans, intensities of 2500 lux full spectrum light (domestic light is around 100 to 500 lux) or light preferably in the blue range (460 to 480 nm) are required to completely suppress melatonin at night, but lower intensities < 200 lux can suppress secretion and shift the rhythm (17-21). Previous photic history influences the response: living in dim light increases sensitivity. Furthermore, the degree of light perception between individuals is related to the incidence of circadian desynchrony; along these lines, blind people with no conscious or unconscious light perception show free-running or abnormally synchronized melatonin and other circadian rhythms (22-24). Some blind subjects retain an intact retinohypothalamic tract and therefore a normal melatonin response despite a lack of conscious light perception (25, 26). It seems clear that an intact innervated pineal gland is necessary for the response to photoperiod change (27). Melatonin functions as a paracrine signal within the retina, it enhances retinal function in low intensity light by inducing photomechanical changes and provides a closed-loop to the pineal-retina-SCN system. All together, they are the basic structures to perceive and transduce non-visual effects of light, and to generate the melatonin rhythm by a closed-loop negative feedback of genes (Clock, “Circadian locomotor output cycles kaput” and Bmal, “Brain and muscle ARNT-like” genes), positive stimulatory elements (Per, “period” and Cry, “Cryptochrome” genes), and negative elements (CCG, clock-controlled genes) of clock gene expression in the SCN (Figure 2).

Figure 2.
Diagrammatic representation of the control of production and the functions of melatonin, regarding seasonal and circadian timing mechanisms. Abbreviations: SCN: suprachiasmatic nucleus, PVN: paraventricular nucleus, SCG: superior cervical ganglion, NA: norepinephrine (noradrenalin), RHT: retino-hypothalamic-tract, CCG: clock-controlled genes. Based on an original diagram by Dr Elisabeth Maywood, MRC Laboratory of Molecular Biology, Neurobiology Division, Hills Road Cambridge, CB2 2QH, UK.
Melatonin Metabolism
Once synthesized, melatonin is released directly into the peripheral circulation (bound to albumin) and to the CSF without being stored. In humans, melatonin’s half-life in blood is around 40 minutes and it is metabolized within the liver, converted to 6-hydroxymelatonin mainly by CYP1A2 and conjugated to 6-sulfatoxymelatonin (aMT6s) for subsequent urinary excretion. The measurement of urinary aMT6s is a good marker of melatonin secretion, since it follows the same pattern with an approximate 2-hours offset (28, 29). Overall, women have slightly higher values of plasma melatonin at night than men (30). On average, the maximum levels of plasma melatonin in adults occur between 02.00 and 04.00 hours and are on average about 60 to 70 pg/mL when measured with high-specificity assays. Concentrations in saliva, like other hormones, are three times lower than in plasma. Minimum concentrations detected are below 1 pg/mL. The plasma melatonin rhythm (timing and amplitude) strongly correlates with urinary aMT6s. Although there is a large variability in amplitude of the rhythm between subjects, the normal human melatonin rhythm is reproducible from day to day intraindividually (Figure 3 and 4).

Figure 3.
Average concentrations of melatonin in human plasma (black, N=133), saliva (blue, N=28) and 6-sulphatoxymelatonin (aMT6s) in urine (red, N=88) using radioimmunoassay measurements. Diagrammatic representation of mean normal values (healthy men and women over 18 years old) from Dr. J. Arendt. Stockgrand Ltd., University of Surrey, UK.

Figure 4.
Plasma melatonin and urinary aMT6s in hourly samples to show the delay in the rhythm of urinary aMT6s compared to plasma melatonin (mean/SEM, N=14). Red lines show typical urine sample collection times over 24-48h to determine timing of the rhythm in out-patient or field studies. Redrawn from R. Naidoo, Thesis, University of Surrey, UK, 1998.
Melatonin Production During Development and Across Life
At birth, melatonin levels are almost undetectable, the only fetal source of melatonin being via the placental circulation. Melatonin levels in fetal umbilical circulation reflect the day-night difference as seen in the maternal circulation. Maternal melatonin sends a temporal circadian signal to the fetus (called “maternal photoperiodic adaptative programming”), preparing the CNS to properly deal with environmental day/night fluctuations after birth. A melatonin rhythm appears around 2 to 3 months of life (31), levels increasing exponentially until a lifetime peak on average in prepubertal children; melatonin concentrations in children are associated with Tanner stages of puberty (32). Thereafter, a steady decrease occurs reaching mean adult concentrations in late teens (33, 34). Values are stable until 35 to 40 years, followed by a decline in amplitude of melatonin rhythm and lower levels with ageing, associated with fragmented sleep-wake patterns (35). In people >90 years, melatonin levels are less than 20% of young adult concentrations (36). The decline in age-related melatonin production is attributed to different reasons; calcification of the pineal gland starting early in life and an impairment in the noradrenergic innervation to the gland or light detection capacity (ocular mydriases, cataracts) (2, 37). Interestingly, pinealectomy accelerates the aging process and several reports suggest that melatonin has anti-aging properties (38).
MELATONIN’S MECHANISMS OF ACTION
Melatonin Target Sites and Receptors
Melatonin’s target sites are both central and peripheral. Binding sites have been found in many areas of the brain, including the pars tuberalis and hypothalamus, but also in the cells of the immune system, gonads, kidney, and the cardiovascular system (39, 40). Melatonin binding sites in the brain might vary according to species. Melatonin exerts both non-receptor and receptor-mediated actions. Non-receptor-mediated actions are due to amphipathic properties (of both lipo- and hydrophilic structure) that allows melatonin to freely cross the cell and nuclear membranes. It can be easily detected in the nucleus of several cells in the brain and peripheral organs (41). Antioxidant properties are one example of non-receptor-mediated actions of melatonin. On the other hand, melatonin has receptor-mediated actions.
Two types of a new family of G protein coupled melatonin receptors (MT) have been cloned in mammals (42). Melatonin receptors are widely expressed, often with overlapping distributions. MT1 is primarily expressed in the pars tuberalis, the SCN together with other hypothalamic areas, pituitary, hippocampus, and adrenal glands, suggesting that circadian and reproductive effects are mediated through this receptor. MT2 is mainly expressed in the SCN, retina, pituitary and the other brain areas, and is associated with phase shifting (43). The affinity of melatonin is five-fold greater for MT1 than MT2. Melatonin administration induces (1) an acute suppression of neuronal firing in the SCN via the MT1 receptor, and (2) a phase-shifting of SCN activity through the MT2 receptor. However, agonists that exclusively act on one or another receptor have not been identified as yet, and the understanding of the role of each receptor in most of the tissues in which both receptors are present is difficult. Also, melatonin receptors are transiently expressed in neuroendocrine tissues during development, suggesting that melatonin has a role as a neuroendocrine synchronizer in developmental physiology (44).
Chronobiotic Effects of Melatonin

Figure 5.
Phase shifting of circadian rhythms. From data in Middleton B. et al., J. Sleep Res, 2002, 11, Suppl 1, p 154, Melatonin phase shifts all measured rhythms abstr. No. 309. Rajaratnam SW, et al., J Clin Endocrinol Metab 2003;88:4303-9. Rajaratnam SW, et al., J Physiol 2004; 561:339-351.
Phase shifting of circadian rhythms by melatonin (Figure 5) was first described in the 1980s (45). Melatonin has chronobiotic effects and is able to synchronize and reset biological oscillations. Melatonin acts on oscillators according to a well-defined phase-response curve (PRC). PRC is characterized by phase-advance zone (early in the evening before the beginning of the nocturnal melatonin production), phase-delay zone (in the late night/early morning hours), and a non-responsive dead. zone (when melatonin levels are high) (46). The melatonin PRC is useful for the clinical administration of melatonin as a chronobiotic agent and treatment of sleep circadian and mood disorders (47). In addition to the circadian chronobiotic effects, melatonin importantly has chronobiotic seasonal effects and acts as a circannual synchronizer, one season determined by increasing duration of the nocturnal melatonin production (in the direction of the winter solstice) and the other season defined by the reduction of nocturnal melatonin production (in the direction of the summer solstice) (8, 48). Interestingly, most of the clock genes are expressed in the pars tuberalis with a 24h rhythmicity different from their expression in the SCN (49), and these clock genes are influenced by melatonin with numerous potential seasonal effects. However, whether melatonin modulates the activity of the SCN via regulation of clock genes is unclear. Also, a central clock, independent of the SCN, can be entrained by food availability, temperature variations, forced activity and rest, drugs (melatonin itself), and timing (50).
MELATONIN PHYSIOLOGY AND PATHOPHYSIOLOGY
As previously stated, melatonin can act through several mechanisms and at almost all levels of the organism. Therefore, it has multiple and diversified actions with immediate (endogenous melatonin, during the night) or prospective effects (exogenous melatonin, during the previous day).
Hypomelatoninemia is more common, and it can be due to factors that affect directly the pineal gland, innervation, melatonin synthesis as a result of congenital disease; or secondary as a consequence of environmental factors and/or medications (shift work, spinal cord cervical transection, sympathectomy, aging, neurodegenerative diseases, genetic diseases, β-blockers, calcium channel blockers, ACE inhibitors). Hypermelatoninemia is less common, and except for pharmacological effects, few conditions have been associated with high melatonin production: spontaneous hypothermia, hyperhidrosis syndrome, polycystic ovary syndrome, hypogonadotropic hypogonadism, anorexia nervosa, and Rabson-Mendenhall syndrome that induces pineal hyperplasia.
Melatonin During Puberty, Menstrual Cycle and Reproductive Function
Neuroendocrine control of sexual maturation is influenced by the pattern of melatonin secretion as a consequence of the light-dark cycle, and in some species the photoperiod via melatonin secretion determines the timing of puberty (51). In vitro studies, in cultured prepuberal rat pituitary gland, showed that melatonin inhibits gonadotrophin-releasing hormone and therefore luteinizing hormone release, providing evidence for a potential causal role of melatonin in the timing of developmental stages (52) (Figure 2). The mechanism by which melatonin inhibits GnRH secretion is not clear; recently, kisspeptin was suggested to mediate this effect. Lower melatonin levels have been associated with precocious puberty and higher levels in delayed puberty and hypothalamic amenorrhea compared to age-matched controls; however, a causal role of melatonin in pubertal development has not been described (53, 54). Data on circulating melatonin, and its variation during the menstrual cycle, are inconsistent (55). In males, high melatonin doses (100 mg daily) potentiate testosterone-induced LH suppression, and a negative correlation between nocturnal serum LH and melatonin have been reported (56, 57). Nevertheless, attempts to develop melatonin as a contraceptive pill in combination with progestin have not been successful (58). Interestingly, people living near the Arctic circle present lower conception rates during winter darkness, when melatonin levels are high, than in summer. In summary, the overall perception in human studies is that melatonin is inhibitory to human reproductive function.
Melatonin and Core Body Temperature
Melatonin plays a role in circadian thermoregulation. In particular, the melatonin peak is associated with the nadir in body temperature, together with maximum tiredness, lowest alertness and performance (59) (Figure 6). Exogenous administration of melatonin during the daytime reproduces this association, increasing fatigue and sleepiness and decreasing body temperature, especially if the subject is seated (posture dependent effect) (59). Along these lines, the rise in temperature during the ovulatory phase of the menstrual cycle is associated with a decline in the amplitude of melatonin.

Figure 6.
Relationship of plasma melatonin to other major circadian rhythms driven by the internal clock. Abbreviations: VAS: visual analogue scale. Reproduced from Rajaratnam SMW and Arendt J. Lancet 358:999-1005, 2001 by permission.
Melatonin and Energy Metabolism and Glucose Homeostasis
Melatonin is an important player in the regulation of energy metabolism and glucose homeostasis. It is responsible for the daily distribution of energy metabolism functions (daily phase of high insulin sensitivity, glycogen synthesis, and lipogenesis and a sleep phase associated with the usage of stored energy) (60). Interestingly, administration of melatonin in post-menopausal women induced a reduction in fat mass and increase in lean mass compared to placebo (61). Nocturnal melatonin secretion facilitates diurnal insulin sensitivity and preservation of beta cell mass and function (62) and low melatonin secretion has independently been associated to a higher risk for type 2 diabetes (T2DM) (63). In short sleepers or when there is a misalignment between waking time and melatonin secretion (melatonin secretion is not interrupted), insulin resistance and hyperglycemia can be observed. Melatonin administration can result in iatrogenic insulin resistance and hyperglycemia in the morning, depending when it is administered and on the metabolizing characteristics of the subject. Moreover, recently, some variants of the gene encoding for the MT-1B have been associated with reduced beta-cell function and increased risk for T2DM (64). Several cardiovascular effects have also been attributed to melatonin, such as, antihypertensive properties, regulation of heart rate, and vascular resistance (65, 66).
Melatonin, Antioxidant Properties and Cancer
Antioxidant and anti-aging properties have also been attributed to melatonin. Melatonin acts as a potent free radical scavenger and antioxidant in vitro, independently of the presence of the receptor (38, 67, 68) protecting lipids, protein and DNA from oxidative damage. It is more effective than glutathione in reducing oxidative stress under many circumstances, being highly concentrated in the mitochondria. Although most of these effects in humans have been observed in supraphysiological doses of melatonin, the quantity of exogenous melatonin required to generate relevant antioxidant activity is not well established. Moreover, the clinical benefits of antioxidant supplements are not clear. Quote “research has not shown antioxidant supplements to be beneficial in preventing diseases”, https://www.nccih.nih.gov/health/antioxidants-in-depth#:~:text=Antioxidants%20are%20man%2Dmade%20or,be%20beneficial%20in%20preventing%20diseases.
Also, there is growing recent evidence for anti-tumoral activity of melatonin (69, 70). Melatonin seems to be useful as an oncostatic agent at the cellular level and slows the progression of cancer (most of the studies are focused on breast and prostate cancer). Interestingly, in rats when a carcinogen is given at night during the highest levels of melatonin, DNA damage is significantly lower (20%) than in rats that receive a carcinogen exposure during the day (71). Pinealectomy stimulates cancer initiation or growth in animals, and pineal calcification, that leads to a decline in melatonin production, have been associated with an increase in pediatric primary brain tumors (72).
An ’Umbrella review’, whereby the results of numerous meta-analyses are combined, has provided important data on which of the numerous claims for melatonin therapeutic benefit stand up to scrutiny. A simplified version is provided here (see below).
As mentioned previously, exposure to light during the “biological night” can suppress melatonin production, and it is also associated to a deleterious effect on health (i.e., increased risk of cancer in most epidemiology studies in night shift workers) (70, 73). Night-shift workers present a higher incidence of hormone-dependent cancer which has been related to light-induced melatonin suppression which consequently increases estrogen production. A 50% increased risk to develop breast cancer in nurses exposed to rotating shift work has been documented (74). In contrast, a reduced risk for breast cancer was observed in blind women, with potentially higher levels of melatonin throughout all day (although there is no evidence that blind people produce more melatonin than sighted people) However, the mechanisms by which melatonin exerts any of oncostatic effects remains to be established.
Miscellaneous
The circadian annual rhythm of prolactin secretion depends on the circadian melatonin signal. Melatonin regulates pars tuberalis timer cells, and coordinates prolactin-secreting cells which together function as an intrapituitary pacemaker timer system. Interestingly, several clock genes are expressed within the pituitary with a circadian rhythm independent of the SCN (75).
Melatonin might act on the adrenal glands as an endogenous pacemaker. Glucocorticoids levels are low after the onset of darkness and rise after the middle of the night concomitantly with a decrease in melatonin levels. This is consistent with an inhibitory effect of melatonin through MT1 on glucocorticoid production. Interestingly, many antidepressant drugs increase the availability of the precursors (tryptophan and serotonin) and the major pineal neurotransmitter NE and therefore stimulate melatonin secretion. A melatonin agonist has been developed as an antidepressant through its actions on serotonin 2C receptors (76). If there is a link between this increase in melatonin production and the efficacy of antidepressant medications this needs further evaluation.
Several conditions (congenital or acquired) might induce a dysregulation of the melatonin synthesis/signaling and affect rhythmic melatonin production:
Pathological or traumatic sympathetic denervation (i.e., injury to the spinal cord) of the pineal gland or administration of β-adrenergic antagonists abolishes the rhythmic synthesis of melatonin and the light-dark control of its production (77, 78). NE is the major transmitter via β1 adrenoceptors with potentiation by α1 stimulation. NE levels are higher at night, approximately 180 degrees out of phase with the serotonin rhythm. Both availability of NE and serotonin are stimulatory for melatonin synthesis. However, several other factors have been related to the secretion and production of melatonin (13) (Table 1). In humans, administration of atenolol suppresses melatonin and enhances the magnitude of light-induced phase shift (79). In fact, several related observations suggest that endogenous melatonin acts to counter undesirable abrupt changes in phase. Pinealectomy i.e., abolishment of the melatonin rhythm, leads to a more rapid circadian adaptation to phase shift in rats (80).
Craniopharyngioma patients often display low melatonin secretion as a result of SCN impairment associated with alterations in sleep pattern (81).
Smith-Magenis syndrome (a congenital disorder due to a haplo-insufficiency of the retinoic acid-induced 1 gene, involved in the regulation of the expression of circadian genes) patients present with an inverted rhythmic melatonin secretion and sleep difficulties that can be managed with melatonin administration in the evening and β-adrenergic blockers during the day to reduce melatonin secretion (82). Low levels of melatonin have been consistently associated with autism spectrum disorders (ASD) (83). The last enzyme in the synthesis of melatonin, acetylserotonin-O-methyltransferase, has been associated with susceptibility for ASD which could be an explanation for low melatonin levels in ASD (84). Interestingly, administration of melatonin has shown to be efficacious for insomnia in children with ASD (85).
MELATONIN, CLINICAL APPLICATION AND THERAPEUTIC USE
Literature on the clinical use of melatonin is extensive and has increased exponentially over the last decade. Melatonin effects on sleep are the most well-known; however, the finding of increased cancer risk in shift workers and that patients with neurodegenerative diseases, autism or depression present abnormal melatonin rhythms, have recently increased attention on the role of melatonin.
Melatonin and Sleep
Numerous factors and comorbidities are associated with chronic insomnia in the adult population, different from the circadian sleep-wake rhythm disorders. Some of these risk factors and comorbidities involve psychiatric conditions (i.e. depression, anxiety, posttraumatic stress disorder), medical conditions (i.e. pulmonary diseases, chronic pain, heart failure, hyperthyroidism, nocturia, gastroesophageal reflux, cancer, pregnancy, pruritus, HIV infection, obstructive sleep apnea syndrome), neurological conditions (i.e. Alzheimer’s, Parkinson’s disease, peripheral neuropathies, strokes, brain tumors, headache syndromes), and medications (i.e. central nervous system stimulants or depressants, bronchodilators, antidepressants, diuretics, glucocorticoids, caffeine, alcohol). All these conditions should be ruled out before establishing the suspicion of a circadian sleep-wake rhythm disorder. The importance of melatonin levels for human sleep was apparently demonstrated by studies on pinealectomized subjects. These patients presented a disrupted 24h circadian rhythm, and a reduction of sleep time and quality, that were reversed after the administration of melatonin (86, 87). However, another careful, prospective study using polysomnography before and following pinealectomy found no effect on sleep and similar data are reported in rats (88-90). Thus, the question is not resolved. Clearly it is not a sleep hormone since in nocturnal animals it is secreted during the active periods. Overall, exogenous melatonin has been shown consistently to reduce sleep latency, and less consistently increase total sleep time, reduce night awakenings, and ultimately improve sleep quality (91). The most obvious action is to optimize sleep timing with respect to the circadian clock: we sleep better when melatonin production (and thus the circadian clock) and sleep are correctly aligned. Diagnostic criteria of every circadian sleep-wake rhythm disorder were fully described by the American Academy of Sleep Medicine (2014) (92).
Circadian sleep wake-rhythm disorders evaluation
The origin of a circadian rhythm disorder can be related either to an intrinsic abnormality in the circadian system itself (misalignment of the intrinsic circadian timing with the desired sleep schedule) or to external factors, as when individuals must be awake at times that are not synchronized with their intrinsic rhythms. Overall, sleep disorders result in clinically significant symptoms of insomnia, daytime sleepiness and impaired physical, neurocognitive (concentration, processing speed, memory), emotional and social functioning, as well as impaired functioning at work or school (92). Age and associated comorbidities can help in the differential diagnoses. Symptoms across sleeping disorders are non-specific; the key to properly identify them is the recognition of abnormal sleep-wake patterns using sleep diaries beyond the complaint of insomnia or daytime sleepiness (93). Time domain is very important for melatonin actions (immediate or prospective effects, chronobiotic, or seasonal effects); therefore, adequate melatonin measurement and time of administration are crucial. Actigraphy can supplement self-reported sleep diary information or be useful in situations of neurodevelopmental disorders where a sleep diary cannot be completed.
Melatonin concentrations typically increase 90 to 120 minutes prior to the habitual bedtime if bright light (>10 lux) is absent. Preferentially frequent blood sampling every 30 to 60 minutes from six hours prior to and one hour following habitual bedtime is collected to assess the individual’s dim light melatonin onset (DLMO) and circadian phase. Samples should be collected in a dark environment (red light <10 lux) without interfering with sleep. In research studies a DLMO protocol is used as a valid and reliable indicator of circadian phase position, according to the time at which melatonin levels rise, and is extremely helpful to guide the timing for melatonin therapy in each individual; however, this is rarely feasible for routine use in clinical settings (94). Alternatively, urinary excretion of 6-sulfatoxymelatonin is a good index of nocturnal melatonin production and it should be collected as sequential urine samples for 48h in order to calculate the timing of the peak or acrophase. Also, it is possible to measure melatonin in saliva, during the evening before sleep and this approach has been frequently used. But, in the case of for example very delayed melatonin rise the time of onset can be missed during sleep. Importantly, melatonin values vary individually and according to age and sex. Although, endogenous melatonin production is closely related with the onset and offset of sleep, few associations have been found between melatonin production and sleep stages (95), and sleep deprivation does not abolish the melatonin rhythm.
Circadian sleep wake-rhythm disorders
The primary goal of treatment of circadian sleep-wake rhythm disorders is to realign the circadian timing of sleep and wake with the required sleep-wake period. Appropriately timed and dosed melatonin, melatonin receptor agonists, and light therapy seem to be useful approaches (96, 97). Following the melatonin PRC and taking the individual DLMO as the reference phase to decide the most suitable time of melatonin administration, according to the desirable effect, seem the most appropriate guide to decide when to start chronic melatonin therapy (98-100).
Delayed and advanced sleep-wake phase disorders, non-24 hour, and irregular sleep-wake rhythm disorders are considered intrinsic circadian disorders; in contrast, jet lag and shift work disorders are due to an environmentally imposed misalignment. The most potent phase shifting factor (zeitgeber) is the environmental light-dark cycle (101).
Light exposure during the last hours of the usual sleep period moves the circadian rhythm forward (phase advanced). On the contrary, light exposure in the evening and the first half of the usual sleep period moves the circadian rhythm back (phase delayed) (Figure 7) (96). Advanced sleep-wake phase disorder is partially due to the physiologic advance that occurs with aging together with the weakening of circadian rhythms observed with aging. It can be genetically determined in some families as well. Delayed sleep-wake phase disorder is more often seen in children with some neurodevelopmental disorders; these patients cannot sleep during the dark time and delay sleep onset until the early hours of the morning, sleeping much of the day. In these situations both bright light in the early morning and evening melatonin 5 hours before endogenous melatonin onset secretion (0.5-5 mg) has been shown to advance sleep time significantly (102), the magnitude of the advanced shift being dose-dependent. In contrast early “biological morning” administration of melatonin induces delayed shifts. The non-24-hour sleep-wake rhythm disorder is most commonly seen in blindness, since the light-dark cycle is the most powerful cue for synchronizing the hypothalamic pacemaker to the 24-hour day (103). Therefore, this disorder is characterized by a failure to maintain stable alignment to the 24-h day, and a “free-running” circadian rhythm system which usually shifts to a later and later phase position. Timed melatonin treatment has been successful in entraining free-running blind patients.
The irregular sleep-wake rhythm disorder is seen in patients with dementia, which a neurodegenerative process might induce a disruption of the circadian system with a consequent loss of modulatory influence on sleep and wakefulness (104).

Figure 7.
Representation of a simplified diagram of phase shifts of the circadian system, as evidenced by changes in melatonin rhythm itself, following oral treatment with fast release melatonin at different times. Biological night” is the time of endogenous melatonin secretion and defines “circadian time” which is independent of clock time.
Jet lag occurs when crossing time zones, and one needs to sleep and be awake at times that are not aligned with one’s own circadian system. It is more severe when more time zones are crossed and if the direction of the travel is eastbound, as it is more difficult to advance than delay the natural circadian cycle. Melatonin accelerates the phase shift if given at the appropriate time prior to bedtime at destination, and the benefits of melatonin administration in the alleviation of jet lag seems to be greater with larger numbers of time zones (105). If melatonin is appropriately time-administered, self-rated jet lag can be reduced by 50 percent (106). The maximum advance shift obtainable with a single treatment of oral melatonin (3-5 mg) is approximately 1-1.5 h.
For night shift workers wakefulness is required at the time that melatonin secretion is rising, and alertness is dissipating, and the opposite for the next day when melatonin secretion decreases, and circadian rhythms promote alertness. While some studies suggested that the use of melatonin improved sleep and increase daytime alertness in night shift workers compared to placebo (107), other studies have not shown beneficial effects (108). More data are needed before recommendations about melatonin as a therapy for shift workers can be made. However anecdotal evidence suggests that it is widely used for daytime sleep.
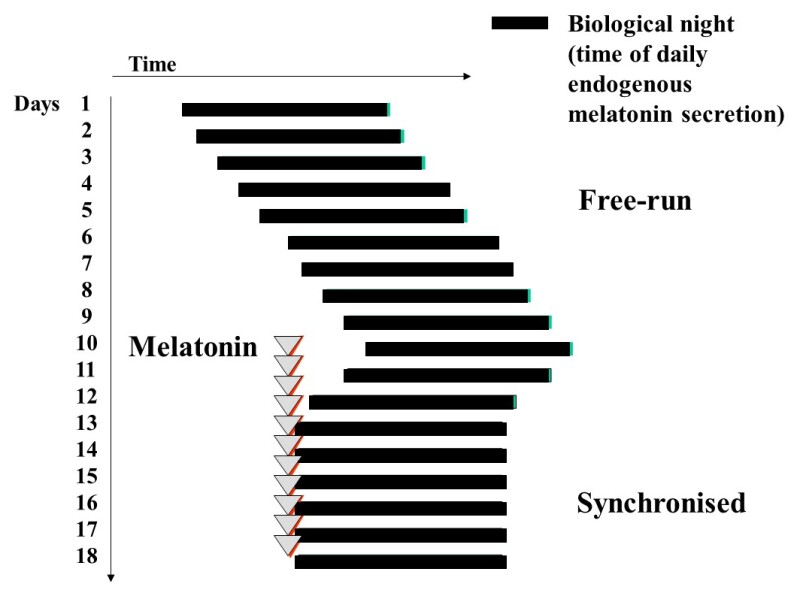
Figure 8.
Diagram to illustrate free-running of the sleep wake cycle and other circadian rhythms in non-24h sleep-wake disorder, frequently seen in totally blind people. Melatonin, usually in doses of 0.5 – 5mg daily can synchronize sleep and the circadian system to the 24h day with benefits for sleep, and daytime alertness.
Melatonin and the CNS
Individuals with neurodegenerative disorders (i.e., Alzheimer’s and Parkinson’s disease, Huntington’s disease, autism) had significant flattened and attenuated melatonin rhythms compared to age-matched controls (37, 109). Melatonin showed efficacy in managing the insomnia in elderly people or improving cognitive function associated with neurodegenerative disorders (110). It has reported effects on anxiety, cognitive function and memory. Knowing the wide distribution of melatonin receptors in the CNS, melatonin seems one of the promising neuroprotective agents to be tested in humans. However other studies have found deleterious effects of melatonin in elderly demented patients (111).
Melatonin: Therapeutic Use
Almost 200 randomized clinical trials on the use of melatonin and evaluation of clinical effects have been published. Moreover, several patents have been registered in relation to the therapeutic applications of melatonin and melatonin analogues (sleep disorders, neuroprotection, cancer). The American Academy of Sleep Science recommends the use of melatonin for jet lag, delayed sleep phase syndrome, and non-24h sleep wake disorder syndrome seen in blind people; however, there were few consensuses acknowledging its therapeutic benefits (112) until an ‘Umbrella’ review of meta-analyses identified consistent therapeutic targets (91). Interestingly, at least in animal models, appropriate melatonin dose administration can reverse most of the effects after a pinealectomy.
Table 2.
Melatonin’s Reported Significant Clinical Effects
Several melatonin receptor agonists have been synthesized, some of them have higher affinity for the receptor than endogenous melatonin. Agomelatine (activity at the serotonin-2C receptor) functions as an anti-depressant, ramelteon (selective MT1/MT2 agonist) is marketed for use in sleep onset insomnia, tasimelteon (MT1/MT2 agonist), is commercialized for the treatment of circadian rhythm disorders particularly non-24h sleep wake disorder (113, 114). Also, slow-release formulations of the natural biologic melatonin have been developed.
The time of melatonin administration is critical, especially in chronic treatments, and the melatonin profile shows large interindividual variation; however, the profile of an individual is highly reproducible from day to day. Also, absorption, metabolism and excretion of melatonin vary between individuals and should be considered to get the desired clinical efficacy of the therapy. Ideally, although not feasible in a daily clinical practice, DLMO should be determined for each individual, and used as a timing reference for the prescription of melatonin. Alternatively, the time each individual goes to sleep could determine the time of administration of melatonin; it is advisable to take oral melatonin around 45 minutes to 1 hour before the usual bedtime (time to reach maximal plasma concentrations following oral immediate-release formulations). Moreover, the duration of the pharmacological profile should last until the usual wake time of the patient; thus, the type of pharmaceutical formulation (slow or fast release) is also an important consideration. However, there is little evidence for greater benefit of slow-release preparations. Route of administration, as well as age, liver function and potential drug interactions (since melatonin is metabolized in the liver), may influence plasma melatonin levels and should be taken into account (115). Sensitivities and pharmacokinetics of melatonin vary between individuals, and a lower dose of 0.3-0.5 mg might be more effective than higher doses in many subjects.
There is no general consensus regarding dosage. A wide range of dose formulations are available, and the usage varies depending on the clinical application. The usual advice is to start with the lowest dose available. Low doses 0.1 to 0.3 mg/d that produce near physiological melatonin concentrations can be used for central clock synchronization; doses ranging from 0.6 to 5 mg/d for sleep disorders, or doses as high as 300 mg/d for neurodegenerative disorders (amyotrophic lateral sclerosis) (116, 117). There is a current tendency to recommend high pharmacological doses for ‘protective’ or antioxidant effects. What consequences these might have for the circadian aspects of melatonin function is not known. It should not be forgotten that melatonin has profound effects on the reproductive function of photoperiodic seasonal breeders and there is good evidence for residual photoperiodicity in humans. However in most clinical trials or studies using melatonin, it is well accepted that in general melatonin lacks toxic adverse events, and it is a safe drug at most of the usual tested doses from 0.5 to 5 mg/d (118). Whether dosage should be changed in chronic melatonin treatment according to the annual season requires further investigation; further studies are needed on the undesirable consequences of melatonin suppression in the long term (for example by beta blocking drugs or shiftwork).
To summarize, benefits of melatonin and its analogues on circadian sleep disorders are consistently reported but for more generalized “insomnia” are often of low strength. The dose must be timed and individually adjusted as optimization will vary among individuals. More studies are required to develop treatment guidelines for different conditions. The long-term consequences of taking high dose melatonin especially in pediatrics need careful evaluation. Melatonin mechanisms of action and effects need much further work, to better understand the therapeutic value of melatonin and its potential applications.
REFERENCES
- 1.
- L V. The Pineal Organ, Handbook of Mikroscopic Anatomy1981.
- 2.
- Tan DX, Xu B, Zhou X, Reiter RJ. Pineal Calcification, Melatonin Production, Aging, Associated Health Consequences and Rejuvenation of the Pineal Gland. Molecules. 2018;23(2) [PMC free article: PMC6017004] [PubMed: 29385085]
- 3.
- Kappers JA. Innervation of the Vertebrate Pineal Organ. In: Axelrod J, Fraschini, F., Velo, G.P. (eds) editor1983.
- 4.
- Kahilogullari G, Ugur HC, Comert A, Brohi RA, Ozgural O, Ozdemir M, et al. Arterial vascularization of the pineal gland. Childs Nerv Syst. 2013;29(10):1835–41. [PubMed: 23334574]
- 5.
- Moller M, Baeres FM. The anatomy and innervation of the mammalian pineal gland. Cell Tissue Res. 2002;309(1):139–50. [PubMed: 12111544]
- 6.
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16(4):283–301. [PubMed: 11506375]
- 7.
- JA. A. Melatonin and the Mammalian Pineal Gland.1995.
- 8.
- Cipolla-Neto J, Amaral FGD. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr Rev. 2018;39(6):990–1028. [PubMed: 30215696]
- 9.
- Gastel JA, Roseboom PH, Rinaldi PA, Weller JL, Klein DC. Melatonin production: proteasomal proteolysis in serotonin N-acetyltransferase regulation. Science (New York, NY). 1998;279(5355):1358–60. [PubMed: 9478897]
- 10.
- Bubenik GA. Localization, physiological significance and possible clinical implication of gastrointestinal melatonin. Biol Signals Recept. 2001;10(6):350–66. [PubMed: 11721091]
- 11.
- Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, et al. Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proc Natl Acad Sci U S A. 2017;114(38):E7997–E8006. [PMC free article: PMC5617277] [PubMed: 28874589]
- 12.
- Lewy AJ, Tetsuo M, Markey SP, Goodwin FK, Kopin IJ. Pinealectomy abolishes plasma melatonin in the rat. J Clin Endocrinol Metab. 1980;50(1):204–5. [PubMed: 7350183]
- 13.
- Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides, and other pineal transmitters. Pharmacol Rev. 2003;55(2):325–95. [PubMed: 12773631]
- 14.
- Zawilska JB, Skene DJ, Arendt J. Physiology and pharmacology of melatonin in relation to biological rhythms. Pharmacol Rep. 2009;61(3):383–410. [PubMed: 19605939]
- 15.
- Takahashi JS, Hong HK, Ko CH, McDearmon EL. The genetics of mammalian circadian order and disorder: implications for physiology and disease. Nat Rev Genet. 2008;9(10):764–75. [PMC free article: PMC3758473] [PubMed: 18802415]
- 16.
- Illnerova H, Vanecek J. Entrainment of the rat pineal rhythm in melatonin production by light. Reprod Nutr Dev (1980). 1988;28(2B):515-26. [PubMed: 3413344]
- 17.
- Laakso ML, Hatonen T, Stenberg D, Alila A, Smith S. One-hour exposure to moderate illuminance (500 lux) shifts the human melatonin rhythm. J Pineal Res. 1993;15(1):21–6. [PubMed: 8229642]
- 18.
- Gooley JJ, Chamberlain K, Smith KA, Khalsa SB, Rajaratnam SM, Van Reen E, et al. Exposure to room light before bedtime suppresses melatonin onset and shortens melatonin duration in humans. J Clin Endocrinol Metab. 2011;96(3):E463–72. [PMC free article: PMC3047226] [PubMed: 21193540]
- 19.
- Reiter RJ. Action spectra, dose-response relationships, and temporal aspects of light's effects on the pineal gland. Ann N Y Acad Sci. 1985;453:215–30. [PubMed: 3907458]
- 20.
- Bojkowski CJ, Aldhous ME, English J, Franey C, Poulton AL, Skene DJ, et al. Suppression of nocturnal plasma melatonin and 6-sulphatoxymelatonin by bright and dim light in man. Horm Metab Res. 1987;19(9):437–40. [PubMed: 3692439]
- 21.
- Zeitzer JM, Dijk DJ, Kronauer R, Brown E, Czeisler C. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. [PMC free article: PMC2270041] [PubMed: 10922269]
- 22.
- Klerman EB, Rimmer DW, Dijk DJ, Kronauer RE, Rizzo JF 3rd, Czeisler CA. Nonphotic entrainment of the human circadian pacemaker. Am J Physiol. 1998;274(4 Pt 2):R991–6. [PubMed: 9575961]
- 23.
- Lewy AJ, Newsome DA. Different types of melatonin circadian secretory rhythms in some blind subjects. J Clin Endocrinol Metab. 1983;56(6):1103–7. [PubMed: 6841552]
- 24.
- Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocrinol Metab. 1997;82(11):3763–70. [PubMed: 9360538]
- 25.
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. The New England journal of medicine. 1995;332(1):6–11. [PubMed: 7990870]
- 26.
- Hull JT, Czeisler CA, Lockley SW. Suppression of Melatonin Secretion in Totally Visually Blind People by Ocular Exposure to White Light: Clinical Characteristics. Ophthalmology. 2018;125(8):1160–71. [PubMed: 29625838]
- 27.
- Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of the pineal gland and melatonin. J Biol Rhythms. 2001;16(4):336–47. [PubMed: 11506379]
- 28.
- Bojkowski CJ, Arendt J, Shih MC, Markey SP. Melatonin secretion in humans assessed by measuring its metabolite, 6-sulfatoxymelatonin. Clin Chem. 1987;33(8):1343–8. [PubMed: 3608151]
- 29.
- Arendt J, Bojkowski C, Franey C, Wright J, Marks V. Immunoassay of 6-hydroxymelatonin sulfate in human plasma and urine: abolition of the urinary 24-hour rhythm with atenolol. J Clin Endocrinol Metab. 1985;60(6):1166–73. [PubMed: 3998065]
- 30.
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, et al. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25(4):288–96. [PMC free article: PMC3792014] [PubMed: 20679498]
- 31.
- Kennaway DJ, Stamp GE, Goble FC. Development of melatonin production in infants and the impact of prematurity. J Clin Endocrinol Metab. 1992;75(2):367–9. [PubMed: 1639937]
- 32.
- Waldhauser F, Weiszenbacher G, Frisch H, Zeitlhuber U, Waldhauser M, Wurtman RJ. Fall in nocturnal serum melatonin during prepuberty and pubescence. Lancet. 1984;1(8373):362–5. [PubMed: 6141425]
- 33.
- Wetterberg L, Bergiannaki JD, Paparrigopoulos T, von Knorring L, Eberhard G, Bratlid T, et al. Normative melatonin excretion: a multinational study. Psychoneuroendocrinology. 1999;24(2):209–26. [PubMed: 10101729]
- 34.
- Kennaway DJ, Lushington K, Dawson D, Lack L, van den Heuvel C, Rogers N. Urinary 6-sulfatoxymelatonin excretion and aging: new results and a critical review of the literature. J Pineal Res. 1999;27(4):210–20. [PubMed: 10551768]
- 35.
- Bojkowski CJ, Arendt J. Factors influencing urinary 6-sulphatoxymelatonin, a major melatonin metabolite, in normal human subjects. Clin Endocrinol (Oxf). 1990;33(4):435–44. [PubMed: 2225488]
- 36.
- Scholtens RM, van Munster BC, van Kempen MF, de Rooij SE. Physiological melatonin levels in healthy older people: A systematic review. J Psychosom Res. 2016;86:20–7. [PubMed: 27302542]
- 37.
- Jengeleski CA, Powers RE, O'Connor DT, Price DL. Noradrenergic innervation of human pineal gland: abnormalities in aging and Alzheimer's disease. Brain Res. 1989;481(2):378–82. [PubMed: 2720390]
- 38.
- Karasek M. Melatonin, human aging, and age-related diseases. Exp Gerontol. 2004;39(11-12):1723–9. [PubMed: 15582288]
- 39.
- Reppert SM, Weaver DR, Godson C. Melatonin receptors step into the light: cloning and classification of subtypes. Trends Pharmacol Sci. 1996;17(3):100–2. [PubMed: 8936344]
- 40.
- Dubocovich ML, Markowska M. Functional MT1 and MT2 melatonin receptors in mammals. Endocrine. 2005;27(2):101–10. [PubMed: 16217123]
- 41.
- Costa EJ, Lopes RH, Lamy-Freund MT. Permeability of pure lipid bilayers to melatonin. J Pineal Res. 1995;19(3):123–6. [PubMed: 8750345]
- 42.
- Dubocovich ML, Delagrange P, Krause DN, Sugden D, Cardinali DP, Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol Rev. 2010;62(3):343–80. [PMC free article: PMC2964901] [PubMed: 20605968]
- 43.
- Reppert SM. Melatonin receptors: molecular biology of a new family of G protein-coupled receptors. J Biol Rhythms. 1997;12(6):528–31. [PubMed: 9406026]
- 44.
- Johnston JD, Klosen P, Barrett P, Hazlerigg DG. Regulation of MT melatonin receptor expression in the foetal rat pituitary. J Neuroendocrinol. 2006;18(1):50–6. [PubMed: 16451220]
- 45.
- Arendt J, Bojkowski C, Folkard S, Franey C, Marks V, Minors D, et al. Some effects of melatonin and the control of its secretion in humans. Ciba Found Symp. 1985;117:266–83. [PubMed: 3836818]
- 46.
- Armstrong SM, Redman JR. Melatonin: a chronobiotic with anti-aging properties? Med Hypotheses. 1991;34(4):300–9. [PubMed: 1865836]
- 47.
- Lewy AJ, Ahmed S, Jackson JM, Sack RL. Melatonin shifts human circadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9(5):380–92. [PubMed: 1394610]
- 48.
- Arendt J, Middleton B. Human seasonal and circadian studies in Antarctica (Halley, 75 degrees S). Gen Comp Endocrinol. 2018;258:250–8. [PubMed: 28526480]
- 49.
- Carr AJ, Johnston JD, Semikhodskii AG, Nolan T, Cagampang FR, Stirland JA, et al. Photoperiod differentially regulates circadian oscillators in central and peripheral tissues of the Syrian hamster. Curr Biol. 2003;13(17):1543–8. [PubMed: 12956958]
- 50.
- Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science (New York, NY). 2008;320(5879):1074–7. [PMC free article: PMC3489954] [PubMed: 18497298]
- 51.
- Ebling FJ, Foster DL. Pineal melatonin rhythms and the timing of puberty in mammals. Experientia. 1989;45(10):946–54. [PubMed: 2680575]
- 52.
- Martin JE, Sattler C. Selectivity of melatonin pituitary inhibition for luteinizing hormone-releasing hormone. Neuroendocrinology. 1982;34(2):112–6. [PubMed: 6122168]
- 53.
- Waldhauser F, Boepple PA, Schemper M, Mansfield MJ, Crowley WF Jr. Serum melatonin in central precocious puberty is lower than in age-matched prepubertal children. J Clin Endocrinol Metab. 1991;73(4):793–6. [PubMed: 1909703]
- 54.
- Berga SL, Mortola JF, Yen SS. Amplification of nocturnal melatonin secretion in women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 1988;66(1):242–4. [PubMed: 3335608]
- 55.
- Parry BL, Berga SL, Mostofi N, Klauber MR, Resnick A. Plasma melatonin circadian rhythms during the menstrual cycle and after light therapy in premenstrual dysphoric disorder and normal control subjects. J Biol Rhythms. 1997;12(1):47–64. [PubMed: 9104690]
- 56.
- Anderson RA, Lincoln GA, Wu FC. Melatonin potentiates testosterone-induced suppression of luteinizing hormone secretion in normal men. Hum Reprod. 1993;8(11):1819–22. [PubMed: 8288743]
- 57.
- Luboshitzky R, Wagner O, Lavi S, Herer P, Lavie P. Abnormal melatonin secretion in hypogonadal men: the effect of testosterone treatment. Clin Endocrinol (Oxf). 1997;47(4):463–9. [PubMed: 9404445]
- 58.
- Voordouw BC, Euser R, Verdonk RE, Alberda BT, de Jong FH, Drogendijk AC, et al. Melatonin and melatonin-progestin combinations alter pituitary-ovarian function in women and can inhibit ovulation. J Clin Endocrinol Metab. 1992;74(1):108–17. [PubMed: 1727807]
- 59.
- Cagnacci A, Elliott JA, Yen SS. Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J Clin Endocrinol Metab. 1992;75(2):447–52. [PubMed: 1639946]
- 60.
- Picinato MC, Haber EP, Carpinelli AR, Cipolla-Neto J. Daily rhythm of glucose-induced insulin secretion by isolated islets from intact and pinealectomized rat. J Pineal Res. 2002;33(3):172–7. [PubMed: 12220333]
- 61.
- Amstrup AK, Sikjaer T, Pedersen SB, Heickendorff L, Mosekilde L, Rejnmark L. Reduced fat mass and increased lean mass in response to 1 year of melatonin treatment in postmenopausal women: A randomized placebo-controlled trial. Clin Endocrinol (Oxf). 2016;84(3):342–7. [PubMed: 26352863]
- 62.
- Costes S, Boss M, Thomas AP, Matveyenko AV. Activation of Melatonin Signaling Promotes beta-Cell Survival and Function. Mol Endocrinol. 2015;29(5):682–92. [PMC free article: PMC4415205] [PubMed: 25695910]
- 63.
- McMullan CJ, Schernhammer ES, Rimm EB, Hu FB, Forman JP. Melatonin secretion and the incidence of type 2 diabetes. JAMA. 2013;309(13):1388–96. [PMC free article: PMC3804914] [PubMed: 23549584]
- 64.
- Prokopenko I, Langenberg C, Florez JC, Saxena R, Soranzo N, Thorleifsson G, et al. Variants in MTNR1B influence fasting glucose levels. Nat Genet. 2009;41(1):77–81. [PMC free article: PMC2682768] [PubMed: 19060907]
- 65.
- Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension. 2004;43(2):192–7. [PubMed: 14732734]
- 66.
- Grossman E, Laudon M, Yalcin R, Zengil H, Peleg E, Sharabi Y, et al. Melatonin reduces night blood pressure in patients with nocturnal hypertension. Am J Med. 2006;119(10):898–902. [PubMed: 17000226]
- 67.
- Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016;61(3):253–78. [PubMed: 27500468]
- 68.
- Tan DX, Reiter RJ, Manchester LC, Yan MT, El-Sawi M, Sainz RM, et al. Chemical and physical properties and potential mechanisms: melatonin as a broad spectrum antioxidant and free radical scavenger. Curr Top Med Chem. 2002;2(2):181–97. [PubMed: 11899100]
- 69.
- Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. 2007;8(12):1065–6. [PubMed: 19271347]
- 70.
- Blask DE, Dauchy RT, Sauer LA. Putting cancer to sleep at night: the neuroendocrine/circadian melatonin signal. Endocrine. 2005;27(2):179–88. [PubMed: 16217131]
- 71.
- Tan D, Reiter RJ, Chen LD, Poeggeler B, Manchester LC, Barlow-Walden LR. Both physiological and pharmacological levels of melatonin reduce DNA adduct formation induced by the carcinogen safrole. Carcinogenesis. 1994;15(2):215–8. [PubMed: 8313511]
- 72.
- Tuntapakul S, Kitkhuandee A, Kanpittaya J, Johns J, Johns NP. Pineal calcification is associated with pediatric primary brain tumor. Asia Pac J Clin Oncol. 2016;12(4):e405–e10. [PubMed: 27461152]
- 73.
- Blask DE, Dauchy RT, Sauer LA, Krause JA, Brainard GC. Light during darkness, melatonin suppression and cancer progression. Neuro Endocrinol Lett. 2002;23 Suppl 2:52–6. [PubMed: 12163849]
- 74.
- Hansen J. Light at night, shiftwork, and breast cancer risk. J Natl Cancer Inst. 2001;93(20):1513–5. [PubMed: 11604468]
- 75.
- Lincoln GA, Clarke IJ, Hut RA, Hazlerigg DG. Characterizing a mammalian circannual pacemaker. Science (New York, NY). 2006;314(5807):1941–4. [PubMed: 17185605]
- 76.
- Mocaer E, Delalleau B, Boyer PA, de Bodinat C. Med Sci (Paris). 2005;21(10):888–93. [Development of a new antidepressant : agomelatine] [PubMed: 16197911]
- 77.
- Scheer FA, Zeitzer JM, Ayas NT, Brown R, Czeisler CA, Shea SA. Reduced sleep efficiency in cervical spinal cord injury; association with abolished night time melatonin secretion. Spinal Cord. 2006;44(2):78–81. [PMC free article: PMC2882209] [PubMed: 16130027]
- 78.
- Zeitzer JM, Ayas NT, Shea SA, Brown R, Czeisler CA. Absence of detectable melatonin and preservation of cortisol and thyrotropin rhythms in tetraplegia. J Clin Endocrinol Metab. 2000;85(6):2189–96. [PubMed: 10852451]
- 79.
- Deacon S, English J, Tate J, Arendt J. Atenolol facilitates light-induced phase shifts in humans. Neuroscience letters. 1998;242(1):53–6. [PubMed: 9510003]
- 80.
- Arendt J. Melatonin: Countering Chaotic Time Cues. Frontiers in endocrinology. 2019;10:391. [PMC free article: PMC6646716] [PubMed: 31379733]
- 81.
- Muller HL, Handwerker G, Wollny B, Faldum A, Sorensen N. Melatonin secretion and increased daytime sleepiness in childhood craniopharyngioma patients. J Clin Endocrinol Metab. 2002;87(8):3993–6. [PubMed: 12161549]
- 82.
- De Leersnyder H. Inverted rhythm of melatonin secretion in Smith-Magenis syndrome: from symptoms to treatment. Trends Endocrinol Metab. 2006;17(7):291–8. [PubMed: 16890450]
- 83.
- Tordjman S, Anderson GM, Pichard N, Charbuy H, Touitou Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biological psychiatry. 2005;57(2):134–8. [PubMed: 15652871]
- 84.
- Melke J, Goubran Botros H, Chaste P, Betancur C, Nygren G, Anckarsater H, et al. Abnormal melatonin synthesis in autism spectrum disorders. Mol Psychiatry. 2008;13(1):90–8. [PMC free article: PMC2199264] [PubMed: 17505466]
- 85.
- Maras A, Schroder CM, Malow BA, Findling RL, Breddy J, Nir T, et al. Long-Term Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder. J Child Adolesc Psychopharmacol. 2018 [PMC free article: PMC6306655] [PubMed: 30132686]
- 86.
- Lehmann ED, Cockerell OC, Rudge P. Somnolence associated with melatonin deficiency after pinealectomy. Lancet. 1996;347(8997):323. [PubMed: 8569375]
- 87.
- Murata J, Sawamura Y, Ikeda J, Hashimoto S, Honma K. Twenty-four hour rhythm of melatonin in patients with a history of pineal and/or hypothalamo-neurohypophyseal germinoma. J Pineal Res. 1998;25(3):159–66. [PubMed: 9745984]
- 88.
- Slawik H, Stoffel M, Riedl L, Vesely Z, Behr M, Lehmberg J, et al. Prospective Study on Salivary Evening Melatonin and Sleep before and after Pinealectomy in Humans. J Biol Rhythms. 2016;31(1):82–93. [PubMed: 26647380]
- 89.
- Mendelson WB, Bergmann BM. Effects of pinealectomy on baseline sleep and response to sleep deprivation. Sleep. 2001;24(4):369–73. [PubMed: 11403520]
- 90.
- Fisher SP, Sugden D. Endogenous melatonin is not obligatory for the regulation of the rat sleep-wake cycle. Sleep. 2010;33(6):833–40. [PMC free article: PMC2881717] [PubMed: 20550025]
- 91.
- Posadzki PP, Bajpai R, Kyaw BM, Roberts NJ, Brzezinski A, Christopoulos GI, et al. Melatonin and health: an umbrella review of health outcomes and biological mechanisms of action. BMC Med. 2018;16(1):18. [PMC free article: PMC5798185] [PubMed: 29397794]
- 92.
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest. 2014;146(5):1387–94. [PubMed: 25367475]
- 93.
- Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287–302. [PMC free article: PMC3250369] [PubMed: 22294820]
- 94.
- Lewy AJ, Sack RL. The dim light melatonin onset as a marker for circadian phase position. Chronobiol Int. 1989;6(1):93–102. [PubMed: 2706705]
- 95.
- Dijk DJ, Shanahan TL, Duffy JF, Ronda JM, Czeisler CA. Variation of electroencephalographic activity during non-rapid eye movement and rapid eye movement sleep with phase of circadian melatonin rhythm in humans. J Physiol. 1997;505(Pt 3):851–8. [PMC free article: PMC1160058] [PubMed: 9457658]
- 96.
- Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical Practice Guideline for the Treatment of Intrinsic Circadian Rhythm Sleep-Wake Disorders: Advanced Sleep-Wake Phase Disorder (ASWPD), Delayed Sleep-Wake Phase Disorder (DSWPD), Non-24-Hour Sleep-Wake Rhythm Disorder (N24SWD), and Irregular Sleep-Wake Rhythm Disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med. 2015;11(10):1199–236. [PMC free article: PMC4582061] [PubMed: 26414986]
- 97.
- Lockley SW, Arendt J, Skene DJ. Visual impairment and circadian rhythm disorders. Dialogues Clin Neurosci. 2007;9(3):301–14. [PMC free article: PMC3202494] [PubMed: 17969867]
- 98.
- Lewy AJ. Clinical applications of melatonin in circadian disorders. Dialogues Clin Neurosci. 2003;5(4):399–413. [PMC free article: PMC3181782] [PubMed: 22033851]
- 99.
- Smits MG, Nagtegaal EE, van der Heijden J, Coenen AM, Kerkhof GA. Melatonin for chronic sleep onset insomnia in children: a randomized placebo-controlled trial. J Child Neurol. 2001;16(2):86–92. [PubMed: 11292231]
- 100.
- van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010;33(12):1605–14. [PMC free article: PMC2982730] [PubMed: 21120122]
- 101.
- Campbell SS. Effects of timed bright-light exposure on shift-work adaptation in middle-aged subjects. Sleep. 1995;18(6):408–16. [PubMed: 7481411]
- 102.
- Nagtegaal JE, Kerkhof GA, Smits MG, Swart AC, Van Der Meer YG. Delayed sleep phase syndrome: A placebo-controlled cross-over study on the effects of melatonin administered five hours before the individual dim light melatonin onset. J Sleep Res. 1998;7(2):135–43. [PubMed: 9682186]
- 103.
- Uchiyama M, Lockley SW. Non-24-Hour Sleep-Wake Rhythm Disorder in Sighted and Blind Patients. Sleep Med Clin. 2015;10(4):495–516. [PubMed: 26568125]
- 104.
- Sack RL, Auckley D, Auger RR, Carskadon MA, Wright KP Jr, Vitiello MV, et al. Circadian rhythm sleep disorders: part II, advanced sleep phase disorder, delayed sleep phase disorder, free-running disorder, and irregular sleep-wake rhythm. An American Academy of Sleep Medicine review. Sleep. 2007;30(11):1484–501. [PMC free article: PMC2082099] [PubMed: 18041481]
- 105.
- Arendt J. Managing jet lag: Some of the problems and possible new solutions. Sleep Med Rev. 2009;13(4):249–56. [PubMed: 19147377]
- 106.
- Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev. 2002;(2):CD001520. [PubMed: 12076414]
- 107.
- Folkard S, Arendt J, Clark M. Can melatonin improve shift workers' tolerance of the night shift? Some preliminary findings. Chronobiol Int. 1993;10(5):315–20. [PubMed: 8261530]
- 108.
- Burgess HJ, Sharkey KM, Eastman CI. Bright light, dark and melatonin can promote circadian adaptation in night shift workers. Sleep Med Rev. 2002;6(5):407–20. [PubMed: 12531129]
- 109.
- Bordet R, Devos D, Brique S, Touitou Y, Guieu JD, Libersa C, et al. Study of circadian melatonin secretion pattern at different stages of Parkinson's disease. Clin Neuropharmacol. 2003;26(2):65–72. [PubMed: 12671525]
- 110.
- Xie Z, Chen F, Li WA, Geng X, Li C, Meng X, et al. A review of sleep disorders and melatonin. Neurol Res. 2017;39(6):559–65. [PubMed: 28460563]
- 111.
- Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJ. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: a randomized controlled trial. JAMA. 2008;299(22):2642–55. [PubMed: 18544724]
- 112.
- Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30(11):1445–59. [PMC free article: PMC2082098] [PubMed: 18041479]
- 113.
- Loiseau F, Le Bihan C, Hamon M, Thiebot MH. Antidepressant-like effects of agomelatine, melatonin and the NK1 receptor antagonist GR205171 in impulsive-related behaviour in rats. Psychopharmacology (Berl). 2005;182(1):24–32. [PubMed: 15986188]
- 114.
- Arendt J, Rajaratnam SM. Melatonin and its agonists: an update. Br J Psychiatry. 2008;193(4):267–9. [PubMed: 18827285]
- 115.
- Harpsoe NG, Andersen LP, Gogenur I, Rosenberg J. Clinical pharmacokinetics of melatonin: a systematic review. Eur J Clin Pharmacol. 2015;71(8):901–9. [PubMed: 26008214]
- 116.
- Weishaupt JH, Bartels C, Polking E, Dietrich J, Rohde G, Poeggeler B, et al. Reduced oxidative damage in ALS by high-dose enteral melatonin treatment. J Pineal Res. 2006;41(4):313–23. [PubMed: 17014688]
- 117.
- Zhdanova IV, Wurtman RJ, Morabito C, Piotrovska VR, Lynch HJ. Effects of low oral doses of melatonin, given 2-4 hours before habitual bedtime, on sleep in normal young humans. Sleep. 1996;19(5):423–31. [PubMed: 8843534]
- 118.
- Seabra ML, Bignotto M, Pinto LR Jr, Tufik S. Randomized, double-blind clinical trial, controlled with placebo, of the toxicology of chronic melatonin treatment. J Pineal Res. 2000;29(4):193–200. [PubMed: 11068941]
Publication Details
Author Information and Affiliations
Publication History
Last Update: October 30, 2022.
Copyright
This electronic version has been made freely available under a Creative Commons (CC-BY-NC-ND) license. A copy of the license can be viewed at http://creativecommons.org/licenses/by-nc-nd/2.0/.
Publisher
MDText.com, Inc., South Dartmouth (MA)
NLM Citation
Arendt J, Aulinas A. Physiology of the Pineal Gland and Melatonin. [Updated 2022 Oct 30]. In: Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.