NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Feingold KR, Anawalt B, Blackman MR, et al., editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-.
ABSTRACT
Dehydroepiandrosterone (DHEA) and its metabolite DHEA sulfate (DHEAS), are steroid pre-hormones synthesized and secreted primarily by the zona reticularis of the adrenal cortex in response to adrenocorticotropic hormone (ACTH). They are both precursor hormones that may be transformed into weak androgens or estrogens. During the last decades, several epidemiologic and cohort studies have shown the age-related circulating levels of DHEA/DHEAS; these first increase in childhood, a process called “adrenarche”, peak in the 3rd decade of life, and progressively decrease in midlife, a phenomenon called “adrenopause”. Some authors have linked obesity in childhood with early adrenarche, i.e., increased circulating levels of adrenal androgens; others have associated low levels in late life with increased frailty and all-cause mortality. The potential clinical and therapeutic roles of DHEA/DHEAS have been studied extensively, but the data remain controversial and largely inconclusive. In this chapter, we provide an overview of the physiology and pathophysiology of adrenal androgen synthesis, secretion, and action and present current evidence regarding their efficacy in the management of adrenal insufficiency or aging-related disorders. For complete coverage of all related areas of Endocrinology, please visit our on-line FREE web-text, WWW.ENDOTEXT.ORG.
INTRODUCTION
Dehydroepiandrosterone (DHEA) and its metabolite DHEA sulfate (DHEAS), are steroid hormones synthesized and secreted primarily by the zona reticularis of the adrenal cortex in response to adrenocorticotropic hormone (ACTH). They exert weak androgenic effects and are therefore considered precursor hormones that need to be transformed to potent androgens or estrogens to exert their effects. The potential clinical roles of DHEA/DHEAS have been studied extensively, as previous epidemiologic and prospective studies associated the age-related decrease of DHEA/DHEAS levels with higher prevalence of degenerative disorders and increased frailty and mortality from all causes in the elderly, attributing to adrenal androgens anti-aging properties. But do they really suggest that they are hormones related to longevity or just another pointless alchemy against aging? This chapter summarizes the physiology and pathophysiology of adrenal androgen synthesis, secretion, and action and provides current evidence regarding their efficacy in the management of aging-related disorders.
THE ADRENAL ANDROGENS
The Adrenal Cortex; Embryology and Normal Structure
The adrenal cortex is derived from the mesoderm lining the posterior abdominal wall. The fetal cortex begins its development in the 5-week-old fetus. At 2 months of gestation, it is already identifiable as a separate organ and is composed of the inner fetal zone (85% of the cortex) and the outer permanent definitive zone. The anatomic relation of the fetal and definitive zones is maintained during gestation; at birth the adrenal glands are 10–20 times larger than the adult gland, relative to kilograms of body weight. After birth, the fetal zone undergoes rapid involution resulting in a rapid decrease of adrenocortical weight in the 3 months following birth. During the next 3 years, the adult adrenal cortex develops from cells of the outer layer of the cortex and differentiates into the three adult zones, the subcapsular zona glomerulosa, the zona fasciculate, which is the thickest zone (70% of the cortex), and the inner zona reticularis.
Biosynthesis of Adrenal Androgens
The adrenal cortex produces many steroid hormones among which the major ones are cortisol, aldosterone, and the adrenal androgens. The subcapsular zona glomerulosa produces aldosterone while the inner two zones fasciculata and reticularis appear to function as a unit and produce cortisol, androgens, and small amounts of estrogens under the regulatory effect of ACTH and maybe of some other factors produced within the adrenal gland, including neurotransmitters, neuropeptides, and nitric oxide. The biosynthetic pathway of the adrenal androgens is shown below (Fig. 1).
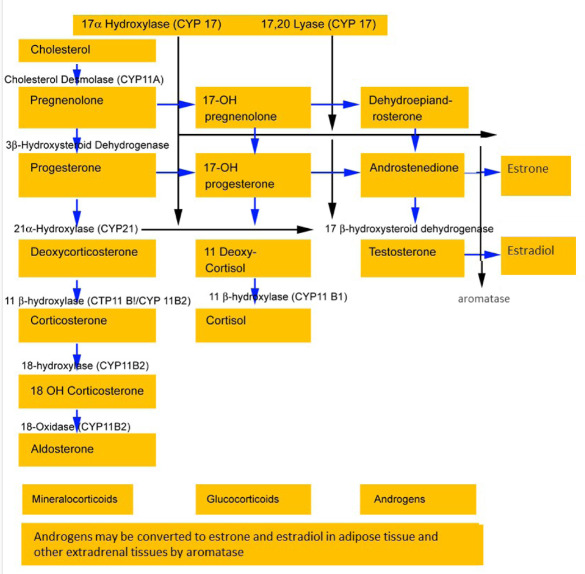
Figure 1.
Steroid biosynthesis in the adrenal cortex.
Quantitatively, the most abundantly produced adrenal androgens are dehydroepiandrosterone (DHEA) and its sulphated form dehydroepiandrosterone sulphate (DHEAS); the latter is the most abundantly produced adrenal steroid. It also has a long half-life and provides a stable pool of circulating DHEA. The ovaries also synthesize DHEA; however, they lack the enzyme DHEA-sulphotransferase so that DHEAS is almost exclusively synthesized and secreted by the adrenals. DHEA is further metabolized to androstenedione (1,2), which may in turn be aromatized to estrone. Whether the adrenals may also produce small amounts of testosterone by further metabolism of androstenedione is controversial (3). Although DHEA and DHEAS are secreted in greater quantities, androstenedione is qualitatively more important since it is more readily converted to testosterone in peripheral tissues. Roughly, the relative androgenic potency of DHEA, androstenedione, testosterone, and dihydrotestosterone (DHT) are 5:10:100:300, respectively. As ACTH is the main regulator of adrenal androgen production in adults, both DHEA and androstenedione exhibit circadian periodicity in concert with ACTH and cortisol and their plasma concentrations increase rapidly following ACTH administration; also, they are suppressed by glucocorticoid administration. Because of its slow metabolic clearance, DHEAS does not exhibit diurnal rhythm variation.
Circulation of Adrenal Androgens
The adrenal androgens are secreted in an unbound state. Soon after their release in the circulation they bind to plasma proteins, chiefly to albumin (90%). Androstenedione, DHEA, and DHEAS circulate weakly bound to albumin, while testosterone is bound extensively to the sex hormone binding globulin (SHBG). Bound steroids are biologically inactive; the unbound steroids are free to interact with target cells either to exert their effects or to be transformed into inactive or active metabolites.
Metabolism of Adrenal Androgens; Gender-Dependent Synthesis Of DHEA/DHEAS
Due to lack or only minor inherent steroidogenic activity, adrenal androgens are precursor hormones (pro-hormones) that need to be transformed to potent androgens or estrogens to exert their effects (4,5). Their transformation into active sex steroids depends upon the level of expression of the various steroidogenic and metabolizing enzymes in each cell type which allows all androgen-sensitive and estrogen-sensitive tissues to have some control over the local levels of sex steroids according to their needs (6). Active androgens and estrogens thus synthesized exert their activity in the target cells with little diffusion, resulting in low levels in the general circulation. This intracrine mechanism serves to eliminate the exposure of other tissues to androgens or estrogens, minimizing unwanted side effects (4,7-9).
In males with normal gonadal function, the conversion of adrenal androgens to testosterone accounts for less than 5% of the total amount of this hormone, and thus the physiologic effect is negligible. In females of reproductive age, the adrenal contribution to total androgen production is more important; during the follicular phase, the adrenal precursors account for 2/3 of total testosterone production and 1/2 of DHT production. During midcycle, the ovarian contribution increases, and the adrenal precursors account for only 40% of testosterone production.
Apart from their peripheral conversion to more potent androgens, the adrenal androgens may be also aromatized to estrogens or undergo degradation and inactivation (4,5) (Fig 2). In more detail, DHEA is readily converted within the adrenal gland to DHEAS. DHEA secreted by the adrenal glands and the ovaries is also converted to DHEAS by the liver and the kidneys or it may be converted to Δ4-androstenedione. The adrenally produced DHEAS may be excreted without further metabolism or it may further undergo limited conversion to DHEA. Both DHEAS and DHEA may be metabolized to 7alpha- and 16alpha-hydroxylated derivatives and by 17β reduction to Δ5-Androstenediol and its sulfate. Androstenedione is converted either to testosterone or by reduction of its 4,5 double bonds to etiocholanolone or androsterone, which may be further converted by 17 alpha reductions to etiocholanediol and androstanediol, respectively. Testosterone is converted to DHT in androgen-sensitive tissues by 5 alpha reduction and it in turn is mainly metabolized by 3 alpha reductions to androstanediol. The metabolites of these androgens are conjugated either as glucuronides or sulfates and excreted in the urine. Regarding aromatization to estrogens, it was shown that not only androstenedione and testosterone, but also DHEA, may be converted to estrogens in peripheral tissues such as brain, bone, breast, and ovaries (6,10); this might be of importance, especially in women during the menopausal transition (see below) (11,12).

Figure 2.
Metabolism of adrenal androgens; 3BHSD, 3β-hydroxysteroid dehydrogenase isozymes; 17BHSD, 17β -hydroxysteroid dehydrogenase isozymes; 5aRed, 5α -reductase isozymesP450 aromatase, steroid sulfatase, STS.
Age-Dependent Synthesis of DHEA/DHEAS
Fetal DHEA and DHEAS fall rapidly after birth and remain low until adrenarche; they then start rising again and peak during the third decade of life after which the serum levels of DHEA and DHEAS progressively decline with advancing age by around 2–5% per year (10,13), so that by menopause the DHEA level has decreased by 60% (14), and by 80-90% of the peak production by the eighth or ninth decade of life (15,16). This decline has been termed “adrenopause”, however, cortisol secretion does not decline with age or may even increase (16,17). Adrenopause is independent of menopause and occurs in both sexes as a gradual process at similar ages. A decrease in 17,20-lyase activity may be responsible for the progressive diminution of DHEA and DHEA-S with advancing age (18,19), although other mechanisms, such as a reduction in the mass of the zona reticularis (20) or a decrease in IGF-I and IGF-II have also been proposed (21). Recent study by Heaney et al. in accordance with previous research found that older subjects exhibited lower plasma and saliva DHEA levels overall, while with increasing age, the DHEA area under the curve was attenuated and the slope of decline became less steep (17,22).
Although DHEAS concentration does not vary throughout the day, DHEA secretion exhibits a diurnal rhythm like that of cortisol. Studies have indicated that DHEA secretion is reduced in the morning period resulting in a flatter diurnal rhythm among the oldest old, in contrast to cortisol which remains stable or even increases in the morning (17,23). The above diurnal rhythms of cortisol and DHEA, lead to an elevated cortisol: DHEA ratio, which is most pronounced in the morning period.
The age-related decline in DHEA/DHEAS levels shows high inter-individual variability (20). There is a clear sex difference in DHEA/DHEAS concentrations with lower DHEAS concentrations in adult women compared to men (24), while there is also a clear genetic component predetermining circulating DHEA/DHEAS. Notably, data from the largest population-based twin study to estimate the genetic and environmental contributions of diurnal DHEAS concentrations demonstrated that salivary DHEAS is a heritable measure, with genetic effects accounting for 37%–46% of the total variance for the late morning and afternoon age-adjusted measures (25).
Since DHEA is the main source of androgens in women, its age-related decline leads to a corresponding decrease in the total androgen pool. Although there is no defined level of androgen below which women can be said to be deficient, the decline of DHEA in postmenopausal women would suggest they are “deficient” in both estrogens and androgens (14). The declining circulating levels of adrenal androgens with advancing age have been related to clinical symptoms and disorders (see below).
In the last few years, the concept that adrenal androgen production gradually declines with advancing age has changed following the analysis of the longitudinal data collected in the Study of Women’s Health Across the Nation (SWAN) (26). When the annual serum levels of DHEAS were aligned according to ovarian status, it was recognized that despite the overall age-related decline in DHEAS, in most women (85% of those studied) the adrenal androgen production rose during the menopausal transition, starting in the early peri-menopause and continuing into the early post-menopause. The DHEAS rise was attributed to the adrenals and not the ovaries, as a similar rise was also observed in intact and ovariectomized women (27); the gender-related rise of adrenal DHEAS and the time course of that rise that returns to a progressive decline following menopause, implies ovarian influences over adrenal steroidogenesis (28). Considering previous failure to adequately attribute phenotype, symptoms, and health trajectories to the observed longitudinal changes in circulating estradiol and progesterone (29), the perimenopausal rise in adrenal androgens could potentially suggest a more important role of these hormones in the occurrence of symptoms during the menopausal transition (30). The observational, epidemiologic, and interventional studies addressing this hypothesis are analyzed below. Some conditions and diseases, like poor life quality, satisfaction and psychosocial, as well as acute stress, severe chronic systemic diseases, anorexia nervosa, Cushing syndrome and chronic administration of glucocorticoids are associated with lower levels of DHEA and DHEAS. Hyperprolactinemia is associated with elevated levels of DHEAS (31,32).
Biologic Effects of Adrenal Androgens; Cellular and Molecular Actions
ROLE AS PRO-HORMONES
DHEA possesses pleiotropic effects. Epidemiologic and prospective studies have associated the decline of circulating levels of androgens with the development and progression of degenerative disorders. The exact mechanism of action and clinical role of DHEA and DHEAS, if any, remain unclear. Due to lack or only minor inherent steroidogenic activity, the adrenal androgens need to be transformed to potent androgens or estrogens to exert their effects on peripheral tissues. Recent data suggest additional direct actions of the adrenal androgens further to those exerted through the androgen and estrogen receptors (see below).
The principal biologic effects of the adrenal androgens typically seen during adrenarche consist mainly of pubic and axillary hair growth and the change of sweat composition that produces adult body odor (33). During the reproductive years, in males with normal gonadal function, the adrenal androgens account for less than 5% of the daily production rate of testosterone and thus the physiologic effect is negligible. DHEA and DHEA-S levels have been shown to be associated with nutritional status. Obese children have higher levels of DHEA and/or DHEA-S and achieve adrenarche earlier than lean children. Indeed, a recent study showed that obese children with higher DHEAS concentrations at the age of seven years had more total and central adiposity and higher insulin than did nonobese children of the same age (34,35). Some research suggests that adrenal androgens directly or after peripheral conversion to estrogen modulate hypothalamic activity influencing the gonadarche. When produced in excess however, the adrenal androgens may have no clinical consequences in adult males or result in LH /FSH suppression and oligospermia/infertility. In boys, the adrenal androgen excess is associated with clinical manifestations including premature penile enlargement, early development of secondary sexual characteristics, premature closure of the epiphyseal growth plates and short final height. In females the excessive production of adrenal steroids as seen in Cushing syndrome, adrenal carcinoma, and congenital adrenal hyperplasia via peripheral conversion to testosterone and eventually to DHT result in acne, hirsutism, and menstrual/fertility defects or even virilization in more severe cases.
MEMBRANE ASSOCIATED DHEA RECEPTORS
Further to their effect via the estrogen and androgen receptors, recent data support direct actions of DHEA through specific G protein-coupled membrane receptors in bovine aortic and primary human umbilical vein endothelial cells (HUVECs) (36-38) through which DHEA activates the endothelial NO synthetase (eNOS) (eNOS/cGMP pathway) [38] and increases the production of nitric oxide (NO), a key modulator of vascular function, by endothelial cells. Such receptors are also seen in the kidney, heart, and liver but at lower level than that in bovine aortic endothelial cells (39) as well as in pulmonary artery smooth muscle cells (PASMCs), where DHEA inhibits voltage-dependent T type Ca-channels (40). In systemic circulation, a plasma membrane receptor has been suggested in the anti-remodeling action of DHEA involving inhibition of the Akt/GSK-3β signaling pathway (41). Other studies have shown inhibitory effect of DHEA on proliferation and apoptosis of endothelial and vascular smooth muscle cells independently of both estrogen and androgen receptors (42,43). The above suggest the presence of a membrane-associated DHEA specific receptor; the molecular structure of this receptor remains to be elucidated.
CYTOSOLIC NUCLEAR RECEPTORS
Steroid action involves cytosolic/nuclear hormone receptors (44); thus, most of the studies looking at the mechanism(s) responsible for DHEA action focused on such receptors (45). However, since DHEA can be metabolized into androgens/estrogens, it is not always easy to determine whether DHEA exerts its effects directly through the estrogen/androgen receptors or after conversion to these hormones. There is some new evidence showing that DHEA and some of its metabolites either bind to or activate nuclear receptors such as pregnane X receptor, constitutive androstanol receptor, estrogen receptor-β, and peroxisome proliferators activated receptors (46-49). Through the activation of peroxisome proliferator-activated receptor alpha for example, DHEA inhibits the activation of nuclear factor-κB and the secretion of interleukin-6 and interleukin-12, through which DHEA exerts anti-inflammatory effects (50,51). 7α-and 7β-hydroxylated derivatives of DHEA also seem to have direct effects on nuclear receptors, but their physiological function is not clear (39). Finally, DHEA inhibits apoptosis and promotes proliferation of osteoblasts in rats through MAPK signaling pathways, independently from androgens and estrogens (52); this action could be beneficial for preservation of bone mass and reduction of fracture risk.
ENDOPLASMIC RETICULUM RECEPTOR SIGMA 1 RECEPTOR
More recently, it has been suggested that DHEA is an agonist of sigma-1 receptor (Sigma-1R) expressed in the endoplasmic reticulum of the heart, kidney, liver, and brain (53,54). Under physiological conditions, the sigma-1 receptor chaperones the functional inositol 1,4,5 trisphosphate receptor at the endoplasmic reticulum participating in the calcium signaling pathway (53,55). Animal studies have shown that via sigma-1R, but also by Akt– eNOs signaling pathway stimulation, DHEA may improve cardiac function (56) and exert vasculo-protective effects (57). There is a great volume of data suggesting antioxidant properties of DHEA; overproduction of oxygen-free radicals (oxidative stress) upregulates inflammation and cellular proliferation and is believed to play a critical role in the development of cancer, atherosclerosis, and Alzheimer's disease, as well as the basic aging process (58-60). DHEA inhibits glucose-6-phosphate dehydrogenase (G-6-PDH) (61,62) and NADPH production. The decrease in NADPH levels results in reduced oxygen-free radical production via NADPH oxidase (62). Moreover, a study found that DHEA treatment of mice increased the number of Brd U-positive neurons co-expressing β-catenin, a downstream GSK-3β target, concluding that sigma-1 receptor stimulation by DHEA led to altered OBX-induced depressive-like behaviors by increasing neurogenesis in the dentate gyrus through activation of the Akt/GSK-3β/β-catenin pathway (63). Increased plasma DHEA and DHEAS have been demonstrated in post-traumatic stress disorder (PTSD), predicting symptom improvement and coping as well as resilience adaptation. The same study suggested that decreased cortisol/DHEA ratio was associated with severe childhood trauma and current symptoms (64).
In summary, DHEA mediates its action via transformation into androgen and estrogen derivatives acting through their specific receptors, but also via multiple (57,65) signaling pathways involving specific membrane, cytosolic/nuclear and endoplasmic reticulum receptors.
POTENTIAL TREATMENT BENEFITS
Data from epidemiologic and prospective studies indicate an inverse relation between low circulating levels of DHEA and DHEA-S and a host of aging-associated pathologies such as sexual dysfunction, mood defects, and poor sense of well-being (27,28), as well as higher risk of hospital admission (66), poor muscle strength (67) and mobility (66,68), and higher prevalence of frailty (69), insulin resistance, obesity, cardiovascular disease (45) and mortality from cardiovascular disease (70). At the same time, a positive relation between higher levels of DHEA-S and better health and well-being was documented (71). Furthermore, animal (primarily rodent) studies have suggested many beneficial effects of DHEA treatment, including improved immune function and prevention of atherosclerosis, cancer, diabetes, and obesity. Therefore, the therapeutic role of DHEA replacement as an anti-aging factor for the prevention and/or treatment of the above conditions was studied; recent systematic reviews of the reports do not seem promising, however (72-78).
Treatment Modalities
DHEA is considered as a hormone in Europe and thus becomes available only by prescription, while in the United States it is considered as a nutritional supplement and is sold over the counter without a prescription. This difference has no scientific foundation and is mostly a matter of declaration. Most DHEA supplements are made in laboratories from a substance called diosgenin, a plant sterol found in soy and wild yams. DHEA supplements were taken off the U.S. market in 1985 because of their unproven safety and effectiveness, but were reintroduced as a dietary supplement after the Dietary Supplement Health and Education Act was passed in 1994. At present, questionable over-the-counter DHEA preparations lacking pharmacokinetic and pharmacodynamic data are widely used in the United States. There is no standard dosage of DHEA replacement; some studies have used between 25 and 200 milligrams a day, or sometimes even higher amounts. DHEA in current preparations has a long half-life (45), which allows a single intake a day. Target levels of DHEA are around the middle of normal range for healthy young subjects, measured in a blood sample 24 hr after the last intake (79).
The adrenal androgens are mainly thought to act as prohormones and exert at least part of their action via conversion to androgens and/or estrogens. Previous studies have shown that the end- products of DHEA supplementation depend on the patient’s gender, with a non-symmetrical transformation of DHEA favoring androgens in women and estrogens in men (72,80,81). The above refer to oral administration of DHEA supplements; percutaneous administration of DHEA seems to provoke similar increases in both estrogens and androgens in the two genders (82).
Adrenal Insufficiency
Adrenal insufficiency, despite supplementation of glucocorticoids, has been associated with decreased quality of life when compared to a healthy population (79,83,84). DHEA supplementation has been suggested as an accessory treatment to conventional adrenal replacement therapy with glucocorticoids and mineralocorticoids. The exact physiological roles of DHEA still remain unclear and the routine therapy of individuals with adrenal insufficiency is still controversial. Some authors reported significant improvements of mood, well-being, sexual thoughts, libido, interest, and satisfaction following DHEA replacement, particularly in females (79,83-85). Other analyses of DHEA administration in women with primary and secondary adrenal insufficiency have resulted in inconsistent and unreproducible results (85). Recently, the supplementation of DHEA was suggested in women with adrenal insufficiency and low libido, depressive symptoms, or low energy levels despite optimal glucocorticoid and mineralocorticoid replacement (86).
LOW DHEA/DHEAS LEVELS AND ASSOCIATED COMORBIDITIES
DHEA And Musculoskeletal Disorders
The increasing incidence of fractures with advancing age has been related, among other factors, with the aging-related reduced muscle mass and strength, that increase the propensity for falling (87). A body of evidence exists on the effect of circulating DHEA/DHEAS on various markers of strength and physical function in older individuals. Studies in elderly individuals support a positive relation between DHEA blood levels and muscle mass (67), muscle strength (67,88), and mobility (68), as well as better self-reported (89) and objectively assessed physical function (90), and measured peak volume of oxygen consumed per minute (91) in elderly with higher DHEA/DHEAS concentrations. In this direction, higher DHEAS levels were associated with increased bone mass density (BMD) in both men (92) and post-menopausal women and inversely related to risk for falls (93). Finally, low DHEAS levels have been associated with a higher prevalence of frailty, a geriatric syndrome of loss of reserve characterized by weight loss, fatigue, weakness, and vulnerability to adverse events (69,94), and low back pain in both genders and slow rehabilitation of low-back pain in women (71,95,96).
Reports from interventional studies support a therapeutic role of DHEA replacement in aging-associated musculoskeletal defects. For example, DHEA exerted positive effects on muscle strength, body composition (97-99), and physical performance (100), as well as on bone mass density (BMD) in both lumbar spine and the hip (15,72,98,101-105) when administered to post-menopausal women and elderly people over a period of 52 weeks. The above positive effects on musculoskeletal system were attributed to the DHEA-related increase of insulin-like growth factor-1 (IGF-1) levels (97,106) and bioavailability (decrease of insulin growth factor binding protein-1 [IGFBP-1]) (106) in both men and women and/or to the increase of androgen levels mostly in women (97,106,107). Some other data also suggest aromatase activity of primary human osteoblasts converting DHEA to estrone (108), while it was shown in vitro that DHEA inhibits apoptosis and promotes proliferation of rat osteoblasts through MAPK signaling pathways, independently from androgen and estrogen effects (52). The above support a positive effect of DHEA on bone through conversion to estrogens, but also independently from its hormonal end-products. Other studies, however, failed to show a beneficial effect of DHEA supplementation on muscle function (109-111) or on BMD (99,100,112); of note all these studies were conducted over a shorter period (26 weeks only). Whether these conflicting data result from DHEA’s mild/moderate effect or from great differences between study designs, such as short duration of treatment and small number of participants, is difficult to say (73). Overall, the effect of DHEA supplementation on BMD is small in relation to other treatments for bone loss, and no fracture data are available. Therefore, its therapeutic utility in rehabilitation and/or fracture/frailty prevention and treatment protocols for older patients remains unclear.
A recent systematic review (73) of the literature (72,83,97,100,113-116) concluded that overall, the benefit (113,116) of DHEA on muscle strength and physical function in older adults remains inconclusive. Some measures of muscle strength may improve, although DHEA does not appear to routinely benefit measures of physical function or performance. Therefore, consensus has not been reached. Further large clinical trials are necessary to better identify the clinical role of DHEA supplementation in this population.
DHEA, Well-Being and Sexual Function
If DHEA’s effects on musculoskeletal disorders are inconclusive, its utility for the management of ageing-related poor sense of well-being and sexual dysfunction is a question for top puzzle solvers. What we know so far from epidemiologic studies is that sexual function problems are common among women and increase with increasing age (117-119). The sex steroid hormones estrogens and androgens seem to play an important role in the sexual life of women; androgens affect the reusability, pleasure, and intensity of orgasm in women and are particularly implicated in the neurovascular smooth muscle response of swelling and lubrication, whereas estrogens contribute to vulval and vaginal congestive response and affect mood and sexual responses (120). Conditions such as menopausal symptoms, loss of libido, vulvovaginal atrophy-related sexual dysfunction, and poor sense of well-being seen in menopausal and peri-menopausal women were related to the age-associated decline in sex steroids (121). Furthermore, interventional studies in postmenopausal women with estrogens have shown much improvement on vaginal atrophy and vasomotor symptoms (121-124); there is also much clinical evidence for the efficacy of testosterone treatment for low sexual function in women (119,125-129).
Given that a) the adrenal steroids are the most abundant sex steroids in post-menopausal women and provide a large reservoir of precursors for the intracellular production of androgens and estrogens in non-reproductive tissues, b) DHEA levels decline with age, c) pre- and post-menopausal women with lower sexual responsiveness have lower levels of serum DHEAS (130) and d) treatment of postmenopausal women with estrogen and testosterone have shown some improvement in sexual function, it was proposed that restoring the circulating levels of DHEA to those found in young women may improve sexual function and well-being in postmenopausal women (131). Some early randomized trials that suffered from methodological issues, such as small number of participants, short treatment duration, and supraphysiological doses, demonstrated positive effects of DHEA replacement on sexual function and well-being (15,130,132-134), as well as on relief of menopausal symptoms (134-136). Similarly, women with adrenal insufficiency treated with oral DHEA replacement demonstrated significant improvement in overall well-being, as well as in frequency of sexual thoughts, sexual interest, and satisfaction (80,84). Other studies, however, failed to show any benefit of DHEA replacement on sexual function, well-being, and menopausal symptoms in peri- and post-menopausal women (74,75,106,137,138) and women with adrenal insufficiency (85,139,140). A recent review of the available data concluded that current evidence does not support the routine use of DHEA in women with adrenal insufficiency (76). Furthermore, the more recent placebo-controlled randomized trials that are of superior design compared to the early trials, as they use validated measures of sexual function, have larger sample sizes, and are of longer duration, failed to document any significant benefit of oral DHEA therapy on well-being or sexual function in women (72-75,77). It has been hypothesized that the efficacy of DHEA to improve sexual function might be dependent on the route of its administration. In women, androgens and estrogens are produced from DHEA in the vagina tissue. As vaginal atrophy and dryness are common symptoms of estrogen deficiency during menopause, causing dyspareunia and sexual dysfunction (141), a possible benefit that emerged is that vaginally administered DHEA may improve the postmenopausal vaginal atrophy-related sexual dysfunction (142) without increasing the circulating levels of estrogen above the postmenopausal range (80,142-144). Despite initial promising, beneficial effects on sexual function, again, even with intravaginally administered DHEA, a study failed to show significant benefits (77).
In men lower circulating levels of DHEA was related to erectile dysfunction. A double-blind, placebo-controlled study that enrolled men with erectile dysfunction treated with oral DHEA 50 mg daily has shown some promise for improving sexual performance in men who had low DHEA blood levels (145). However, high-quality studies have demonstrated inconsistent results regarding DHEA supplementation for improving sexual function, libido, and erectile dysfunction. Although research in this area is promising, additional well-designed studies are required.
DHEA And Mood Disorders
The prevalence of depression increases in cohorts of the elderly and has been independently related to high morbidity and mortality (146). In the central nervous system, DHEA is considered a neurosteroid with a wide range of functions. Animal studies demonstrated several DHEA-modulated neurotransmitters, including dopamine, glutamate, and c-amino butyric acid (39), as well as DHEA-induced increased activity of 5-hydroxytryptamine (5-HT) neurons (147), providing the cellular basis for a potential antidepressant effect of DHEA. Furthermore, typical neuroleptic-like effects of DHEA were displayed in animal models of schizophrenia suggesting potential role of DHEA replacement in the treatment of schizophrenia (148).
Previous studies suggested a strong relation between low levels of DHEA/DHEA-S and major depression in children and adolescents (149), as well as adults and the elderly (150,151). On the contrary, higher DHEA-S levels were positively associated with depressive symptoms during the menopausal transition (152) and depression in patients with major depression (153,154); whether the elevated DHEA-S levels in the above studies represent increased adrenal activity that could explain the depressive symptoms is not clear, as cortisol was not measured. Moreover, successful treatment of depression was followed by reductions in both DHEA-S (153-155) and DHEA levels (155), making the relation between DHEA/DHEAS and depression even more confusing.
Several interventional studies have shown that DHEA replacement may improve negative and depressive symptoms (132,133,156-158). In women with adrenal insufficiency, oral DHEA replacement significantly improved the overall well-being, as well as scores for depression and anxiety (110); similar results were found in the management of the negative symptoms of schizophrenia (158). Recent placebo-controlled randomized trials, however, failed to demonstrate a beneficial effect of DHEA therapy on mood, quality of life, perceptions of physical and emotional health, and life satisfaction in postmenopausal women (72,74,75). However, recent data have suggested that increased circulating DHEA(S) levels may predict SSRI-associated remission in major depression (159). Thus, the therapeutic role of DHEA on mood disorders remains unclear.
DHEA and Age-Related Cognitive Decline
The incidence of dementia increases exponentially with increasing age in both men and women (172). The number of elderly people nowadays is the fastest growing segment of the population, which means the related personal, social, and economic burdens of dementia are extremely high and could increase dramatically over the next few decades. Therefore, effective prevention/treatment of neurodegenerative disorders is imperative. It has been proposed that DHEA and DHEAS may exert neuroprotective effects in the brain mainly through DHEA-dependent neural stem cell stimulation, genomic activity modulation, and upregulation of androgen receptor levels (173,174), as well as via the DHEA-induced inhibition of pro-inflammatory factor production, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) (39) that are involved in the pathogenesis of the amyloid plaques of Alzheimer disease (175). Higher serum levels of DHEAS have been related to more favorable cognitive function in older people, such as better concentration and working memory (176,177) and higher scores on the Mini Mental State Examination (178). In this direction, low DHEA/DHEAS levels in particular brain regions were thought to play a role in the development of Parkinson disease, which is the second most common neurodegenerative disorder, just behind Alzheimer (179), while DHEA administration showed some beneficial effect in a primate model of Parkinson disease (180,181). Inverse relations between DHEAS levels in saliva (181) and circulation (176) and some domains of memory impairment were also documented, supporting the hypothesis that DHEA supplementation may improve cognition in the elderly; yet solid evidence of associations between the endogenous levels of these steroids and measures of cognitive function is lacking.
No studies with DHEA replacement, either acute administration or chronic (up to 12 months) supplementation, have shown a benefit in cognitive function in healthy elderly populations (74,137,181-185). Furthermore, DHEA supplementation failed to show any benefit in patients with Alzheimer disease (186) and had only minimal beneficial effect on specific cognitive domains such as the verbal fluency in older women with mild to moderate cognitive impairment (187). Remarkably, some other studies have shown a negative effect of DHEAS replacement on cognitive performance (183,188,189). It should be noted however, that most studies included only small groups of patients and were up to a yearlong, which is probably not enough time to address the potential role of DHEA / DHEAS in neurodegenerative disorders.
DHEA and Metabolism
LIPIDS
In women, the effects of sex steroids on lipid profile differ according to the steroid treatment (estrogen or androgen) and to the route of administration. Thus, oral methyltestosterone lowers high-density lipoprotein (HDL)-cholesterol (190), and oral estrogen increases HDL-cholesterol and triglycerides and lowers low-density lipoprotein (LDL)-cholesterol and total cholesterol (191,192), while transdermal estradiol and transdermal testosterone have little or no effect on lipids (193). Combined oral estrogen and methyltestosterone is associated with lowering of HDL-cholesterol (194,195). Considering that DHEA can be converted intracellularly to estrogens and androgens, the effect on the lipid profile could be mixed and may vary between individuals. Most of the recent well-designed studies, addressing this issue report no association or even an adverse association (at least in women) (196,197) between plasma levels of DHEA (198,199) or DHEA administration (97,109,192,200,201) and the lipid profile.
BODY MASS INDEX (BMI)
Animal studies support a beneficial effect of DHEA administration on obesity (202-204). In humans, two sets of longitudinal analyses of studies with women in menopausal transition showed that elevated DHEAS level is negatively related to BMI (27,28). On the other hand, baseline analyses by Santoro et al [209] did not find much association between DHEAS and BMI, waist-hip ratio, or waist. Childhood obesity is associated with higher levels of DHEAS (34). Similarly, a 2-year, placebo-controlled, randomized, double-blind study involving elderly men and women with low levels of DHEAS, showed no significant effect of DHEA replacement (75 mg per day orally) on body composition measurements (72). Interestingly, a, recent meta-analysis of intervention studies showed that DHEA supplementation in elderly men can induce only a small positive effect on body composition which is strictly dependent on DHEA conversion into its bioactive metabolites such as androgens or estrogens (205). Putting together these results, current data regarding DHEA effect on BMI contradict each other, and its usage in clinical practice for body weight management is not suggested or recommended at the present.
INSULIN RESISTANCE
DHEA may at least theoretically improve endothelial function (43), and ameliorate local/systemic inflammation (50,51) and oxidative stress (58-60,206). These effects in association with DHEAS’s inverse relation with body mass index (BMI) (23,27,28,207) would most probably suggest beneficial effect of DHEA/DHEAS supplement on insulin sensitivity (207). This hypothesis was confirmed by reports from animal studies in which DHEA replacement had a beneficial effect on insulin sensitivity (202,203). In human studies, however, the results are rather inconsistent. In some studies, the lower levels of DHEA seen with aging have been associated with impaired glucose tolerance, insulin resistance, and diabetes (208-211), while in another (212) exactly the opposite relation was shown as higher levels of DHEA were associated with impaired glucose tolerance and diabetes mellitus in post-menopausal women. The truth regarding DHEA/DHEAS and insulin resistance and its associated conditions gets even more complicated considering conflicting results from interventional studies with DHEA replacement. Thus, an ameliorating effect of long-term treatment with DHEA on insulin resistance was described in a group of middle-aged hypo-adrenal women treated with DHEA (213), but also in groups of elderly men (214) and postmenopausal women (213-217) replaced with DHEA. The DHEA dose used ranged between 25 and 100 mg/day oally and the duration of treatment varied between 3 and 12 months; in one study transdermal DHEA was used (217). Most other interventional studies addressing this issue, failed to demonstrate any significant effect of DHEA on insulin resistance/sensitivity (72,97,110,112,113,139) and so did a recent review of the available data regarding use of DHEA in women with adrenal insufficiency (76). Remarkably, Mortola and Yen (84) reported worsening insulin resistance with DHEA replacement in postmenopausal women; in this study however, the number of participants was small (n=6), the duration of treatment short (28 days), and the DHEA dosage supraphysiological (1600 mg/day orally). Putting together the above, the relation between DHEA and carbohydrate metabolism is still uncertain.
DHEA and Cardiovascular Disease (CVD)
CVD represents a serious public health problem; its prevalence increases with advancing age (218). Low androgen levels have been related to atherogenic profile in men (219,220), while data from acute coronary units have shown a transient fall of the testosterone levels in the first 24 hours after myocardial infarction (MI) (221,222), which probably deprives these patients of testosterone’s pro-fibrinolytic activity (223-225) and may actually result in increased 30-day mortality rate following acute MI (226); the above findings suggest a strong relation between sex steroid hormones and CVD morbidity and mortality. Many studies have previously documented a significant inverse relation between low DHEA/DHEAS levels and key elements involved in the development of atherosclerosis and CVD, including carotid artery intima-media thickness (IMT) (227,228), oxidative stress (58,59), and endothelial dysfunction (229), independent of other coronary risk factors. Low DHEAS levels (201,230-233) were also predictive of severe coronary atherosclerosis on coronary angiography (234), but also of earlier cardiac allograft vasculopathy development in heart transplant patients (235).
These findings are suggestive of anti-atherogenic and cardioprotective effect of DHEA/DHEAS. Numerous epidemiological studies have, therefore, looked at the specific relation between plasma levels of adrenal androgens and CVD. Most have shown that low plasma levels of DHEA/DHEA-S were clearly associated with increased incidence of atherosclerotic vascular diseases (91,234,236-238) and cardiovascular morbidity (234,239-245), independently from classic cardiovascular risk factors, as well as with increased CVD-related mortality in elderly men but not in postmenopausal women, unless they had pre-existing coronary disease (16,70,199,241,246-248).
The plasma levels of DHEA were also inversely associated with the progression (249) and prognosis of heart failure (250), at least in men. The exact pathophysiologic background is still more or less unclear. Some preliminary data in patients with type 2 diabetes mellitus suggest that the adrenal androgens may increase the generation of activated protein C, an important anticoagulant protein that protects from acute coronary events (228). Furthermore, DHEA may directly stimulate eNOS phosphorylation/activation in endothelial cells and NO production (36,39,251), which in turn induces vasodilation, and preserves myocardial perfusion (252). DHEA may also exert anti-inflammatory actions (39,253), through which it may alleviate endothelial dysfunction, atherogenesis (254), and the acute thrombotic complications of atheroma (39,253,255-258) enhanced by systemic inflammation. The protective effects of DHEA on endothelium were also shown in several in vitro studies in which DHEA increased endothelial proliferation (43) and protected endothelial cells against apoptosis (59,259). Finally, DHEA can alleviate oxidative stress and inflammation in vascular smooth muscle cells (VSMCs) via ERK1/2 and NF-κB signaling pathways, although it has no effect on their phenotype transition (260).
Other studies, however, have failed to show a significant relation between DHEA/DHEA-S and CVD. In men for example, myocardial infarction occurrence was not altered by DHEA-S levels, and acute myocardial infarctions were seen in patients with either low or high DHEA-S levels (261-264). Similarly in women, lower DHEA-S levels in ischemic heart disease patients versus control were observed in some studies, but not in others (238,265,266). The reasons that account for the discrepancies among the above studies are not clear. It can be argued that smoking could be a possible confounding variable for both DHEA-S levels and CVD, as smoking increases DHEA-S levels but also increases the incidence of adverse cardiovascular events (267,268). The discrepancies among the above studies may also be attributed to population variability; for example, in the study by Mazat et al. the relative risk of an 8-year mortality associated with low DHEA-S was 3.4 times higher in males under 70 years compared to older men (odds ratio of 6.5 versus 1.9) (16). Finally, DHEA-S was checked just once in some retrospective studies, often several years before the adverse cardiovascular events (269).
Whether exogenously administered DHEA could ameliorate key elements involved in the generation and progression of the atherosclerotic process was addressed in humans with atherosclerosis and experimental animal models. The human studies have shown a beneficial effect of DHEA on angiographic evidence of atherosclerosis and improvement of vascular endothelial function (43,234,270). Several animal studies have also clearly demonstrated the inhibitory effect of orally administered DHEA on atherosclerosis and plaque progression (271,272) as well as beneficial effects on ischemia–reperfusion injury in the microcirculation (273,274) and cardiac dysfunction (56,57). Arterial stiffness, which is also considered a risk factor for CVD, significantly improved after DHEA replacement in both elderly men and women (275,276). Whether the above findings could be translated into DHEA administration in clinical practice for the reduction of CVD morbidity and/or mortality is not well documented and supported by current reports. However, since DHEA is a well-tolerated molecule and an inexpensive drug, additional large multi-centric clinical studies could address its role in the prevention and/or management of CVD.
DHEA and Cerebrovascular Disease
Stroke is the third-leading cause for disability worldwide (277); therefore, early risk stratification for an optimized allocation of health care resources is imperative. The ischemic strokes that account for the great majority of all stroke cases (87 percent) occur as a result of acute obstruction of atherosclerotic blood vessels supplying blood to the brain (278). Considering DHEAS has neuroprotective and antiatherosclerotic properties (243,279,280) and its synthesis has been documented in the brain (175,281,282), the role of DHEA/DHEAS in acute stroke incidence and outcome was investigated. Interestingly, in women from Nurses’ Health Study, lower DHEAS levels were associated with a greater risk of ischemic stroke (283). In addition, it was suggested that DHEAS levels in the blood may predict the severity and functional outcome of acute strokes (284,285). Whether the above findings suggest baseline DHEAS levels could alter stroke management in clinical practice or whether DHEA replacement has a therapeutic potential role in stroke management need to be addressed.
DHEA and Pulmonary Hypertension
The previously described vasorelaxant properties of DHEA in systemic circulation were also investigated in pulmonary hypertension in animal models and in humans. Several studies have shown that DHEA replacement could effectively prevent and reverse hypoxic pulmonary hypertension, pulmonary arterial remodeling, and right ventricular hypertrophy in rats (286-288) in a dose-dependent manner (289) and also prevent the age-related frailty induced by hypoxic pulmonary hypertension in older mice (288). The effect of DHEA is selective to the pulmonary circulation since the systemic blood pressure was not altered. It was shown that the beneficial effects of DHEA on pulmonary hypertension were at least partly independent of its conversion to estrogen/testosterone and eNOS activation. Some of the potential molecular mechanism by which DHEA promotes pulmonary artery relaxation appears to involve K+ channel activation, upregulation of soluble guanylate cyclase (287,290,291), downregulation of hypoxia inducible factor 1a (HIF-1a) (292), and by NADPH oxidation-elicited subunit dimerization of protein kinase G 1α (293).
As previously discussed, DHEA may inhibit and reverse chronic hypoxia-induced pulmonary hypertension in rats. Little is known, however, about the effects of DHEA on the pulmonary circulation in humans. The levels of DHEA/DHEA-S in patients with pulmonary hypertension over time have not been determined, but the recent Multi-Ethnic Study of Atherosclerosis (MESA) - Right Ventricle (RV) Study found that higher DHEA levels were associated with increased RV mass and stroke volume in women (294). Another prospective study suggested a strong inverse correlation between natural DHEA/DHEA-S blood levels and the ten-year mortality in old male smokers and ex-smokers (16). Prompted by the experimental findings in the pulmonary circulation, a recent study investigated whether DHEA can improve the clinical and hemodynamic status of patients with pulmonary hypertension associated to chronic obstructive pulmonary disease; eight patients with the disease were treated with DHEA (200mg daily orally) for 3 months. The results were very promising as DHEA treatment significantly improved the pulmonary hemodynamics and the physical performance of the patients, without worsening gas exchange, as do other pharmacological treatments of pulmonary hypertension (295).
Putting together the above evidence, there are experimental data to support the beneficial role of DHEA treatment in models with pulmonary hypertension, but only a few studies supporting its beneficial effect in patients with pulmonary hypertension associated with chronic obstructive pulmonary disease. Further clinical studies would probably clarify its therapeutic role in the management of pulmonary hypertension in clinical practice.
DHEA and Autoimmune Disorders
INFLAMMATORY BOWEL DISEASE (IBD)
DHEA has anti-inflammatory properties (50,51). Its levels appear to be low in people with ulcerative colitis and Crohn’s disease, irrespective of the patient’s age (296,297). A phase II small pilot trial in patients with active inflammatory bowel disease refractory to other drugs, treated with 200 mg dehydroepiandrosterone per day orally for 56 days (298) showed that DHEA may decrease the clinical activity of the disease and may even cause a remission. More studies are needed before saying for sure whether DHEA helps IBD or not.
SYSTEMIC LUPUS ERYTHEMATOSUS (SLE)
Several randomized controlled clinical studies have reported that regardless of the patient’s age, taking DHEA (50-200mg/day) along with other medications improves quality of life for people with mild to moderate SLE, decreases corticosteroid requirements, and reduces the frequency of flare-ups, though it probably does not change the overall course of their disease (84,299-303). A study had shown DHEA replacement may increase bone mass in women with lupus (302). A 2007 report in the Cochrane Database of Systematic Reviews (78) suggests a "modest but clinically significant impact" of DHEA replacement on health-related quality of life in the short-term for people with SLE; the impact on disease activity was inconsistent. Long- term outcomes and safety remain unstudied.
RHEUMATOID ARTHRITIS (RA)
DHEA levels have been found to be low in people with rheumatoid arthritis (304,305) and decrease further with glucocorticoid therapy (306). Considering the well-demonstrated immune-suppressive activities exerted by the adrenal androgens and their derivatives (307-309), the utility of DHEA as potential therapy for management of male and female RA patients was studied. Preliminary data from animal studies showed benefits of DHEA treatment in collagen-induced arthritis (310-312). However, in carefully controlled human clinical trials, DHEA treatment produced only modest benefits (313), probably with the exception of female-treated RA patients who benefit the most by DHEA replacement (314). The noted discrepancy in benefits from DHEA treatment between animals and humans may be related to the low endogenous DHEA in rodents relative to humans because of low levels of cytochrome P450 17α-hydroxylase (175), but also because of different DHEA metabolism between species; remarkably, in rodents DHEA has many highly oxygenated metabolites and a surprisingly complex metabolism that results in production of a multitude highly oxygenated species that may exert the beneficial effects on arthritis (315).
DHEA AND ADVERSE HEALTH OUTCOMES
DHEA supplements are generally well tolerated in studies using oral or percutaneous administration, with daily doses ranging from 25 mg to 1,600 mg. DHEA is an important precursor for estrogen and androgen production. In women DHEA when administered orally is mainly converted to androgen metabolites. As a result, some minimal androgenic adverse effects have been reported, including mild acne, seborrhea, facial hair growth, and ankle swelling (45,75,315).
A hormonal etiology has long been suspected for breast and endometrial cancer as several risk factors for each cancer, such as obesity, nulliparity, and early menarche are hormonally related (78,316-318). The plasma concentrations of the adrenal androgens in premenopausal women were previously associated with higher risk for development of breast cancer (319-321). Furthermore, DHEA-S levels above a cut off limit predicted disease progression in hypoestrogenized women treated for breast cancer (322). On the other hand, in vitro studies support an inhibitory effect of DHEA on the growth of human mammary cancer cells and the growth of chemically-induced mammary cancer in rats (10,62,323). It was shown that the effect of DHEA in mammary tissue depends on the level of plasma estrogens. Thus, growth inhibition occurs only in the presence of high estrogen concentrations, and growth stimulation occurs in the presence of a low-level estrogen milieu (12,324). The exact role of DHEA supplementation on breast cancer in humans has not been fully studied. A previous review of clinical, epidemiological, and experimental studies suggests late promotion of breast cancer in postmenopausal women by prolonged intake of DHEA, especially if central obesity coexists, and suggests extra caution when DHEA supplements are used by obese postmenopausal women (325). A more recent review of the medical literature investigating DHEA physiology and randomized controlled trials of the use of DHEA in postmenopausal women, however, did not find any adverse effect of DHEA supplementation (31).
Unopposed estrogen is also known to be associated with an increased risk of endometrial carcinoma (316). DHEA supplementation did not increase the endometrial thickness in postmenopausal women treated with 25 mg/day DHEA orally for 6 months (136) or 50 mg daily for 12 months (135,136,326). In addition, DHEA administered percutaneously for 12 months to postmenopausal women was shown to have an estrogenic effect on the vagina without affecting the endometrium that remained atrophic (105).
In men, DHEA supplements are mainly transformed to estrogen metabolites but also to more potent androgens. As a result, concerns regarding the effect of DHEA supplementation on prostate were raised, especially after the finding that about 15% of DHT present in the prostate comes from DHEA metabolism (327). A 2-year, placebo-controlled, randomized, double blind study involving elderly men receiving DHEA did not show any adverse effects in prostate (72).
As long as long-term safety data for DHEA supplementation are lacking, the American Cancer Society advices caution in its use in people who have cancer, especially types of cancer that respond to hormones, such as certain types of breast cancer, prostate cancer, and endometrial cancer (328). The authors of a Cochrane Systematic Review regarding the supplementation of DHEA in peri- and post-menopausal women, questioned the effectiveness of DHEA in women, and concluded that the overall quality of the studies analyzed was moderate to low and that the study outcomes were inconsistent and could not be pooled to obtain an overall effect due to the diversity of the measurement methods employed (329).
CONCLUSIONS
Theoretically, supplementing a pre-hormone is extremely interesting as it would provide peripheral tissues with levels of sex steroids according to local needs and would eliminate the exposure of other tissues to androgens or estrogens, minimizing unwanted side effects. Therefore, DHEA administration is closer to ‘‘hormonal optimization’’ than hormonal supplementation. In older people, lower than normal levels of DHEA/DHEAS were previously related to aging-associated degenerative disorders, including metabolic and cardiovascular diseases, poor physical performance, mood and memory defects, sexual dysfunction, and poor sense of wellbeing. Whether this is just a statistical finding with no practical clinical meaning has been investigated by many interventional studies most of which, however, were of short duration and had a small number of participants. Without exception, all recent reviews of the available data regarding DHEA replacement utility for the management of aging-related disorders do not support its use in clinical practice (72-78); no significant adverse or negative side effects of DHEA were reported in clinical studies, but also no significant evidence that low levels of DHEA cause the aging-related degenerative disorders or that taking DHEA can help prevent/treat them. Thus, current clinical modalities with DHEA supplements do not comply with evidence-based medicine. Since there are several known biochemical actions by which DHEA could ameliorate disorders affecting the elderly population and is a well-tolerated molecule and an inexpensive drug, additional large multi-center clinical studies would probably give us a better understanding of its clinical utility in the management of aging-related disorders. Till then, we should probably reconsider suggesting patients to start on a pro-hormone that would help them only as much as a placebo would help.
REFERENCES
- 1.
- Auchus RJ. Overview of dehydroepiandrosterone biosynthesis. Seminars in reproductive medicine. 2004;22(4):281–288. [PubMed: 15635496]
- 2.
- Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocrine reviews. 2004;25(6):947–970. [PubMed: 15583024]
- 3.
- Davis SR. Androgens and female sexuality. The journal of gender-specific medicine : JGSM : the official journal of the Partnership for Women's Health at Columbia. 2000;3(1):36-40. [PubMed: 11253235]
- 4.
- Labrie F. Extragonadal synthesis of sex steroids: intracrinology. undefined. 2003. [PubMed: 12773942]
- 5.
- Labrie F, Bélanger A, Cusan L, Candas B. Physiological changes in dehydroepiandrosterone are not reflected by serum levels of active androgens and estrogens but of their metabolites: intracrinology. The Journal of clinical endocrinology and metabolism. 1997;82(8):2403–2409. [PubMed: 9253308]
- 6.
- Intracrinology Labrie F. Molecular and cellular endocrinology. 1991;78(3) [PubMed: 1838082]
- 7.
- Labrie C, Belanger A, Labrie F. Androgenic activity of dehydroepiandrosterone and androstenedione in the rat ventral prostate. Endocrinology. 1988;123(3):1412–1417. [PubMed: 2969802]
- 8.
- Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22(4):299–309. [PubMed: 15635498]
- 9.
- Labrie F. DHEA as physiological replacement therapy at menopause. Journal of endocrinological investigation. 1998;21(6):399–401. [PubMed: 9699133]
- 10.
- Labrie F, Luu-The V, Labrie C, Belanger A, Simard J, Lin SX, Pelletier G. Endocrine and intracrine sources of androgens in women: inhibition of breast cancer and other roles of androgens and their precursor dehydroepiandrosterone. Endocr Rev. 2003;24(2):152–182. [PubMed: 12700178]
- 11.
- Poulin R, Labrie F. Stimulation of cell proliferation and estrogenic response by adrenal C19-delta 5-steroids in the ZR-75-1 human breast cancer cell line. Cancer Res. 1986;46(10):4933–4937. [PubMed: 2944574]
- 12.
- Seymour-Munn K, Adams J. Estrogenic effects of 5-androstene-3 beta, 17 beta-diol at physiological concentrations and its possible implication in the etiology of breast cancer. Endocrinology. 1983;112(2):486–491. [PubMed: 6848360]
- 13.
- Labrie F, Bélanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. The Journal of clinical endocrinology and metabolism. 1997;82(8):2396–2402. [PubMed: 9253307]
- 14.
- Labrie F, Bélanger A, Bélanger P, Bérubé R, Martel C, Cusan L, Gomez J, Candas B, Castiel I, Chaussade V, Deloche C, Leclaire J. Androgen glucuronides, instead of testosterone, as the new markers of androgenic activity in women. The Journal of steroid biochemistry and molecular biology. 2006;99(4-5):182–188. [PubMed: 16621522]
- 15.
- Baulieu EE, Thomas G, Legrain S, Lahlou N, Roger M, Debuire B, Faucounau V, Girard L, Hervy MP, Latour F, Leaud MC, Mokrane A, Pitti-Ferrandi H, Trivalle C, De Lacharrière O, Nouveau S, Rakoto-Arison B, Souberbielle JC, Raison J, Le Bouc Y, Raynaud A, Girerd X, Forette F. Dehydroepiandrosterone (DHEA), DHEA sulfate, and aging: contribution of the DHEAge Study to a sociobiomedical issue. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(8):4279–4284. [PMC free article: PMC18228] [PubMed: 10760294]
- 16.
- Mazat L, Lafont S, Berr C, Debuire B, Tessier JF, Dartigues JF, Baulieu EE. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(14):8145–8150. [PMC free article: PMC35482] [PubMed: 11427700]
- 17.
- Heaney JL, Phillips AC, Carroll D. Ageing, physical function, and the diurnal rhythms of cortisol and dehydroepiandrosterone. Psychoneuroendocrinology. 2012;37(3):341–349. [PubMed: 21802858]
- 18.
- Laughlin GA, Barrett-Connor E. Sexual Dimorphism in the Influence of Advanced Aging on Adrenal Hormone Levels: The Rancho Bernardo Study*. The Journal of Clinical Endocrinology & Metabolism Printed. 2000;85(10) [PubMed: 11061502]
- 19.
- Baulieu EE. Androgens and aging men. Molecular and Cellular Endocrinology. 2002;198(1-2):41–49. [PubMed: 12573813]
- 20.
- Parker CR. Dehydroepiandrosterone and dehydroepiandrosterone sulfate production in the human adrenal during development and aging. Steroids. 1999;64(9):640–647. [PubMed: 10503722]
- 21.
- Yen SSC, Laughlin GA. Aging and the adrenal cortex. Experimental Gerontology. 1998;33(7-8):897–910. [PubMed: 9951633]
- 22.
- Ahu RS, Lee YJ, Choi JY, Kwon HB, Chun SI. Salivary cortisol and DHEA levels in the Korean population: age-related differences, diurnal rhythm, and correlations with serum levels. Yonsei medical journal. 2007;48(3):379–388. [PMC free article: PMC2628086] [PubMed: 17594144]
- 23.
- Liu CH, Laughlin GA, Fischer UG, Yen SSC. Marked attenuation of ultradian and circadian rhythms of dehydroepiandrosterone in postmenopausal women: evidence for a reduced 17,20-desmolase enzymatic activity. The Journal of clinical endocrinology and metabolism. 1990;71(4):900–906. [PubMed: 2169480]
- 24.
- Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. The Journal of clinical endocrinology and metabolism. 1984;59(3):551–555. [PubMed: 6235241]
- 25.
- Prom-Wormley EC, York TP, Jacobson KC, Eaves LJ, Mendoza SP, Hellhammer D, Maninger N, Levine S, Lupien S, Lyons MJ, Hauger R, Xian H, Franz CE, Kremen WS. Genetic and environmental effects on diurnal dehydroepiandrosterone sulfate concentrations in middle-aged men. Psychoneuroendocrinology. 2011;36(10):1441–1452. [PMC free article: PMC3183407] [PubMed: 21570195]
- 26.
- Lasley BL, Santoro N, Randolf JF, Gold EB, Crawford S, Weiss G, McConnell DS, Sowers MF. The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. The Journal of clinical endocrinology and metabolism. 2002;87(8):3760–3767. [PubMed: 12161507]
- 27.
- Lasley BL, Crawford SL, Laughlin GA, Santoro N, McConnell DS, Crandall C, Greendale GA, Polotsky AJ, Vuga M. Circulating dehydroepiandrosterone sulfate levels in women who underwent bilateral salpingo-oophorectomy during the menopausal transition. Menopause (New York, NY). 2011;18(5):494–498. [PMC free article: PMC3123411] [PubMed: 21178790]
- 28.
- Crawford S, Santoro N, Laughlin GA, Sowers MF, McConnell D, Sutton-Tyrrell K, Weiss G, Vuga M, Randolph J, Lasley B. Circulating dehydroepiandrosterone sulfate concentrations during the menopausal transition. J Clin Endocrinol Metab. 2009;94(8):2945–2951. [PMC free article: PMC2730879] [PubMed: 19470626]
- 29.
- Randolph JF, Sowers M, Gold EB, Mohr BA, Luborsky J, Santoro N, McConnell DS, Finkelstein JS, Korenman SG, Matthews KA, Sternfeld B, Lasley BL. Reproductive hormones in the early menopausal transition: relationship to ethnicity, body size, and menopausal status. The Journal of clinical endocrinology and metabolism. 2003;88(4):1516–1522. [PubMed: 12679432]
- 30.
- Lasley BL, Crawford S, McConnell DS. Adrenal androgens and the menopausal transition. Obstetrics and gynecology clinics of North America. 2011;38(3):467–475. [PMC free article: PMC3185242] [PubMed: 21961714]
- 31.
- Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011;96(6):1642–1653. [PubMed: 21411558]
- 32.
- Pluchino N, Drakopoulos P, Bianchi-Demicheli F, Wenger JM, Petignat P, Genazzani AR. Neurobiology of DHEA and effects on sexuality, mood and cognition. J Steroid Biochem Mol Biol. 2015;145:273–280. [PubMed: 24892797]
- 33.
- Parker LN. Adrenarche. Endocrinologist. 1993;3(6):385–391.
- 34.
- Corvalan C, Uauy R, Mericq V. Obesity is positively associated with dehydroepiandrosterone sulfate concentrations at 7 y in Chilean children of normal birth weight. Am J Clin Nutr. 2013;97(2):318–325. [PMC free article: PMC3545681] [PubMed: 23283497]
- 35.
- Rabijewski M, Papierska L, Binkowska M, Maksym R, Jankowska K, Skrzypulec-Plinta W, Zgliczynski W. Supplementation of dehydroepiandrosterone (DHEA) in pre- and postmenopausal women - position statement of expert panel of Polish Menopause and Andropause Society. Ginekol Pol. 2020;91(9):554–562. [PubMed: 33030737]
- 36.
- Liu D, Dillon JS. Dehydroepiandrosterone stimulates nitric oxide release in vascular endothelial cells: Evidence for a cell surface receptor. Steroids. 2004;69(4):279–289. [PubMed: 15183694]
- 37.
- Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Galpha(i2,3). The Journal of biological chemistry. 2002;277(24):21379–21388. [PubMed: 11934890]
- 38.
- Simoncini T, Mannella P, Fornari L, Varone G, Caruso A, Genazzani AR. Dehydroepiandrosterone modulates endothelial nitric oxide synthesis via direct genomic and nongenomic mechanisms. Endocrinology. 2003;144(8):3449–3455. [PubMed: 12865324]
- 39.
- Traish AM, Kang HP, Saad F, Guay AT. Dehydroepiandrosterone (DHEA)-A precursor steroid or an active hormone in human physiology (CME). Journal of Sexual Medicine. 2011;8(11):2960–2982. [PubMed: 22032408]
- 40.
- Chevalier M, Gilbert G, Lory P, Marthan R, Quignard JF, Savineau JP. Dehydroepiandrosterone (DHEA) inhibits voltage-gated T-type calcium channels. Biochemical pharmacology. 2012;83(11):1530–1539. [PubMed: 22391268]
- 41.
- Bonnet S, Paulin R, Sutendra G, Dromparis P, Roy M, Watson KO, Nagendran J, Haromy A, Dyck JRB, Michelakis ED. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-{beta}/NFAT axis. Circulation. 2009;120(13):1231–1240. [PubMed: 19752325]
- 42.
- Williams MRI, Ling S, Dawood T, Hashimura K, Dai A, Li H, Liu JP, Funder JW, Sudhir K, Komesaroff PA. Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. The Journal of clinical endocrinology and metabolism. 2002;87(1):176–181. [PubMed: 11788644]
- 43.
- Williams MRI, Dawood T, Ling S, Dai A, Lew R, Myles K, Funder JW, Sudhir K, Komesaroff PA. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. The Journal of clinical endocrinology and metabolism. 2004;89(9):4708–4715. [PubMed: 15356084]
- 44.
- Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Molecular Endocrinology. 2005;19(8):1951–1959. [PMC free article: PMC1249516] [PubMed: 15705661]
- 45.
- Legrain S, Massien C, Lahlou N, Roger M, Debuire B, Diquet B, Chatellier G, Azizi M, Faucounau V, Porchet H, Forette F, Baulieu EE. Dehydroepiandrosterone replacement administration: Pharmacokinetic and pharmacodynamic studies in healthy elderly subjects. Journal of Clinical Endocrinology and Metabolism. 2000;85(9):3208–3217. [PubMed: 10999810]
- 46.
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, Collins JL, Kliewer SA. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. Journal of Biological Chemistry. 2000;275(20):15122–15127. [PubMed: 10748001]
- 47.
- Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocrine reviews. 2002;23(5):687–702. [PubMed: 12372848]
- 48.
- Goodwin B, Redinbo MR, Kliewer SA. Regulation of CYP3A gene transcription by the pregnane X receptor. Annual Review of Pharmacology and Toxicology. 2002;42:1–23. [PubMed: 11807162]
- 49.
- Ripp SL, Fitzpatrick JL, Peters JM, Prough RA. Induction of CYP3A expression by dehydroepiandrosterone: involvement of the pregnane X receptor. Drug metabolism and disposition: the biological fate of chemicals. 2002;30(5):570–575. [PubMed: 11950789]
- 50.
- Straub RH, Konecna L, Hrach S, Rothe G, Kreutz M, Schölmerich J, Falk W, Lang B. Serum dehydroepiandrosterone (DHEA) and DHEA sulfate are negatively correlated with serum interleukin-6 (IL-6), and DHEA inhibits IL-6 secretion from mononuclear cells in man in vitro: possible link between endocrinosenescence and immunosenescence. The Journal of clinical endocrinology and metabolism. 1998;83(6):2012–2017. [PubMed: 9626133]
- 51.
- Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor alpha activation modulates cellular redox status, represses nuclear factor-kappaB signaling, and reduces inflammatory cytokine production in aging. The Journal of biological chemistry. 1998;273(49):32833–32841. [PubMed: 9830030]
- 52.
- Wang L, Wang YD, Wang WJ, Zhu Y, Li DJ. Dehydroepiandrosterone improves murine osteoblast growth and bone tissue morphometry via mitogen-activated protein kinase signaling pathway independent of either androgen receptor or estrogen receptor. Journal of molecular endocrinology. 2007;38(4):467–479. [PubMed: 17446236]
- 53.
- Maurice T, Grégoire C, Espallergues J. Neuro(active)steroids actions at the neuromodulatory sigma1 (sigma1) receptor: biochemical and physiological evidences, consequences in neuroprotection. Pharmacology, biochemistry, and behavior. 2006;84(4):581–597. [PubMed: 16945406]
- 54.
- Cheng ZX, Lan DM, Wu PY, Zhu YH, Dong Y, Ma L, Zheng P. Neurosteroid dehydroepiandrosterone sulphate inhibits persistent sodium currents in rat medial prefrontal cortex via activation of sigma-1 receptors. Experimental neurology. 2008;210(1):128–136. [PubMed: 18035354]
- 55.
- Tsai S-Y, Hayashi T, Mori T, Su T-P. Sigma-1 receptor chaperones and diseases. Central nervous system agents in medicinal chemistry. 2009;9(3):184–189. [PMC free article: PMC3150837] [PubMed: 20021352]
- 56.
- Tagashira H, Bhuiyan S, Shioda N, Fukunaga K. Distinct cardioprotective effects of 17β-estradiol and dehydroepiandrosterone on pressure overload-induced hypertrophy in ovariectomized female rats. Menopause (New York, NY). 2011;18(12):1317–1326. [PubMed: 21844826]
- 57.
- Bhuiyan MS, Tagashira H, Fukunaga K. Dehydroepiandrosterone-mediated stimulation of sigma-1 receptor activates Akt-eNOS signaling in the thoracic aorta of ovariectomized rats with abdominal aortic banding. Cardiovascular therapeutics. 2011;29(4):219–230. [PubMed: 20553277]
- 58.
- Camporez JP, Akamine EH, Davel AP, Franci CR, Rossoni LV, Carvalho CR. Dehydroepiandrosterone protects against oxidative stress-induced endothelial dysfunction in ovariectomized rats. J Physiol. 2011;589(Pt 10):2585–2596. [PMC free article: PMC3115827] [PubMed: 21486789]
- 59.
- Nheu L, Nazareth L, Xu GY, Xiao FY, Luo RZ, Komesaroff P, Ling S. Physiological effects of androgens on human vascular endothelial and smooth muscle cells in culture. Steroids. 2011;76(14):1590–1596. [PubMed: 22019845]
- 60.
- Jacob MH, Janner Dda R, Bello-Klein A, Llesuy SF, Ribeiro MF. Dehydroepiandrosterone modulates antioxidant enzymes and Akt signaling in healthy Wistar rat hearts. J Steroid Biochem Mol Biol. 2008;112(1-3):138–144. [PubMed: 18848627]
- 61.
- Levy HR, Daouk GH. Simultaneous analysis of NAD- and NADP-linked activities of dual nucleotide-specific dehydrogenases. Application to Leuconostoc mesenteroides glucose-6-phosphate dehydrogenase. J Biol Chem. 1979;254(11):4843–4847. [PubMed: 35541]
- 62.
- Schwartz AG, Pashko LL. Dehydroepiandrosterone, glucose-6-phosphate dehydrogenase, and longevity. Ageing Res Rev. 2004;3(2):171–187. [PubMed: 15177053]
- 63.
- Moriguchi S, Shinoda Y, Yamamoto Y, Sasaki Y, Miyajima K, Tagashira H, Fukunaga K. Stimulation of the sigma-1 receptor by DHEA enhances synaptic efficacy and neurogenesis in the hippocampal dentate gyrus of olfactory bulbectomized mice. PLoS One. 2013;8(4):e60863. [PMC free article: PMC3620380] [PubMed: 23593332]
- 64.
- Yehuda R, Brand SR, Golier JA, Yang RK. Clinical correlates of DHEA associated with post-traumatic stress disorder. Acta Psychiatr Scand. 2006;114(3):187–193. [PubMed: 16889589]
- 65.
- Bhuiyan MS, Fukunaga K. Stimulation of Sigma-1 receptor by dehydroepiandrosterone ameliorates hypertension-induced kidney hypertrophy in ovariectomized rats. Experimental biology and medicine (Maywood, NJ). 2010;235(3):356–364. [PubMed: 20404054]
- 66.
- Forti P, Maltoni B, Olivelli V, Pirazzoli GL, Ravaglia G, Zoli M. Serum dehydroepiandrosterone sulfate and adverse health outcomes in older men and women. Rejuvenation Res. 2012;15(4):349–358. [PubMed: 22524205]
- 67.
- Valenti G, Denti L, Maggio M, Ceda G, Volpato S, Bandinelli S, Ceresini G, Cappola A, Guralnik JM, Ferrucci L. Effect of DHEAS on skeletal muscle over the life span: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59(5):466–472. [PubMed: 15123757]
- 68.
- Ravaglia G, Forti P, Maioli F, Boschi F, Cicognani A, Bernardi M, Pratelli L, Pizzoferrato A, Porcu S, Gasbarrini G. Determinants of functional status in healthy Italian nonagenarians and centenarians: a comprehensive functional assessment by the instruments of geriatric practice. J Am Geriatr Soc. 1997;45(10):1196–1202. [PubMed: 9329480]
- 69.
- Leng SX, Cappola AR, Andersen RE, Blackman MR, Koenig K, Blair M, Walston JD. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004;16(2):153–157. [PubMed: 15195991]
- 70.
- Ohlsson C, Labrie F, Barrett-Connor E, Karlsson MK, Ljunggren O, Vandenput L, Mellstrom D, Tivesten A. Low serum levels of dehydroepiandrosterone sulfate predict all-cause and cardiovascular mortality in elderly Swedish men. J Clin Endocrinol Metab. 2010;95(9):4406–4414. [PubMed: 20610590]
- 71.
- Kroboth PD, Salek FS, Pittenger AL, Fabian TJ, Frye RF. DHEA and DHEA-S: a review. J Clin Pharmacol. 1999;39(4):327–348. [PubMed: 10197292]
- 72.
- Nair KS, Rizza RA, O'Brien P, Dhatariya K, Short KR, Nehra A, Vittone JL, Klee GG, Basu A, Basu R, Cobelli C, Toffolo G, Dalla Man C, Tindall DJ, Melton LJ 3rd, Smith GE, Khosla S, Jensen MD. DHEA in elderly women and DHEA or testosterone in elderly men. N Engl J Med. 2006;355(16):1647–1659. [PubMed: 17050889]
- 73.
- Baker WL, Karan S, Kenny AM. Effect of dehydroepiandrosterone on muscle strength and physical function in older adults: a systematic review. J Am Geriatr Soc. 2011;59(6):997–1002. [PubMed: 21649617]
- 74.
- Kritz-Silverstein D, von Muhlen D, Laughlin GA, Bettencourt R. Effects of dehydroepiandrosterone supplementation on cognitive function and quality of life: the DHEA and Well-Ness (DAWN) Trial. J Am Geriatr Soc. 2008;56(7):1292–1298. [PMC free article: PMC2574781] [PubMed: 18482290]
- 75.
- Panjari M, Bell RJ, Jane F, Wolfe R, Adams J, Morrow C, Davis SR. A randomized trial of oral DHEA treatment for sexual function, well-being, and menopausal symptoms in postmenopausal women with low libido. J Sex Med. 2009;6(9):2579–2590. [PubMed: 19619146]
- 76.
- Alkatib AA, Cosma M, Elamin MB, Erickson D, Swiglo BA, Erwin PJ, Montori VM. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA treatment effects on quality of life in women with adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(10):3676–3681. [PubMed: 19773400]
- 77.
- Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dube R, Cote I, Labrie C, Lavoie L, Berger L, Gilbert L, Martel C, Balser J. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause. 2009;16(5):923–931. [PubMed: 19424093]
- 78.
- van den Brandt PA, Spiegelman D, Yaun SS, Adami HO, Beeson L, Folsom AR, Fraser G, Goldbohm RA, Graham S, Kushi L, Marshall JR, Miller AB, Rohan T, Smith-Warner SA, Speizer FE, Willett WC, Wolk A, Hunter DJ. Pooled analysis of prospective cohort studies on height, weight, and breast cancer risk. Am J Epidemiol. 2000;152(6):514–527. [PubMed: 10997541]
- 79.
- Arlt W. The approach to the adult with newly diagnosed adrenal insufficiency. J Clin Endocrinol Metab. 2009;94(4):1059–1067. [PubMed: 19349469]
- 80.
- Panjari M, Davis SR. DHEA for postmenopausal women: a review of the evidence. Maturitas. 2010;66(2):172–179. [PubMed: 20089375]
- 81.
- Labrie F, Belanger A, Belanger P, Berube R, Martel C, Cusan L, Gomez J, Candas B, Chaussade V, Castiel I, Deloche C, Leclaire J. Metabolism of DHEA in postmenopausal women following percutaneous administration. J Steroid Biochem Mol Biol. 2007;103(2):178–188. [PubMed: 17084625]
- 82.
- Labrie F, Cusan L, Gomez JL, Martel C, Berube R, Belanger P, Chaussade V, Deloche C, Leclaire J. Changes in serum DHEA and eleven of its metabolites during 12-month percutaneous administration of DHEA. J Steroid Biochem Mol Biol. 2008;110(1-2):1–9. [PubMed: 18359622]
- 83.
- Arlt W, Callies F, Koehler I, van Vlijmen JC, Fassnacht M, Strasburger CJ, Seibel MJ, Huebler D, Ernst M, Oettel M, Reincke M, Schulte HM, Allolio B. Dehydroepiandrosterone supplementation in healthy men with an age-related decline of dehydroepiandrosterone secretion. J Clin Endocrinol Metab. 2001;86(10):4686–4692. [PubMed: 11600526]
- 84.
- Arlt W, Callies F, van Vlijmen JC, Koehler I, Reincke M, Bidlingmaier M, Huebler D, Oettel M, Ernst M, Schulte HM, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency. N Engl J Med. 1999;341(14):1013–1020. [PubMed: 10502590]
- 85.
- Lovas K, Gebre-Medhin G, Trovik TS, Fougner KJ, Uhlving S, Nedrebo BG, Myking OL, Kampe O, Husebye ES. Replacement of dehydroepiandrosterone in adrenal failure: no benefit for subjective health status and sexuality in a 9-month, randomized, parallel group clinical trial. J Clin Endocrinol Metab. 2003;88(3):1112–1118. [PubMed: 12629093]
- 86.
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, Husebye ES, Merke DP, Murad MH, Stratakis CA, Torpy DJ. Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. [PMC free article: PMC4880116] [PubMed: 26760044]
- 87.
- Guideline for the prevention of falls in older persons. American Geriatrics Society, British Geriatrics Society, and American Academy of Orthopaedic Surgeons Panel on Falls Prevention. J Am Geriatr Soc. 2001;49(5):664–672. [PubMed: 11380764]
- 88.
- Kostka T, Arsac LM, Patricot MC, Berthouze SE, Lacour JR, Bonnefoy M. Leg extensor power and dehydroepiandrosterone sulfate, insulin-like growth factor-I and testosterone in healthy active elderly people. Eur J Appl Physiol. 2000;82(1-2):83–90. [PubMed: 10879447]
- 89.
- Berkman LF, Seeman TE, Albert M, Blazer D, Kahn R, Mohs R, Finch C, Schneider E, Cotman C, McClearn G, et al. High, usual and impaired functioning in community-dwelling older men and women: findings from the MacArthur Foundation Research Network on Successful Aging. J Clin Epidemiol. 1993;46(10):1129–1140. [PubMed: 8410098]
- 90.
- Morrison MF, Katz IR, Parmelee P, Boyce AA, TenHave T. Dehydroepiandrosterone sulfate (DHEA-S) and psychiatric and laboratory measures of frailty in a residential care population. Am J Geriatr Psychiatry. 1998;6(4):277–284. [PubMed: 9793575]
- 91.
- Abbasi A, Duthie EH Jr, Sheldahl L, Wilson C, Sasse E, Rudman I, Mattson DE. Association of dehydroepiandrosterone sulfate, body composition, and physical fitness in independent community-dwelling older men and women. J Am Geriatr Soc. 1998;46(3):263–273. [PubMed: 9514370]
- 92.
- Clarke BL, Ebeling PR, Jones JD, Wahner HW, O'Fallon WM, Riggs BL, Fitzpatrick LA. Predictors of bone mineral density in aging healthy men varies by skeletal site. Calcif Tissue Int. 2002;70(3):137–145. [PubMed: 11907709]
- 93.
- Bischoff-Ferrari HA, Orav EJ, Dawson-Hughes B. Additive benefit of higher testosterone levels and vitamin D plus calcium supplementation in regard to fall risk reduction among older men and women. Osteoporos Int. 2008;19(9):1307–1314. [PMC free article: PMC2680613] [PubMed: 18351428]
- 94.
- Voznesensky M, Walsh S, Dauser D, Brindisi J, Kenny AM. The association between dehydroepiandosterone and frailty in older men and women. Age Ageing. 2009;38(4):401–406. [PMC free article: PMC2720687] [PubMed: 19276095]
- 95.
- Hasselhorn HM, Theorell T, Vingard E. Musculoskeletal Intervention Center -Norrtalje Study G. Endocrine and immunologic parameters indicative of 6-month prognosis after the onset of low back pain or neck/shoulder pain. Spine (Phila Pa 1976). 2001;26(3):E24–29. [PubMed: 11224875]
- 96.
- Schell E, Theorell T, Hasson D, Arnetz B, Saraste H. Stress biomarkers' associations to pain in the neck, shoulder and back in healthy media workers: 12-month prospective follow-up. Eur Spine J. 2008;17(3):393–405. [PMC free article: PMC2270377] [PubMed: 18075764]
- 97.
- Morales AJ, Haubrich RH, Hwang JY, Asakura H, Yen SS. The effect of six months treatment with a 100 mg daily dose of dehydroepiandrosterone (DHEA) on circulating sex steroids, body composition and muscle strength in age-advanced men and women. Clin Endocrinol (Oxf). 1998;49(4):421–432. [PubMed: 9876338]
- 98.
- Villareal DT, Holloszy JO, Kohrt WM. Effects of DHEA replacement on bone mineral density and body composition in elderly women and men. Clin Endocrinol (Oxf). 2000;53(5):561–568. [PubMed: 11106916]
- 99.
- Kaiman DS, Colker CM, Swain MA, Torina GC, Shi Q. A randomized, double-blind, placebo-controlled study of 3-acetyl-7-oxo-dehydroepiandrosterone in healthy overweight adults. Current Therapeutic Research. 2000;61(7):435–442.
- 100.
- Kenny AM, Boxer RS, Kleppinger A, Brindisi J, Feinn R, Burleson JA. Dehydroepiandrosterone combined with exercise improves muscle strength and physical function in frail older women. J Am Geriatr Soc. 2010;58(9):1707–1714. [PubMed: 20863330]
- 101.
- Weiss EP, Shah K, Fontana L, Lambert CP, Holloszy JO, Villareal DT. Dehydroepiandrosterone replacement therapy in older adults: 1- and 2-y effects on bone. Am J Clin Nutr. 2009;89(5):1459–1467. [PMC free article: PMC2677000] [PubMed: 19321570]
- 102.
- von Muhlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R. Effect of dehydroepiandrosterone supplementation on bone mineral density, bone markers, and body composition in older adults: the DAWN trial. Osteoporos Int. 2008;19(5):699–707. [PMC free article: PMC2435090] [PubMed: 18084691]
- 103.
- Jankowski CM, Gozansky WS, Kittelson JM, Van Pelt RE, Schwartz RS, Kohrt WM. Increases in bone mineral density in response to oral dehydroepiandrosterone replacement in older adults appear to be mediated by serum estrogens. J Clin Endocrinol Metab. 2008;93(12):4767–4773. [PMC free article: PMC2626446] [PubMed: 18812486]
- 104.
- Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, Van Pelt RE, Kohrt WM. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab. 2006;91(8):2986–2993. [PubMed: 16735495]
- 105.
- Labrie F, Diamond P, Cusan L, Gomez JL, Belanger A, Candas B. Effect of 12-month dehydroepiandrosterone replacement therapy on bone, vagina, and endometrium in postmenopausal women. J Clin Endocrinol Metab. 1997;82(10):3498–3505. [PubMed: 9329392]
- 106.
- Morales AJ, Nolan JJ, Nelson JC, Yen SS. Effects of replacement dose of dehydroepiandrosterone in men and women of advancing age. J Clin Endocrinol Metab. 1994;78(6):1360–1367. [PubMed: 7515387]
- 107.
- Yen SS, Morales AJ, Khorram O. Replacement of DHEA in aging men and women. Potential remedial effects. Ann N Y Acad Sci. 1995;774:128–142. [PubMed: 8597453]
- 108.
- Nawata H, Tanaka S, Tanaka S, Takayanagi R, Sakai Y, Yanase T, Ikuyama S, Haji M. Aromatase in bone cell: association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol. 1995;53(1-6):165–174. [PubMed: 7626449]
- 109.
- Jedrzejuk D, Medras M, Milewicz A, Demissie M. Dehydroepiandrosterone replacement in healthy men with age-related decline of DHEA-S: effects on fat distribution, insulin sensitivity and lipid metabolism. Aging Male. 2003;6(3):151–156. [PubMed: 14628495]
- 110.
- Callies F, Fassnacht M, van Vlijmen JC, Koehler I, Huebler D, Seibel MJ, Arlt W, Allolio B. Dehydroepiandrosterone replacement in women with adrenal insufficiency: effects on body composition, serum leptin, bone turnover, and exercise capacity. J Clin Endocrinol Metab. 2001;86(5):1968–1972. [PubMed: 11344193]
- 111.
- Flynn MA, Weaver-Osterholtz D, Sharpe-Timms KL, Allen S, Krause G. Dehydroepiandrosterone replacement in aging humans. J Clin Endocrinol Metab. 1999;84(5):1527–1533. [PubMed: 10323374]
- 112.
- Casson PR, Santoro N, Elkind-Hirsch K, Carson SA, Hornsby PJ, Abraham G, Buster JE. Postmenopausal dehydroepiandrosterone administration increases free insulin-like growth factor-I and decreases high-density lipoprotein: a six-month trial. Fertil Steril. 1998;70(1):107–110. [PubMed: 9660430]
- 113.
- Igwebuike A, Irving BA, Bigelow ML, Short KR, McConnell JP, Nair KS. Lack of dehydroepiandrosterone effect on a combined endurance and resistance exercise program in postmenopausal women. J Clin Endocrinol Metab. 2008;93(2):534–538. [PMC free article: PMC2729150] [PubMed: 18029465]
- 114.
- Muller M, van den Beld AW, van der Schouw YT, Grobbee DE, Lamberts SW. Effects of dehydroepiandrosterone and atamestane supplementation on frailty in elderly men. J Clin Endocrinol Metab. 2006;91(10):3988–3991. [PubMed: 16804050]
- 115.
- Villareal DT, Holloszy JO. DHEA enhances effects of weight training on muscle mass and strength in elderly women and men. Am J Physiol Endocrinol Metab. 2006;291(5):E1003–1008. [PubMed: 16787962]
- 116.
- Percheron G, Hogrel JY, Denot-Ledunois S, Fayet G, Forette F, Baulieu EE, Fardeau M, Marini JF. Double-blind placebo-controlled t. Effect of 1-year oral administration of dehydroepiandrosterone to 60- to 80-year-old individuals on muscle function and cross-sectional area: a double-blind placebo-controlled trial. Arch Intern Med. 2003;163(6):720–727. [PubMed: 12639206]
- 117.
- Nijland E, Davis S, Laan E, Schultz WW. Female sexual satisfaction and pharmaceutical intervention: a critical review of the drug intervention studies in female sexual dysfunction. J Sex Med. 2006;3(5):763–777. [PubMed: 16942521]
- 118.
- Hayes RD, Dennerstein L, Bennett CM, Koochaki PE, Leiblum SR, Graziottin A. Relationship between hypoactive sexual desire disorder and aging. Fertil Steril. 2007;87(1):107–112. [PubMed: 17081522]
- 119.
- Shifren JL, Braunstein GD, Simon JA, Casson PR, Buster JE, Redmond GP, Burki RE, Ginsburg ES, Rosen RC, Leiblum SR, Caramelli KE, Mazer NA. Transdermal testosterone treatment in women with impaired sexual function after oophorectomy. N Engl J Med. 2000;343(10):682–688. [PubMed: 10974131]
- 120.
- Genazzani AD, Lanzoni C, Genazzani AR. Might DHEA be considered a beneficial replacement therapy in the elderly? Drugs Aging. 2007;24(3):173–185. [PubMed: 17362047]
- 121.
- Nappi RE, Polatti F. The use of estrogen therapy in women's sexual functioning (CME). J Sex Med. 2009;6(3):603–616. [PubMed: 19284468]
- 122.
- Bachmann GA, Leiblum SR. The impact of hormones on menopausal sexuality: a literature review. Menopause. 2004;11(1):120–130. [PubMed: 14716193]
- 123.
- Alexander JL, Kotz K, Dennerstein L, Kutner SJ, Wallen K, Notelovitz M. The effects of postmenopausal hormone therapies on female sexual functioning: a review of double-blind, randomized controlled trials. Menopause. 2004;11(6 Pt 2):749–765. [PubMed: 15543027]
- 124.
- Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188(1):286–293. [PubMed: 12548231]
- 125.
- Goldstat R, Briganti E, Tran J, Wolfe R, Davis SR. Transdermal testosterone therapy improves well-being, mood, and sexual function in premenopausal women. Menopause. 2003;10(5):390–398. [PubMed: 14501599]
- 126.
- Braunstein GD, Sundwall DA, Katz M, Shifren JL, Buster JE, Simon JA, Bachman G, Aguirre OA, Lucas JD, Rodenberg C, Buch A, Watts NB. Safety and efficacy of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Arch Intern Med. 2005;165(14):1582–1589. [PubMed: 16043675]
- 127.
- Davis SR, van der Mooren MJ, van Lunsen RH, Lopes P, Ribot C, Rees M, Moufarege A, Rodenberg C, Buch A, Purdie DW. Efficacy and safety of a testosterone patch for the treatment of hypoactive sexual desire disorder in surgically menopausal women: a randomized, placebo-controlled trial. Menopause. 2006;13(3):387–396. [PubMed: 16735935]
- 128.
- Buster JE, Kingsberg SA, Aguirre O, Brown C, Breaux JG, Buch A, Rodenberg CA, Wekselman K, Casson P. Testosterone patch for low sexual desire in surgically menopausal women: a randomized trial. Obstet Gynecol. 2005;105(5 Pt 1):944–952. [PubMed: 15863529]
- 129.
- Davis S, Papalia MA, Norman RJ, O'Neill S, Redelman M, Williamson M, Stuckey BG, Wlodarczyk J, Gard'ner K, Humberstone A. Safety and efficacy of a testosterone metered-dose transdermal spray for treating decreased sexual satisfaction in premenopausal women: a randomized trial. Ann Intern Med. 2008;148(8):569–577. [PubMed: 18413618]
- 130.
- Davis SR, Davison SL, Donath S, Bell RJ. Circulating androgen levels and self-reported sexual function in women. JAMA. 2005;294(1):91–96. [PubMed: 15998895]
- 131.
- Panjari M, Davis SR. DHEA therapy for women: effect on sexual function and wellbeing. Hum Reprod Update. 2007;13(3):239–248. [PubMed: 17208951]
- 132.
- Schmidt PJ, Daly RC, Bloch M, Smith MJ, Danaceau MA, St Clair LS, Murphy JH, Haq N, Rubinow DR. Dehydroepiandrosterone monotherapy in midlife-onset major and minor depression. Arch Gen Psychiatry. 2005;62(2):154–162. [PubMed: 15699292]
- 133.
- Bloch M, Schmidt PJ, Danaceau MA, Adams LF, Rubinow DR. Dehydroepiandrosterone treatment of midlife dysthymia. Biol Psychiatry. 1999;45(12):1533–1541. [PubMed: 10376113]
- 134.
- Hackbert L, Heiman JR. Acute dehydroepiandrosterone (DHEA) effects on sexual arousal in postmenopausal women. J Womens Health Gend Based Med. 2002;11(2):155–162. [PubMed: 11975863]
- 135.
- Genazzani AR, Stomati M, Valentino V, Pluchino N, Pot E, Casarosa E, Merlini S, Giannini A, Luisi M. Effect of 1-year, low-dose DHEA therapy on climacteric symptoms and female sexuality. Climacteric. 2011;14(6):661–668. [PubMed: 21942655]
- 136.
- Stomati M, Monteleone P, Casarosa E, Quirici B, Puccetti S, Bernardi F, Genazzani AD, Rovati L, Luisi M, Genazzani AR. Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause. Gynecol Endocrinol. 2000;14(5):342–363. [PubMed: 11109974]
- 137.
- Mortola JF, Yen SS. The effects of oral dehydroepiandrosterone on endocrine-metabolic parameters in postmenopausal women. J Clin Endocrinol Metab. 1990;71(3):696–704. [PubMed: 2144295]
- 138.
- Wolf OT, Neumann O, Hellhammer DH, Geiben AC, Strasburger CJ, Dressendorfer RA, Pirke KM, Kirschbaum C. Effects of a two-week physiological dehydroepiandrosterone substitution on cognitive performance and well-being in healthy elderly women and men. J Clin Endocrinol Metab. 1997;82(7):2363–2367. [PubMed: 9215320]
- 139.
- Johannsson G, Burman P, Wiren L, Engstrom BE, Nilsson AG, Ottosson M, Jonsson B, Bengtsson BA, Karlsson FA. Low dose dehydroepiandrosterone affects behavior in hypopituitary androgen-deficient women: a placebo-controlled trial. J Clin Endocrinol Metab. 2002;87(5):2046–2052. [PubMed: 11994339]
- 140.
- van Thiel SW, Romijn JA, Pereira AM, Biermasz NR, Roelfsema F, van Hemert A, Ballieux B, Smit JW. Effects of dehydroepiandrostenedione, superimposed on growth hormone substitution, on quality of life and insulin-like growth factor I in patients with secondary adrenal insufficiency: a randomized, placebo-controlled, cross-over trial. J Clin Endocrinol Metab. 2005;90(6):3295–3303. [PubMed: 15797966]
- 141.
- Avis NE, Brockwell S, Randolph JF Jr, Shen S, Cain VS, Ory M, Greendale GA. Longitudinal changes in sexual functioning as women transition through menopause: results from the Study of Women's Health Across the Nation. Menopause. 2009;16(3):442–452. [PMC free article: PMC2908487] [PubMed: 19212271]
- 142.
- Labrie F, Cusan L, Gomez JL, Cote I, Berube R, Belanger P, Martel C, Labrie C. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol. 2008;111(3-5):178–194. [PubMed: 18598765]
- 143.
- Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dube R, Cote I, Labrie C, Lavoie L, Berger L, Martel C, Balser J. High internal consistency and efficacy of intravaginal DHEA for vaginal atrophy. Gynecol Endocrinol. 2010;26(7):524–532. [PubMed: 20459349]
- 144.
- Ibe C, Simon JA. Vulvovaginal atrophy: current and future therapies (CME). J Sex Med. 2010;7(3):1042–1050. [PubMed: 20500443]
- 145.
- Reiter WJ, Pycha A, Schatzl G, Pokorny A, Gruber DM, Huber JC, Marberger M. Dehydroepiandrosterone in the treatment of erectile dysfunction: a prospective, double-blind, randomized, placebo-controlled study. Urology. 1999;53(3):590–594. [PubMed: 10096389]
- 146.
- Steffens DC. A multiplicity of approaches to characterize geriatric depression and its outcomes. Curr Opin Psychiatry. 2009;22(6):522–526. [PMC free article: PMC2833219] [PubMed: 19625967]
- 147.
- Robichaud M, Debonnel G. Modulation of the firing activity of female dorsal raphe nucleus serotonergic neurons by neuroactive steroids. J Endocrinol. 2004;182(1):11–21. [PubMed: 15225127]
- 148.
- Kilic FS, Kulluk D, Musmul A. Effects of dehydroepiandrosterone in amphetamine-induced schizophrenia models in mice. Neurosciences (Riyadh). 2014;19(2):100–105. [PubMed: 24739405]
- 149.
- Goodyer IM, Herbert J, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996;26(2):245–256. [PubMed: 8685281]
- 150.
- Barrett-Connor E, von Muhlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999;47(6):685–691. [PubMed: 10366167]
- 151.
- Michael A, Jenaway A, Paykel ES, Herbert J. Altered salivary dehydroepiandrosterone levels in major depression in adults. Biol Psychiatry. 2000;48(10):989–995. [PubMed: 11082473]
- 152.
- Morrison MF, Freeman EW, Lin H, Sammel MD. Higher DHEA-S (dehydroepiandrosterone sulfate) levels are associated with depressive symptoms during the menopausal transition: results from the PENN Ovarian Aging Study. Arch Womens Ment Health. 2011;14(5):375–382. [PMC free article: PMC3690802] [PubMed: 21773816]
- 153.
- Takebayashi M, Kagaya A, Uchitomi Y, Kugaya A, Muraoka M, Yokota N, Horiguchi J, Yamawaki S. Plasma dehydroepiandrosterone sulfate in unipolar major depression. Short communication. J Neural Transm (Vienna). 1998;105(4-5):537–542. [PubMed: 9720981]
- 154.
- Assies J, Visser I, Nicolson NA, Eggelte TA, Wekking EM, Huyser J, Lieverse R, Schene AH. Elevated salivary dehydroepiandrosterone-sulfate but normal cortisol levels in medicated depressed patients: preliminary findings. Psychiatry Res. 2004;128(2):117–122. [PubMed: 15488954]
- 155.
- Fabian TJ, Dew MA, Pollock BG, Reynolds CF 3rd, Mulsant BH, Butters MA, Zmuda MD, Linares AM, Trottini M, Kroboth PD. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biol Psychiatry. 2001;50(10):767–774. [PubMed: 11720695]
- 156.
- Wolkowitz OM, Reus VI, Roberts E, Manfredi F, Chan T, Raum WJ, Ormiston S, Johnson R, Canick J, Brizendine L, Weingartner H. Dehydroepiandrosterone (DHEA) treatment of depression. Biol Psychiatry. 1997;41(3):311–318. [PubMed: 9024954]
- 157.
- Wolkowitz OM, Reus VI, Keebler A, Nelson N, Friedland M, Brizendine L, Roberts E. Double-blind treatment of major depression with dehydroepiandrosterone. Am J Psychiatry. 1999;156(4):646–649. [PubMed: 10200751]
- 158.
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60(2):133–141. [PubMed: 12578430]
- 159.
- Hough CM, Lindqvist D, Epel ES, Denis MS, Reus VI, Bersani FS, Rosser R, Mahan L, Burke HM, Wolkowitz OM, Mellon SH. Higher serum DHEA concentrations before and after SSRI treatment are associated with remission of major depression. Psychoneuroendocrinology. 2017;77:122–130. [PMC free article: PMC5336487] [PubMed: 28038403]
- 160.
- Kaplan JR, Manuck SB, Clarkson TB, Lusso FM, Taub DM. Social status, environment, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1982;2(5):359–368. [PubMed: 6889852]
- 161.
- Kivimaki M, Nyberg ST, Batty GD, et al. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380(9852):1491–1497. [PMC free article: PMC3486012] [PubMed: 22981903]
- 162.
- Marmot M. Psychosocial factors and cardiovascular disease: epidemiological approaches. Eur Heart J. 1988;9(6):690–697. [PubMed: 3409903]
- 163.
- Epel ES, Blackburn EH, Lin J, Dhabhar FS, Adler NE, Morrow JD, Cawthon RM. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci U S A. 2004;101(49):17312–17315. [PMC free article: PMC534658] [PubMed: 15574496]
- 164.
- Wolkowitz OM, Epel ES, Reus VI, Mellon SH. Depression gets old fast: do stress and depression accelerate cell aging? Depress Anxiety. 2010;27(4):327–338. [PubMed: 20376837]
- 165.
- Jeckel CM, Lopes RP, Berleze MC, Luz C, Feix L, Argimon II, Stein LM, Bauer ME. Neuroendocrine and immunological correlates of chronic stress in 'strictly healthy' populations. Neuroimmunomodulation. 2010;17(1):9–18. [PubMed: 19816052]
- 166.
- Izawa S, Saito K, Shirotsuki K, Sugaya N, Nomura S. Effects of prolonged stress on salivary cortisol and dehydroepiandrosterone: a study of a two-week teaching practice. Psychoneuroendocrinology. 2012;37(6):852–858. [PubMed: 22047956]
- 167.
- Brzoza Z, Kasperska-Zajac A, Badura-Brzoza K, Matysiakiewicz J, Hese RT, Rogala B. Decline in dehydroepiandrosterone sulfate observed in chronic urticaria is associated with psychological distress. Psychosom Med. 2008;70(6):723–728. [PubMed: 18606731]
- 168.
- Lac G, Dutheil F, Brousse G, Triboulet-Kelly C, Chamoux A. Saliva DHEAS changes in patients suffering from psychopathological disorders arising from bullying at work. Brain Cogn. 2012;80(2):277–281. [PubMed: 22940752]
- 169.
- Du CL, Lin MC, Lu L, Tai JJ. Correlation of Occupational Stress Index with 24-hour Urine Cortisol and Serum DHEA Sulfate among City Bus Drivers: A Cross-sectional Study. Saf Health Work. 2011;2(2):169–175. [PMC free article: PMC3431900] [PubMed: 22953199]
- 170.
- Kim MS, Lee YJ, Ahn RS. Day-to-day differences in cortisol levels and molar cortisol-to-DHEA ratios among working individuals. Yonsei Med J. 2010;51(2):212–218. [PMC free article: PMC2824866] [PubMed: 20191012]
- 171.
- Lennartsson AK, Theorell T, Rockwood AL, Kushnir MM, Jonsdottir IH. Perceived stress at work is associated with lower levels of DHEA-S. PLoS One. 2013;8(8):e72460. [PMC free article: PMC3756071] [PubMed: 24015247]
- 172.
- Jorm AF, Jolley D. The incidence of dementia: a meta-analysis. Neurology. 1998;51(3):728–733. [PubMed: 9748017]
- 173.
- Lu SF, Mo Q, Hu S, Garippa C, Simon NG. Dehydroepiandrosterone upregulates neural androgen receptor level and transcriptional activity. J Neurobiol. 2003;57(2):163–171. [PubMed: 14556282]
- 174.
- Suzuki M, Wright LS, Marwah P, Lardy HA, Svendsen CN. Mitotic and neurogenic effects of dehydroepiandrosterone (DHEA) on human neural stem cell cultures derived from the fetal cortex. Proc Natl Acad Sci U S A. 2004;101(9):3202–3207. [PMC free article: PMC365767] [PubMed: 14973190]
- 175.
- Lang PO, Mitchell WA, Lapenna A, Pitts D, Aspinall R. Immunological pathogenesis of main age-related diseases and frailty: Role of immunosenescence. European Geriatric Medicine. 2010;1(2):112–121.
- 176.
- Davis SR, Shah SM, McKenzie DP, Kulkarni J, Davison SL, Bell RJ. Dehydroepiandrosterone sulfate levels are associated with more favorable cognitive function in women. J Clin Endocrinol Metab. 2008;93(3):801–808. [PubMed: 18073302]
- 177.
- Hildreth KL, Gozansky WS, Jankowski CM, Grigsby J, Wolfe P, Kohrt WM. Association of serum dehydroepiandrosterone sulfate and cognition in older adults: sex steroid, inflammatory, and metabolic mechanisms. Neuropsychology. 2013;27(3):356–363. [PMC free article: PMC4316210] [PubMed: 23688217]
- 178.
- Valenti G, Ferrucci L, Lauretani F, Ceresini G, Bandinelli S, Luci M, Ceda G, Maggio M, Schwartz RS. Dehydroepiandrosterone sulfate and cognitive function in the elderly: The InCHIANTI Study. J Endocrinol Invest. 2009;32(9):766–772. [PMC free article: PMC6106776] [PubMed: 19620821]
- 179.
- Weill-Engerer S, David JP, Sazdovitch V, Liere P, Eychenne B, Pianos A, Schumacher M, Delacourte A, Baulieu EE, Akwa Y. Neurosteroid quantification in human brain regions: comparison between Alzheimer's and nondemented patients. J Clin Endocrinol Metab. 2002;87(11):5138–5143. [PubMed: 12414884]
- 180.
- Belanger N, Gregoire L, Bedard PJ, Di Paolo T. DHEA improves symptomatic treatment of moderately and severely impaired MPTP monkeys. Neurobiol Aging. 2006;27(11):1684–1693. [PubMed: 16253392]
- 181.
- van Niekerk JK, Huppert FA, Herbert J. Salivary cortisol and DHEA: association with measures of cognition and well-being in normal older men, and effects of three months of DHEA supplementation. Psychoneuroendocrinology. 2001;26(6):591–612. [PubMed: 11403980]
- 182.
- Bielohuby M, Roemmler J, Manolopoulou J, Johnsen I, Sawitzky M, Schopohl J, Reincke M, Wolf E, Hoeflich A, Bidlingmaier M. Chronic growth hormone excess is associated with increased aldosterone: a study in patients with acromegaly and in growth hormone transgenic mice. Experimental biology and medicine (Maywood, NJ). 2009;234(8):1002–1009. [PubMed: 19491373]
- 183.
- Wolf OT, Kudielka BM, Hellhammer DH, Hellhammer J, Kirschbaum C. Opposing effects of DHEA replacement in elderly subjects on declarative memory and attention after exposure to a laboratory stressor. Psychoneuroendocrinology. 1998;23(6):617–629. [PubMed: 9802132]
- 184.
- Wolf OT, Kirschbaum C. Actions of dehydroepiandrosterone and its sulfate in the central nervous system: effects on cognition and emotion in animals and humans. Brain Res Brain Res Rev. 1999;30(3):264–288. [PubMed: 10567728]
- 185.
- Grimley Evans J, Malouf R, Huppert F, van Niekerk JK. Dehydroepiandrosterone (DHEA) supplementation for cognitive function in healthy elderly people. Cochrane Database Syst Rev. 2006;2006(4):CD006221. [PMC free article: PMC8988513] [PubMed: 17054283]
- 186.
- Wolkowitz OM, Kramer JH, Reus VI, Costa MM, Yaffe K, Walton P, Raskind M, Peskind E, Newhouse P, Sack D, De Souza E, Sadowsky C, Roberts E. Research DH-AsDC. DHEA treatment of Alzheimer's disease: a randomized, double-blind, placebo-controlled study. Neurology. 2003;60(7):1071–1076. [PubMed: 12682308]
- 187.
- Yamada S, Akishita M, Fukai S, Ogawa S, Yamaguchi K, Matsuyama J, Kozaki K, Toba K, Ouchi Y. Effects of dehydroepiandrosterone supplementation on cognitive function and activities of daily living in older women with mild to moderate cognitive impairment. Geriatr Gerontol Int. 2010;10(4):280–287. [PubMed: 20497239]
- 188.
- Parsons TD, Kratz KM, Thompson E, Stanczyk FZ, Buckwalter JG. Dhea supplementation and cognition in postmenopausal women. Int J Neurosci. 2006;116(2):141–155. [PubMed: 16393880]
- 189.
- Morrison MF, Redei E, TenHave T, Parmelee P, Boyce AA, Sinha PS, Katz IR. Dehydroepiandrosterone sulfate and psychiatric measures in a frail, elderly residential care population. Biol Psychiatry. 2000;47(2):144–150. [PubMed: 10664831]
- 190.
- Somboonporn W, Davis S, Seif MW, Bell R. Testosterone for peri- and postmenopausal women. Cochrane Database Syst Rev. 2005;(4):CD004509. [PubMed: 16235365]
- 191.
- Darling GM, Johns JA, McCloud PI, Davis SR. Estrogen and progestin compared with simvastatin for hypercholesterolemia in postmenopausal women. N Engl J Med. 1997;337(9):595–601. [PubMed: 9271481]
- 192.
- Barnhart KT, Freeman E, Grisso JA, Rader DJ, Sammel M, Kapoor S, Nestler JE. The effect of dehydroepiandrosterone supplementation to symptomatic perimenopausal women on serum endocrine profiles, lipid parameters, and health-related quality of life. J Clin Endocrinol Metab. 1999;84(11):3896–3902. [PubMed: 10566625]
- 193.
- Davis SR, Goldstat R, Newman A, Berry K, Burger HG, Meredith I, Koch K. Differing effects of low-dose estrogen-progestin therapy and pravastatin in postmenopausal hypercholesterolemic women. Climacteric. 2002;5(4):341–350. [PubMed: 12626213]
- 194.
- Floter A, Nathorst-Boos J, Carlstrom K, von Schoultz B. Serum lipids in oophorectomized women during estrogen and testosterone replacement therapy. Maturitas. 2004;47(2):123–129. [PubMed: 14757271]
- 195.
- Leao LM, Duarte MP, Silva DM, Bahia PR, Coeli CM, de Farias ML. Influence of methyltestosterone postmenopausal therapy on plasma lipids, inflammatory factors, glucose metabolism and visceral fat: a randomized study. Eur J Endocrinol. 2006;154(1):131–139. [PubMed: 16382002]
- 196.
- Srinivasan M, Irving BA, Dhatariya K, Klaus KA, Hartman SJ, McConnell JP, Nair KS. Effect of dehydroepiandrosterone replacement on lipoprotein profile in hypoadrenal women. J Clin Endocrinol Metab. 2009;94(3):761–764. [PMC free article: PMC2681274] [PubMed: 19066301]
- 197.
- Srinivasan M, Irving BA, Frye RL, O'Brien P, Hartman SJ, McConnell JP, Nair KS. Effects on lipoprotein particles of long-term dehydroepiandrosterone in elderly men and women and testosterone in elderly men. J Clin Endocrinol Metab. 2010;95(4):1617–1625. [PMC free article: PMC2853990] [PubMed: 20139233]
- 198.
- Bell RJ, Davison SL, Papalia MA, McKenzie DP, Davis SR. Endogenous androgen levels and cardiovascular risk profile in women across the adult life span. Menopause. 2007;14(4):630–638. [PubMed: 17224854]
- 199.
- Shufelt C, Bretsky P, Almeida CM, Johnson BD, Shaw LJ, Azziz R, Braunstein GD, Pepine CJ, Bittner V, Vido DA, Stanczyk FZ, Bairey Merz CN. DHEA-S levels and cardiovascular disease mortality in postmenopausal women: results from the National Institutes of Health--National Heart, Lung, and Blood Institute (NHLBI)-sponsored Women's Ischemia Syndrome Evaluation (WISE). J Clin Endocrinol Metab. 2010;95(11):4985–4992. [PMC free article: PMC2968728] [PubMed: 20739385]
- 200.
- Christiansen JJ, Andersen NH, Sorensen KE, Pedersen EM, Bennett P, Andersen M, Christiansen JS, Jorgensen JO, Gravholt CH. Dehydroepiandrosterone substitution in female adrenal failure: no impact on endothelial function and cardiovascular parameters despite normalization of androgen status. Clin Endocrinol (Oxf). 2007;66(3):426–433. [PubMed: 17302879]
- 201.
- Panjari M, Bell RJ, Jane F, Adams J, Morrow C, Davis SR. The safety of 52 weeks of oral DHEA therapy for postmenopausal women. Maturitas. 2009;63(3):240–245. [PubMed: 19410392]
- 202.
- Mohan PF, Ihnen JS, Levin BE, Cleary MP. Effects of dehydroepiandrosterone treatment in rats with diet-induced obesity. J Nutr. 1990;120(9):1103–1114. [PubMed: 2144587]
- 203.
- Cleary MP, Zisk JF. Anti-obesity effect of two different levels of dehydroepiandrosterone in lean and obese middle-aged female Zucker rats. Int J Obes. 1986;10(3):193–204. [PubMed: 2944850]
- 204.
- Hansen PA, Han DH, Nolte LA, Chen M, Holloszy JO. DHEA protects against visceral obesity and muscle insulin resistance in rats fed a high-fat diet. Am J Physiol. 1997;273(5):R1704–1708. [PubMed: 9374813]
- 205.
- Corona G, Rastrelli G, Giagulli VA, Sila A, Sforza A, Forti G, Mannucci E, Maggi M. Dehydroepiandrosterone supplementation in elderly men: a meta-analysis study of placebo-controlled trials. J Clin Endocrinol Metab. 2013;98(9):3615–3626. [PubMed: 23824417]
- 206.
- Jia C, Chen X, Li X, Li M, Miao C, Sun B, Fan Z, Ren L. The effect of DHEA treatment on the oxidative stress and myocardial fibrosis induced by Keshan disease pathogenic factors. J Trace Elem Med Biol. 2011;25(3):154–159. [PubMed: 21602037]
- 207.
- Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97(11):2601–2610. [PMC free article: PMC507347] [PubMed: 8647954]
- 208.
- Labrie F, Luu-The V, Belanger A, Lin SX, Simard J, Pelletier G, Labrie C. Is dehydroepiandrosterone a hormone? J Endocrinol. 2005;187(2):169–196. [PubMed: 16293766]
- 209.
- Wellman M, Shane-McWhorter L. Orlando, Jennings JP. The role of dehydroepiandrosterone in diabetes mellitus. Pharmacotherapy. 1999;19(5):582–591. [PubMed: 10331821]
- 210.
- Livingstone C, Collison M. Sex steroids and insulin resistance. Clin Sci (Lond). 2002;102(2):151–166. [PubMed: 11834135]
- 211.
- Golden SH, Dobs AS, Vaidya D, Szklo M, Gapstur S, Kopp P, Liu K, Ouyang P. Endogenous sex hormones and glucose tolerance status in postmenopausal women. J Clin Endocrinol Metab. 2007;92(4):1289–1295. [PubMed: 17244779]
- 212.
- Barrett-Connor E, Ferrara A. Dehydroepiandrosterone, dehydroepiandrosterone sulfate, obesity, waist-hip ratio, and noninsulin-dependent diabetes in postmenopausal women: the Rancho Bernardo Study. J Clin Endocrinol Metab. 1996;81(1):59–64. [PubMed: 8550794]
- 213.
- Dhatariya K, Bigelow ML, Nair KS. Effect of dehydroepiandrosterone replacement on insulin sensitivity and lipids in hypoadrenal women. Diabetes. 2005;54(3):765–769. [PubMed: 15734854]
- 214.
- Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292(18):2243–2248. [PubMed: 15536111]
- 215.
- Lasco A, Frisina N, Morabito N, Gaudio A, Morini E, Trifiletti A, Basile G, Nicita-Mauro V, Cucinotta D. Metabolic effects of dehydroepiandrosterone replacement therapy in postmenopausal women. Eur J Endocrinol. 2001;145(4):457–461. [PubMed: 11581005]
- 216.
- Gomez-Santos C, Larque E, Granero E, Hernandez-Morante JJ, Garaulet M. Dehydroepiandrosterone-sulphate replacement improves the human plasma fatty acid profile in plasma of obese women. Steroids. 2011;76(13):1425–1432. [PubMed: 21840329]
- 217.
- Diamond P, Cusan L, Gomez JL, Belanger A, Labrie F. Metabolic effects of 12-month percutaneous dehydroepiandrosterone replacement therapy in postmenopausal women. J Endocrinol. 1996;150 Suppl:S43–50. [PubMed: 8943786]
- 218.
- Paul S Jellinger DAS, Adi E Mehta, Om Ganda, Yehuda Handelsman, Helena W Rodbard, Mark D Shepherd, John A Seibel, AACE Task Force for Management of Dyslipidemia and Prevention of Atherosclerosis. American Association of Clinical Endocrinologists' Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis. Endocrine Practice. 2012(Mar-Apr;18):1-78. [PubMed: 22522068]
- 219.
- Phillips GB, Pinkernell BH, Jing TY. Are major risk factors for myocardial infarction the major predictors of degree of coronary artery disease in men? Metabolism. 2004;53(3):324–329. [PubMed: 15015144]
- 220.
- Freedman DS, O'Brien TR, Flanders WD, DeStefano F, Barboriak JJ. Relation of serum testosterone levels to high density lipoprotein cholesterol and other characteristics in men. Arterioscler Thromb. 1991;11(2):307–315. [PubMed: 1998648]
- 221.
- Tripathi Y, Hegde BM. Serum estradiol and testosterone levels following acute myocardial infarction in men. Indian J Physiol Pharmacol. 1998;42(2):291–294. [PubMed: 10225060]
- 222.
- Pugh PJ, Channer KS, Parry H, Downes T, Jone TH. Bio-available testosterone levels fall acutely following myocardial infarction in men: association with fibrinolytic factors. Endocr Res. 2002;28(3):161–173. [PubMed: 12489566]
- 223.
- Glueck CJ, Glueck HI, Stroop D, Speirs J, Hamer T, Tracy T. Endogenous testosterone, fibrinolysis, and coronary heart disease risk in hyperlipidemic men. J Lab Clin Med. 1993;122(4):412–420. [PubMed: 8228555]
- 224.
- Caron P, Bennet A, Camare R, Louvet JP, Boneu B, Sie P. Plasminogen activator inhibitor in plasma is related to testosterone in men. Metabolism. 1989;38(10):1010–1015. [PubMed: 2507874]
- 225.
- De Pergola G, De Mitrio V, Sciaraffia M, Pannacciulli N, Minenna A, Giorgino F, Petronelli M, Laudadio E, Giorgino R. Lower androgenicity is associated with higher plasma levels of prothrombotic factors irrespective of age, obesity, body fat distribution, and related metabolic parameters in men. Metabolism. 1997;46(11):1287–1293. [PubMed: 9361687]
- 226.
- Militaru C, Donoiu I, Dracea O, Ionescu DD. Serum testosterone and short-term mortality in men with acute myocardial infarction. Cardiol J. 2010;17(3):249–253. [PubMed: 20535714]
- 227.
- Fukui M, Kitagawa Y, Nakamura N, Kadono M, Yoshida M, Hirata C, Wada K, Hasegawa G, Yoshikawa T. Serum dehydroepiandrosterone sulfate concentration and carotid atherosclerosis in men with type 2 diabetes. Atherosclerosis. 2005;181(2):339–344. [PubMed: 16039288]
- 228.
- Suzuki T, Yano Y, Sakamoto M, Uemura M, Yasuma T, Onishi Y, Sasaki R, Matsumoto K, Hayashi T, Maruyama-Furuta N, Akatsuka H, Gabazza EC, Sumida Y, Takei Y. Correlation of circulating dehydroepiandrosterone with activated protein C generation and carotid intima-media thickness in male patients with type 2 diabetes. Diabet Med. 2012;29(7):e41–46. [PubMed: 22248365]
- 229.
- Akishita M, Hashimoto M, Ohike Y, Ogawa S, Iijima K, Eto M, Ouchi Y. Association of plasma dehydroepiandrosterone-sulfate levels with endothelial function in postmenopausal women with coronary risk factors. Hypertens Res. 2008;31(1):69–74. [PubMed: 18360020]
- 230.
- Gebre-Medhin G, Husebye ES, Mallmin H, Helstrom L, Berne C, Karlsson FA, Kampe O. Oral dehydroepiandrosterone (DHEA) replacement therapy in women with Addison's disease. Clin Endocrinol (Oxf). 2000;52(6):775–780. [PubMed: 10848883]
- 231.
- Nestler JE, Barlascini CO, Clore JN, Blackard WG. Dehydroepiandrosterone reduces serum low density lipoprotein levels and body fat but does not alter insulin sensitivity in normal men. J Clin Endocrinol Metab. 1988;66(1):57–61. [PubMed: 2961787]
- 232.
- Casson PR, Faquin LC, Stentz FB, Straughn AB, Andersen RN, Abraham GE, Buster JE. Replacement of dehydroepiandrosterone enhances T-lymphocyte insulin binding in postmenopausal women. Fertil Steril. 1995;63(5):1027–1031. [PubMed: 7720912]
- 233.
- Basu R, Dalla Man C, Campioni M, Basu A, Nair KS, Jensen MD, Khosla S, Klee G, Toffolo G, Cobelli C, Rizza RA. Two years of treatment with dehydroepiandrosterone does not improve insulin secretion, insulin action, or postprandial glucose turnover in elderly men or women. Diabetes. 2007;56(3):753–766. [PubMed: 17327446]
- 234.
- Herrington DM. Dehydroepiandrosterone and coronary atherosclerosis. Ann N Y Acad Sci. 1995;774:271–280. [PubMed: 8597465]
- 235.
- Herrington DM, Nanjee N, Achuff SC, Cameron DE, Dobbs B, Baughman KL. Dehydroepiandrosterone and cardiac allograft vasculopathy. J Heart Lung Transplant. 1996;15(1 Pt 1):88–93. [PubMed: 8820087]
- 236.
- Shono N, Kumagai S, Higaki Y, Nishizumi M, Sasaki H. The relationships of testosterone, estradiol, dehydroepiandrosterone-sulfate and sex hormone-binding globulin to lipid and glucose metabolism in healthy men. J Atheroscler Thromb. 1996;3(1):45–51. [PubMed: 9225239]
- 237.
- Bernini GP, Moretti A, Sgro M, Argenio GF, Barlascini CO, Cristofani R, Salvetti A. Influence of endogenous androgens on carotid wall in postmenopausal women. Menopause. 2001;8(1):43–50. [PubMed: 11201514]
- 238.
- Bernini GP, Sgro M, Moretti A, Argenio GF, Barlascini CO, Cristofani R, Salvetti A. Endogenous androgens and carotid intimal-medial thickness in women. J Clin Endocrinol Metab. 1999;84(6):2008–2012. [PubMed: 10372702]
- 239.
- Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis. 1996;125(1):1–13. [PubMed: 8831922]
- 240.
- Feldman HA, Johannes CB, Araujo AB, Mohr BA, Longcope C, McKinlay JB. Low dehydroepiandrosterone and ischemic heart disease in middle-aged men: prospective results from the Massachusetts Male Aging Study. Am J Epidemiol. 2001;153(1):79–89. [PubMed: 11159150]
- 241.
- Trivedi DP, Khaw KT. Dehydroepiandrosterone sulfate and mortality in elderly men and women. J Clin Endocrinol Metab. 2001;86(9):4171–4177. [PubMed: 11549645]
- 242.
- Hak AE, Witteman JC, de Jong FH, Geerlings MI, Hofman A, Pols HA. Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: the Rotterdam study. J Clin Endocrinol Metab. 2002;87(8):3632–3639. [PubMed: 12161487]
- 243.
- Herrington DM, Gordon GB, Achuff SC, Trejo JF, Weisman HF, Kwiterovich PO Jr, Pearson TA. Plasma dehydroepiandrosterone and dehydroepiandrosterone sulfate in patients undergoing diagnostic coronary angiography. J Am Coll Cardiol. 1990;16(6):862–870. [PubMed: 2146310]
- 244.
- Ishihara F, Hiramatsu K, Shigematsu S, Aizawa T, Niwa A, Takasu N, Yamada T, Matsuo K. Role of adrenal androgens in the development of arteriosclerosis as judged by pulse wave velocity and calcification of the aorta. Cardiology. 1992;80(5-6):332–338. [PubMed: 1451120]
- 245.
- Mitchell LE, Sprecher DL, Borecki IB, Rice T, Laskarzewski PM, Rao DC. Evidence for an association between dehydroepiandrosterone sulfate and nonfatal, premature myocardial infarction in males. Circulation. 1994;89(1):89–93. [PubMed: 8281699]
- 246.
- Barrett-Connor E, Goodman-Gruen D. Dehydroepiandrosterone sulfate does not predict cardiovascular death in postmenopausal women. The Rancho Bernardo Study. Circulation. 1995;91(6):1757–1760. [PubMed: 7882484]
- 247.
- Barrett-Connor E, Goodman-Gruen D. The epidemiology of DHEAS and cardiovascular disease. Ann N Y Acad Sci. 1995;774:259–270. [PubMed: 8597464]
- 248.
- Berr C, Lafont S, Debuire B, Dartigues JF, Baulieu EE. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: a French community-based study. Proc Natl Acad Sci U S A. 1996;93(23):13410–13415. [PMC free article: PMC24107] [PubMed: 8917605]
- 249.
- Nakamura S, Yoshimura M, Nakayama M, Ito T, Mizuno Y, Harada E, Sakamoto T, Saito Y, Nakao K, Yasue H, Ogawa H. Possible association of heart failure status with synthetic balance between aldosterone and dehydroepiandrosterone in human heart. Circulation. 2004;110(13):1787–1793. [PubMed: 15364798]
- 250.
- Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M, Anker SD, Banasiak W, Poole-Wilson PA, Ponikowski P. Anabolic deficiency in men with chronic heart failure: prevalence and detrimental impact on survival. Circulation. 2006;114(17):1829–1837. [PubMed: 17030678]
- 251.
- Formoso G, Chen H, Kim JA, Montagnani M, Consoli A, Quon MJ. Dehydroepiandrosterone mimics acute actions of insulin to stimulate production of both nitric oxide and endothelin 1 via distinct phosphatidylinositol 3-kinase- and mitogen-activated protein kinase-dependent pathways in vascular endothelium. Mol Endocrinol. 2006;20(5):1153–1163. [PubMed: 16373398]
- 252.
- Duncker DJ, Bache RJ. Inhibition of nitric oxide production aggravates myocardial hypoperfusion during exercise in the presence of a coronary artery stenosis. Circ Res. 1994;74(4):629–640. [PubMed: 8137499]
- 253.
- Wang L, Hao Q, Wang YD, Wang WJ, Li DJ. Protective effects of dehydroepiandrosterone on atherosclerosis in ovariectomized rabbits via alleviating inflammatory injury in endothelial cells. Atherosclerosis. 2011;214(1):47–57. [PubMed: 21071029]
- 254.
- Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340(2):115–126. [PubMed: 9887164]
- 255.
- Libby P, Geng YJ, Aikawa M, Schoenbeck U, Mach F, Clinton SK, Sukhova GK, Lee RT. Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol. 1996;7(5):330–335. [PubMed: 8937525]
- 256.
- Libby P. Current concepts of the pathogenesis of the acute coronary syndromes. Circulation. 2001;104(3):365–372. [PubMed: 11457759]
- 257.
- Libby P, Simon DI. Inflammation and thrombosis: the clot thickens. Circulation. 2001;103(13):1718–1720. [PubMed: 11282900]
- 258.
- Belosjorow S, Bolle I, Duschin A, Heusch G, Schulz R. TNF-alpha antibodies are as effective as ischemic preconditioning in reducing infarct size in rabbits. Am J Physiol Heart Circ Physiol. 2003;284(3):H927–930. [PubMed: 12578818]
- 259.
- Liu D, Si H, Reynolds KA, Zhen W, Jia Z, Dillon JS. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Galphai protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology. 2007;148(7):3068–3076. [PubMed: 17395704]
- 260.
- Chen J, Xu L, Huang C. DHEA inhibits vascular remodeling following arterial injury: a possible role in suppression of inflammation and oxidative stress derived from vascular smooth muscle cells. Mol Cell Biochem. 2014;388(1-2):75–84. [PubMed: 24287563]
- 261.
- Hautanen A, Manttari M, Manninen V, Tenkanen L, Huttunen JK, Frick MH, Adlercreutz H. Adrenal androgens and testosterone as coronary risk factors in the Helsinki Heart Study. Atherosclerosis. 1994;105(2):191–200. [PubMed: 8003095]
- 262.
- LaCroix AZ, Yano K, Reed DM. Dehydroepiandrosterone sulfate, incidence of myocardial infarction, and extent of atherosclerosis in men. Circulation. 1992;86(5):1529–1535. [PubMed: 1423966]
- 263.
- Zumoff B, Troxler RG, O'Connor J, Rosenfeld RS, Kream J, Levin J, Hickman JR, Sloan AM, Walker W, Cook RL, Fukushima DK. Abnormal hormone levels in men with coronary artery disease. Arteriosclerosis. 1982;2(1):58–67. [PubMed: 6460495]
- 264.
- Slowinska-Srzednicka J, Zgliczynski S, Ciswicka-Sznajderman M, Srzednicki M, Soszynski P, Biernacka M, Woroszylska M, Ruzyllo W, Sadowski Z. Decreased plasma dehydroepiandrosterone sulfate and dihydrotestosterone concentrations in young men after myocardial infarction. Atherosclerosis. 1989;79(2-3):197–203. [PubMed: 2532016]
- 265.
- Haffner SM, Moss SE, Klein BE, Klein R. Sex hormones and DHEA-SO4 in relation to ischemic heart disease mortality in diabetic subjects. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Diabetes Care. 1996;19(10):1045–1050. [PubMed: 8886548]
- 266.
- Golden SH, Maguire A, Ding J, Crouse JR, Cauley JA, Zacur H, Szklo M. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol. 2002;155(5):437–445. [PubMed: 11867355]
- 267.
- Khaw KT, Tazuke S, Barrett-Connor E. Cigarette smoking and levels of adrenal androgens in postmenopausal women. N Engl J Med. 1988;318(26):1705–1709. [PubMed: 2967432]
- 268.
- Salvini S, Stampfer MJ, Barbieri RL, Hennekens CH. Effects of age, smoking and vitamins on plasma DHEAS levels: a cross-sectional study in men. J Clin Endocrinol Metab. 1992;74(1):139–143. [PubMed: 1530789]
- 269.
- Wu FC, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24(2):183–217. [PubMed: 12700179]
- 270.
- Kawano H, Yasue H, Kitagawa A, Hirai N, Yoshida T, Soejima H, Miyamoto S, Nakano M, Ogawa H. Dehydroepiandrosterone supplementation improves endothelial function and insulin sensitivity in men. J Clin Endocrinol Metab. 2003;88(7):3190–3195. [PubMed: 12843164]
- 271.
- Eich DM, Nestler JE, Johnson DE, Dworkin GH, Ko D, Wechsler AS, Hess ML. Inhibition of accelerated coronary atherosclerosis with dehydroepiandrosterone in the heterotopic rabbit model of cardiac transplantation. Circulation. 1993;87(1):261–269. [PubMed: 8419015]
- 272.
- Gordon GB, Bush DE, Weisman HF. Reduction of atherosclerosis by administration of dehydroepiandrosterone. A study in the hypercholesterolemic New Zealand white rabbit with aortic intimal injury. J Clin Invest. 1988;82(2):712–720. [PMC free article: PMC303568] [PubMed: 2969922]
- 273.
- Aragno M, Parola S, Brignardello E, Mauro A, Tamagno E, Manti R, Danni O, Boccuzzi G. Dehydroepiandrosterone prevents oxidative injury induced by transient ischemia/reperfusion in the brain of diabetic rats. Diabetes. 2000;49(11):1924–1931. [PubMed: 11078461]
- 274.
- Ayhan S, Tugay C, Norton S, Araneo B, Siemionow M. Dehydroepiandrosterone protects the microcirculation of muscle flaps from ischemia-reperfusion injury by reducing the expression of adhesion molecules. Plast Reconstr Surg. 2003;111(7):2286–2294. [PubMed: 12794471]
- 275.
- Kinlay S, Creager MA, Fukumoto M, Hikita H, Fang JC, Selwyn AP, Ganz P. Endothelium-derived nitric oxide regulates arterial elasticity in human arteries in vivo. Hypertension. 2001;38(5):1049–1053. [PubMed: 11711496]
- 276.
- Weiss EP, Villareal DT, Ehsani AA, Fontana L, Holloszy JO. Dehydroepiandrosterone replacement therapy in older adults improves indices of arterial stiffness. Aging Cell. 2012;11(5):876–884. [PMC free article: PMC3444670] [PubMed: 22712469]
- 277.
- Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2197–2223. [PubMed: 23245608]
- 278.
- Donkor ES. Stroke in the 21(st) Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res Treat. 2018;2018:3238165. [PMC free article: PMC6288566] [PubMed: 30598741]
- 279.
- Baulieu EE, Robel P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc Natl Acad Sci U S A. 1998;95(8):4089–4091. [PMC free article: PMC34265] [PubMed: 9539693]
- 280.
- Lapchak PA, Chapman DF, Nunez SY, Zivin JA. Dehydroepiandrosterone sulfate is neuroprotective in a reversible spinal cord ischemia model: possible involvement of GABA(A) receptors. Stroke. 2000;31(8):1953–1956. [PubMed: 10926963]
- 281.
- Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78(8):4704–4707. [PMC free article: PMC320231] [PubMed: 6458035]
- 282.
- Baulieu EE. Neuroactive neurosteroids: dehydroepiandrosterone (DHEA) and DHEA sulphate. Acta Paediatr Suppl. 1999;88(433):78–80. [PubMed: 10626550]
- 283.
- Jimenez MC, Sun Q, Schurks M, Chiuve S, Hu FB, Manson JE, Rexrode KM. Low dehydroepiandrosterone sulfate is associated with increased risk of ischemic stroke among women. Stroke. 2013;44(7):1784–1789. [PMC free article: PMC3811081] [PubMed: 23704104]
- 284.
- Pappa T, Vemmos K, Saltiki K, Mantzou E, Stamatelopoulos K, Alevizaki M. Severity and outcome of acute stroke in women: relation to adrenal sex steroid levels. Metabolism. 2012;61(1):84–91. [PubMed: 21820139]
- 285.
- Blum CA, Mueller C, Schuetz P, Fluri F, Trummler M, Mueller B, Katan M, Christ-Crain M. Prognostic value of dehydroepiandrosterone-sulfate and other parameters of adrenal function in acute ischemic stroke. PLoS One. 2013;8(5):e63224. [PMC free article: PMC3641134] [PubMed: 23650556]
- 286.
- Hampl V, Bibova J, Povysilova V, Herget J. Dehydroepiandrosterone sulphate reduces chronic hypoxic pulmonary hypertension in rats. Eur Respir J. 2003;21(5):862–865. [PubMed: 12765434]
- 287.
- Bonnet S, Dumas-de-La-Roque E, Begueret H, Marthan R, Fayon M, Dos Santos P, Savineau JP, Baulieu EE. Dehydroepiandrosterone (DHEA) prevents and reverses chronic hypoxic pulmonary hypertension. Proc Natl Acad Sci U S A. 2003;100(16):9488–9493. [PMC free article: PMC170945] [PubMed: 12878719]
- 288.
- Debonneuil EH, Quillard J, Baulieu EE. Hypoxia and dehydroepiandrosterone in old age: a mouse survival study. Respir Res. 2006;7(1):144. [PMC free article: PMC1764736] [PubMed: 17176479]
- 289.
- Oka M, Karoor V, Homma N, Nagaoka T, Sakao E, Golembeski SM, Limbird J, Imamura M, Gebb SA, Fagan KA, McMurtry IF. Dehydroepiandrosterone upregulates soluble guanylate cyclase and inhibits hypoxic pulmonary hypertension. Cardiovasc Res. 2007;74(3):377–387. [PMC free article: PMC1950784] [PubMed: 17346686]
- 290.
- Farrukh IS, Peng W, Orlinska U, Hoidal JR. Effect of dehydroepiandrosterone on hypoxic pulmonary vasoconstriction: a Ca(2+)-activated K(+)-channel opener. Am J Physiol. 1998;274(2):L186–195. [PubMed: 9486202]
- 291.
- Gupte SA, Li KX, Okada T, Sato K, Oka M. Inhibitors of pentose phosphate pathway cause vasodilation: involvement of voltage-gated potassium channels. J Pharmacol Exp Ther. 2002;301(1):299–305. [PubMed: 11907187]
- 292.
- Dessouroux A, Akwa Y, Baulieu EE. DHEA decreases HIF-1alpha accumulation under hypoxia in human pulmonary artery cells: potential role in the treatment of pulmonary arterial hypertension. J Steroid Biochem Mol Biol. 2008;109(1-2):81–89. [PubMed: 18261897]
- 293.
- Patel D, Kandhi S, Kelly M, Neo BH, Wolin MS. Dehydroepiandrosterone promotes pulmonary artery relaxation by NADPH oxidation-elicited subunit dimerization of protein kinase G 1alpha. Am J Physiol Lung Cell Mol Physiol. 2014;306(4):L383–391. [PMC free article: PMC3920223] [PubMed: 24375799]
- 294.
- Ventetuolo CE, Ouyang P, Bluemke DA, Tandri H, Barr RG, Bagiella E, Cappola AR, Bristow MR, Johnson C, Kronmal RA, Kizer JR, Lima JA, Kawut SM. Sex hormones are associated with right ventricular structure and function: The MESA-right ventricle study. Am J Respir Crit Care Med. 2011;183(5):659–667. [PMC free article: PMC3081282] [PubMed: 20889903]
- 295.
- Dumas de La Roque E, Savineau JP, Metivier AC, Billes MA, Kraemer JP, Doutreleau S, Jougon J, Marthan R, Moore N, Fayon M, Baulieu EE, Dromer C. Dehydroepiandrosterone (DHEA) improves pulmonary hypertension in chronic obstructive pulmonary disease (COPD): a pilot study. Ann Endocrinol (Paris). 2012;73(1):20–25. [PubMed: 22280813]
- 296.
- Straub RH, Vogl D, Gross V, Lang B, Scholmerich J, Andus T. Association of humoral markers of inflammation and dehydroepiandrosterone sulfate or cortisol serum levels in patients with chronic inflammatory bowel disease. Am J Gastroenterol. 1998;93(11):2197–2202. [PubMed: 9820396]
- 297.
- de la Torre B, Hedman M, Befrits R. Blood and tissue dehydroepiandrosterone sulphate levels and their relationship to chronic inflammatory bowel disease. Clin Exp Rheumatol. 1998;16(5):579–582. [PubMed: 9779307]
- 298.
- Andus T, Klebl F, Rogler G, Bregenzer N, Scholmerich J, Straub RH. Patients with refractory Crohn's disease or ulcerative colitis respond to dehydroepiandrosterone: a pilot study. Aliment Pharmacol Ther. 2003;17(3):409–414. [PubMed: 12562454]
- 299.
- van Vollenhoven RF, Engleman EG, McGuire JL. An open study of dehydroepiandrosterone in systemic lupus erythematosus. Arthritis Rheum. 1994;37(9):1305–1310. [PubMed: 7945493]
- 300.
- van Vollenhoven RF, Engleman EG, McGuire JL. Dehydroepiandrosterone in systemic lupus erythematosus. Results of a double-blind, placebo-controlled, randomized clinical trial. Arthritis Rheum. 1995;38(12):1826–1831. [PubMed: 8849355]
- 301.
- van Vollenhoven RF, Morabito LM, Engleman EG, McGuire JL. Treatment of systemic lupus erythematosus with dehydroepiandrosterone: 50 patients treated up to 12 months. J Rheumatol. 1998;25(2):285–289. [PubMed: 9489820]
- 302.
- van Vollenhoven RF, Park JL, Genovese MC, West JP, McGuire JL. A double-blind, placebo-controlled, clinical trial of dehydroepiandrosterone in severe systemic lupus erythematosus. Lupus. 1999;8(3):181–187. [PubMed: 10342710]
- 303.
- Chang DM, Lan JL, Lin HY, Luo SF. Dehydroepiandrosterone treatment of women with mild-to-moderate systemic lupus erythematosus: a multicenter randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(11):2924–2927. [PubMed: 12428233]
- 304.
- Wilder RL. Adrenal and gonadal steroid hormone deficiency in the pathogenesis of rheumatoid arthritis. J Rheumatol Suppl. 1996;44:10–12. [PubMed: 8833045]
- 305.
- Kanik KS, Chrousos GP, Schumacher HR, Crane ML, Yarboro CH, Wilder RL. Adrenocorticotropin, glucocorticoid, and androgen secretion in patients with new onset synovitis/rheumatoid arthritis: relations with indices of inflammation. J Clin Endocrinol Metab. 2000;85(4):1461–1466. [PubMed: 10770182]
- 306.
- Yukioka M, Komatsubara Y, Yukioka K, Toyosaki-Maeda T, Yonenobu K, Ochi T. Adrenocorticotropic hormone and dehydroepiandrosterone sulfate levels of rheumatoid arthritis patients treated with glucocorticoids. Mod Rheumatol. 2006;16(1):30–35. [PubMed: 16622721]
- 307.
- Danenberg HD, Alpert G, Lustig S, Ben-Nathan D. Dehydroepiandrosterone protects mice from endotoxin toxicity and reduces tumor necrosis factor production. Antimicrob Agents Chemother. 1992;36(10):2275–2279. [PMC free article: PMC245489] [PubMed: 1444309]
- 308.
- Daynes RA, Araneo BA, Ershler WB, Maloney C, Li GZ, Ryu SY. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J Immunol. 1993;150(12):5219–5230. [PubMed: 8515056]
- 309.
- Keller ET, Chang C, Ershler WB. Inhibition of NFkappaB activity through maintenance of IkappaBalpha levels contributes to dihydrotestosterone-mediated repression of the interleukin-6 promoter. J Biol Chem. 1996;271(42):26267–26275. [PubMed: 8824277]
- 310.
- Williams PJ, Jones RH, Rademacher TW. Reduction in the incidence and severity of collagen-induced arthritis in DBA/1 mice, using exogenous dehydroepiandrosterone. Arthritis Rheum. 1997;40(5):907–911. [PubMed: 9153553]
- 311.
- Kobayashi Y, Tagawa N, Muraoka K, Okamoto Y, Nishida M. Participation of endogenous dehydroepiandrosterone and its sulfate in the pathology of collagen-induced arthritis in mice. Biol Pharm Bull. 2003;26(11):1596–1599. [PubMed: 14600408]
- 312.
- Rontzsch A, Thoss K, Petrow PK, Henzgen S, Brauer R. Amelioration of murine antigen-induced arthritis by dehydroepiandrosterone (DHEA). Inflamm Res. 2004;53(5):189–198. [PubMed: 15105968]
- 313.
- Giltay EJ, van Schaardenburg D, Gooren LJ, Dijkmans BA. Dehydroepiandrosterone sulfate in patients with rheumatoid arthritis. Ann N Y Acad Sci. 1999;876:152–154. [PubMed: 10415604]
- 314.
- Cutolo M. Sex hormone adjuvant therapy in rheumatoid arthritis. Rheum Dis Clin North Am. 2000;26(4):881–895. [PubMed: 11084949]
- 315.
- Marwah A, Marwah P, Lardy H., Ergosteroids VI. Metabolism of dehydroepiandrosterone by rat liver in vitro: a liquid chromatographic-mass spectrometric study. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;767(2):285–299. [PubMed: 11885858]
- 316.
- Gambacciani M, Monteleone P, Sacco A, Genazzani AR. Hormone replacement therapy and endometrial, ovarian and colorectal cancer. Best Pract Res Clin Endocrinol Metab. 2003;17(1):139–147. [PubMed: 12763517]
- 317.
- Hankinson SE, Colditz GA, Willett WC. Towards an integrated model for breast cancer etiology: the lifelong interplay of genes, lifestyle, and hormones. Breast Cancer Res. 2004;6(5):213–218. [PMC free article: PMC549181] [PubMed: 15318928]
- 318.
- Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev. 2002;11(12):1531–1543. [PubMed: 12496040]
- 319.
- Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC). J Natl Cancer Inst. 2005;97(10):755–765. [PubMed: 15900045]
- 320.
- Tworoger SS, Missmer SA, Eliassen AH, Spiegelman D, Folkerd E, Dowsett M, Barbieri RL, Hankinson SE. The association of plasma DHEA and DHEA sulfate with breast cancer risk in predominantly premenopausal women. Cancer Epidemiol Biomarkers Prev. 2006;15(5):967–971. [PubMed: 16702378]
- 321.
- Key T, Appleby P, Barnes I, Reeves G, Endogenous H. Breast Cancer Collaborative G. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. [PubMed: 11959894]
- 322.
- Morris KT, Toth-Fejel S, Schmidt J, Fletcher WS, Pommier RF. High dehydroepiandrosterone-sulfate predicts breast cancer progression during new aromatase inhibitor therapy and stimulates breast cancer cell growth in tissue culture: a renewed role for adrenalectomy. Surgery. 2001;130(6):947–953. [PubMed: 11742322]
- 323.
- Schwartz AG, Pashko LL. Cancer prevention with dehydroepiandrosterone and non-androgenic structural analogs. J Cell Biochem Suppl. 1995;22:210–217. [PubMed: 8538200]
- 324.
- Maggiolini M, Donze O, Jeannin E, Ando S, Picard D. Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor alpha. Cancer Res. 1999;59(19):4864–4869. [PubMed: 10519397]
- 325.
- Stoll BA. Dietary supplements of dehydroepiandrosterone in relation to breast cancer risk. Eur J Clin Nutr. 1999;53(10):771–775. [PubMed: 10556982]
- 326.
- Genazzani AD, Stomati M, Bernardi F, Pieri M, Rovati L, Genazzani AR. Long-term low-dose dehydroepiandrosterone oral supplementation in early and late postmenopausal women modulates endocrine parameters and synthesis of neuroactive steroids. Fertil Steril. 2003;80(6):1495–1501. [PubMed: 14667889]
- 327.
- Arnold JT. DHEA metabolism in prostate: For better or worse? Mol Cell Endocrinol. 2009;301(1-2):83–88. [PMC free article: PMC2667103] [PubMed: 19013497]
- 328.
- Schwartz AG. Dehydroepiandrosterone, Cancer, and Aging. Aging Dis. 2022;13(2):423–432. [PMC free article: PMC8947821] [PubMed: 35371612]
- 329.
- Scheffers CS, Armstrong S, Cantineau AEP, Farquhar C. Dehydroepiandrosterone for menopausal women. Cochrane Database of Systematic Reviews. 2014(4). [PMC free article: PMC10662543] [PubMed: 25879093]
- Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause.[Gynecol Endocrinol. 2000]Six-month oral dehydroepiandrosterone supplementation in early and late postmenopause.Stomati M, Monteleone P, Casarosa E, Quirici B, Puccetti S, Bernardi F, Genazzani AD, Rovati L, Luisi M, Genazzani AR. Gynecol Endocrinol. 2000 Oct; 14(5):342-63.
- Review [Adrenopause].[Pol Merkur Lekarski. 2008]Review [Adrenopause].Szkróbka W, Krysiak R, Okopień B. Pol Merkur Lekarski. 2008 Jul; 25(145):77-82.
- Review The Utilization of Dehydroepiandrosterone as a Sexual Hormone Precursor in Premenopausal and Postmenopausal Women: An Overview.[Pharmaceuticals (Basel). 2021]Review The Utilization of Dehydroepiandrosterone as a Sexual Hormone Precursor in Premenopausal and Postmenopausal Women: An Overview.Tang J, Chen LR, Chen KH. Pharmaceuticals (Basel). 2021 Dec 29; 15(1). Epub 2021 Dec 29.
- Review [Current views on the role of dehydroepiandrosterone in physiology, pathology and therapy].[Pol Merkur Lekarski. 2008]Review [Current views on the role of dehydroepiandrosterone in physiology, pathology and therapy].Krysiak R, Frysz-Naglak D, Okopień B. Pol Merkur Lekarski. 2008 Jan; 24(139):66-71.
- Human chorionic gonadotropin does not alter patterns of adrenal androgen secretion in primary human adrenal reticularis and fasciculata cell culture.[Menopause. 2007]Human chorionic gonadotropin does not alter patterns of adrenal androgen secretion in primary human adrenal reticularis and fasciculata cell culture.Casson PR, Buster JE, Callas PM, Hornsby PJ. Menopause. 2007 Mar-Apr; 14(2):316-9.
- Adrenal Androgens and Aging - EndotextAdrenal Androgens and Aging - Endotext
- Homo sapiens RELB proto-oncogene, NF-kB subunit (RELB), mRNAHomo sapiens RELB proto-oncogene, NF-kB subunit (RELB), mRNAgi|317575683|ref|NM_006509.3|Nucleotide
Your browsing activity is empty.
Activity recording is turned off.
See more...
