NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Androgens are an important class of C19 steroid hormones that control normal male development and reproductive function. The main circulating androgen is testosterone, which is produced in the Leydig cells of the testis and can also act as a pro-hormone after being metabolized to dihydrotestosterone (DHT) or estradiol (E2). The biological actions of testosterone and DHT are mediated by the androgen receptor, a member of the nuclear receptor superfamily, which in response to hormone regulates gene expression in target tissues. In this chapter the biosynthesis of androgens, receptor structure/function, and the consequences of genetic changes impacting on receptor expression and signaling in disorders of male development are discussed. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Androgens are important hormones for expression of the male phenotype. They have characteristic roles during male sexual differentiation, but also during development and maintenance of secondary male characteristics and during initiation and maintenance of spermatogenesis (1, 2). The two most important androgens in this respect are testosterone and 5α-dihydrotestosterone [Figure 1].
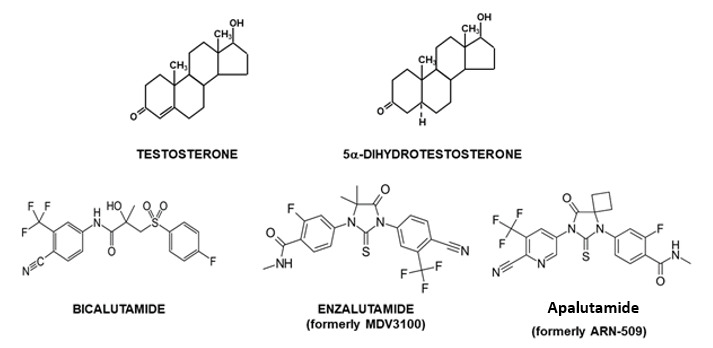
Figure 1.
Structure of testosterone and 5α-dihydrotestosterone and anti-androgens.
While acting through the same androgen receptor, each androgen has its own specific role during male sexual differentiation: testosterone is directly involved in development and differentiation of Wolffian duct derived structures (epididymides, vasa deferentia, seminal vesicles and ejaculatory ducts) [Figure 2A], whereas 5α-dihydrotestosterone, a metabolite of testosterone, is the active ligand in a number of other androgen target tissues, like urogenital sinus and tubercle and their derived structures (prostate gland, scrotum, urethra, penis) [Figure 2B] (3, 4).
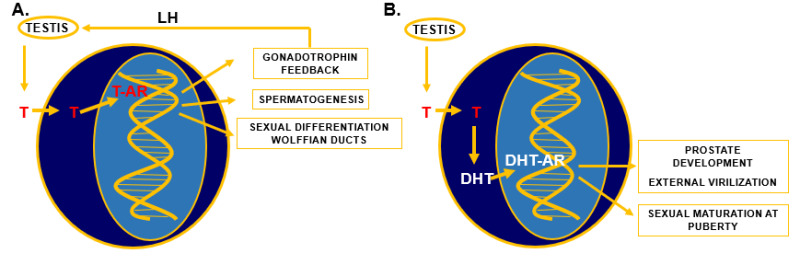
Figure 2.
Specific actions of testosterone (T) and 5α-dihydrotestosterone (DHT). A) Testosterone is synthesized in the testis under the control of luteinizing hormone (LH) from the pituitary. After entering target cells in the hypothalamus, pituitary, testis, and Wolffian duct, T binds to the androgen receptor (AR) and the T-AR complex binds to specific DNA sequences and regulates gene transcription, which can result in negative feedback regulation of gonadotrophin synthesis and secretion, in initiation and regulation of spermatogenesis, and in differentiation and development of Wolffian ducts. B) T is synthesized in the testis under the control of LH, enters target cells in urogenital sinus, urogenital tubercle, and several other androgen target tissues and is metabolized to DHT by the enzyme 5α-reductase type 2. DHT binds directly to the AR and the DHT-AR complex interacts with specific DNA sequences and regulates gene transcription resulting in differentiation and development of the prostate, the external genitalia, and during puberty several secondary male sex characteristics.
The interaction of both androgens with the androgen receptor is different. Testosterone has a twofold lower affinity than 5α-dihydrotestosterone for the androgen receptor, while the dissociation rate of testosterone from the receptor is five-fold faster than of 5α-dihydrotestosterone (5). However, testosterone can compensate for this "weaker" androgenic potency during sexual differentiation and development of Wolffian duct structures via high local concentrations due to diffusion from the nearby positioned testis. In more distally located structures, like the urogenital sinus and urogenital tubercle the testosterone signal is amplified via conversion to 5α-dihydrotestosterone.
ANDROGEN BIOSYNTHESIS
Androgens (testosterone and 5α-dihydrotestosterone) belong to the group of steroid hormones. The major circulating androgen is testosterone, which is synthesized from cholesterol in the Leydig cells in the testis. Testosterone production in the fetal human testis starts during the sixth week of pregnancy. Leydig cell differentiation and the initial early testosterone biosynthesis in the fetal testis are independent of luteinizing hormone (LH) (6-8). During testis development production of testosterone comes under the control of LH which is produced by the pituitary gland. Synthesis and release of LH is under control of the hypothalamus through gonadotropin-releasing hormone (GnRH) and inhibited by testosterone via a negative feedback mechanism [Figure 2A] (9).The biosynthetic conversion of cholesterol to testosterone involves several discrete steps, of which the first one includes the transfer of cholesterol from the outer to the inner mitochondrial membrane by the steroidogenic acute regulatory protein (Star) and the subsequent side chain cleavage of cholesterol by the enzyme P450scc (10). This conversion, resulting in the synthesis of pregnenolone, is the rate-limiting step in testosterone biosynthesis. Subsequent steps require several enzymes including, 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase/C17-20-lyase and 17β-hydroxysteroid dehydrogenase type 3 [Figure 3] (11).
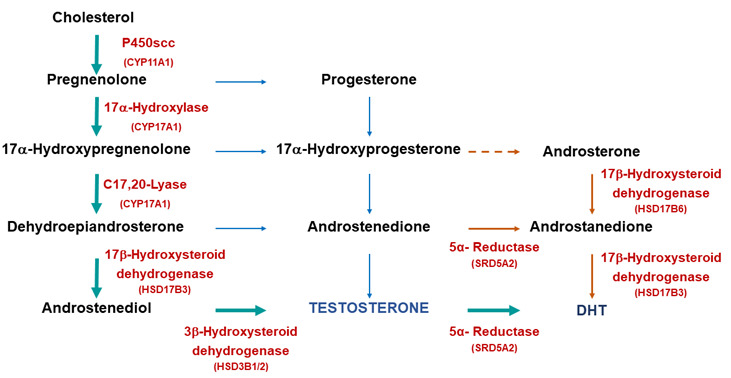
Figure 3.
Biosynthetic pathways for testosterone and DHT synthesis. The classic pathway show testosterone synthesized from cholesterol with further metabolism to DHT. The alternative or “backdoor” pathway shows DHT production without going through testosterone. Note only some of the enzymes are shown for clarity.
METABOLISM OF TESTOSTERONE TO 5ΑΑ-DIHYDRO-TESTOSTERONE
Metabolism of testosterone to 5α-dihydrotestosterone occurs through the classical pathway [Figure 3] and is essential for initiation of the differentiation and development of the urogenital sinus into the prostate [Figure 2B]. Differentiation of male external genitalia (penis, scrotum and urethra) also strongly depends on the conversion of testosterone to 5α-dihydrotestosterone in the urogenital tubercle, labioscrotal swellings, and urogenital folds (1). In recent research there has been considerable interest in the alternative or ‘backdoor’ pathway of DHT production (12 and references therein). This pathway has been found to have a significant role in the normal masculinization of the male fetus (see 13) and abnormal virilization of the female fetus in cases of congenital adrenal hyperplasia resulting from mutations in the enzyme P450 oxidoreductase (14).
The irreversible conversion of testosterone to 5α-dihydrotestosterone is catalyzed by the microsomal enzyme 5α-reductase type 2 (SRD5A2) and is NADPH dependent [Figure 4] (15). The cDNA of the gene for 5α-reductase type 2 codes for a protein of 254 amino acid residues with a predicted molecular mass of 28,398 Dalton (16, 17).

Figure 4.
Metabolism of testosterone to DHT by the enzyme 5α-reductase type 2 (SDR5A2).
The NH2-terminal part of the protein contains a subdomain proposed to be involved in testosterone binding, while the COOH-terminal region is involved in NADPH-binding (3). The enzyme 5α-reductase type 2 belongs to the 5α-reductase family which is composed of 3 subfamilies with a total of 5 members (18). There are three isozymes: type 1, type 2 and the more recently discovered type 3, which has a role in the conversion of polyprenols to dolichols (important step in protein N-glycosylation) (19, 20). The other members are the proteins glycoprotein synaptic 2 (GPSN2) and glycoprotein synaptic 2 like (GPNS2L) and are most likely involved in double bond reduction during fatty acid elongation (21).
ANDROGEN ACTION
The Androgen Receptor and the Nuclear Receptor Family
Actions of androgens are mediated by the androgen receptor (NR3C4; Nuclear Receptor subfamily 3, group C, gene 4). This ligand-dependent transcription factor belongs to the superfamily of 48 known human nuclear receptors (22). This family includes receptors for steroid hormones, thyroid hormones, all-trans and 9-cis retinoic acid, 1,25 dihydroxy-vitamin D, ecdysone and activators of peroxisome proliferation (23-25). An increasing number of nuclear proteins have been identified with a protein structure homologous with that of nuclear receptors, but without a known ligand. These so-called "orphan" receptors form an important subfamily of transcription factors acting either in the absence of any ligand or with yet unknown endogenous ligands (26). Comparative structural and functional analysis of nuclear hormone receptors has revealed a common structural organization in 4 different functional domains: a NH2-Terminal Domain, a DNA-Binding Domain, a Hinge Region and a Ligand Binding Domain [Figure 5].

Figure 5.
Steroid hormone receptor family. Sequence homologies between the human androgen receptor (hAR), human progesterone receptor (hPR), human glucocorticoid receptor (hGR), human mineralocorticoid receptor (hMR), and the human estrogen receptor alpha (hERα) and beta (hERβ).
The current model for androgen action involves a multi-step mechanism as depicted in Figure 6. Upon entry of testosterone into the androgen target cell, binding occurs to the androgen receptor either directly or after its conversion to 5α-dihydrotestosterone. Binding to the receptor is followed by dissociation of chaperone protein complexes (e.g., heat shock proteins) in the cytoplasm, simultaneously accompanied by a conformational change of the receptor protein resulting in a transformation and a translocation to the nucleus. The complex of chaperone and chaperone-associated proteins is collectively called the ‘foldosome’ and has functions beyond the classical role in the cytosol. The foldosome can for instance affect nuclear translocation and target gene expression (27, 28). Upon binding in the nucleus to specific DNA-sequences the receptor dimerizes with a second molecule and the homodimer entity recruits further additional proteins (e.g., coactivators, general transcription factors, RNA-polymerase II) via specific interaction motifs (29). This finally results in transcriptional activation or suppression of specific androgen responsive genes (30).

Figure 6.
Simplified model of androgen action in an androgen target cell. The androgen receptor (AR) binds testosterone or its active metabolite DHT. After disassociation of heat shock proteins (hsp) the receptor enters the nucleus via an intrinsic nuclear localization signal and binds as a homodimer to specific DNA elements present as enhances upstream of androgen target genes. The next step is recruitment of coactivators, which form the communication bridge between the receptor and several components of the transcription machinery. The direct and indirect binding of the androgen receptor with several components of the transcription machinery (e.g., RNA polymerase II (RNA Pol II), general transcription factors (GTFs)) are key events in nuclear signaling. This communication triggers subsequent mRNA synthesis and consequently protein synthesis resulting in androgen responses. A non-genomic pathway involving the AR via cross-talk with the Src/Raf-1Erk-2 pathway is also known.
Interestingly androgen signaling via the androgen receptor can also occur in a non-genomic, rapid and sex-nonspecific way by crosstalk with the Scr, Raf-1, Erk-2 pathway [Figure 6] (31, 32). The classical androgen receptor is also involved in androgen-mediated induction of Xenopus oocyte maturation via the (MAPK)-signaling cascade in a transcription independent way (33, 34).
Cloning and Structural Organization of the Androgen Receptor Gene
Since cloning of the human androgen receptor cDNA our insights into the mechanism of androgen action have increased tremendously. Only one androgen receptor cDNA has been identified and cloned, despite the two different ligands (35-38). The concept of two hormones and one receptor to explain the different actions of androgens has been generally accepted and, according to the information available from the human genome project, it is very unlikely that additional genes exist coding for a functional nuclear receptor with androgen receptor-like properties (25).
The androgen receptor gene is located on the X-chromosome at Xq11.2 -12. The gene spans 186,587 kilobases (kb) in total and codes for a protein with a molecular mass of approximately 110 kDa [Figure 7] (39, 40). The gene consists of 8 coding exons and the structural organization of the coding exons is essentially identical to those of the genes coding for the other steroid hormone receptors (e.g., exon/intron boundaries are highly conserved) and is characterized by unusually long 5’- and 3’-UTRs [Figure 7] (36, 41-43, 47). As a result of differential splicing in the 3' - untranslated region two androgen receptor mRNA species (of around 7.5 and 10 kb, respectively) have been identified in several human tissues and cell lines (36): only the larger transcript is seen in rodent tissues (36, 43, 47). There is no structural indication in the androgen receptor mRNA for any preferential use of either of the two transcripts or transcript specific functions, but it can be speculated that tissue-specific factors may determine which transcript is present in which androgen target tissue. In the human prostate and in genital skin fibroblasts the 10 kb size mRNA is predominantly expressed (43). It may also be significant that a number of micro-RNAs have been identified and validated that target the 3’-UTR that are likely to contribute to the regulation of receptor levels (44-46) [Figure 7].

Figure 7.
Human androgen receptor gene was mapped to the long arm of the X chromosome. The human androgen receptor gene consists of coding exons and unusually long 5’- and 3’ UTRs. These have been shown to be important for transcriptional regulation (binding sites for both positive and negative regulatory factors) in the case of the 5’UTR. The 3’UTR region of the mRNA is targeted by a number of microRNAs (miRNAs). The androgen receptor has been shown to downregulate its own mRNA through response elements located in the 5’UTR and exon 2.
Regulation and Expression of the Androgen Receptor Gene
The promoter for the androgen receptor gene lacks TATA and CCAAT elements and transcription is driven primarily by the Zn-finger transcription factor Sp1. Sp1 binds to GC-boxes upstream of the transcription start site (-46 to -41 bps) and within the 5’UTR (+429 to +442) (47-52) [Figure 7]. In addition, the promoter and the region spanning the 5’-UTR and exon 1 contains a CpG island that demonstrates tissue-selective methylation patterns (53) and to be associated with loss of AR expression in prostate cancer (54).
Transcription of the receptor gene is under both positive and negative regulation (55, 58). Recent studies have focused on the auto-down regulation of the receptor mRNA in prostate cells. Balk and co-workers (56) identified, using chromatin immunoprecipitation (ChIP), binding sites for ligand bound androgen receptor within the second intron and a second negative androgen response elements has been characterized in the 5’UTR (+611 bp) of the human receptor gene (57). Unravelling the molecular mechanism(s) for androgen-dependent down regulation, including possible synergy between the identified elements, in different cell types and tissues is an active area of research (58).
In addition to regulation by hormone, recent work has also highlighted the importance of the balance between positive (Sp1) and negative (Purα) transcription factors binding to the 5’UTR of the human gene in determining the expression of receptor mRNA in different prostate cancer cell models (52 and references therein).
Androgen Receptor Polymorphisms
The androgen receptor DNA-binding and ligand-binding domains have a high homology with the corresponding domains of the other members of the steroid receptor subfamily (59) [Figure 5].
There is a remarkably low homology between the androgen receptor NH2-terminal domain and that of the other steroid receptors [Figure 5, see above] (60-65). A poly-glutamine stretch, encoded by a polymorphic (CAG)nCAA - repeat is present in the NH2-terminal domain (66). The length of the repeat has been used for identification of X-chromosomes for carrier detection in pedigree analyses (67, 68). Variation in length (9 - 38 glutamine residues) is observed in the normal population and has been suggested to be associated with a very mild modulation of androgen receptor activity (69). This assumption is based on in vitro experiments after transient transfection of androgen receptor cDNA's containing (CAG)nCAA - repeats of different lengths (70, 71). In translating this finding to the in vivo situation, it can be envisaged that either shorter or longer repeat lengths can result in a relevant biologic effect during life. This concept has been explored in epidemiological studies of men with prostate cancer or infertility. With respect to prostate cancer, a clear picture has not emerged, and controversy persists. In several studies, shortening of the (CAG)nCAA repeat length in exon 1 of the androgen receptor gene was found to correlate with an earlier age of onset of prostate cancer, and a higher tumor grade and aggressiveness (72-74). However, in other epidemiological studies in prostate cancer patients these associations were not confirmed (75, 76).
In several investigations in male infertile patients an association was found between a longer (CAG)nCAA repeat and the risk of defective spermatogenesis (77-79). This suggests that a less active androgen receptor, due to a moderate expanded repeat length, may be a factor in the etiology of male infertility.
The (CAG)nCAA - repeat in exon 1 of the androgen receptor gene is expanded in patients with spinal and bulbar muscular atrophy (SBMA) and varies between 38 and 75 repeat units (69, 80, 81). Spinal and bulbar muscular atrophy is characterized by progressive muscle weakness and atrophy and is associated with nuclear accumulation of androgen receptor protein with the expanded polyglutamine stretch in motor neurons. Clinical symptoms usually manifest in the third to fifth decade and result from severe depletion of lower motor nuclei in the spinal cord and brainstem (69, 82, 83). SBMA patients frequently exhibit endocrinological abnormalities including testicular atrophy, infertility, gynecomastia, and elevated LH, FSH and estradiol levels. Sex differentiation proceeds normally, and characteristics of mild androgen insensitivity appear later in life.
The neurotoxicity of the polyglutamine androgen receptor may involve generation of NH2-terminal truncation fragments, as such peptides occur in SBMA patients, but several other mechanisms have also been suggested for the molecular basis of SBMA (84, 85). Therapeutic approaches in SBMA are focusing on reducing nuclear localized mutant androgen receptor via enhanced mutant androgen receptor degradation or by disrupting the interaction with androgen receptor coregulators (86, 87). In a phase 3 clinical trial it was shown that the use of leuprorelin, a synthetic neuropeptide with an inhibitory action on LH secretion and consequently on testicular testosterone synthesis, is associated with improved swallowing function in SBMA patients, suggesting that interference by a pharmacon in testosterone-mediated AR aggregation can be a potential therapy in SBMA patients (88). The selective action of dutasteride (a 5α-reductase inhibitor) in motor neurons, by reducing significantly the formation of the active androgen 5α-dihydrotestosterone, resulted in a slowdown of the progression of SBMA and illustrated that active androgen depleting therapies can be promising in the treatment of SBMA (89).
In general patients with an expanded CAG repeat are expected to have a low incidence of prostate cancer. However, a rare case has been reported in which a high stage prostate cancer has been detected in a SBMA patient, which responded to a maximal androgen blockade therapy (90).
An important step in the receptor-mediated mechanism of action of androgens involves the NH2-terminal domain interacting with the COOH-terminal ligand binding domain (N/C interaction). (See details below under ‘Androgen Receptor Functional Domain Structure’). This N/C interaction is also a prerequisite for androgen receptor aggregation and toxicity in SBMA. Interference of the N/C interaction by selective androgen receptor modulators ameliorates aggregation and toxicity (91).
The androgen receptor is a substrate for numerous post-translational modifications (see below) and phosphorylation of serine 516 has been associated with cleavage of the receptor and cytotoxicity (92). In contrast, phosphorylation of serines 215 and 793, by Akt kinase, was found to prevent nuclear translocation and receptor transactivation (93). Interestingly, methylation on arginine residues 210, 212, 787, 789 enhanced cytotoxicity and the authors proposed that this was as a consequence of mutual antagonism of phosphorylation (serines 215, 792) and arginine methylation (94). Similarly, prevention of SUMOylation rescues the SBMA phenotype in a mouse model by enhancing receptor-dependent transcriptional activity (95).
The isoflavone genistein, which is derived from soy, is a potential therapeutic agent in SBMA, because this androgen receptor modulator can effectively disrupt the interaction between the co-regulator ARA70 and the androgen receptor and promotes the degradation of the mutant receptor in neuronal cells. (96). Similarly, targeting molecular chaperone complexes with small molecule modulators (e.g., 17-AAG, YM-1) has been shown to reduce neurotoxicity and enhance receptor-dependent degradation (reviewed in 81).
Several therapeutic approaches have been investigated at different levels in the androgen receptor signaling pathway and aggregation process, in SBMA mouse models. However, translating these results to the human situation in SBMA patients has its limitations and is far from a complete cure of SBMA patients (97, 98).
ANDROGEN RECEPTOR AMINO ACID NUMBERING
The current sequence of the androgen receptor cDNA and the amino acid numbering of the corresponding protein is based on the NCBI reference sequence NM_000044.3. This is different from the original numbering scheme used over the past 20 years that was based on Gen-Bank mRNA sequence M20132.1 (36).
In order to correctly identify mutations previously published, the following changes should be kept in mind: the variable polyglutamine tract length is two longer (23 instead of 21), whereas the variable polyglycine tract length is one shorter (23 instead of 24) for NM_000044.3 versus M20132.1, respectively. Consequently, the androgen receptor protein of the new reference sequence is one amino acid longer, that is, 920 residues, leading to a +2 shift in amino acid numbering between residues 78 and 449 and to a +1 shift between residues 472 and 919 compared with the previously used standard reference sequence. The +1 shift involves all the amino acid residues in the DNA-binding domain (DBD) and ligand-binding domain (LBD). The new reference numbering is further explained and illustrated in Figure 8 and will be used throughout the text.

Figure 8.
Reference numbering of the androgen receptor (AR) of protein. The numbering of the amino acid residues is according to National Center for Biotechnology Information (NCBI) reference sequence number NM_000044.3, which refers to a gene size of 187,246 nucleotides and an AR protein of 920 amino acid residues with a polyglutamine tract of 23 and a polyglycine tract of 23 (110). Amino acid numbering +2 between 78 and 449; Amino acid numbering +1 between 472 and 919. In addition, a number of splice variants of the AR have been identified in prostate cancer cell lines and patient samples. These splice variants lack most or all of the LBD but retain a functional DBD and NTD with unique C-terminal sequences derived from cryptic exons (CE) (e.g., AR-v7).
ANDROGEN RECEPTOR: FUNCTIONAL DOMAIN STRUCTURE
The NH2-terminal Domain
The androgen receptor NH2-terminal domain (NTD) harbors the major transcription activation functions and several structural subdomains. The NTD of the androgen receptor, as that of the other steroid receptors, can be considered as an intrinsically disordered protein domain, existing as an ensemble of conformers. It has a structure between a fully unfolded state and a structured folded conformation: this molten-globule-like conformation has the propensity to form helical structures, despite its structurally plasticity (99-102). Within its 539 amino acids, two independent activation domains have been identified: activation function 1 (AF-1) (located between residues 103 and 372) that is essential for transcriptional activity of full-length androgen receptor, and activation function 5 (AF-5) (located between residues 362-486) that is required for transactivity of a constitutively active androgen receptor, which lacks its LBD (103). Evidence is available now that the AF-5 region in the receptor NH2-terminal domain interacts with a glutamine rich domain in p160 cofactors like SRC-1 and TIF2/GRIP1 and not with their LxxLL-like protein interacting motifs (104-107).
Recent years have seen further structural and functional insights into the intrinsically disordered NTD. Key discoveries include the high-resolution mapping of helical regions within the AF1 domain (108) that were in very good agreement with previous predictions (109); and the identification of a helical segment involving the WHTLF motif responsible for TFIIF binding (110). Also of note are helical regions mapping to the poly-Q and adjacent leucine stretch (111) and the sequence immediately preceding the DBD (112). Collectively, these studies emphasize the presence of helical regions within the NTD and its propensity to adopt a more helical structure underpinning function.
Another function of the androgen receptor NH2-terminal domain is its binding to the COOH-terminal LBD (N/C interaction) (113, 114). The NH2-terminal regions required for the binding of the LBD have been mapped to two essential units: the first 36 amino acids and residues 372-495 (115).
The hormone dependent interaction of the NH2-terminal domain with the ligand binding domain can play a role in stabilization of the androgen receptor dimer complex and in stabilization of the ligand receptor complex by slowing the rate of ligand dissociation and decreasing receptor degradation (116, 117). Agonists like T and DHT, but not antagonists like hydroxyflutamide or bicalutamide induce the N/C interaction in full length receptor. In a FRET (fluorescence resonance energy transfer) study it was clearly shown that the androgen receptor N/C interaction is rapidly initiated in the cytoplasm after hormone binding as an intramolecular interaction and is followed by an intermolecular N/C interaction in the nucleus, contributing to receptor dimerization (118). The N/C interaction occurs preferentially in the mobile androgen receptor, where it protects the coactivator binding groove for ultimately unfavorable protein-protein interactions. Specifically bound to DNA, the N/C interaction is lost allowing cofactor binding (119). Several mutations in the ligand binding domain, detected in patients with the syndrome of androgen insensitivity, negatively affect the interaction of the NH2-terminal domain with the ligand binding domain, while androgen binding was impaired, indicating the importance of this interaction (120).
In addition to the role of the NH2-terminal domain in protein-protein interactions it has also been reported to modulate DNA binding, leading to a lower apparent binding affinity for both selective and non-selective response elements (see also below) (121). These findings suggest a further role of the NH2-terminal domain, in interdomain interactions and allosteric regulation of receptor activity.
The DNA-Binding Domain
The DNA-binding domain is the best conserved among the members of the receptor superfamily [Figure 5]. It is characterized by a high content of basic amino acids and by nine conserved cysteine residues [Figure 9A]. Detailed structural information has been published on the crystal structure of the DNA-binding domain of the glucocorticoid receptor complexed with DNA (122). 3D-information is also available for the androgen receptor-DNA interaction on an artificial DNA response element (123) [Figure 9B]. The folding of the DBD is similar to that reported for the glucocorticoid and estrogen receptor DBDs.
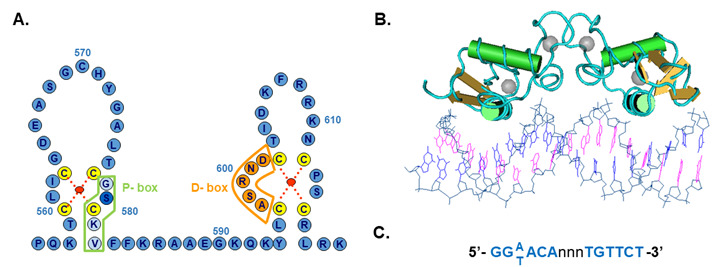
Figure 9.
Structure of the DNA binding domain of the androgen receptor. A) The protein structure is represented in the one letter code. The domain consists of two zinc cluster modules, which are stabilized by the coordination binding of a zinc atom (red dot) by 4 cysteine residues (yellow). The first zinc cluster contains the P-box (proximal box) of which three residues determine androgen response element recognition. The second zinc cluster contains the D-box (distal box) in which amino acids are located that are involved in protein-protein interactions with a second receptor molecule in the homodimer complex. B) Structure of the AR-DBD bound to DNA (Pdb 1R41). C) Consensus androgen receptor response element.
Briefly, the DNA-binding domain has a compact, globular structure in which three substructures can be distinguished: two zinc clusters and a more loosely structured carboxy terminal extension (CTE) (124). Both zinc substructures contain centrally one zinc atom which interacts via coordination bonds with four cysteine residues [Figure 9].
The two zinc coordination centers are both C-terminally flanked by an α-helix (122, 123). The two zinc clusters are structurally and functionally different and are encoded by two different exons [see Figures 7 and 8]. The α-helix of the most N-terminal located zinc cluster interacts directly with nucleotides of the hormone response element in the major groove of the DNA. Three amino acid residues at the N-terminus of this α-helix are responsible for the specific recognition of the DNA-sequence of the responsive element [Figure 9A]. These three amino acid residues, the so-called P(proximal)-box [Gly; Ser; Val;] are identical in the androgen, progesterone, glucocorticoid and mineralocorticoid receptors, and differ from the residues at homologous positions in the estradiol receptor. It is not surprising therefore, that the androgen, progesterone, glucocorticoid and mineralocorticoid receptors can recognize the same response element. The receptor DNA binding domain requires a CTE of minimally four residues (amino acids 626 – TLGA – 629) for proper binding to an ARE (androgen response element) with an inverted repeat of high affinity ARE-half sites and a CTE of at least twelve residues (amino acids 626 – TLGARKLKKLGN – 637) for binding to an ARE with one high and one low affinity half site (125). For the hormone and tissue-specific responses of the different receptors additional determinants are needed. Important in this respect are DNA-sequences flanking the hormone response element, receptor interactions with other proteins and receptor concentrations. The second zinc cluster motif is involved in protein-protein interactions such as receptor dimerization via the so-called D(distal)-box [Figure 9A and B] (122, 123).
DNA Response Elements for the Androgen Receptor
In vitro the androgen receptor binds to 15 bp palindromic sequences [Figure 9C]. These non-selective elements are also recognized and bound by the glucocorticoid, mineralocorticoid and progesterone receptors. In contrast, androgen response elements demonstrate selectivity for the receptor. In an animal model, termed Specificity-affecting androgen receptor Knock-in or SPARKI, where the androgen receptor-DBD has been replaced by that of the glucocorticoid receptor-DBD, binding to selective AREs is disrupted (126). These mice have a reproductive phenotype, with male reproductive tissues having reduced weight and size and the animals showing reduced fertility. Interestingly the SPARKI males also demonstrated differential gene expression with the Rhox5 mRNA significantly reduced which correlated with a role for a selective ARE, necessary for receptor-dependent transcription of this gene (126).
More recently a number of genome-wide studies, using chromatin immunoprecipitation (ChIP), have increased our knowledge of androgen-regulated genes and have demonstrated a significant variability in DNA response element architecture, with imperfect palindromic sequences and half-sites identified as potential receptor binding sites (30, 127-131). These studies have also highlighted the enrichment of pioneering factors, such as FOXA1 and GATA2 in close proximity to receptor binding sites (30, 127-131).
The Hinge Region
Between the DNA-binding domain and the ligand binding domain is located a non-conserved hinge region, which is also variable in size in different steroid receptors [Figure 5]. The hinge region can be considered as a flexible linker between the ligand binding domain and the rest of the receptor molecule. The hinge region is important for nuclear localization and contains a bipartite nuclear localization signal. Co-repressor binding can also occur via the hinge region (125). In some nuclear receptors, including the androgen receptor, acetylation can occur in the hinge region at a highly conserved consensus site [KLLKK] [Figure 11, see below] (132, 133).
The Ligand Binding Domain
Finally, the second-best conserved region is the hormone binding domain. This domain is encoded by approximately 250 amino acid residues in the C-terminal end of the molecule [Figure 5, see above] (37, 60-63, 134). The crystal structure of the human androgen receptor ligand binding in complex with the synthetic ligand methyltrienolone (R1881) and 5α-dihydrotestosterone, respectively, have been determined [Figure 10A and B] (135, 136).

Figure 10.
Structure of the ligand binding domain of the human androgen receptor. A) The crystal structure of the LBD with DHT bound (pdb 1137). Specific amino acid- hormone interactions are illustrated in the right-hand panel. B) The LBD structure with the synthetic agonist R1881 and a coactivator peptide with an FxxLF motif bound to AF2 region (pink oval) (pdb 1XOW). C) Structure of the LBD showing the location of the BF3 pocket (blue oval) with triiodothyroacetic acid/TRIAC bound (pdb 2PKL).
The 3-dimensional structure has the typical nuclear receptor ligand binding domain fold (59). Interestingly the ligand binding pocket consists of 18 amino acid residues interacting more or less directly with the bound ligand, with a relatively small number of specific hydrogen-bonds and hydrophobic interactions determining hormone-selectivity [Figure 10A] (135). The ligand binding pocket is somewhat flexible and can accommodate ligands with different structures. The structural data are being used in designing optimized selective androgen receptor modulators (SARMs) (137, 138). Several AR mutations found in prostate tumors have been investigated functionally, including T878S, T878A, H875T, V716M, W742C, and L702H as a single mutation or in combination with T878A. Similar to T878A these AR mutations have a broadened ligand specificity and are activated by different low affinity ligands like estradiol, progesterone, glucocorticoids and different partial and full antagonists (139-146).
Crystallographic data on the ligand binding domain complexed with agonist predict 11 helices (no helix 2) with two anti-parallel β-sheets arranged in a so-called helical sandwich pattern. In the agonist-bound conformation the carboxy-terminal helix 12 is positioned in an orientation allowing a closure of the ligand binding pocket. Upon hormone binding the fold of the ligand binding domain results in a globular structure with an interaction surface for binding of interacting proteins like co-activators (AF2) [Figure 10B]. In this way the androgen receptor selectively recruits a number of proteins and can communicate with other partners of the transcription initiation complex. Crystallization studies of wild type androgen receptor ligand binding domain with antagonists have not been reported so far. However, the structural consequences of surface modulatory compounds on the receptor LBD crystals complexed with DHT are promising for future developments of new androgen receptor modulators including a new type of androgen receptor antagonists (147).
The androgen receptor can use different transactivation domains (AF1 and AF5, respectively, in the NH2-terminal domain and AF2 in the COOH-terminal domain) depending on the "form" of the receptor protein [Figure 8, see above] (103). The AF2 function in the ligand binding domain is strongly dependent on the presence of nuclear receptor coactivators. In vivo experiments favor a ligand-dependent functional interaction between the AF-2 region in the ligand-binding domain with the NH2-terminal domain (113, 115). In fact, the AF2 surface demonstrates a preference for more bulky hydrophobic amino acids over the LxxLL motif and the structural basis for this has been described (148-150). Thus, the receptor NTD FxxLF motif [Figure 10B] is more effective at forming a charge clamp with Glu898 and Lys721 and burying the phenylalanine residues into the AF2 pocket, whereas peptides containing the sequence LxxLL make weaker and fewer contacts with the LBD.
Interestingly, a previously unknown regulatory surface cleft, named BF-3, has been identified in the receptor LBD (147) [Figure 10C]. BF-3 comprises of Ile-673, Phe-674, Pro-724, Gly-725, Asn-728, Phe-827, Glu-830, Asn-834, Glu-838 and Arg-841. The androgen receptor transcriptional activity and co-activator binding can be decreased by binding of thyroid hormones triiodothyronine (T3) and TRIAC and three non-steroidal anti-inflammatory drugs to the BF-3 pocket. In addition, several mutations of the amino acid residues of BF-3 have been found in subjects with either androgen insensitivity syndrome (AIS, loss of function mutation) or in prostate cancer (gain of function mutation) (151). Mutational analyses have shown the requirement of several of these amino acid residues for receptor-dependent transcriptional activity. However, these analyses have been performed only in the presence of DHT (147). The influence of each of these residues in the presence of T3, TRIAC or other nonsteroidal anti-inflammatory drugs is therefore unknown.
A long-standing question in the field concerning dimerization of the AR-LBD has recently been resolved with new crystallographic studies (152, 153). The work from the Estébanez-Perpiñá group identified sequences in helix 5 as novel dimerization interface. Significantly, a number of point mutations associated with androgen insensitivity or prostate cancer map to this region emphasizing its functional importance for AR signaling (152).
Androgen Receptor Splice Variants Lacking the LBD
Deletions in the ligand binding domain abolish hormone binding completely (154). Deletions in the N-terminal domain and DNA-binding domain do not affect hormone binding. Deletion of the ligand binding domain leads to a constitutively active androgen receptor protein with trans-activation capacity comparable to the full-length androgen receptor (154). Thus, it appears that the hormone binding domain acts as a repressor of the trans-activation function in the absence of hormone. This regulatory function of the androgen receptor ligand binding domain in the absence of hormone, is not unique for the androgen receptor and has been reported also for the glucocorticoid receptor (155).
The generation of NH2-terminal splice variants involves the use of cryptic exons (AR-v1and -v7) or exon skipping (AR-v12) [Figure 8] (156). Androgen receptor variants have been shown to regulate similar patterns of gene expression to the full-length hormone-bound receptor (157). However, intriguingly there are a growing number of studies reporting unique sets of genes expressed by AR-v7 (157, 158), both expected and variant-specific target genes for AR-v12 (159) or differential regulation of classical androgen receptor-target genes (160). Importantly, these constitutively active splice variants have been identified in prostate cancer cell-lines, xenographs and prostate cancer patients undergoing androgen ablation therapy (157-159, 161-163).
Structural Insights from a DNA-bound Complex of Full-length Androgen Receptor
A major development in our understanding of AR mechanism of action has been the recent description of the structure of the full-length receptor bound to DNA and co-regulatory proteins (SRC-3 and p300) (164). The structures of the receptor alone or in a transcriptional active complex were solved by cryo-EM at resolution of 12 to 20 Å and reveal several interesting features. Particularly striking is the folding of the NTD of each monomer into a ‘life buoy ring’ surround the LBD-DBD dimer, creating a platform for SRC-3 and p300 binding, as well as N/C interaction and contacts between the NTDs (164). In contrast to a similar structure of the estrogen receptor α (165), only one molecule of SRC-3 is bound to the AR and the conformation of each NTD is proposed to be different based on visualizing antibodies recognizing the very N-terminus and AF1 regions resulting in an asymmetric appearance. This could have implications for protein-protein interactions and transcriptional regulation: for, example does the conformation of the NTDs change depending on the nature of the DNA binding site? It was also of note that the binding of p300 was enhanced in the presence of SRC-3, suggesting the latter stabilized the binding of the former. However, it is worth noting that previous biochemical studies demonstrated folding of the AR-AF1, using a chemical chaperone (TMAO) or an SRC-1 polypeptide, similarly enhanced subsequent co-regulatory protein binding (166) supporting a model of induced folding of AF1 and assembly of transcription complexes.
ANDROGEN RECEPTOR POSTTRANSLATIONAL MODIFICATIONS
Methylation, Acetylation, Ubiquitination and SUMOylation
The androgen receptor protein can be extensively covalently modified either by methylation, acetylation, ubiquitination, SUMOylation or phosphorylation [Figure 11] (132, 133, 167-171).
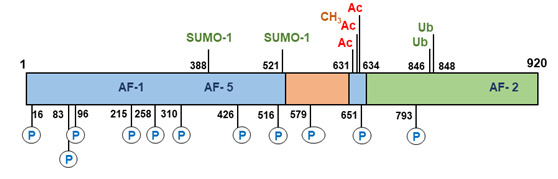
Figure 11.
Post-translational modifications of the human androgen receptor. AC, acetylation of lysine residues (631, 633, and 634); CH3, methylation of lysine (633); P, phosphorylation of serines (16, 83, 96, 215, 258, 310, 426, 516, 651, and 792); SUMO-1, sumoylation on lysines (388 and 521); Ub, ubiquitination of lysines (846 and 848).
All these reactions are reversible and consequently enzymes that mediate dephosphorylation, deacetylation, deubiquitination, demethylation and de-SUMOylation are also potential regulators of androgen receptor activity. A total of 23 sites in the androgen receptor protein have been identified undergoing direct modification (170). These posttranslational modifications can contribute significantly to androgen receptor structure, activity and stability. It has been shown for instance that the histone methyltransferase SET9 is able to methylate the receptor in the hinge region at the Lysine residues 631 and 633 resulting in enhancement of transcriptional activity of the receptor (172, 173). The same Lysine residues together with Lysine 634 can be acetylated and the acetylation-deficient mutants have a decreased transcriptional activity, while the acetylation-mimetic mutations showed an enhanced transcriptional activity (132, 174). Recently, phosphorylation of serine 83 was observed to result in recruitment of the histone acetyltransferase p300, acetylation of the receptor and enhanced receptor stabilization and transcriptional activity (175).
Conversely, disruption of acetylation, through mutating the lysine residues or knock-down of p300 resulted in receptor ubiquitination and degradation. This study elegantly demonstrates how different post-translational modifications of the androgen receptor can work in concert to regulate receptor expression and activity. RNF6 dependent ubiquitination of Lysine residues 846 and 848 in the receptor protein results in recruitment of the coregulator ARA54 by the androgen receptor and directly determines promoter selectivity and specificity of the receptor (176).
SUMOylation of the androgen receptor occurs at two sites Lysine residues 388 and 521, but SUMOylation only at Lysine 388 results in a significant reduction of transcriptional activity (177). However, recent, whole genome analysis revealed that SUMOylation regulated both receptor recruitment to DNA and target gene selection (178). Significantly, the physiological importance of SUMOylation has been demonstrated in a knock-in mouse model, ARKI, where the SUMOylation sites were mutated to arginine (179). Male animals developed normally but were found to be infertile due to defects in epididymal sperm maturation. Crucially, In the ARKI animals the AR-dependent transcriptional activity was impaired in the epididymis and there was an absence of receptor SUMOylation linking this PTM to normal male reproduction and fertility (179).
Phosphorylation
The androgen receptor can be phosphorylated at serine, threonine and tyrosine residues (170, 171, 180, 181). Immediately after translation the androgen receptor becomes phosphorylated resulting in the appearance of two isoforms separable by SDS-polyacrylamide gel electrophoresis (182). The non-phosphorylated faster migrating 110 kDa isoform is converted into a 112 kDa phospho-isoform. Mutational analysis of serine 83 or serine 96 in the androgen receptor NH2-terminal domain abolishes this up-shift indicating that phosphorylation of these serine residues likely contributes to the phosphorylation of the 112 kDa androgen receptor isoform (70, 183). Phosphorylation of Serine 83 by CDK9 stabilizes androgen receptor chromatin binding, mediates transcriptional activity and can influence prostate cancer cell growth (184, 185). This serine residue is also phosphorylated after stimulation of Plexin-B1 resulting in nuclear translocation of the receptor protein (186). Three other androgen receptor phosphorylation sites have been identified using mutational analysis and trypsin-digestion of 32P-labelled androgen receptor followed by HPLC analysis and Edman degradation (183, 187, 188). These include the serine residues at position 516, 651, and 663. Ser-516 phosphorylation by MAP kinase is linked to altering the nuclear cytoplasmic shuttling and to the EGF-induced increase in androgen receptor transcriptional activity (189). Furthermore, androgen receptor intranuclear localization and transcriptional activity has been correlated with phosphorylation of serine 310 by CDK1, demonstrating a role for phosphorylation in regulating the receptor in a cell-cycle-dependent manner (181, 190). Transcription factor TFIIH also phosphorylates the receptor at Ser516 and is an essential partner in the cyclic recruitment of the transcription machinery (191). Substitution of serine 651 reduced androgen receptor activity by up to 30%. Furthermore, dephosphorylation of receptor phosphorylated at serine 651 by protein phosphatase 1 (PP1) can modulate androgen receptor translocation to the nucleus (192). More recently, PP1α has been shown to bind to the receptor-LBD and prevent ubiquitination and receptor degradation (193). Several other sites have been identified in the NH2-terminal domain at positions S16, S215, S258, S310, and S426 (180, 194-196). The function of phosphorylation of these sites is in the majority of the cases unknown or controversial. Two additional sites (S579 and S792) have been identified and characterized in the DNA-binding and ligand binding domains, respectively (189, 197). Phosphorylation of serine 579 by PKC kinase alters the nuclear cytoplasmic shuttling and elimination of phosphorylation at serine 579 eliminates EGF-induced transcriptional activation (189).
Besides the basal phosphorylation resulting in the 110-112 kDa doublet, addition of androgen induces another shift and the generation of a 110-112-114 kDa androgen receptor triplet (70). This triplet is the result of both an addition and a redistribution of phosphorylated sites, however, it is unknown which exact residues are involved (198). Interestingly, mutations that inactivate androgen receptor function, such as mutations resulting in loss of DNA binding or transactivation, inhibit the formation of the 114 kDa isoform. This suggests that part of the androgen - induced phosphorylation occurs during or after androgen receptor transcription regulation (70).
Functional phosphorylation at three tyrosine residues has also been demonstrated and extensively studied. The androgen receptor tyrosine residue 536 is highly phosphorylated. This phosphorylation is induced by EGF via activation of Src tyrosine kinase and may be important for prostate cancer cell growth under androgen-depleted conditions (199, 200). Activation of Cdc42-associated tyrosine kinase Ack1 can result in phosphorylation of tyrosine residues 269 and 365 enhancing androgen receptor transcriptional function and promoting androgen independent prostate cell growth (200, 201) and disrupting phosphorylation primarily of tyrosine 269 results in impaired nuclear localization (202). Recently it was reported that threonine phosphorylation of the receptor can also occur. Aurora A induces androgen receptor transactivation activity by phosphorylation of Threonine residue 284 (203).
In conclusion, phosphorylation of the androgen receptor can occur at serine, threonine and tyrosine residues by specific kinases and can be directly or indirectly linked to activation upon hormone binding, altering of nuclear cytoplasmic shuttling, modulation of DNA binding and transcriptional activity (168, 170, 181, 199, 204). Furthermore, phosphorylation of the androgen receptor can play an essential role in the hormone-independent activation of the androgen receptor by protein kinases in the MAPK and AKT (protein kinase B) signaling pathways, activated either through HER-2/neu or growth factors (205, 206).
ANTI-ANDROGENS AND SELECTIVE ANDROGEN RECEPTOR MODULATORS
Androgen receptor antagonists are compounds that interfere in some way in the biological effects of androgens and are frequently used in the treatment of androgen-based pathologies. The steroidal anti-androgens, cyproterone acetate (CPA) and RU38486 (RU486; mifepristone), have partial agonistic and antagonistic actions. Interestingly both compounds also display partial progestational and glucocorticoid actions and are therefore not considered to be pure anti-androgens. The non-steroidal anti-androgens hydroxyflutamide, nilutamide and bicalutamide [see Figure 1] are pure antiandrogens (207-209). Recent developments have led to the generation and marketing of second-generation non-steroidal antiandrogens, such as enzalutamide (formerly called MDV3100) [Figure 1], which have been reported to be more effective at blocking receptor nuclear translocation and activity (210). Recently, two new anti-androgens apalutamide and darolutamide have received FDA approval for treatment of non-metastatic castrate resistant prostate cancer (see 211, 212). However, resistance to enzalutamide has now also been identified as a result of an Phe876Leu point mutation in the LBD (213) and the expression of NH2-terminal domain splice variants (163) in CRPC, emphasizing the need for continued research and development of strategies to switch off androgen receptor signaling.
Mechanism of Action of Antiandrogens
In contrast to the full antagonists hydroxyflutamide and bicalutamide, CPA and RU486 can partially activate the androgen receptor with respect to transcription activation (214). With a limited proteolytic protection assay, it was demonstrated that binding of androgens by the androgen receptor results in two consecutive conformational changes of the receptor molecule. Initially, a fragment of 35 kDa, spanning the complete ligand binding domain and part of the hinge region, is protected from digestion by the ligand. After prolonged incubation times with the ligand a second conformational change occurs resulting in protection of a smaller fragment of 29 kDa (214, 215). In the presence of several anti-androgens (e.g., cyproterone acetate, hydroxyflutamide and bicalutamide) only the 35 kDa fragment is protected from proteolytic digestion, and no smaller fragments are detectable upon longer incubations. Obviously, the 35 kDa fragment can be associated with an inactive conformation, whereas the second conformational change, only inducible by agonists and considered as the necessary step for transcription activation, is lacking upon binding of anti-androgens.
During treatment of advanced prostate cancers, resistance develops to several of the above-mentioned anti-androgens, mostly due to mutations rendering the receptor protein less sensitive to anti-androgens. Promising results were reported for a newly developed second generation of antiandrogens for castration resistant prostate cancer (CRPC): ASC-J19, enzalutamide (MDV3100), apalutamide (ARN-509), AZD 3514, Compound30 and VPC-3033. (87, 210, 216-220). Characteristics of this new generation of anti-androgens are androgen displacement, inhibition of receptor- mediated transcription and enhancement of androgen receptor degradation. Clinical applications in prostate cancer were reported for enzalutamide (221-223). However, resistance to enzalutamide and apalutamide has been reported in prostate cancer due to a mutation at residue Phe877Leu (213, 224). Interestingly this mutation is located in a residue next to the LNCaP prostate cancer cell line mutation Thr878Ala (139, 225), supporting the view that this region in the ligand binding domain of the androgen receptor is very susceptible to mutagenesis in prostate cancer, which may lead to the tumor becoming resistant to hormone-based therapies.
Selective Androgen Receptor Modulation (SARMs)
Androgen signaling via the androgen receptor can occur in a non-genomic, rapid and sex-nonspecific way by crosstalk with the Scr, Raf-1, Erk-2 pathway [Figure 6, see above] (31, 32, 226). The anti-apoptotic action via the androgen receptor in bone cells (osteocytes, osteoblasts), and also in HeLa cells, could be induced by androgens and estrogens and inhibited by antiandrogens as well as anti-estrogens. The anti-apoptotic action appeared to be dissociated from the genomic action of the androgen receptor. The progesterone-induced oocyte maturation in Xenopus laevis also appears to be mediated in a non-genomic way by androgens and the androgen receptor via activating the MAPK pathway after the rapid conversion of progesterone to androstenedione and testosterone (33). These findings stimulated the development of new compounds (SARMs) which can selectively activate the androgen receptor either in a non-genomic pathway or in a genotropic transcriptional activation pathway. The term SARM (= Selective Androgen Receptor Modulator) was introduced in 1999 in analogy of the term SERM (Selective Estrogen Receptor Modulator) (227). A SARM can be defined as a molecule that targets the androgen receptor, and elicits a biological response, in a tissue-specific way. In a sense, anti-androgens (molecules that specifically target the androgen receptor pathway resulting in inhibition of the biological effects of androgens) can be considered as a special subtype of SARMs. Extensive overviews of current clinical trials with newly developed SARMs by several different pharmaceutical companies have been presented (228-230).
The structural basis for SARM binding and activity has been reviewed (138). Based on the conformational changes of the androgen receptor ligand binding domain induced by androgens or anti-androgens, it can be concluded that the different transcriptional activities displayed by either full agonists (testosterone, 5α-dihydrotestosterone, methyltrienolone), partial agonists (RU486 and CPA) or full antagonists (hydroxyflutamide, bicalutamide, enzalutamide) are the result of recruitment of a different repertoire of co-regulators (coactivators or corepressors) as a consequence of these conformational changes. The differential recruitment of co-regulators can be considered as a special form of ligand-selective modulation of the androgen receptor ligand binding domain and can also be applied in a broader sense to the tissue selective modulation of androgen action, where levels of co-activators and co-repressors may ultimately determine the final activity (229-232).
TISSUE-SPECIFIC ANDROGEN RECEPTOR MEDIATED ACTIONS IN MOUSE MODELS
Genetic mouse models in which the androgen receptor gene has been inactivated (so-called ARKO [androgen receptor knock-out] mouse models) are valuable tools to understand in detail the role of receptor-mediated pathways in male and female reproductive functions. For this purpose several different mouse models have been developed for studying androgen receptor mediated tissue-specific action in almost all known androgen target tissues, although the application of the mouse findings to the human situation has its limitation (233-238). Furthermore, the development of a mouse model for imaging of luciferase activity under control of endogenous androgen receptor activity has contributed to a further elucidation of tissue-specific receptor action (239).
ANDROGEN RECEPTOR DISORDERS
There is growing evidence for the involvement of the androgen receptor in the gender biases seen in a wide range of pathological conditions, from cancers of non-reproductive tissues (i.e., bladder, liver) (see 240, 241) to cardiovascular and metabolic disease (see 242-244). However, in this review we will focus on receptor mutations leading to defects of male development and fertility.
Androgen Insensitivity Syndrome
It has been known for quite some time that defects in male sexual differentiation in 46, XY individuals have an X-linked pattern of inheritance. It was Reifenstein who reported in 1947 on families with severe hypospadias, infertility, and gynecomastia (245). The end-organ resistance to androgens has been designated as androgen insensitivity syndrome (AIS) and is distinct from other XY disorders of sex development (XY, DSD; formerly named male pseudohermaphroditism) like 17β-hydroxy-steroid dehydrogenase type 3 deficiency or 5α-reductase type 2 deficiency (3, 246-248). It is generally accepted that defects in the androgen receptor gene can prevent the normal development of both internal and external male structures in 46, XY individuals and information on the molecular structure of the human androgen receptor gene has facilitated the study of molecular defects associated with androgen insensitivity. Due to the X-linked character of the syndrome, only 46, XY individuals are affected, while in female carriers only sporadic reports are available on delayed menarche (249). Naturally occurring mutations in the androgen receptor gene are an interesting source for the investigation of receptor structure-function relationships. In addition, the variation in clinical phenotypes provides the opportunity to correlate a mutation in the androgen receptor structure with the impairment of a specific physiological function.
Clinical Features of the Complete Androgen Insensitivity Syndrome (CAIS)
The main phenotypic characteristics of individuals with the complete androgen insensitivity syndrome (CAIS) are: female external genitalia, a short, blind ending vagina, absence of Wolffian duct derived structures like epididymides, vasa deferentia, and seminal vesicles, the absence of a prostate, the absence of pubic and axillary hair and the development of gynecomastia (250, 251). Müllerian duct derived structures are usually absent because anti-Mullerian hormone action is normal due to the presence of both testes in the abdomen or in the inguinal canals. Usually, testosterone levels are within the normal range (10 - 40 nmol/L) or elevated, while elevated luteinizing hormone (LH) levels (> 10 IU/L) are also found indicating androgen resistance at the hypothalamic-pituitary level. The high testosterone levels are also substrate for aromatase activity, resulting in substantial amounts of estrogens, which are responsible for further feminization in CAIS individuals.
Clinical Features of the Partial Androgen Insensitivity Syndrome (PAIS)
In the partial androgen insensitivity syndrome (PAIS) several phenotypes ranging from individuals with predominantly a female appearance (e.g., external female genitalia and pubic hair at puberty, or with mild clitoromegaly, and some fusion of the labia) to persons with ambiguous genitalia or individuals with a predominantly male phenotype (also called Reifenstein syndrome) (250, 251). Patients from this latter group can present with a micropenis, perineal hypospadias, and cryptorchidism. In the group of PAIS individuals, Wolffian duct derived structures can be partially to fully developed, depending on the biochemical phenotype of the androgen receptor mutation. At puberty, elevated luteinizing hormone, testosterone, and estradiol levels are observed, but in general, the degree of feminization is less as compared to individuals with CAIS. Individuals with mild symptoms of undervirilization (mild androgen insensitivity syndrome) and infertility have been described as well. Phenotypic variation between individuals in different families has been described for several mutations (251-254). However, in cases of CAIS no phenotypic variation has been described within one single family, in contrast to families with individuals with PAIS (255).
Genetics of Androgen Insensitivity Syndrome (AIS)
Since the cloning of the androgen receptor cDNA in 1988 and the subsequent elucidation of the genomic organization of the androgen receptor gene, molecular diagnostic tools have been available for the molecular analysis of the androgen receptor gene in individuals with AIS. In addition to endocrinology data, such as levels of testosterone, luteinizing hormone, androstenedione, and 5α-dihydrotestosterone, which can vary widely in AIS individuals, the most reliable approach is the sequencing of each individual androgen receptor exon and the flanking intron sequences. In general, AIS can be routinely analyzed and separated from entirely different syndromes presenting with similar phenotypes including testicular enzyme deficiencies, 5α-reductase type 2 deficiency, and Leydig cell hypoplasia due to inactivating luteinizing hormone receptor mutations. Furthermore, in pedigree analysis intragenic polymorphisms like the highly polymorphic (CAG)nCAA repeat encoding a poly-glutamine stretch, the polymorphic GGN repeat encoding a poly-glycine stretch, the HindIII polymorphism [Figure 8, see above] (39) and the StuI polymorphism (256), can be used as X-chromosomal markers (67, 257, 258). Extensive general information can be obtained at the internet site, www.genecards.org for the androgen receptor gene (NR3C4) and on the 233 identified single nucleotide polymorphisms (SNP’s).
Mutations in the Androgen Receptor Gene
In the androgen receptor gene, 4 different types of mutations have been detected in 46, XY individuals with AIS: single point mutations resulting in amino acid substitutions or premature stop codons, nucleotide insertions or deletions most often leading to a frame shift and premature termination, complete or partial gene deletions (>10 nucleotides), and intronic mutations in either splice donor or splice acceptor sites which affect the splicing of androgen receptor RNA (151). In general, in 70% of the cases, androgen receptor gene mutations are transmitted in an X-linked recessive manner, but in 30% the mutations arise de novo. When de novo mutations occur after the zygotic stage, they result in somatic mosaicisms (259). The most recent update on androgen receptor gene mutations is available at http://www.mcgill.ca/androgendb/ (151).
MUTATIONS IN THE NH2-TERMINAL DOMAIN
Mutations in the NH2-terminal domain (exon 1 of the gene) do not occur frequently and the vast majority of the mutations result directly in a stop codon or in premature termination due to frameshifts caused by nucleotide insertions or deletions. Mutations in 103 different codons have been reported in the NH2-terminal domain, which is approximately 18% of all codons in exon 1 (http://androgendb.mcgill.ca/ ) (151, 260-264).
An interesting mutation is described in the fourth nucleotide, which results in a decreased translational efficiency of the androgen receptor mRNA in an individual with PAIS (265). Three other missense mutations were reported in combination with mosaicism or with a mutation in another region of the gene. In a family with PAIS associated with severe hypospadias, the length of the androgen receptor NH2-terminal poly-glutamine repeat has been reported to be shortened to only 12 glutamine residues (266). The shortened glutamine stretch as such is not the cause for the androgen resistance, but it seems to increase the thermolability of the androgen receptor in combination with a point mutation in exon 5 (Y764C) in the ligand binding domain. This point mutation causes rapid dissociation of hormone, but no thermolability. These data support a functional interaction of the two separated regions in the androgen receptor and indicates further that the defect becomes critical in only some of the androgen target tissues because of the partial character of the androgen resistance found in this family (266).
MUTATIONS IN THE DNA-BINDING DOMAIN
In general, mutations in the DNA binding domain (e.g., single nucleotide substitutions) result in a normal hormone-binding protein, which is defective in DNA-binding/dimerization and consequently in transcription activation. In total, 71 different mutations have been reported in 38 different codons in the DNA-binding domain, which is approximately 43% of all codons in exons 2 and 3 (http://androgendb.mcgill.ca/ ) (151, 260, 264, 267, 268). Thirty-four mutations were observed in the first zinc cluster and thirty-two in the second zinc cluster. Since the 3D structure of the DNA-binding domain of several nuclear receptors have been published earlier than that of the androgen receptor DNA-binding domain, the consequence of several mutations in the androgen receptor DNA-binding domain have been predicted initially on basis of the structure of the glucocorticoid receptor DNA-binding domain (122, 123).This is illustrated in two studies in which 3D-modelling of the mutated DNA binding domain of the androgen receptor predicts the functional activity of mutant receptors (269, 270). A mutation (G578R) in the so-called P-box [Figure 9, see above], which is involved in androgen response element recognition, was found in a PAIS individual. This mutation differentially affects transactivation of several natural and synthetic promoters, suggesting that androgen target genes may be differentially affected by this mutation (271). An interesting observation was made with respect to the second zinc cluster in which either one of two adjacent arginine residues (Arg608 & Arg609) were found to be mutated in PAIS individuals who developed breast cancer [Figure 9, see above] (272, 273). It is speculated that a decrease in androgen action within the breast cells could account for the development of male breast cancer by the loss of a protective effect of androgens. However, the same mutations in several other PAIS individuals did not result in breast cancer development.
The mutation Ala597Thr in the second zinc cluster in the so-called D-box resulted in abolishment of dimerization in a PAIS individual [Figure 9, see above] (274). A similar mutation at an identical position in the second zinc cluster of the glucocorticoid receptor DNA-binding domain has been created to discriminate between dimerization/DNA binding of the glucocorticoid receptor and protein-protein interactions with other transcription factors such as the AP-1 transcription complex (275). It appeared that the dimerization mutant did not affect the cross-talk with other transcription factors. In this way, a tissue-specific response can be influenced by a single amino acid change and if this is also true for the mutant androgen receptor then the partial phenotype can be explained. Interestingly a Ser580Arg, also located in the D-box can cause significantly different phenotypes ranging from under-virilization to a normal male phenotype (276).
MUTATIONS IN THE HINGE REGION
In the so-called hinge region, located between amino acid residues 623 and 671 [Figure 8, see above], only nine mutations have been reported. The relatively low number of mutations in the hinge region (only in 18% of all codons) indicates that this region might be very flexible and that some variation in composition and length of this region is not detrimental for androgen receptor function (http://www.mcgill.ca/androgendb/) (151). Four amino acid substitutions within the hinge region have been described that resulted in CAIS, four in PAIS and one in MAIS (http://www.mcgill.ca/androgendb/ (151). The Ile665Asn substitution on the border of the hinge region and ligand-binding domain, resulted in a decreased hormone binding (277).
MUTATIONS IN THE LIGAND-BINDING DOMAIN
It can be expected that mutations in the ligand binding domain might affect different functional aspects (e.g., loss of ligand binding, changes in ligand binding affinity and specificity, changes in co-activator receptor interactions, changes in receptor stability and thermolability). A large number of mutations (283 different mutations in 164 codons, which is in 66% of all codons of the ligand binding domain) in the ligand binding domain have been reported in all 5 exons in individuals with either CAIS, PAIS or MAIS (http://androgendb.mcgill.ca/ ) (151, 260, 265, 278-286). Most mutations are located in exons 4 (62 mutations), 5 (77 mutations) and 7 (54 mutations). Interestingly mutations are found in 13 of the 18 amino acid residues considered to interact with the ligand directly (120). For some mutations (in total 25, distributed over the whole ligand binding domain) either a complete (CAIS) as well as a partial (PAIS) phenotype (13 cases) or a CAIS and a PAIS and a mild (MAIS) phenotype (4 cases) or a PAIS and a MAIS phenotype (8 cases) has been described, indicating that phenotype does not always match with genotype. In the AF-2 core region (894-EMMAEIIS-901) of the androgen receptor ligand-binding domain a relatively low number of mutations have been reported [see Figure 10 for location of AF-2]. At positions methionine 895 (deletion), Met896, Ala897, Glu898 and Ile899 (all substitutions) have been described in individuals with the complete syndrome (287, 288). It can be speculated that in this part of helix 12 mutations in the androgen receptor ligand-binding domain are very deleterious for androgen receptor function as well as those in helix 5 and in the β-turn, wherein almost every amino acid residue has been found to be mutated in AIS individuals (http://androgendb.mcgill.ca/ ) (151). Functional analysis of an androgen receptor mutation, Gln903Lys in helix 12, in an individual with partial androgen insensitivity, indicated that this residue is important for modulation of NH2/COOH terminal interaction and TIF-2 activation (289). Interestingly a mutation, Phe827Leu, found in a PAIS patient, displayed an unexpected increased N/C interaction and TIF2 coactivation (290). An explanation for the phenotype of the patient could be that the receptor mutant recruits a different repertoire of co-activators absent in genital tissues. Alternatively, an altered conformation of the ligand binding domain may enhance preferential recruitment of co-repressors.
Several reports have established the pathogenic nature of androgen receptor mutations found in AIS individuals with different functional assays (260, 289-292). In order to optimize molecular diagnosis an extensive functional analysis of receptor mutations is desired. For counselling strategies and for future outcome predictions a correct functional diagnosis is very important as well as for prognosis on the risks of gonadal malignancy (293). A combination of different functional analyses, designed to test androgen receptor mutations at different stages in receptor functioning (e.g., hormone binding, transcriptional activation, cofactor binding, translocation to the nucleus and nuclear dynamics) will provide a more accurate prediction of androgen receptor action and will help to establish a more exact phenotypic characterization.
DELETIONS AND DUPLICATIONS OF THE ANDROGEN RECEPTOR GENE
Only a few cases (8 different deletions in 15 different patients) have been reported on partial or complete androgen receptor gene deletions, indicating the relatively low frequency of this type of androgen receptor defect (http://androgendb.mcgill.ca/) (151, 294). All cases reported are found in CAIS individuals, with the exception of two cases, one in which an exon 4 deletion was found in a person with azoospermia (295) and another one in which a large intron 2 deletion of at least 6 kb was reported involving a branch point site, which resulted in a partial exon 3 skipping during the splicing process (294).
Deletion of either exon 3 or exon 4 occur both in-frame and result in a non-functional protein lacking either the second zinc cluster or the hinge region and the NH2-terminal part of the ligand-binding domain [see Figure 7 for genomic organization of the androgen receptor gene]. In case of an exon 3 deletion, an intact and functional ligand-binding domain is present [Figure 7]. So far, functionally significant mutations in the androgen receptor promoter region or in the 5'- and 3'- untranslated regions of the gene have not been reported.
SPLICE SITE MUTATIONS AFFECTING ANDROGEN RECEPTOR RNA SPLICING
A special group of interesting, but rare, mutations are the splice donor and splice acceptor site mutations in the androgen receptor gene in AIS individuals (http://androgendb.mcgill.ca/ ) (151). For all splice donor sites in the gene, the consensus splice donor site sequence GTAAG/A is present. The twelve reported mutations in donor splice sites are all substitutions either at position +1 (G → A or G → T), position +2 (T → C), position +3 (A → T), position + 4 (A→T) or position + 5 (G → A) and result in defective splicing with the consequence of one or more exons spliced out, or the use of a cryptic splice donor site within the preceding exon (264, 296-301). In 11 of the reported cases, the phenotype is complete androgen insensitivity. In one case, an insertion of one nucleotide (T) at position + 4 in the splice donor site of intron 6 has been reported, resulting in a partial androgen insensitive phenotype (300). Only 5 mutations have been reported in splice acceptor sites, which all affect the splicing of the androgen receptor RNA. Interestingly, a substitution at position -11 (T →G) has been found in the pyrimidine-rich region of the splice acceptor site of intron 2, resulting in the activation of a cryptic splice acceptor site at position -70/-69 and consequently in the insertion of 69 nucleotides (corresponding to 23 additional amino acid residues) in the mRNA between exons 2 and 3 (302). The corresponding protein is defective in DNA-binding because the insertion has occurred between the first and second zinc cluster. In another CAIS patient a splice junction mutation at the intron2/exon3 splice acceptor site resulted in the utilization of the same cryptic splice acceptor site and also in the insertion of 69 bp in the mRNA, predicting the insertion of 23 amino acid residues in frame between the two zinc clusters (303).
Androgen Receptor Gene Mutations in Cancers
Mutations in the androgen receptor gene have also been reported to be associated with prostate cancers, breast cancers, larynx cancers, liver cancers and testicular cancers (http://androgendb.mcgill.ca/ ) (151).
ANDROGEN METABOLISM DISORDERS
The metabolism of testosterone to 5α-dihydrotestosterone by the enzyme 5α-reductase type 2 (SRD5A2) is essential for the initiation of the differentiation and development of the urogenital sinus into the prostate. The differentiation of the male external genitalia (penis, scrotum and urethra) also strongly depends on the conversion of testosterone to 5α-dihydrotestosterone in the urogenital tubercle, labioscrotal swellings and urogenital folds, respectively [Figure 2B, see above] (3, 4). Interestingly in the SPARKI mouse expression of Srd5α2 gene is significantly impaired in the epididymis and the androgen-regulation of the gene was demonstrated to involve three selective AREs (304).
Clinical Features of the Syndrome of 5α-reductase Type 2 Deficiency
46, XY individuals with impairment of 5α-reductase type 2 have normally virilized Wolffian duct derived structures, with seminal vesicles (although small seminal vesicles have been reported as well), with vasa deferentia, epididymides and ejaculatory ducts and no Mullerian duct derived structures (3, 305, 306). However, differentiation of the urogenital sinus and genital tubercle is not observed, resulting in absence of the prostate and in ambiguous or in female external genitalia at birth (3, 305, 306). Affected 46, XY individuals are therefore often raised as girls. At puberty all affected individuals show some or a severe degree of virilization often resulting in deepening of the voice, an increased muscle mass, growth of the penis, scrotal development, testicular descent and sometimes leading to a gender change (3, 307).
Gynecomastia in adulthood does not occur. The additional virilization may result from the action of testosterone because testosterone is available at high levels during puberty. In addition, some testosterone may be converted to 5α-dihydrotestosterone by some residual 5α-reductase activity and by the action of 5α-reductase type 1, which is expressed in non-genital skin, pubic skin, liver and certain brain regions. In the affected 46, XY individuals a typical female pubic hair pattern develops, while the facial and body hair amount is absent or reduced (4). This last observation points to a role for 5α-reductase type 2 in the normal development of this type of body hair. Male pattern baldness has never been observed. 5α-reductase type 2 deficient patients are usually infertile due to the absence or underdevelopment of the prostate and seminal vesicles, in addition to oligospermia or azoospermia due to maldescent of the testes. However, paternity has been reported in some cases, either by intrauterine insemination or after in vitro fertilization in combination with intracytoplasmic sperm injection (3, 305, 308-310). 46, XX female carriers have normal fertility, decreased body hair and delayed menarche, normal sebum production but no history of acne (3, 305). This suggests a role of 5α-reductase type 2 enzyme in females in the physiology and pathophysiology of body hair growth, menarche and follicular development (305).
Molecular Basis for the Syndrome of 5α-Reductase Type 2 Deficiency
A reflection of defective or absence 5α-reductase type 2 enzyme activity can be obtained in patients’ serum and urine samples by measuring testosterone levels (elevated), 5α-dihydrotestosterone levels (decreased) and by measuring the ratio of testosterone/5α-dihydrotestosterone (increased after hCG stimulation) (3). Furthermore, in cultured genital skin fibroblasts (if available) the conversion of testosterone to 5α-dihydrotestosterone can be assessed and is an option for establishing a defective enzyme. In broken cell preparations at pH 5.5, the type 2 isozyme activity is measured more specifically and can be compared with a preparation from a normal person (3).
Genetic analysis of 5α-reductase type 2 deficiency has become possible since the cloning of the cDNA (17). The gene is located on chromosome 2 at locus 2p23. The enzyme is encoded by 5 exons and the most reliable approach to detect gene mutations is the sequencing of each individual exon and the flanking intron sequences [Figure 12]. A relatively large number of loss of function mutations in the type 2 steroid 5α-reductase has been identified in 46XY individuals with this rare autosomal recessive disorder of sex development (46XY, DSD).

Figure 12.
Mutations in the5α-reductase type 2 gene (SDR5A2) reported in patients with the syndrome of 5α-reductase deficiency. The 5α-reductase type 2 enzyme is encoded by 5 different exons and mutations have been reported in all 5 exons, as well as a complete gene deletion, small deletions of nucleotides, and splice site mutations.
Interestingly worldwide 87 different mutations have been detected in the 5α-reductase type 2 gene in patients with the syndrome of 5α-reductase type 2 deficiency in several different ethnic groups [Figure 12] (3, 4, 285, 305-307, 311-339). Identical mutations have been reported in different ethnic groups and some of them can be considered to be due to a founder effect and some to have occurred de novo (340-342). The mutations were found in all five exons of the gene, although the majority of the mutations are reported in exons 1 and 4 [Figure 12].
The mutations comprise of 57 amino acid substitutions (65.5%), one complete gene deletion (3, 306), one complete exon 1 deletion (16), one substitution at stop codon 255 resulting in a Serine residue (336), ten small deletions resulting in either a premature stop codon or in an in-frame amino acid residue deletion, four small insertions (335), nine nonsense mutations and four splice site mutations, resulting in aberrant splicing [Figure 12]. The majority of the reported patients are homozygous for one of the mutations. A smaller number of patients appeared to be compound heterozygous, while a small group of patients are heterozygous (331, 340, 341).
In general male carriers of a single mutant allele have normal fertility as is the case for female carriers. The largest investigated kindreds were found in the Dominican Republic, in Turkey and in New Guinea (3, 305, 333). In all three kindreds the high incidence can be directly related to a founder affect in geographical isolated populations with a high degree of inbreeding. For other cases also a large incidence of proven consanguinity is reported (3, 305).
In prostate cancer de novo mutations in the 5α-reductase type 2 have been reported, resulting in increased 5α-reductase activity (317, 333, 343, 344). This finding indicates a role for increased 5α-dihydrotestosterone levels in the prostate, during prostate cancer progression in a subset of patients. The V89L mutant significantly reduced SRD5A2 enzymatic activity by almost 30% (316, 342, 343). The rare allele frequency of the V89L variant is 22%, 23,5%, and 46,1% for African Americans, Caucasians, and Asians, respectively, paralleling a substantial racial/ethnic variation in prostate cancer risk, indicating that this polymorphism might be implicated in prostate cancer carcinogenesis (343-346).
CONCLUSIONS-KEY POINTS
Androgenic steroids are important for normal development and function of male reproductive tissues and for anabolic actions in muscle and bone. The multiple actions of the main circulating androgen testosterone and the more potent metabolite DHT are mediated by a single intracellular receptor protein, the androgen receptor. The hormone-bound receptor acts primarily to differentially regulate gene expression in target tissues and its encoding gene is located on the X chromosome, making it a single-copy gene in males. Thus, genetic changes affecting expression or structure/function of the receptor protein will lead to a range of diseases associated with loss or impaired androgen signaling, including disruption of male development, infertility or a late onset neurodegenerative disease (SBMA). Furthermore, altered expression and genetic changes in the receptor are also key drivers in progression of prostate cancer to a therapy-resistant stage.
Since the first cloning of the androgen receptor cDNA, over thirty years ago, considerable progress has been made in our understanding of receptor structure and function. Advances include: the availability of 3D-structures of the isolated LBD with different ligands bound and the isolated DBD; structural characterization of the intrinsically disordered NH2-terminal domain; first glimpse of the structure of full-length AR transcriptional complex on DNA; the identification of a plethora of co-regulatory proteins binding to the ligand- and NH2-terminal domains; identification of gene regulatory pathways in target cells; and a better understanding of the impact of genetic changes affecting receptor structure/function. Future research will likely focus on the mechanisms determining cell/tissue-selective expression and function of the androgen receptor in both normal and pathophysiological conditions and a more complete structural descriptions of the full-length receptor bound to different DNA response elements and co-regulatory proteins.
REFERENCES
- 1.
- George FW, Wilson JD 1994 Sex determination and differentiation. In: Knobil E, Neill JD, eds. The Physiology of Reproduction. New York.: Raven Press Ltd.; Chapter 1.
- 2.
- Brinkmann AO. Molecular mechanisms of androgen action--a historical perspective. Methods Mol Biol. 2011;776:3–24. [PubMed: 21796517]
- 3.
- Wilson JD, Griffin JE, Russell DW. Steroid 5 alpha-reductase 2 deficiency. Endocr Rev. 1993;14(5):577–593. [PubMed: 8262007]
- 4.
- Randall VA. Role of 5 alpha-reductase in health and disease. Baillieres Clin Endocrinol Metab. 1994;8(2):405–431. [PubMed: 8092979]
- 5.
- Grino PB, Griffin JE, Wilson JD. Testosterone at high concentrations interacts with the human androgen receptor similarly to dihydrotestosterone. Endocrinology. 1990;126(2):1165–1172. [PubMed: 2298157]
- 6.
- El-Gehani F, Zhang FP, Pakarinen P, Rannikko A, Huhtaniemi I. Gonadotropin-independent regulation of steroidogenesis in the fetal rat testis. Biol Reprod. 1998;58(1):116–123. [PubMed: 9472931]
- 7.
- Brinkmann AO, van Straalen RJ. Development of the LH-response in fetal guinea pig testes. Biol Reprod. 1979;21(4):991–997. [PubMed: 230862]
- 8.
- O'Shaughnessy PJ, Baker P, Sohnius U, Haavisto AM, Charlton HM, Huhtaniemi I. Fetal development of leydig cell activity in the mouse is independent of pituitary gonadotroph function. Endocrinology. 1998;139(3):1141–1146. [PubMed: 9492048]
- 9.
- Curtin D, Jenkins S, Farmer N, Anderson AC, Haisenleder DJ, Rissman E, Wilson EM, Shupnik MA. Androgen suppression of GnRH-stimulated rat LHbeta gene transcription occurs through Sp1 sites in the distal GnRH-responsive promoter region. Mol Endocrinol. 2001;15(11):1906–1917. [PubMed: 11682622]
- 10.
- Stocco DM, Clark BJ. Regulation of the acute production of steroids in steroidogenic cells. Endocr Rev. 1996;17(3):221–244. [PubMed: 8771357]
- 11.
- Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9(3):295–318. [PubMed: 3061784]
- 12.
- Fukami M, Homma K, Hasegawa T, Ogata T. Backdoor pathway for dihydrotestosterone biosynthesis: implications for normal and abnormal human sex development. Dev Dyn. 2013;242(4):320–9. [PubMed: 23073980]
- 13.
- O'Shaughnessy PJ, Antignac JP, Le Bizec B, Morvan ML, Svechnikov K, Söder O, Savchuk I, Monteiro A, Soffientini U, Johnston ZC, Bellingham M, Hough D, Walker N, Filis P, Fowler PA. Alternative (backdoor) androgen production and masculinization in the human fetus. PLoS Biol. 2019;17(2):e3000002. [PMC free article: PMC6375548] [PubMed: 30763313]
- 14.
- Reisch N, Taylor AE, Nogueira EF, Asby DJ, Dhir V, Berry A, Krone N, Auchus RJ, Shackleton CHL, Hanley NA, Arlt W. Alternative pathway androgen biosynthesis and human fetal female virilization. Proc Natl Acad Sci U S A. 2019;116(44):22294–22299. [PMC free article: PMC6825302] [PubMed: 31611378]
- 15.
- Russell DW, Wilson JD. Steroid 5 alpha-reductase: Two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. [PubMed: 7979239]
- 16.
- Andersson S, Berman DM, Jenkins EP, Russell DW. Deletion of steroid 5 alpha-reductase 2 gene in male pseudohermaphroditism. Nature. 1991;354(6349):159–161. [PMC free article: PMC4451825] [PubMed: 1944596]
- 17.
- Andersson S, Bishop RW, Russell DW. Expression cloning and regulation of steroid 5 alpha-reductase, an enzyme essential for male sexual differentiation. J Biol Chem. 1989;264(27):16249–16255. [PMC free article: PMC4448950] [PubMed: 2476440]
- 18.
- Azzouni F, Godoy A, Li Y, Mohler J. The 5 alpha-reductase isozyme family: A review of basic biology and their role in human diseases. Adv Urol. 2012;2012:530121. [PMC free article: PMC3253436] [PubMed: 22235201]
- 19.
- Stiles AR, Russell DW. SRD5A3: A surprising role in glycosylation. Cell. 2010;142(2):196–198. [PMC free article: PMC3104503] [PubMed: 20655462]
- 20.
- Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E, De Brouwer AP, Blumel P, Sykut-Cegielska J, Houliston S, Swistun D, Ali BR, Dobyns WB, Babovic-Vuksanovic D, van Bokhoven H, Wevers RA, Raetz CR, Freeze HH, Morava E, Al-Gazali L, Gleeson JG. SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell. 2010;142(2):203–217. [PMC free article: PMC2940322] [PubMed: 20637498]
- 21.
- Moon YA, Horton JD. Identification of two mammalian reductases involved in the two-carbon fatty acyl elongation cascade. J Biol Chem. 2003;278(9):7335–7343. [PubMed: 12482854]
- 22.
- Nuclear Receptors Nomenclature Committee. A unified nomenclature system for the nuclear receptor superfamily. Cell. 1999;97(2):161–163. [PubMed: 10219237]
- 23.
- Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240(4854):889–895. [PMC free article: PMC6159881] [PubMed: 3283939]
- 24.
- Laudet V, Hanni C, Coll J, Catzeflis F, Stehelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992;11(3):1003–1013. [PMC free article: PMC556541] [PubMed: 1312460]
- 25.
- Robinson-Rechavi M, Carpentier AS, Duffraisse M, Laudet V. How many nuclear hormone receptors are there in the human genome? Trends Genet. 2001;17(10):554–556. [PubMed: 11585645]
- 26.
- Enmark E, Gustafsson JA. Orphan nuclear receptors--the first eight years. Mol Endocrinol. 1996;10(11):1293–1307. [PubMed: 8923456]
- 27.
- Hutchison KA, Dittmar KD, Pratt WB. All of the factors required for assembly of the glucocorticoid receptor into a functional heterocomplex with heat shock protein 90 are preassociated in a self-sufficient protein folding structure, a "foldosome". J Biol Chem. 1994;269(45):27894–27899. [PubMed: 7961721]
- 28.
- Cano LQ, Lavery DN, Bevan CL. Mini-review: Foldosome regulation of androgen receptor action in prostate cancer. Mol Cell Endocrinol. 2013;369(1-2):52–62. [PubMed: 23395916]
- 29.
- Heemers HV, Tindall DJ. Androgen receptor (AR) coregulators: A diversity of functions converging on and regulating the AR transcriptional complex. Endocr Rev. 2007;28(7):778–808. [PubMed: 17940184]
- 30.
- Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, Chen Z, Beroukhim R, Wang H, Lupien M, Wu T, Regan MM, Meyer CA, Carroll JS, Manrai AK, Janne OA, Balk SP, Mehra R, Han B, Chinnaiyan AM, Rubin MA, True L, Fiorentino M, Fiore C, Loda M, Kantoff PW, Liu XS, Brown M. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138(2):245–256. [PMC free article: PMC2726827] [PubMed: 19632176]
- 31.
- Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F. Steroid-induced androgen receptor-oestradiol receptor beta-src complex triggers prostate cancer cell proliferation. EMBO J. 2000;19(20):5406–5417. [PMC free article: PMC314017] [PubMed: 11032808]
- 32.
- Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: Dissociation from transcriptional activity. Cell. 2001;104(5):719–730. [PubMed: 11257226]
- 33.
- Lutz LB, Cole LM, Gupta MK, Kwist KW, Auchus RJ, Hammes SR. Evidence that androgens are the primary steroids produced by xenopus laevis ovaries and may signal through the classical androgen receptor to promote oocyte maturation. Proc Natl Acad Sci U S A. 2001;98(24):13728–13733. [PMC free article: PMC61109] [PubMed: 11707587]
- 34.
- Sen A, Prizant H, Hammes SR. Understanding extranuclear (nongenomic) androgen signaling: What a frog oocyte can tell us about human biology. Steroids. 2011;76(9):822–828. [PMC free article: PMC4972037] [PubMed: 21354434]
- 35.
- Chang CS, Kokontis J, Liao ST. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988;240(4850):324–326. [PubMed: 3353726]
- 36.
- Lubahn DB, Joseph DR, Sar M, Tan J, Higgs HN, Larson RE, French FS, Wilson EM. The human androgen receptor: Complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol. 1988;2(12):1265–1275. [PubMed: 3216866]
- 37.
- Trapman J, Klaassen P, Kuiper GG, van der Korput JA, Faber PW, van Rooij HC, Geurts van Kessel A, Voorhorst MM, Mulder E, Brinkmann AO. Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem Biophys Res Commun. 1988;153(1):241–248. [PubMed: 3377788]
- 38.
- Tilley WD, Marcelli M, Wilson JD, McPhaul MJ. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989;86(1):327–331. [PMC free article: PMC286457] [PubMed: 2911578]
- 39.
- Brown CJ, Goss SJ, Lubahn DB, Joseph DR, Wilson EM, French FS, Willard HF. Androgen receptor locus on the human X chromosome: Regional localization to Xq11-12 and description of a DNA polymorphism. Am J Hum Genet. 1989;44(2):264–269. [PMC free article: PMC1715398] [PubMed: 2563196]
- 40.
- van Laar JH, Bolt-de Vries J, Voorhorst-Ogink MM, Brinkmann AO. The human androgen receptor is a 110 kDa protein. Mol Cell Endocrinol. 1989;63(1-2):39–44. [PubMed: 2787763]
- 41.
- Kuiper GG, Faber PW, van Rooij HC, van der Korput JA, Ris-Stalpers C, Klaassen P, Trapman J, Brinkmann AO. Structural organization of the human androgen receptor gene. J Mol Endocrinol. 1989;2(3):R1–4. [PubMed: 2546571]
- 42.
- Lubahn DB, Brown TR, Simental JA, Higgs HN, Migeon CJ, Wilson EM, French FS. Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proc Natl Acad Sci U S A. 1989;86(23):9534–9538. [PMC free article: PMC298531] [PubMed: 2594783]
- 43.
- Gaspar ML, Meo T, Tosi M. Structure and size distribution of the androgen receptor mRNA in wild-type and Tfm/Y mutant mice. Mol Endocrinol. 1990;4(10):1600–10. [PubMed: 2178222]
- 44.
- Ebron JS, Shukla GC. Molecular characterization of a novel androgen receptor transgene responsive to MicroRNA mediated post-transcriptional control exerted via 3'-untranslated region. Prostate. 2016;76(9):834–44. [PubMed: 26988939]
- 45.
- Östling P, Leivonen SK, Aakula A, Kohonen P, Mäkelä R, Hagman Z, Edsjö A, Kangaspeska S, Edgren H, Nicorici D, Bjartell A, Ceder Y, Perälä M, Kallioniemi O. Systematic analysis of microRNAs targeting the androgen receptor in prostate cancer cells. Cancer Res. 2011;71(5):1956–67. [PubMed: 21343391]
- 46.
- Liu C, Chen Z, Hu X, Wang L, Li C, Xue J, Zhang P, Chen W, Jiang A. MicroRNA-185 downregulates androgen receptor expression in the LNCaP prostate carcinoma cell line. Mol Med Rep. 2015;11(6):4625–32. [PubMed: 25673182]
- 47.
- Faber PW, King A, van Rooij HC, Brinkmann AO, de Both NJ, Trapman J 1991 The mouse androgen receptor. functional analysis of the protein and characterization of the gene. Biochem J 278 (Pt 1)(Pt 1):269-278. [PMC free article: PMC1151478] [PubMed: 1883336]
- 48.
- Faber PW, van Rooij HC, van der Korput HA, Baarends WM, Brinkmann AO, Grootegoed JA, Trapman J. Characterization of the human androgen receptor transcription unit. J Biol Chem. 1991;266(17):10743–10749. [PubMed: 1710213]
- 49.
- Faber PW, van Rooij HC, Schipper HJ, Brinkmann AO, Trapman J. Two different, overlapping pathways of transcription initiation are active on the TATA-less human androgen receptor promoter. the role of Sp1. J Biol Chem. 1993;268(13):9296–9301. [PubMed: 8486625]
- 50.
- Takane KK, McPhaul MJ. Functional analysis of the human androgen receptor promoter. Mol Cell Endocrinol. 1996;119(1):83–93. [PubMed: 8793857]
- 51.
- Wang LG, Ferrari AC. Mithramycin targets sp1 and the androgen receptor transcription level-potential therapeutic role in advanced prostate cancer. Transl Oncogenomics. 2006;1:19–31. [PMC free article: PMC3642134] [PubMed: 23662037]
- 52.
- Hay CW, Hunter I, MacKenzie A, McEwan IJ. An Sp1 modulated regulatory region unique to higher primates regulates human androgen receptor promoter activity in prostate cancer cells. PLoS One. 2015;10(10):e0139990. [PMC free article: PMC4598089] [PubMed: 26448047]
- 53.
- Jarrard DF, Kinoshita H, Shi Y, Sandefur C, Hoff D, Meisner LF, Chang C, Herman JG, Isaacs WB, Nassif N. Methylation of the androgen receptor promoter CpG island is associated with loss of androgen receptor expression in prostate cancer cells. Cancer Res. 1998;58(23):5310–4. [PubMed: 9850055]
- 54.
- Kinoshita H, Shi Y, Sandefur C, Meisner LF, Chang C, Choon A, Reznikoff CR, Bova GS, Friedl A, Jarrard DF. Methylation of the androgen receptor minimal promoter silences transcription in human prostate cancer. Cancer Res. 2000;60(13):3623–30. [PubMed: 10910077]
- 55.
- Burnstein KL. Regulation of androgen receptor levels: Implications for prostate cancer progression and therapy. J Cell Biochem. 2005;95(4):657–669. [PubMed: 15861399]
- 56.
- Cai C, Chen S, Ng P, Bubley GJ, Nelson PS, Mostaghel EA, Marck B, Matsumoto AM, Simon NI, Wang H, Chen S, Balk SP. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 2011;71(20):6503–6513. [PMC free article: PMC3209585] [PubMed: 21868758]
- 57.
- Hay CW, Watt K, Hunter I, Lavery DN, MacKenzie A, McEwan IJ. Negative regulation of the androgen receptor gene through a primate-specific androgen response element present in the 5' UTR. Horm Cancer. 2014;5(5):299–311. [PMC free article: PMC4164857] [PubMed: 24895212]
- 58.
- Hunter I, Hay CW, Esswein B, Watt K, McEwan IJ. Tissue control of androgen action: The ups and downs of androgen receptor expression. Mol Cell Endocrinol. 2018;465:27–35. [PubMed: 28789969]
- 59.
- Huang P, Chandra V, Rastinejad F. Structural overview of the nuclear receptor superfamily: Insights into physiology and therapeutics. Annu Rev Physiol. 2010;72:247–272. [PMC free article: PMC3677810] [PubMed: 20148675]
- 60.
- Hollenberg SM, Weinberger C, Ong ES, Cerelli G, Oro A, Lebo R, Thompson EB, Rosenfeld MG, Evans RM. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318(6047):635–641. [PMC free article: PMC6165583] [PubMed: 2867473]
- 61.
- Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: Sequence, expression and homology to v-erb-A. Nature. 1986;320(6058):134–139. [PubMed: 3754034]
- 62.
- Misrahi M, Atger M, d'Auriol L, Loosfelt H, Meriel C, Fridlansky F, Guiochon-Mantel A, Galibert F, Milgrom E. Complete amino acid sequence of the human progesterone receptor deduced from cloned cDNA. Biochem Biophys Res Commun. 1987;143(2):740–748. [PubMed: 3551956]
- 63.
- Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, Evans RM. Cloning of human mineralocorticoid receptor complementary DNA: Structural and functional kinship with the glucocorticoid receptor. Science. 1987;237(4812):268–275. [PubMed: 3037703]
- 64.
- Faber PW, Kuiper GG, van Rooij HC, van der Korput JA, Brinkmann AO, Trapman J. The N-terminal domain of the human androgen receptor is encoded by one, large exon. Mol Cell Endocrinol. 1989;61(2):257–262. [PubMed: 2917688]
- 65.
- Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93(12):5925–5930. [PMC free article: PMC39164] [PubMed: 8650195]
- 66.
- Sleddens HF, Oostra BA, Brinkmann AO, Trapman J. Trinucleotide repeat polymorphism in the androgen receptor gene (AR). Nucleic Acids Res. 1992;20(6):1427. [PMC free article: PMC312201] [PubMed: 1561105]
- 67.
- Boehmer AL, Brinkmann AO, Niermeijer MF, Bakker L, Halley DJ, Drop SL. Germ-line and somatic mosaicism in the androgen insensitivity syndrome: Implications for genetic counseling. Am J Hum Genet. 1997;60(4):1003–1006. [PMC free article: PMC1712473] [PubMed: 9106550]
- 68.
- Li SL, Ting SS, Lindeman R, Ffrench R, Ziegler JB. Carrier identification in X-linked immunodeficiency diseases. J Paediatr Child Health. 1998;34(3):273–279. [PubMed: 9633977]
- 69.
- Nance MA. Clinical aspects of CAG repeat diseases. Brain Pathol. 1997;7(3):881–900. [PMC free article: PMC8098176] [PubMed: 9217974]
- 70.
- Jenster G, de Ruiter PE, van der Korput HA, Kuiper GG, Trapman J, Brinkmann AO. Changes in the abundance of androgen receptor isotypes: Effects of ligand treatment, glutamine-stretch variation, and mutation of putative phosphorylation sites. Biochemistry. 1994;33(47):14064–14072. [PubMed: 7947816]
- 71.
- Kazemi-Esfarjani P, Trifiro MA, Pinsky L. Evidence for a repressive function of the long polyglutamine tract in the human androgen receptor: Possible pathogenetic relevance for the (CAG)n-expanded neuronopathies. Hum Mol Genet. 1995;4(4):523–527. [PubMed: 7633399]
- 72.
- Hardy DO, Scher HI, Bogenreider T, Sabbatini P, Zhang ZF, Nanus DM, Catterall JF. Androgen receptor CAG repeat lengths in prostate cancer: Correlation with age of onset. J Clin Endocrinol Metab. 1996;81(12):4400–4405. [PubMed: 8954049]
- 73.
- Giovannucci E, Stampfer MJ, Krithivas K, Brown M, Dahl D, Brufsky A, Talcott J, Hennekens CH, Kantoff PW. The CAG repeat within the androgen receptor gene and its relationship to prostate cancer. Proc Natl Acad Sci U S A. 1997;94(7):3320–3323. [PMC free article: PMC20367] [PubMed: 9096391]
- 74.
- Cude KJ, Montgomery JS, Price DK, Dixon SC, Kincaid RL, Kovacs KF, Venzon DJ, Liewehr DJ, Johnson ME, Reed E, Figg WD. The role of an androgen receptor polymorphism in the clinical outcome of patients with metastatic prostate cancer. Urol Int. 2002;68(1):16–23. [PubMed: 11803263]
- 75.
- Correa-Cerro L, Wohr G, Haussler J, Berthon P, Drelon E, Mangin P, Fournier G, Cussenot O, Kraus P, Just W, Paiss T, Cantu JM, Vogel W. (CAG)nCAA and GGN repeats in the human androgen receptor gene are not associated with prostate cancer in a french-german population. Eur J Hum Genet. 1999;7(3):357–362. [PubMed: 10234512]
- 76.
- Freedman ML, Pearce CL, Penney KL, Hirschhorn JN, Kolonel LN, Henderson BE, Altshuler D. Systematic evaluation of genetic variation at the androgen receptor locus and risk of prostate cancer in a multiethnic cohort study. Am J Hum Genet. 2005;76(1):82–90. [PMC free article: PMC1196436] [PubMed: 15570555]
- 77.
- Dowsing AT, Yong EL, Clark M, McLachlan RI, de Kretser DM, Trounson AO. Linkage between male infertility and trinucleotide repeat expansion in the androgen-receptor gene. Lancet. 1999;354(9179):640–643. [PubMed: 10466666]
- 78.
- Mifsud A, Sim CK, Boettger-Tong H, Moreira S, Lamb DJ, Lipshultz LI, Yong EL. Trinucleotide (CAG) repeat polymorphisms in the androgen receptor gene: Molecular markers of risk for male infertility. Fertil Steril. 2001;75(2):275–281. [PubMed: 11172827]
- 79.
- Wallerand H, Remy-Martin A, Chabannes E, Bermont L, Adessi GL, Bittard H. Relationship between expansion of the CAG repeat in exon 1 of the androgen receptor gene and idiopathic male infertility. Fertil Steril. 2001;76(4):769–774. [PubMed: 11591412]
- 80.
- La Spada AR, Wilson EM, Lubahn DB, Harding AE, Fischbeck KH. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991;352(6330):77–79. [PubMed: 2062380]
- 81.
- Orafidiya FA, McEwan IJ. Trinucleotide repeats and protein folding and disease: the perspective from studies with the androgen receptor. Future Sci OA. 2015 Sep 1;1(2):FSO47. [PMC free article: PMC5137883] [PubMed: 28031874] [CrossRef]
- 82.
- Kennedy WR, Alter M, Sung JH. Progressive proximal spinal and bulbar muscular atrophy of late onset. A sex-linked recessive trait. Neurology. 1968;18(7):671–680. [PubMed: 4233749]
- 83.
- Robitaille Y, Lopes-Cendes I, Becher M, Rouleau G, Clark AW. The neuropathology of CAG repeat diseases: Review and update of genetic and molecular features. Brain Pathol. 1997;7(3):901–926. [PMC free article: PMC8098401] [PubMed: 9217975]
- 84.
- Young JE, Garden GA, Martinez RA, Tanaka F, Sandoval CM, Smith AC, Sopher BL, Lin A, Fischbeck KH, Ellerby LM, Morrison RS, Taylor JP, La Spada AR. Polyglutamine-expanded androgen receptor truncation fragments activate a bax-dependent apoptotic cascade mediated by DP5/Hrk. J Neurosci. 2009;29(7):1987–1997. [PMC free article: PMC2746676] [PubMed: 19228953]
- 85.
- Beitel LK, Alvarado C, Mokhtar S, Paliouras M, Trifiro M. Mechanisms mediating spinal and bulbar muscular atrophy: Investigations into polyglutamine-expanded androgen receptor function and dysfunction. Front Neurol. 2013;4:53. [PMC free article: PMC3654311] [PubMed: 23720649]
- 86.
- Adachi H, Waza M, Tokui K, Katsuno M, Minamiyama M, Tanaka F, Doyu M, Sobue G. CHIP overexpression reduces mutant androgen receptor protein and ameliorates phenotypes of the spinal and bulbar muscular atrophy transgenic mouse model. J Neurosci. 2007;27(19):5115–5126. [PMC free article: PMC6672370] [PubMed: 17494697]
- 87.
- Yang Z, Chang YJ, Yu IC, Yeh S, Wu CC, Miyamoto H, Merry DE, Sobue G, Chen LM, Chang SS, Chang C. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat Med. 2007;13(3):348–353. [PubMed: 17334372]
- 88.
- Banno H, Katsuno M, Suzuki K, Tanaka F, Sobue G. Pathogenesis and molecular targeted therapy of spinal and bulbar muscular atrophy (SBMA). Cell Tissue Res. 2012;349(1):313–320. [PubMed: 22476656]
- 89.
- Fernandez-Rhodes LE, Kokkinis AD, White MJ, Watts CA, Auh S, Jeffries NO, Shrader JA, Lehky TJ, Li L, Ryder JE, Levy EW, Solomon BI, Harris-Love MO, La Pean A, Schindler AB, Chen C, Di Prospero NA, Fischbeck KH. Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: A randomised placebo-controlled trial. Lancet Neurol. 2011;10(2):140–147. [PMC free article: PMC3056353] [PubMed: 21216197]
- 90.
- Kosaka T, Miyajima A, Kikuchi E, Takahashi S, Suzuki N, Oya M. A case of spinal and bulbar muscular atrophy with high-stage and high-gleason score prostate cancer responded to maximal androgen blockade therapy. J Androl. 2012;33(4):563–565. [PubMed: 21940987]
- 91.
- Orr CR, Montie HL, Liu Y, Bolzoni E, Jenkins SC, Wilson EM, Joseph JD, McDonnell DP, Merry DE. An interdomain interaction of the androgen receptor is required for its aggregation and toxicity in spinal and bulbar muscular atrophy. J Biol Chem. 2010;285(46):35567–35577. [PMC free article: PMC2975181] [PubMed: 20826791]
- 92.
- LaFevre-Bernt MA, Ellerby LM. Kennedy's disease. phosphorylation of the polyglutamine-expanded form of androgen receptor regulates its cleavage by caspase-3 and enhances cell death. J Biol Chem. 2003;278(37):34918–34924. [PubMed: 12824190]
- 93.
- Palazzolo I, Burnett BG, Young JE, Brenne PL, La Spada AR, Fischbeck KH, Howell BW, Pennuto M. Akt blocks ligand binding and protects against expanded polyglutamine androgen receptor toxicity. Hum Mol Genet. 2007;16(13):1593–1603. [PubMed: 17470458]
- 94.
- Scaramuzzino C, Casci I, Parodi S, Lievens PM, Polanco MJ, Milioto C, Chivet M, Monaghan J, Mishra A, Badders N, Aggarwal T, Grunseich C, Sambataro F, Basso M, Fackelmayer FO, Taylor JP, Pandey UB, Pennuto M. Protein arginine methyltransferase 6 enhances polyglutamine-expanded androgen receptor function and toxicity in spinal and bulbar muscular atrophy. Neuron. 2015;85(1):88–100. [PMC free article: PMC4305189] [PubMed: 25569348]
- 95.
- Chua JP, Reddy SL, Yu Z, Giorgetti E, Montie HL, Mukherjee S, Higgins J, McEachin RC, Robins DM, Merry DE, Iniguez-Lluhi JA, Lieberman AP. Disrupting SUMOylation enhances transcriptional function and ameliorates polyglutamine androgen receptor-mediated disease. J Clin Invest. 2015;125(2):831–845. [PMC free article: PMC4319414] [PubMed: 25607844]
- 96.
- Qiang Q, Adachi H, Huang Z, Jiang YM, Katsuno M, Minamiyama M, Doi H, Matsumoto S, Kondo N, Miyazaki Y, Iida M, Tohnai G, Sobue G. Genistein, a natural product derived from soybeans, ameliorates polyglutamine-mediated motor neuron disease. J Neurochem. 2013;126(1):122–130. [PubMed: 23363377]
- 97.
- Ranganathan S, Fischbeck KH. Therapeutic approaches to spinal and bulbar muscular atrophy. Trends Pharmacol Sci. 2010;31(11):523–527. [PMC free article: PMC2963653] [PubMed: 20863580]
- 98.
- Rocchi A, Pennuto M. New routes to therapy for spinal and bulbar muscular atrophy. J Mol Neurosci. 2013;50(3):514–523. [PubMed: 23420040]
- 99.
- Lavery DN, McEwan IJ. Structural characterization of the native NH2-terminal transactivation domain of the human androgen receptor: A collapsed disordered conformation underlies structural plasticity and protein-induced folding. Biochemistry. 2008;47(11):3360–3369. [PubMed: 18284208]
- 100.
- Kumar R, Thompson EB. Transactivation functions of the N-terminal domains of nuclear hormone receptors: Protein folding and coactivator interactions. Mol Endocrinol. 2003;17(1):1–10. [PubMed: 12511601]
- 101.
- McEwan IJ. Intrinsic disorder in the androgen receptor: Identification, characterisation and drugability. Mol Biosyst. 2012;8(1):82–90. [PubMed: 21822504]
- 102.
- Kumar R, McEwan IJ. Allosteric modulators of steroid hormone receptors: Structural dynamics and gene regulation. Endocr Rev. 2012;33(2):271–299. [PMC free article: PMC3596562] [PubMed: 22433123]
- 103.
- Jenster G, van der Korput HA, Trapman J, Brinkmann AO. Identification of two transcription activation units in the N-terminal domain of the human androgen receptor. J Biol Chem. 1995;270(13):7341–7346. [PubMed: 7706276]
- 104.
- Christiaens V, Bevan CL, Callewaert L, Haelens A, Verrijdt G, Rombauts W, Claessens F. Characterization of the two coactivator-interacting surfaces of the androgen receptor and their relative role in transcriptional control. J Biol Chem. 2002;277(51):49230–49237. [PubMed: 12370175]
- 105.
- Claessens F, Denayer S, Van Tilborgh N, Kerkhofs S, Helsen C, Haelens A. Diverse roles of androgen receptor (AR) domains in AR-mediated signaling. Nucl Recept Signal. 2008;6:e008. [PMC free article: PMC2443950] [PubMed: 18612376]
- 106.
- Reid J, Murray I, Watt K, Betney R, McEwan IJ. The androgen receptor interacts with multiple regions of the large subunit of general transcription factor TFIIF. J Biol Chem. 2002;277(43):41247–41253. [PubMed: 12181312]
- 107.
- Lavery DN, McEwan IJ. Functional characterization of the native NH2-terminal transactivation domain of the human androgen receptor: Binding kinetics for interactions with TFIIF and SRC-1a. Biochemistry. 2008;47(11):3352–3359. [PubMed: 18284209]
- 108.
- De Mol E, Fenwick RB, Phang CT, Buzón V, Szulc E, de la Fuente A, Escobedo A, García J, Bertoncini CW, Estébanez-Perpiñá E, McEwan IJ, Riera A, Salvatella X. EPI-001, A Compound Active against Castration-Resistant Prostate Cancer, Targets Transactivation Unit 5 of the Androgen Receptor. ACS Chem Biol. 2016;11(9):2499–505. [PMC free article: PMC5027137] [PubMed: 27356095]
- 109.
- Reid J, Kelly SM, Watt K, Price NC, McEwan IJ. Conformational analysis of the androgen receptor amino-terminal domain involved in transactivation. Influence of structure-stabilizing solutes and protein-protein interactions. J Biol Chem. 2002;277(22):20079–86. [PubMed: 11896058]
- 110.
- De Mol E, Szulc E, Di Sanza C, Martínez-Cristóbal P, Bertoncini CW, Fenwick RB, Frigolé-Vivas M, Masín M, Hunter I, Buzón V, Brun-Heath I, García J, De Fabritiis G, Estébanez-Perpiñá E, McEwan IJ, Nebreda ÁR, Salvatella X. Regulation of Androgen Receptor Activity by Transient Interactions of Its Transactivation Domain with General Transcription Regulators. Structure. 2018;26(1):145–152. [PMC free article: PMC5830076] [PubMed: 29225078]
- 111.
- Eftekharzadeh B, Piai A, Chiesa G, Mungianu D, García J, Pierattelli R, Felli IC, Salvatella X. Sequence Context Influences the Structure and Aggregation Behavior of a PolyQ Tract. Biophys J. 2016;110(11):2361–2366. [PMC free article: PMC4900447] [PubMed: 27276254]
- 112.
- Meyer S, Ying-Hui Wang Y-H, Pau Pérez-Escrivà P, Bruno Kieffer B. Backbone 1H, 15N, 13C NMR assignment of the 518-627 fragment of the androgen receptor encompassing N-terminal and DNA binding domains. Biomol NMR Assign. 2016;10(1):175–8. [PubMed: 26732902]
- 113.
- Langley E, Zhou ZX, Wilson EM. Evidence for an anti-parallel orientation of the ligand-activated human androgen receptor dimer. J Biol Chem. 1995;270(50):29983–29990. [PubMed: 8530400]
- 114.
- Doesburg P, Kuil CW, Berrevoets CA, Steketee K, Faber PW, Mulder E, Brinkmann AO, Trapman J. Functional in vivo interaction between the amino-terminal, transactivation domain and the ligand binding domain of the androgen receptor. Biochemistry. 1997;36(5):1052–1064. [PubMed: 9033395]
- 115.
- Berrevoets CA, Doesburg P, Steketee K, Trapman J, Brinkmann AO. Functional interactions of the AF-2 activation domain core region of the human androgen receptor with the amino-terminal domain and with the transcriptional coactivator TIF2 (transcriptional intermediary factor2). Mol Endocrinol. 1998;12(8):1172–1183. [PubMed: 9717843]
- 116.
- Zhou ZX, Lane MV, Kemppainen JA, French FS, Wilson EM. Specificity of ligand-dependent androgen receptor stabilization: Receptor domain interactions influence ligand dissociation and receptor stability. Mol Endocrinol. 1995;9(2):208–218. [PubMed: 7776971]
- 117.
- Centenera MM, Harris JM, Tilley WD, Butler LM. The contribution of different androgen receptor domains to receptor dimerization and signaling. Mol Endocrinol. 2008;22(11):2373–2382. [PubMed: 18617596]
- 118.
- Schaufele F, Carbonell X, Guerbadot M, Borngraeber S, Chapman MS, Ma AA, Miner JN, Diamond MI. The structural basis of androgen receptor activation: Intramolecular and intermolecular amino-carboxy interactions. Proc Natl Acad Sci U S A. 2005;102(28):9802–9807. [PMC free article: PMC1168953] [PubMed: 15994236]
- 119.
- van Royen ME, Cunha SM, Brink MC, Mattern KA, Nigg AL, Dubbink HJ, Verschure PJ, Trapman J, Houtsmuller AB. Compartmentalization of androgen receptor protein-protein interactions in living cells. J Cell Biol. 2007;177(1):63–72. [PMC free article: PMC2064112] [PubMed: 17420290]
- 120.
- Thompson J, Saatcioglu F, Janne OA, Palvimo JJ. Disrupted amino- and carboxyl-terminal interactions of the androgen receptor are linked to androgen insensitivity. Mol Endocrinol. 2001;15(6):923–935. [PubMed: 11376111]
- 121.
- Brodie J, McEwan IJ. Intra-domain communication between the N-terminal and DNA-binding domains of the androgen receptor: Modulation of androgen response element DNA binding. J Mol Endocrinol. 2005;34(3):603–615. [PubMed: 15956332]
- 122.
- Luisi BF, Xu WX, Otwinowski Z, Freedman LP, Yamamoto KR, Sigler PB. Crystallographic analysis of the interaction of the glucocorticoid receptor with DNA. Nature. 1991;352(6335):497–505. [PubMed: 1865905]
- 123.
- Shaffer PL, Jivan A, Dollins DE, Claessens F, Gewirth DT. Structural basis of androgen receptor binding to selective androgen response elements. Proc Natl Acad Sci U S A. 2004;101(14):4758–4763. [PMC free article: PMC387321] [PubMed: 15037741]
- 124.
- Jakob M, Kolodziejczyk R, Orlowski M, Krzywda S, Kowalska A, Dutko-Gwozdz J, Gwozdz T, Kochman M, Jaskolski M, Ozyhar A. Novel DNA-binding element within the C-terminal extension of the nuclear receptor DNA-binding domain. Nucleic Acids Res. 2007;35(8):2705–2718. [PMC free article: PMC1885670] [PubMed: 17426125]
- 125.
- Haelens A, Tanner T, Denayer S, Callewaert L, Claessens F. The hinge region regulates DNA binding, nuclear translocation, and transactivation of the androgen receptor. Cancer Res. 2007;67(9):4514–4523. [PubMed: 17483368]
- 126.
- Schauwaers K, De Gendt K, Saunders PT, Atanassova N, Haelens A, Callewaert L, Moehren U, Swinnen JV, Verhoeven G, Verrijdt G, Claessens F. Loss of androgen receptor binding to selective androgen response elements causes a reproductive phenotype in a knockin mouse model. Proc Natl Acad Sci U S A. 2007;104(12):4961–4966. [PMC free article: PMC1829247] [PubMed: 17360365]
- 127.
- Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE, Mills IG. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007;8(9):871–878. [PMC free article: PMC1950328] [PubMed: 17721441]
- 128.
- Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21(16):2005–2017. [PMC free article: PMC1948856] [PubMed: 17699749]
- 129.
- Eacker SM, Shima JE, Connolly CM, Sharma M, Holdcraft RW, Griswold MD, Braun RE. Transcriptional profiling of androgen receptor (AR) mutants suggests instructive and permissive roles of AR signaling in germ cell development. Mol Endocrinol. 2007;21(4):895–907. [PubMed: 17244764]
- 130.
- Wang Q, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27(3):380–392. [PMC free article: PMC3947890] [PubMed: 17679089]
- 131.
- Chen Z, Lan X, Thomas-Ahner JM, Wu D, Liu X, Ye Z, Wang L, Sunkel B, Grenade C, Chen J, Zynger DL, Yan PS, Huang J, Nephew KP, Huang TH, Lin S, Clinton SK, Li W, Jin VX, Wang Q. Agonist and antagonist switch DNA motifs recognized by human androgen receptor in prostate cancer. EMBO J. 2015;34(4):502–516. [PMC free article: PMC4331004] [PubMed: 25535248]
- 132.
- Fu M, Wang C, Zhang X, Pestell RG. Acetylation of nuclear receptors in cellular growth and apoptosis. Biochem Pharmacol. 2004;68(6):1199–1208. [PubMed: 15313417]
- 133.
- Clinckemalie L, Vanderschueren D, Boonen S, Claessens F. The hinge region in androgen receptor control. Mol Cell Endocrinol. 2012;358(1):1–8. [PubMed: 22406839]
- 134.
- Brinkmann AO, Trapman J. Genetic analysis of androgen receptors in development and disease. Adv Pharmacol. 2000;47:317–341. [PubMed: 10582090]
- 135.
- Matias PM, Donner P, Coelho R, Thomaz M, Peixoto C, Macedo S, Otto N, Joschko S, Scholz P, Wegg A, Basler S, Schafer M, Egner U, Carrondo MA. Structural evidence for ligand specificity in the binding domain of the human androgen receptor. implications for pathogenic gene mutations. J Biol Chem. 2000;275(34):26164–26171. [PubMed: 10840043]
- 136.
- Sack JS, Kish KF, Wang C, Attar RM, Kiefer SE, An Y, Wu GY, Scheffler JE, Salvati ME, Krystek SR Jr, Weinmann R, Einspahr HM. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc Natl Acad Sci U S A. 2001;98(9):4904–4909. [PMC free article: PMC33136] [PubMed: 11320241]
- 137.
- Pereira de Jesus-Tran K, Cote PL, Cantin L, Blanchet J, Labrie F, Breton R. Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci. 2006;15(5):987–999. [PMC free article: PMC2242507] [PubMed: 16641486]
- 138.
- McEwan IJ. Androgen receptor modulators: A marriage of chemistry and biology. Future Med Chem. 2013;5(10):1109–1120. [PubMed: 23795968]
- 139.
- Veldscholte J, Berrevoets CA, Ris-Stalpers C, Kuiper GG, Jenster G, Trapman J, Brinkmann AO, Mulder E. The androgen receptor in LNCaP cells contains a mutation in the ligand binding domain which affects steroid binding characteristics and response to antiandrogens. J Steroid Biochem Mol Biol. 1992;41(3-8):665–669. [PubMed: 1562539]
- 140.
- Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332(21):1393–1398. [PubMed: 7723794]
- 141.
- Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59(11):2511–2515. [PubMed: 10363963]
- 142.
- Zhao XY, Boyle B, Krishnan AV, Navone NM, Peehl DM, Feldman D. Two mutations identified in the androgen receptor of the new human prostate cancer cell line MDA PCa 2a. J Urol. 1999;162(6):2192–2199. [PubMed: 10569618]
- 143.
- Zhao XY, Malloy PJ, Krishnan AV, Swami S, Navone NM, Peehl DM, Feldman D. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat Med. 2000;6(6):703–706. [PubMed: 10835690]
- 144.
- Krishnan AV, Zhao XY, Swami S, Brive L, Peehl DM, Ely KR, Feldman D. A glucocorticoid-responsive mutant androgen receptor exhibits unique ligand specificity: Therapeutic implications for androgen-independent prostate cancer. Endocrinology. 2002;143(5):1889–1900. [PubMed: 11956172]
- 145.
- Shi XB, Ma AH, Xia L, Kung HJ, de Vere White RW. Functional analysis of 44 mutant androgen receptors from human prostate cancer. Cancer Res. 2002;62(5):1496–1502. [PubMed: 11888926]
- 146.
- Hay CW, McEwan IJ. The impact of point mutations in the human androgen receptor: Classification of mutations on the basis of transcriptional activity. PLoS One. 2012;7(3):e32514. [PMC free article: PMC3293822] [PubMed: 22403669]
- 147.
- Estebanez-Perpina E, Arnold LA, Nguyen P, Rodrigues ED, Mar E, Bateman R, Pallai P, Shokat KM, Baxter JD, Guy RK, Webb P, Fletterick RJ. A surface on the androgen receptor that allosterically regulates coactivator binding. Proc Natl Acad Sci U S A. 2007;104(41):16074–16079. [PMC free article: PMC1999396] [PubMed: 17911242]
- 148.
- Dubbink HJ, Hersmus R, Verma CS, van der Korput HA, Berrevoets CA, van Tol J, Ziel-van der Made AC, Brinkmann AO, Pike AC, Trapman J. Distinct recognition modes of FXXLF and LXXLL motifs by the androgen receptor. Mol Endocrinol. 2004;18(9):2132–2150. [PubMed: 15178743]
- 149.
- He B, Gampe RT Jr, Kole AJ, Hnat AT, Stanley TB, An G, Stewart EL, Kalman RI, Minges JT, Wilson EM. Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol Cell. 2004;16(3):425–438. [PubMed: 15525515]
- 150.
- Hur E, Pfaff SJ, Payne ES, Gron H, Buehrer BM, Fletterick RJ. Recognition and accommodation at the androgen receptor coactivator binding interface. PLoS Biol. 2004;2(9):E274. [PMC free article: PMC509409] [PubMed: 15328534]
- 151.
- Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat. 2012;33(5):887–894. [PubMed: 22334387]
- 152.
- Nadal M, Prekovic S, Gallastegui N, Helsen C, Abella M, Zielinska K, Gay M, Vilaseca M, Taulès M, Houtsmuller AB, van Royen ME, Claessens F, Fuentes-Prior P, Estébanez-Perpiñá E. Structure of the homodimeric androgen receptor ligand-binding domain. . Nat Commun. 2017;8:14388. [PMC free article: PMC5303882] [PubMed: 28165461]
- 153.
- Jiménez-Panizo A, Pérez P, Rojas AM, Fuentes-Prior P, Estébanez-Perpiñá E. Non-canonical dimerization of the androgen receptor and other nuclear receptors: implications for human disease. Endocr Relat Cancer. 2019;26(8):R479–R497. [PubMed: 31252411]
- 154.
- Jenster G, van der Korput HA, van Vroonhoven C, van der Kwast TH, Trapman J, Brinkmann AO. Domains of the human androgen receptor involved in steroid binding, transcriptional activation, and subcellular localization. Mol Endocrinol. 1991;5(10):1396–1404. [PubMed: 1775129]
- 155.
- Hollenberg SM, Evans RM. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988;55(5):899–906. [PubMed: 3191531]
- 156.
- Lu J, Van der Steen T, Tindall DJ. Are androgen receptor variants a substitute for the full-length receptor? Nat Rev Urol. 2015;12(3):137–144. [PubMed: 25666893]
- 157.
- Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011 [PMC free article: PMC3360954] [PubMed: 21446008]
- 158.
- Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. [PMC free article: PMC2672822] [PubMed: 19244107]
- 159.
- Sun S, Sprenger CC, Vessella RL, Haugk K, Soriano K, Mostaghel EA, Page ST, Coleman IM, Nguyen HM, Sun H, Nelson PS, Plymate SR. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J Clin Invest. 2010;120(8):2715–2730. [PMC free article: PMC2912187] [PubMed: 20644256]
- 160.
- Krause WC, Shafi AA, Nakka M, Weigel NL. Androgen receptor and its splice variant, AR-V7, differentially regulate FOXA1 sensitive genes in LNCaP prostate cancer cells. Int J Biochem Cell Biol. 2014;54:49–59. [PMC free article: PMC4160387] [PubMed: 25008967]
- 161.
- Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. [PMC free article: PMC2663383] [PubMed: 18593950]
- 162.
- Hornberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, Bergh A, Wikstrom P. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6(4):e19059. [PMC free article: PMC3084247] [PubMed: 21552559]
- 163.
- Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. [PMC free article: PMC4201502] [PubMed: 25184630]
- 164.
- Yu X, Yi P, Hamilton RA, Shen H, Chen M, Foulds CE, Mancini MA, Ludtke SJ, Wang Z, O'Malley BW. Structural Insights of Transcriptionally Active, Full-Length Androgen Receptor Coactivator Complexes. Mol Cell. 2020;79(5):812–823.e4. [PMC free article: PMC7483370] [PubMed: 32668201]
- 165.
- Yi P, Wang Z, Feng Q, Chou CK, Pintilie GD, Shen H, Foulds CE, Fan G, Serysheva I, Ludtke SJ, Schmid MF, Hung MC, Chiu W, O'Malley BW. Structural and Functional Impacts of ER Coactivator Sequential Recruitment. Mol Cell. 2017;67(5):733–743. [PMC free article: PMC5657569] [PubMed: 28844863]
- 166.
- Kumar R, Betney R, Li J, Thompson EB, McEwan IJ. Induced alpha-helix structure in AF1 of the androgen receptor upon binding transcription factor TFIIF. Biochemistry. 2004;43(11):3008–13. [PubMed: 15023052]
- 167.
- Lavery DN, Bevan CL. Androgen receptor signalling in prostate cancer: The functional consequences of acetylation. J Biomed Biotechnol. 2011;2011:862125. [PMC free article: PMC3022265] [PubMed: 21274273]
- 168.
- Anbalagan M, Huderson B, Murphy L, Rowan BG. Post-translational modifications of nuclear receptors and human disease. Nucl Recept Signal. 2012;10:e001. [PMC free article: PMC3309075] [PubMed: 22438791]
- 169.
- Coffey K, Robson CN. Regulation of the androgen receptor by post-translational modifications. J Endocrinol. 2012;215(2):221–237. [PubMed: 22872761]
- 170.
- Gioeli D, Paschal BM. Post-translational modification of the androgen receptor. Mol Cell Endocrinol. 2012;352(1-2):70–78. [PubMed: 21820033]
- 171.
- van der Steen T, Tindall DJ, Huang H. Posttranslational modification of the androgen receptor in prostate cancer. Int J Mol Sci. 2013;14(7):14833–14859. [PMC free article: PMC3742275] [PubMed: 23863692]
- 172.
- Gaughan L, Stockley J, Wang N, McCracken SR, Treumann A, Armstrong K, Shaheen F, Watt K, McEwan IJ, Wang C, Pestell RG, Robson CN. Regulation of the androgen receptor by SET9-mediated methylation. Nucleic Acids Res. 2011;39(4):1266–1279. [PMC free article: PMC3045589] [PubMed: 20959290]
- 173.
- Ko S, Ahn J, Song CS, Kim S, Knapczyk-Stwora K, Chatterjee B. Lysine methylation and functional modulation of androgen receptor by Set9 methyltransferase. Mol Endocrinol. 2011;25(3):433–444. [PMC free article: PMC3045741] [PubMed: 21273441]
- 174.
- Fu M, Rao M, Wang C, Sakamaki T, Wang J, Di Vizio D, Zhang X, Albanese C, Balk S, Chang C, Fan S, Rosen E, Palvimo JJ, Janne OA, Muratoglu S, Avantaggiati ML, Pestell RG. Acetylation of androgen receptor enhances coactivator binding and promotes prostate cancer cell growth. Mol Cell Biol. 2003;23(23):8563–8575. [PMC free article: PMC262657] [PubMed: 14612401]
- 175.
- Zhong J, Ding L, Bohrer LR, Pan Y, Liu P, Zhang J, Sebo TJ, Karnes RJ, Tindall DJ, van Deursen J, Huang H. p300 acetyltransferase regulates androgen receptor degradation and PTEN-deficient prostate tumorigenesis. Cancer Res. 2014;74(6):1870–1880. [PMC free article: PMC3971883] [PubMed: 24480624]
- 176.
- Xu K, Shimelis H, Linn DE, Jiang R, Yang X, Sun F, Guo Z, Chen H, Li W, Chen H, Kong X, Melamed J, Fang S, Xiao Z, Veenstra TD, Qiu Y. Regulation of androgen receptor transcriptional activity and specificity by RNF6-induced ubiquitination. Cancer Cell. 2009;15(4):270–282. [PMC free article: PMC2848969] [PubMed: 19345326]
- 177.
- Kaikkonen S, Jaaskelainen T, Karvonen U, Rytinki MM, Makkonen H, Gioeli D, Paschal BM, Palvimo JJ. SUMO-specific protease 1 (SENP1) reverses the hormone-augmented SUMOylation of androgen receptor and modulates gene responses in prostate cancer cells. Mol Endocrinol. 2009;23(3):292–307. [PMC free article: PMC5428156] [PubMed: 19116244]
- 178.
- Sutinen P, Malinen M, Heikkinen S, Palvimo JJ. SUMOylation modulates the transcriptional activity of androgen receptor in a target gene and pathway selective manner. Nucleic Acids Res. 2014;42(13):8310–8319. [PMC free article: PMC4117771] [PubMed: 24981513]
- 179.
- Zhang FP, Malinen M, Mehmood A, Lehtiniemi T, Jääskeläinen T, Niskanen EA, Korhonen H, Laiho A, Elo LL, Ohlsson C, Kotaja N, Poutanen M, Sipilä P, Palvimo JJ. Lack of androgen receptor SUMOylation results in male infertility due to epididymal dysfunction. Nat Commun. 2019;10(1):777. [PMC free article: PMC6377611] [PubMed: 30770815]
- 180.
- Gioeli D, Ficarro SB, Kwiek JJ, Aaronson D, Hancock M, Catling AD, White FM, Christian RE, Settlage RE, Shabanowitz J, Hunt DF, Weber MJ. Androgen receptor phosphorylation. regulation and identification of the phosphorylation sites. J Biol Chem. 2002;277(32):29304–29314. [PubMed: 12015328]
- 181.
- Koryakina Y, Ta HQ, Gioeli D. Androgen receptor phosphorylation: Biological context and functional consequences. Endocr Relat Cancer. 2014;21(4):T131–45. [PMC free article: PMC4437516] [PubMed: 24424504]
- 182.
- Kuiper GG, de Ruiter PE, Grootegoed JA, Brinkmann AO. Synthesis and post-translational modification of the androgen receptor in LNCaP cells. Mol Cell Endocrinol. 1991;80(1-3):65–73. [PubMed: 1955082]
- 183.
- Zhou ZX, Kemppainen JA, Wilson EM. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol Endocrinol. 1995;9(5):605–615. [PubMed: 7565807]
- 184.
- Gordon V, Bhadel S, Wunderlich W, Zhang J, Ficarro SB, Mollah SA, Shabanowitz J, Hunt DF, Xenarios I, Hahn WC, Conaway M, Carey MF, Gioeli D. CDK9 regulates AR promoter selectivity and cell growth through serine 81 phosphorylation. Mol Endocrinol. 2010;24(12):2267–2280. [PMC free article: PMC2999477] [PubMed: 20980437]
- 185.
- Chen S, Gulla S, Cai C, Balk SP. Androgen receptor serine 81 phosphorylation mediates chromatin binding and transcriptional activation. J Biol Chem. 2012;287(11):8571–8583. [PMC free article: PMC3318700] [PubMed: 22275373]
- 186.
- Williamson M, de Winter P, Masters JR. Plexin-B1 signalling promotes androgen receptor translocation to the nucleus. Oncogene. 2016;35(8):1066–1072. [PubMed: 25982277]
- 187.
- Blok LJ, de Ruiter PE, Brinkmann AO. Forskolin-induced dephosphorylation of the androgen receptor impairs ligand binding. Biochemistry. 1998;37(11):3850–3857. [PubMed: 9521705]
- 188.
- Wong HY, Burghoorn JA, Van Leeuwen M, De Ruiter PE, Schippers E, Blok LJ, Li KW, Dekker HL, De Jong L, Trapman J, Grootegoed JA, Brinkmann AO. Phosphorylation of androgen receptor isoforms. Biochem J. 2004;383(Pt 2):267–276. [PMC free article: PMC1134067] [PubMed: 15239671]
- 189.
- Ponguta LA, Gregory CW, French FS, Wilson EM. Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J Biol Chem. 2008;283(30):20989–21001. [PMC free article: PMC2475695] [PubMed: 18511414]
- 190.
- Koryakina Y, Knudsen KE, Gioeli D. Cell-cycle-dependent regulation of androgen receptor function. Endocr Relat Cancer. 2015;22(2):249–264. [PMC free article: PMC4382102] [PubMed: 25691442]
- 191.
- Chymkowitch P, Le May N, Charneau P, Compe E, Egly JM. The phosphorylation of the androgen receptor by TFIIH directs the ubiquitin/proteasome process. EMBO J. 2011;30(3):468–479. [PMC free article: PMC3034013] [PubMed: 21157430]
- 192.
- Chen S, Kesler CT, Paschal BM, Balk SP. Androgen receptor phosphorylation and activity are regulated by an association with protein phosphatase 1. J Biol Chem. 2009;284(38):25576–25584. [PMC free article: PMC2757959] [PubMed: 19622840]
- 193.
- Liu X, Han W, Gulla S, Simon NI, Gao Y, Cai C, Yang H, Zhang X, Liu J, Balk SP, Chen S. Protein phosphatase 1 suppresses androgen receptor ubiquitylation and degradation. Oncotarget. 2016;7(2):1754–1764. [PMC free article: PMC4811495] [PubMed: 26636645]
- 194.
- Yang CS, Xin HW, Kelley JB, Spencer A, Brautigan DL, Paschal BM. Ligand binding to the androgen receptor induces conformational changes that regulate phosphatase interactions. Mol Cell Biol. 2007;27(9):3390–3404. [PMC free article: PMC1899975] [PubMed: 17325038]
- 195.
- Taneja SS, Ha S, Swenson NK, Huang HY, Lee P, Melamed J, Shapiro E, Garabedian MJ, Logan SK. Cell-specific regulation of androgen receptor phosphorylation in vivo. J Biol Chem. 2005;280(49):40916–40924. [PubMed: 16210317]
- 196.
- Zong H, Chi Y, Wang Y, Yang Y, Zhang L, Chen H, Jiang J, Li Z, Hong Y, Wang H, Yun X, Gu J. Cyclin D3/CDK11p58 complex is involved in the repression of androgen receptor. Mol Cell Biol. 2007;27(20):7125–7142. [PMC free article: PMC2168904] [PubMed: 17698582]
- 197.
- Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, Hung MC. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the akt pathway. Cancer Res. 2000;60(24):6841–6845. [PubMed: 11156376]
- 198.
- Kuiper GG, De Ruiter PE, Brinkmann AO. Androgen receptor phosphorylation. Ann N Y Acad Sci. 1993;684:224–226. [PubMed: 8317838]
- 199.
- Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, Nesheiwat I, Kong X, Melamed J, Handratta VD, Njar VC, Brodie AM, Yu LR, Veenstra TD, Chen H, Qiu Y. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell. 2006;10(4):309–319. [PubMed: 17045208]
- 200.
- Liu Y, Karaca M, Zhang Z, Gioeli D, Earp HS, Whang YE. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and src kinases. Oncogene. 2010;29(22):3208–3216. [PMC free article: PMC2880659] [PubMed: 20383201]
- 201.
- Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, Earp HS, Whang YE. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc Natl Acad Sci U S A. 2007;104(20):8438–8443. [PMC free article: PMC1895968] [PubMed: 17494760]
- 202.
- Karaca M, Liu Y, Zhang Z, De Silva D, Parker JS, Earp HS, Whang YE. Mutation of androgen receptor N-terminal phosphorylation site tyr-267 leads to inhibition of nuclear translocation and DNA binding. PLoS One. 2015;10(5):e0126270. [PMC free article: PMC4423977] [PubMed: 25950519]
- 203.
- Shu SK, Liu Q, Coppola D, Cheng JQ. Phosphorylation and activation of androgen receptor by aurora-A. J Biol Chem. 2010;285(43):33045–33053. [PMC free article: PMC2963369] [PubMed: 20713353]
- 204.
- Koryakina Y, Ta HQ, Gioeli D. Phosphorylation of the Androgen Receptor. Endocr Relat Cancer. 2014 2014 Aug;21(4):T131–T145. [PMC free article: PMC4437516] [PubMed: 24424504]
- 205.
- Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5(3):280–285. [PubMed: 10086382]
- 206.
- Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: A novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96(10):5458–5463. [PMC free article: PMC21881] [PubMed: 10318905]
- 207.
- Neumann F, Topert M. Pharmacology of antiandrogens. J Steroid Biochem. 1986;25(5B):885–895. [PubMed: 2949114]
- 208.
- Raynaud JP, Ojasoo T. The design and use of sex-steroid antagonists. J Steroid Biochem. 1986;25(5B):811–833. [PubMed: 3543501]
- 209.
- Furr BJ, Valcaccia B, Curry B, Woodburn JR, Chesterson G, Tucker H. ICI 176,334: A novel non-steroidal, peripherally selective antiandrogen. J Endocrinol. 1987;113(3):R7–9. [PubMed: 3625091]
- 210.
- Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. [PMC free article: PMC2981508] [PubMed: 19359544]
- 211.
- Rathkopf DE, Scher HI. Apalutamide for the treatment of prostate cancer. Expert Rev Anticancer Ther. 2018;18(9):823–836. [PMC free article: PMC6643296] [PubMed: 30101644]
- 212.
- Sugawara T, Baumgart SJ, Nevedomskaya E, Reichert K, Steuber H, Lejeune P, Mumberg D, Haendler B. Darolutamide is a potent androgen receptor antagonist with strong efficacy in prostate cancer models. Int J Cancer. 2019;145(5):1382–1394. [PMC free article: PMC6766977] [PubMed: 30828788]
- 213.
- Korpal M, Korn JM, Gao X, Rakiec DP, Ruddy DA, Doshi S, Yuan J, Kovats SG, Kim S, Cooke VG, Monahan JE, Stegmeier F, Roberts TM, Sellers WR, Zhou W, Zhu P. An F876L mutation in androgen receptor confers genetic and phenotypic resistance to MDV3100 (enzalutamide). Cancer Discov. 2013;3(9):1030–1043. [PubMed: 23842682]
- 214.
- Kuil CW, Mulder E. Mechanism of antiandrogen action: Conformational changes of the receptor. Mol Cell Endocrinol. 1994;102(1-2):R1–5. [PubMed: 7926260]
- 215.
- Kuil CW, Berrevoets CA, Mulder E. Ligand-induced conformational alterations of the androgen receptor analyzed by limited trypsinization. studies on the mechanism of antiandrogen action. J Biol Chem. 1995;270(46):27569–27576. [PubMed: 7499218]
- 216.
- Clegg NJ, Wongvipat J, Joseph JD, Tran C, Ouk S, Dilhas A, Chen Y, Grillot K, Bischoff ED, Cai L, Aparicio A, Dorow S, Arora V, Shao G, Qian J, Zhao H, Yang G, Cao C, Sensintaffar J, Wasielewska T, Herbert MR, Bonnefous C, Darimont B, Scher HI, Smith-Jones P, Klang M, Smith ND, De Stanchina E, Wu N, Ouerfelli O, Rix PJ, Heyman RA, Jung ME, Sawyers CL, Hager JH. ARN-509: A novel antiandrogen for prostate cancer treatment. Cancer Res. 2012;72(6):1494–1503. [PMC free article: PMC3306502] [PubMed: 22266222]
- 217.
- Lin TH, Lee SO, Niu Y, Xu D, Liang L, Li L, Yeh SD, Fujimoto N, Yeh S, Chang C. Differential androgen deprivation therapies with anti-androgens casodex/bicalutamide or MDV3100/Enzalutamide versus anti-androgen receptor ASC-J9(R) lead to promotion versus suppression of prostate cancer metastasis. J Biol Chem. 2013;288(27):19359–19369. [PMC free article: PMC3707641] [PubMed: 23687298]
- 218.
- Loddick SA, Ross SJ, Thomason AG, Robinson DM, Walker GE, Dunkley TP, Brave SR, Broadbent N, Stratton NC, Trueman D, Mouchet E, Shaheen FS, Jacobs VN, Cumberbatch M, Wilson J, Jones RD, Bradbury RH, Rabow A, Gaughan L, Womack C, Barry ST, Robson CN, Critchlow SE, Wedge SR, Brooks AN. AZD3514: A small molecule that modulates androgen receptor signaling and function in vitro and in vivo. Mol Cancer Ther. 2013;12(9):1715–1727. [PMC free article: PMC3769207] [PubMed: 23861347]
- 219.
- Kuruma H, Matsumoto H, Shiota M, Bishop J, Lamoureux F, Thomas C, Briere D, Los G, Gleave M, Fanjul A, Zoubeidi A. A novel antiandrogen, compound 30, suppresses castration-resistant and MDV3100-resistant prostate cancer growth in vitro and in vivo. Mol Cancer Ther. 2013;12(5):567–576. [PubMed: 23493310]
- 220.
- Li H, Hassona MD, Lack NA, Axerio-Cilies P, Leblanc E, Tavassoli P, Kanaan N, Frewin K, Singh K, Adomat H, Bohm KJ, Prinz H, Guns ET, Rennie PS, Cherkasov A. Characterization of a new class of androgen receptor antagonists with potential therapeutic application in advanced prostate cancer. Mol Cancer Ther. 2013;12(11):2425–2435. [PubMed: 23939374]
- 221.
- Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E, Rathkopf D, Shelkey J, Yu EY, Alumkal J, Hung D, Hirmand M, Seely L, Morris MJ, Danila DC, Humm J, Larson S, Fleisher M, Sawyers CL., Prostate Cancer Foundation/Department of Defense Prostate Cancer Clinical Trials Consortium. Antitumour activity of MDV3100 in castration-resistant prostate cancer: A phase 1-2 study. Lancet. 2010;375(9724):1437–1446. [PMC free article: PMC2948179] [PubMed: 20398925]
- 222.
- Hoffman-Censits J, Kelly WK. Enzalutamide: A novel antiandrogen for patients with castrate-resistant prostate cancer. Clin Cancer Res. 2013;19(6):1335–1339. [PubMed: 23300275]
- 223.
- Menon MP, Higano CS. Enzalutamide, a second generation androgen receptor antagonist: Development and clinical applications in prostate cancer. Curr Oncol Rep. 2013;15(2):69–75. [PubMed: 23341368]
- 224.
- Joseph JD, Lu N, Qian J, Sensintaffar J, Shao G, Brigham D, Moon M, Maneval EC, Chen I, Darimont B, Hager JH. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 2013;3(9):1020–1029. [PubMed: 23779130]
- 225.
- Veldscholte J, Berrevoets CA, Brinkmann AO, Grootegoed JA, Mulder E. Anti-androgens and the mutated androgen receptor of LNCaP cells: Differential effects on binding affinity, heat-shock protein interaction, and transcription activation. Biochemistry. 1992;31(8):2393–2399. [PubMed: 1540595]
- 226.
- Lamont KR, Tindall DJ. Minireview: Alternative activation pathways for the androgen receptor in prostate cancer. Mol Endocrinol. 2011;25(6):897–907. [PMC free article: PMC3100605] [PubMed: 21436259]
- 227.
- Negro-Vilar A. Selective androgen receptor modulators (SARMs): A novel approach to androgen therapy for the new millennium. J Clin Endocrinol Metab. 1999;84(10):3459–3462. [PubMed: 10522980]
- 228.
- Narayanan R, Mohler ML, Bohl CE, Miller DD, Dalton JT. Selective androgen receptor modulators in preclinical and clinical development. Nucl Recept Signal. 2008;6:e010. [PMC free article: PMC2602589] [PubMed: 19079612]
- 229.
- Haendler B, Cleve A. Recent developments in antiandrogens and selective androgen receptor modulators. Mol Cell Endocrinol. 2012;352(1-2):79–91. [PubMed: 21704118]
- 230.
- Berrevoets CA, Umar A, Trapman J, Brinkmann AO. Differential modulation of androgen receptor transcriptional activity by the nuclear receptor co-repressor (N-CoR). Biochem J. 2004;379(Pt 3):731–738. [PMC free article: PMC1224119] [PubMed: 14744261]
- 231.
- Augello MA, Hickey TE, Knudsen KE. FOXA1: Master of steroid receptor function in cancer. EMBO J. 2011;30(19):3885–3894. [PMC free article: PMC3209791] [PubMed: 21934649]
- 232.
- Grosse A, Bartsch S, Baniahmad A. Androgen receptor-mediated gene repression. Mol Cell Endocrinol. 2012;352(1-2):46–56. [PubMed: 21784131]
- 233.
- Wang RS, Yeh S, Tzeng CR, Chang C. Androgen receptor roles in spermatogenesis and fertility: Lessons from testicular cell-specific androgen receptor knockout mice. Endocr Rev. 2009;30(2):119–132. [PMC free article: PMC2662628] [PubMed: 19176467]
- 234.
- Walters KA, Simanainen U, Handelsman DJ. Molecular insights into androgen actions in male and female reproductive function from androgen receptor knockout models. Hum Reprod Update. 2010;16(5):543–558. [PubMed: 20231167]
- 235.
- De Gendt K, Verhoeven G. Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol Cell Endocrinol. 2012;352(1-2):13–25. [PubMed: 21871526]
- 236.
- Robins DM. Androgen receptor gene polymorphisms and alterations in prostate cancer: Of humanized mice and men. Mol Cell Endocrinol. 2012;352(1-2):26–33. [PMC free article: PMC3188356] [PubMed: 21689727]
- 237.
- Matsumoto T, Sakari M, Okada M, Yokoyama A, Takahashi S, Kouzmenko A, Kato S. The androgen receptor in health and disease. Annu Rev Physiol. 2013;75:201–224. [PubMed: 23157556]
- 238.
- O'Hara L, Smith LB. Development and Characterization of Cell-Specific Androgen Receptor Knockout Mice. Methods Mol Biol. 2016;1443:219–48. [PubMed: 27246343]
- 239.
- Dart DA, Waxman J, Aboagye EO, Bevan CL. Visualising androgen receptor activity in male and female mice. PLoS One. 2013;8(8):e71694. [PMC free article: PMC3737126] [PubMed: 23940781]
- 240.
- Zhang H, Li XX, Yang Y, Zhang Y, Wang HY, Zheng XFS. Significance and mechanism of androgen receptor overexpression and androgen receptor/mechanistic target of rapamycin cross-talk in hepatocellular carcinoma. Hepatology. 2018;67(6):2271–2286. [PMC free article: PMC6106789] [PubMed: 29220539]
- 241.
- Li P, Chen J, Miyamoto H. Androgen Receptor Signaling in Bladder Cancer. Cancers (Basel). 2017;9(2):20. [PMC free article: PMC5332943] [PubMed: 28241422]
- 242.
- Huang CK, Lee SO, Chang E, Pang H, Chang C. Androgen receptor (AR) in cardiovascular diseases. J Endocrinol. 2016;229(1):R1–R16. [PMC free article: PMC4932893] [PubMed: 26769913]
- 243.
- Agiannitopoulos K, Bakalgianni A, Marouli E, Zormpa I, Manginas A, Papamenzelopoulos S, Lamnissou K.J. Gender Specificity of a Genetic Variant of Androgen Receptor and Risk of Coronary Artery Disease. Clin Lab Anal. 2016;30(3):204–7. [PMC free article: PMC6807026] [PubMed: 25716092]
- 244.
- Takov K, Wu J, Denvir MA, Smith LB, Hadoke PWF. The role of androgen receptors in atherosclerosis. Mol Cell Endocrinol. 2018;465:82–91. [PubMed: 29024781]
- 245.
- Reifenstein EC Jr. Hereditary familial hypogonadism. Proc Am Fed Clin Res. 1947;3:86. [PubMed: 18909356]
- 246.
- Wilson JD, Harrod MJ, Goldstein JL, Hemsell DL, MacDonald PC. Familial incomplete male pseudohermaphroditism, type 1: evidence for androgen resistance and variable clinical manifestations in a family with the reifenstein syndrome. N Engl J Med. 1974;290(20):1097–1103. [PubMed: 4821173]
- 247.
- Geissler WM, Davis DL, Wu L, Bradshaw KD, Patel S, Mendonca BB, Elliston KO, Wilson JD, Russell DW, Andersson S. Male pseudohermaphroditism caused by mutations of testicular 17 beta-hydroxysteroid dehydrogenase 3. Nat Genet. 1994;7(1):34–39. [PubMed: 8075637]
- 248.
- Hughes IA. Disorders of sex development: A new definition and classification. Best Pract Res Clin Endocrinol Metab. 2008;22(1):119–134. [PubMed: 18279784]
- 249.
- Sai TJ, Seino S, Chang CS, Trifiro M, Pinsky L, Mhatre A, Kaufman M, Lambert B, Trapman J, Brinkmann AO. An exonic point mutation of the androgen receptor gene in a family with complete androgen insensitivity. Am J Hum Genet. 1990;46(6):1095–1100. [PMC free article: PMC1683844] [PubMed: 2339702]
- 250.
- Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: Historical, clinical, and molecular perspectives. Endocr Rev. 1995;16(3):271–321. [PubMed: 7671849]
- 251.
- Boehmer AL, Brinkmann AO, Nijman RM, Verleun-Mooijman MC, de Ruiter P, Niermeijer MF, Drop SL. Phenotypic variation in a family with partial androgen insensitivity syndrome explained by differences in 5alpha dihydrotestosterone availability. J Clin Endocrinol Metab. 2001;86(3):1240–1246. [PubMed: 11238515]
- 252.
- Batch JA, Davies HR, Evans BA, Hughes IA, Patterson MN. Phenotypic variation and detection of carrier status in the partial androgen insensitivity syndrome. Arch Dis Child. 1993;68(4):453–457. [PMC free article: PMC1029262] [PubMed: 8099270]
- 253.
- Imasaki K, Hasegawa T, Okabe T, Sakai Y, Haji M, Takayanagi R, Nawata H. Single amino acid substitution (840Arg-->His) in the hormone-binding domain of the androgen receptor leads to incomplete androgen insensitivity syndrome associated with a thermolabile androgen receptor. Eur J Endocrinol. 1994;130(6):569–574. [PubMed: 8205256]
- 254.
- Evans BA, Hughes IA, Bevan CL, Patterson MN, Gregory JW. Phenotypic diversity in siblings with partial androgen insensitivity syndrome. Arch Dis Child. 1997;76(6):529–531. [PMC free article: PMC1717223] [PubMed: 9245853]
- 255.
- Boehmer AL, Brinkmann O, Bruggenwirth H, van Assendelft C, Otten BJ, Verleun-Mooijman MC, Niermeijer MF, Brunner HG, Rouwe CW, Waelkens JJ, Oostdijk W, Kleijer WJ, van der Kwast TH, de Vroede MA, Drop SL. Genotype versus phenotype in families with androgen insensitivity syndrome. J Clin Endocrinol Metab. 2001;86(9):4151–4160. [PubMed: 11549642]
- 256.
- Lu J, Danielsen M. A stu I polymorphism in the human androgen receptor gene (AR). Clin Genet. 1996;49(6):323–324. [PubMed: 8884086]
- 257.
- Davies HR, Hughes IA, Patterson MN. Genetic counselling in complete androgen insensitivity syndrome: Trinucleotide repeat polymorphisms, single-strand conformation polymorphism and direct detection of two novel mutations in the androgen receptor gene. Clin Endocrinol (Oxf). 1995;43(1):69–77. [PubMed: 7641413]
- 258.
- Ris-Stalpers C, Hoogenboezem T, Sleddens HF, Verleun-Mooijman MC, Degenhart HJ, Drop SL, Halley DJ, Oosterwijk JC, Hodgins MB, Trapman J. A practical approach to the detection of androgen receptor gene mutations and pedigree analysis in families with x-linked androgen insensitivity. Pediatr Res. 1994;36(2):227–234. [PubMed: 7970939]
- 259.
- Kohler B, Lumbroso S, Leger J, Audran F, Grau ES, Kurtz F, Pinto G, Salerno M, Semitcheva T, Czernichow P, Sultan C. Androgen insensitivity syndrome: Somatic mosaicism of the androgen receptor in seven families and consequences for sex assignment and genetic counseling. J Clin Endocrinol Metab. 2005;90(1):106–111. [PubMed: 15522944]
- 260.
- Elfferich P, van Royen ME, van de Wijngaart DJ, Trapman J, Drop SL, van den Akker EL, Lusher SJ, Bosch R, Bunch T, Hughes IA, Houtsmuller AB, Cools M, Faradz SM, Bisschop PH, Bunck MC, Oostdijk W, Bruggenwirth HT, Brinkmann AO. Variable loss of functional activities of androgen receptor mutants in patients with androgen insensitivity syndrome. Sex Dev. 2013;7(5):223–234. [PubMed: 23774508]
- 261.
- Topcu V, Ilgin-Ruhi H, Siklar Z, Karabulut HG, Berberoglu M, Hacihamdioglu B, Savas-Erdeve S, Aycan Z, Peltek-Kendirci HN, Ocal G, Tukun FA. Investigation of androgen receptor gene mutations in a series of 21 patients with 46,XY disorders of sex development. J Pediatr Endocrinol Metab. 2015;28(11-12):1257–1263. [PubMed: 26197461]
- 262.
- Phelan N, Williams EL, Cardamone S, Lee M, Creighton SM, Rumsby G, Conway GS. Screening for mutations in 17beta-hydroxysteroid dehydrogenase and androgen receptor in women presenting with partially virilised 46,XY disorders of sex development. Eur J Endocrinol. 2015;172(6):745–751. [PubMed: 25740850]
- 263.
- Paris F, Gaspari L, Mbou F, Philibert P, Audran F, Morel Y, Biason-Lauber A, Sultan C. Endocrine and molecular investigations in a cohort of 25 adolescent males with prominent/persistent pubertal gynecomastia. Andrology. 2016;4(2):263–269. [PubMed: 26845730]
- 264.
- Doehnert U, Bertelloni S, Werner R, Dati E, Hiort O. Characteristic features of reproductive hormone profiles in late adolescent and adult females with complete androgen insensitivity syndrome. Sex Dev. 2015;9(2):69–74. [PubMed: 25613104]
- 265.
- Choong CS, Quigley CA, French FS, Wilson EM. A novel missense mutation in the amino-terminal domain of the human androgen receptor gene in a family with partial androgen insensitivity syndrome causes reduced efficiency of protein translation. J Clin Invest. 1996;98(6):1423–1431. [PMC free article: PMC507569] [PubMed: 8823308]
- 266.
- McPhaul MJ, Marcelli M, Tilley WD, Griffin JE, Isidro-Gutierrez RF, Wilson JD. Molecular basis of androgen resistance in a family with a qualitative abnormality of the androgen receptor and responsive to high-dose androgen therapy. J Clin Invest. 1991;87(4):1413–1421. [PMC free article: PMC295186] [PubMed: 2010552]
- 267.
- Dougan GC, Uli N, Shulman DI. Progressive central puberty in a toddler with partial androgen insensitivity. J Pediatr. 2014;164(3):655–657. [PubMed: 24367986]
- 268.
- de Silva KS, Sirisena ND, Wijenayaka HK, Cooray JG, Jayasekara RW, Dissanayake VH. Androgen insensitivity syndrome in a cohort of sri lankan children with 46, XY disorders of sex development (46, XY DSD). Ceylon Med J. 2015;60(4):139–142. [PubMed: 26778393]
- 269.
- Lobaccaro JM, Poujol N, Chiche L, Lumbroso S, Brown TR, Sultan C. Molecular modeling and in vitro investigations of the human androgen receptor DNA-binding domain: Application for the study of two mutations. Mol Cell Endocrinol. 1996;116(2):137–147. [PubMed: 8647313]
- 270.
- Bruggenwirth HT, Boehmer AL, Lobaccaro JM, Chiche L, Sultan C, Trapman J, Brinkmann AO. Substitution of Ala564 in the first zinc cluster of the deoxyribonucleic acid (DNA)-binding domain of the androgen receptor by asp, asn, or leu exerts differential effects on DNA binding. Endocrinology. 1998;139(1):103–110. [PubMed: 9421404]
- 271.
- Nguyen D, Steinberg SV, Rouault E, Chagnon S, Gottlieb B, Pinsky L, Trifiro M, Mader S. A G577R mutation in the human AR P box results in selective decreases in DNA binding and in partial androgen insensitivity syndrome. Mol Endocrinol. 2001;15(10):1790–1802. [PubMed: 11579211]
- 272.
- Wooster R, Mangion J, Eeles R, Smith S, Dowsett M, Averill D, Barrett-Lee P, Easton DF, Ponder BA, Stratton MR. A germline mutation in the androgen receptor gene in two brothers with breast cancer and reifenstein syndrome. Nat Genet. 1992;2(2):132–134. [PubMed: 1303262]
- 273.
- Lobaccaro JM, Lumbroso S, Belon C, Galtier-Dereure F, Bringer J, Lesimple T, Heron JF, Pujol H, Sultan C. Male breast cancer and the androgen receptor gene. Nat Genet. 1993;5(2):109–110. [PubMed: 8252034]
- 274.
- Gast A, Neuschmid-Kaspar F, Klocker H, Cato AC. A single amino acid exchange abolishes dimerization of the androgen receptor and causes reifenstein syndrome. Mol Cell Endocrinol. 1995;111(1):93–98. [PubMed: 7649358]
- 275.
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93(4):531–541. [PubMed: 9604929]
- 276.
- Giwercman YL, Ivarsson SA, Richthoff J, Lundin KB, Giwercman A. A novel mutation in the D-box of the androgen receptor gene (S597R) in two unrelated individuals is associated with both normal phenotype and severe PAIS. Horm Res. 2004;61(2):58–62. [PubMed: 14646391]
- 277.
- Pinsky L, Trifiro M, Kaufman M, Beitel LK, Mhatre A, Kazemi-Esfarjani P, Sabbaghian N, Lumbroso R, Alvarado C, Vasiliou M. Androgen resistance due to mutation of the androgen receptor. Clin Invest Med. 1992;15(5):456–472. [PubMed: 1458719]
- 278.
- Lottrup G, Jorgensen A, Nielsen JE, Jorgensen N, Duno M, Vinggaard AM, Skakkebaek NE, Rajpert-De Meyts E. Identification of a novel androgen receptor mutation in a family with multiple components compatible with the testicular dysgenesis syndrome. J Clin Endocrinol Metab. 2013;98(6):2223–2229. [PubMed: 23589523]
- 279.
- Tordjman KM, Yaron M, Berkovitz A, Botchan A, Sultan C, Lumbroso S. Fertility after high-dose testosterone and intracytoplasmic sperm injection in a patient with androgen insensitivity syndrome with a previously unreported androgen receptor mutation. Andrologia. 2014;46(6):703–706. [PubMed: 23808476]
- 280.
- Petroli RJ, Hiort O, Struve D, Maciel-Guerra AT, Guerra-Junior G, Palandi de Mello M, Werner R. Preserved fertility in a patient with gynecomastia associated with the p.Pro695Ser mutation in the androgen receptor. Sex Dev. 2014;8(6):350–355. [PubMed: 25401426]
- 281.
- Li Y, Qu S, Li P. A novel mutation of the androgen receptor gene in familial complete androgen insensitivity syndrome. Eur Rev Med Pharmacol Sci. 2015;19(21):4146–4152. [PubMed: 26592841]
- 282.
- Rajender S, Gupta NJ, Chakrabarty B, Singh L, Thangaraj K. L712V mutation in the androgen receptor gene causes complete androgen insensitivity syndrome due to severe loss of androgen function. Steroids. 2013;78(12-13):1288–1292. [PubMed: 24055831]
- 283.
- Franasiak JM, Yao X, Ashkinadze E, Rosen T, Scott RT Jr. Discordant embryonic aneuploidy testing and prenatal ultrasonography prompting androgen insensitivity syndrome diagnosis. Obstet Gynecol. 2015;125(2):383–386. [PubMed: 25569013]
- 284.
- Nam H, Kim CH, Cha MY, Kim JM, Kang BM, Yoo HW. Polycystic ovary syndrome woman with heterozygous androgen receptor gene mutation who gave birth to a child with androgen insensitivity syndrome. Obstet Gynecol Sci. 2015;58(2):179–182. [PMC free article: PMC4366873] [PubMed: 25798434]
- 285.
- Akcay T, Fernandez-Cancio M, Turan S, Guran T, Audi L, Bereket A. AR and SRD5A2 gene mutations in a series of 51 turkish 46,XY DSD children with a clinical diagnosis of androgen insensitivity. Andrology. 2014;2(4):572–578. [PubMed: 24737579]
- 286.
- Mazen I, Soliman H, El-Gammal M, Torky A, Mekkawy M, Abdel-Hamid MS, Essawi M. A novel mutation (c.2735_2736delTC) in the androgen receptor gene in 46,XY females with complete androgen insensitivity syndrome in an egyptian family. Horm Res Paediatr. 2014;82(6):411–414. [PubMed: 25034089]
- 287.
- Hiort O, Sinnecker GH, Holterhus PM, Nitsche EM, Kruse K. Inherited and de novo androgen receptor gene mutations: Investigation of single-case families. J Pediatr. 1998;132(6):939–943. [PubMed: 9627582]
- 288.
- Lundberg Giwercman Y, Nikoshkov A, Lindsten K, Bystrom B, Pousette A, Chibalin AV, Arvidsson S, Tiulpakov A, Semitcheva TV, Peterkova V, Hagenfeldt K, Ritzen EM, Wedell A. Functional characterisation of mutations in the ligand-binding domain of the androgen receptor gene in patients with androgen insensitivity syndrome. Hum Genet. 1998;103(4):529–531. [PubMed: 9856504]
- 289.
- Umar A, Berrevoets CA, Van NM, van Leeuwen M, Verbiest M, Kleijer WJ, Dooijes D, Grootegoed JA, Drop SL, Brinkmann AO. Functional analysis of a novel androgen receptor mutation, Q902K, in an individual with partial androgen insensitivity. J Clin Endocrinol Metab. 2005;90(1):507–515. [PubMed: 15486055]
- 290.
- Wong HY, Hoogerbrugge JW, Pang KL, van Leeuwen M, van Royen ME, Molier M, Berrevoets CA, Dooijes D, Dubbink HJ, van de Wijngaart DJ, Wolffenbuttel KP, Trapman J, Kleijer WJ, Drop SL, Grootegoed JA, Brinkmann AO. A novel mutation F826L in the human androgen receptor in partial androgen insensitivity syndrome; increased NH2-/COOH-terminal domain interaction and TIF2 co-activation. Mol Cell Endocrinol. 2008;292(1-2):69–78. [PubMed: 18656523]
- 291.
- Elfferich P, Juniarto AZ, Dubbink HJ, van Royen ME, Molier M, Hoogerbrugge J, Houtsmuller AB, Trapman J, Santosa A, de Jong FH, Drop SL, Faradz SM, Bruggenwirth H, Brinkmann AO. Functional analysis of novel androgen receptor mutations in a unique cohort of indonesian patients with a disorder of sex development. Sex Dev. 2009;3(5):237–244. [PubMed: 19851057]
- 292.
- Tadokoro R, Bunch T, Schwabe JW, Hughes IA, Murphy JC. Comparison of the molecular consequences of different mutations at residue 754 and 690 of the androgen receptor (AR) and androgen insensitivity syndrome (AIS) phenotype. Clin Endocrinol (Oxf). 2009;71(2):253–260. [PubMed: 19178528]
- 293.
- Cools M, Wolffenbuttel KP, Drop SL, Oosterhuis JW, Looijenga LH. Gonadal development and tumor formation at the crossroads of male and female sex determination. Sex Dev. 2011;5(4):167–180. [PubMed: 21791949]
- 294.
- Ris-Stalpers C, Verleun-Mooijman MC, de Blaeij TJ, Degenhart HJ, Trapman J, Brinkmann AO. Differential splicing of human androgen receptor pre-mRNA in X-linked reifenstein syndrome, because of a deletion involving a putative branch site. Am J Hum Genet. 1994;54(4):609–617. [PMC free article: PMC1918097] [PubMed: 8128958]
- 295.
- Akin JW, Behzadian A, Tho SP, McDonough PG. Evidence for a partial deletion in the androgen receptor gene in a phenotypic male with azoospermia. Am J Obstet Gynecol. 1991;165(6 Pt 1):1891–1894. [PubMed: 1750490]
- 296.
- Ris-Stalpers C, Kuiper GG, Faber PW, Schweikert HU, van Rooij HC, Zegers ND, Hodgins MB, Degenhart HJ, Trapman J, Brinkmann AO. Aberrant splicing of androgen receptor mRNA results in synthesis of a nonfunctional receptor protein in a patient with androgen insensitivity. Proc Natl Acad Sci U S A. 1990;87(20):7866–7870. [PMC free article: PMC54851] [PubMed: 2236003]
- 297.
- Ahmed SF, Cheng A, Dovey L, Hawkins JR, Martin H, Rowland J, Shimura N, Tait AD, Hughes IA. Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J Clin Endocrinol Metab. 2000;85(2):658–665. [PubMed: 10690872]
- 298.
- Yong EL, Chua KL, Yang M, Roy A, Ratnam S. Complete androgen insensitivity due to a splice-site mutation in the androgen receptor gene and genetic screening with single-stranded conformation polymorphism. Fertil Steril. 1994;61(5):856–862. [PubMed: 8174721]
- 299.
- Avila DM, Wilson CM, Nandi N, Griffin JE, McPhaul MJ. Immunoreactive AR and genetic alterations in subjects with androgen resistance and undetectable AR levels in genital skin fibroblast ligand-binding assays. J Clin Endocrinol Metab. 2002;87(1):182–188. [PubMed: 11788645]
- 300.
- Trifiro MA, Lumbroso R, Beitel LK, Vasiliou DM, Bouchard J, Deal C, Van Vliet G, Pinsky L. Altered mRNA expression due to insertion or substitution of thymine at position +3 of two splice-donor sites in the androgen receptor gene. Eur J Hum Genet. 1997;5(1):50–58. [PubMed: 9156321]
- 301.
- Infante JB, Alvelos MI, Bastos M, Carrilho F, Lemos MC 2016 Complete androgen insensitivity syndrome caused by a novel splice donor site mutation and activation of a cryptic splice donor site in the androgen receptor gene. J Steroid Biochem Mol Biol 155(Pt A):63-66. [PubMed: 26435450]
- 302.
- Bruggenwirth HT, Boehmer AL, Ramnarain S, Verleun-Mooijman MC, Satijn DP, Trapman J, Grootegoed JA, Brinkmann AO. Molecular analysis of the androgen-receptor gene in a family with receptor-positive partial androgen insensitivity: An unusual type of intronic mutation. Am J Hum Genet. 1997;61(5):1067–1077. [PMC free article: PMC1716041] [PubMed: 9345099]
- 303.
- Jaaskelainen J, Mongan NP, Harland S, Hughes IA. Five novel androgen receptor gene mutations associated with complete androgen insensitivity syndrome. Hum Mutat. 2006;27(3):291. [PubMed: 16470553]
- 304.
- Kerkhofs S, Dubois V, De Gendt K, Helsen C, Clinckemalie L, Spans L, Schuit F, Boonen S, Vanderschueren D, Saunders PT, Verhoeven G, Claessens F. A role for selective androgen response elements in the development of the epididymis and the androgen control of the 5alpha reductase II gene. FASEB J. 2012;26(10):4360–4372. [PubMed: 22798427]
- 305.
- Zhu YS, Katz MD, Imperato-McGinley J. Natural potent androgens: Lessons from human genetic models. Baillieres Clin Endocrinol Metab. 1998;12(1):83–113. [PubMed: 9890063]
- 306.
- Sinnecker GH, Hiort O, Dibbelt L, Albers N, Dorr HG, Hauss H, Heinrich U, Hemminghaus M, Hoepffner W, Holder M, Schnabel D, Kruse K. Phenotypic classification of male pseudohermaphroditism due to steroid 5 alpha-reductase 2 deficiency. Am J Med Genet. 1996;63(1):223–230. [PubMed: 8723114]
- 307.
- Forti G, Falchetti A, Santoro S, Davis DL, Wilson JD, Russell DW. Steroid 5 alpha-reductase 2 deficiency: Virilization in early infancy may be due to partial function of mutant enzyme. Clin Endocrinol (Oxf). 1996;44(4):477–482. [PubMed: 8706317]
- 308.
- Katz MD, Kligman I, Cai LQ, Zhu YS, Fratianni CM, Zervoudakis I, Rosenwaks Z, Imperato-McGinley J. Paternity by intrauterine insemination with sperm from a man with 5alpha-reductase-2 deficiency. N Engl J Med. 1997;336(14):994–997. [PubMed: 9077378]
- 309.
- Matsubara K, Iwamoto H, Yoshida A, Ogata T 2010 Semen analysis and successful paternity by intracytoplasmic sperm injection in a man with steroid 5alpha-reductase-2 deficiency. Fertil Steril 94(7):2770.e7-2770.10. [PubMed: 20493473]
- 310.
- Kang HJ, Imperato-McGinley J, Zhu YS, Cai LQ, Schlegel P, Palermo G, Rosenwaks Z. The first successful paternity through in vitro fertilization-intracytoplasmic sperm injection with a man homozygous for the 5alpha-reductase-2 gene mutation. Fertil Steril. 2011;95(6):2125.e5–2125.e8. [PubMed: 21334614]
- 311.
- Canto P, Vilchis F, Chavez B, Mutchinick O, Imperato-McGinley J, Perez-Palacios G, Ulloa-Aguirre A, Mendez JP. Mutations of the 5 alpha-reductase type 2 gene in eight mexican patients from six different pedigrees with 5 alpha-reductase-2 deficiency. Clin Endocrinol (Oxf). 1997;46(2):155–160. [PubMed: 9135696]
- 312.
- Hiort O, Sinnecker GH, Willenbring H, Lehners A, Zollner A, Struve D. Nonisotopic single strand conformation analysis of the 5 alpha-reductase type 2 gene for the diagnosis of 5 alpha-reductase deficiency. J Clin Endocrinol Metab. 1996;81(9):3415–3418. [PubMed: 8784107]
- 313.
- Boudon C, Lobaccaro JM, Lumbroso S, Ogur G, Ocal G, Belon C, Sultan C. A new deletion of the 5 alpha-reductase type 2 gene in a turkish family with 5 alpha-reductase deficiency. Clin Endocrinol (Oxf). 1995;43(2):183–188. [PubMed: 7554313]
- 314.
- Vilchis F, Mendez JP, Canto P, Lieberman E, Chavez B. Identification of missense mutations in the SRD5A2 gene from patients with steroid 5alpha-reductase 2 deficiency. Clin Endocrinol (Oxf). 2000;52(3):383–387. [PubMed: 10718838]
- 315.
- Silver RI, Rodriguez R, Chang TS, Gearhart JP. In vitro fertilization is associated with an increased risk of hypospadias. J Urol. 1999;161(6):1954–1957. [PubMed: 10332480]
- 316.
- Anwar R, Gilbey SG, New JP, Markham AF. Male pseudohermaphroditism resulting from a novel mutation in the human steroid 5 alpha-reductase type 2 gene (SRD5A2). Mol Pathol. 1997;50(1):51–52. [PMC free article: PMC379579] [PubMed: 9208814]
- 317.
- Makridakis N, Ross RK, Pike MC, Chang L, Stanczyk FZ, Kolonel LN, Shi CY, Yu MC, Henderson BE, Reichardt JK. A prevalent missense substitution that modulates activity of prostatic steroid 5alpha-reductase. Cancer Res. 1997;57(6):1020–1022. [PubMed: 9067262]
- 318.
- Mazen I, Gad YZ, Hafez M, Sultan C, Lumbroso S. Molecular analysis of 5alpha-reductase type 2 gene in eight unrelated egyptian children with suspected 5alpha-reductase deficiency: Prevalence of the G34R mutation. Clin Endocrinol (Oxf). 2003;58(5):627–631. [PubMed: 12699446]
- 319.
- Sasaki G, Nakagawa K, Hashiguchi A, Hasegawa T, Ogata T, Murai M. Giant seminoma in a patient with 5 alpha-reductase type 2 deficiency. J Urol. 2003;169(3):1080–1081. [PubMed: 12576851]
- 320.
- Hiort O, Schutt SM, Bals-Pratsch M, Holterhus PM, Marschke C, Struve D. A novel homozygous disruptive mutation in the SRD5A2-gene in a partially virilized patient with 5alpha-reductase deficiency. Int J Androl. 2002;25(1):55–58. [PubMed: 11869378]
- 321.
- Hafez M, Mazen I, Ghali I, Sultan C, Lumbroso S. A new mutation of 5-alpha-reductase type 2 (A62E) in a large egyptian kindred. Horm Res. 2003;59(6):281–284. [PubMed: 12784092]
- 322.
- Bahceci M, Ersay AR, Tuzcu A, Hiort O, Richter-Unruh A, Gokalp D. A novel missense mutation of 5-alpha reductase type 2 gene (SRD5A2) leads to severe male pseudohermaphroditism in a turkish family. Urology. 2005;66(2):407–410. [PubMed: 16098368]
- 323.
- Bertelloni S, Scaramuzzo RT, Parrini D, Baldinotti F, Tumini S, Ghirri P. Early diagnosis of 5alpha-reductase deficiency in newborns. Sex Dev. 2007;1(3):147–151. [PubMed: 18391525]
- 324.
- Baldinotti F, Majore S, Fogli A, Marrocco G, Ghirri P, Vuerich M, Tumini S, Boscherini B, Vetri M, Scommegna S, Rinaldi R, Simi P, Grammatico P. Molecular characterization of 6 unrelated italian patients with 5alpha-reductase type 2 deficiency. J Androl. 2008;29(1):20–28. [PubMed: 17609295]
- 325.
- Chan AO, But BW, Lau GT, Lam AL, Ng KL, Lam YY, Lee CY, Shek CC. Diagnosis of 5alpha-reductase 2 deficiency: A local experience. Hong Kong Med J. 2009;15(2):130–135. [PubMed: 19342739]
- 326.
- Sahakitrungruang T, Wacharasindhu S, Yeetong P, Snabboon T, Suphapeetiporn K, Shotelersuk V. Identification of mutations in the SRD5A2 gene in thai patients with male pseudohermaphroditism. Fertil Steril. 2008;90(5):2015.e11–2015.e15. [PubMed: 18314109]
- 327.
- Sahu R, Boddula R, Sharma P, Bhatia V, Greaves R, Rao S, Desai M, Wakhlu A, Phadke S, Shukla M, Dabadghao P, Mehrotra RN, Bhatia E. Genetic analysis of the SRD5A2 gene in indian patients with 5alpha-reductase deficiency. J Pediatr Endocrinol Metab. 2009;22(3):247–254. [PubMed: 19492581]
- 328.
- Vilchis F, Valdez E, Ramos L, Garcia R, Gomez R, Chavez B. Novel compound heterozygous mutations in the SRD5A2 gene from 46,XY infants with ambiguous external genitalia. J Hum Genet. 2008;53(5):401–406. [PubMed: 18350250]
- 329.
- Kim SH, Kim KS, Kim GH, Kang BM, Yoo HW. A novel frameshift mutation in the 5alpha-reductase type 2 gene in korean sisters with male pseudohermaphroditism. Fertil Steril. 2006;85(3):750.e9–750.e12. [PubMed: 16500352]
- 330.
- Di Marco C, Bulotta AL, Varetti C, Dosa L, Michelucci A, Baldinotti F, Meucci D, Castagnini C, Lo Rizzo C, Di Maggio G, Simi P, Mari F, Bertelloni S, Renieri A, Messina M. Ambiguous external genitalia due to defect of 5-alpha-reductase in seven iraqi patients: Prevalence of a novel mutation. Gene. 2013;526(2):490–493. [PubMed: 23664981]
- 331.
- Zhang M, Yang J, Zhang H, Ning G, Li X, Sun S. A novel SRD5A2 mutation with loss of function identified in chinese patients with hypospadias. Horm Res Paediatr. 2011;76(1):44–49. [PubMed: 21540559]
- 332.
- Berra M, Williams EL, Muroni B, Creighton SM, Honour JW, Rumsby G, Conway GS. Recognition of 5alpha-reductase-2 deficiency in an adult female 46XY DSD clinic. Eur J Endocrinol. 2011;164(6):1019–1025. [PubMed: 21402750]
- 333.
- Maimoun L, Philibert P, Cammas B, Audran F, Bouchard P, Fenichel P, Cartigny M, Pienkowski C, Polak M, Skordis N, Mazen I, Ocal G, Berberoglu M, Reynaud R, Baumann C, Cabrol S, Simon D, Kayemba-Kay's K, De Kerdanet M, Kurtz F, Leheup B, Heinrichs C, Tenoutasse S, Van Vliet G, Gruters A, Eunice M, Ammini AC, Hafez M, Hochberg Z, Einaudi S, Al Mawlawi H, Nunez CJ, Servant N, Lumbroso S, Paris F, Sultan C 2011 Phenotypical, biological, and molecular heterogeneity of 5alpha-reductase deficiency: An extensive international experience of 55 patients. J Clin Endocrinol Metab 96(2):296-307. [PubMed: 21147889]
- 334.
- Maimoun L, Philibert P, Bouchard P, Ocal G, Leheup B, Fenichel P, Servant N, Paris F, Sultan C. Primary amenorrhea in four adolescents revealed 5alpha-reductase deficiency confirmed by molecular analysis. Fertil Steril. 2011;95(2):804.e1–804.e5. [PubMed: 20850730]
- 335.
- Maimoun L, Philibert P, Cammas B, Audran F, Pienkowski C, Kurtz F, Heinrich C, Cartigny M, Sultan C. Undervirilization in XY newborns may hide a 5alpha-reductase deficiency: Report of three new SRD5A2 gene mutations. Int J Androl. 2010;33(6):841–847. [PubMed: 20132346]
- 336.
- Fenichel P, Paris F, Philibert P, Hieronimus S, Gaspari L, Kurzenne JY, Chevallier P, Bermon S, Chevalier N, Sultan C. Molecular diagnosis of 5alpha-reductase deficiency in 4 elite young female athletes through hormonal screening for hyperandrogenism. J Clin Endocrinol Metab. 2013;98(6):E1055–9. [PubMed: 23633205]
- 337.
- Nie M, Zhou Q, Mao J, Lu S, Wu X. Five novel mutations of SRD5A2 found in eight chinese patients with 46,XY disorders of sex development. Mol Hum Reprod. 2011;17(1):57–62. [PubMed: 20736251]
- 338.
- Savas Erdeve S, Aycan Z, Berberoglu M, Siklar Z, Hacihamdioglu B, Sipahi K, Akar N, Ocal G. A novel mutation of 5alpha-steroid reductase 2 deficiency (CD 65 ALA-PRO) with severe virilization defect in a turkish family and difficulty in gender assignment. Eur J Pediatr. 2010;169(8):991–995. [PubMed: 20179965]
- 339.
- Tsai MC, Chou YY, Lin SJ, Tsai LP. A novel SRD5A2 mutation in a taiwanese newborn with ambiguous genitalia. Kaohsiung J Med Sci. 2012;28(4):231–235. [PubMed: 22453073]
- 340.
- Shabir I, Khurana ML, Marumudi E, Khadgawat R, Ammini AC. Novel nucleotide insertions in two unrelated indian patients with 5alpha reductase 2 deficiency leading to premature termination of SRD5A2 enzyme. Steroids. 2013;78(12-13):1159–1163. [PubMed: 24012728]
- 341.
- Shabir I, Khurana ML, Joseph AA, Eunice M, Mehta M, Ammini AC. Phenotype, genotype and gender identity in a large cohort of patients from india with 5alpha-reductase 2 deficiency. Andrology. 2015;3(6):1132–1139. [PubMed: 26453174]
- 342.
- Zhu H, Liu W, Han B, Fan M, Zhao S, Wang H, Lu Y, Pan C, Chen F, Chen M, Song H, Cheng K, Qiao J. Phenotypic and molecular characteristics in eleven chinese patients with 5alpha-reductase type 2 deficiency. Clin Endocrinol (Oxf). 2014;81(5):711–720. [PubMed: 24665940]
- 343.
- Makridakis NM, Ross RK, Pike MC, Crocitto LE, Kolonel LN, Pearce CL, Henderson BE, Reichardt JK. Association of mis-sense substitution in SRD5A2 gene with prostate cancer in african-american and hispanic men in los angeles, USA. Lancet. 1999;354(9183):975–978. [PubMed: 10501358]
- 344.
- Makridakis N, Akalu A, Reichardt JK. Identification and characterization of somatic steroid 5alpha-reductase (SRD5A2) mutations in human prostate cancer tissue. Oncogene. 2004;23(44):7399–7405. [PubMed: 15326487]
- 345.
- Makridakis NM, di Salle E, Reichardt JK. Biochemical and pharmacogenetic dissection of human steroid 5 alpha-reductase type II. Pharmacogenetics. 2000;10(5):407–413. [PubMed: 10898110]
- 346.
- Wang C, Tao W, Chen Q, Hu H, Wen XY, Han R. SRD5A2 V89L polymorphism and prostate cancer risk: A meta-analysis. Prostate. 2010;70(2):170–178. [PubMed: 19760631]
- ABSTRACT
- INTRODUCTION
- ANDROGEN BIOSYNTHESIS
- METABOLISM OF TESTOSTERONE TO 5ΑΑ-DIHYDRO-TESTOSTERONE
- ANDROGEN ACTION
- ANDROGEN RECEPTOR AMINO ACID NUMBERING
- ANDROGEN RECEPTOR: FUNCTIONAL DOMAIN STRUCTURE
- ANDROGEN RECEPTOR POSTTRANSLATIONAL MODIFICATIONS
- ANTI-ANDROGENS AND SELECTIVE ANDROGEN RECEPTOR MODULATORS
- TISSUE-SPECIFIC ANDROGEN RECEPTOR MEDIATED ACTIONS IN MOUSE MODELS
- ANDROGEN RECEPTOR DISORDERS
- ANDROGEN METABOLISM DISORDERS
- CONCLUSIONS-KEY POINTS
- REFERENCES
- Review Androgen Physiology, Pharmacology, Use and Misuse.[Endotext. 2000]Review Androgen Physiology, Pharmacology, Use and Misuse.Handelsman DJ. Endotext. 2000
- Review Prolactin influences upon androgen action in male accessory sex organs.[Adv Sex Horm Res. 1976]Review Prolactin influences upon androgen action in male accessory sex organs.Thomas JA, Keenan EJ. Adv Sex Horm Res. 1976; 2:425-70.
- Review Androgens and Cardiovascular Disease in Men.[Endotext. 2000]Review Androgens and Cardiovascular Disease in Men.Yeap BB, Dwivedi G. Endotext. 2000
- The role of gonadotropins in testicular and adrenal androgen biosynthesis pathways-Insights from males with congenital hypogonadotropic hypogonadism on hCG/rFSH and on testosterone replacement.[Clin Endocrinol (Oxf). 2021]The role of gonadotropins in testicular and adrenal androgen biosynthesis pathways-Insights from males with congenital hypogonadotropic hypogonadism on hCG/rFSH and on testosterone replacement.Rohayem J, Zitzmann M, Laurentino S, Kliesch S, Nieschlag E, Holterhus PM, Kulle A. Clin Endocrinol (Oxf). 2021 Jan; 94(1):90-101. Epub 2020 Sep 24.
- Androgen-uterine interaction: nuclear translocation of the estrogen receptor and induction of the synthesis of the uterine-induced protein (IP) by high concentrations of androgens in vitro but not in vivo.[Endocrinology. 1976]Androgen-uterine interaction: nuclear translocation of the estrogen receptor and induction of the synthesis of the uterine-induced protein (IP) by high concentrations of androgens in vitro but not in vivo.Schmidt WN, Sadler MA, Katzenellenbogen BS. Endocrinology. 1976 Mar; 98(3):702-16.
- Androgen Physiology: Receptor and Metabolic Disorders - EndotextAndrogen Physiology: Receptor and Metabolic Disorders - Endotext
Your browsing activity is empty.
Activity recording is turned off.
See more...
