NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Care Services; National Cancer Policy Forum. Long-Term Survivorship Care After Cancer Treatment: Proceedings of a Workshop. Washington (DC): National Academies Press (US); 2018 Apr 27.

Long-Term Survivorship Care After Cancer Treatment: Proceedings of a Workshop.
Show detailsWORKSHOP OVERVIEW1
The 2006 Institute of Medicine (IOM) consensus study report From Cancer Patient to Cancer Survivor: Lost in Transition made recommendations to improve the quality of care that cancer survivors receive, in recognition that cancer survivors are at risk for significant physical, psychosocial, and financial repercussions from cancer and its treatment (IOM and NRC, 2006). Since then, efforts to recognize and address the unique needs of cancer survivors have increased, including an emphasis on improving the evidence base for cancer survivorship care and identifying best practices in the delivery of high-quality cancer survivorship care.
To examine progress in cancer survivorship care since the From Cancer Patient to Cancer Survivor report, the National Cancer Policy Forum of the National Academies of Sciences, Engineering, and Medicine held a workshop, Long-Term Survivorship Care After Cancer Treatment, on July 24 and July 25, 2017, in Washington, DC. Workshop presentations and discussions highlighted potential opportunities to improve the planning, management, and delivery of cancer survivorship care. Invited experts described the current evidence base on physical well-being in cancer survivorship; psychosocial well-being and family considerations in cancer survivorship; socioeconomic considerations; models of survivorship care delivery; and policy opportunities to improve cancer survivorship care. Throughout the workshop, cancer survivors and caregivers shared their perspectives and personal insights based on their experiences.
This proceedings chronicles the presentations and discussions at the workshop. A broad range of views and ideas were presented, and Box 1 highlights suggestions from individual participants to improve cancer survivorship care. The workshop Statement of Task can be found in Appendix A and the workshop agenda can be found in Appendix B. The speakers' presentations (as PDF and video files) have been archived online.2
BOX 1
Suggestions from Individual Workshop Participants to Improve Cancer Survivorship Care.
To summarize suggestions made by many participants throughout the workshop to accelerate progress in cancer survivorship, Patricia Ganz, director of the Center for Cancer Prevention and Control Research at the University of California, Los Angeles, Jonsson Comprehensive Cancer Center, presented several goals for the next decade:
- Providing survivorship care that is accessible, affordable, and equitable;
- Improving ways to reduce suffering and mortality among survivors, and promoting return to life, work, and school;
- Testing models of care delivery and approaches to risk stratification that take into account the whole person—all of one's health conditions and social conditions, not just one's cancer;
- Enhancing the education of survivors and all clinicians;
- Focusing on the needs of caregivers;
- Collecting better data on diverse populations with cancer in research studies;
- Integrating evidence-based psychosocial services into standard care;
- Eliminating services when evidence indicates there is no benefit (e.g., certain surveillance tests); and
- Developing and implementing quality measures for survivorship care.
OVERVIEW OF CANCER SURVIVORSHIP CARE
Ganz described the cancer survivorship care continuum, starting with risk assessment and intervention at the time of diagnosis (see Figure 1). Patients need to know how treatments may affect their life, she said, if care providers are to deliver “the right therapy to the right person at the right time.” Ganz added that survivorship care should focus on palliation of symptoms, prevention of late effects, and health promotion.
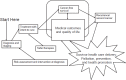
FIGURE 1
The cancer care trajectory. SOURCES: Ganz presentation, July 25, 2017; IOM and NRC, 2006.
Ganz noted the challenges in defining survivorship and the different points of view on the question of “When does survivorship begin?” For some, and as defined by the National Coalition for Cancer Survivorship (NCCS), it begins at the time of diagnosis, when treatment decisions are being made. For others, survivorship refers to the time after cancer treatment, when some of the late effects become apparent. Some patients and clinicians are generally uncomfortable with the term “survivor” (see Box 2). Workshop discussions underscored the breadth of interpretation of the word “survivorship,” due to the uniqueness of one's experiences with cancer and the complexities in modern-day cancer care and treatment. For example, Catherine Alfano, vice president of survivorship at the American Cancer Society (ACS), suggested that survivorship language be inclusive of the full range of cancer experiences and trajectories, including patients who are posttreatment and have no evidence of disease; those with cancer and on active surveillance; patients treated intermittently for relapsing/remitting disease; and those living with chronic metastatic disease.
BOX 2
A Patient's Voice: The Language of Cancer Survivorship.
Ganz recalled her involvement with IOM's 2006 report From Cancer Patient to Cancer Survivor: Lost in Transition and said at that time, scant attention had been paid to the 10 million survivors of cancer. She credited fellow committee members, the late Ellen Stovall, a tireless advocate of survivorship, and the late Rodger Winn, an eminent oncologist, with insights that led to that report's success. She acknowledged that progress has been made since the report, but emphasized that survivorship remains a neglected phase of the cancer care trajectory. Ganz observed that the needs of many cancer survivors are not being met and emphasized that opportunities to intervene in the lives of cancer survivors are often missed. She said care is often not coordinated, and although models of survivorship care are emerging, many have not been well tested. The pace of improvements in these areas has been disappointing, said Ganz. She also noted that since the 2006 IOM report, additional challenges to survivorship care planning have emerged. Cancer treatments have become even more complex, with many new targeted therapies and complicated and lengthy treatment regimens. The cost of care has increased exponentially and the term “financial toxicity” has been coined to reflect the damaging effects of the financial hardships introduced by treatment, including medically related bankruptcy.
Ganz described the many ways that cancer is different from many chronic diseases. Cancer care is very complex, with multimodal therapies and multidisciplinary care providers. Often, primary treatment may take a year or more and treatment can be very toxic and very high risk. Cancer therapies tend to be very costly and services are often poorly coordinated during and following treatment. Furthermore, cancer treatment usually occurs in isolation from primary health care delivery. Cancer care poses particularly difficult communication challenges because a patient's medical records come from multiple specialists practicing in different environments, but Ganz expressed optimism that advances in electronic health records (EHRs) will improve communication and facilitate the use of survivorship care plans.
Ganz noted that the conversation about definitions and care models will continue, but she emphasized the need to focus on improving survivorship care through more training and educational programs and through better systems of chronic care for individuals who have been diagnosed with cancer and who have ongoing needs. Better survivorship care also includes providing greater support for family caregivers and greater recognition of their role, Ganz said.
The Needs of Cancer Survivors
Perspectives on survivorship care from the lens of a cancer survivor were provided by Neeraj Arora, associate director in the Healthcare Delivery and Disparities Research program at the Patient-Centered Outcomes Research Institute, and former program director at the National Cancer Institute (NCI). Arora shared his personal cancer experiences and offered his vision for the future of cancer survivorship care.
“Cancer begins and ends with people. In the midst of scientific abstraction, it is sometimes possible to forget this one basic fact,” Arora stated, quoting June Goodfield, a scientist, writer, and cancer survivor (Goodfield, 1975). He added that behind every cancer patient or research data point, there is a story that includes family members. Two questions are paramount to people who receive a diagnosis of cancer, Arora said. The first is “What is going to happen to me?” In response, people try to seek care from a health care system that will maximize their chance of survival. The second question is, “Will I get care from a health care system that will help me through the crisis?” Arora said people seek a range of support to address a variety of needs following a cancer diagnosis, including
- Informational support to understand complex medical information;
- Decision-making support to make informed decisions;
- Emotional support to adjust to, and cope with, illness;
- Appraisal support to help deal with uncertainty;
- Instrumental support to assist in navigating the health care system and coordinating care; and
- Self-management support to take care of health outside the health care interaction.
In addition to measuring whether patients are offered evidence-based treatments and tests, Arora said that systematic quality assessment of survivorship care should address how well the health care system meets the needs of patients and their families. He said greater focus should be placed on indicators of patient-centered care, and to emphasize this point, he referenced the 2013 IOM consensus study report Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis, which stated, “Patients are at the center of the committee's conceptual framework, recognizing that the system's most important goal is to meet the care needs of patients with cancer and their families, through patient-centered communication and shared decision making” (IOM, 2013b, p. 35).
Arora summarized findings from two population-based studies on cancer survivorship that he worked on while at the NCI that quantified the degree of unmet needs in the cancer survivor population (see Table 1). The studies included survivors who were 2 to 5 years postdiagnosis (Beckjord et al., 2008), as well as long-term cancer survivors who were 4 to 14 years postdiagnosis (Kent et al., 2012). Across several domains, a large majority of cancer survivors said they had unmet needs for information. They wanted more information about how to deal with late- and long-term effects of their cancer treatment and which follow-up tests they should be getting, as well as information about complementary and alternative strategies that could potentially help improve outcomes. These studies also found that survivors reported many unmet needs on issues related to health promotion, such as diet and physical activity. Unmet needs were also evident for interpersonal and emotional issues, for example, dealing with anxiety about recurrence, and having concerns about the cancer risk of other members of the family (Beckjord et al., 2008; Kent et al., 2012). Arora suggested that there is significant room for improvement in how well cancer patients are prepared for life after cancer treatment.
TABLE 1
Information Needs Among Cancer Survivors.
Arora described his vision for the future of survivorship as a system that coordinates care and facilitates patient engagement. He added that increasing the pace with which care innovations are translated from research into community-based practice, where most cancer patients receive their care, should be a national priority. For example, he said that research findings on the use of patient-reported outcomes (PROs) in survivorship care and the optimal processes for survivorship care planning have not been widely disseminated or used by clinicians.
Arora said cancer patients and survivors often seek a “quarterback” who can help coordinate care, noting that care coordination is more complex for older cancer survivors. Research shows that at least one in four cancer survivors ages 65 or older has five or more comorbid conditions (IOM and NRC, 2006), and that Medicare beneficiaries who have five or more chronic conditions see many different physicians, often in multiple organizations, each year (Pham et al., 2007). He emphasized that the enormous care coordination challenges for older survivors with complex health needs are further complicated by the likelihood that the caregiver is also elderly, with his or her own chronic conditions.
In Arora's view, the ideal to strive for in survivorship care is the medical home model of care, which puts a patient at the center of her or his care and coordinates the appropriate medical and psychosocial interventions across care providers. He said the primary-care based patient-centered medical home3 is one type of medical home, but other models include medical home neighborhoods where different specialties co-manage patients, and specialty medical homes where specialists manage a significant portion of the care of patients (e.g., cardiology or oncology).
Arora reflected on his own experience as a cancer survivor (see Box 3) and concluded, “On the road to delivering high-quality care, always walk in the shoes of the patient.”
BOX 3
A Patient's Voice: Challenges in Care Coordination.
A Review of the History and Progress in Cancer Survivorship Care
Several workshop participants reviewed the progress made in cancer survivorship care since the release of the 2006 IOM report From Cancer Patient to Cancer Survivor: Lost in Transition (IOM and NRC, 2006). Ganz and Larissa Nekhlyudov, associate professor of medicine at Harvard Medical School, clinical director of Internal Medicine for Cancer Survivors at the Dana-Farber Cancer Institute, and internist at the Brigham and Women's Hospital, described the current state of survivorship care and progress in implementing the recommendations from that consensus study report (see Box 4).
BOX 4
Recommendations from the 2006 Institute of Medicine Consensus Study Report From Cancer Patient to Cancer Survivor: Lost in Transition.
Awareness of the Needs of Cancer Survivors
Since 2006, Nekhlyudov said there has been an increase in programs dedicated to cancer survivorship. Public awareness has also been raised as public officials, actors, and newsmakers have shared their survivorship experiences. In addition, information about survivorship is increasingly available through books (Coscarelli et al., 2011; Feuerstein, 2007; Ganz, 2007; Miller, 2010; O'Dell and Stubblefield, 2009), special reports (IOM, 2007), conferences, survivorship guidelines, and survivor advocacy organizations. However, Nekhlyudov said that information about survivorship has not been uniformly transferred to community and academic health care settings. Ganz added that although some public attention is paid to the concerns of cancer survivors, many survivors remain unaware of their risk of recurrence and late effects, and have no plan for follow-up care.
Survivorship Care Plans
As described in From Cancer Patient to Cancer Survivor, the survivorship care plan is a vehicle to facilitate informed communications between the patient and the care team (IOM and NRC, 2006). In discussing the need for survivorship care plans, Ganz indicated that clinicians can benefit from knowing the types of cancer treatment the survivor had, the potential late effects associated with those treatments, and their expected time course. For example, if a survivor had radiation directed to the chest, any clinician seeing the survivor needs to know about the risk of cardiac late effects. The risks of late effects need to be communicated to the survivor as well, in a way that he or she can understand, said Ganz. She emphasized that the ideal care plan also includes steps for positive health promotion and disease prevention, noting that patients find this to be empowering as it represents an aspect of their health care that they can control.
Ganz discussed survivorship care plans in the context of the Chronic Care Model, an evidence-based, conceptual framework that “describes changes to the health care system that help practices—particularly those in primary care settings—to improve outcomes among patients with chronic illness” (Epping-Jordan et al., 2004). She said an important finding in From Cancer Patient to Cancer Survivor was that the Chronic Care Model applies to the care of cancer survivors because nearly all of them need chronic monitoring and follow-up. The model posits that the quality of care benefits from an interaction between the community (e.g., resources and policies) and the health systems organization of health care (e.g., self-management support, delivery systems design, decision support, clinical information systems) (see Figure 2). Ganz noted that the advent of many electronic tools should make these communications easier than they were more than a decade ago.
Nekhlyudov explained that the use of survivorship care plans has been facilitated by care plan toolkits available from several organizations,4 such as the American Society of Clinical Oncology (ASCO), Journey Forward, and Oncolink, as well as by the Commission on Cancer (CoC) standard requiring organizations to provide a survivorship care plan to cancer patients completing treatment. Nekhlyudov said that “care plans cannot be just a piece of paper;” to be effective, a focus on the care planning process is essential (Earle, 2006; Parry et al., 2013). In developing a survivorship care plan, Ganz said there is some uncertainty about when the process should start. For example, when providing follow-up for a patient with a high-grade lymphoma, the clinician might be inclined to wait 12 to 18 months in case the cancer recurs. For patients with breast cancer, some clinicians are comfortable preparing a care plan when women have finished their primary treatment and are starting endocrine therapy. Ganz noted that this represents a good time to talk about wellness and adherence to medications. She added that it can be difficult to discuss health promotion and disease prevention when the patient's prognosis is uncertain.
Nekhlyudov acknowledged that the provision of a survivorship care plan is not routine among clinician practices. She said the reasons for the lack of widespread adoption include the time and labor needed to prepare care plans and a lack of research on the impact of survivorship care plans on patient outcomes (Brennan et al., 2014; Klemanski et al., 2016; Mayer et al., 2015; Spears et al., 2017). She noted that research about the effectiveness of care plans is under way.
Clinical Practice Guidelines for Cancer Survivorship Care
Ganz said that in 2006, there were few clinical practice guidelines on follow-up care for survivors, and health care professionals generally lacked survivorship education and training. Ganz observed that while progress has been made in this regard, professional education and training in survivorship is often still inadequate, and current survivorship care guidelines vary, which can leave clinicians uncertain about follow-up care. Nekhlyudov said that, increasingly, guidelines have become evidence based, and efforts are under way to harmonize international guidelines. Nonetheless, she said many disease-based guidelines do not fully recognize and highlight the complex needs of cancer survivors.
Nekhlyudov identified organizations leading the way in the development of survivorship care guidelines. The Children's Oncology Group created consensus-based guidelines for survivors of childhood, adolescent, and young adult cancers, and she said these guidelines include a growing evidence base.5 The National Comprehensive Cancer Network (NCCN) has survivorship care guidelines reported by site of cancer and by topics, such as cancer prevention and screening and supportive care.6 The ACS developed survivorship care guidelines addressing breast, prostate, colorectal, and head and neck cancers7 and has worked to disseminate them to primary care clinicians who often see cancer survivors in community-based practices. ASCO's survivorship care guidelines focus on screening and management of late- and long-term effects of cancer therapy.8 Nekhlyudov noted that mobile apps may be used to disseminate cancer survivorship guidelines to clinicians.
Quality Assessment and Models of Care
Nekhlyudov said that assessing the quality of survivorship care remains a work in progress, with a need for further research and development. She pointed out that ASCO's Quality Oncology Practice Initiative (QOPI®) has made advances in measuring quality of care, for example, but she noted that the metrics are mainly focused on cancer treatment and not on survivorship care.
Ganz said the essential components of survivorship care identified in the 2006 report—prevention, surveillance, intervention, and coordination—are lacking in many care settings, and that systems of care remain inadequate for cancer survivors in terms of the ability to intervene early to prevent the many sequelae of treatment, both medical and psychosocial. However, the stratification of survivors into low-, intermediate-, or high-risk categories has been helpful in the design of models of follow-up care, said Ganz.
Ganz also summarized the findings from the 2013 IOM consensus study report Delivering High-Quality Cancer Care: Charting a New Course for a System in Crisis (IOM, 2013b). She explained that the consensus committee concluded that cancer care is often not as patient-centered, accessible, coordinated, or evidence-based as it could be. A conceptual framework was developed that illustrates the importance of patient-centered care in the delivery of high-quality cancer care (see Figure 3).
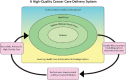
FIGURE 3
A conceptual framework of the components of a high-quality cancer care delivery system. SOURCES: Ganz presentation, July 25, 2017; IOM, 2013b.
Ganz said the consensus committee's first goal stated, “The cancer care team should provide patients and their families with understandable information about cancer prognosis, treatment benefits and harms, palliative care, psychosocial support, and estimates of the total and out-of-pocket costs of cancer care” (IOM, 2013b, p. 8). To achieve this goal, the IOM committee recommended that:
- The federal government and other stakeholders should improve the development and dissemination of this information and decision aids.
- Professional educational programs for cancer care teams should train clinicians in communication.
- The cancer care team should communicate and personalize this information for their patients and collaborate with their patients to develop care plans.
- The Centers for Medicare & Medicaid Services (CMS) and other payers should design, implement, and evaluate innovative payment models.
Thirteen items were identified in the 2013 IOM report as critical information to be included in a cancer care plan:
- 1.
Patient information;
- 2.
Information on diagnosis;
- 3.
Prognosis;
- 4.
Treatment goals;
- 5.
Initial plan for treatment and duration;
- 6.
Expected response to treatment;
- 7.
Treatment benefits and harms;
- 8.
Information on quality of life and a patient's likely experience with treatment;
- 9.
Identifying who is responsible for care;
- 10.
Advance care plans;
- 11.
Costs of cancer treatment;
- 12.
A plan for addressing psychosocial health; and
- 13.
A survivorship plan.
In response to the committee's recommendation, Ganz said that CMS initiated the Oncology Care Model (OCM).9 Oncology practices receive $160 per Medicare beneficiary in additional payment to deliver a more comprehensive set of services in a more integrated way. These practices are obligated to create this 13-point structured plan. Private payers are also participating in OCM. Ganz described this CMS initiative as a significant step forward.
State Cancer Control Plans
A review of state comprehensive cancer control programs undertaken by the George Washington University (GW) Cancer Institute found mention of cancer survivorship across most of the cancer control plan objectives (Underwood et al., 2015), but to date there has been no systematic review of the outcomes reported, Nekhlyudov said. In her experience as a member of a committee in Massachusetts working to define quality metrics to integrate into the state plan, she said it was difficult to find outcomes that were clinically meaningful, measureable, and available.
In an effort to assist state planners, the GW Cancer Institute published a cancer plan resource guide and tool (GW Cancer Institute, 2015). Nekhlyudov said it will be instructive to see what works at the state level and whether successes can be replicated across the country.
Educational Opportunities for Health Care Providers
Nekhlyudov described some of the resources and activities available to health care providers to help them meet the complex needs of cancer survivors. In recognition of the important role that primary care clinicians play in caring for cancer survivors, the ACS and the GW Cancer Institute collaborated with the Centers for Disease Control and Prevention (CDC) on a free online cancer survivorship e-learning series for primary care clinicians.10 Unfortunately, the target audience for the series has not taken full advantage of this valuable resource, she said.
ASCO also has resources for survivorship care, including toolkits, available through an online survivorship compendium,11 and has released a core curriculum for cancer survivorship education, a resource that can be taken to local communities for dissemination (Shapiro et al., 2016). ASCO University is developing a presentation slide set about survivorship issues for nationwide dissemination, according to Nekhlyudov. In addition, ASCO holds an annual cancer survivorship workshop that brings the oncology and primary care communities together.
Ganz said the 2013 IOM report recommended educational approaches that focus on all members of the oncology team, including nursing, social work, psychology, and rehabilitation (IOM, 2013b). She added that efforts in this area are in progress and there has been movement toward multi-professional education at many medical schools.
Employment Challenges
Nekhlyudov cited some of the many studies on the topic of employment-related issues for cancer survivors (Ekwueme et al., 2014; Guy et al., 2014; Nekhlyudov et al., 2016; Short et al., 2008; Yabroff et al., 2016; Zafar and Abernethy, 2013a,b). Many studies have addressed the implications of cancer for work, employment, and physical function, but she concluded that there is more work to be done in this area.
Access to Health Insurance
Ganz said the Patient Protection and Affordable Care Act (ACA)12 was a positive development for cancer survivors because it expanded access to health insurance and it removed the lifetime caps on coverage. Without such protection, Ganz said, even patients with a generous insurance plan could easily meet a $1 million cap after a cancer diagnosis. Ganz said other ACA provisions removed an insurer's ability to exclude people on the basis of preexisting conditions, and extended the coverage on a family insurance plan for children up to age 26, which Ganz noted has been very important for adolescents and young adults (AYA) facing cancer.
Nekhlyudov added that the ACA also benefits cancer survivors by focusing on preventative care. She mentioned one study that attempted to assess the impact of the ACA on cancer survivorship; the investigators concluded that while there is some suggestion of benefit, there are currently few data available with which to fully evaluate the ACA's effect (Leopold et al., 2017).
Survivorship Research
Nekhlyudov reported on a marked increase in survivorship research published from 1984 to 2010 (Harrop et al., 2011). She said several federal agencies (CMS, the NCI, the Agency for Healthcare Research and Quality, and the Department of Veterans Affairs) have funded studies addressing survivorship care delivery. However, she emphasized that there is a lack of population-based research in this area. She added that much of the research has focused on quality of life, but other important domains of cancer survivorship are not yet well addressed. Nekhlyudov cited a review (Jacobsen et al., 2016) demonstrating that there has been a relative lack of research involving:
- Common cancers other than breast cancer;
- Older cancer survivors (greater than 65 years old);
- Long-term cancer survivors (more than 5 years);
- Interventional studies with younger cancer survivors (less than 21 years old);
- Biologic mechanisms and genetic factors related to recurrence and adverse effects; and
- Patterns and quality of survivorship care.
Nekhlyudov said that in light of the increasing number of long-term cancer survivors, additional investments in research are imperative.
Components of Survivorship Care Delivery
Deborah Mayer, a 10-year cancer survivor and director of cancer survivorship at the University of North Carolina Lineberger Comprehensive Cancer Center, explained that in 2011, the LIVESTRONG Foundation held a consensus meeting to determine essential elements of survivorship care delivery, which were organized into three tiers of care (consensus elements, high-need elements, and strive elements, respectively) (see Table 2). Mayer said there is some evidence that progress is being made in delivering tier one services to survivors. However, she said there has been little success in delivering tier two and three services.
TABLE 2
LIVESTRONG Essential Elements of Survivorship Care Delivery, 2011.
As an oncology nurse since 1975, Mayer reflected on nursing practice in the 1970s, when there were no real models of shared care, and the oncology team took care of the whole person for the rest of a patient's life. This practice still remains to some extent, Mayer said, but as the population of cancer survivors ages and the prevalence of comorbidities rises, oncologists will no longer be able to manage all care. Nurse practitioners will, by necessity, extend and enhance the care that needs to be provided in any delivery model, including tasks such as clinical management of diabetes, hypertension, and other chronic diseases, said Mayer.
Mayer discussed a recent literature review on the relationship between primary care clinicians and cancer specialists (Dossett et al., 2017), which indicated that:
- There is poor and delayed communication between primary care clinicians and cancer specialists;
- Cancer specialists endorse a specialist-based model of care;
- Primary care clinicians believe they play an important role in the cancer continuum and are willing to perform that role;
- Cancer specialists and primary care clinicians themselves are uncertain whether primary care clinicians have the training or knowledge to provide care across the cancer continuum; and
- There is discordance among cancer specialists and primary care clinicians about expectations and perceived roles.
Mayer said that within complex health care systems, there are too many failures in handoffs and transitions. She said some patients report that information in the EHR does not get to the primary care clinician, who then depends on the patient to relay information about his or her cancer care. Mayer said that primary care clinicians do believe they have an important role in survivorship care and are very open to education to help them fulfill that role (Dossett et al., 2017).
Mayer concluded by listing key questions that need to be answered in survivorship care: What care is needed? Who needs that care? Who delivers care? Where should care be delivered? She suggested optimizing the functionality and use of data and electronic tools (e.g., EHRs, apps, eHealth) to extend the ability to deliver quality cancer care, noting that it might be possible, for example, to give every cancer survivor their own disease-specific virtual assistant, which they could talk to and get answers to some of their basic questions. To extend and enhance the care delivery system, Mayer emphasized a need to fully use advanced practice clinicians for survivorship care, as well as nurse and lay navigators. She added that care has moved from the hospital, to outpatient settings, and now into the home, so family members will need support, and those without home support will need attention.
PHYSICAL WELL-BEING IN CANCER SURVIVORSHIP
Several workshop participants discussed research, clinical practices, and interventions related to the various physical health issues that cancer survivors may face following treatment.
Late- and Long-Term Health Risks
Kevin Oeffinger, director of the Duke Center for Onco-Primary Care and the Duke Cancer Institute Cancer Supportive Care and Survivorship Center, summarized the health risks that cancer survivors face following treatment and discussed the research on interventions for improving the physical well-being of cancer survivors. Oeffinger provided an overview of the many potential late effects of cancer treatment (see Table 3), and said many factors may contribute to the development of these late effects (Hudson, 2005), including
TABLE 3
Potential Late Effects After Cancer, by System and Exposure.
- Premorbid conditions;
- Genetics (e.g., BReast CAncer gene [BRCA], ataxia-telangiectasia mutated, p53, and polymorphisms);
- Tumor factors (e.g., histology, site, biology, and response);
- Treatment factors (e.g., surgery, chemotherapy, and radiation therapy);
- Treatment events;
- Host factors (e.g., age, gender, and race);
- Health behaviors (e.g., tobacco use, diet, alcohol use, exercise, and sun exposure); and
- Aging.
Oeffinger said research shows that other physical problems in addition to a patient's primary cancer also contribute to the mortality risk of long-term cancer survivors:
- An analysis of late mortality in approximately 700 patients diagnosed as adults with Hodgkin's lymphoma indicated that by about 23 years after a cancer diagnosis, the risk of death from the original cancer was exceeded by deaths due to cardiac disease, second cancers, and other problems (Matasar et al., 2015).
- In a study of Stage 1 testicular seminomas, a highly curable cancer, the risk of death from second primary cancers or cardiovascular disease—perhaps in part related to cancer treatment exposures or to genetics—was reported to be elevated (Beard et al., 2013).
- An analysis showed that among women aged 50 and older with estrogen receptor (ER) positive breast cancer, the probability of dying from breast cancer is relatively low and the risk of dying from other causes increases over time. These deaths are largely attributable to aging and often a common insulin-resistance pathway that leads to both cardiovascular disease and breast cancer, and generally not the late effects of cancer therapy (Hanrahan et al., 2007).
To further illustrate the physical issues affecting cancer survivors, Oeffinger summarized research findings related to subsequent primary cancers and cardiovascular disease, which are the greatest contributors to morbidity and premature mortality apart from the primary cancer, as well as accelerated aging.
Subsequent Cancers
Oeffinger said one out of every five new cancers diagnosed in the United States is a second, third, or fourth cancer for the patient (Travis et al., 2018). He said the factors contributing to a subsequent primary cancer include unhealthy behaviors (e.g., tobacco use, sedentary lifestyle, obesity), aging (patients 65 and older account for two-thirds of cancers), genetic factors, treatment exposures from the first cancer, and interactions among these factors.
Oeffinger said studies involving patients with the highest risk for lifestyle-related second primary cancers (individuals with head and neck cancer) indicated that:
- The risk for a death due to a second primary cancer at 13 years following diagnosis was similar to the risk of death from the original head and neck cancer (Baxi et al., 2014).
- The 10-year cumulative risk of having any type of second primary cancer among patients aged 55 to 64 years was nearly one in five, which is substantially higher than the risk in the general population (Moitry et al., 2017).
- The risk of second primary cancer of the head and neck and esophagus is high (Morris et al., 2011), and because of the tobacco exposures in this population, there is also an elevated risk of lung cancer. Oeffinger concluded that lung cancer screening should be considered for this very high-risk population.
Aging is also a risk factor for a second primary cancer, Oeffinger reported (Donin et al., 2016). He said a modeling study indicated that among women with a primary breast or colorectal cancer, the 10-year cumulative risk of a second primary cancer increases with age and is higher than that expected in the general population (Moitry et al., 2017). At ages 75 and older, approximately 10 percent of women with either primary breast or colorectal cancer would experience a second cancer. Among men with a primary prostate or colorectal cancer, the 10-year cumulative risk of a second primary cancer increases to about 16 percent after age 75, and men with a primary colorectal cancer face a 10-year cumulative risk of a second primary cancer of more than 20 percent at ages 65 and older, Oeffinger said (Moitry et al., 2017).
Other studies have shown the role of genetics in second primary cancers, said Oeffinger. For example, 50 percent of patients with Li-Fraumeni syndrome (i.e., those with a TP53 gene mutation) have a second primary cancer within 10 years following the diagnosis of a first cancer (Mai et al., 2016). In addition, he said people with mismatch repair gene mutations—a large subset of patients with hereditary non-polyposis colorectal cancer—have an elevated risk for a variety of different cancers (e.g., prostate cancer or endometrial cancer) (Win et al., 2012).
Oeffinger also described some studies of patients treated for Hodgkin's lymphoma. One study showed that among patients diagnosed between ages 15 to 50, the risk of second primary cancers did not change much over different eras of therapy (i.e., 1965–1976, 1977–1988, 1989–2000) (Schaapveld et al., 2015). He noted that these patients often have third, fourth, and subsequent primaries (van Eggermond et al., 2014). He also cited a case-controlled, population-based study of patients with Hodgkin's lymphoma that found the risk of subsequent lung cancer diagnosis was much higher among moderate to heavy smokers as compared to non- or light smokers (Travis et al., 2002). The difference was observed across treatment groups (i.e., exposure to alkylating agents or to radiation to the chest area), and the risk was exponentially higher among those receiving both treatment modalities, indicating an interaction between lifestyle behaviors and treatment exposures.
Cardiovascular Disease
Oeffinger explained that advances in radiation therapy (e.g., the use of smaller, more focused fields that spare normal surrounding tissues) can help to mitigate cardiovascular late effects. He said the decreased radiation exposure from newer techniques has led to a decline in the risk of second breast cancers and pulmonary problems in breast cancer patients, but that even with the use of more targeted fields, Hodgkin's lymphoma patients face cardiovascular risks because many have mediastinal lymph node involvement near the proximal coronary arteries. He said there is an ongoing international effort to define dose–response relationships to better quantify cardiovascular risks associated with radiation therapy.
According to Oeffinger, studies show a marked elevation in risk of coronary artery disease among both men and women treated with mediastinal radiotherapy. The risk for women aged 35, 45, and 50 is as high as for men of the same age, and there is a one in five chance of having symptomatic coronary artery disease by 20 years following moderate dose radiation to the mediastinal area, he said (Reinders et al., 1999). By 30 years, the chance of having a myocardial infarction (MI) is about 13 percent (Aleman et al., 2007). Among those experiencing an MI following mediastinal radiation, the risk of death is three times greater than for those having an MI without a history of radiation to the chest, he added (Swerdlow et al., 2007).
Oeffinger said a study by van Nimwegen et al. (2016) estimated that a woman treated for Hodgkin's lymphoma at age 20 with 21 grays13 to the mediastinum has a 5.5 percent cumulative risk of having coronary heart disease by age 40. Over the next 10 years, she has an absolute risk of about 10 to 12 percent of having a serious cardiac event (van Nimwegen et al., 2016). This risk is in sharp contrast to the 1 percent risk a woman without the history of Hodgkin's lymphoma and few other risk factors (e.g., is normotensive, not diabetic, and a non-smoker).14 He said that among men treated contemporarily for testicular cancer, the risk of cardiovascular deaths appears to be primarily during chemotherapy or soon thereafter (Fung et al., 2015). The older the patient was at the time of treatment, especially with multiple comorbidities, the higher the risk was of cardiac death.
Among the largest population of cancer survivors, women treated for breast cancer, Oeffinger said studies show that more than half of those ages 65 or older have hypertension or lipid disorders, and a sizable fraction have diabetes (Chen et al., 2012). Hypertension, either before, during, or after cancer therapy, is the single most important predictor of heart failure in breast cancer survivors treated with anthracyclines and trastuzumab, he noted (Chen et al., 2012; Jawa et al., 2016). Oeffinger emphasized that for women with breast cancer, continued monitoring of hypertension, diabetes, and lipid disorders is important for their longevity and quality of life, and that standardized approaches to managing these common comorbidities are needed.
Oeffinger added that for most cancer survivors, hypertension is now recognized as the single most important driver of long-term cardiovascular outcomes, both heart failure and coronary artery disease. He also noted that prevention and monitoring guidelines for cardiovascular dysfunction were recently developed (Armenian et al., 2016).
Accelerated Aging
Oeffinger said the acceleration of aging varies among cancer survivors and across body systems. He said a useful geriatric assessment tool, the deficit-accumulation frailty index, includes a variety of domains (e.g., comorbidities, psychosocial status, lab values) to measure degree of frailty. In a study that applied this index to a cohort of 500 cancer patients ages 65 and older, half of the patients were classified as non-frail, 197 (39 percent) were pre-frail, and 52 (11 percent) were frail (Cohen et al., 2016). He said subsequent studies of older breast cancer patients showed a higher risk of all-cause mortality and breast cancer mortality among those women classified as frail or pre-frail relative to the robust cohort (Mandelblatt et al., 2017). This same trend of higher risk of death among those categorized as frail has also been observed in adult survivors of childhood cancer, he noted (Ness et al., 2013).
Research on Interventions for Improved Physical Well-Being
Oeffinger discussed research on strategies for mitigating late- and long-term physical health risks in adult cancer survivors. Although some consensus-based screening guidelines exist for high-risk groups (e.g., patients with Li-Fraumeni syndrome and women with BRCA2 mutations), he said that survivors at higher risk for second primary cancers are generally not screened more aggressively than the average-risk general population, and new screening intervals might be necessary. Oeffinger added that more research on effective strategies for improving screening rates among high-risk survivors is needed, as is more evidence to support risk-stratified surveillance.
He emphasized that risk prediction models incorporating treatment exposures are needed for a range of outcomes so that absolute risks can be calculated and used to inform patients and plan survivorship care. He and other investigators are working on developing risk prediction models for patients who are treated with Hodgkin's and non-Hodgkin's lymphoma during their adult years. He also noted that there is scant research on variations in the risk of late effects among different racial and ethnic groups or by gender, although some evidence suggests that women are at a greater risk for many of the late effects compared to men. For example, women have a higher risk of second primary cancers than men who have had the same treatment exposures, he said (Turcotte et al., 2017).
Few studies exist to guide preventive strategies for posttreatment effects, Oeffinger said, but he noted the success of exercise training among the sedentary elderly in the general population in improving mobility and functioning and preventing frailty (Pahor et al., 2014). Whether such interventions would be successful among cancer survivors remains to be tested, he said.
He also identified some of the research challenges in generating evidence about posttreatment interventions. In his view, funders and grant reviewers tend to favor interventional studies over the observational studies that could help answer some of the survivorship care questions. There is also difficulty in determining the point at which an early intervention improves outcomes. For example, in the case of cardiovascular disease, Oeffinger said that interventions are ideally targeted to those who are asymptomatic, but who have evidence of risk (e.g., a low ejection fraction).
Oeffinger also described new technologies in development for cancer detection. He noted the promise of “liquid biopsies” that use circulating cell-free DNA or circulating tumor cells for prognosis and for monitoring that potentially could be used as tools to screen high-risk populations (Vockley and Niederhuber, 2015). He also referred to cancer interception—the ability to identify and treat a mutation before the cells have progressed to cancer (Albini et al., 2016; Li and Marchenko, 2017)—which he said could potentially augment or replace other currently used strategies (e.g., the use of Tamoxifen in chemoprevention). Oeffinger anticipated that these innovations will be further evaluated to assess their potential benefits and harms, and then disseminated over the next 5 to 10 years.
Sleep, Fatigue, and Cognitive Functioning
Paul Jacobsen, associate director of the NCI Division of Cancer Control and Population Science's Healthcare Delivery Research Program, said that addressing fatigue, sleep, and cognitive functioning is essential to the comprehensive management of symptoms that cancer survivors experience.
He said studies show that the burden of symptoms for people in the posttreatment period is substantial: In one survey of more than 2,800 patients with breast, colorectal, prostate, or lung cancer, approximately 33 percent of patients who had completed primary cancer therapy reported three or more moderate to severe symptoms (Cleeland et al., 2013). Fatigue and disturbed sleep were the most common symptoms reported out of 13 possible symptoms. The study did not assess cognitive dysfunction, but other research suggests it is also common (Janelsins et al., 2014).
Following cancer treatment, poorly controlled symptoms contribute to poor quality of life and impaired physical and social functioning, Jacobsen said (Kim et al., 2009). Furthermore, they can contribute to non-adherence with or the discontinuation of oral therapies, including aromatase inhibitors (Henry et al., 2012) and imatinib (Murphy et al., 2012). Poorly controlled symptoms also contribute to lower rates of return to work following cancer treatment and to impaired abilities among people who attempt to return to work (Duijts et al., 2014; Sun et al., 2017).
Posttreatment Fatigue
PRO measures, such as the Brief Fatigue Inventory (Mendoza et al., 1999), are commonly used to assess posttreatment fatigue, Jacobsen said. A semi-structured interview based on a case definition of cancer-related fatigue helps to identify more severe cases (Andrykowski et al., 2005; Donovan et al., 2013).
He said that risk factors for posttreatment fatigue include the presence of pretreatment fatigue (Goedendorp et al., 2013), greater body mass index (Andrykowski et al., 2010), and the type of cancer treatment. For example, among women with early-stage breast cancer, those exposed to chemotherapy versus radiotherapy as their initial cancer treatment are more likely to have persistent posttreatment fatigue (Donovan et al., 2004). Another risk factor for fatigue is the presence of polymorphisms in inflammation-related genes (i.e., IL-1B, IL-6, and tumor necrosis factor alpha or TNFα) (Bower, 2014), which can lead to persistent inflammation (Bower and Lamkin, 2013). According to Jacobsen, one of the best studied biological explanations for persistent fatigue is inappropriate activation of the cytokine network, either because of the disease or the treatment (Bower and Lamkin, 2013).
Jacobsen described a model that distinguishes the precipitating factors that give rise to symptoms from the perpetuating factors that sustain symptoms, including cognitive and behavioral responses. For example, fatigue may activate cognitive responses that can lead to feelings of hopelessness and helplessness in the face of continuing severe fatigue (Donovan et al., 2007). A behavioral response to fatigue is the natural inclination to be less physically active, which may exacerbate the problem.
Jacobsen reviewed the results of a recent meta-analysis of the effectiveness of various interventions for cancer-related fatigue. The analysis included 113 randomized clinical trials with a large number of studies focused primarily on women with early-stage breast cancer; 53 of the studies included patients who had completed treatment (Mustian et al., 2017). The research showed that exercise and psychological interventions (mostly cognitive behavioral therapy), or a combination of the two, were beneficial in the management of cancer-related fatigue. Patients who had completed primary treatment reported the greatest benefit (Mustian et al., 2017). Jacobsen said studies of pharmacologic interventions, primarily psychostimulants, have not been shown to be beneficial for posttreatment fatigue. However, they may be of some benefit in the treatment of fatigue in patients with advanced disease.
Based on the body of research, Jacobsen said the ASCO guidelines (Bower et al., 2014) and the Pan-Canadian guidelines15 recommend exercise, cognitive behavioral therapy, and psychoeducation for all patients who are at risk for persistent fatigue. The guidelines note that there is limited evidence for mindfulness-based approaches, yoga, and acupuncture, but there is no evidence to support the use of psychostimulant medications.
Jacobsen said future research about fatigue should address genetic risk factors and clarify the underlying biological mechanisms of fatigue to inform intervention development. In addition, he said research should identify the appropriate intensity of exercise needed to manage fatigue, as well as the effectiveness of interventions that may allow more patients to be treated (e.g., home-based walking exercise programs, telemedicine, and other Web-based strategies). New intervention strategies are also needed for patients who do not respond to exercise or cognitive behavioral therapy, Jacobsen said.
Posttreatment Sleep Problems
To assess posttreatment sleep problems, the Pittsburgh Sleep Quality Index is often used in cancer-related research, Jacobsen said (Buysse et al., 1989). Polysomnography, which involves home-based equipment to monitor motor functioning and cardiac and respiratory function, is the gold standard assessment tool, but because of its expense it is not widely used in cancer research. A less expensive monitoring technique is actigraphy, which uses accelerometers worn on the wrist to measure night-time motor activity and provide an objective measure of certain aspects of sleep quality (Ancoli-Israel et al., 2003).
Jacobsen said that risk factors for posttreatment sleep problems include the type of cancer treatment, arousability (individual differences in responsiveness to environmental stimuli, e.g., noise), and pretreatment sleep problems (Savard et al., 2001, 2009). He said that factors contributing to posttreatment sleep problems include cognitive responses (dysfunctional beliefs about sleep) and behavioral responses such as daytime napping, which is known to interfere with sleep at night (Savard et al., 2009).
Jacobsen said that cognitive behavioral therapy is the most widely studied intervention for insomnia in cancer survivors. This therapy addresses dysfunctional beliefs (e.g., “I cannot cope without a good night's sleep”), includes relaxation training, and helps people associate bed with sleep and not with other activities, such as watching television or eating. He said a meta-analysis of eight randomized controlled trials found cognitive behavioral therapy for insomnia to be effective (Johnson et al., 2016).
Jacobsen reported that the NCCN Survivorship Guidelines16 and Pan-Canadian Guidelines17 recommend the following interventions for insomnia in cancer survivors (Howell et al., 2013):
- Sleep hygiene measures (e.g., avoiding caffeine during the day and daytime napping);
- Cognitive behavioral therapy;
- Short-term intermittent use of hypnotic medication; and
- Psychoeducation for patients at risk for posttreatment sleep problems.
The guidelines state that although there is insufficient evidence to recommend exercise, there is enough evidence to suggest exercise as an intervention for sleep problems. Jacobsen suggested several areas for future research into sleep problems. One is to expand investigations into other sleep disorders, particularly sleep apnea. Risk factors for apnea include older age and being overweight, which are also risk factors for cancer. He said another focus of sleep research should be to clarify the underlying biological mechanisms by which chemotherapy and other cancer treatments might interfere with the structural organization of sleep (i.e., rapid eye movement [REM] and non-REM sleep). He also emphasized the need to adapt effective interventions for more widespread dissemination and implementation. For example, he said cognitive behavioral therapy is effective and could be disseminated further through Web-based approaches. Lastly, Jacobsen stated that the implications of a symptom cluster concept, such as the co-occurrence of fatigue and sleep problems, should be considered because they might have a common underlying mechanism, or the treatment of one symptom could have a cascade effect on the other.
Posttreatment Cognitive Problems
Compared with posttreatment fatigue and sleep disorders, much less is known about cognitive problems following cancer treatment, said Jacobsen. Patient reports and concerns that may suggest problems in executive functioning have prompted the research community to focus on this problem.18
Jacobsen discussed four modalities used to assess posttreatment cognitive problems:
- 1.
PRO measures, such as the Functional Assessment of Cancer Therapy-Cognition (Wagner et al., 2009);
- 2.
Neuropsychological tests, standard tests of major domains of cognitive functioning (e.g., learning and memory, processing speed, executive function) in which performance is evaluated relative to reference norms (Wefel et al., 2011);
- 3.
Functional imaging studies, such as magnetic resonance imaging and positron emission tomography (PET) (Wefel et al., 2015); and
- 4.
Qualitative electroencephalography often used in combination with other measures to more directly assess brain function (Hunter et al., 2014).
Jacobsen said PROs and neuropsychological tests are only modestly correlated in many studies, and that some researchers speculate that self-reported measures might be more sensitive.
He said that risk factors for posttreatment cognitive problems include older age (Ahles et al., 2010), cognitive reserve (i.e., premorbid intellectual ability) (Ahles et al., 2010), and genetic polymorphisms (e.g., in the apolipoprotein E gene) that have been implicated as a risk factor for dementia (Ahles et al., 2003) or in the catechol-O-methyltransferase (COMT) gene, which is known to regulate several neurotransmitters (Small et al., 2011).
The direct neurotoxic effect of treatment is one mechanism implicated in posttreatment cognitive problems, Jacobsen said (Bray et al., 2017; Janelsins et al., 2014; Joly et al., 2015). Animal models suggest that cancer drugs may cross the blood–brain barrier in very small amounts, leading to both gray and white matter volume loss, reduced white matter integrity, and altered neurochemistry and metabolism. Cytokine deregulation and treatment-induced hormonal changes have also been implicated, he said (Bray et al., 2017; Janelsins et al., 2014; Joly et al., 2015). For example, he said that lower testosterone levels in older men are a factor in age-related cognitive decline and that the use of androgen deprivation therapy for prostate cancer is associated with cognitive problems and with increased risk for dementia.
Jacobsen noted that the NCCN Survivorship Guidelines for evaluating cognitive problems do not recommend specific interventions. He said more research is needed and several promising interventions merit full-scale trials, but some of the interventions that could potentially be used to address cognitive functioning in cancer survivors include
- Cognitive training (Zeng et al., 2016);
- Memory and attention adaptation training that focuses on developing strategies to cope with loss (e.g., mnemonic devices) (Zeng et al., 2016);
- Cognitive rehabilitation (Zeng et al., 2016);
- Electroencephalography neurofeedback (Zeng et al., 2016);
- Exercise and integrative medicine techniques such as yoga, Tai Chi, and Qigong (Wefel et al., 2015; Zimmer et al., 2016);
- Psychostimulant medications, like methylphenidate (Wefel et al., 2015); and
- Acetylcholinesterase inhibitors (which have been used in the treatment of biocognitive impairment in early Alzheimer's) (Wefel et al., 2015).
Jacobsen suggested future research should seek to assess and integrate the different modalities used in the assessment of posttreatment cognitive problems, and to gain a better understanding of the genetic risk factors and mechanisms underlying structural and functional brain changes. Chemotherapy exposure has been the primary focus of the research, but studies on other therapies, including hormonal therapies and other oral agents, are also needed, Jacobsen said. In addition, he said studies are needed to explore the possibility of preventing cognitive changes by examining whether variations in therapeutic exposures or modifications of treatment regimens affect cognitive outcomes, or by focusing on the development and evaluation of neuro-protective agents. Ultimately, the goal of research in this area should be to have evidence-based treatment guidelines for the large population of survivors who are experiencing cognitive problems, Jacobsen said.
Translating Research into Practice to Improve Posttreatment Symptoms
Jacobsen stated that systematic efforts to translate research and guidelines into clinical practice are lacking. He emphasized the need for better dissemination of clinical practice recommendations for posttreatment symptoms to ensure appropriate: (1) screening (e.g., a universal brief screen to identify a clinically significant symptom); (2) assessment (including a focused history, symptom evaluation, and identification of reversible contributing factors); (3) management and treatment (including education, support, and self-management strategies and psychological, psychosocial, and pharmacologic interventions); and (4) follow-up care and ongoing reassessment.
According to Jacobsen, posttreatment symptoms are not systematically assessed and reported. PRO measures to identify the presence of symptoms are underused at the point of care, many providers are not aware of clinical practice guidelines for symptom management, and practice settings often do not have the resources to support symptom management. He said several randomized controlled trials have examined the utility of integrated symptom monitoring and management systems and found that such systems can improve symptom control and health care usage (Basch et al., 2016; Mooney et al., 2017). Success rests on the routine assessment of symptoms, providing that information to clinicians, outlining a treatment and follow-up plan, and offering guidance on a plan for patient self-management, he said.
Jacobsen described the recommendations for symptom management issued by the Blue Ribbon Panel convened by the National Cancer Advisory Board under the Cancer Moonshot initiative.19 The panel recommended a strategic research investment using implementation science to accelerate the adoption of integrated systems that gather and monitor patient-reported symptoms and provide decision support and care using evidence-based symptom management guidelines. Jacobsen concluded by showing a schema illustrating the phases of translational research and the gaps that need to be filled at each phase (see Figure 4).

FIGURE 4
The phases in translational research. SOURCE: Jacobsen presentation, July 24, 2017.
Jacobsen also discussed the need for triage and step-care models that use available resources efficiently to gain maximal benefit. In the triage model, survivors with mild to moderate symptoms could be given self-management strategies supported by informational materials and recommendations for online support. In a stepped-care approach, if people do not respond to those interventions, then more resource-intensive interventions can be marshaled, either via face-to-face encounters or telehealth approaches. Jacobsen suggested that regional centers could be developed to provide telehealth and online resources to patients in community-based care settings. Alternatively, centers that have the expertise could potentially contract with community-based practices to provide the specialized care. However, Jacobsen identified cross-state reimbursement policies as a barrier to these approaches.
Lifestyle Interventions and Physical Health
Wendy Demark-Wahnefried, professor and Webb Endowed chair of nutrition sciences at The University of Alabama at Birmingham, reviewed relevant data on weight management, physical activity, diet, smoking, and alcohol use. She said that studies show associations among a variety of factors—such as obesity, physical activity, tobacco use, and alcohol use—and cancer risk (IOM, 2012). Obesity is associated with increased risk for 13 different cancers (Lauby-Secretan et al., 2016) and obesity rates among cancer survivors have increased from 1992 to 2015 (NCI, 2017a). High levels of leisure-time physical activity are associated with lower risks of several cancers (Moore et al., 2016). Tobacco and alcohol use are both associated with increased risk for several cancers, including head and neck cancers and esophageal cancer (Bagnardi et al., 2015; Gandini et al., 2008). Rates of smoking and physical inactivity among cancer survivors have declined in recent decades (NCI, 2017b,c; Underwood et al., 2012), but there are areas in the United States, particularly in the southeast, where rates are still relatively high. Younger cancer survivors (ages 18 to 40) may be at the greatest risk for continued smoking (Bellizzi et al., 2005).
Poor diets are also associated with increased cancer risk, said Demark-Wahnefried. A national study found that cancer survivors have poorer adherence to the recommended nutritional goals in the U.S. Department of Agriculture 2010 Dietary Guidelines for Americans, especially for fiber, vitamin D, vitamin E, calcium, and potassium (Zhang et al., 2015), compared with non-cancer survivors. This dietary profile increases the risk of osteoporosis because many of the nutrients lacking in survivors' diets support skeletal health. Moreover, she said that higher intakes of saturated fats, alcohol, added sugars, and salt contribute to greater risks for cardiovascular disease.
The potential for behavioral changes in diet, exercise, and smoking cessation to improve the health of cancer survivors is reflected in the ACS guidelines for nutrition and physical activity, Demark-Wahnefried said (Rock et al., 2012). She added that a cancer diagnosis can provide impetus for behavior change, but few individuals can make necessary changes of substantive duration without support. For example, a review of 13 studies about smoking cessation interventions found that the perioperative period provides a good teachable moment for patients (Nayan et al., 2013), but continued support is necessary to assure that individuals remain smoke-free, Demark-Wahnefried said.
Research on Weight Loss Interventions
Demark-Wahnefried said several research trials have assessed the benefit of weight loss interventions in cancer survivors (NASEM, 2018a). For example, while the Women's Intervention Nutrition Study was not explicitly focused on weight loss, the trial examined the effects of a low-fat diet on recurrence rates among 2,437 early-stage breast cancer survivors (Chlebowski et al., 2006). After 5 years, the women in the low-fat diet had a significantly reduced rate of recurrence, especially among women with ER-negative cancer. The women who were on the low-fat diet lost about 6 pounds over the course of the trial, which reinforces the notion that weight loss may improve outcomes for cancer survivors, Demark-Wahnefried reported. She said a summary of 14 research trials evaluating the effect of weight loss interventions on breast cancer survivors concluded that approximately 60 percent of enrollees had weight loss of at least 5 percent, even with relatively short-term interventions (2 to 18 months in duration) (Reeves et al., 2014). Those who lost weight had clinically significant benefits, such as lowered hemoglobin A1C, insulin, inflammatory markers, and lipids; lowered blood pressure; improvements in quality of life; and increases in physical functioning. Demark-Wahnefried said that 11 studies of weight loss intervention in breast cancer survivors are either in progress or have been published in the past 2 years, including some that aim to learn more about diverse populations (e.g., breast cancer survivors who are African American or Hispanic).
Improving Delivery of Lifestyle Interventions
Demark-Wahnefried noted three key elements of behavior change: self-monitoring (e.g., keeping dietary logs, weighing regularly); self-efficacy (e.g., improving confidence through incremental behavior change and modeling); and support (long term and by many). She added that behavioral change may be more attainable if addressed at multiple levels (e.g., the individual, interpersonal, organizational, community, and public policy). She also described the 5As model of behavioral change, an evidence-based approach for a range of different behaviors and health conditions (Glasgow and Nutting, 2004):
- 1.
Ask patients if they know about the relationship between behavior and cancer;
- 2.
Advise them on what they should do and provide educational materials;
- 3.
Assess if they are ready to make lifestyle changes;
- 4.
Assist them if they are ready, for example, by having them set a start date or identifying incremental changes; and
- 5.
Arrange support for that cancer survivor (e.g., referral, prescription).
If the individual is not ready to change, then continue to reinforce the message of the importance of a healthy lifestyle.
Demark-Wahnefried said more research is needed to optimize the delivery of various interventions; for example, identifying combinations that work, setting preferable timing for interventions, discovering the best delivery channels, and crafting the most effective messaging. Research is also needed to identify triaging approaches that recognize the diversity in readiness to change: Some people need a light touch to move them forward, while others need more intensive and long-lasting support, she said.
Demark-Wahnefried said Web-based wellness resources, such as the American Institute for Cancer Research website,20 the LIVESTRONG website,21 and the SurvivorSHINE website22 are available, but in her view an infrastructure that better reflects the value of these interventions, including a reimbursement structure, is needed. She added that there is a need to train clinicians and allied health practitioners in lifestyle guidance for cancer survivors, pointing out, for example, that there are only approximately 750 dietitians who are certified specialists in oncology in the United States and Canada (Oncology Nutrition, 2018) and approximately 465 certified exercise trainers nationally. In a survey of physicians, nurse practitioners, and nurses about half said weight management is important for improving health, and about half said they would value training in weight management interventions (Anderson et al., 2013).
Eric Vinson, project coordinator at the Northwest Portland Area Indian Health Board, said there is also a need to improve the delivery of smoking cessation services among cancer survivors. He said that many cancer centers do not have tobacco cessation counselors on staff, noting that reimbursement is a barrier, and patients are being referred to tobacco quit lines that are ill-equipped to address the needs of cancer survivors. Jacobsen said the NCI had recently provided supplements to several major cancer centers to improve smoking cessation services, but he added that successful models need to be disseminated to community-based centers.
Prehabilitation and Rehabilitation
Julie Silver, associate professor, associate chair, and cancer rehabilitation director in the Department of Physical Medicine and Rehabilitation at Harvard Medical School and the Spaulding Rehabilitation Network, discussed the importance of making rehabilitation services an integral part of survivorship care. She also described her personal perspective on the need for rehabilitation services, based on her own experience with cancer (see Box 5).
BOX 5
A Patient's Voice: Cancer Rehabilitation.
Silver defined cancer rehabilitation as:
medical care that should be integrated throughout the oncology care continuum and delivered by trained rehabilitation professionals who have it within their scope of practice to diagnose and treat patients' physical, psychological, and cognitive impairments in an effort to maintain or restore function, reduce symptom burden, maximize independence, and improve quality of life in this medically complex population. (Silver et al., 2015, p. 3636)
She explained that following the publication of the 2006 IOM From Cancer Patient to Cancer Survivor report, the oncology community recognized the need for rehabilitation services—for example, physiatry and physical, occupational, and speech therapy—among cancer patients23 (Silver et al., 2013). She said studies show that cancer survivors frequently experience functional loss and disability as a result of side effects that often are untreated. In one study, 163 women with metastatic breast cancer reported a total of 530 physical impairments that were not detected during hospitalization, but which required physical therapy and/or occupational therapy (Cheville et al., 2008). In another study of 529 older adults with cancer, 341 (approximately 64 percent) had potentially modifiable functional deficits and needed physical or occupational therapy, but only 9 percent of the patients with these impairments received such therapy (Pergolotti et al., 2015). Silver said the lack of needed rehabilitation services leads to unnecessary physical and psychological suffering; several studies have documented significant levels of distress and disability among cancer survivors (Banks et al., 2010; Bevans et al., 2014; Penttinen et al., 2011; Weaver et al., 2012).
Improving Rehabilitation Services Delivery
Silver highlighted two recommendations that emerged from a National Institutes of Health (NIH) panel on cancer rehabilitation (Stout et al., 2016):
- 1.
Provide rehabilitation screening and assessment as part of a comprehensive cancer care plan, from the time of diagnosis throughout the course of illness and recovery, to address the functional needs of patients. These services should be provided by trained rehabilitation professionals who use evidence-based best practices to diagnose and treat the many physical, cognitive, and functional impairments associated with this medically complex population.
- 2.
In selected cancers, rehabilitation services should be offered pretreatment to optimize tolerance to surgical intervention and adjuvant treatment in order to minimize toxicity and improve outcomes.
Silver pointed out that cancer rehabilitation is generally reimbursable care, unlike some other survivorship services, but she said that better integration of cancer rehabilitation into the care continuum is needed, and that every cancer center should have a rehabilitation professional (e.g., a physiatrist or a physical therapist) in a leadership role to enable delivery of rehabilitation services as a part of usual cancer care.
In addition, Silver said that access to cancer rehabilitation services could be improved by providing more information to patients. She said more than 90 percent of the websites for NCI-designated cancer centers did not have an easily identifiable patient-focused description of, or link to, cancer rehabilitation services, and only 8 percent included accurate and detailed information about core rehabilitation services (physiatry and physical, occupational, and speech therapy) (Silver et al., 2017).
Silver also described prehabilitation, which is designed to increase a patient's functional capacity prior to cancer treatment, as a relatively new approach in cancer rehabilitation. She defined prehabilitation as:
a process on the cancer continuum of care that occurs between the time of diagnosis and the beginning of acute treatment and includes physical and psychological assessments that establish a baseline functional level, identify impairments, and provide targeted interventions that promote physical and psychological health to reduce the incidence and/or severity of future impairments. (Silver et al., 2013, p. 307)
For example, she said that with the advent of low-dose computed tomography (CT) screening for people at high risk for lung cancer, patients are diagnosed early and need to prepare for surgery. Prehabilitation can address risk factors such as smoking and diet and thereby lower the risk of thoracic surgery and improve functional outcomes. She said an expert panel recently proposed recommendations for future research on prehabilitation for patients with cancer (Carli et al., 2017).
Otis Brawley, chief medical officer at the ACS, raised the point that there is some commonality between palliative care and rehabilitation medicine, and that access to supportive care could be improved if these two disciplines worked together.
PSYCHOSOCIAL WELL-BEING AND FAMILY CONSIDERATIONS IN CANCER SURVIVORSHIP
Julia Rowland, director of the NCI's Office of Cancer Survivorship,24 explained that when the office was created in 1996 (in response to advocacy on the part of survivors and those caring for them), there was recognition that more individuals were living long term following cancer diagnosis, but little was known about the long-term health and quality of life of cancer survivors. Rowland noted that although significant advances have been made in understanding medical long-term and late sequelae, major gaps remain for psychosocial aspects of care.
Darci Graves, special assistant to the director in the Office of Minority Health at CMS, described the difficult transition to survivorship care after being treated for breast cancer (see Box 6). Graves said there was no real discussion about her mental health during her cancer treatment. In her view, the integration of physical, mental, spiritual, and social well-being represents the largest opportunity for improving survivorship care across the phases of cancer care. This should be accomplished, she said, by ensuring that care visits do not elicit unnecessary psychosocial distress and by delivering care, following policies, and conducting procedures that are culturally and linguistically appropriate, and which takes into account both the patient and the family.
BOX 6
A Patient's Voice: Posttreatment.
Psychosocial Issues Across the Life Span
Several workshop participants discussed the range of psychosocial needs faced by individuals who have received a cancer diagnosis. While some psychosocial issues are common to having a cancer diagnosis regardless of when the diagnosis was received, there are some unique psychosocial issues faced by young adults and long-term adult survivors of childhood cancer.
Adult Cancer Survivors
Barbara L. Andersen, distinguished professor of psychology in the Department of Psychology at The Ohio State University, addressed stress, depression, and anxiety (e.g., panic disorder or posttraumatic stress disorder) in adult cancer survivors. According to Andersen, these conditions are commonly comorbid with a cancer diagnosis and their presence significantly affects other emotions, behaviors, biology, and health outcomes. She explained that the prevalence of depression and anxiety among cancer patients is significantly greater than that seen in the general population (see Table 4).
TABLE 4
Depression and Anxiety Comorbidities in Cancer Patients.
Cancer patients who are most at risk of psychological and/or behavioral difficulties include (Andersen et al., 2014):
- Those with a prior history of anxiety or mood disorders;
- Men;
- The aged (risk rises at age 55 and older);
- Those who are alone, either actually or functionally (many people have partners, but that relationship may not be supportive);
- Individuals who are unemployed or are of low socioeconomic status;
- Patients receiving the most toxic therapies; and
- Individuals with progressive disease or recurrence.
Andersen noted that these risk factors are well documented, can be identified at the time of diagnosis, and thus provide opportunities for intervention. In discussing these various risk factors, Andersen indicated that 5- and 10-year survival is significantly greater for women and for those who are married (Goodwin et al., 1987; Ortmeyer, 1974). She also noted that use of taxanes for adjuvant therapy improves breast cancer outcomes (Anampa et al., 2015), but women exposed to these drugs often experience not only posttreatment neurotoxicity, pain, and peripheral neuropathy, but also significant depressive symptoms during and after treatment (Thornton et al., 2008). She said that a meta-analysis of 50 studies showed a significantly heightened risk (hazard ratio of 1.34) for premature mortality among patients with breast, lung, stomach, or colorectal cancer who were also diagnosed with depression (Chida et al., 2008). Andersen said some cancer patients have depression so severe that they consider suicide if untreated. Other studies have shown lower survival among those who describe themselves as having poor social functioning (Park et al., 2008) or psychosocial well-being (Kaasa et al., 1989).
Andersen said effective psychosocial interventions that reduce emotional distress and improve quality of life in high-risk patients typically include some sort of stress reduction strategy (e.g., relaxation training), information, behavioral and cognitive coping strategies (e.g., dealing with fatigue), social support, and focused interventions (e.g., sun protection for melanoma survivors) (Andersen, 1992). She summarized findings from six meta-analyses that found moderate to very large effect sizes, particularly for depression. Positive outcomes were found with psycho-education, cognitive behavioral therapy, relaxation, and mindfulness-based stress reduction (see Table 5). Andersen pointed out that these studies may underestimate the effect sizes associated with these interventions because study participants were not screened for anxiety and/or depression. In studies that limit enrollment to individuals with depression, outcomes include reductions in depression, related biologic changes (e.g., reduction in markers of inflammation), and changes in disease endpoints (e.g., reductions in cancer recurrence) (Andersen et al., 2008; Brothers et al., 2011).
TABLE 5
Efficacy of Psychological Interventions: Meta-Analyses.
Andersen said that over the past 40 years, psychosocial research has identified effective intervention strategies for cancer survivors, and she suggested ways to improve the delivery of psychosocial interventions for cancer survivors going forward. First, she said patients should be screened, noting that guidelines exist to support the screening and assessment of depression and distress in adult patients with cancer (Andersen et al., 2014). She said a stepped-care model can be used to screen individuals and stratify them by the degree of their symptoms, then target resources according to the degree of risk. Second, Andersen emphasized the need to disseminate and implement current evidence-based interventions (Neta et al., 2015), noting that only about 15 percent of patients receive care at a major cancer center. Training professionals on how to employ empirically based interventions in their practices is also needed, she said. Third, Andersen stressed the need to optimize existing interventions and expand outcomes beyond the psychological well-being (e.g., health care outcomes, costs). Finally, she said we need to embrace the “whole” cancer patient. Recognizing the diversity of patients is an imperative, she stressed; it may be necessary to test interventions targeted to certain subgroups of patients (e.g., racial/ethnic groups, those living in poverty, or individuals with substance use disorders). Vinson said that American and Alaskan Natives have the lowest survival rate of all races and he hoped that survivorship issues could be addressed within this population to reduce their cancer burden.
Childhood Cancer Survivors
Christopher Recklitis, director of research and support services for the Perini Family Survivors' Center at the Dana-Farber Cancer Institute and associate professor of pediatrics at Harvard Medical School, explained that a large proportion of pediatric cancer patients will be cured by their treatment, and because of their age, will live for a very long time with late effects. He summarized the research results on the psychological late effects on adult survivors of childhood cancers. The Childhood Cancer Survivor Study (CCSS)25 is a multicenter study of cancer survivors of 5 years or more that includes self-reported psychological and quality of life outcomes for more than 7,000 adults, reports from parents on more than 2,900 adolescents (age under 18), and the involvement of more than 5,000 sibling controls for comparisons.
Findings from the CCSS raise concerns about the psychosocial health of adult survivors of childhood cancers, Recklitis said (Hudson et al., 2003; Recklitis et al., 2010; Stuber et al., 2010; Zeltzer et al., 2009). Adult survivors are twice as likely as sibling controls to report significant psychological symptoms, particularly anxiety and depression (Hudson et al., 2003). They are almost twice as likely to report suicide ideation (Recklitis et al., 2010) and impaired mental health (Zeltzer et al., 2009), and they are nearly four times more likely to be diagnosed with posttraumatic stress disorder symptoms compared with their siblings (Stuber et al., 2010). According to the CCSS parent questionnaire results, adolescent survivors were more likely to demonstrate signs of anxiety and depression (1.5 times the risk) compared with their siblings (Jacola et al., 2016; Schultz et al., 2007). Recklitis added that these findings are consistent across diagnostic groups and types of treatment, although there is a higher prevalence of major mental illness in survivors of childhood brain tumor (Ross et al., 2003).
In terms of social adaptation, Recklitis said studies indicate that compared with siblings, adult survivors of childhood cancers are:
- Approximately five times more likely to report functional impairment in health-related quality of life (Hudson et al., 2003);
- About three times more likely to report using special education services (Mitby et al., 2003);
- Less likely to attend college (Mitby et al., 2003);
- Six times more likely to report unemployment related to health (Kirchhoff et al., 2010); and
- More likely to be unmarried (Janson et al., 2009).
These findings of increased psychological distress, educational impairments, and social attainment limitations are also found in other large international cohort studies of adult survivors of childhood cancers, he added (Frobisher et al., 2007; Koch et al., 2004; Langeveld et al., 2003; Lorenzi et al., 2009; Michel et al., 2010; Stam et al., 2005).
Nonetheless, Recklitis said findings from the large cohort studies suggest that most survivors are adapting well (Brinkman et al., 2016; Gunst et al., 2016; Gurney et al., 2009; Janson et al., 2009; Kirchhoff et al., 2010; Mertens and Marchak, 2015; Willard et al., 2017; Zeltzer et al., 2009). For example, 60 to 75 percent of childhood cancer survivors are not reporting psychological problems, approximately 40 percent marry (Gurney et al., 2009; Janson et al., 2009), and approximately 80 percent find employment (Gurney et al., 2009; Kirchhoff et al., 2010). When asked, almost half reported that cancer had little impact on them in their adult life and many also reported positive consequences of their cancer experience (Gunst et al., 2016).
Recklitis pointed out that symptom scales used clinically and in research do not always tell the whole story. For example, in one study of young adult survivors, about 40 percent reported symptoms, but only 18 percent of these survivors received a psychiatric diagnosis (Recklitis et al., 2016). He said that 25 to 40 percent of childhood cancer survivors may have psychosocial needs and that symptoms are predominately mild to moderate in severity. He indicated that, relative to the general population, adult survivors of childhood cancer have similar rates of high distress and impairment, somewhat higher rates of moderate distress and impairment, and somewhat lower rates of low distress and impairment (Recklitis et al., 2016).
Recklitis discussed two significant risk factors for poor psychosocial outcomes among cancer survivors. The first is medical late effects, including pain (D'Agostino et al., 2016), disfigurement (Kinahan et al., 2012), chronic conditions (Vuotto et al., 2017), and impaired overall health (Vuotto et al., 2017). The second is therapy directed to the central nervous system (Schultz et al., 2007). Brain tumor survivors are particularly at risk for poor psychosocial outcomes, in part, because of neuropsychological and neuropsychiatric problems (King et al., 2017). Having certain symptoms can interfere with a young person's functional abilities, such as driving a car, having a relationship, and going to college, which in turn can have an impact on one's emotional health, Recklitis noted. When cancer onset predates attainment of adult capacities and roles, the impact is going to be much greater, he said. Vulnerable periods can occur around life transitions, such as a move to a new environment, a new relationship, or a career change (Coscarelli et al., 2011; Liptak et al., 2016).
Recklitis noted that there has been modest uptake of psychosocial services among survivors of childhood cancer and there may be ways to enhance participation. Recklitis described how he supports the front-line medical clinicians at the Dana-Farber Cancer Institute by suggesting screening measures and how to interpret them, by providing consultation in real time at the clinic, either to the clinicians or to the patients, and by providing urgent/emergent backup services when needed. He raised the question about where psychosocial services should be provided, saying that the cancer center or clinic may be well-suited for delivering some psychosocial intervention, but not others. On one hand, oncology clinicians understand the survivor's treatment and late effects, have the patient's trust, and can integrate other interventions with medical care, but on the other hand, oncology care settings may lack the necessary expertise and resources to meet the psychosocial needs of all survivors.
Recklitis discussed how survivors with low levels of distress can benefit from routine supportive and preventive care (Husson et al., 2011; Jacobson et al., 2009; Recklitis and Syrjala, 2017; Stanton, 2012)—with a focus on education, monitoring, providing self-help and supportive resources, and reassurance and anticipatory guidance—that could be provided in the context of survivorship care planning. He said the challenge is having sufficient programs and personnel to deliver care consistently across different groups and geographic areas.
At the other end of the spectrum, Recklitis described the needs of survivors with major mental illness who may need psychiatric care based outside the cancer center. Cancer-focused clinicians can help educate psychiatric and community-based clinicians about the effects of cancer treatment, and in some cases help with case management and serve as liaison between the mental health and oncology professionals, he said.
Recklitis described how those survivors in the middle range of distress are perhaps the most difficult and complex to manage, in large part because they have a range of symptoms and medical late effects. They could benefit from more intensive evaluation, symptom-related care, and targeted programs at the cancer center or clinic and in the community. Screening can occur through medical encounters, the use of health history forms, and self-report screening forms, but he said that commonly used standard screens may miss 20 to 40 percent of young survivors with a psychiatric diagnosis (Recklitis et al., 2016, 2017). For that reason, he cautioned against use of screening measures as the sole method of identifying survivors' distress.
For those survivors with moderate levels of distress, Recklitis described both low- and high-intensity interventions (Alfano and Rowland, 2006; Andersen et al., 2014; Recklitis and Syrjala, 2017; Stanton, 2012; Stanton et al., 2015). The interventions could range in focus to include
- Social activities, social support, and survivor activism;
- Mental health and coping with medical illness; and
- Biopsychosocial interventions (e.g., to address sleep disorders, fatigue, and sexual issues).
The primary care behavioral health model has relevance to survivorship care, according to Recklitis. This model focuses on early identification, quick resolution of identified problems, long-term problem prevention, and wellness promotion (Funderburk et al., 2013; Robinson and Reiter, 2007; VA Healthcare Network Upstate New York, 2005).
Family Caregiving Issues
As a spouse and a caregiver of a cancer survivor, Hedy Wald, clinical professor of family medicine at the Warren Alpert Medical School of Brown University, and director of Resident Resilience and Wellbeing for Residency Programs in Child Neurology and Neurodevelopmental Disabilities at Boston Children's Hospital-Harvard Medical School, discussed the needs of the families who support cancer survivors and the challenges of meeting those needs. She noted that caregiver distress impacts patients, and cancer-specific stress can have a measurable impact on caregivers' own physical and mental health and immune functioning. Wald described her experience of becoming a caregiver to her husband Mark, a neurologist who was diagnosed with brain cancer (see Box 7).
BOX 7
A Caregiver's Voice: Living with Cancer.
Wald conveyed that the cancer caregiving experience has some unique features that distinguish it from other chronic care caregiving experiences (Kent et al., 2016). Cancer caregivers typically spend more hours per day providing care, provide more intense care over a shorter period of time, and are often more likely to incur out-of-pocket expenses than caregivers of individuals with other chronic illnesses. Concerns about disease recurrence and patterns of progression are stressful, and there is often financial stress. Wald observed that the statistics on cancer caregivers are a call to action to address the needs of caregivers:
- There were approximately 43.5 million adults in the United States providing care to an adult or child in the preceding 12 months, and cancer was identified as the fourth main reason for which people needed a family caregiver (National Alliance for Caregiving and AARP Public Policy Institute, 2015).
- The vast majority (88 percent) of cancer caregivers provide care to a relative (National Alliance for Caregiving, 2016).
- Caregiving services in 2006 were valued at approximately $350 billion (Gibson and Houser, 2007) and rose to approximately $470 billion in 2013 (Reinhard et al., 2015).
Wald said NCCS includes family members in the definition of a cancer survivor, recognizing the essential role of family members. Caregivers are part of the health care team, and their efforts should be coordinated with the other members, including nurse practitioners, psychologists, and social workers, she emphasized.
Wald went on to say that communications skill training for clinicians to help them address patients with serious illness should also include communicating with caregivers. For example, when overwhelmed, a caregiver may not be able to process or cope well with what is being conveyed. Wald suggested that support for caregivers at early stages of the cancer care process might prevent clinical depression or severe anxiety and help boost resilience. She said often caregivers need assistance with child care, financial difficulties, insurance, as well as with diet and other aspects of wellness; thus greater access is needed for interventions such as individual counseling, support groups, caregiver websites and social network groups, other psychosocial services (e.g., art and music therapy, writing groups), as well as options for telehealth and other online support. Wald advocated for incorporating the caregiver voice into conferences and on research review panels to bring greater attention to the psychosocial needs of caregivers, identify best practices, and raise questions and concerns that could be addressed by the research community.
State of Knowledge About Caregiving
Barbara Given, university distinguished professor and interim associate dean for research and the Ph.D. program at the Michigan State University College of Nursing, said caregivers perform many tasks in support of a survivor's health and well-being, including (van Ryn et al., 2011):
- Making decisions and problem solving;
- Managing symptoms;
- Providing emotional support;
- Administering medications and managing complex medication regimens;
- Coordinating care;
- Performing complex tasks without prior preparation; and
- Ensuring follow-up care.
She summarized the state of knowledge about family caregiving based on her review of the research literature (Fox and Brenner, 2012). According to Given:
- Spouses provide most of the care and 50 to 65 percent of caregivers are employed and under age 65.
- The demands on caregivers have increased because of advances in treatment (there are more survivors and they are older) and because more care is provided on an outpatient basis and at home.
- Caregivers are not fully acknowledged as being members of the health care team or included in survivorship care plans.
- The role of caregivers and their needs go unrecognized in the care system.
- Caregivers often do not know how and when to access community resources.
Given described many gaps and limitations in the research on caregiving. Much of the research has focused on caregivers of breast and prostate cancer survivors. There have been few longitudinal studies of caregivers' needs; for example, stress and depression have been well researched, but not during the survivorship period, even though many caregivers assist patients beyond the initial cancer treatment. Given said research has documented the interdependence between the patient and caregiver—for example, one's depression or anxiety can affect the other—but there are few studies on caregivers' social relationships or occupational circumstances.
There is a lack of clarity regarding the knowledge and set of skills caregivers need to be successful, both in terms of managing treatments and coordinating care, said Given (Reed et al., 2017; Shaffer et al., 2016). Likewise, how roles and role relationships vary across the phases of care and over time is not well understood. Also lacking are studies of possible interventions during the survivorship phase that might affect the caregiver or the patient's outcomes, or relieve family caregivers' burden. Given said, “Family caregivers are the hidden cancer care team members. We need to recognize them, we need to value them, we need to include them in the provision of care.”
Effects of Caregiving
Given said the available research shows that more attention is needed on caregivers' health and psychosocial comorbidities. She cited research indicating that emotional distress such as anxiety and depression are high (approximately 38 percent) among caregivers at 2 years postdiagnosis and persist at 5 years (approximately 21 percent) (Kim et al., 2010). The rates of anxiety and depression were higher among caregivers of cancer patients than they were for caregivers of other types of patients (Lambert et al., 2017). She described research showing caregivers need help coping with uncertainty, managing their own health issues and emotions, and finding support and community resources. The effects on finances, lifestyle, and employment were also major challenges for caregivers 2 years after diagnosis (Lambert et al., 2017).
Meeting Caregiver's Needs
Given found few examples of caregiver training in her review of comprehensive cancer centers. She said studies indicate that caregivers do not have important information about follow-up care and treatment late effects, and about the availability of support. In addition, clinicians often are not aware of caregiver resources such as community-based aging services that provide transportation, respite, meals, and other types of supportive care to patients and their families (Adams et al., 2009; Hodgkinson et al., 2007; Lambert et al., 2017; Shaffer et al., 2016). Given explained the importance of having the clinician who is managing the patient's survivorship care assess the caregiver's circumstances and needs related to mental and physical health, life roles, decisional and family conflicts, transitions in care (e.g., need for rehabilitation), and follow-up care (e.g., for late effects, multiple complex comorbidities).
Interventions include referrals to community-based support groups, individualized therapy from psychosocial providers, or online and telephone-based support. Given said 79 percent of caregivers have access to the internet; of those, 88 percent looked online for health information (Fox and Brenner, 2012). Given mentioned instructional videos on the AARP website26 for caregivers. For example, the videos explain how to prepare the home for safe mobility and how to move someone from a vehicle to a wheelchair. Given suggested that a toolbox of interventions and resources be developed for caregivers, noting that resources for caregivers of individuals with dementia could serve as models for the cancer community.
Guidelines, standards, and benchmarks could also be developed to aid clinicians and family caregivers and facilitate assessing the quality of care that caregivers provide. Underlying all of these efforts is professional education, said Given, noting that ASCO has developed resources for caregivers27 and is working on curriculum development and continuing education.
Lifestyle Support for Caregivers
Given said studies have shown that individuals who had healthy lifestyles that include activities such as running and yoga often do not resume those activities once they become caregivers (Adams et al., 2015). Poor habits such as consuming fast food or comfort food and a lack of exercise persist in these studies when followed up at 2, 3, and 5 years. Rowland mentioned research involving caregivers of cancer patients from the Cancer Care Outcomes Research and Surveillance Consortium, which found concordance between caregiver stress and smoking.28 Wald suggested that lifestyle interventions, such as a nutrition consult, be presented to both the patient and the caregiver. Wald added that early interventions, such as group sessions for caregivers, can mitigate distress before it becomes severe and debilitating.
Areas for Research and Policy
Given advocated for a large study of how caregiving affects a survivor's quality of care in terms of patient outcomes and safety, for example. Caregivers can play important roles in identifying complications and getting help promptly, thereby reducing patient morbidity and reducing costs of care, according to Given. Other areas of research she recommended include
- Caregiver surveillance at intervals of survivorship (e.g., 1, 3, and 5 years);
- High-risk situations and optimal interventions for them;
- Vulnerable caregiver populations (e.g., rural, racial, and ethnic groups);
- Quality measurement (e.g., quality and safety of patient/caregiver care, coordination of care, patient/caregiver outcomes);
- Family navigation (e.g., psychosocial coaches);
- Effects of interventions on patient/caregiver employment; and
- Caregiver health care utilization.
In the area of policy, Given said the Caregiver Advise, Record and Enable Act, which has been passed in 39 states,29 says a caregiver must be identified for patients admitted to a hospital, and that the caregiver must receive instruction at discharge and into survivorship. Given said other policies that support caregivers include tax credits and Social Security credits, workplace accommodations, and payment for caregiving services.
Challenges to Implementing Psychosocial Interventions
Many workshop participants discussed the lack of progress in getting evidence-based psychosocial services into practice and offered views on various barriers to making beneficial psychosocial interventions a part of routine care. Rowland acknowledged that despite the screening tools for distress that are available, it remains a challenge to identify individuals at risk and intervene early to prevent catastrophic outcomes such as suicide. She added that there is a Healthy People 2020 goal to improve the physical and emotional well-being of survivors,30 but little direction on how to meet this goal. Karen Basen-Engquist, professor of behavioral science and the director of the Center for Energy Balance in Cancer Prevention and Survivorship at The University of Texas MD Anderson Cancer Center, suggested that the integration of various screening instruments that identify risks for psychosocial distress and impairments, as well as lifestyle risks, might result in a more comprehensive screening approach that could affect the subsequent referral path. Andersen cited screening assessments within cardiology practices that incorporate comorbidity, obesity, and other risk factors. Rowland mentioned the NCCN survivorship care plan includes items on health behaviors and global function (Denlinger et al., 2017) and she said that there is a survey tool31 used for individuals entering clinical trials that assesses global quality of life, comorbidity, and health behaviors.
Recklitis underscored the need for more research on the effectiveness of screening, assessment, and treatment approaches, and said the general state of mental health care in the nation is inadequate, noting most people with a psychiatric disorder do not receive care. Andersen pointed to four problems: (1) shortages of professionals, and insufficient integration of psychosocial professionals within the medical care team; (2) the lack of funding for and poor dissemination of empirically supported psychosocial treatments throughout the various training programs; (3) a lack of experience with the screening and triage approach to survivorship care; and (4) reimbursement systems that do not support the provision of psychosocial services. Andersen added that the next generation of interventions needs to incorporate new technologies, for example, e-health, which have the capacity to target certain groups of clinicians and have a broader reach.
Vinson raised the issue of cultural competence in the context of risk assessment and triage. For example, an individual may have high needs, but they may not be identified because certain cultures are not as communicative about their needs as others. Graves cited the National Standards for Culturally and Linguistically Appropriate Services in Health and Healthcare,32 a resource that provides guidance in 15 areas where organizations can work to improve cultural and linguistic competency. Wald added that workshops on cultural competency at national conferences and elsewhere would help oncology clinicians address the needs of the diverse community of cancer survivors. In Wald's view, medical and nursing schools should teach psychosocial approaches as a standard of care for patients, families, and caregivers, and the curriculum should include mandatory communication skills training and assessment to boost competency in effectively delivering psychosocial care to patients and their caregivers.
Ganz raised issues related to payment mechanisms and quality of care. She observed that some survivors are receiving unnecessary blood tests and scans during their follow-up care while necessary psychosocial services are not provided or paid for. Brenda Nevidjon, chief executive officer of the Oncology Nursing Society (ONS), discussed problems in the delivery system environment, specifically clinician burnout and compassion fatigue due to insufficient staffing, that impede progress in delivering comprehensive and compassionate care to survivors and their caregivers.
SOCIOECONOMIC CONSIDERATIONS IN CANCER SURVIVORSHIP
Workshop participants discussed employment, health insurance, and financial status of patients and survivors and examined the interrelationships among them and other aspects of well-being and health.
Economic Impacts of Cancer
Employment and Insurance
Cathy J. Bradley, associate director of cancer prevention and control at the University of Colorado Cancer Center, reviewed the research on cancer and employment and discussed its implications. Among the approximately 15.5 million survivors in the United States (ACS, 2017), about 7 million are of working age, Bradley reported (Howlader et al., 2014). Employment experiences reflect trends in cancer survivor characteristics and modern cancer treatment practices; for example, a high percentage of women survivors (58 percent), a shift toward a younger age of diagnosis, greater use of outpatient care, a wide range in treatment duration, and increasing use of oral agents, often on a continuing basis.
Bradley observed that these treatment trends coincide with dramatic changes in the American workforce: Individuals are remaining in the workforce longer, the number of women in the workforce is increasing, and the way in which work is being performed is changing. For example, for high-skilled workers, there has been an increase in the so-called “gig economy,” which is characterized by people working at home, working for multiple employers, and having more flexibility, but virtually no benefits. These freelance workers are not covered by some of the laws that offer some protection to those facing illness and disability, such as the Family and Medical Leave Act (FMLA)33 or the Americans with Disabilities Act (ADA).34 But in general, she said the skilled workforce can compete for work and negotiate for better work environments and benefits. Bradley noted that the two top demands of employees following pay and salary are health care coverage and work flexibility.
At the other end of the spectrum, Bradley described the circumstances facing low-skilled laborers. Overall, there is a loss of middle-skilled jobs (e.g., cashier) contributing to more men moving into low-skilled jobs and more women shifting to high-skilled jobs. Cost-cutting measures used by employers include eliminating health care coverage and other benefits and cutting back work hours, leading people to work multiple jobs or to withdraw from the labor force.
These changes in the workforce affect cancer survivors as they try to maintain their employment status. Bradley observed that survivors are very motivated to keep working to retain their health insurance and to combat financial toxicity, as well as to maintain a sense of normalcy, self-worth, and accomplishment that can come from work. Bradley added, “When somebody says, ‘How is he doing?' and the answer is ‘He is back to work,' that immediately sends a positive signal of recovery.”
Even though return to work is a key element of quality of life, it has not been included as an outcome measure in any clinical trials or drug studies, Bradley said. Employment-related outcomes that have been studied include:
- Labor supply (employment and hours worked) for both the patient and the spouse;
- Wages;
- Benefit retention and “hours” lock;
- Job mobility (whether or not the employee is able to move jobs following a diagnosis and treatment); and
- Work limitations.
Bradley said research studies indicate that many factors affect employment-related outcomes and return to work. These factors are related to treatment characteristics, the work environment, and patient characteristics (Bradley et al., 2007b) (see Table 6).
TABLE 6
Risks Affecting Return to Work.
For example, patients with higher levels of education generally experience better employment-related outcomes, Bradley said (Bradley et al., 2005). Regarding treatment factors, in one study, about half of women with breast cancer reported that cancer treatment interfered with their physical efforts at work (Bradley et al., 2007b). These women also reported difficulty with concentration (31 percent), the ability to do analysis (28 percent), keeping the pace set by others (39 percent), and learning new things (20 percent) (Bradley et al., 2007b). She said studies have shown that the longer the treatment goes on, the harder it is for the patient to cope with treatment and the accumulation of side effects (Barnes et al., 2014). For example, among breast cancer survivors who had lengthy periods of treatment, women who were highly educated and in cognitively demanding jobs tended to suffer the most depression and anxiety, largely because they were highly invested in intensive jobs, but were unable to perform them.
Studies have also shown that many work environment factors affect employment-related outcomes (Barnes et al., 2014). For example, survivors who start off with low job satisfaction have even lower job satisfaction over time. This tendency is correlated with poor outcomes, such as very low ratings of quality of life and high rates of depression and anxiety.
Employers play a major role in enabling cancer patients to return to work. In a study conducted by Bradley and colleagues, nearly 90 percent of breast cancer patients and 85 percent of prostate cancer patients reported that their employer was accommodating to their need for treatment and time away from work (Bouknight et al., 2006; Bradley et al., 2007b). Low-income, multiethnic women treated for breast cancer were less likely to report having an accommodating employer (Blinder et al., 2017). Bradley also noted studies showing that the longer the treatment goes on, the greater the tendency for employers to become less understanding and for workplace conflicts to arise.
Jacobsen noted the importance of return to work as a clinical and psychosocial outcome for those who are working before treatment, pointing out that a more expeditious return to work among those who want to go back to work could be an indicator of the quality of care. Bradley described the challenge of trying to capture this information from EHRs, using a change in insurance as an imperfect indicator of return to work. Shelley Fuld Nasso, chief executive officer of NCCS, suggested that a better marker of quality of care would be return to functional status and quality of life. She cautioned that return to work may be a marker, but it is not sufficient, noting that an individual could be back at work and be miserable if still dealing with side effects. Bradley added that measures of how individuals are doing medically and psychosocially upon return to work would be helpful. In her research, she found that about two-thirds of the women discussed their employment situation with their physician, but usually only when they started to experience difficulties.
Kim Hall Jackson, cancer survivor and advocate, shared her experience of workplace conflict that arose following her diagnosis of colorectal cancer at age 45 in 2008 (see Box 8).
BOX 8
A Patient's Voice: Challenges at Work During and After Treatment.
Bradley observed that there is little research on spouse employment, but some studies have shown that employed spouses of breast cancer survivors do not change their labor force participation and tend to continue working, largely because women rely on various other types of caregivers, whereas men who are ill and married tend to rely on their wives to be the caregiver (Bradley and Dahman, 2013). There was some decrease in hours worked during the active treatment period, but men who provided insurance for their wives through a family policy were less likely to decrease their hours in order to maintain their health insurance.
Bradley has found that type of health insurance greatly affects employment (Bradley et al., 2007a,b). Individuals who have a family policy are more likely to work, and cancer patients and survivors who carry the insurance themselves are more likely to work as well. Some study participants reported that they would miss a chemotherapy appointment before they would jeopardize their work because of concerns about maintaining health insurance. These concerns were particularly acute for those with dependents (children or a spouse) on their policy. She noted that the consequences of this dependency on employer-based health insurance may include health sacrifices for those who continue working and the loss of coverage for those who cannot continue working.
Research on cancer survivorship and employment has clinical, employment, and policy implications, Bradley said. At the clinical level, she suggested that employment outcomes should be integrated into clinical studies to answer questions about the impact of different kinds of treatment on one's ability to work. Improving the control of symptoms that affect work is also important; a survivor's ability to work can be enhanced when sleep aids, antidepressants, physical therapy, and cognitive reconditioning are available, she emphasized. Clinicians need be mindful, however, of how symptom control interventions affect patients' ability to work; for example, drugs for insomnia may make the patient sleepy during the work day.
Bradley said more workplace interventions are also needed, noting that employers and employees do not fully understand the long-term effects of cancer and how cancer and its treatment can diminish the ability to work long after treatment ends. In her view, employers need to be educated so they can do a better job of providing the kinds of accommodations that survivors need. The accommodation needs that Bradley heard from breast cancer survivors most often were: flexible schedules; reduced hours, especially during active treatment; and special equipment (e.g., a laptop that could facilitate working at home).
Examining the extent to which the employer is “health centered” or offers a “well-being” environment is a new area of research, said Bradley. She described a CDC-funded initiative known as Total Worker Health35 to examine employers' knowledge and understanding of worker well-being, identify mechanisms promoting a health-centered workplace, and evaluate whether a business case can be made for the adoption of this approach.
At the policy level, Bradley said the research on survivorship and health insurance suggests a need to broaden the options for insurance outside the workplace. In addition, paid sick leave is important, especially during the active treatment phase. Bradley mentioned that FMLA does not apply to small employers (with less than 50 employees), and most of today's workforce is employed by small employers or are working independently as freelancers and contractors. Bradley concluded that solutions are needed across the employment spectrum to improve the experience of cancer patients and survivors.
Financial Hardship
Robin Yabroff, social science analyst in the Office of Health Policy at the Office of the Assistant Secretary for Planning and Evaluation in the Department of Health and Human Services,36 described key research findings regarding financial hardship among cancer survivors. For context, Yabroff explained that there have been dramatic increases in the price of cancer drugs (Bach, 2009; IOM, 2014; NASEM, 2018b). Some immunotherapies and targeted agents now cost $25,000 or more per month, she said, and even with insurance, cost-sharing provisions can mean significant out-of-pocket expenses for patients treated with these drugs, which may not offer a cure or even significant improvement in survival. Additional costs might include supportive agents, advanced imaging tests, and hospitalizations.
According to the research, Yabroff said that financial hardship is relatively common among cancer survivors, even many years after treatment. Risk factors for financial hardship include
- Younger age (Banegas et al., 2016; Kent et al., 2013; Shankaran et al., 2012; Yabroff et al., 2016);
- Being female (Yabroff et al., 2016);
- A recent cancer diagnosis or treatment (Kent et al., 2013; Yabroff et al., 2016);
- Having lower socioeconomic status (Banegas et al., 2016; Shankaran et al., 2012; Yabroff et al., 2016); and
- Being unemployed or changing employment because of cancer (Yabroff et al., 2016).
Compared to individuals without a cancer history, cancer survivors have greater health care expenditures and more financial difficulties because they are unable to work, or have work limitations (Finkelstein et al., 2009; Guy et al., 2013; Short et al., 2011; Yabroff et al., 2008). Research also shows higher bankruptcy rates among cancer patients compared to people without a cancer diagnosis, controlling for age, residence, and socioeconomic status (2.65 times higher from 1995 to 2009) (Ramsey et al., 2013). She added that among cancer survivors, bankruptcy has been associated with increased mortality risk, perhaps due to lower quality of life and overall well-being among those who experience bankruptcy, and/or to an inability to adhere to a treatment plan because of the financial hardship (Ramsey et al., 2016).
Yabroff discussed findings from studies of financial hardship that conceptualize hardship in terms of material hardship, psychological hardship, and behavioral hardship (Altice et al., 2017; Tucker-Seeley and Yabroff, 2016; Yabroff et al., 2016). Material financial hardship relates to having trouble paying medical bills or having medical debt. Psychological financial hardship relates to the distress or worry that may accompany having medical bills. Behavioral financial hardship occurs when care is delayed or foregone because of cost (e.g., skipping medication doses to save money).
Estimates from the nationally representative 2011 Medical Expenditure Panel Survey (MEPS) on Experiences with Cancer, Yabroff explained, suggest a high level of financial hardship among cancer survivors (Yabroff et al., 2016) (see Table 7). Approximately 20 percent of survivors reported they had experienced any aspect of material financial hardship and approximately 23 percent were worried about paying for their medical care. Most respondents with a cancer history were long-term cancer survivors, reporting they had been diagnosed more than 5 years ago, and Yabroff said this suggests that the financial hardships reported are long lasting. In a study of cancer survivors in health plans, individuals with lung or breast cancer had greater material financial hardship compared to those with colorectal cancer, prostate cancer, or melanoma (Nekhlyudov et al., 2016).
TABLE 7
Financial Hardships Associated with Cancer.
Yabroff described other findings based on data from the MEPS on Experiences with Cancer (Yabroff et al., 2016):
- Among those 18 to 64 years, cancer survivors with private health insurance report significantly less material financial hardship than those who are uninsured or have public insurance (approximately 25 percent versus 35 to 40 percent). About 13 percent of survivors ages 65 years and over said they were having trouble paying their medical bills.
- Psychological financial hardship was highest among adults ages 18 to 64 who were uninsured (about 50 percent). Rates for those in this age group with any insurance (public or private) were about 30 percent. For the population ages 65 and over, about 13 percent experienced psychological financial hardship.
- There are greater reports of either material or psychological financial hardship among working age adults age 18 to 64 years (about 40 percent) compared to those age 65 years and older (about 20 percent) (see Figure 5).
According to Yabroff, findings from a LIVESTRONG Foundation study of 4,719 working-age adults with a previous diagnosis of cancer indicate high levels of material hardship, with approximately one-third of survivors saying they had borrowed money or gone into debt (Banegas et al., 2016). Among that group, the out-of-pocket spending was primarily for medical expenses, transportation, lodging, child care, and home/respite care. Approximately 64 percent of respondents reported psychological financial hardship associated with cancer (e.g., worrying about paying medical bills) (Banegas et al., 2016).
Yabroff said findings from the National Health Interview Survey37 show that adults ages 18 to 64 with a history of cancer are much more likely than those without a cancer history to say that they delayed filling a prescription, took less medication, and skipped medication doses because of cost (Zheng et al., 2017). These analyses adjusted for age, sex, race/ethnicity, socioeconomic status, type of health insurance, residence, and number of comorbid conditions.
Yabroff concluded that it is important to distinguish the material, psychological, and behavioral aspects of financial hardship in order to intervene more effectively to relieve some of the consequences of this hardship. For example, if someone is having difficulty paying bills, distress counseling may not be helpful in alleviating that person's burden, and a very different intervention may be needed. She said for individuals facing financial hardship, resources may be available, such as financial navigators and special programs. For example, the Cancer Care Equity Program at the Dana-Farber Cancer Institute aims to reduce cancer disparities.38
Financial hardship research continues to evolve, Yabroff said, with ongoing measure development, primary data collection, and use of the MEPS Experiences with Cancer Survey (the 2016–2017 MEPS Experiences with Cancer Survey was in the field at the time of the workshop). Other national surveys are also relevant. For example, the Behavioral Risk Factor Surveillance System Survey has questions about financial hardship such as household instability and food insecurity. Another resource Yabroff mentioned was the NCI Physician Data Query, which includes a summary of evidence on financial toxicity that is continually updated.39
Patient Perspectives on Costs of Care and Financial Toxicity
Using her experience as a cancer survivor and advocate for 20 years, Mary Jackson Scroggins, co-founder of Pinkie Hugs, LLC, and In My Sister's Care, discussed issues of financial toxicity from the patient perspective. According to Scroggins, financial toxicity is inadequately defined, explained, and studied. She said when asked about finances, patients can feel stigmatized and embarrassed and therefore, try to make their answers acceptable to the person who is asking the questions. Others may fear they will receive inferior care if their financial vulnerability is disclosed or have concerns about how their financial information will be used.
Scroggins said health care professionals may find it uncomfortable to ask questions about a patient's financial circumstances, but it is important for them to facilitate an open and honest conversation with patients. She added that patients need to feel comfortable telling their clinicians that the reason that they are not taking their medication or adhering to some aspect of their care regimen is because they are having financial difficulties. Only then can assistance be offered, she said.
Scroggins said tools are needed to help health care professionals who are reluctant and unprepared to discuss financial matters. Researchers and clinicians should anticipate patients' concerns and provide clear information about disclosure and privacy policies, explain how their information will be used, avoid verbal language and body language that suggests to the patients that they are being judged, and provide private areas to have conversations, said Scroggins. In research studies, Scroggins noted the importance of participation by individuals of all income and demographic groups so that results can be generalized. Scroggins emphasized opportunities in the health care system for:
- Standardizing and conducting more research on financial toxicity;
- Assessing patient needs and educating and learning from patients and the community;
- Engaging the patient/survivor and advocacy communities to develop and validate research tools/questions and interpretations;
- Eliminating perceived stigma around financial disclosures; and
- Enhancing patient care and outcomes.
Scroggins stressed the need for additional evidence surrounding the impact of finances on patient care in order to advance the development of effective interventions.
“e-Patient Dave” deBronkart, co-founder and chair emeritus of the Society for Participatory Medicine and patient advocate, described the voice of patients as “ground truth” and referenced the IOM consensus study report Best Care at Lower Cost (IOM, 2013a), which identified engaged, empowered patients as an essential characteristic of a continuously learning health care system. He stressed the need for patients to use the Internet to learn about their health, treatment options, and cost of care. deBronkart described these “e-patients” as being “empowered, engaged, equipped, and enabled.” deBronkart pointed out that more information and incentives to use the information are needed. In his personal story of financial toxicity, deBronkart described having to make treatment decisions for his skin cancer and the challenge of obtaining information about the cost of care (see Box 9).
BOX 9
A Patient's Voice: The Cost of Care.
Legal Considerations in Employment and Education
Barbara Hoffman, founding chair of NCCS and assistant teaching professor of law at Rutgers Law School, described some of the legal consequences of cancer (Marmot et al., 2008; Zevon et al., 2007). Cancer can affect employment and the ability to obtain a good education, and can contribute to housing insecurity and transportation challenges. There are many impacts on insurance, including not only health insurance, but also life and disability insurance. There are also legal issues related to dependent care and end-of-life planning. Hoffman said these legal implications add to the many other challenges facing survivors and often have a negative effect on health. The stress and financial difficulties that arise from these legal issues can increase family tensions, reduce quality of life, and decrease treatment compliance (Ko et al., 2016; Zevon et al., 2007).
Legal Issues Related to Employment
The ADA prohibited some types of employment discrimination and created a legal right for people with disabilities to receive workplace accommodations. But Hoffman said that courts often did not consider cancer survivors as members of one of the protected classes of the ADA: (1) a person with a disability; (2) a person with a history of a disability; or (3) a person who was regarded as having a disability (Hoffman, 2000).
Hoffman explained that in 2008, the ADA was amended to cover individuals with a cancer history. According to Hoffman, the law shifted from “having to prove you are covered” to “how is the law going to protect you?” After the 2008 amendment, there was an increase in the number of cancer survivors who had favorable outcomes for discrimination claims filed with the federal U.S. Equal Employment Opportunity Commission (EEOC), Hoffman said. Most of the cases were resolved with a focus on appropriate accommodations for the cancer survivor, and overall, employers were less likely to discriminate, Hoffman said (Hoffman, 2014).
Hoffman indicated that the ADA has provided a means for cancer survivors to demand reasonable accommodations and to insist on being treated by employers according to their ability to work, not on the basis of having a disability or cancer. Under the ADA, “If you are able to work, you are entitled to a reasonable accommodation that enables you to do your job,” Hoffman said. She added that the most common accommodation that survivors use is time off for treatment or follow-up care. Flexible work hours are another common accommodation, for example, to include rest into the workday.
Hoffman said the growth of patient advocacy in the past 10 to 15 years has advanced legal protections and more legal resources are available, such as patient navigators, social workers, and others familiar with legal issues in clinical settings. The ADA currently covers all employers who have at least 15 workers, but Hoffman said the law would need to be amended to ensure coverage of those in the new economy (e.g., independent workers).
Hoffman also described the FMLA as another law relevant to cancer survivors. The law requires up to 12 weeks of unpaid leave to care for yourself or, if you are a caregiver, to care for a spouse or a child who has a serious medical condition. She said most cancer diagnoses meet the definition of a serious medical condition, but this law only applies to employers who have at least 50 employees. Hoffman said another shortcoming of the law is that it does not require paid leave, noting that the United States is one of the few countries in the Western world that does not have paid family leave. There have been attempts in Congress to amend the law to include this provision, but they have failed. Hoffman added that some state laws have provisions for paid leave and some employers provide such leave as part of their benefit package.
Hoffman explained that the Genetic Information Nondiscrimination Act of 2008 prohibits some forms of discrimination based on the results of a genetic test or on the basis of having a family member with a genetic-based disease or condition, but the law does not prohibit discrimination against an employee who has a genetic-based disease.40 Hoffman said that one aim of the law is to encourage people to get genetic testing so they can be proactive with their health care.
Hoffman noted that a range of protections is provided by state law as well. Every state has a law similar to the ADA that prohibits some kinds of workplace discrimination and requires some types of reasonable accommodation. Hoffman added that progressive states like California and New Jersey have laws with more extensive protections than the federal ADA, while other states, particularly those in the south, have laws with more limited protections.
There is also variation, Hoffman said, in the extent to which state laws provide medical leave, with some mandating more medical leave than required under the FMLA. In the area of privacy, again, Hoffman mentioned the variation in protections afforded by state laws, with some states offering more protections than those under the Genetic Information Nondiscrimination Act of 2008. In summary, Hoffman observed that there is a “layering of federal and state legal rights for cancer survivors.”
Legal Issues Related to Education
Hoffman described the many ways that a history of cancer can affect educational attainment and the quality of education. She said the effect of cancer on education is especially salient for survivors of childhood cancer because the late effects of radiation, chemotherapy, and surgery can lead to neurocognitive deficits, growth retardation, cardiac dysfunction, and subsequent malignancies. In addition, there can be serious psychosocial late effects and symptoms, such as fatigue, that can impair learning and academic achievement. Hoffman noted that these late effects could make it very difficult to be a full-time student.
Hoffman described the protections offered by the Individuals with Disabilities Education Act (IDEA). This law applies to children and young adults from ages 3 to 21 and requires schools to have an individual education plan tailored to the needs of children with disabilities, including cancer survivors. These plans are developed by multidisciplinary teams, and in the case of pediatric cancer, the teams should include a professional with knowledge of the late effects of cancer and its treatment. When individuals age out of the protections offered by the IDEA, and enter college or graduate school, the ADA provides protections. Hoffman added that both the IDEA and the ADA require individually tailored accommodations to help the survivor go to and stay in school. These accommodations could be as simple as making provisions for breaks while at school or allowing for extra time when taking an exam. The accommodations can also be more resource intensive, for example, providing an aide to accompany the student to school to take notes, open doors, and record lesson plans.
Hoffman described some concerns regarding the future legal rights of cancer survivors. There is a potential, she said, for Congress to amend laws to weaken existing protections and for federal agencies, such as the EEOC, the Department of Labor, and the Department of Education, to reduce their enforcement efforts. Hoffman added that reductions in support of legal services could hamper the ability of cancer survivors in financial distress to get assistance. She encouraged workshop participants to pursue opportunities in their communities; for example, a cancer center might be interested in starting a medical–legal partnership.
In Hoffman's view, the marked increase in advocacy, informed by advances in survivorship research, has led to a growth in awareness of the needs of cancer survivors among employers, educators, the health care community, and the general public. She added that with this awareness will come recognition of the need to maintain and improve employment and educational legal protections for cancer survivors. Hoffman said multidisciplinary strategies, such as the formation of medical–legal partnerships within patient-centered medical homes, also help support survivors' legal rights and can potentially prevent problems in the workplace or school. Lastly, Hoffman discussed the need for multidisciplinary training that includes oncology and legal professionals, which in her view would promote more holistic care of survivors to address their physical, mental, financial, and legal health.
MODELS OF SURVIVORSHIP CARE DELIVERY
Workshop participants discussed various models of survivorship care and the science behind effective program implementation. In addition, representatives of various survivorship care programs and patient advocacy programs and individual survivors discussed experiences and learned lessons and shared their views on improving the delivery of survivorship care.
Overview of Models of Care
Mary McCabe, former Clinical Director of the Survivorship Center at Memorial Sloan Kettering Cancer Center, described types of survivorship care delivery models in existence today. She indicated that at the time of the 2006 From Cancer Patient to Cancer Survivor report (IOM and NRC, 2006), most services for cancer survivors and their families were single services focused on one aspect of care, such as psychosocial support groups; models of survivorship care delivery, such as the pediatric oncology multidisciplinary model, were only beginning to emerge.
In the past decade, McCabe said there has been a progression from a one-size-fits-all approach to survivorship care to one that looks at “who needs what and for how long and with what provider.” The structure of the model and type of clinician depend on the survivors' risk of recurrence and late effects, the types of services needed for comprehensive care, and the timing of those services, whether it is a one-time consultative visit or ongoing care (McCabe et al., 2013). McCabe described the various models of care that correspond to survivors' risk profiles:
- A multidisciplinary care model, such as the pediatric survivorship model, supports patients at high risk of late effects and recurrence. Clinicians provide care with expertise in long-term effects, such as cardiology, psychology, and endocrinology. Care takes place in a setting separate from the oncology practice.
- Patients with a moderate risk of recurrence and late effects, which includes most patients, McCabe said, can benefit from one of three models of follow-up care:
- A disease-/treatment-specific clinic where the type and intensity of follow-up care are determined by the type of cancer treatment received.
- An integrated care clinic embedded in the oncology practice of a cancer center, community hospital, or private practice. Care is coordinated with the patient's primary care clinician.
- A consultative approach that could entail a one-time consultation to coordinate the survivorship plan of care. Follow-up may be with the oncologist or primary care clinicians.
- Low-risk patients can benefit from a community generalist model of care in which the transition of the patient occurs early, with follow-up care provided by a primary care clinician. McCabe said in other countries, care is often shared between the generalist and the oncology specialist, with an early hand-off for patient follow-up care to a generalist, but that in the United States this approach is impeded by the reluctance of oncologists to refer to primary care clinicians and the shortage of these generalist clinicians.
More research is needed to fully understand which approach is most effective for the various survivor risk profiles, said McCabe. She mentioned some of the research findings to date that indicate that nurse-led and primary care clinician follow-up may be equivalent to oncologist care in detecting recurrence (Gates and Krishnasamy, 2009; Grunfeld et al., 2011; Howell et al., 2012; Knowles et al., 2007). She added that the outcomes have been measured across models under study, but they often focus on recurrence and quality-of-life endpoints. Most models of care assume a role for the primary care clinician; however, more clarity is needed on the written information necessary during transitions of care, she said.
McCabe described three other models of care that may offer guidance in the context of survivorship care:
- Self-management: Evidence from other chronic care disciplines demonstrates that the self-management model promotes patient skills in problem solving, decision making, and taking action (in particular, initiating timely communications with health professionals) (Beckmann et al., 2007; Krouse et al., 2016; Wilson, 2008). McCabe added that this approach could also be used for psychosocial support, managing symptoms, and encouraging and promoting healthy behaviors.
- Group visits: Well-established evidence in the context of various chronic conditions demonstrate that group visits are cost-effective interventions. Group visits can address symptom management and psychosocial support and have the advantage of being less resource intensive than other approaches (Trotter et al., 2011; Vachon et al., 2007; Wagner et al., 2001). Groups can also be tailored to meet the needs of families and other caregivers.
- Virtual follow-up care: There are many potential applications of telemedicine (e.g., symptom management, counseling, and monitoring of the survivorship care plan), and the interest in virtual follow-up care is growing, McCabe said (Cole-Vadjic and Crews, 2016; Warrington et al., 2015). She added that while high-tech interventions hold promise, telephone counseling has value as a low-cost intervention.
McCabe outlined some areas of possible future research as the oncology community implements, evaluates, revises, and improves survivorship care (Halpern et al., 2015):
- Developing a common understanding of “usual care” to compare with survivorship models, a common taxonomy for the various models of survivorship care, and a minimal set of outcome measures that are relevant to survivors in each risk group;
- Applying a common set of characteristics for evaluation of survivorship care models;
- Evaluating the content, delivery, and utility of care plans to the recipients;
- Assessing the cost of formal survivorship care and services in order to determine their value in terms of the individual/family and the health care system; and
- Identifying the unique needs of survivors in underserved populations.
In closing, McCabe recalled some of the comments made by workshop participants: “Implement what we know; resist the impulse to provide all care to all survivors; think about using team-based care; practice differently; rethink how we deliver care; and we can do it.”
A Patient Perspective on Survivorship Care Delivery
Martha Gaines, ovarian cancer survivor, and founder and director of the Center for Patient Partnerships and distinguished clinical professor of law at the University of Wisconsin Law School, presented a patient perspective on survivorship care delivery. The Center she directs is interdisciplinary and trains future professionals in law, medicine, nursing, pharmacy, genetic counseling, social work, and industrial engineering. Interdisciplinary teams respond to calls from patients from around the world and address questions about diagnoses and treatments, as well as employment- and insurance-related concerns of cancer patients and survivors.
Gaines's primary recommendation to organizations that support survivors is to effectively engage patients from the very beginning. Patients need to be fully engaged stakeholders in their own care, she said. Gaines acknowledged that scientists and clinicians are not always successful in their attempts to engage patients. However, in her view engagement can be improved when patients and clinicians learn together. Gaines suggested that a clinician should learn as much about a patient as he or she knows about the patient's diagnosis. She quoted physician Sir William Osler, who said, “It is far more important to know what patient has the disease than to know what disease the patient has.” Gaines emphasized that a true partnership exists only when there is familiarity and trust. In her view, a survivorship care plan is a way to document what is understood by both the patient and his or her clinicians and it helps ensure the involvement of the primary care clinician.
On the macro level, the patient experience is incorporated into care systems through surveys such as the Hospital Consumer Assessment of Healthcare Providers and Systems.41 But Gaines noted that the complexity of the health care system impedes information sharing among clinicians and patients and makes creating guidelines difficult. In her view, EHRs are potentially great sources of information, however, because they are proprietary, they cannot be fully explored to learn more about what works.
Survivorship care plans vary widely, and Gaines observed that this reflects creativity on the part of the various programs, but it does impede sharing information and learning across programs. Given the diversity of care plans, it would be helpful to have researchers, clinicians, and survivors share their experiences with the plans and identify what seems to be working best.
Gaines said that in studies about what is important to patients, patients consistently report, “I want to have a relationship with the people who care for me.” She added that this holds true in the diagnosis and treatment phases of care and in posttreatment survivorship care. The true value of a survivorship care plan, according to Gaines, comes from its joint creation. She explained that a survivorship care plan is an important decision tool and conversation facilitator, but it is not the focus of care. She said, “It is not about the document. It is about the relationships, and it is about having a willing co-creator who is actually going to view the plan as a covenant.” She observed that participation in care creates agency on the part of the patient and this, in turn, usually leads to engagement, adherence to treatment, and improved outcomes.
In stressing the importance of patient engagement, Gaines said, the health care system produces treatment protocols, survivorship care plans, and entire cancer centers without ever asking the users. Instead of asking, “On a scale of one to seven, how satisfied are you with this care plan?” Gaines suggests asking instead, “What are three ways this plan could be better?” and “What are three ways it really works well?” Gaines encouraged workshop participants to tap into the rapidly evolving science of patient engagement. She mentioned a valuable resource, the HIPxChange42 from the Institute for Clinical and Translational Research at the University of Wisconsin, adding that the program website includes many free tools on how to engage patients in many areas, such as research and organization and system redesign.
Reaching Rural Cancer Survivors
Keith Argenbright, professor and chief of community health sciences at The University of Texas Southwestern Medical Center and director at Moncrief Cancer Institute, described the challenges of providing care for rural cancer survivors, noting that about one in five cancer survivors live in rural settings. When rural residents get cancer, he said they are often in poorer health and face more limited access to health care resources than survivors in non-rural areas. Compared to metropolitan areas in the United States, in rural areas there is (Henley et al., 2017):
- A higher average annual age-adjusted death rate for all cancer sites combined;
- A higher incidence and death rates for cancers related to smoking;
- A higher incidence and death rates for cancers that can be prevented by screening (e.g., colorectal and cervical cancer); and
- A higher incidence of cancers related to human papillomavirus.
Poor access to health care in rural areas can be traced to the closure of many hospitals and the subsequent loss of physicians, Argenbright said. As hospitals close, there is a loss of the ancillary services, including advanced imaging and physical rehabilitation. He said that compared to urban residents, rural residents have to travel 6 to 10 times farther for chemotherapy and 2 to 3 times farther for radiation therapy (Chan et al., 2006). To expand its outreach to rural cancer survivors, the Moncrief Cancer Institute in Fort Worth, Texas, launched a first-of-its-kind mobile survivorship clinic to provide prevention, early detection, patient navigation, and survivorship services to the medically underserved in 35 mostly rural counties in north Texas (see Box 10).
BOX 10
Rural Care Delivery Innovation: Mobile Survivorship Clinic.
A Survivorship Clinic in a Community Cancer Center
Mark O'Rourke, medical director, and Regina Franco, director, both of the Center for Integrative Oncology and Survivorship (CIOS) at the Greenville Health System Cancer Institute, described the challenges and opportunities of providing survivorship care in a South Carolina community cancer center.
O'Rourke said more than 3,100 patients within the Greenville Health System are diagnosed with cancer each year. Some of the impetus for the establishment of the survivorship care program was the 2006 IOM From Cancer Patient to Cancer Survivor report and the 2012 CoC accreditation requirements for distress screening and survivorship care planning. He said when the survivorship clinic was initiated in 2012, cancer patients in the hospital's catchment area were invited to come for a 45-minute survivorship care plan visit to discuss issues related to late effects and recurrence, complete a distress screen, and address lifestyle and health promotion. The addition of other services to the program followed, including lymphedema management, oncology rehabilitation, nutrition, social worker counseling, genetic counseling, a lifetime cancer surveillance clinic, and a smoking cessation clinic. O'Rourke mentioned that early in the program's development the nurse practitioner, social worker, and dietitian engaged in one comprehensive visit, but later a decision was made to separate the visits. More recently the survivorship clinic started offering oncofertility referrals; cancer screenings (lung, breast, cervical, and colon cancers); an AYA cancer clinic; and a high-risk clinic for cancer prevention and risk analysis (e.g., for people who test BRCA positive). Services under development include sexual health visits and spiritual navigation with the hospital's interdenominational chaplain to address patients' spiritual concerns. Franco added that most of the clinic activity is led by an advanced practice registered nurse rather than by a physician. Physicians work in the clinic two afternoons per week, but the care is delivered primarily by dietitians, social workers, and nurse practitioners.
O'Rourke reported that there are 600 to 700 visits per month in the CIOS, with 30 to 50 survivor care plan visits monthly. A patient can opt for a second survivorship program visit at the end of treatment, and about 50 percent of patients schedule a second care plan visit, he said. Franco added that most patients who opt for the second visit are considered to be high risk (e.g., those undergoing multimodality treatments). O'Rourke said the survivorship care clinic creates two to four referrals per patient for other integrative and/or survivorship services in the center.
There have been challenges in using the care plans, O'Rourke explained. Initially, “A Journey Forward” template was used, but then the program switched to the ASCO template and developed disease-focused, detailed plans with survivorship information pertinent to each major cancer. “For a while we had care plans for each different diagnosis and we would have a nurse navigator develop these care plans, and then we would deliver the care plans individually. We found that it was just too time consuming. It was just overwhelming,” he said.
As a result, in 2014 the program adopted a generic, shorter survivorship care plan template that focuses on psychosocial and lifestyle issues. Since 2015, cancer-related and other information for the care plan has been generated from the tumor registry database. Although the hospital implemented the Epic EHR, the survivorship program used its own template, which is scanned into the EHR. O'Rourke said the creation of the care plan and monitoring for CoC compliance is time consuming and that EHRs are still suboptimal for survivorship care planning. Franco added that some information has to be entered manually into the EHR and it takes many calls and much effort to populate the entire care plan.
Franco described the program's use of surveys to assess patient satisfaction and engagement. The results are analyzed on a quarterly basis to identify needed changes. For example, Franco said that survey respondents indicated a need for assistance and support during treatment, so the timing of the first survivorship meeting was moved to approximately 4 to 6 weeks after treatment begins. Franco added that a completed survivorship care plan is mailed to the survivor at the end of treatment or at the conclusion of their second survivorship clinic visit. Other strategies for engaging patients include a survivorship registry that is used to follow up with patients and inform them of new developments or supportive programs that may be of interest. The registry is approved by the Institutional Review Board and patients sign a five-page consent form if they agree to being contacted, Franco said.
Other evaluation tools include focus groups and staff feedback. Clinic statistics are reviewed on a monthly basis to track growth patterns, the origins of referrals, the level of participation in groups, and the rate of no-shows and rescheduled appointments. Compliance with screening guidelines and the CoC accreditation requirements are also monitored.
Franco summarized several lessons learned about survivorship care plans from the Center's experiences, noting that the adage “one size does not fit all” applies to the development of survivorship services. She said each survivorship visit needs to be patient specific because each cancer survivor has a unique personal experience, and staff training is essential.
Franco said that highly detailed care plans are time consuming to prepare but educational for survivors. To reduce the burden, the program adopted a streamlined care plan template that allows clinicians to focus on lifestyles—exercise, nutrition, weight control, and smoking—because these areas reduce cancer risk and improve overall general health. The plan also designates which clinician is responsible for the various aspects of follow-up care.
Psychosocial concerns are prevalent and deserve attention, said Franco. Many patient concerns revolve around non-cancer–related issues and there is a lack of mental health services, especially for those who are under-insured or uninsured. In addition, health maintenance and screening are often neglected among cancer survivors. Franco has found that explaining the value of screening and assisting with scheduling helps with compliance. Many cancer survivors lack primary care medical management to guide non-oncological health care, so the survivorship clinic often makes referrals to establish a primary care relationship.
Franco shared her vision of future potential survivorship care:
- Embedded satellite clinics to complement destination programs;
- Telemedicine for genetic counseling and other services that do not require a physical examination;
- Self-management with support from credible advocacy organizations (e.g., Cancer Support Community, the ACS); and
- Lifetime cancer surveillance clinics where annual visits with the nurse practitioner are integrated with primary care.
Franco identified several aspects to sustaining survivorship clinics: a marketing budget; billable models for physician/advanced practice nurse visits; and documentation of the downstream revenue of the survivorship care-planning visit. O'Rourke noted collaborations that have benefited the CIOS. The South Carolina Cancer Alliance,43 an approximately 800-member group representing the state's medical community, academic institutions, nonprofit organizations, and various community groups, has a survivorship care subgroup that meets periodically and holds annual evidence academies where representatives of 17 CoC-accredited hospitals share information and experiences. The CIOS also collaborates with the Cancer Support Community,44 a national advocacy organization that has assisted the CIOS with the development of programs tailored to the needs of cancer survivors and helps patients with online and telephone counseling to address personal and financial concerns.
Survivorship Care Delivered by Independent Medical and Nonprofit Organizations
Jay Burton, founder and president of the Primary Care Cancer Survivorship Program of Western Massachusetts, is a physician and a survivor of acute myeloid leukemia and a stem cell transplant. After Burton recovered from his treatment, he created a survivorship clinic that provides primary care services and coordinates survivorship services.45 He also formed a nonprofit organization to provide psychosocial services to cancer survivors and their caregivers.46
Burton said he started with no in-house survivorship services, mental health care providers, or educational programs, and he had no marketing team or help with fundraising. He said that primary care medical charts do not effectively capture the cancer patient's experience, so he created a primary care chart that covers cancer diagnosis, treatment, complications, surveillance, and late effects. Burton has educated his practice's staff on cancer survivorship, with a focus on prompt access to care. His outreach extends to family and caregivers, health care practitioners, care managers, employers, and others in the community, and this outreach has been facilitated by a local television station.
Burton could not get support within his medical practice for psychosocial services, so he said he started a nonprofit organization called Survivor Journeys, with a mission to provide social and emotional support services to cancer survivors, their families, and caregivers. The survivorship program includes cancer type–specific support groups that are composed of a facilitator, behavioral specialist, physician or nurse practitioner, and a survivor of that particular cancer. Groups are held in community settings, such as libraries, houses of worship, and senior centers. A mentoring program has been developed for survivors, and representatives of regional cancer centers provide education to the local survivors. The program also provides assistance for transportation to group sessions, but he said fundraising continues to be a challenge.
He said the program is part of a national organization for volunteer management in cancer care, whose members include the Dana-Farber Cancer Institute, The University of Texas MD Anderson Cancer Center, and the Cleveland Clinic. Burton and colleagues are also working on developing a speaker's bureau to meet the needs of survivors and providers in communities distant from cancer centers, noting the value of the GW collaborative e-learning series on survivorship.47
Dissemination and Implementation Science in Cancer Survivorship Care Delivery
David Chambers, deputy director for implementation science in the division of cancer control and population sciences at the NCI, described dissemination and implementation science and its role in cancer survivorship care delivery. Chambers cited a classic study that found it takes an average of 17 years for a fraction of research findings to be adopted into clinical practice (Balas and Boren, 2000). He elaborated on this point by saying there is a lengthy pathway from research study completion to the publication of findings, and their incorporation into practice guidelines and textbooks, and ultimately implementation in practice. In his view, one reason for this lag in implementation is the ongoing development of many models of care and interventions without careful consideration of the demand for them. Taking demand into account is complicated, he said, involving consideration of clinicians, administrators, payers, and most importantly, patients and families. Chambers conveyed that an evidence-based program for survivorship care is only beneficial if it is adopted within systems of care, clinicians are trained to deliver it appropriately and can incorporate it into their practice, and eligible patients receive it (Belza et al., 2007).
Chambers said the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework (Glasgow et al., 1999) is often used to understand the various outcomes needed to move interventions into clinical practice settings to improve population health (Belza et al., 2007) (see Figure 6). The model takes the following factors into account: how well we are reaching the population that we think is going to benefit from a program; how effective is the program; whether organizations can be supported to deliver the intervention; whether the intervention can be implemented effectively; and finally, whether it can be maintained within systems over the longer term. Taken together, these elements represent the overall public health impact of a program or policy. Chambers said, “To maximize overall impact, an intervention must perform well across all five elements; significant program weakness in any of the elements may adversely affect impact” (Belza et al., 2007).
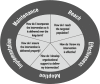
FIGURE 6
Elements of the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework. SOURCES: Chambers presentation, July 25, 2017; Belza et al., 2007. Reprinted with permission from the National Council on Aging.
Referencing some key terms in the field (see Box 11), Chambers said the NCI refers to this work as “implementation science,” while the NIH-level activities have typically fallen under the heading of “dissemination and implementation research.”
BOX 11
Dissemination and Implementation Key Terms.
Implementation research contrasts with intervention research by shifting away from the typical question of “what do I provide to a patient” (e.g., empirically supported treatments or evidence-based practices) to an emphasis on “how do I get that intervention delivered within health systems,” emphasizing implementation outcomes like feasibility, acceptability and cost, and service outcomes (e.g., efficiency and safety). The ultimate aims of implementation efforts are to create positive health outcomes in patient satisfaction, overall function, and health status at a population level, by identifying strategies that will result in higher rates of program adoption and sustainability.
Chambers described a review of 61 dissemination and implementation models, suggesting that a substantial body of knowledge exists for dissemination and implementation science (Tabak et al., 2012). The various models had different perspectives; some focus on change at the individual level, while others examine change at the organizational or broader system level. Chambers provided examples of two key dissemination and implementation models. One of these most cited models is Everett Rogers's diffusion of innovations that illustrates how and whether innovations get diffused into the broader marketplace (Krein et al., 2006) (see Figure 7). According to this model, diffusion is dependent on the characteristics of the intervention; for example, does it have a comparative advantage over current practice and is it simple or complex? The model also highlights the importance of the characteristics of local organizations and the broader context, such as the importance of care financing.
Another model, the Consolidated Framework for Implementation Research (CFIR) from Damschroder et al. (2009), reflects an effort to synthesize a range of different models. Key components of the CFIR model take into account the individual, the local environment, and the broader policy environment. In this model, successful interventions are adaptable so that they may more likely fit with the cultural, organizational, and financing context of the local setting, according to Chambers.
Chambers also described funding opportunities for dissemination and implementation research, including trans-NIH announcements that address cancer and other health conditions. He added that more than 150 grants have been funded since 2006. In 2010, the Center for Scientific Review48 assembled a standing committee to focus on dissemination and implementation research and bring that level of expertise into the grant review process; this committee provides peer review for the majority of studies coming through NIH.
Chambers described some priority areas for research:
- Studies of the local adaptation of evidence-based practices in the context of implementation;
- Longitudinal and follow-up studies on the factors that contribute to the sustainability of evidence-based interventions;
- Scaling up health care interventions across health plans, systems, and networks; and
- De-implementation of ineffective or suboptimal care.
Pointing to the NCI's interest in building the capacity of dissemination and implementation investigators, Chambers described an NCI-funded R25 research education grant that supports a 2-year training program with a focus on dissemination and implementation research in cancer, including survivorship. In addition, an annual training institute is held in concert with the annual Conference on the Science of Dissemination and Implementation in Health,49 and the NIH Center for Global Health50 also provides training opportunities in implementation science.
Chambers stressed the importance of considering dissemination and implementation as early in the developmental process as possible. Consideration should be given early to who is ultimately going to deliver the intervention, where within the system the intervention will be delivered, and which community could benefit most from the intervention.
An NCI-piloted program called SPeeding Research-tested INTerventions (SPRINT)51 encourages those who are developing interventions to think about the broader marketplace for their intervention, or simply, what happens after their trial is completed. When asked to consider implementation issues early in their design of clinical trials, some investigators have found some key mismatches between their hypotheses regarding implementation and the reality of how the intervention was ultimately used. Chambers also described several NCI-funded projects52 under way that are related to cancer survivorship in the areas of care coordination, delivery systems, and communication. The NCI maintains an inventory of evidence-based practices through its Research-Tested Intervention Programs website,53 including some related to survivorship and supportive care.
Developing a Novel Survivorship Program
Rebekkah Schear, director of mission delivery at the LIVESTRONG Foundation,54 said the Foundation is in the process of co-creating a new model of care with patients and community members building on the work from the LIVESTRONG Survivorship Centers of Excellence Network and patient-centered research. The Foundation's partner is the new Dell Medical School at The University of Texas at Austin. It is based on human-centered design plus evidence-based practices with a focus on innovation and the values of patients. Schear said that comprehensive patient and caregiver support will be offered through a model of care with a broad-based team, technology, telemedicine, PROs in real time (using wearable devices to track PROs), early coordinated care with primary care clinicians, palliative care and symptom management from the moment of diagnosis, and a focus on reducing financial toxicity. Caregivers will receive their own care plan.
Because the LIVESTRONG program is under development, Schear asked the panel what they might recommend in designing a “blue-sky, dream” program. O'Rourke suggested having sufficient capacity to allow for easy access to behavioral and mental health services and having those services integrated throughout the care system, including primary care, specialty care in the hospitals, and palliative care. Burton suggested that medical and nursing schools offer survivorship education to raise awareness and build the workforce. Building relationships and partnerships with community groups are also necessary, he said.
Franco mentioned a few integrative programs that have been well received at their survivorship program, including restorative yoga, nutrition and “cancer fighting” cooking classes, and demonstrations with video capability to reach outlying areas. Gaines discussed the need to engage patients fully and cited the Health Canada policy toolkit that describes a patient engagement ladder with engagement from the ground up and reaching the highest levels of planning and design (Health Canada, 2000). Chambers mentioned the benefit of having systems in place early to monitor outcomes and learn from experiences so that programs can be refined, strengthened, and improved.
POLICY OPTIONS TO IMPROVE CANCER SURVIVORSHIP CARE
Workshop participants discussed existing policy initiatives in the public and private sectors. Many suggested areas where new policy development is needed to address current gaps in survivorship care and service delivery to improve care for cancer survivors.
Federal Policy
Ronald Kline, medical officer in the CMS Center for Medicare & Medicaid Innovation (CMMI), described the implementation of OCM. CMMI is developing several new payment and delivery models, including OCM, which are designed to improve the effectiveness and efficiency of specialty care.55 Under OCM, physician practices have entered into payment arrangements that include financial and performance accountability for episodes of care surrounding chemotherapy administration to cancer patients. The model is being tested by 192 U.S. oncology practices that serve roughly 150,000 Medicare beneficiaries annually.56 These practices represent about 20 percent of the fee-for-service oncology care covered by Medicare. The practices participating in OCM have committed to providing enhanced services to Medicare beneficiaries such as care coordination, navigation, and national treatment guidelines for care. Practices within OCM are required to use the 13-point care plan that incorporates the 2013 IOM recommendations (IOM, 2013b). The 13-point care plan, which he said EHR vendors have been integrating into their systems, includes a survivorship plan and information on prognosis, advance care directives, and out-of-pocket costs (to address some of the financial toxicity issues). Under OCM, clinicians are also required to administer depression screening at every visit, and he said some oncologists have told him that they had not realized how depressed their patients were until they started asking about it at each visit.
R. Adams Dudley, professor of medicine and health policy and director of the Center for Healthcare Value at the University of California, San Francisco, recommended that the National Academies of Sciences, Engineering, and Medicine host a workshop focused on payment issues. In his experience, payment systems do not address many of the needs of patients. He suggested asking patients to define what should be measured and rewarded, and asking oncologists who are not providing survivorship care plans to explain why they are not, and then what would compel them to adopt the practice. Payment experts could then build a payment system that would work for both patients and providers. Dudley observed that the reason many of the needs of survivors are not being met can be traced to payment policy and incentive systems (e.g., public reporting, accreditation programs). Ganz agreed and added that our health care payment system needs to change and that “we need to think about very good payment mechanisms to get the services that we need.”
Kline observed that a large body of evidence has accumulated on effective interventions to improve survivorship care; however, many have not been widely adopted. Kline cited writer Johann Wolfgang von Goethe: “Knowing is not enough; we must apply. Willing is not enough; we must do.”
Standards for Physician Care
Richard Baron, president and chief executive officer of the American Board of Internal Medicine (ABIM) and the ABIM Foundation, said there are 13,251 oncologists board-certified by ABIM, and nearly 60 percent of those oncologists were trained before the From Cancer Patient to Cancer Survivor report was released in 2006 (IOM and NRC, 2006). Baron emphasized the importance of training and educational opportunities for clinicians, including actions to reach clinicians already in practice to keep them updated on the ever-expanding body of science and evidence.
Baron described an ABIM Foundation initiative, Choosing Wisely®, which is intended to spur conversation about what comprises appropriate and necessary treatment, and to curb common practices that are not supported by evidence. Choosing Wisely® promotes patient–clinician conversations to help patients avoid unnecessary medical tests and procedures.57 Baron said there are two recommendations from ASCO that are relevant to breast cancer survivorship among the various practice recommendations included in Choosing Wisely®. One of these recommendations advises against performing surveillance testing (biomarkers) or imaging (PET, CT, and radionuclide bone scans) for asymptomatic individuals who have been treated for breast cancer with curative intent; and the other advises against performing PET, CT, and radionuclide bone scans in the staging of early breast cancer at low risk for metastasis.
Baron also mentioned ASCO's QOPI®, which provides quality assessment tools and quality measures for oncology practices to assess care and improve patient outcomes.58 For example, one QOPI® measure states that infertility risks should be discussed with patients of reproductive age prior to chemotherapy, said Baron.
Baron discussed the concept of moving away from lifetime certification, where physicians get certified once in their career, toward a system that assures competence on an ongoing basis, beyond what is now required as part of continuing medical education. To address this issue, ABIM is working closely with ASCO to develop maintenance of certification assessments.59 Baron added that both organizations acknowledge that in the rapidly evolving field of oncology, a flexible credentialing process is needed to make sure that clinicians keep up with the current knowledge base. He added that ABIM is moving toward more frequent assessments that can be integrated into practice. But Baron acknowledged that the maintenance of certification program is controversial, as demonstrated by pending legislation in 25 states, advanced by state medical societies, to make it illegal for hospitals or medical groups to use the certification or maintenance of certification credential in hiring, credentialing, privileging, or payment. In Baron's view, this represents a strong backlash against accountability.
Baron said that in developing the maintenance of certification exam for medical oncology, all U.S. board-certified oncologists were asked to identify important content.60 The current blueprint for the exam includes content on fertility, primary and secondary cancers, secondary cancer prevention, non-malignant sequelae, and surveillance, but he noted that survivorship issues represent less than 2 percent of the total blueprint because of the broad range of subjects in which oncologists are expected to be knowledgeable. In closing, Baron said it is important “to maintain an oversight regime of professional self-regulation to help oncologists stay current in their field, and to represent to their patients and their colleagues that they have done so.”
Nekhlyudov asked Baron whether modules could be developed that focus on survivorship issues such as surveillance and late effects, or whether a cancer survivorship subspecialty might be considered. Baron replied that any conversation about the possibility of a survivorship subspecialty would most likely begin within ASCO and the oncology community; the role of ABIM in recognizing this level of expertise would be by making a credential available, should this be identified as a need by the oncology community. Baron also said that although there is value in creating a discipline that focuses on training and research, it is probably more beneficial to expect a set of core skills across the wide spectrum of clinicians caring for cancer survivors. Nekhlyudov pointed out that there is a specialty of palliative care, but it is also important to have palliative care skills across nearly all physician groups.
Nursing Care Training and Scope of Practice
Susan Schneider, associate professor at the Duke University School of Nursing and ONS president, discussed issues in nursing education and the role of nurses in survivorship care. She said there is a general shortage of nurses, including advanced practice nurses, adding that about half of the nurses currently working are over the age of 50 and nearing retirement (AACN, 2017). Schneider reported that the ability to train additional nurses is hampered by a national shortage of nursing faculty and clinical training sites; U.S. nursing schools turned away 64,067 qualified applicants for nurses in 2016 (AACN, 2017). She pointed out that there is scant coverage of survivorship in the curricula of undergraduate nursing programs and that creative models are needed to integrate such content into nursing training. In addition, Schneider indicated that advanced practice nurses are often prepared as generalists in primary care or geriatric care and are not usually providing specialty care. If they are to have a role in survivorship care, she said that educational programs will have to be developed, perhaps a post-master's certificate or other specialty education program. Internet-based modules could possibly be embedded into existing coursework.
Schneider said that care provided to cancer survivors by advanced practice nurses is associated with improvements in patient satisfaction and compliance (Nevidjon et al., 2010), and from an administrative perspective, such care is cost-effective because it is associated with fewer hospital and emergency department admissions and decreased lengths of stay (Donald et al., 2015). In light of these findings, Schneider said that nurses need to be able to practice to the full extent of their training and license. Some laws limit the role of nurses by defining the scope of practice. In addition, some reimbursement policies restrict payment to physicians. Schneider said that reimbursement models should support the role of advanced practice nurses in survivorship care. For example, in North Carolina, a bill is being considered to give nurse practitioners the authority to sign for handicap parking permits.61 The law currently restricts this practice to physicians.
Schneider said the traditional sources of support for nursing education are also being eroded. For example, she said there is a proposed 8 percent cut from 2017 in Title 8,62 which is administered by the Health Resources and Services Administration and provides nursing education grants, nursing workforce diversity grants, the Nurse Corps Loan Repayment and Scholarship Program, and the Nurse Faculty Loan Program. Schneider recommended advocating for more Title 8 funding.
Schneider added that nurses are actively engaged and play a critical role in conducting symptom management research. Schneider noted that a large part of survivorship care is related to symptom management. She called for additional research on both interventions to reduce these symptoms, and also on how symptom management care is delivered.
Jean Rosiak, ONS director-at-large and nurse practitioner at Aurora Medical Group, said a solution to many of the challenges of survivorship care could be met if the nursing scope of practice was standardized and nursing educational programs were expanded to include survivorship training.
Fertility Issues in Adolescent and Young Adult Cancer Survivorship
Brandon Hayes-Lattin, associate professor of medicine in the division of hematology and medical oncology at Oregon Health & Science University and medical director of the AYA Oncology Program at the university's Knight Cancer Institute, discussed policies affecting fertility, one of the defining issues in cancer care for AYA. A previous workshop summary from the National Cancer Policy Forum reviewed the many unique needs of AYA with cancer.63
Hayes-Lattin said AYA survivors have been defined as those diagnosed between ages 15 and 39. Their risk of infertility is associated with treatment exposure, as well as the underlying cancer. In discussing data collection opportunities, Hayes-Lattin cited the Stem Cell Therapeutic and Research Act of 2005,64 which created a nationwide transplant outcomes registry that requires participation from all transplant centers. This registry can help ascertain the number of young adults with cancer or patients at risk of infertility and can provide evidence on how certain treatment exposures relate to pregnancy outcomes, according to Hayes-Lattin. Those performing bone marrow transplants currently report 100-day survival outcomes, and soon will be reporting 3-year outcomes to the registry. He noted that this change has provided resources for the program, as well as a renewed focus on long-term follow-up care.
In AYA survivorship, Hayes-Lattin said there are opportunities for interdisciplinary education and training, including certifications and recognition for special training in AYA fertility, which is available in both pediatric and adult medical oncology. Some physicians specialize in oncofertility, and are trained in both oncology and reproductive endocrinology. For bone marrow transplant specialists, there is no formal certification; however, most programs have specialized training.
Hayes-Lattin also gave examples that illustrate the importance of working toward legislative and executive solutions to overcome fertility-related challenges in AYA survivorship care. In 2017, Connecticut passed a bill that amended the requirement stipulating that insurance companies provide infertility services as part of their insurance package.65 In addition to requiring certain coverage for individuals who had tried to achieve pregnancy for 1 year and were unable to become pregnant, language was added to clarify that the same services must be covered when they are “medically necessary.” This language extends coverage to patients who are diagnosed with cancer and who are not necessarily infertile at the time of diagnosis, but who may become infertile following treatment. Hayes-Lattin also mentioned a U.S. House of Representatives bill, the Deferment for Active Cancer Treatment Act of 2017,66 that would require the deferment of interest and payments to federal student loans when someone is undergoing active cancer treatment. He viewed this bill as representing a creative solution to the potential for financial toxicity that clinicians and advocacy groups can support.
The Role of Advocacy Organizations
Laurie Isenberg, cancer survivor and board member at NCCS, noted the long-standing role of NCCS in advocating for policies in support of survivorship, such as the CoC's accreditation requirement for a survivorship care plan at the conclusion of active treatment and for adequate reimbursement for the creation of survivorship care plans.
She said NCCS has advocated for the Planning Actively for Cancer Treatment Act,67 which would establish a cancer care planning service in the Medicaid program. The service would be available to cancer patients in active treatment and beyond, into long-term survivorship. The care plan envisioned in this Act would facilitate shared decision making and encourage a multidisciplinary approach to treatment and symptom management. She said NCCS plans to pursue the introduction of the Act in 2018.
In 2017, NCCS advocated for affordable health insurance coverage for those with preexisting conditions. Isenberg said this is critical to those who are diagnosed with cancer. NCCS has coordinated with more than 30 advocacy and care professional groups to promote the concept that improving the quality of care for survivors begins with access to affordable care.
To support survivorship care policy, NCCS convenes an annual meeting with patient advocates to help them work on survivorship policy issues at both local and national levels, Isenberg said. Experts are invited to present on the key issues related to survivorship (e.g., health care financing and patient–provider communications). The advocates also learn how to effectively share their stories with their congressional representatives and others to promote the advancement of survivorship policies. Medical students who have expressed an interest in oncology care are also invited to the annual meetings to meet with advocates and share their stories about communication, with the hope that these conversations will foster better communication skills within the new cohort of physicians.
Isenberg described working with the University of California, San Francisco, Medical Center, which lacked an integrated survivorship program and turned to her to review existing programs at other cancer centers and offer advice. Through this review, Isenberg said she learned that all of the successful survivorship programs in large institutions had a major source of funding to jump start their efforts. The successful programs also had a philosophy and culture that recognized survivorship as a critical part of long-term patient care. She observed that successful programs had at least one passionate champion who listened to patients and families as they developed services and incorporated research into the program. Patience was a key feature of program leaders, said Isenberg; many of those interviewed said, “Start with what you have.”
Rebecca Kirch, executive vice president of health care quality and value for the National Patient Advocate Foundation, identified several “policy levers,” including professional education and training, advocacy training for patients and caregivers, public awareness campaigns, and professional and institutional performance and accreditation standards.
Kirch said her organization focuses policy activity on advancing three strategies for achieving value-based and person-centered care. First is assessing people's needs by asking what is important, what is valued, and then addressing these needs. She identified psychosocial support, rehabilitation care, and palliative care as essential aspects of survivorship care. She advocated for assessing the need for these supportive services alongside disease-directed treatment. Attending to the needs of caregivers and the extended family is also key to survivorship care, said Kirch.
The second strategy is incorporating meaningful survivor-reported and caregiver-reported outcomes into quality measures used in value assessments. Kirch said input from survivors about what matters to them in terms of important outcomes is needed early. There is also a need to go beyond the traditional standard measures, such as disease-free progression.
The third strategy is developing positive clinical team–patient/family relationships. The foundation of such relationships is a focus on person-centered care, said Kirch. She added that effective communication is a learned skill and needs to be incorporated into education and training programs and reinforced through quality measures.
Program Accreditation Standards
Lawrence Shulman, director of the Center for Global Cancer Medicine and deputy director for clinical services of the Abramson Cancer Center at the University of Pennsylvania, is also the chair of the CoC, an organization that accredits hospital cancer programs. He described the CoC as an amalgam of 57 organizations, including medical professional organizations and patient advocate groups. In 2012, the CoC examined its existing standards and based on the IOM's 2005 report From Cancer Patient to Cancer Survivor and the recommendation of medical professionals and patient advocacy groups, developed a standard for the provision of survivorship care plans beginning in 2015 (see Box 12). At the time, few of the hospitals accredited by the CoC had survivorship programs.
BOX 12
Commission on Cancer Standard 3.3.
The CoC's standard was intended to stimulate the creation of survivorship programs and promote the documentation of care. To ease the implementation of the standard, the CoC initially targeted a subset of patients—those treated for potentially curable diseases—and then phased in the standard over a 4-year period.
Shulman reported that there were 1,500 accredited hospital oncology programs as of 2016, which provided care for approximately 70 percent of U.S. cancer patients. Programs are accredited for 3 years and every year about 500 programs are accredited. The 500 programs accredited in 2016 were surveyed and asked to report on their experience with survivorship care plans in 2015, when the requirement was for 10 percent of the patients to receive a survivorship care plan. There were 399,859 patients eligible to have received a care plan, and of these 79,120 (approximately 20 percent) had received one. When projected nationally, an estimated 237,000 patients seen in the 1,500 accredited programs received a care plan. This level of compliance to the standard was viewed as less than optimal, but “not a bad start,” said Shulman. He noted that some programs have been able to integrate survivorship care plans into their EHRs; however, some of these successes have not been shared with others because people are “working in silos.”
A number of forums have been held at the CoC to reflect on these results and discuss whether the care planning standard is a valuable measure of quality survivorship care. Shulman noted that the standard had put survivorship care “on the radar” of the CoC's 1,500 hospital cancer programs. However, questions have been raised regarding the feasibility of implementing the standard. Creating the plans is time intensive and technically challenging, and there are concerns that the focus should be “on the care and not on the plan.” Shulman acknowledged that the care plans are an important way to communicate with the patient and the family and, importantly, with the patient's primary care and other providers.
In Shulman's view, the experience with the survivorship care plan recommendation has been mixed. In some programs, the production of the care plan is accompanied by effective communication and therefore a good measure of the survivorship program. There are other programs, however, that generate a “piece of paper and give it to a patient and feel like they have done their work,” he said. Shulman mentioned that some have recommended that the CoC eliminate the numerical targets and focus on the components of a hospital's survivorship program. Others have said that the numerical targets push programs to more seriously invest in survivorship. He observed that it is difficult for a surveyor to go into a hospital for a day or two and evaluate every aspect of the cancer program. Shulman also acknowledged the lack of evidence on whether and how survivorship care plans improve care, and he said the CoC would decide whether or not to maintain the survivorship care planning standard in the fall of 2017.68 He added that the input from the National Cancer Policy Forum's workshop would be invaluable, and other input is welcomed.
The Biden Cancer Initiative
Gregory Simon, president of the Biden Cancer Initiative, described his personal experience with cancer and the lack of attention to survivorship issues following his treatment for chronic lymphocytic leukemia. Simon emphasized the importance of identifying individuals who can change the assumptions about what it means to take care of people with cancer. A cancer center can spend hundreds of millions of dollars to get certified as a center of excellence, and yet many do not have smoking prevention or survivorship programs. He observed that most cancer care occurs in a very distressing environment, a hospital or a clinician's office, so it is not surprising that patients have trouble recalling the content of their visit. Simon suggested that is not a lack of money that is getting in the way of innovation, but rather a lack of will to change the present caregiving culture.
Simon said the Biden Cancer Initiative is exploring ways to change the expectations and assumptions that prevent survivors from getting the same intensity of care that they experience during their initial treatment. He added that survivors need to know how to take care of themselves and recognize the early signs of recurrence or late effects of treatment. Cancer survivors need to be treated holistically, said Simon, with support from not only physicians, but also nurses, social workers, financial navigators, and others. Simon said he did not benefit from a cancer survivorship program upon completion of his treatment, but received appropriate care though the advice of knowledgeable friends and by having a good health insurance plan. He suggested that there are likely thousands and maybe millions of individuals who have not been followed up with the same vigor because of their lack of resources.
Former Vice President Joe Biden has a sense of urgency and is committed to changing the cancer care culture, said Simon. “We have to change our expectations and the normal way of doing things because that is not working,” Simon said, and added, “we need to start with fighting for our insurance and then start fighting for our humanity.” He suggested that reimbursement for cancer care be tied to the existence of a survivorship care program.
ADVANCING PROGRESS IN CANCER SURVIVORSHIP CARE
Alfano outlined a blueprint for what she said is needed in the next decade in the context of five factors, which she described as a “perfect storm.” First, the number of survivors is increasing. There are now more than 15.5 million cancer survivors in the United States and approximately 1.7 million new cancer cases are expected to be diagnosed in 2017 (ACS, 2017). Second, she emphasized the morbidity associated with the chronic and late effects of cancer and its treatment. These include physical and psychosocial problems, functional impairments, and social concerns, which may affect a survivor's finances, work, education, and relationships. Third, Alfano said there are clinician shortages in oncology, primary care, and nursing. Fourth, she said there are knowledge deficits among clinicians regarding the late- and long-term effects of cancer and its treatment, especially in the primary care workforce. She added that oncologists also often lack the knowledge and skills necessary to address survivors' psychosocial and sexual health issues. The fifth factor Alfano mentioned is the skyrocketing cost of cancer care, as well as the medical costs associated with managing the comorbidities suffered by cancer survivors.
Risk Stratification
Alfano suggested the use of a risk-stratified follow-up strategy employed in the United Kingdom,69 where the number of clinicians, the kind of clinicians seen, and the intensity of the interventions vary according to the needs of the patient. She stressed that as people transition away from chemotherapy and radiotherapy into follow-up care, one size does not fit all. She discussed three levels of follow-up care (Nekhlyudov et al., 2017) (see Figure 8). One group, patients at very high risk for not only disease recurrence, but also for morbidity related to late effects, could have comorbid conditions managed by a multidisciplinary team of clinicians with oncology expertise. These patients could be followed within this oncology-focused model in perpetuity. At the other end of the spectrum, a survivor's primary care clinician could follow patients at low risk of disease recurrence, average risk of second cancers, and low risk of cancer morbidity. An intermediate risk group might have a shared care model between primary care and oncology, said Alfano. Mayer also advocated to have risk stratification models operationalized and evaluated. Until they are, Mayer suggested that risk stratification will not be widely implemented because clinicians will not have the confidence to effectively triage their patients.
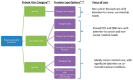
FIGURE 8
Survivorship care strategies using risk stratification. * Five years is based on general recommendations in the cancer community, transition of care may vary. ** Risk categories, as examples:
Data Analyses
Mayer said more data are needed to help triage patients into risk-based models of survivorship care, and she expressed hope that some of the analyses planned as part of the Cancer Moonshot70 initiative will help in this regard. Alfano described how “precision medicine lens” could help personalize care in a risk-stratification model. She suggested leveraging the diverse data that are available to understand the risk of late- and long-term effects, and the mechanisms underlying that risk. Data could include laboratory and genetic information, as well as the survivor's psychosocial history, health behaviors, living situation and environment, and material resources. She added that a business case could be made that a risk stratification approach to care improves patient functioning and quality of life and thus preserves a patient's ability to work or resume life roles; improves clinic flow and clinician availability; and reduces health care usage and cost.
System Changes
Alfano also proposed strategies to promote changes in the mindset about survivorship, in survivorship care delivery models, and in the language used to talk about cancer survivorship. To change the mindset about what survivorship is and when it starts, Alfano suggested that survivorship be conceptually defined as starting at diagnosis, with a focus on prevention, such as screening for and prompt management of potential toxicities, second cancers, and recurrences. Early intervention is essential because it can forestall impairment and disability related to cancer, Alfano explained. Furthermore, she said an expanded team of clinicians, far beyond oncology, is needed for this model of care delivery to succeed. Alfano suggested that algorithms could be developed to help oncologists refer patients to a multidisciplinary team of specialists (e.g., cancer rehabilitation, psychosocial care, and palliative care). Alfano said the primary care clinician, when available, should always be a part of the team and that engaging advanced practice clinicians and patient navigators could help expand the workforce. Online or virtual tools could also assist with surveillance and patient self-management.
WRAP-UP
In pursuing approaches and strategies for improving survivorship care going forward, Ganz spoke to the importance of patient-centered care, saying that “the patient is the center of all of our activities as we deliver cancer care and try to improve the cancer care system.” She encouraged the survivorship care community to focus efforts on all aspects of the cancer care trajectory (see Figure 1), and stressed that “these are things that we can actually do.” Specifically, Ganz suggested that the survivorship care community should consider the cancer journey as starting at the time of diagnosis and should use risk assessment and interventions at diagnosis to better understand a person's experiences and how treatments may affect his or her life as a survivor. She indicated there is a need for safer therapies and for giving the right therapy to the right person at the right time, and finally, Ganz stressed the need to deliver survivorship health care focusing on palliation, prevention, and health promotion.
Footnotes
- 1
The planning committee's role was limited to planning the workshop, and the Proceedings of a Workshop was prepared by the workshop rapporteurs as a factual summary of what occurred at the workshop. Statements, recommendations, and opinions expressed are those of individual presenters and participants, and are not necessarily endorsed or verified by the National Academies of Sciences, Engineering, and Medicine, and they should not be construed as reflecting any group consensus.
- 2
See http://www
.nationalacademies .org/hmd/Activities /Disease/NCPF/2017-July-24.aspx (accessed September 13, 2017). - 3
See https://www
.pcmh.ahrq .gov/sites/default/files /attachments/creating-patient-centered-team-based-primary-care-white-paper .pdf and https://www .pcmh.ahrq.gov (accessed September 29, 2017). - 4
See https://www
.cancer.org /treatment/survivorship-during-and-after-treatment /survivorship-care-plans .html (accessed February 9, 2018). - 5
See https:
//childrensoncologygroup .org/index .php/survivorshipguidelines (accessed August 12, 2017). - 6
See https://www
.nccn.org /professionals/physician_gls /f_guidelines.asp (accessed August 12, 2017). - 7
See https://www
.cancer.org /health-care-professionals /american-cancer-society-survivorship-guidelines .html (accessed August 12, 2017). - 8
See https://www
.asco.org /practice-guidelines /cancer-care-initiatives /prevention-survivorship /survivorship-compendium-0 (accessed August 12, 2007). - 9
See https://www
.cms.gov/Newsroom /MediaReleaseDatabase /Fact-sheets /2016-Factsheets-items/2016-06-29.html (accessed September 12, 2017). - 10
See https://smhs
.gwu.edu /gwci/survivorship/ncsrc/elearning (accessed August 12, 2017). - 11
- 12
Public Law Number 111-148.
- 13
Gray is “the international system unit of radiation dose expressed in terms of absorbed energy per unit mass of tissue. The gray is the unit of absorbed dose and has replaced the rad. 1 gray = 1 joule/kilogram and also equals 100 rad.” See http://hps
.org/publicinformation /radterms/radfact79.html (accessed March 15, 2018). - 14
See http://clincalc
.com/cardiology /ascvd/pooledcohort.aspx (accessed March 15, 2018). - 15
See http://www
.capo.ca/pdf/CRF_Guideline.pdf (accessed September 22, 2017). - 16
See https://www
.nccn.org /professionals/physician_gls /f_guidelines.asp (accessed August 23, 2017). - 17
See https://link
.springer .com/article/10.1007 %2Fs00520-013-1823-6 (accessed February 9, 2018). - 18
For example, see http://www
.nytimes.com /2007/04/29/health/29chemo .html?mcubz=0 (accessed August 23, 2017). - 19
- 20
See http://www
.aicr.org (accessed August 23, 2017). - 21
See http://www
.livestrong.org (accessed August 23, 2017). - 22
See https:
//survivorshine.org (accessed August 23, 2017). - 23
- 24
As of the time of publication, Julia Rowland is the senior strategic advisor at the Smith Center for Healing and the Arts.
- 25
See https://ccss
.stjude.org (accessed August 29, 2017). - 26
See http://www
.aarp.org/ppi /info-2017/home-alone-alliance.html (accessed October 2, 2017). - 27
See http://www
.cancer.net/sites/cancer .net/files /asco_answers_guide_caregiving.pdf (accessed August 28, 2017). - 28
See https://www
.ncbi.nlm .nih.gov/pmc/articles/PMC3035926 (accessed October 3, 2017). - 29
See http://www
.aarp.org/content /dam/aarp/ppi /2017/08/from-home-alone-to-the-care-act.pdf (accessed October 2, 2017). - 30
See https://www
.healthypeople .gov/2020/topics-objectives /topic/cancer (accessed October 3, 2017). - 31
See http://www
.mayo.edu/research /clinical-trials /cls-20306498#overview (accessed March 11, 2018). - 32
See https://www
.thinkculturalhealth .HHS.gov/assets /pdfs/EnhancedNationalCLASStandards.pdf (accessed October 3, 2017). - 33
See https://www
.dol.gov/whd/fmla (accessed February 16, 2018). - 34
See https://www
.ada.gov (accessed February 16, 2018). - 35
Total Worker Health “is defined as policies, programs, and practices that integrate protection from work-related safety and health hazards with promotion of injury and illness prevention efforts to advance worker well-being.” See https://www
.cdc.gov/niosh /twh/totalhealth.html (accessed October 3, 2017). - 36
At the time of publication, Robin Yabroff is the strategic director of economic burden of cancer at the American Cancer Society.
- 37
The National Health Interview Survey is a nationally representative household survey.
- 38
See www
.dana-farber.org/Research /Departments-and-Centers /Cancer-Care-Equity-Program .aspx (accessed August 31, 2017). - 39
See www
.cancer.gov/about-cancer /managing-care /track-care-costs/financial-toxicity-pdq (accessed August 31, 2017). - 40
See https://www
.eeoc.gov/laws/statutes/gina .cfm (accessed February 16, 2018). - 41
See http://www
.hcahpsonline.org (accessed February 16, 2018). - 42
See https://www
.hipxchange .org/PatientEngagement (accessed September 1, 2017). - 43
See https://www
.sccancer.org (accessed September 5, 2017). - 44
See https://www
.cancersupportcommunity.org (accessed September 1, 2017). - 45
See www
.springfieldmed.com /specialty/primary-care-cancer-survivorship-program (accessed September 1, 2017). - 46
See www
.survivorjourneys.org (accessed September 8, 2017). - 47
See https://smhs
.gwu.edu /gwci/survivorship/ncsrc/elearning (accessed September 1, 2017). - 48
See https://public
.csr.nih .gov/pages/default.aspx (accessed February 16, 2018). - 49
See http://www
.academyhealth .org/events/site /10th-annual-conference-science-dissemination-and-implementation-health (accessed September 1, 2017). - 50
See https://www
.cancer.gov /about-nci/organization/cgh (accessed February 16, 2018). - 51
See http://www
.nci-sprint.com (accessed September 1, 2017). - 52
See https:
//cancercontrol .cancer.gov/is/funding.html (accessed September 1, 2017). - 53
See https://rtips
.cancer.gov/rtips/index.do (accessed March 16, 2018). - 54
At the time of publication, Rebekkah Schear is the associate director of patient experience at the LIVESTRONG Cancer Institutes at the Dell Medical School of The University of Texas at Austin.
- 55
See https://innovation
.cms .gov/initiatives/oncology-care (accessed September 8, 2017). - 56
Ibid.
- 57
See http://www
.choosingwisely.org (accessed September 8, 2017). - 58
See www
.instituteforquality .org/qopi/measures (accessed September 8, 2017). - 59
See www
.asco.org/about-asco /press-center/news-releases /internal-medicine-organizations-explore-new-options-physicians (accessed February 16, 2018). - 60
See www
.abim.org/~/media /ABIM%20Public/Files /pdf/exam-blueprints /maintenance-of-certification /medical-oncology.pdf (accessed February 16, 2018). - 61
The bill was signed by North Carolina Governor Roy Cooper on July 12, 2017. See www
.ncleg.net/gascripts /BillLookUp/BillLookUp .pl?Session=2017&BillID =S160 (accessed February 7, 2018). - 62
See www
.aamc.org/advocacy /washhigh/highlights2017 /481490/072117houseappropriationscommitteeapprovesfundingcutsforhealthwo .html (accessed February 7, 2018). - 63
See www
.nap.edu/catalog/18547 (accessed April 2, 2018). - 64
See www
.congress.gov/bill /109th-congress/house-bill/2520 (accessed February 7, 2018). - 65
See www
.cga.ct.gov/2017/act /pa/2017PA-00055-R00HB-07124-PA.htm (accessed February 7, 2018). - 66
See www
.congress.gov/bill /115th-congress/house-bill /2976/text?format=txt (accessed September 26, 2017). - 67
See www
.congress.gov/bill /114th-congress/house-bill/2846 (accessed September 26, 2017). - 68
As of December 11, 2017, “the percentage of delivered survivorship care plans to eligible patients required for Commission on Cancer (CoC) compliance with Standard 3.3 has been lowered to 50 percent for 2018. All CoC-accredited programs will be expected to meet or exceed the delivery of survivorship care plans to 50 percent of eligible patients by the end of 2018.” See www
.facs.org/quality-programs /cancer/news/survivorship (accessed February 16, 2018). - 69
See www
.england.nhs.uk/wp-content /uploads/2016 /04/stratified-pathways-update.pdf (accessed September 29, 2017). - 70
See www
.cancer.gov/research /key-initiatives/moonshot-cancer-initiative (accessed August 23, 2017).
REFERENCES
- AACN (American Association of Colleges of Nursing). Fact sheet: Nursing shortage. 2017. [February 16, 2018]. http://www
.aacnnursing .org/Portals/42/News /Factsheets/Nursing-ShortageFactsheet-2017.pdf. - ACS (American Cancer Society). Cancer facts & figures 2017. 2017. [September 29, 2017]. https://www
.cancer.org /content/dam/cancer-org /research/cancer-facts-and-statistics /annual-cancer-facts-and-figures /2017/cancer-facts-and-figures-2017.pdf. - Adams E, Boulton M, Watson E. The information needs of partners and family members of cancer patients: A systematic literature review. Patient Education and Counseling. 2009;77(2):179–186. [PubMed: 19406609]
- Adams RN, Mosher CE, Blair CK, Snyder DC, Sloane R, Demark-Wahnefried W. Cancer survivors' uptake and adherence in diet and exercise intervention trials: An integrative data analysis. Cancer. 2015;121(1):77–83. [PMC free article: PMC4270838] [PubMed: 25155573]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psycho-Oncology. 2003;12(6):612–619. [PubMed: 12923801]
- Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, Mulrooney TJ, Schwartz GN, Kaufman PA. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: Impact of age and cognitive reserve. Journal of Clinical Oncology. 2010;28(29):4434–4440. [PMC free article: PMC2988635] [PubMed: 20837957]
- Albini A, DeCensi A, Cavalli F, Costa A. Cancer prevention and interception: A new era for chemopreventive approaches. Clinical Cancer Research. 2016;22(17):4322. [PubMed: 27220959]
- Aleman BMP, van den Belt-Dusebout AW, De Bruin ML, van't Veer MB, Baaijens MHA, de Boer JP, Hart AAM, Klokman WJ, Kuenen MA, Ouwens GM, Bartelink H, van Leeuwen FE. Late cardiotoxicity after treatment for Hodgkin lymphoma. Blood. 2007;109(5):1878. [PubMed: 17119114]
- Alfano CM, Rowland JH. Recovery issues in cancer survivorship: A new challenge for supportive care. The Cancer Journal. 2006;12(5):432–443. [PubMed: 17034679]
- Altice CK, Banegas MP, Tucker-Seeley RD, Yabroff KR. Financial hardships experienced by cancer survivors: A systematic review. Journal of the National Cancer Institute. 2017;109(2):djw205. [PMC free article: PMC6075571] [PubMed: 27754926]
- Anampa J, Makower D, Sparano JA. Progress in adjuvant chemotherapy for breast cancer: An overview. BMC Medicine. 2015;13(1):195. [PMC free article: PMC4538915] [PubMed: 26278220]
- Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26(3):342–392. [PubMed: 12749557]
- Andersen BL. Psychological interventions for cancer patients to enhance the quality of life. Journal of Consulting and Clinical Psychology. 1992;60(4):552–568. [PMC free article: PMC2743106] [PubMed: 1506503]
- Andersen BL, Yang H-C, Farrar WB, Golden-Kreutz DM, Emery CF, Thornton LM, Young DC, Carson WE. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113(12):3450–3458. [PMC free article: PMC2661422] [PubMed: 19016270]
- Andersen BL, DeRubeis RJ, Berman BS, Gruman J, Champion VL, Massie MJ, Holland JC, Partridge AH, Bak K, Somerfield MR, Rowland JH. Screening, assessment, and care of anxiety and depressive symptoms in adults with cancer: An American Society of Clinical Oncology guideline adaptation. Journal of Clinical Oncology. 2014;32(15):1605–1619. [PMC free article: PMC4090422] [PubMed: 24733793]
- Anderson AS, Caswell S, Wells M, Steele RJC. Obesity and lifestyle advice in colorectal cancer survivors—how well are clinicians prepared? Colorectal Disease. 2013;15(8):949–957. [PubMed: 23480570]
- Andrykowski MA, Schmidt JE, Salsman JM, Beacham AO, Jacobsen PB. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. Journal of Clinical Oncology. 2005;23(27):6613–6622. [PMC free article: PMC2562283] [PubMed: 16170168]
- Andrykowski MA, Donovan KA, Laronga C, Jacobsen PB. Prevalence, predictors, and characteristics of off-treatment fatigue in breast cancer survivors. Cancer. 2010;116(24):5740–5748. [PMC free article: PMC4048855] [PubMed: 20734399]
- Armenian SH, Lacchetti C, Barac A, Carver J, Constine LS, Denduluri N, Dent S, Douglas PS, Durand J-B, Ewer M, Fabian C, Hudson M, Jessup M, Jones LW, Ky B, Mayer EL, Moslehi J, Oeffinger K, Ray K, Ruddy K, Lenihan D. Prevention and monitoring of cardiac dysfunction in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. Journal of Clinical Oncology. 2016;35(8):893–911. [PubMed: 27918725]
- Bach PB. Limits on Medicare's ability to control rising spending on cancer drugs. New England Journal of Medicine. 2009;360(6):626–633. [PubMed: 19176475]
- Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Galeone C, Bellocco R, Negri E, Corrao G, Boffetta P, La Vecchia C. Alcohol consumption and site-specific cancer risk: A comprehensive dose–response meta-analysis. British Journal of Cancer. 2015;112(3):580–593. [PMC free article: PMC4453639] [PubMed: 25422909]
- Balas EA, Boren SA. Managing clinical knowledge for health care improvement. IMIA Yearbook. 2000:65–70. [PubMed: 27699347]
- Banegas MP, Guy GP, de Moor JS, Ekwueme DU, Virgo KS, Kent EE, Nutt S, Zheng Z, Rechis R, Yabroff KR. For working-age cancer survivors, medical debt and bankruptcy create financial hardships. Health Affairs. 2016;35(1):54–61. [PMC free article: PMC6057727] [PubMed: 26733701]
- Banks E, Byles JE, Gibson RE, Rodgers B, Latz IK, Robinson IA, Williamson AB, Jorm LR. Is psychological distress in people living with cancer related to the fact of diagnosis, current treatment or level of disability? Findings from a large Australian study. The Medical Journal of Australia. 2010;193(5):62–67. [PubMed: 21542449]
- Barnes AJ, Robert N, Bradley CJ. Job attributes, job satisfaction and the return to health after breast cancer diagnosis and treatment. Psycho-Oncology. 2014;23(2):158–164. [PMC free article: PMC4573637] [PubMed: 24000141]
- Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, Rogak L, Bennett AV, Dueck AC, Atkinson TM, Chou JF, Dulko D, Sit L, Barz A, Novotny P, Fruscione M, Sloan JA, Schrag D. Symptom monitoring with patient-reported outcomes during routine cancer treatment: A randomized controlled trial. Journal of Clinical Oncology. 2016;34(6):557–565. [PMC free article: PMC4872028] [PubMed: 26644527]
- Baxi SS, Pinheiro LC, Patil SM, Pfister DG, Oeffinger KC, Elkin EB. Causes of death in long-term survivors of head and neck cancer. Cancer. 2014;120(10):1507–1513. [PMC free article: PMC4101810] [PubMed: 24863390]
- Beard CJ, Travis LB, Chen M-H, Arvold ND, Nguyen PL, Martin NE, Kuban DA, Ng AK, Hoffman KE. Outcomes in Stage I testicular seminoma: A population-based study of 9193 patients. Cancer. 2013;119(15):2771–2777. [PubMed: 23633409]
- Beckjord EB, Arora NK, McLaughlin W, Oakley-Girvan I, Hamilton AS, Hesse BW. Health-related information needs in a large and diverse sample of adult cancer survivors: Implications for cancer care. Journal of Cancer Survivorship. 2008;2(3):179–189. [PubMed: 18792791]
- Beckmann K, Strassnick K, Abell L, Hermann J, Oakley B. Is a chronic disease self-management program beneficial to people affected by cancer? Australian Journal of Primary Health. 2007;13(1):36–44.
- Bellizzi KM, Rowland JH, Jeffery DD, McNeel T. Health behaviors of cancer survivors: Examining opportunities for cancer control intervention. Journal of Clinical Oncology. 2005;23(34):8884–8893. [PubMed: 16314649]
- Belza B, Toobert D, Glasgow R. Re-aim for program planning: Overview and applications. 2007. [September 28, 2017]. https:
//fromhungertohealth .files.wordpress .com/2013/02/re-aim_issue_brief.pdf. - Bevans MF, Mitchell SA, Barrett J, Bishop MR, Childs R, Fowler D, Krumlauf M, Prince P, Shelburne N, Wehrlen L, Yang L. Symptom distress predicts long-term health and well-being in allogeneic stem cell transplant survivors. Journal of the American Society for Blood and Marrow Transplantation. 2014;20(3):387–395. [PMC free article: PMC3962950] [PubMed: 24355521]
- Blinder V, Eberle C, Patil S, Gany FM, Bradley CJ. Women with breast cancer who work for accommodating employers more likely to retain jobs after treatment. Health Affairs. 2017;36(2):274–281. [PMC free article: PMC5559299] [PubMed: 28167716]
- Bouknight RR, Bradley CJ, Luo Z. Correlates of return to work for breast cancer survivors. Journal of Clinical Oncology. 2006;24(3):345–353. [PubMed: 16421415]
- Bower JE. Cancer-related fatigue: Mechanisms, risk factors, and treatments. Nature Reviews Clinical Oncology. 2014;11(10):597–609. [PMC free article: PMC4664449] [PubMed: 25113839]
- Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain, Behavior, and Immunity. 2013;30:S48–S57. [PMC free article: PMC3978020] [PubMed: 22776268]
- Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA, Schnipper HH, Lacchetti C, Ligibel JA, Lyman GH, Ogaily MS, Pirl WF, Jacobsen PB. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical Oncology clinical practice guideline adaptation. Journal of Clinical Oncology. 2014;32(17):1840–1850. [PMC free article: PMC4039870] [PubMed: 24733803]
- Bradley CJ, Dahman B. Time away from work: Employed husbands of women treated for breast cancer. Journal of Cancer Survivorship. 2013;7(2):227–236. [PMC free article: PMC3624054] [PubMed: 23400733]
- Bradley CJ, Neumark D, Bednarek HL, Schenk M. Short-term effects of breast cancer on labor market attachment: Results from a longitudinal study. Journal of Health Economics. 2005;24(1):137–160. [PubMed: 15617792]
- Bradley CJ, Neumark D, Luo Z, Bednarek HL. Employment-contingent health insurance, illness, and labor supply of women: Evidence from married women with breast cancer. Health Economics. 2007a;16(7):719–737. [PubMed: 17177273]
- Bradley CJ, Neumark D, Luo Z, Schenk M. Employment and cancer: Findings from a longitudinal study of breast and prostate cancer survivors. Cancer Investigation. 2007b;25(1):47–54. [PubMed: 17364557]
- Bray VJ, Dhillon HM, Vardy JL. Cancer-related cognitive impairment in adult cancer survivors: A review of the literature. Cancer Forum. 2017;41(1):46–54.
- Brennan ME, Gormally JF, Butow P, Boyle FM, Spillane AJ. Survivorship care plans in cancer: A systematic review of care plan outcomes. British Journal of Cancer. 2014;111(10):1899–1908. [PMC free article: PMC4229639] [PubMed: 25314068]
- Brinkman TM, Li C, Vannatta K, Marchak JG, Lai J-S, Prasad PK, Kimberg C, Vuotto S, Di C, Srivastava D, Robison LL, Armstrong GT, Krull KR. Behavioral, social, and emotional symptom comorbidities and profiles in adolescent survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2016;34(28):3417–3425. [PMC free article: PMC5035122] [PubMed: 27432919]
- Brothers BM, Yang H-C, Strunk DR, Andersen BL. Cancer patients with major depressive disorder: Testing a biobehavioral/cognitive behavior intervention. Journal of Consulting and Clinical Psychology. 2011;79(2):253–260. [PMC free article: PMC3752923] [PubMed: 21341891]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. [PubMed: 2748771]
- Carli F, Silver JK, Feldman LS, McKee A, Gilman S, Gillis C, Scheede-Bergdahl C, Gamsa A, Stout N, Hirsch B. Surgical prehabilitation in patients with cancer: State-of-the-science and recommendations for future research from a panel of subject matter experts. Physical Medicine and Rehabilitation Clinics of North America. 2017;28(1):49–64. [PubMed: 27913000]
- Chan L, Hart LG, Goodman DC. Geographic access to health care for rural Medicare beneficiaries. The Journal of Rural Health. 2006;22(2):140–146. [PubMed: 16606425]
- Chen J, Long JB, Hurria A, Owusu C, Steingart RM, Gross CP. Incidence of heart failure or cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. Journal of the American College of Cardiology. 2012;60(24):2504–2512. [PubMed: 23158536]
- Cheville AL, Troxel AB, Basford JR, Kornblith AB. Prevalence and treatment patterns of physical impairments in patients with metastatic breast cancer. Journal of Clinical Oncology. 2008;26(16):2621–2629. [PMC free article: PMC4524509] [PubMed: 18509174]
- Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nature Clinical Practice Oncology. 2008;5(8):466–475. [PubMed: 18493231]
- Chlebowski RT, Blackburn GL, Thomson CA, Nixon DW, Shapiro A, Hoy MK, Goodman MT, Giuliano AE, Karanja N, McAndrew P, Hudis C, Butler J, Merkel D, Kristal A, Caan B, Michaelson R, Vinciguerra V, Del Prete S, Winkler M, Hall R, Simon M, Winters BL, Elashoff RM. Dietary fat reduction and breast cancer outcome: Interim efficacy results from the Women's Intervention Nutrition Study. Journal of the National Cancer Institute. 2006;98(24):1767–1776. [PubMed: 17179478]
- Cleeland CS, Zhao F, Chang VT, Sloan JA, O'Mara AM, Gilman PB, Weiss M, Mendoza TR, Lee J-W, Fisch MJ. The symptom burden of cancer: Evidence for a core set of cancer-related and treatment-related symptoms from the Eastern Cooperative Oncology Group's Symptom Outcomes and Practice Patterns study. Cancer. 2013;119(24):4333–4340. [PMC free article: PMC3860266] [PubMed: 24114037]
- Cohen HJ, Smith D, Sun C-L, Tew W, Mohile SG, Owusu C, Klepin HD, Gross CP, Lichtman SM, Gajra A, Filo J, Katheria V, Hurria A. Frailty as determined by a comprehensive geriatric assessment-derived deficit-accumulation index in older patients with cancer who receive chemotherapy. Cancer. 2016;122(24):3865–3872. [PMC free article: PMC5138076] [PubMed: 27529755]
- Cole-Vadjic KK, Crews JR. Use of telemedicine to expand access to survivorship care. Journal of Clinical Oncology. 2016;34(3 Suppl):26.
- Commission on Cancer. Cancer program standards: Ensuring patient-centered care. 2016. [February 26, 2018]. https://www
.facs.org /~/media/files/quality%20programs /cancer /coc/2016%20coc%20standards %20manual_interactive%20pdf.ashx. - Coscarelli A, Recklitis C, Ahmed K. Long-term psychological well-being: Strategies for assessment and intervention. In: Feuerstein M, Ganz PA, editors. Health services for cancer survivors: Practice, policy and research. New York: Springer; 2011.
- D'Agostino NM, Edelstein K, Zhang N, Recklitis CJ, Brinkman TM, Srivastava D, Leisenring WM, Robison LL, Armstrong GT, Krull KR. Comorbid symptoms of emotional distress in adult survivors of childhood cancer. Cancer. 2016;122(20):3215–3224. [PMC free article: PMC5048494] [PubMed: 27391586]
- Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implementation Science. 2009;4(1):50. [PMC free article: PMC2736161] [PubMed: 19664226]
- Denlinger CS, Sanft T, Baker KS, Baxi S, Broderick G, Demark-Wahnefried W, Friedman DL, Goldman M, Hudson M, Khakpour N, King A, Koura D, Kvale E, Lally RM, Langbaum TS, Melisko M, Montoya JG, Mooney K, Moslehi JJ, O'Connor T, Overholser L, Paskett ED, Peppercorn J, Rodriguez MA, Ruddy KJ, Silverman P, Smith S, Syrjala KL, Tevaarwerk A, Urba SG, Wakabayashi MT, Zee P, Freedman-Cass DA, McMillian NR. Survivorship, version 2.2017, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network. 2017;15(9):1140–1163. [PMC free article: PMC5865602] [PubMed: 28874599]
- Donald F, Kilpatrick K, Reid K, Carter N, Bryant-Lukosius D, Martin-Misener R, Kaasalainen S, Harbman P, Marshall D, DiCenso A. Hospital to community transitional care by nurse practitioners: A systematic review of cost-effectiveness. International Journal of Nursing Studies. 2015;52(1):436–451. [PubMed: 25443307]
- Donin N, Filson C, Drakaki A, Tan H-J, Castillo A, Kwan L, Litwin M, Chamie K. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122(19):3075–3086. [PMC free article: PMC6192520] [PubMed: 27377470]
- Donovan KA, Jacobsen PB, Andrykowski MA, Winters EM, Balducci L, Malik U, Kenady D, McGrath P. Course of fatigue in women receiving chemotherapy and/or radiotherapy for early stage breast cancer. Journal of Pain and Symptom Management. 2004;28(4):373–380. [PMC free article: PMC2398710] [PubMed: 15471655]
- Donovan KA, Small BJ, Andrykowski MA, Munster P, Jacobsen PB. Utility of a cognitive–behavioral model to predict fatigue following breast cancer treatment. Health Psychology. 2007;26(4):464–472. [PMC free article: PMC2383279] [PubMed: 17605566]
- Donovan KA, McGinty HL, Jacobsen PB. A systematic review of research using the diagnostic criteria for cancer-related fatigue. Psycho-Oncology. 2013;22(4):737–744. [PubMed: 22544488]
- Dossett LA, Hudson JN, Morris AM, Lee MC, Roetzheim RG, Fetters MD, Quinn GP. The primary care provider (PCP)–cancer specialist relationship: A systematic review and mixed-methods meta-synthesis. CA: A Cancer Journal for Clinicians. 2017;67(2):156–169. [PMC free article: PMC5342924] [PubMed: 27727446]
- Duijts SFA, van Egmond MP, Spelten E, van Muijen P, Anema JR, van der Beek AJ. Physical and psychosocial problems in cancer survivors beyond return to work: A systematic review. Psycho-Oncology. 2014;23(5):481–492. [PubMed: 24375630]
- Earle CC. Failing to plan is planning to fail: Improving the quality of care with survivorship care plans. Journal of Clinical Oncology. 2006;24(32):5112–5116. [PubMed: 17093272]
- Ekwueme DU, Yabroff KR, Guy GP Jr, Banegas MP, de Moor JS, Li C, Han X, Zheng Z, Soni A, Davidoff A, Rechis R, Virgo KS. Centers for Disease Control and Prevention. Medical costs and productivity losses of cancer survivors—United States, 2008–2011. Morbidity and Mortality Weekly Report. 2014;63(23):505–510. [PMC free article: PMC5779368] [PubMed: 24918485]
- Epping-Jordan J, Pruitt S, Bengoa R, Wagner E. Improving the quality of health care for chronic conditions. Quality & Safety in Health Care. 2004;13(4):299–305. [PMC free article: PMC1743863] [PubMed: 15289634]
- Feuerstein M. Handbook of cancer survivorship. New York: Springer; 2007.
- Finkelstein EA, Tangka FK, Trogdon JG, Sabatino SA, Richardson LC. The personal financial burden of cancer for the working-aged population. The American Journal of Managed Care. 2009;15(11):801–806. [PubMed: 19895184]
- Fox S, Brenner J. Family caregivers online. 2012. [March 11, 2018]. http://www
.pewinternet .org/2012/07/12/family-caregivers-online. - Frobisher C, Lancashire ER, Winter DL, Jenkinson HC, Hawkins MM. Long-term population-based marriage rates among adult survivors of childhood cancer in Britain. International Journal of Cancer. 2007;121(4):846–855. [PubMed: 17450524]
- Funderburk JS, Dobmeyer AC, Hunter CL, Walsh CO, Maisto SA. Provider practices in the Primary Care Behavioral Health (PCBH) model: An initial examination in the Veterans Health Administration and United States Air Force. Families, Systems, & Health. 2013;31(4):341–353. [PubMed: 24377767]
- Fung C, Fossa SD, Milano MT, Sahasrabudhe DM, Peterson DR, Travis LB. Cardiovascular disease mortality after chemotherapy or surgery for testicular nonseminoma: A population-based study. Journal of Clinical Oncology. 2015;33(28):3105–3115. [PMC free article: PMC4582143] [PubMed: 26240226]
- Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: A meta-analysis. International Journal of Cancer. 2008;122(1):155–164. [PubMed: 17893872]
- Ganz P. Cancer survivorship: Today and tomorrow. New York: Springer; 2007.
- Gates P, Krishnasamy M. Nurse-led survivorship care. Cancer Forum. 2009;33(3):176–179.
- Gibson MJ, Houser A. Valuing the invaluable: A new look at the economic value of family caregiving. 2007. [October 2, 2017]. https://assets
.aarp.org /rgcenter/il/ib82_caregiving.pdf. [PubMed: 17612038] - Glasgow RE, Nutting PA. Diabetes. In: Haas LJ, editor. Handbook of primary care psychology. New York: Oxford University Press; 2004. pp. 299–311.
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. American Journal of Public Health. 1999;89(9):1322–1327. [PMC free article: PMC1508772] [PubMed: 10474547]
- Goedendorp MM, Gielissen MFM, Verhagen CAHHVM, Bleijenberg G. Development of fatigue in cancer survivors: A prospective follow-up study from diagnosis into the year after treatment. Journal of Pain and Symptom Management. 2013;45(2):213–222. [PubMed: 22926087]
- Goodfield J. The siege of cancer. New York: Random House; 1975.
- Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258(21):3125–3130. [PubMed: 3669259]
- Grunfeld E, Julian JA, Pond G, Maunsell E, Coyle D, Folkes A, Joy AA, Provencher L, Rayson D, Rheaume DE, Porter GA, Paszat LF, Pritchard KI, Robidoux A, Smith S, Sussman J, Dent S, Sisler J, Wiernikowski J, Levine MN. Evaluating survivorship care plans: Results of a randomized, clinical trial of patients with breast cancer. Journal of Clinical Oncology. 2011;29(36):4755–4762. [PubMed: 22042959]
- Gunst DCM, Kaatsch P, Goldbeck L. Seeing the good in the bad: Which factors are associated with posttraumatic growth in long-term survivors of adolescent cancer? Supportive Care in Cancer. 2016;24(11):4607–4615. [PubMed: 27349524]
- Gurney JG, Krull KR, Kadan-Lottick N, Nicholson HS, Nathan PC, Zebrack B, Tersak JM, Ness KK. Social outcomes in the Childhood Cancer Survivor Study cohort. Journal of Clinical Oncology. 2009;27(14):2390–2395. [PMC free article: PMC2677924] [PubMed: 19224833]
- Guy GP, Ekwueme DU, Yabroff KR, Dowling EC, Li C, Rodriguez JL, de Moor JS, Virgo KS. Economic burden of cancer survivorship among adults in the United States. Journal of Clinical Oncology. 2013;31(30):3749–3757. [PMC free article: PMC3795887] [PubMed: 24043731]
- Guy GP, Yabroff KR, Ekwueme DU, Smith AW, Dowling EC, Rechis R, Nutt S, Richardson LC. Estimating the health and economic burden of cancer among those diagnosed as adolescents and young adults. Health Affairs (Project Hope). 2014;33(6):1024–1031. [PMC free article: PMC4582764] [PubMed: 24889952]
- GW (George Washington University) Cancer Institute. State cancer plans priority alignment resource guide & tool. 2015. [September 26, 2017]. https://smhs
.gwu.edu /cancercontroltap/sites /cancercontroltap /files/PriorityAlignmentToolCombined-FINAL_20150302.pdf. - Halpern MT, Viswanathan M, Evans TS, Birken SA, Basch E, Mayer DK. Models of cancer survivorship care: Overview and summary of current evidence. Journal of Oncology Practice. 2015;11(1):e19–e27. [PubMed: 25205779]
- Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, Valero V. Overall survival and cause-specific mortality of patients with stage T1a, bN0M0 breast carcinoma. Journal of Clinical Oncology. 2007;25(31):4952–4960. [PubMed: 17971593]
- Harrop JP, Dean JA, Paskett ED. Cancer survivorship research: A review of the literature and summary of current NCI-designated cancer center projects. Cancer Epidemiology, Biomarkers & Prevention. 2011;20(10):2042–2047. [PMC free article: PMC3191883] [PubMed: 21980012]
- Health Canada. Health Canada policy toolkit for public involvement in decision making. 2000. [March 11, 2018]. https://www
.canada.ca /content/dam/hc-sc/migration /hc-sc/ahc-asc /alt_formats/pacrb-dgapcr /pdf/public-consult /2000decision-eng.pdf. - Henley S, Anderson R, Thomas C, Massetti G, Peaker B, Richardson L. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in nonmetropolitan and metropolitan counties—United States. Morbidity and Mortality Weekly Report Surveillance Summaries. 2017;66(14):1–13. [PMC free article: PMC5879727] [PubMed: 28683054]
- Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, Hayden J, Tarpinian K, Yakim E, Flockhart DA, Stearns V, Hayes DF, Storniolo AM. Predictors of aromatase inhibitor discontinuation as a result of treatment-emergent symptoms in early-stage breast cancer. Journal of Clinical Oncology. 2012;30(9):936–942. [PMC free article: PMC3341106] [PubMed: 22331951]
- Hodgkinson K, Butow P, Hunt GE, Wyse R, Hobbs KM, Wain G. Life after cancer: Couples' and partners' psychological adjustment and supportive care needs. Supportive Care in Cancer. 2007;15(4):405–415. [PubMed: 17043776]
- Hoffman B. Between a disability and a hard place: The cancer survivors' catch-22 of proving disability status under the Americans with Disabilities Act. 2000. [September 28, 2017]. http:
//digitalcommons .law.umaryland.edu/cgi/viewcontent .cgi?article =3086&context=mlr. - Hoffman B. The law of intended consequences: Did the Americans with Disabilities Act Amendments Act make it easier for cancer survivors to prove disability status? NYU Annual Survey of American Law. 2014;68:843–890.
- Howell D, Hack TF, Oliver TK, Chulak T, Mayo S, Aubin M, Chasen M, Earle CC, Friedman AJ, Green E, Jones GW, Jones JM, Parkinson M, Payeur N, Sabiston CM, Sinclair S. Models of care for post-treatment follow-up of adult cancer survivors: A systematic review and quality appraisal of the evidence. Journal of Cancer Survivorship. 2012;6(4):359–371. [PubMed: 22777364]
- Howell D, Oliver TK, Keller-Olaman S, Davidson J, Garland S, Samuels C, Savard J, Harris C, Aubin M, Olson K, Sussman J, MacFarlane J, Taylor C. A pan-Canadian practice guideline: Prevention, screening, assessment, and treatment of sleep disturbances in adults with cancer. Supportive Care in Cancer. 2013;21(10):2695–2706. [PubMed: 23708820]
- Howlader N, Noone AM, Krapcho M, Garshell J, Miller D, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA, editors. SEER cancer statistics review, 1975–2011. 2014. [March 11, 2018]. http://seer
.cancer.gov/csr/1975_2011. - Hudson MM. A model for care across the cancer continuum. Cancer. 2005;104(S11):2638–2642. [PubMed: 16258932]
- Hudson MM, Mertens AC, Yasui Y, Hobbie W, Chen H, Gurney JG, Yeazel M, Recklitis CJ, Marina N, Robison LR, Oeffinger KC. the Childhood Cancer Survivor Study Investigators. Health status of adult long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. [PubMed: 14506117]
- Hunter AM, Kwan L, Ercoli LM, Mills BK, Cook IA, Ganz PA, Leuchter AF. Quantitative electroencephalography biomarkers of cognitive complaints after adjuvant therapy in breast cancer survivors: A pilot study. Psycho-Oncology. 2014;23(6):713–715. [PubMed: 24890579]
- Husson O, Mols F, van de Poll-Franse LV. The relation between information provision and health-related quality of life, anxiety and depression among cancer survivors: A systematic review. Annals of Oncology. 2011;22(4):761–772. [PMC free article: PMC3065875] [PubMed: 20870912]
- IOM (Institute of Medicine). Implementing cancer survivorship care planning: Workshop summary. Washington, DC: The National Academies Press; 2007.
- IOM. The role of obesity in cancer survival and recurrence: Workshop summary. Washington, DC: The National Academies Press; 2012. [PubMed: 24830056]
- IOM. Best care at lower cost: The path to continuously learning health care in America. Washington, DC: The National Academies Press; 2013a. [PubMed: 24901184]
- IOM. Delivering high-quality cancer care: Charting a new course for a system in crisis. Washington, DC: The National Academies Press; 2013b. [PubMed: 24872984]
- IOM. Ensuring patient access to affordable cancer drugs: Workshop summary. Washington, DC: The National Academies Press; 2014. [PubMed: 25590110]
- IOM and NRC (National Research Council). From cancer patient to cancer survivor: Lost in transition. Washington, DC: The National Academies Press; 2006.
- Jacobsen PB, Rowland JH, Paskett ED, Van Leeuwen F, Moskowitz C, Katta S, Wollins D, Robison LL. Identification of key gaps in cancer survivorship research: Findings from the American Society of Clinical Oncology survey. Journal of Oncology Practice. 2016;12(3):190–193. [PubMed: 26907451]
- Jacobson JO, Polovich M, McNiff KK, LeFebvre KB, Cummings C, Galioto M, Bonelli KR, McCorkle MR. American Society of Clinical Oncology/Oncology Nursing Society chemotherapy administration safety standards. Journal of Clinical Oncology. 2009;27(32):5469–5475. [PubMed: 19786650]
- Jacola LM, Edelstein K, Liu W, Pui C-H, Hayashi R, Kadan-Lottick NS, Srivastava D, Henderson T, Leisenring W, Robison LL, Armstrong GT, Krull KR. Cognitive, behaviour, and academic functioning in adolescent and young adult survivors of childhood acute lymphoblastic leukaemia: A report from the Childhood Cancer Survivor Study. The Lancet Psychiatry. 2016;3(10):965–972. [PMC free article: PMC5056029] [PubMed: 27639661]
- Jagsi R, Pottow JAE, Griffith KA, Bradley C, Hamilton AS, Graff J, Katz SJ, Hawley ST. Long-term financial burden of breast cancer: Experiences of a diverse cohort of survivors identified through population-based registries. Journal of Clinical Oncology. 2014;32(12):1269–1276. [PMC free article: PMC3986387] [PubMed: 24663041]
- Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. International Review of Psychiatry. 2014;26(1):102–113. [PMC free article: PMC4084673] [PubMed: 24716504]
- Janson C, Leisenring W, Cox C, Termuhlen AM, Mertens AC, Whitton JA, Goodman P, Zeltzer L, Robison LL, Krull KR, Kadan-Lottick NS. Predictors of marriage and divorce in adult survivors of childhood cancers: A report from the Childhood Cancer Survivor Study. Cancer Epidemiology, Biomarkers & Prevention. 2009;18(10):2626–2635. [PMC free article: PMC2768276] [PubMed: 19815636]
- Jawa Z, Perez RM, Garlie L, Singh M, Qamar R, Khandheria BK, Jahangir A, Shi Y. Risk factors of trastuzumab-induced cardiotoxicity in breast cancer: A meta-analysis. Medicine. 2016;95(44):e5195. [PMC free article: PMC5591107] [PubMed: 27858859]
- Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M, Carlson LE, Garland SN. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Medicine Reviews. 2016;27(Suppl C):20–28. [PubMed: 26434673]
- Joly F, Giffard B, Rigal O, De Ruiter MB, Small BJ, Dubois M, LeFel J, Schagen SB, Ahles TA, Wefel JS, Vardy JL, Pancré V, Lange M, Castel H. Impact of cancer and its treatments on cognitive function: Advances in research from the Paris international cognition and cancer task force symposium and update since 2012. Journal of Pain and Symptom Management. 2015;50(6):830–841. [PubMed: 26344551]
- Kaasa S, Mastekaasa A, Lund E. Prognostic factors for patients with inoperable non-small cell lung cancer, limited disease. Radiotherapy and Oncology. 1989;15(3):235–242. [PubMed: 2549582]
- Kent EE, Arora NK, Rowland JH, Bellizzi KM, Forsythe LP, Hamilton AS, Oakley-Girvan I, Beckjord EB, Aziz NM. Health information needs and health-related quality of life in a diverse population of long-term cancer survivors. Patient Education and Counseling. 2012;89(2):345–352. [PMC free article: PMC4560240] [PubMed: 23021856]
- Kent EE, Forsythe LP, Yabroff KR, Weaver KE, de Moor JS, Rodriguez JL, Rowland JH. Are survivors who report cancer-related financial problems more likely to forgo or delay medical care? Cancer. 2013;119(20):3710–3717. [PMC free article: PMC4552354] [PubMed: 23907958]
- Kent EE, Rowland JH, Northouse L, Litzelman K, Chou W-YS, Shelburne N, Timura C, O'Mara A, Huss K. Caring for caregivers and patients: Research and clinical priorities for informal cancer caregiving. Cancer. 2016;122(13):1987–1995. [PMC free article: PMC5597246] [PubMed: 26991807]
- Kim J-EE, Dodd MJ, Aouizerat BE, Jahan T, Miaskowski C. A review of the prevalence and impact of multiple symptoms in oncology patients. Journal of Pain and Symptom Management. 2009;37(4):715–736. [PMC free article: PMC2688644] [PubMed: 19019626]
- Kim Y, Kashy DA, Spillers RL, Evans TV. Needs assessment of family caregivers of cancer survivors: Three cohorts comparison. Psycho-Oncology. 2010;19(6):573–582. [PubMed: 19582798]
- Kinahan KE, Sharp LK, Seidel K, Leisenring W, Didwania A, Lacouture ME, Stovall M, Haryani A, Robison LL, Krull KR. Scarring, disfigurement, and quality of life in long-term survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2012;30(20):2466–2474. [PMC free article: PMC3397782] [PubMed: 22614987]
- King AA, Seidel K, Di C, Leisenring WM, Perkins SM, Krull KR, Sklar CA, Green DM, Armstrong GT, Zeltzer LK, Wells E, Stovall M, Ullrich NJ, Oeffinger KC, Robison LL, Packer RJ. Long-term neurologic health and psychosocial function of adult survivors of childhood medulloblastoma/PNET: A report from the Childhood Cancer Survivor Study. Neuro-Oncology. 2017;19(5):689–698. [PMC free article: PMC5464442] [PubMed: 28039368]
- Kirchhoff AC, Leisenring W, Krull KR, Ness KK, Friedman DL, Armstrong GT, Stovall M, Park ER, Oeffinger KC, Hudson M, Robison LL, Wickizer T. Unemployment among adult survivors of childhood cancer: A report from the Childhood Cancer Survivors Study. Medical Care. 2010;48(11):1015–1025. [PMC free article: PMC3428202] [PubMed: 20940653]
- Klemanski DL, Browning KK, Kue J. Survivorship care plan preferences of cancer survivors and health care providers: A systematic review and quality appraisal of the evidence. Journal of Cancer Survivorship. 2016;10(1):71–86. [PubMed: 25911150]
- Knowles G, Sherwood L, Dunlop MG, Dean G, Jodrell D, McLean C, Preston E. Developing and piloting a nurse-led model of follow-up in the multidisciplinary management of colorectal cancer. European Journal of Oncology Nursing. 2007;11(3):212–223. [PubMed: 17188938]
- Ko NY, Battaglia TA, Gupta-Lawrence R, Schiller J, Gunn C, Festa K, Nelson K, Flacks J, Morton SJ, Rosen JE. Burden of socio-legal concerns among vulnerable patients seeking cancer care services at an urban safety-net hospital: A cross-sectional survey. BMC Health Services Research. 2016;16:196. [PMC free article: PMC4906581] [PubMed: 27296566]
- Koch SV, Kejs AMT, Engholm G, Johansen C, Schmiegelow K. Educational attainment among survivors of childhood cancer: A population-based cohort study in Denmark. British Journal of Cancer. 2004;91(5):923–928. [PMC free article: PMC2409866] [PubMed: 15292930]
- Krein SL, Olmsted RN, Hofer TP, Kowalski C, Forman J, Banaszak-Holl J, Saint S. Translating infection prevention evidence into practice using quantitative and qualitative research. American Journal of Infection Control. 2006;34(8):507–512. [PubMed: 17015156]
- Krouse RS, Grant M, McCorkle R, Wendel CS, Cobb MD, Tallman NJ, Ercolano E, Sun V, Hibbard JH, Hornbrook MC. A chronic care ostomy self-management program for cancer survivors. Psycho-Oncology. 2016;25(5):574–581. [PMC free article: PMC4833624] [PubMed: 26804708]
- Lambert S, Girgis A, Descallar J, Levesque JV, Jones B. Trajectories of mental and physical functioning among spouse caregivers of cancer survivors over the first five years following the diagnosis. Patient Education and Counseling. 2017;100(6):1213–1221. [PubMed: 28089132]
- Langeveld NE, Ubbink MC, Last BF, Grootenhuis MA, VoÛte PA, de Haan RJ. Educational achievement, employment and living situation in long-term young adult survivors of childhood cancer in the Netherlands. Psycho-Oncology. 2003;12(3):213–225. [PubMed: 12673806]
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC working group. New England Journal of Medicine. 2016;375(8):794–798. [PMC free article: PMC6754861] [PubMed: 27557308]
- Leopold C, Park ER, Nekhlyudov L. The impact of the Affordable Care Act on cancer survivorship. The Cancer Journal. 2017;23(3):181–189. [PubMed: 28537964]
- Li D, Marchenko ND. ErbB2 inhibition by lapatinib promotes degradation of mutant p53 protein in cancer cells. Oncotarget. 2017;8(4):5823–5833. [PMC free article: PMC5351592] [PubMed: 27791982]
- Liptak C, Brinkman T, Bronson A, Delaney B, Chordas C, Brand S, Patenaude AF, Muriel AC, Manley P. A social program for adolescent and young adult survivors of pediatric brain tumors: The power of a shared medical experience. Journal of Psychosocial Oncology. 2016;34(6):493–511. [PubMed: 27541834]
- Lorenzi M, McMillan AJ, Siegel LS, Zumbo BD, Glickman V, Spinelli JJ, Goddard KJ, Pritchard SL, Rogers PC, McBride ML. Educational outcomes among survivors of childhood cancer in British Columbia, Canada. Cancer. 2009;115(10):2234–2245. [PubMed: 19326396]
- Mai PL, Best AF, Peters JA, DeCastro R, Khincha PP, Loud JT, Bremer RC, Rosenberg PS, Savage SA. Risks of first and subsequent cancers among tp53 mutation-carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer. 2016;122(23):3673–3681. [PMC free article: PMC5115949] [PubMed: 27496084]
- Mandelblatt JS, Cai L, Luta G, Kimmick G, Clapp J, Isaacs C, Pitcher B, Barry W, Winer E, Sugarman S, Hudis C, Muss H, Cohen HJ, Hurria A. Frailty and long-term mortality of older breast cancer patients: CALGB 369901 (alliance). Breast Cancer Research and Treatment. 2017;164(1):107–117. [PMC free article: PMC5479131] [PubMed: 28364214]
- Marmot M, Friel S, Bell R, Houweling TAJ, Taylor S. Closing the gap in a generation: Health equity through action on the social determinants of health. The Lancet. 2008;372(9650):1661–1669. [PubMed: 18994664]
- Matasar MJ, Ford JS, Riedel ER, Salz T, Oeffinger KC, Straus DJ. Late morbidity and mortality in patients with Hodgkin's lymphoma treated during adulthood. Journal of the National Cancer Institute. 2015;107(4):djv018. [PMC free article: PMC6281032] [PubMed: 25717170]
- Mayer DK, Birken SA, Check DK, Chen RC. Summing it up: An integrative review of studies of cancer survivorship care plans (2006–2013). Cancer. 2015;121(7):978–996. [PMC free article: PMC4948720] [PubMed: 25252164]
- McCabe MS, Partridge A, Grunfeld E, Hudson MM. Risk-based health care, the cancer survivor, the oncologist and the primary care physician. Seminars in Oncology. 2013;40(6):804–812. [PMC free article: PMC4465133] [PubMed: 24331199]
- Mendoza TR, Wang XS, Cleeland CS, Morrissey M, Johnson BA, Wendt JK, Huber SL. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85(5):1186–1196. [PubMed: 10091805]
- Mertens AC, Marchak JG. Mental health status of adolescent cancer survivors. Clinical Oncology in Adolescents and Young Adults. 2015;2015(5):87–95.
- Michel G, Rebholz CE, von der Weid NX, Bergstraesser E, Kuehni CE. Psychological distress in adult survivors of childhood cancer: The Swiss Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2010;28(10):1740–1748. [PubMed: 20194864]
- Miller KD. Medical and psychosocial care of the cancer survivor. Boston, MA: Jones & Bartlett Learning; 2010.
- Mitby PA, Robison LL, Whitton JA, Zevon MA, Gibbs IC, Tersak JM, Meadows AT, Stovall M, Zeltzer LK, Mertens AC. Utilization of special education services and educational attainment among long-term survivors of childhood cancer. Cancer. 2003;97(4):1115–1126. [PubMed: 12569614]
- Moitry M, Velten M, Trétarre B, Bara S, Daubisse-Marliac L, Lapôtre-Ledoux B, Troussard X, Molinié F, Ligier K, Woronoff A-S, Bouvier V, Colonna M, Klein D, Guizard A-V, Jégu J. Development of a model to predict the 10-year cumulative risk of second primary cancer among cancer survivors. Cancer Epidemiology. 2017;47(Suppl C):35–41. [PubMed: 28113110]
- Mooney K, Berry DL, Whisenant M, Sjoberg D. Improving cancer care through the patient experience: How to use patient-reported outcomes in clinical practice. American Society of Clinical Oncology Educational Book. 2017;37:695–704. [PubMed: 28561689]
- Moore SC, Lee I, Weiderpass E, Campbell PT, Sampson JN, Kitahara CM, Keadle SK, Arem H, Berrington de Gonzalez A, Hartge P. Association of leisure-time physical activity with risk of 26 types of cancer in 1.44 million adults. JAMA Internal Medicine. 2016;176(6):816–825. [PMC free article: PMC5812009] [PubMed: 27183032]
- Morris LGT, Sikora AG, Patel SG, Hayes RB, Ganly I. Second primary cancers after an index head and neck cancer: Subsite-specific trends in the era of human papillomavirus-associated oropharyngeal cancer. Journal of Clinical Oncology. 2011;29(6):739–746. [PMC free article: PMC3056657] [PubMed: 21189382]
- Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: A systematic review. Breast Cancer Research and Treatment. 2012;134(2):459–478. [PMC free article: PMC3607286] [PubMed: 22689091]
- Mustian KM, Alfano CM, Heckler C, Kleckner AS, Kleckner IR, Leach CR, Mohr D, Palesh OG, Peppone LJ, Piper BF, Scarpato J, Smith T, Sprod LK, Miller SM. Comparison of pharmaceutical, psychological, and exercise treatments for cancer-related fatigue: A meta-analysis. JAMA Oncology. 2017;3(7):961–968. [PMC free article: PMC5557289] [PubMed: 28253393]
- NASEM (National Academies of Sciences, Engineering, and Medicine). Incorporating weight management and physical activity throughout the cancer care continuum: Proceedings of a workshop. Washington, DC: The National Academies Press; 2018a. [PubMed: 29206390]
- NASEM. Making medicines affordable: A national imperative. Washington, DC: The National Academies Press; 2018b. [PubMed: 29620830]
- National Alliance for Caregiving. Cancer caregiving in the U.S.: An intense, episodic, and challenging care experience. 2016. [April 3, 2018]. http://www
.caregiving .org/wp-content/uploads /2016/06/CancerCaregivingReport _FINAL_June-17-2016.pdf. - National Alliance for Caregiving and AARP Public Policy Institute. Caregiving in the U.S. 2015—executive summary. 2015. [October 2, 2017]. http://www
.caregiving .org/wp-content/uploads /2015/05/2015_CaregivingintheUS _Executive-Summary-June-4_WEB.pdf. - Nayan S, Gupta MK, Strychowsky JE, Sommer DD. Smoking cessation interventions and cessation rates in the oncology population. Otolaryngology-Head and Neck Surgery. 2013;149(2):200–211. [PubMed: 23715685]
- NCI (National Cancer Institute). Cancer survivors and obesity. 2017a. [October 23, 2017]. https:
//progressreport .cancer.gov/after/obesity. - NCI. Cancer survivors and physical activity. 2017b. [October 23, 2017]. https:
//progressreport .cancer.gov/after/physical_activity. - NCI. Cancer survivors and smoking. 2017c. [October 23, 2017]. https:
//progressreport .cancer.gov/after/smoking. - Nekhlyudov L, Walker R, Ziebell R, Rabin B, Nutt S, Chubak J. Cancer survivors' experiences with insurance, finances, and employment: Results from a multisite study. Journal of Cancer Survivorship. 2016;10(6):1104–1111. [PubMed: 27277896]
- Nekhlyudov L, O'Malley DM, Hudson SV. Integrating primary care providers in the care of cancer survivors: Gaps in evidence and future opportunities. The Lancet Oncology. 2017;18(1):e30–e38. [PMC free article: PMC5553291] [PubMed: 28049575]
- Ness KK, Krull KR, Jones KE, Mulrooney DA, Armstrong GT, Green DM, Chemaitilly W, Smith WA, Wilson CL, Sklar CA, Shelton K, Srivastava DK, Ali S, Robison LL, Hudson MM. Physiologic frailty as a sign of accelerated aging among adult survivors of childhood cancer: A report from the St. Jude lifetime cohort study. Journal of Clinical Oncology. 2013;31(36):4496–4503. [PMC free article: PMC3871511] [PubMed: 24248696]
- Neta G, Sanchez MA, Chambers DA, Phillips SM, Leyva B, Cynkin L, Farrell MM, Heurtin-Roberts S, Vinson C. Implementation science in cancer prevention and control: A decade of grant funding by the National Cancer Institute and future directions. Implementation Science. 2015;10:4. [PMC free article: PMC4296529] [PubMed: 25567702]
- Nevidjon BR, Miller P, Murphy C, Rosenzweig MQ, McCorkle MR, Baileys K. Filling the gap: Development of the oncology nurse practitioner workforce. Journal of Oncology Practice. 2010;6(1):1–6. [PMC free article: PMC2805339] [PubMed: 20539723]
- O'Dell M, Stubblefield M. Cancer rehabilitation: Principles and practice. New York: Springer Publishing Company; 2009.
- Oncology Nutrition. 2016–2017 annual report: Oncology Nutrition Dietetic Practice Group. 2018. [March 11, 2018]. http://dpg-storage
.s3 .amazonaws.com/ondpg /documents/45f85c2eabb54f1e /ON_DPG_Annual_Report_2016-2017 .pdf. - Ortmeyer CE. Variations in mortality, morbidity and health care by marital status. In. In: Erhard CL, Berlin JE, editors. Mortality and morbidity in the United States. Cambridge, MA: Harvard University Press; 1974. pp. 159–199.
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, Espeland MA, Fielding RA, Gill TM, Groessl EJ, King AC, Kritchevsky SB, Manini TM, McDermott MM, Miller ME, Newman AB, Rejeski WJ, Sink KM, Williamson JD. Effect of structured physical activity on prevention of major mobility disability in older adults: The life study randomized clinical trial. JAMA. 2014;311(23):2387–2396. [PMC free article: PMC4266388] [PubMed: 24866862]
- Park SH, Cho MS, Kim YS, Hong J, Nam E, Park J, Cho EK, Shin DB, Lee JH, Lee WK. Self-reported health-related quality of life predicts survival for patients with advanced gastric cancer treated with first-line chemotherapy. Quality of Life Research. 2008;17(2):207–214. [PubMed: 18224458]
- Parry C, Kent EE, Forsythe LP, Alfano CM, Rowland JH. Can't see the forest for the care plan: A call to revisit the context of care planning. Journal of Clinical Oncology. 2013;31(21):2651–2653. [PMC free article: PMC3709053] [PubMed: 23796989]
- Penttinen HM, Saarto T, Kellokumpu-Lehtinen P, Blomqvist C, Huovinen R, Kautiainen H, Järvenpää S, Nikander R, Idman I, Luoto R, Sievänen H, Utriainen M, Vehmanen L, Jääskeläinen AS, Elme A, Ruohola J, Luoma M, Hakamies-Blomqvist L. Quality of life and physical performance and activity of breast cancer patients after adjuvant treatments. Psycho-Oncology. 2011;20(11):1211–1220. [PubMed: 20878646]
- Pergolotti M, Deal AM, Lavery J, Reeve B, Muss HB. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. Journal of Geriatric Oncology. 2015;6(3):194–201. [PMC free article: PMC4459887] [PubMed: 25614296]
- Pham HH, Schrag D, O'Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. New England Journal of Medicine. 2007;356(11):1130–1139. [PubMed: 17360991]
- Ramsey S, Blough D, Kirchhoff A, Kreizenbeck K, Fedorenko C, Snell K, Newcomb P, Hollingworth W, Overstreet K. Washington cancer patients found to be at greater risk for bankruptcy than people without a cancer diagnosis. Health Affairs. 2013;32(6):1143–1152. [PMC free article: PMC4240626] [PubMed: 23676531]
- Ramsey SD, Bansal A, Fedorenko CR, Blough DK, Overstreet KA, Shankaran V, Newcomb P. Financial insolvency as a risk factor for early mortality among patients with cancer. Journal of Clinical Oncology. 2016;34(9):980–986. [PMC free article: PMC4933128] [PubMed: 26811521]
- Rechis R, Beckjord EB, Arvey SR, Reynolds KA, McGoldrick D. The essential elements of survivorship care: A LIVESTRONG brief. 2011. [September 27, 2017]. https:
//d1un1nybq8gi3x .cloudfront.net/sites /default/files/what-we-do /reports/EssentialElementsBrief .pdf. - Recklitis CJ, Syrjala KL. Provision of integrated psychosocial services for cancer survivors post-treatment. The Lancet Oncology. 2017;18(1):e39–e50. [PMC free article: PMC5865587] [PubMed: 28049576]
- Recklitis CJ, Diller LR, Li X, Najita J, Robison LL, Zeltzer L. Suicide ideation in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2010;28(4):655–661. [PMC free article: PMC2816000] [PubMed: 19841325]
- Recklitis CJ, Blackmon JE, Chang G. Screening young adult cancer survivors for distress with the distress thermometer (DT): Comparisons with a structured clinical diagnostic interview. Cancer. 2016;122(2):296–303. [PMC free article: PMC4707976] [PubMed: 26457669]
- Recklitis CJ, Blackmon JE, Chang G. Validity of the brief symptom inventory-18 (bsi-18) for identifying depression and anxiety in young adult cancer survivors: Comparison with a structured clinical diagnostic interview. Psychological Assessment. 2017;29(10):1189–1200. [PMC free article: PMC5507754] [PubMed: 28080106]
- Reed SC, Bell JF, Whitney R, Lash R, Kim KK, Bold RJ, Joseph JG. Psychosocial outcomes in active treatment through survivorship. Psycho-Oncology. 2017;27(1):279–285. [PubMed: 28429466]
- Reeves MM, Terranova CO, Eakin EG, Demark-Wahnefried W. Weight loss intervention trials in women with breast cancer: A systematic review. Obesity Reviews. 2014;15(9):749–768. [PubMed: 24891269]
- Reinders JG, Heijmen BJM, Olofsen-van Acht MJJ, van Putten WLJ, Levendag PC. Ischemic heart disease after mantlefield irradiation for Hodgkin's disease in long-term follow-up. Radiotherapy and Oncology. 1999;51(1):35–42. [PubMed: 10386715]
- Reinhard SC, Feinberg LF, Choula R, Houser A. Valuing the invaluable: 2015 update: Undeniable progress, but big gaps remain. 2015. [October 2, 2017]. http://www
.aarp.org/content /dam/aarp/ppi /2015/valuing-the-invaluable-2015-update-new.pdf. - Robinson P, Reiter J. Behavioral consultation and primary care: A guide to integrating services. New York: Springer US; 2007.
- Rock CL, Doyle C, Demark-Wahnefried W, Meyerhardt J, Courneya KS, Schwartz AL, Bandera EV, Hamilton KK, Grant B, McCullough M, Byers T, Gansler T. Nutrition and physical activity guidelines for cancer survivors. CA: A Cancer Journal for Clinicians. 2012;62(4):242–274. [PubMed: 22539238]
- Ross L, Johansen C, Dalton SO, Mellemkjær L, Thomassen LH, Mortensen PB, Olsen JH. Psychiatric hospitalizations among survivors of cancer in childhood or adolescence. New England Journal of Medicine. 2003;349(7):650–657. [PubMed: 12917301]
- Savard J, Simard S, Blanchet J, Ivers H, Morin CM. Prevalence, clinical characteristics, and risk factors for insomnia in the context of breast cancer. Sleep. 2001;24(5):583–590. [PubMed: 11480655]
- Savard J, Villa J, Ivers H, Simard S, Morin CM. Prevalence, natural course, and risk factors of insomnia comorbid with cancer over a 2-month period. Journal of Clinical Oncology. 2009;27(31):5233–5239. [PubMed: 19738124]
- Schaapveld M, Aleman BMP, van Eggermond AM, Janus CPM, Krol ADG, van der Maazen RWM, Roesink J, Raemaekers JMM, de Boer JP, Zijlstra JM, van Imhoff GW, Petersen EJ, Poortmans PMP, Beijert M, Lybeert ML, Mulder I, Visser O, Louwman MWJ, Krul IM, Lugtenburg PJ, van Leeuwen FE. Second cancer risk up to 40 years after treatment for Hodgkin's lymphoma. New England Journal of Medicine. 2015;373(26):2499–2511. [PubMed: 26699166]
- Schultz KAP, Ness KK, Whitton J, Recklitis C, Zebrack B, Robison LL, Zeltzer L, Mertens AC. Behavioral and social outcomes in adolescent survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2007;25(24):3649–3656. [PubMed: 17704415]
- Shaffer KM, Kim Y, Carver CS. Physical and mental health trajectories of cancer patients and caregivers across the year post-diagnosis: A dyadic investigation. Psychology & Health. 2016;31(6):655–674. [PMC free article: PMC4930392] [PubMed: 26680247]
- Shankaran V, Jolly S, Blough D, Ramsey SD. Risk factors for financial hardship in patients receiving adjuvant chemotherapy for colon cancer: A population-based exploratory analysis. Journal of Clinical Oncology. 2012;30(14):1608–1614. [PubMed: 22412136]
- Shapiro CL, Jacobsen PB, Henderson T, Hurria A, Nekhlyudov L, Ng A, Surbone A, Mayer DK, Rowland JH, Shapiro CL, Jacobsen PB, Henderson T, Hurria A, Nekhlyudov L, Ng A, Surbone A, Mayer DK, Rowland JH. Recap: ASCO core curriculum for cancer survivorship education. Journal of Oncology Practice. 2016;12(2):145. [PubMed: 26813926]
- Short PF, Vasey JJ, Moran JR. Long-term effects of cancer survivorship on the employment of older workers. Health Services Research. 2008;43(1 Pt 1):193–210. [PMC free article: PMC2323147] [PubMed: 18211525]
- Short PF, Moran JR, Punekar R. Medical expenditures of adult cancer survivors under age 65 in the U.S. Cancer. 2011;117(12):2791–2800. [PMC free article: PMC4124459] [PubMed: 21656757]
- Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: An essential component of quality care and survivorship. CA: A Cancer Journal for Clinicians. 2013;63(5):295–317. [PubMed: 23856764]
- Silver JK, Raj VS, Fu JB, Wisotzky EM, Smith SR, Kirch RA. Cancer rehabilitation and palliative care: Critical components in the delivery of high-quality oncology services. Supportive Care in Cancer. 2015;23(12):3633–3643. [PubMed: 26314705]
- Silver JK, Raj VS, Fu JB, Wisotzky EM, Smith SR, Knowlton SE, Silver AJ. Journal of Cancer Education. 2017. Most National Cancer Institute-designated cancer center websites do not provide survivors with information about cancer rehabilitation services. Epub ahead of print. 1007/s13187-016-1157-4. [PubMed: 28064402]
- Small BJ, Rawson KS, Walsh E, Jim HSL, Hughes TF, Iser L, Andrykowski MA, Jacobsen PB. Catechol-o-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer. 2011;117(7):1369–1376. [PubMed: 21425136]
- Spears JA, Craft M, White S. Outcomes of cancer survivorship care provided by advanced practice RNs compared to other models of care: A systematic review. Oncology Nursing Forum. 2017;44(1):34. [PubMed: 28067032]
- Stam H, Grootenhuis MA, Last BF. The course of life of survivors of childhood cancer. Psycho-Oncology. 2005;14(3):227–238. [PubMed: 15386772]
- Stanton AL. What happens now? Psychosocial care for cancer survivors after medical treatment completion. Journal of Clinical Oncology. 2012;30(11):1215–1220. [PubMed: 22412133]
- Stanton A, Rowland J, Ganz P. Life after diagnosis and treatment of cancer in adulthood: Contributions from psychosocial oncology research. The American Psychologist. 2015;70(2):159–174. [PubMed: 25730722]
- Stout NL, Silver JK, Raj VS, Rowland J, Gerber L, Cheville A, Ness KK, Radomski M, Nitkin R, Stubblefield MD, Morris GS, Acevedo A, Brandon Z, Braveman B, Cunningham S, Gilchrist L, Jones L, Padgett L, Wolf T, Winters-Stone K, Campbell G, Hendricks J, Perkin K, Chan L. Toward a national initiative in cancer rehabilitation: Recommendations from a subject matter expert group. Archives of Physical Medicine and Rehabilitation. 2016;97(11):2006–2015. [PubMed: 27237580]
- Stuber ML, Meeske KA, Krull KR, Leisenring W, Stratton K, Kazak AE, Huber M, Zebrack B, Uijtdehaage SH, Mertens AC, Robison LL, Zeltzer LK. Prevalence and predictors of posttraumatic stress disorder in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatrics. 2010;125(5):e1124–e1134. [PMC free article: PMC3098501] [PubMed: 20435702]
- Sun Y, Shigaki CL, Armer JM. Return to work among breast cancer survivors: A literature review. Supportive Care in Cancer. 2017;25(3):709–718. [PubMed: 27873016]
- Swerdlow AJ, Higgins CD, Smith P, Cunningham D, Hancock BW, Horwich A, Hoskin PJ, Lister A, Radford JA, Rohatiner AZS, Linch DC. Myocardial infarction mortality risk after treatment for Hodgkin disease: A collaborative British cohort study. Journal of the National Cancer Institute. 2007;99(3):206–214. [PubMed: 17284715]
- Tabak RG, Khoong EC, Chambers D, Brownson RC. Bridging research and practice: Models for dissemination and implementation research. American Journal of Preventive Medicine. 2012;43(3):337–350. [PMC free article: PMC3592983] [PubMed: 22898128]
- Thornton LM, Carson WE, Shapiro CL, Farrar WB, Andersen BL. Delayed emotional recovery after taxane-based chemotherapy. Cancer. 2008;113(3):638–647. [PMC free article: PMC2746480] [PubMed: 18521922]
- Travis LB, Gospodarowicz M, Curtis RE, Clarke EA, Andersson M, Glimelius B, Joensuu T, Lynch CF, van Leeuwen FE, Holowaty E, Storm H, Glimelius I, Pukkala E, Stovall M, Fraumeni JF Jr, Boice JD Jr, Gilbert E. Lung cancer following chemotherapy and radiotherapy for Hodgkin's disease. Journal of the National Cancer Institute. 2002;94(3):182–192. [PubMed: 11830608]
- Travis LB, Bhatia S, Allan JM, Oeffinger KC, Ng A. Second primary cancers. In: Devita VT Jr, Lawrence TS, Rosenberg SA, editors. Cancer: Principles and practice of oncology. 11th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2018.
- Trotter K, Frazier A, Hendricks CK, Scarsella H. Innovation in survivor care: Group visits. Clinical Journal of Oncology Nursing. 2011;15(2):24–33. [PubMed: 21444277]
- Tucker-Seeley RD, Yabroff KR. Minimizing the “financial toxicity” associated with cancer care: Advancing the research agenda. Journal of the National Cancer Institute. 2016;108(5):djv410. [PubMed: 26657336]
- Turcotte LM, Liu Q, Yasui Y, Arnold MA, Hammond S, Howell RM, Smith SA, Weathers RE, Henderson TO, Gibson TM, Leisenring W, Armstrong GT, Robison LL, Neglia JP. Temporal trends in treatment and subsequent neoplasm risk among 5-year survivors of childhood cancer, 1970–2015. JAMA. 2017;317(8):814–824. [PMC free article: PMC5473951] [PubMed: 28245323]
- Underwood J, Townsend J, Stewart S, Buchannan N, Ekwueme D, Hawkins N, Li J, Peaker B, Pollack L, Richards T, Rim S, Rohan E, Sabatino S, Smith J, Tai E, Townsend G, White A, Fairley T. the Centers for Disease Control and Prevention. Surveillance of demographic characteristics and health behaviors among adult cancer survivors—Behavioral Risk Factor Surveillance System, United States, 2009. Morbidity and Mortality Weekly Report Surveillance Summaries. 2012;61(SS01):1–23. [PubMed: 22258477]
- Underwood JM, Lakhani N, Rohan E, Moore A, Stewart SL. An evaluation of cancer survivorship activities across National Comprehensive Cancer Control programs. Journal of Cancer Survivorship. 2015;9(3):554–559. [PMC free article: PMC4591926] [PubMed: 25732543]
- VA (Veterans Affair) Healthcare Network Upstate New York. Integrated primary care behavioral health services: Operations manual. 2005. [March 11, 2018]. http://www
.google.com /url?sa=t&rct =j&q=&esrc =s&source =web&cd =1&cad=rja&uact =8&ved =0ahUKEwjDn-botuXZAhWnTd8KHUTqBVAQFggnMAA&url =http%3A%2F%2Fwww .avapl .org%2Fpub%2FPrimaryCare %2FIntegrated%2520Primary %2520Care--Operations %2520Manual%25202-10-05 .doc&usg =AOvVaw0POxIX6zmY1o6x8ChZ6Lxd. - Vachon GC, Ezike N, Brown-Walker M, Chhay V, Pikelny I, Pendergraft TB. Improving access to diabetes care in an inner-city, community-based outpatient health center with a monthly open-access, multistation group visit program. Journal of the National Medical Association. 2007;99(12):1327–1336. [PMC free article: PMC2575933] [PubMed: 18229769]
- van Eggermond AM, Schaapveld M, Lugtenburg PJ, Krol ADG, de Boer JP, Zijlstra JM, Raemaekers JMM, Kremer LCM, Roesink JM, Louwman MWJ, Aleman BMP, van Leeuwen FE. Risk of multiple primary malignancies following treatment of Hodgkin lymphoma. Blood. 2014;124(3):319. [PubMed: 24740811]
- van Nimwegen FA, Schaapveld M, Cutter DJ, Janus CPM, Krol ADG, Hauptmann M, Kooijman K, Roesink J, van der Maazen R, Darby SC, Aleman BMP, van Leeuwen FE. Radiation dose–response relationship for risk of coronary heart disease in survivors of Hodgkin lymphoma. Journal of Clinical Oncology. 2016;34(3):235–243. [PubMed: 26573075]
- van Ryn M, Sanders S, Kahn K, van Houtven C, Griffin JM, Martin M, Atienza AA, Phelan S, Finstad D, Rowland J. Objective burden, resources, and other stressors among informal cancer caregivers: A hidden quality issue? Psycho-Oncology. 2011;20(1):44–52. [PMC free article: PMC4479404] [PubMed: 20201115]
- Vockley JG, Niederhuber JE. Diagnosis and treatment of cancer using genomics. British Medical Journal. 2015;350:1–9.
- Vuotto SC, Krull KR, Li C, Oeffinger KC, Green DM, Patel SK, Srivastava D, Stovall M, Ness KK, Armstrong GT, Robison LL, Brinkman TM. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2017;123(3):521–528. [PMC free article: PMC5258824] [PubMed: 27764524]
- Wagner EH, Grothaus LC, Sandhu N, Galvin MS, McGregor M, Artz K, Coleman EA. Chronic care clinics for diabetes in primary care: A system-wide randomized trial. Diabetes Care. 2001;24(4):695–700. [PubMed: 11315833]
- Wagner L, Sweet J, Butt Z, Lai J, Cella D. Measuring patient self-reported cognitive function: Development of the functional assessment of cancer therapy–cognitive function instrument. The Journal of Supportive Oncology. 2009;7:W32–W39.
- Wald H. Poised at the abyss: A wife faces her neurologist husband's brain cancer. 2016. [March 11, 2018]. https://www
.kevinmd.com /blog/2016/07/poised-abyss-wife-faces-neurologist-husbands-brain-cancer.html. - Warrington L, Absolom K, Velikova G. Integrated care pathways for cancer survivors—a role for patient-reported outcome measures and health informatics. Acta Oncologica. 2015;54(5):600–608. [PubMed: 25751758]
- Weaver KE, Forsythe LP, Reeve BB, Alfano CM, Rodriguez JL, Sabatino SA, Hawkins NA, Rowland JH. Mental and physical health-related quality of life among U.S. cancer survivors: Population estimates from the 2010 National Health Interview Survey. Cancer Epidemiology, Biomarkers & Prevention. 2012;21(11):2108–2117. [PMC free article: PMC3645867] [PubMed: 23112268]
- Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. The Lancet Oncology. 2011;12(7):703–708. [PubMed: 21354373]
- Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA: A Cancer Journal for Clinicians. 2015;65(2):123–138. [PMC free article: PMC4355212] [PubMed: 25483452]
- Willard VW, Klosky JL, Li C, Srivastava DK, Brinkman TM, Robison LL, Hudson MM, Phipps S. The impact of childhood cancer: Perceptions of adult survivors. Cancer. 2017;123(9):1625–1634. [PMC free article: PMC5400693] [PubMed: 28098955]
- Wilson PM. The UK expert patients program: Lessons learned and implications for cancer survivors' self-care support programs. Journal of Cancer Survivorship. 2008;2(1):45–52. [PubMed: 18648986]
- Win AK, Lindor NM, Young JP, Macrae FA, Young GP, Williamson E, Parry S, Goldblatt J, Lipton L, Winship I, Leggett B, Tucker KM, Giles GG, Buchanan DD, Clendenning M, Rosty C, Arnold J, Levine AJ, Haile RW, Gallinger S, Marchand LL, Newcomb PA, Hopper JL, Jenkins MA. Risks of primary extracolonic cancers following colorectal cancer in Lynch syndrome. Journal of the National Cancer Institute. 2012;104(18):1363–1372. [PMC free article: PMC3529597] [PubMed: 22933731]
- Yabroff KR, Lamont EB, Mariotto A, Warren JL, Topor M, Meekins A, Brown ML. Cost of care for elderly cancer patients in the United States. Journal of the National Cancer Institute. 2008;100(9):630–641. [PubMed: 18445825]
- Yabroff KR, Dowling EC, Guy GP, Banegas MP, Davidoff A, Han X, Virgo KS, McNeel TS, Chawla N, Blanch-Hartigan D, Kent EE, Li C, Rodriguez JL, de Moor JS, Zheng Z, Jemal A, Ekwueme DU. Financial hardship associated with cancer in the United States: Findings from a population-based sample of adult cancer survivors. Journal of Clinical Oncology. 2016;34(3):259–267. [PMC free article: PMC4872019] [PubMed: 26644532]
- Zafar SY, Abernethy AP. Financial toxicity, part I: A new name for a growing problem. Oncology. 2013a;27(2):80–149. [PMC free article: PMC4523887] [PubMed: 23530397]
- Zafar SY, Abernethy AP. Financial toxicity, part II: How can we help with the burden of treatment-related costs? Oncology. 2013b;27(4):253–254. [PubMed: 23781687]
- Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JCI, Lu Q, Krull K. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27(14):2396–2404. [PMC free article: PMC2677925] [PubMed: 19255309]
- Zeng Y, Cheng ASK, Chan CCH. Meta-analysis of the effects of neuropsychological interventions on cognitive function in non-central nervous system cancer survivors. Integrative Cancer Therapies. 2016;15(4):424–434. [PMC free article: PMC5739163] [PubMed: 27151596]
- Zevon MA, Schwabish S, Donnelly JP, Rodabaugh KJ. Medically related legal needs and quality of life in cancer care. Cancer. 2007;109(12):2600–2606. [PubMed: 17487862]
- Zhang FF, Liu S, John E, Must A, Demark-Wahnefried W. Diet quality of cancer survivors and non-cancer individuals: Results from a national survey. Cancer. 2015;121(23):4212–4221. [PMC free article: PMC4667562] [PubMed: 26624564]
- Zheng Z, Han X, Guy GP, Davidoff AJ, Li C, Banegas MP, Ekwueme DU, Yabroff KR, Jemal A. Do cancer survivors change their prescription drug use for financial reasons? Findings from a nationally representative sample in the United States. Cancer. 2017;123(8):1453–1463. [PMC free article: PMC6080209] [PubMed: 28218801]
- WORKSHOP OVERVIEW
- OVERVIEW OF CANCER SURVIVORSHIP CARE
- PHYSICAL WELL-BEING IN CANCER SURVIVORSHIP
- PSYCHOSOCIAL WELL-BEING AND FAMILY CONSIDERATIONS IN CANCER SURVIVORSHIP
- SOCIOECONOMIC CONSIDERATIONS IN CANCER SURVIVORSHIP
- MODELS OF SURVIVORSHIP CARE DELIVERY
- POLICY OPTIONS TO IMPROVE CANCER SURVIVORSHIP CARE
- ADVANCING PROGRESS IN CANCER SURVIVORSHIP CARE
- WRAP-UP
- REFERENCES
- Proceedings of a Workshop - Long-Term Survivorship Care After Cancer TreatmentProceedings of a Workshop - Long-Term Survivorship Care After Cancer Treatment
Your browsing activity is empty.
Activity recording is turned off.
See more...