Clinic Exam: Interview, Physical Exam, ECG, Gen 3 Exam 2, New Offspring Spouse Exam 2, Omni 2 Exam 2: Protocol
Clinic Exam: Interview, Physical Exam, ECG, Gen 3 Exam 2, New Offspring Spouse Exam 2, Omni 2 Exam 2: Protocol
15 May 2008Framingham Phenotypic Identifier: e_exam_2011_m_0017s
Table of Contents
- General Information
- Generation 3 Exam 2 Complete Examination
- Generation 3 Exam 2: Short Examination/Split Exam
- Equipment for Exam 3 Procedures
- Annual/Monthly/Daily Equipment Calibration Protocol
- All Equipment Calibration Time Table: 2008-2009
- MONTHLY/ANNUALLY CALIBRATIONS – “YEAR”
- SCALE Calibration Log Room #
- MANOMETER Calibration Log Room #
- JAMAR HANDGRIP Calibration Log Room #
- HeartStream AED
- Guidelines for Coding Accuracy
- Exam Referral Forms & Medical Encounter Forms
- Clinical Measurements & Procedures - Part 1
- FHS GEN 3 CYCLE 2 Clinic Flow Sheet
- "Ankle Brachial Index Combined With Framingham Risk Score to Predict Cardiovascular Events and Mortality: A Meta-analysis"
- THIGH AND ABDOMINAL CIRCUMFERENCE
- NHANES III - Body Measurements (Anthropometry)
- 1 INTRODUCTION TO ANTHROPOMETRY
- 2 EQUIPMENT
- 3 EXAMINATION PROTOCOL
- 3.1 Eligibility Criteria
- 3.2 Pre-Examination Procedures
- 3.3 Examination Procedures
- 3.3.1 Protocol Procedures
- 3.3.1.1 Weight
- 3.3.1.2 Standing Height
- 3.3.1.3 Sitting Height
- 3.3.1.4 Upper Leg Length
- 3.3.1.5 Knee Height
- 3.3.1.6 Biacromial Breadth
- 3.3.1.7 Biiliac Breadth
- 3.3.1.8 Upper Arm Length
- 3.3.1.9 Arm Circumference
- 3.3.1.10 Abdominal (Waist) Circumference
- 3.3.1.11 Buttocks (Hip) Circumference
- 3.3.1.12 Thigh Circumference
- 3.3.1.13 Skinfolds
- 3.3.1.14 Elbow Breadth
- 3.3.1.15 Wrist Breadth
- 3.3.1.16 Sequence of Measurement Components, SP Position, and Examiner Equipment for Adults 20+
- 3.3.1.17 Measuring Children under Eight Years of Age
- 3.3.1.18 Measuring Handicapped SP’s
- 3.3.2 Examination Form
- 3.3.1 Protocol Procedures
- 3.4 Postexamination Procedures
- 4 LOGS AND RECORDS
- 5 QUALITY CONTROL
- 6 SAFETY PROCEDURES
- "Performance-Based Measures of Physical Function for High-Function Populations"
- Gait Measurement Timer User's Guide
- "Television viewing, computer use, obesity, and adiposity in US preschool children"
- "Peripheral Arterial Disease: Identification and Implications"
- "Physical Activity and Sedentary Behavior: A Population-Based Study of Barriers, Enjoyment, and Preference"
- "Validation and Utility of a Self-report Version of PRIME-MD (Primary Care Evaluation of Mental Disorders) - The PHQ Primary Care Study"
- "Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults"
- "Is Television Viewing Time a Marker of a Broader Pattern of Sedentary Behavior?"
- Short Physical Performance Battery
- "Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents"
- Informed Consent & Tracking Procedures
- Informed Consent
- Consent by Substituted Judgment
- HIPPA: Research Subject’s Authorization for Release of Health Information for Research Purposes
- RESEARCH SUBJECT'S AUTHORIZATION FOR RELEASE OF HEALTH INFORMATION FOR RESEARCH PURPOSES
- Call Backs/ Split Exams
- Admitting Protocol
- Consenting Protocol
- Consent Protocol for Third Generation Study
- Reminder Calls Script
- Call Backs/ Split Exams
- Protocol for Consent for Call Backs / Split Exams
- Phlebotomy
- Clinical Measurements & Procedures - Part 2
- Weight Measurement (Clinic)
- Weight Measurement (Offsite)
- Standing Height Measurement (clinic only)
- Neck Circumference
- Waist Girth at Umbilicus
- Hip Circumference Measurement
- Thigh Circumference - from the Health ABC Study
- Standard ECG & P-Hi Res Protocol
- P-Wave Signal Averaged Electrocardiogram Notes:
- Exit Interview Procedures
- Chart Completion
- Instruction Manual: RESPIRONICS Actical Physical Activity Monitoring System
- Actical Protocol (Use, Download and Creation of Data Sets)
- ACTICAL Training - Framingham Heart Study, May 5, 2008
- Accelerometer Script:
- Physical Activity Monitor Instructions
- Actical® Instruction Manual
- Accelerometer feedback forms
- Pulmonary Function Test Manual of Procedures
- FVC chart
- "Standardisation of spirometry"
- Spirometry Quality Control Program
- "Spirometric Reference Values from a Sample of the General U.S. Population"
- PFT DAILY CALIBRATION
- Manual of Procedures for Pulmonary Function Testing – Generation 3 Cohort, Exam 2
- 1 Overview
- 2 Background and glossary of terms
- 3 Staff training requirements
- 4 Subject selection for bronchodilator testing
- 5 Pulmonary function protocol summary
- 6 Pulmonary function equipment and technical support contact information
- 7 Supply list
- 8 Pulmonary function equipment daily procedures
- 9 Pulmonary function equipment calibration protocol
- 10 Participant testing – detailed procedures
- 11 Quality control observations of pulmonary function technicians by supervisor
- 12 Pulmonary function data back-up
- 13 Pulmonary function equipment maintenance schedule
- 14 Calibration syringe exchange
- 15 Appendix
- Tech-Administered Questionnaires
- Frequency response card
- Rosow-Breslau Questions
- Fractures
- CES-D Scale
- ADMINISTRATION INSTRUCTIONS
- CERAD Word List Memory Task
- Victoria Stroop Test (VST)
- CERAD Word List Memory Task: Learning Trials
- Victoria Stroop Test (VST) – Parts 1-3
- Procedures administered during CERAD Word List delay
- CERAD Word List Memory Task: Recall
- CERAD Word List Memory Task: Recognition
- Factors Affecting the Validity of Data
- Self-Administered Questionnaires
- Physician-Administered Medical History
CLINIC PROTOCOL MANUAL
Generation 3 Cycle 2
General Information
Generation 3 Exam 2 Complete Examination
- Informed Consent & Tracking Procedures
- Informed Consent
- Consent by Substituted Judgment
- HIPPA - Release of Health Information for Research Purposes
- FHS Follow-up by Proxy
- Admitting Procedures
- Phlebotomy
- Urine Specimen
- Blood Draw
- Glucose Tolerance Test
- Pregnancy testing
- Clinical Measurements & Procedures
- Anthropometrics
- Weight
- Height
- Neck Circumference
- Waist Girth at Umbilicus
- Hip Circumference
- Thigh Circumference
- Sagittal Abdominal Diameter (SAD)
- Electrocardiogram (ECG)
- Ankle Arm Doppler (AAD) (participants 40 years and older)
- Observed Physical Performance
- Jamar Handgrip Strength
- Chair Stands (10)
- Fast Walk
- Pulmonary Function
- Spirometry (FVC)
- Diffusion Capacity
- Post-Albuterol Spirometry (select participants)
- Respiratory Questionnaire
- Tonometry and orthostatic blood pressure measurement
- Anthropometrics
- Tech-Administered Questionnaires
- Rosow-Breslau Functional Health Scale
- Fractures
- CES-D
- Exercise Questionnaire
- Neurocognitive Questionnaire
- CERAD
- Victoria Stroop
- Self-Administered Questionnaires
- Socio-demographics
- SF-12 Health Survey
- Appetite Questionnaire
- Willett Dietary Questionnaire
- Physician-Administered Medical History
- Medical History
- Resting Blood Pressure
- Physical Exam
- Other
- Accelerometer
Generation 3 Exam 2: Short Examination/Split Exam
A short exam is completed when a participant requests an abbreviated exam (usually up to 2 hours of testing). A split exam is completed when a participant requests to do an examination in 2 visits.
The priority of exam procedures is listed below:
- Informed Consent & Tracking Procedures
- Informed Consent
- HIPPA-Release of Health Information for Research Purposes
- Tracking Information Form
- Clinical Measurements & Procedures
- Lab
- Blood
- Urine
- Glucose Tolerance Test
- Anthropometrics
- Weight
- Height
- Neck
- Waist at Umbilicus
- Hip
- Thigh
- Sagittal Abdominal Diameter (SAD)
- ECG
- Lab
- Physician-Administered Medical History and Physical Exam
- Medical History
- Resting Blood Pressure
- Physical Exam
- Non-Invasive Vascular Testing
- Tonometry
If time permits for a short exam, the participant will undergo PFT and other measurement of vascular function (Ankle-Arm Doppler).
If the participant chooses to have a split exam a second date will be arranged to complete all of the remaining testing for the exam cycle.
Equipment for Exam 3 Procedures
- Scale to measure body weight in lbs.: (2) Detecto Scale/ (1) Seca 700
Worcester Scale Co., Inc.
228 Brooks Street
Worcester, MA
508-853-2886 - Weight to calibrate scale: 50 lbs.
Worcester Scale Co., Inc. (See address above)
- Accu Hite Stadiometer- (3) Seca 216
Quick Medical
425-831-5963
888-345-4858 - Heart Square by Heartware Inc.
Purchased from: Nova Heart
- Marquette Mac5000 (electrocardiogram cart)
eGalEthCHare Technologies
1701 Military Trail Suite 150
Jupiter, FL 33468-9100Tech support:
Sales Rep:
Clinical Applications: 800-531-5613
- Acquisition Module for Mac5000
Cam-14 (see address above)
- Mac PC (see information for Mac5000 above)
backup portable ECG machine
- Standard mercury column sphygmomanometer: Desk Top Baumanometer, (E98169)
W.A. Baum Co., Inc
620 OakStreet
Copiague, NY 1172
631-226-3940
888-281-6061 - Baumanometer blood pressure cuffs in four sizes: regular adult, large adult, pediatric and thigh.
Inflation systems supplied by Moore Medical.
- Litman stethoscope tubing and earpieces with bell: Classic II
- Gulick retractable tape measure
Novell Products
P.O. Box 408
Rockton, IL 61072
815-624-4888
800-323-5143 - (2) Tailor’s fiberglass tape measure
- Stopwatch -Water Resistant/Shock Resistant VCAT: 1045
Fisher Scientific
Atlanta, GA
(800) 766-7000Calibration Only:
Control Company
4455 Rex Road
Friendswood, TX, 77546
281-482-1714
Email: service/at/control3.com - Pulmonary Function Test (PFT), please see: Manual of Operations: Spirometry and Diffusion Capacity
E-mail:
- Spirometer: Collins CPL pf
Collins Medical, Inc.
220 Wood Road
Braintree, MA 02184-2403
800-225-5157Sales Rep/Customer Service:
- 3 Liter Calibration Syringe S/N # 3729
nSpire Health
1830 Lefthand Circle,
Longmont, CO 80501
303-666-5555 - Parts for Spirometer: (Nspire/Ferraris and Somerset Medical)
- KoKo Moe Disposable Filter and Mouthpiece w/ noseclips #810050
- Nafion Tubing #K381248
- PFT Adaptor #212147
- Disposable Hydrous Dessicator Column #K211501UK
- CO2 Absorbent Granules #K022556
- MicroTach #330672
- Balloons with O-Rings #K022355
- Gases
- Oxygen Gas:
- Lung Diffusion Mix; .3%CO, .3%CH4,21% O2, BALN2
Air Gas East
27 North Western Drive
Salem, NH 03079
- Gait Timer System
Multifunction Timer/Counter
Model # 54035A - Laser Walk Test Contacts:
- BU Sargent College
635 Commonwealth Ave.
Boston, MA 02215 - BU Electronics Design Facility
590 Commonwealth Ave.
Boston, MA 02215 - BU School of Medicine
Framingham Heart Study
715 Albany St. B-608B
Boston, MA 02118-2526 - Boston University
Production Engineer CAS/ Electronic Shop
590 Commonwealth Ave.
Physics Room 2551
Boston, MA 02215
- BU Sargent College
- JAMAR dynamometer
Model #5030J1
Sales Address:
Sammons Preston
14 N Peoria B-101
Chicago, IL, 60607Calibration and Repair Address:
Sammons Preston/ JLW Instruments, INC
14 N Peoria B-101
Chicago, IL 60607
1-800-323-5547 - Holtain Kahn Abdominal Caliper: ( Description Seritex Inc. 77748)
Seritex Inc.
1 Madison Street
E. Rutherford, NJ, 07073
(973) 472-4200
Fax: (973) 472-0222 - Actical Software- Version 2.1 and ActiReader with Instruction Manual
Mini Mitter, a Respironics Company
20300 Empire Avenue, Bldg. B-3
Bend, Oregon 97701Customer Service: 800-685-2999
Tech Support:
- Accelerometer
Mini Mitter, a Respironics Company
20300 Empire Avenue, Bldg. B-3
Bend, Oregon 97701
www.minimitter.comClinic Consultant:
Annual/Monthly/Daily Equipment Calibration Protocol
- Scales: Annually/Monthly/Daily (during clinic)
- Room 100- Detecto
- Room 101- Detecto
- Room 102- Seca 700
Protocol:
- Once a month scales are to be calibrated.
- Place a 50 lb weight onto the scale.
- Set the scale at zero.
- If scale is balanced then calibration is done.
- If scale is unbalanced then turn knob slightly on the side of the zero bar.
- Mark the date in the calibration log book located in the clinic office.
- Furthermore, scales must be certified on a yearly basis. This information can be found in the Clinic Equipment Book located in the clinic office.
- Stadiometers: Monthly
- Room 100- Seca Model # 216 1914009
- Room 101- Seca Model # 216 1914009
- Room 102- Seca Model # 216 1914009
Protocol:
- Using the purple measuring tape located in the clinic office.
- Line up against the meter to determine correct marker points.
- Make sure to move up and down at different spots along the meter.
- If lines do not match up then a new stadiometer must be ordered.
- Mark date in calibration log book once a month.
- Manometers: Monthly/ Daily (during clinic)
- Room 100- V88290
- Room 102- CE3201
- Room 101- T30928
- Room 107- T30927
- Room 109- T30906
- Spare- E95102 (located in clinic office rm.114)
Protocol:
- Use the blood pressure calibrator located in the clinic office in the calibration equipment box.
- Make sure to place manometer on a flat surface and at eye level.
- Connect pieces making sure there is no leak and inflate.
- Slowly release pressure stopping along the way at random places.
- Make sure both meters read the exact same.
- Repeat for all five then mark in the Calibration Log Book located in the clinic office.
- If meter is off, the spare will be used while repairs are being made.
- Calibrate once a month.
- Measuring Tape: Monthly
- Room 100
- Room 102
- (2) Offsite
Protocol:
- Use Purple Measuring tape located in the clinic office.
- Match up the two making sure they are exactly lined up.
- Move around the whole tape at random spots.
- If lines do not match then the tape has been stretched and a new one must be ordered.
- Once a month mark in Calibration Log Book.
- Hand Grips : Annually/ Daily (during clinic)
- Room 100- 10322924
- Room 102- 30208097
- Offsite- 10593388
Protocol:
- The hand grips must be certified on a yearly basis.
- Use the Clinic Equipment Book for contact info.
- Handgrip should be at zero and documented on the daily log sheet.
- Offsite will be checked on a monthly basis and logged in the calibration book.
- AED: Monthly
- The AED is located in the clinic hall on stand outside of Room 109.
Protocol:
- Once a month regular status (readiness) indicator checks are performed and recorded in Log Book.
- Also check and make sure that the spare battery and pads have not exceeded their expiration date.
- Digital Timers: When certificate expires (around every two years)
- Clinic #1- 72318431
- Clinic #2- 72318449
- Clinic #3- 22087033
- Offsite #1- 22086935
- Offsite #2- 61768767
Protocol:
- Digital timers are calibrated about every two years.
- All information can be found in the Clinic Equipment Book.
- Holtain Kahn Abdominal Caliper: Monthly
- (2) In Clinic
- (1) Offsite
Protocol:
- Put Caliper on a FLAT Surface.
- Check to see if the Spirit Level (located at the top of the Caliper) is centered and the upper arm is even with the lower arm.
- If the Level is not centered and/or the arms are not even than the white screws on all four sides of the upper arm can be adjusted using a small screw driver located in the clinic office.
- There are also screws on the bottom that can be adjusted if arms are not leveling with the Spirit Level.
- Mark date in calibration log book once a month.
- Digital Offsite Scales: Monthly
- (2) Offsite Digital Scales
Protocol:
- Use a 50 lb weight.
- Place scale on solid flat surface then place weight onto scale.
- Weight must be 50 lbs exact.
- Mark the date in Log Book once a month.
- Blood Pressure Gauge: Monthly
- (2) Offsite
- (1) Used as a calibrator
Protocol:
- All three gauges are Lifetime Certified, but they are still compared on a monthly basis for consistency.
- Attach pressure gauges and inflate pressure.
- Slowly release pressure making sure to stop at random spots for exact comparison.
- Accelerometer: Every 6 Months
- (100) devices
Protocol:
- Replace batteries every 6 months.
- Replace rubber backing every 6 months.
- Time changes can be found in the program data.
All Equipment Calibration Time Table: 2008-2009
| Activity | Daily | Monthly | Yearly | 2 Years |
|---|---|---|---|---|
| Scale (Onsite) | ||||
| Zero Reading | X | |||
| 50 lb Weight | X | |||
| Professionally Calibrated | X | |||
| Digital Scale (Offsite) | ||||
| 50 lb Weight | X | |||
| Stadiometer (Onsite) | ||||
| Check w/ Purple measuring tape | X | |||
| Tape Measure (Onsite & Offsite) | ||||
| Check w/ Purple measuring tape | X | |||
| Mercury Manometer (Onsite) | ||||
| Zero Reading | X | |||
| Check Inflation System | X | |||
| Aneroid-Gauge Type (Offsite & Onsite) | ||||
| Check Inflation System | X | |||
| Hand Grip (Onsite) | ||||
| Zero Reading | X | |||
| Professional Calibration | X | |||
| Hand Grip (Offsite) | ||||
| Zero Reading | X | |||
| Professional Calibration | X | |||
| Digital Timer (Onsite & Offsite) | ||||
| Professional Calibration | X | |||
| 3 Liter Syringe | ||||
| Replacement | X | |||
| PFT Machine | ||||
| Leak Check | X | |||
| Balloon Check | X | |||
| Barometric Pressure Check | X | |||
| Volume Cal Check | X | |||
| Pneumotach | X | |||
| Gas Analyzer | X | |||
| Professional InspireHealth Calibration | X | |||
| Abdominal Caliper | ||||
| Level Check | X | |||
| Accelerometer Device | 6 Months | |||
| -Battery Change -Rubber band Change | X |
MONTHLY/ANNUALLY CALIBRATIONS – “YEAR”
| JAN. | FEB. | MARCH | APRIL | MAY | JUNE | |
|---|---|---|---|---|---|---|
| Room 100 (ECG) | ||||||
| Manometer V88290 | ||||||
| Stadiometer Level | ||||||
| Scale Detecto | ||||||
| Measuring Tape | ||||||
| Hand Grip 10322924 | ||||||
| Room 102 (ECG) | ||||||
| Manometer CE3201 | ||||||
| Stadiometer Level | ||||||
| Scale Seca 700 | ||||||
| Measuring Tape | ||||||
| Hand Grip 30208097 | ||||||
| Room 101 (PFT) | ||||||
| Manometer T30928 | ||||||
| Stadiometer Broken-level | ||||||
| Scale Detecto | ||||||
| Syringe | ||||||
| Room 109 (MD) | ||||||
| Manometer T30906 | ||||||
| Room 107 (MD) | ||||||
| Manometer T30927 | ||||||
| Offsite- Hand Grip 10593388 | ||||||
| Spare- Manometer E95102 | ||||||
| Holtain Kahn Ab. Caliper | ||||||
| Room 100- Ab. Caliper | ||||||
| Room 102- Ab. Caliper |
| JULY | AUG. | SEPT. | OCT. | NOV. | DEC. | |
|---|---|---|---|---|---|---|
| Room 100 (ECG) | ||||||
| Manometer V88290 | ||||||
| Stadiometer Level | ||||||
| Scale Detecto | ||||||
| Measuring Tape | ||||||
| Hand Grip 10322924 | ||||||
| Room 102 (ECG) | ||||||
| Manometer CE3201 | ||||||
| Stadiometer Level | ||||||
| Scale Seca 700 | ||||||
| Measuring Tape | ||||||
| Hand Grip 10100317 | ||||||
| Room 101 (PFT) | ||||||
| Manometer T30928 | ||||||
| Stadiometer Broken-level | ||||||
| Scale Detecto | ||||||
| Syringe | ||||||
| Room 109 (MD) | ||||||
| Manometer T30906 | ||||||
| Room 107 (MD) | ||||||
| Manometer T30927 | ||||||
| Offsite- Hand Grip 10593388 | ||||||
| Spare- Manometer E95102 | ||||||
| Holtain Kahn Ab. Caliper | ||||||
| Room 100- Ab. Caliper | ||||||
| Room 102- Ab. Caliper |
SCALE Calibration Log Room #
MANOMETER Calibration Log Room #
JAMAR HANDGRIP Calibration Log Room #
HeartStream AED
CF Medical Inc,
12 Lakeview Ave.
Danvers, MA, 01923
978-750-1899
Guidelines for Coding Accuracy
To ensure accuracy and legibility for persons performing data entry, adhere to the following guidelines:
- Use a red or blue pen or any other pen which will stand out from the page (pencil or black ball-point pens are unacceptable).
- Make sure all numerals are unmistakably clear.
- If measurements are not taken enter 9’s (if the coding option is available) and document the reason. If the coding option of 9 is not available, leave blank and write any comments on why the questions were not asked. Comments are helpful at any point of the exam where data is not recorded in the standard manner.
- If an error is made, cross the old value out with one line so original value is still visible, write the correct information in the margin, and initial and put the date the change was made. Do not superimpose numerals one on top of the other. It is always okay to write the reason for the change especially if it is not clear why.
- Make sure both sides of the examination form are completed.
- At the end of the exam a technician will review the entire exam and make sure all questions have been answered and that there are no blanks left on the form.
- If a questionnaire was not administered check the following item at the top of the page and write in the reason why:
|__| Check here if whole page is blank. Reason why___________________________________
Exam Referral Forms & Medical Encounter Forms
Cognitive Clinic Referral Form

RECORD OF IN-CLINIC MEDICAL ENCOUNTER
DEPARTMENT OF HEALTH & HUMAN SERVICES
Public Health Service
FRAMINGHAM HEART STUDY
73 Mt. Wayte Avenue
Framingham, MA 01702
RECORD OF IN-CLINIC MEDICAL ENCOUNTER
(to be filed in chart)
Participant’s ID#:____________ Participant’s Name:___________________________
Date of incident:______________
Description of incident:
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
Physician:__________________________________________
Follow up (if any)
Date of follow-up:__________________
___________________________________________________________________________
___________________________________________________________________________
___________________________________________________________________________
Physician/Staff:______________________________________
Record of Telephone Encounter
(to be filed in chart)
Participant’s ID#: ___-______ Participant’s Name:_____________________________
Date of Incident: ___/___/___
Person Contacted:_________________________________________________________
________________________________________________________________________
Regarding:_______________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
Contact Made By:_________________________________________________________
Referral Tracking
- Was further medical evaluation recommended for this participant?
This question is to be answered by the physician completing the chart.
In addition to the physician writing in their physician ID number on the form, he/she will code this question using the following codes:
- Coding
- 0= No
- 1= Yes
- 9= Unknown
If No, go to the next section.
If Yes, the MD is to code the reason for further evaluation:
- Blood Pressure result ___/___
Phone call > 200/110
Expedite ≥180/100
Elevated > 140/90
Write in abnormality
- ECG abnormality _________________________________________
- Clinical Physician identified medical problem _________________________________________
- Other____________________________________________________
_________________________________________________________
- Was there an adverse event in clinic/offsite exam that does not require further medical evaluation?
This question is to be completed by a staff member completing the exit interview.
In addition to the staff member writing in their technician ID number on the form, he/she will code this question using the following codes:
- Coding
- 0= No
- 1= Yes
- 9= Unknown
If Yes, write in any comments and photocopy this form and give to .
- Was a FHS physician contacted during the examination due to adverse exam findings?
This question is to be completed at offsite exams only by the staff member completing interview.
In addition to the staff member writing in their technician ID number on the form, he/she will code this question using the following codes:
- Coding
- 0= No
- 1= Yes
- 9= Unknown
If Yes, write in any comments regarding the telephone encounter.
- Method used to inform participants of need for further medical evaluation
This information is to be coded by the physician completed the chart.
Circle ALL that apply
- 1= Face-to-face in clinic
- 2= Phone call
- 3= Result letter
- 4= Other
- Method used to inform participant’s personal physician of need for further medical evaluation.
This information is to be coded by the physician completed the chart.
Circle ALL that apply
- 1= Phone call
- 2= Results letter mailed
- 3= Results letter FAX’d
- 4= Other
Date referral made: ___- -___- -__ __ __ __ Use 4 digits for year
ID number of person completing this referral:________
Notes documenting conversation with participant or participant’s personal
physician:_______________________________________________________________
_______________________________________________________________________
_______________________________________________________________________
Home Visit/Nursing Home Visit Routing Sheet
Participant Label: _____________
Date of Visit: ___/___/___
Offsite Technician: ________________________________
Stroke Tracking Referral Form
The Framingham Study
* Please complete the upper portion of this form if you identify a new neurological event.
ID#:______________________ Name:______________________________
Date Opened: ___/___/___
Date of Event: ___/___/___ Date Type: ___ (0=Exact, 1= Approximate)
Source of Referral:__________
- 1 = Hospital Admission
- 2 = Biennial Exam
- 3 = Offspring Exam
- 4 = Family
- 5 = Medical Records
- 6 = Review
- 7 = Other (Please specify)
Initials:_________
Reason for Referral:_______________________________________________________
Reason for Hospitalization:_________________ (1=Neurology, 2=Other, 3=N/A)
Comments:______________________________________________________________
________________________________________________________________________
________________________________________________________________________
DISPOSITION (FOR TRACKING PERSONNEL TO COMPLETE)
- Dictation:______________ (0=Awaiting, 1=In)
- To be Scheduled in Stroke Clinic:___________ (0=No, 1= Yes, 2=Pending)
- Date Seen in Stroke Clinic: ___/___/___
- Reason Not Seen in Clinic:_________________(1=N/A, 2=Refused, 3=Deceased, 4=Out of State)
- Part of PSIP Follow-Up Protocol:____________ (0=No, 1=Yes, 9=Unknown)
- Previously Seen:____________ (0=No, 1=Stroke, 2=Dementia, 3=Other)
- Medical Records needed: _________ (0=No, 1=Yes)
- Date: ___/___/___
- CT/MRI/MRA to be obtained:____________ (0=No, 1= Yes)
- Date: ___/___/___
- Review Status:_____________ (1=Awaiting Review, 2=Reviewed, 3=Need Info)
- Date Reviewed:___/___/___
- Status of Case:______________ (0=Opened, 1=Closed)
- Date:___/___/___
- Diagnosis:_________________________________________________________
(1=Stroke, 2=TIA, 3=?TIA, 4=Parkinson’s, 5=No CVA, 6=Other Neuro, 7=Migraine, 10=?Stroke, 20=Recurrent TIA, 9=Unknown, 11=Multiple Sclerosis)
Dear___________________________:
We thank you for participating in the Framingham Heart Study. We have reserved a space for you in our clinic on ___________at _____________.
The Framingham Heart Study’s address is 73 Mt.Wayte Avenue, in the Perini Building. The Framingham Heart Study offices are located in the wing at the Franklin Street side of the Building. There is reserved parking for participants behind the Franklin Street wing. Please see the enclosed map. The building is handicap accessible.
You should bring slippers and if you choose, bring your own robe. In order to perform certain tests, we ask that you NOT eat after 8:00 P.M. the previous evening. You may have water, decaffeinated black coffee or tea (no creamer, milk or sugar) that evening and again in the morning before your appointment. A urine sample will be collected when you arrive.
Please do not wear jewelry because of the Bone Density Scan.
Please take any prescription medications, as you normally would.
Using the enclosed MEDICATION BAG, please bring all prescription and nonprescription medications you currently take or have taken in the past month in their original containers.
ON THE BACK OF THIS SHEET, please list information regarding hospitalizations and major illnesses you have experienced since your last exam or health history update.
PLEASE BRING THIS LETTER WITH YOU TO THE CLINIC. If you need help completing this form, Clinic staff can assist you at the time of your appointment.
If you have any questions, please call , Project Coordinator at locally and for long distance at
Sincerely yours,
Director
Framingham Heart Study
FHS REPORT GOES TO:
OVER →
| Doctor’s Name | Doctor’s Address & Phone # |
| __________________________ | _______________________________________ |
| __________________________ | _______________________________________ |
| __________________________ | _______________________________________ |
Hospitalizations, Emergency Room Visits, or Day Surgery Since Your Last Clinic Visit
| Date | Reason | Hospital Name & Address | Doctor’s Name |
|---|---|---|---|
| _____ | _______________ | ________________________ | ____________________ |
| _____ | _______________ | ________________________ | ____________________ |
| _____ | _______________ | ________________________ | ____________________ |
| _____ | _______________ | ________________________ | ____________________ |
Doctor Office Visits:
| Date | Reason | Doctor’s Name |
|---|---|---|
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
Clinical Measurements & Procedures - Part 1
GENERATION 3 EXAM 2
(Inside Golden Folder)
- CHART ORDER:
- Consent Form
- Summary Sheet
- ECG
- Summary of Findings (Yellow)
- Main Exam Form
- Bone Study Packet (2)
- Numerical Data Sheet
- Second Visit Sheet
- Ankle Arm Doppler
- Exit Interview
- Respiratory Disease Questionnaire
- PFT White Sheet
- STAPLED- TECH QUESTIONNAIRES
- CES-D
- Rosow-Breslau & Fractures
- Physical Activity Questionnaire
- Observed Performance
- STAPLED
- CERAD & STROOP
- STAPLED-SELF ADMIN QUESTIONNAIRES
- SOCIODEMOGRAPHIC
- SF-12
- SLEEP QUESTIONNAIRE
- REFERRAL TRACKING
- ADMITTING FORM
- PARTICIPANT LETTER (FROM DR)
- HIPPA MEDICAL RELEASE FORM
- APPOINTMENT LETTER
- LAB TEST REQUEST FORM
(ORANGE INSIDE OF BROWN FOLDER)
COHORT EXAM 31
(Inside Purple Folder)
- CHART ORDER:
- Summary Sheet
- ECG
- Summary of Findings
- Main Exam Form
- Numerical Data Sheet
- Observed Performance
- STAPLED
- MMSE
- Sociodemographics
- IADL’s
- Nagi
- Rosow-Breslau
- Katz
- Falls
- Activity Questions
- Berkman
- Leisure Time Cognitive
- CES-D
- Proxy
- Sentence/Drawing handout
- Referral Tracking
- Admit Form (Salmon Sheet)
- Participant Letter
- HIPPA Medical Release Form
- Appointment Letter
- All remaining forms except Exam 30
(PURPLE FOLDER INSIDE OF WHITE FOLDER)
TECH ID #’S
FHS GEN 3 CYCLE 2 Clinic Flow Sheet
"Ankle Brachial Index Combined With Framingham Risk Score to Predict Cardiovascular Events and Mortality: A Meta-analysis"
Publication omitted
Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodríguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008 Jul 9;300(2):197-208. [PubMed ID: 18612117], [ID : PMC2932628]
THIGH AND ABDOMINAL CIRCUMFERENCE
Health ABC - Operations Manual Vol. III - Chapter 3N, page 1
1. Background and Rationale
1.1. Thigh circumference.
Thigh circumference is an important indicator of muscle atrophy due to disease or injury. In addition, it may be a useful indicator of adiposity or lean body mass. Mid-thigh circumference will be measured using the right thigh at the midpoint between the inguinal crease and the proximal border of the patella.
1.2. Abdominal circumference.
The abdominal circumference is an anthropometric indicator of subcutaneous and deep adipose tissue. In past studies, measurements have been taken at the level of the umbilicus and natural waistline. In Health ABC, the measurement will be made at the maximum circumference because it may be a better indicator of adipose tissue. This level is the area between the lower rib and the iliac crest, usually, but not always, at the level of the umbilicus,
2. Equipment and Supplies
- A flexible inelastic fiberglass tape (about 0.7 cm wide) that is marked in centimeters alone on one side. (Confusion may arise if the tape is marked in centimeters and inches on the same side).
- Grease pencil
- Chair
- Mirror hanging at waist level in clinic room
3. Safety Issues and Exclusions
The measurements of thigh and abdominal circumference should not pose any safety problems to the study participants provided that they can stand independently.
4. Participant and Exam Room Preparation
Study participants should be encouraged to empty their bladder and/or bowels prior to these measurements.
Script: "The measurement that we are about to take is more accurate if you use the bathroom before we measure you. If you need to use the bathroom it is down the hall."
The measurements will be taken over bare skin. Participants should be dressed in a clinic gown so that appropriate landmarks can be located and should be instructed prior to the visit not to wear restricting or compressing undergarments, such as girdles or panty hose, which could interfere with the measurement. The thigh measurement is taken on the same side as the quadriceps strength measurement, generally the right side.
5. Detailed Measurement Procedures
5.1. Thigh Circumference
Measure the thigh on the same side used for the isokinetic dynamometer (Kin-Com) strength measurement. This will be the right side unless contraindicated for the Kin- Com test.
- The participant should start out sitting on a chair, with the knees flexed to about 90 degrees and the thighs
horizontal.
Script: "I am going to measure your right thigh circumference. In order to do that I first have to mark your thigh with a cosmetic pencil to determine where to do the measurement."
- Mark the proximal border of the patella (knee cap). To help locate the landmark, ask the participant to straighten their leg to about a 120 degree angle while keeping their heel on the floor with the thigh muscles relaxed.
- Locate the midpoint of the inguinal crease (see figure below). This is easily located if the hips are flexed as they
will be with the patient seated. To help locate the landmark, ask the participant to lift the thigh about 1
cm. The point where the muscle and tendon contract is the midpoint of the crease.

- With the patient seated and the thigh muscles relaxed, measure, and record on the form, the distance between the
inguinal crease and the proximal border of the patella and divide by two to get the midpoint (record the
midpoint on the form also). Mark the location with a grease pencil.

- Ask the participant to stand up and place the heels about 20 cm apart.
The weight should be evenly distributed over both feet, both feet flat on the floor. If balance is a problem, the participant may hold onto a chair or wall. The examiner should be squatting so that their eyes are at the level of the mid thigh.
- The circumference is measured at the marked midpoint with the measuring tape placed horizontally (that is,
perpendicular to the long axis of the thigh) around the thigh. View the thigh from the front and both sides to
check this. The tape should be in complete contact with the skin, without compressing the soft tissue. The
midpoint mark should be visible in the gap made by the upper and lower wrap of the tape. Make sure that the
lower edge of the tape at the zero mark sits directly at the top of the midpoint mark. Make sure that the top
edge of the lower wrap of the tape sits directly below the midpoint mark. Read the value directly below the
zero mark (see example in figure on next page: measurement is 51.4 cm).
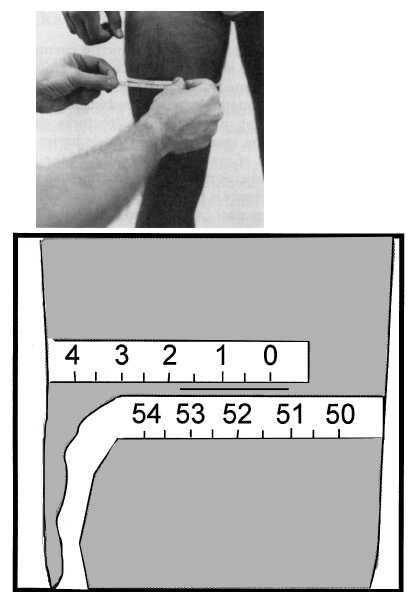
- Record the circumference to the nearest 0.1 cm and mark which thigh was measured.
- Remove and reposition the tape. Repeat the measurement. If the difference between the measurements is > 1 cm, a third and fourth measurement should be obtained. Record all the measurements. The computed value will be the mean of the two or four recorded values.
5.2. Abdominal circumference
- Ask the participant to stand with their weight equally distributed on both feet, arms hanging at their side, and head
facing straight ahead. They should relax their abdomen and breath normally. The examiner should be sitting or
squatting at the side of the participant so that their eyes are at the level of the waist. It may be necessary
to view the participant alternately from the front and the side to locate the largest circumference.
Measure the abdominal circumference directly over bare skin. If necessary, lower pants so that waist bands do not produce a bulge in tissue.
- Pull the tape around the participant's middle at the level of largest circumference with the tape in a horizontal
plane. This tends to coincide with the greatest protuberance of the abdomen, as seen from the side. The
greatest protuberance is usually at, or near, the level of the umbilicus.
If the largest circumference is obstructed by e.g., stomach pouch, obtain measurement, and mark on the form. In very obese participants the maximal truncal circumference may be at hip level. In this case, measure the widest circumference in the region between the lower ribs and the iliac crest and indicate on the form that the actual maximal circumference was at hip level.
Use a wall mirror hanging at waist level to be sure the tape is in the same horizontal plane all around. An assistant may sometimes be needed to help position the tape behind the participant. Alternately, have the participant help hold the tape in position. Bending their arm slightly should not affect the measurement as long as they maintain an erect posture.
Hold the tape snug against the skin, without compressing the tissue, and with its zero end below the value to be recorded.
- Make the measurement at the end of a normal expiration to the nearest 0.1 cm.
Script: "I'd like to take a measurement around your middle where it is the largest. I may need to move some of your clothing out of the way. Breath normally. Don't hold your stomach in. Just relax.";
- Remove and reposition the tape. Repeat the measurement. If the difference between the measurements is > 1 cm, a third and fourth measurement should be obtained. Record all the measurements. The computed value will be the mean of the two or four recorded values.
6. Procedures for Performing the Measurement at Home
Same as described above.
7. Alert Values/Follow-up/Reporting to Participants
If they request, the participants will be told of the measurements at the time of the exam.
8. Quality Assurance
8.1. Training Requirements
No special qualifications or experience are required to perform this assessment. Training should include:
- Read and study manual
- Attend HABC training session on techniques (or observe administration by experienced examiner)
- Practice on volunteers with a special emphasis on obese participants (Goal: differences between repeat measurements ≤ 1 cm)
- Compare measurements with those made by experienced colleagues (Goal: keep differences in any measurement ≤ 1 cm)
- Discuss problems and questions with local expert or QC officer
8.2. Certification Requirements
- Complete training requirements
- Conduct exam on 2 volunteers:
- According to protocol, as demonstrated by completed QC checklist
- Differences between repeat measurements ≤ 1 cm
- Differences between trainee's and QC Officer's measurements should be ≤ 1 cm.
8.3. Quality Assurance Checklist
□ Tape is inelastic, marked in cm only on one side
Thigh circumference
| □ | Measurement made on same side as quadriceps strength | ||||||
| □ | Side measured indicated on form | ||||||
| □ | Records distance between inguinal crease and proximal border of patella | ||||||
| □ | Locates the landmarks correctly
| ||||||
| □ | Records thigh length and correctly calculates midpoint | ||||||
| □ | Checks for tape in same horizontal plane all around (perpendicular to long axis of thigh) |
Waist circumference
| □ | Asks participant to use the bathroom, if necessary |
| □ | Places tape at the largest circumference of the abdomen, viewed from the side |
| □ | Examiner eye level at participant's waist |
| □ | Checks for tape in same horizontal plane all around |
| □ | Measurement taken after normal expiration |
| □ | Clothing is not producing a bulge in tissue |
| □ | Tape snug against bare skin, but does not compress skin |
| □ | If the first two measurements differ by > 1 cm, performs third and fourth measurements |
| □ | Reviews form for completeness |
| □ | Correctly completes form |
9. Form
NHANES III - Body Measurements (Anthropometry)
NATIONAL HEALTH AND NUTRITION EXAMINATION SURVEY III
Body Measurements (Anthropometry)
Westat, Inc.
1650 Research Boulevard
Rockville, MD 20850
(301) 251-1500
October 1988
1. INTRODUCTION TO ANTHROPOMETRY
1.1. Overview of Anthropometry
Nutrition is a major determinant of health, and the resolution of many nutritional issues of public health concern requires survey data. One of the major aims of NHANES III is to provide information useful for studying the relationship among diet, nutritional status, and health. In addition to dietary intake methodologies, questionnaire material, hematological tests, and nutritional biochemistries, the assessment of nutritional status requires a series of stature, weight, and other anthropometric dimensions.
Anthropometry is the study of the measurement of the human body in terms of the dimensions of bone, muscle, and adipose (fat) tissue. Measures of subcutaneous adipose tissue are important because individuals with large values are reported to be at increased risks for hypertension, adult-onset diabetes mellitus, cardiovascular disease, gallstones, arthritis, and other disease, and forms of cancer. Combined with the dietary and related questionnaire data, and the biochemical determinations, anthropometry is essential and critical information needed to assist in describing the data collected from persons in the NHANES III sample.
1.2. Purpose of Anthropometrics
Actual stature, weight, and body measurements including skinfolds, girths, and breadths will be collected in the MEC for purposes of assessing growth, body fat distribution, and for provision of reference data. Measurements of stature and weight will allow for a revision of the child growth charts which are based in part on data collected in NHES cycles II and III and data from the Fels Longitudinal Study. Anthropometric measurements such as skinfolds and circumferences and bioelectrical impedance (a method used to estimate the amount of lean tissue) will allow cross-sectional analysis of the relationship between obesity and risk of disease. Therefore, many of the measurements included in NHANES III will repeat ones made in previous NHANES and HHANES so that trend analyses can be conducted. Some measures have been added to provide further information on body frame size and fat distribution, while others have been dropped because new data have determined that other measures are more informative.
2. EQUIPMENT
2.1. Description of Exam Room in MEC
The body measurement room is located in trailer #4 of the MEC. The room is equipped with some unique features designed to facilitate an accurate and efficient measurement procedure. These features include strategically placed mirrors and a custom-built table for SP’s to sit on. The Toledo scale, stadiometer, and infant measurement board are all contained in this room. The body measurement room is shared with the allergy component and the MEC computer.
2.2. Description of Equipment and Supplies
The equipment and supplies necessary for body measurements are as follows:
- Body measurement table
- Toledo self-zeroing weight scale
- Stadiometer
- Infant measuring board
- Measurement box for sitting height
- Insertion tape
- Steel measuring tape
- Holtain skinfold caliper
- Holtain small sliding breadth caliper
- 2 Mediform large sliding calipers
- Polaroid Land camera with close-up photographic lens
- Special light attachment for camera
- Polaroid film
- Calculator
- Computer terminal
- Weights for scale calibration
- Calibrations rods
- Step wedge standards
- Cosmetic pencils (wax base)
- Footstool
- Scissors - blunt edge
- Paper tape
- White vinegar
- Alcohol
- Baby oil
- Gauze 4x4
Inventory of Equipment and Supplies
At the beginning of each stand and at the end of each stand, the health technician should take an inventory of the equipment and supplies needed for the body measurement examination component as discussed in Standardized Procedures. Any pieces of equipment that are missing should be reported to the MEC manager.
2.3. Start of Stand Procedures
Unpack the calipers and supplies and arrange accordingly in the room. Clean and calibrate the equipment as discussed in this chapter.
- Plug in the power cord.
- Untape the weight blocks and move to the front of the scale.
- Remobilize the scale platform by removing the table paper between the platform and scale.
The printer comprises a bank of numbers and letters that indicate, from left to right, time (AM or PM), date and weight. To set the time/date function displayed in the LED on the front panel, do the following:
- Plug the power cord into the power outlet.
- Find the two push buttons on the panel of the printer above the attached power cable. The top one is the "set" button; the bottom one is the "advance" button.
- Press the "set" button to cause the right most LED digit to begin blinking. Press the "advance" button to advance the numerals until the correct year designation appears. Press "set" once again to fix that numeral in the LED and cause the second digit from the right to begin blinking.
- Follow the above process through the six-digit field that represents the date and the fourdigit field that represents the time. Although the time must be set according to a 24-hour clock, time will appear on the AM and PM.
- When all the digits have been correctly set, press the "set" button twice to start the timing operation.
- Plug the light into the power outlet.
- Untape the light from the camera-holding bar.
- Untape the horizontal bar.
- The caliper is calibrated at the beginning of the stand and once every two weeks during the stand.
- Use the small and medium calibration rods.
- The caliper reading should agree with the known values of the calibration bars.
- Record the caliper readings on the Equipment Calibration Log under the appropriate headings.
- If the two readings do not agree, inform the MEC manager and use the spare set of calipers.
- Calibrate the small sliding breadth calipers at the beginning of the stand and once every two weeks during the stand using the step wedge standard.
- The caliper reading should agree with the known values of the step wedge standard measurements.
- Record the caliper readings on the Equipment Calibration Log.
- If any abnormality is noticed, use the spare set of small sliding breadth calipers and notify the MEC manager.
- The infant measuring board is checked at the beginning of each stand and once every two weeks by placing the calibration rod(s) on the board to check that the board has not been damaged during transit. Check the digital counter reading against the known values of the calibration rod(s) to make sure they agree.
- Record the counter reading on the Equipment Calibration Log under the appropriate heading. If the two readings do not agree, inform the MEC manager.
- Calibrate the height scale at the beginning of each stand, once every two weeks, and at the end of each stand after all examinations.
- Place the calibration rods separately on the floor of the stadiometer.
- Place the horizontal bar of the stadiometer firmly against the top of each calibration rod.
- Take a Polaroid photograph of the stadiometer tape. The measurement should be equal to the known value of each calibration rod. If it is not, adjust the sighting window on the height scale until the measurement does agree and rephotograph the stadiometer tape.
- Calibrate the weight scale at the beginning of each stand and at the end of each stand.
- Place the electronic digital system in the pound mode by pressing the LB/KG button the keyboard until the readout is in tenths. If the digital readout does not register "000.0," press the zero key to automatically balance the scale at zero.
- After zeroing the scale properly, print the zero weight on a sheet of 8 1/2 x 11 paper.
- Place calibration weights on the scale in increments of 25 pounds, starting with 25 and continuing to 250.
- Print the weight in pounds at each increment on the calibration paper by pressing the PRINT key on the time/date unit. At 100 pounds, print the weight in pounds and in kilograms to attest to the accuracy of the pound/kilogram conversion.
- If the scale is out of calibration by at least one-half pound at more than three levels, inform the MEC manager.
- When a satisfactory calibration is obtained, record the stand number, stand location, date, and tech number on the paper and save it with the Equipment Calibration Log.
- Calibrate the Holtain skinfold calipers at the beginning and end of each stand and once a week during each stand using the step wedge standard.
- Zero the calipers before starting the calibration procedures. Place the step wedge standard between the caliper arms at each of the five steps, and check that the reading on the scale corresponds to the standard measurement.
- Record the measurement taken at each step on the Equipment Calibration Log under the appropriate heading. An identical calibration should be done on the spare set of skinfold calipers and the corresponding measurements also recorded on the calibration log sheet. Be careful to record the caliper’s values on the correct device identification line. (The spare is not always the B instrument.)
- If the calipers are 1 mm or more out of calibration at any level, use the other set of calipers and inform the MEC manager. They will be returned to the manufacturer for adjustment.
- If the calipers become too loose, use the spare set of calipers and inform the MEC manager.
Before each examining session, the calipers should be "zeroed." Check to make sure the pointer is clearly reading zero. If not, loosen the flat screw on top of the dial, turn the dial slowly and gently until the pointer reads zero and then turn the screw tight again.
- The technician assigned to the body measurement station should apply a random set of the standard weights daily to roughly check the accuracy of the weight scales. This check is noted in the Equipment Calibration Log.
- If there is any reason to believe that the scales are not accurate, do a complete recalibration. The recording of the calibration should be recorded in the Equipment Calibration Log and the MEC manager should be contacted.
- Each day check that the digital counter and the foot board are operating smoothly. If they are not, a small amount of lubrication can be applied. IF the operation is still not smooth, inform the MEC manager.
- Each day check that the upright bar and attached tape measure have not been damaged. This check is noted in the Equipment Calibration Log.
- Check that the horizontal bar is firmly attached to the upright sliding section and that the section operates smoothly. If it does not, clean the upright bar with white vinegar.
- Check the Polaroid camera and light to see that they produce optimum photos. This check is noted in the Equipment Calibration Log.
2.4. Care and Maintenance
- At the beginning of each stand and during the stand as necessary, wipe the surfaces of the sliding calipers, skinfold calipers, and tape measures with alcohol.
- Clean the equipment with alcohol at the end of each examining day.
- Clean the camera roller bars periodically according to the following instructions to assure uniform
spreading of the photo developing agent.
- Open the back of the camera by releasing the lever at the bottom panel of the camera.
- Grasp the roller springs on the top and bottom of the roller assembly and pull them straight outward, thus allowing the roller bars to swing free of the inside camera body.
- Clean the roller bars thoroughly using alcohol on gauze to remove the chemical residue.
- Put the roller assembly against the back panel of the camera body, and press firmly at the center of the roller bars to reseat the rollers.
- Place the back of the camera against the main body of the camera, and press on it firmly to close the camera.
Report any malfunctions of the body measurement equipment to the MEC manager. Back-up equipment (i.e., calipers) are provided in each MEC to be used until malfunctioning equipment can be repaired or replaced.
2.5. End of Stand Procedures
At the end of each stand, it is the responsibility of the health technicians to prepare the body measurement room and equipment for moving. The following procedures are to be followed.
- Calibrate completely the weight scale and stadiometer as described in this chapter.
- Send the Equipment Calibration Log to Westat at the end of the stand.
- Place the mediform calipers and the elbow breadth calipers in the traveling case. Store the case in the body measurement cabinets.
- Place the skinfold calipers in their protective case, and store them in the body measurement cabinets.
- Unplug the power cord, and check that the weight scale is in a vertical position.
- Move the weight blocks on the front of the scale to the far right side, and tape them in position.
- Immobilize the scale platform by inserting table paper snugly between the platform and the scale base.
- Unplug the power cord from the wall outlet.
- Disconnect the input cable to the scale, and tape the cable onto the printer shelf.
- Put the printer on the floor.
- Unplug the light from the power outlet.
- Place the light against the camera-holding bar and tape it into position.
- Raise the horizontal bar to the top of the upright bar and tape it into position.
- Be sure that the camera is securely fastened down for transit.
Close and lock the drawers and cabinet doors.
3. EXAMINATION PROTOCOL
3.1. Eligibility Criteria
All SP’s aged two months and older are eligible for the body measurement component. Specific measurements are completed dependent on the age of the SP. Table 3-1 lists the SP age groups and the corresponding measurements.
3.2. Pre-Examination Procedures
The collection of anthropometric data requires two health technicians for the roles of examiner and recorder. Health technicians for NHANES III are trained to perform both roles. However, the original examiner and recorder should complete an examination once it is started.
The examiner is responsible for positioning the SP, taking each measurement, and saying the measurement aloud to the recorder. The recorder repeats the number, enters it into the automated system (or hardcopy form), and says the name of the next measurement listed on the computer screen. The examiner should keep the measuring instrument set on the SP until the recorder repeats the number.
It is the recorders role to "assist" the examiner in obtaining correct measurements. This includes helping the examiner correctly position the SP and checking to make sure the SP is standing or sitting erect for specified measurements. The recorder also assists the examiner by checking the tension and horizontal position of the steel measuring tape for girth measurements.
The recorder has the responsibility of ensuring that correct data are entered into the automated system (or recorded on the hardcopy form). The recorder, having had the same training as the examiner, should recognize a gross error in measurement or in reading the different instruments. When an error is recognized, the recorder should call it to the examiner’s attention and the measurement should be repeated.
Body measurements are always taken on the right side of the body. However, some measurements may be taken on the left side of the body because of casts, amputation, or other reasons. When this occurs, the reason is noted in the comments section on the body measurement results screen or hardcopy form by the recorder.
All measurements, except skinfolds, should be taken to the nearest tenth of a centimeter or 1.0 millimeter. Skinfold measurements are taken to the nearest 0.1 millimeter. Measures that exceed specific limits on the computer will be repeated by each technician.
All skinfold measurements will be done in duplicate (i.e., by two different technicians or twice by the same technician) since these measures have the most variability.
If a skinfold is too tight to be measured the code for "tight skin" should be recorded in the space for that skinfold on the computer or hardcopy form. If a skinfold is above the measurable limits of the calipers, the code for "50+" should be entered in the recording space for that skinfold.
3.3. Examination Procedures
The electronic digital scale should be in the kilogram mode. It is not, press the LB/KG key on the keyboard face. The digital LED readout should show 000.00 before weighing a sampled person. If it does not, press the zero key on the keyboard scale to zero the scale.
Have the sampled person stand on the center of the weight scale platform. Record the weight in kilograms in the automated system or on the body measurement exam form in the appropriate space.
Since the scale printer will only print to 250 pounds, the following procedure must be followed if an SP weighs more than 250 pounds:
- If the SP weighs more than 250 pounds, but no more than 350 pounds:
- Move the bottom weight on the notched bar on the front of the scale to 100 pounds (far right).
- Weigh the examinee and press the KG key on the keyboard just as though he weighed less than 250 pounds.
- Add 45.36 kilograms (100 pounds) to the weight.
- Record the total weight (stamped weight plus 45.36 kilograms) in the automated system or in the proper space on the body measurement exam form.
- If the examinee weighs more than 350 pounds, but no more than 400 pounds:
- Move the bottom weight on the notched bar to 100 pounds.
- Move the top weight on the numbered bar to 50 pounds (far right).
- Weigh the examinee and press the KG key just as though he weighed less than 250 pounds.
- Add 68.04 kilograms (150 pounds) to the weight.
- Record the total weight (stamped weight plus 68.04 kilograms) in the automated system or in the proper space on the body measurement exam form.
- If the examinee weighs more than 400 pounds ask him to estimate his weight and document this estimation in the comments section of the automated system.
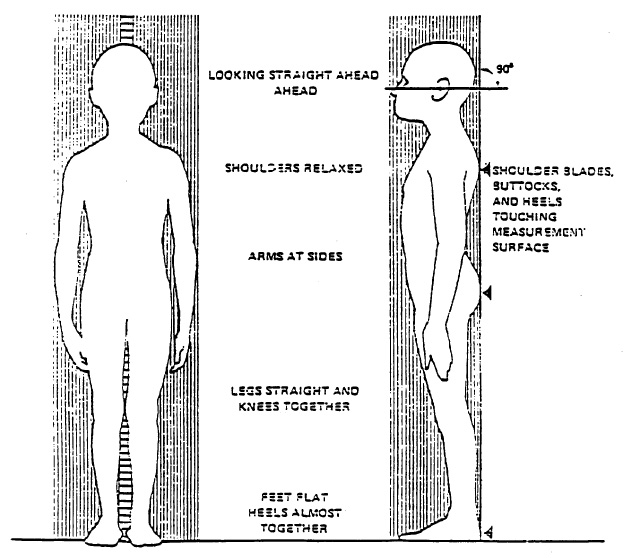
Have the SP stand erect on the floor board of the stadiometer with his or her back to the vertical backboard of the stadiometer. The weight of the participant is evenly distributed on both feet. The heels of the feet are placed together with both heels touching the base of the vertical board. Place the feet pointed slightly outward at a 60 degree angle (see Exhibit 3-1). If the SP has knock knees, the feet are separated so that the inside of the knees are in contact but not overlapping. The buttocks, scapulae, and head are positioned in contact with the vertical backboard. It may not be possible for some children and most adults to place their heels, buttocks, scapulae and the posterior aspect of the head against the backboard while maintaining normal stature. Such SP’s are positioned so that only the heels and buttocks are in contact with the vertical board, and the body is positioned vertically above the waist. The arms hang freely by the sides of the trunk with palms facing the thighs.
The SP is asked to inhale deeply and to stand fully erect without altering the position of the heels. The SP’s head is maintained in the Frankfort Horizontal Plane position while the examiner lowers the horizontal bar snugly to the crown of the head with sufficient pressure to compress the hair. Hair ornaments, buns, braids, etc. must be removed to obtain an accurate measurement. The bar is locked in place and one of the sample number labels placed next to the tape on the upright bar so that the label can be read on the standing height measurement photograph. The examiner needs to make sure that the hair of the SP does not obscure the scale when the photograph is taken. After the measurement is read from the photograph by the examiner and recorded by the recorder to the nearest 0.1 cm.
For measuring sitting height, the examiner moves the specially-made measurement box onto the floor board of the stadiometer. The right leg of the SP’s exam pants need to be cut up the leg so that the skin can be marked for thigh and calf measurements. Reassure the sampled person that the pant leg will be re-taped after the body measurements are completed. The SP then sits on the box with his or her back and buttocks to the backboard of the stadiometer. The SP sits as erect as possible with the head in the Frankfort Horizontal Plane. The knees are directed straight ahead with the arms and hands resting at the sides (see Exhibit 3-2). Ask the SP to sit tall, take a deep breath, and then bring the horizontal bar down snugly to the head. The SP’s head is maintained in the Frankfort Horizontal Plane position while the examiner lowers the horizontal bar snugly to the crown of the head with sufficient pressure to compress the hair. The bar is locked in place and one of the sample number labels placed next to the tape on the upright bar so that the label can be read on the measurement photograph. The examiner needs to make sure that the hair of the SP does not obscure the scale when the photograph is taken. After the sitting height measurement is photographed and the film processed, the sample number label from the upright bar is placed on the photo. The measurement is read from the photograph by the examiner and recorded by the recorder to the nearest 0.1 cm.
The SP sits straight on the measuring box with the right knee bent at a 90 degree angle. The small sliding caliper (used to measure elbow breadth) is positioned as if one were to measure the breadth of the patella. The blades of the caliper are positioned against the distal end of the femur on either side of the patella. The horizontal bar of the caliper should be touching, or close to the anterior surface of the thigh, proximal to the patella. Using the superior edge of the horizontal bar of the caliper as a guide, mark a line on the anterior surface of the thigh. The steel measuring tape is placed at the inguinal crease which is easily located if the hips are in a sitting position. No pressure is to be applied at the inguinal crease; however, folds of fat tissue may have to be lifted on some obese SP’s to measure at the crease. The exam gown should be lifted and the pants slightly pulled to smooth out gathers. The tape is extended along the midline of the thigh to the line just proximal to the patella (see Exhibit 3-3). The length of the upper leg is called to the recorder and the examiner also makes a (+) at the mid point of the thigh with the cosmetic marker. This point will be used at a later time for the thigh circumference and the thigh skinfold.
Knee height is only measured on adults 60 years of age and older. To obtain the measurement, the SP sits on the examination table with both legs dangling. The SP may require the assistance of the examiner to help him onto the table. The examiner places the fixed blade of the large sliding caliper under the heel of the right leg just below the lateral malleolus of the fibula. From a squatting position, the examiner raises the leg so that the knee and ankle are both at a 90 degree angle (see Exhibit 3-4). This is best accomplished by resting the SP’s foot in the palm of the examiner’s hand. The moveable blade of the caliper is placed on the anterior surface of the right thigh, above the condyles of the femur, about two inches above the patella. The shaft of the caliper is held parallel to the shaft of the tibia so that the shaft of the caliper passes over the lateral malleolus of the fibula and just posterior to the head of the fibula. Pressure is applied to compress the tissue. The recorder checks the positioning of the leg and the caliper. Knee height is recorded to the nearest 0.1 cm.
The SP sits on the body measurement table which is approximately chair height. The SP is asked to sit erect with the arms hanging freely at the sides. The examiner stands behind the SP with the mediform sliding calipers. The examiner checks the posture of the SP making sure that the shoulders are neither too far back nor forward, and that there is a noticeable curvature in the lower back. The objective is to have the SP relaxed with the shoulders downward and slightly forward so that the reading is maximal. The sleeves of the exam gown are pulled up towards the neck rather than pulling the gown down over the shoulders. The examiner then locates the acromial process. The caliper rests gently between the thumb and forefinger of the examiner. This allows the examiner to palpate the bony ridges with his other fingers. The examiner locates the lateral border of the acromial process on each shoulder. The arms of the sliding caliper are placed directly on the skin next to the lateral border of each acromial process and pressure is applied to compress the soft tissue over the acromial processes without hurting the SP. The maximum breadth across the lateral borders of the acromial processes is measured to the nearest 0.1 cm.
The SP stands erect with feet together. The SP needs to hold the examination gown up so that the waist and top of hips are exposed. The examiner stands behind the SP holding the large sliding calipers. At the same time, the examiner locates the right side of the iliac crest at its highest point. At this point, the arms of the sliding caliper are placed on the lateral borders of each iliac crest. The soft tissue is compressed to obtain the bone measurement without hurting the SP. The maximum breadth at the highest point of the iliac crests is measured to the nearest 0.1 cm.
Have the SP stand erect with feet together and the right arm flexed 90 degrees at the elbow with the palm facing up. The examiner is positioned behind the SP. The most upper edge of the posterior border of the acromion process of the scapula is located and marked (see Exhibit 3-5). Hold the zero end of the measuring tape at this mark and extend the tape down the posterior surface of the arm to the tip of the olecranon process (the bony part of the mid-elbow). Read the measurement aloud to the recorder. Keep the tape in position and locate half the distance from the acromion to the olecranon processes, i.e., the midpoint of the upper arm. With the cosmetic pencil, mark (+) at the midpoint on the posterior of the arm. This location will be the site for the mid arm circumference and triceps skinfold.
The SP is standing with the elbow relaxed so that the right arm hangs freely to the side. The examiner stands facing the SP’s right side. The measuring tape is placed around the upper arm at the marked point perpendicular to the long axis of the upper arm (+ from upper arm length). The tape is again held so that the zero end is held below the measurement value. The tape rests on the skin surface, but is not pulled tight enough to compress the skin. The arm circumference is recorded to the nearest 0.1 cm.
The SP is in a standing position. The SP is asked to hold up his gown. The examiner stands behind the SP and palpates the hip area for the right iliac crest (see Exhibit 3-6). The examiner marks a horizontal line at the high point of the iliac crest and then crosses the line to indicate the midaxillary line of the body. The pants and underclothing of the SP must be lowered slightly for the examiner to palpate directly on the hip area for the iliac crest. The examiner then stands on the SP’s right side and places the measuring tape around the trunk in a horizontal plane at this level marked on the right side of the trunk. The recorder walks around the SP to make sure that the tape is parallel to the floor and that the tape is snug, but does not compress the skin. The measurement is made at minimal respiration to the nearest 0.1 cm.
The SP stands erect with feet together and weight evenly distributed on both feet. The SP is holding up the examination gown. The recorder stands in back of the SP and gathers the side seams of the exam pants together above the hips and places the thumb in the fabric to make a fold. The recorder holds the folded sides of the pants snugly while the examiner squats on the right side of the SP and places the measuring tape around the buttocks. The tape is placed at the maximum extension of the buttocks (see Exhibit 3-7). The recorder then adjusts the sides of the tape and checks the front and sides so that the plane of the tape is horizontal. The zero end of the tape is held under the measurement value. The tape is held snug but not tight. The examiner takes the measurement from the right side and calls it to the recorder.
The SP is standing with the right leg just in front of the left leg and the weight shifted back to the left leg. This instruction should be demonstrated by the examiner. The edge of the examining table may be used for the SP to hold onto to maintain his balance. The examiner stands on the SP’s right side and the measuring tape is placed around the midthigh at the point that is already marked by a (+). The tape is positioned perpendicular to the long axis of the thigh with the zero end of the tape held below the measurement value. The tape rests firmly on the skin without compressing the skin. The recorder checks to make sure the tape is positioned correctly. The thigh circumference is measured to the nearest 0.1 cm.
All skinfolds are measured with the Holtain skinfold calipers. The measurements are taken on the right side of the body. The fold of skin and underlying subcutaneous adipose tissue should be gently grasped between the examiner’s left thumb and forefingers. The amount grasped depends upon the thickness of the subcutaneous adipose tissue. The examiner grasps enough skin and adipose tissue to form a distinct fold that separates from the underlying muscle. The sides of the fold should be roughly parallel. The skinfold is grasped 2.0 cm above the place the measurement is to be taken and is held gently with the thumb and forefinger. The jaws of the calipers are placed at the marked level, perpendicular to the length of the fold, and the skinfold thickness is measured to the nearest 0.1 mm while the fingers continue to hold the skinfold. The actual measurement is read from the caliper about 3 seconds after the caliper tension is released. A young child is unfamiliar with this procedure; therefore explain the procedure and demonstrate the use of the caliper on the child’s palm. All skinfolds are recorded to the nearest 0.1 mm.
- Thigh Skinfold
The thigh skinfold is measured in the midline of the anterior aspect of the right thigh. This level has already been marked from the thigh circumference measurement. The SP stands with his weight shifted back on the left leg with the right leg forward, knee slightly flexed, foot flat on the floor. Some SP’s will need to hold onto the edge of the table to maintain their balance in this position. This is the same position used for measuring the thigh circumferences. A fold of skin and subcutaneous tissue is grasped in the midline about 2.0 cm above the marked point. The jaws of the skinfold calipers are placed perpendicular to the length of the fold and the shaft of the thigh over the marked point. Skinfolds are recorded to the nearest 0.1 mm.
- Triceps Skinfold
The SP stands erect with feet together, shoulders relaxed and the arms hanging freely at the sides. The examiner stands behind the SP’s right side. The point on the posterior surface of the right upper arm is located in the same area as the marked midpoint for the upper arm circumference. A fold of skin and subcutaneous adipose tissue is grasped gently with thumb and fingers approximately 2.0 cm above the marked level with the skinfold parallel to the long axis of the arm (see Exhibit 3-8). The jaws of the calipers are placed at the marked level, perpendicular to the length of the fold, and the skinfold thickness is measured to the nearest 0.1 mm while the fingers continue to hold the skinfold.
- Subscapular Skinfold
The SP stands erect with shoulders and arms relaxed at the side. The examiner opens the back of the examination gown and palpates for the inferior angle (or triangle portion) of the right scapula. The examiner makes a (+) on the inferior angle of the scapula with the cosmetic pencil marker. The examiner grasps a fold of skin and subcutaneous adipose tissue directly below (1.0 cm) and medial to the inferior angle. The skinfold forms a line about 45 degrees below the horizontal extending diagonally toward the right elbow (see Exhibit 3-9). The jaws of the caliper are placed perpendicular to the length of the fold about 2.0 cm lateral to the fingers with the top jaw of the caliper on the mark over the inferior angle of the scapula. The skinfold thickness is measured to the nearest 0.1 mm while the fingers continue to hold the skinfold.
- Suprailiac Skinfold
The SP stands and holds the right side of the examining gown up so that the right hip area is exposed. It may be necessary to lower the exam pants slightly to expose the area. The iliac crest had already been marked from previous measurements. The examiner places his/her thumb (left) on the intersecting marks and picks up the skinfold with the thumb and fingers. The skinfold should slope downward and forward at a 45 degree angle extending toward the pubic symphysis (see Exhibit 3-10). The caliper is placed perpendicular to the skinfold about 2.0 cm medial to the fingers and the skinfold is measured to the nearest 0.1 mm.
The SP stands erect with feet together facing the examiner. The right arm is extended forward until it is perpendicular to the body. The examiner then flexes the right arm of the SP so that the elbow forms a 90 degree angle with the fingers pointing up and the posterior part of the wrist toward the examiner. With the small sliding caliper held at a 45 degree angle to the plane of the long axis of the upper arm, the greatest breadth across the epicondyles of the elbow are measured to the nearest 0.1 cm. This measurement is taken with the calipers at a slight angle because the medial condyle is more distal than the lateral condyle (see Exhibit 3-11).
The SP stands and extends the right arm keeping the arm straight and near the side of the chest. The examiner stands to the right side of the SP and guides the blades of the small sliding caliper with the thumb and first finger of each hand. The examiner palpates the most prominent aspect of the ulnar styloid process with the middle or index finger of the right hand and slides the right blade of the caliper on to this landmark (see Exhibit 3-12). The most prominent aspect of the radial styloid process is located with the middle or index finger of the left hand. Firm pressure is applied and the breadth is recorded to the nearest 0.1 cm.
| Measurement | SP Position | Equipment | |
|---|---|---|---|
| 1. | Weight | Standing | Scale |
| 2. | Standing height | Standing | Stadiometer Polaroid Camera |
| 3. | Sitting height | Sitting right pant leg open | Measurement box Stadiometer |
| 4. | Upper leg length (mid mark is placed on SP) | Sitting on box right pant leg open | Measurement box Small sliding caliper Insertion tape Cosmetic pencil |
| 5. | Knee height *60+ only | Sitting on exam table | Mediform sliding caliper |
| 6. | Biacromial breadth | Sitting on exam table | Mediform sliding caliper |
| 7. | Biiliac breadth | Standing hold gown up | Mediform sliding caliper |
| 8. | Upper arm length (mid mark is placed on SP) | Standing | Insertion tape Steel tape Cosmetic pencil |
| 9. | Arm circumference | Standing | Steel tape |
| 10. | Abdominal circumference (mark iliac crest) | Standing hold gown up | Steel tape Cosmetic pencil |
| 11. | Buttocks circumference | Standing hold gown up | Steel tape |
| 12. | Thigh circumference | Standing hold gown up right pant leg open | Steel tape |
| 13. | Thigh skinfold | Standing hold gown up right pant leg open | Skinfold caliper |
| 14. | Triceps skinfold | Standing | Skinfold caliper |
| 15. | Subscapular skinfold | Standing | Skinfold caliper |
| 16. | Suprailiac skinfold | Standing hold up gown | Skinfold caliper |
| 17. | Elbow breadth | Standing | Small sliding caliper |
| 18. | Wrist breadth | Standing gown down | Small sliding caliper |
The same procedures are followed for measuring stature and weight of children aged two through seven years as used for older SP’s. For measuring breadths, circumferences, or skinfolds, the child may stand on a footstool placed in the center of the measuring room. If the child is too young to sit or stand by himself, the measurements are taken with the child sitting in the parent’s lap. The examiner’s eyes must be level with the calipers to prevent parallax. Otherwise, use the same procedures as that for older SP’s.
- Recumbent Length
Place children 3 years of age and younger supine on the infant measuring board. The recorder holds the child’s head in the Frankfort plane and applies gentle traction to bring the head into contact with the fixed headboard. The examiner holds the child’s legs by placing one hand gently but firmly over the knees with the child’s toes pointing directly upward. By applying gentle pressure to the legs to prevent the knees from flexing, the examiner brings the movable footboard to rest firmly against the child’s heels. The measurement is recorded to the nearest 0.1 cm from the digital counter on the measuring board. It may be necessary to have a third person help with restless infants to take the measurement as quickly as possible while maintaining accuracy.
- Head Circumference
This measurement is done on children 2 months to 7 years of age. The child either sits in the parent’s lap, on the footstool, or stands depending upon age and activity level. The tape is placed across the frontal bones just above the eyebrows, around the head above the ears on each side, and over the occipital prominence at the back of the head. The examiner holds the tape snugly around the head. Hair ornaments and braids should be removed. The tape is moved up and down over the back of the head to locate the maximal circumference of the head. The tape should be perpendicular to the long axis of the face and should be pulled firmly to compress the hair and underlying soft tissues. Record the measurement to the nearest 0.1 cm.
For those SP’s that are handicapped (in wheelchairs) only limited anthropometric data can be collected.
- Weight can be recorded using the tared wheelchair in the MEC.
- Upper arm length is measured as if the SP were standing. It is necessary to position the SP over to the right side of the wheelchair so that the right arm is not restricted by the arm of the chair.
- Arm circumference is also measured as if the SP were standing. The SP should be in the same position in the wheelchair as for measuring upper arm length. Again, it is important that the right arm is extended so that it is not restricted by the arm of the wheelchair.
- Triceps skinfold is measured on the back of the right arm as if the SP were standing. The position of the SP and the right arm are the same as for measuring arm circumference.
- Knee height can be recorded from the right lower leg as if the SP were ambulatory. It is necessary to remove or rearrange parts of the wheelchair so that there is no interference with the measurements. Again it is important that both the knee and ankle are each at 90° angles.
- Head circumference can be recorded in the same manner as an ambulatory child.
- Wrist and elbow breadths can also be easily recorded from an SP in a wheelchair. The same procedures are followed but the SP is seated rather than standing.
The automated system for the Body Measurement examination will consist of several screens: an introductory screen, data collection screens and an exit screen. Data collection screens are designed to display descriptions of the measurements to be done for each age group. If an SP is less than two years of age, only the measurements applicable to that age group will be displayed on the screen. Likewise, if an SP is 2+ years of age, only the measurements applicable to each age group will be displayed on the screen. Different measurements are collected for SP’s 2 months or greater, 2 years or greater, 60 years or greater, 2 months to 3 years and 2 months to seven years of age.
All measurement entries will be automatically edited for entry of a decimal point. If a decimal point is missing or incorrectly placed, a message will be displayed and the cursor will not advance until the decimal problem is corrected. Measurement entries will also be edited for length. If the number of positions entered for a measurement exceeds the number of positions allowed for that measurement, a message will be displayed and the cursor will not advance until the problem is resolved. Values which fall outside of specified boundaries for body measurements will be flagged for immediate verification. The technician will either verify the original value or enter the second "correct" value.
Every other day, one SP will be selected by the computer to have body measurements replicated by another technician. This selection will be made after the first set of measurements are completed. All body measurements will be repeated for these SP’s.
- Introductory Screen
Five procedures or functions are automated for the body measurement examination room. These functions will be performed under the control of a menu system. When a function is selected from the main menu, screens for the selected application will be displayed.
Selecting "data collection" from the main menu will bring up the introductory screen and allow entry into the data collection forms. The purpose of the introductory screen is to establish the identity of both the technician and the SP and to establish examinations required of the SP for each MEC room. After the technician enters the SP ID number, identification data for the SP will be sent to the body measurement screen by the scheduling system.
Only procedures required for the SP will be displayed on the introductory screen. Although allergy and body measures will be done in the same room, only SP’s ages 6-19 and SP’s ages 20-59 with an even NCHS number will receive an allergy examination. All SP’s will receive a body measurement examination.
The Introductory Screen appears as follows:

- Data Collection Screens
After selecting the appropriate procedure for an SP, the data collection screens will be displayed. If an SP is ages 12-16, the first data collection screen will be as follows:

The technician will ask the SP these two questions before obtaining any measurements. Again, this screen is only displayed and completed for SP’s 12-16 years old.
These data collection screens will be displayed for SP’s less than two years of age.

Technicians are required to enter a decimal point for each measurement entry.
The replicate skinfold screen follows the measurement screens. Only two skinfolds are repeated for SP’s less than two years of age.

The second technician (Tech B) will measure the skinfolds and record the values. Discrepancies between the skinfold measures of two technicians are allowed within certain tolerance levels. If the discrepancy exceeds these levels, the measurement will be flagged with a "*" and a message will be displayed at the bottom of the screen to measure and record the skinfold measurement again.

These are screens for two year olds. Similar screens will be displayed for other ages. The cursor will move from measurement field to next measurement field. If the technician wants to enter a comment (i.e., tight skin) the "Insert Here" key will move the cursor to the comment field and then back to the measurement screen. A list of comments and codes will be placed near the terminal for reference and are also listed on the hardcopy exam form. If a code is not available, specific comments may be entered at the end of the exit screen.

After the measurement screens are completed, the height photo screen will be displayed for all SP’s ages 2 years and older. This screen verifies that height photos were obtained and this information will also create a shipping record for the photos.
- Results Screens
The results of examination screen displays at the conclusion of every MEC examination.

If "Test Done" is selected, the automated system will return to the introductory screen. If "Test Incomplete" or "Test Not Done" is selected, the "Reasons Test Incomplete or Not Done" screen will be displayed.

The technician will select the most appropriate reason for an incomplete or unattempted exam. If the reasons displayed do not apply, select "Other" and record reason in "Comments." The "Comments" field can always be utilized for any additional comments the technician thinks is applicable to the body measurement examination.
When the automated system is not functioning, the body measurement data are recorded on hardcopy data forms (see Exhibit 3-13). The recorder enters the SP’s identification number, or label at the top of the form. The examiner ID# is also recorded. The recorder must then verbally prompt the examiner as to which measurements are applicable to the SP according to age. All of the possible exam components are listed on the hardcopy data form. It is the responsibility of the recorder to carefully monitor the form to make sure only the correct measurements are performed.
The "Results of Examination" code should be selected after the measurements are obtained. If "Test Incomplete" or "Test Not Done" is selected, a reason from the "Reasons Test Incomplete or Not Done" list must be selected.
Page two of the hardcopy data form also includes the list of codes that are used when a measurement is unable to be taken. These codes are the same comment codes that are entered into the automated system for unobtained measurements.
After the exam is completed, hardcopy data forms are placed in the SP chart with other completed data forms. If hardcopy data forms are used because the automated system is not functioning, the data from the hardcopy forms must be carefully enterd into the computer when it returns to working order as per instructions from the MEC manager.
3.4. Postexamination Procedures
After completion of the body measurement examination, the technician should remove all cosmetic pencil marks off the SP with some alcohol or baby oil on a piece of gauze. Technicians should direct or accompany SP’s to their next examination destination as per MEC procedures.
This section describes additional procedures for the Body Measurement component that are the responsibility of the health technicians. These procedures include completing records, logs, and transmittal forms for the recording and shipment of examination data. The accurate completion of these procedures help facilitate an organized data collection process.
4. LOGS AND RECORDS
4.1. Daily Appointment Schedule
The Daily Appointment Schedule will provide information for several uses. A copy of the schedule will be given by the coordinator to the health technicians for the next day. the form from the automated system will provide a copy with the name, age, and sample numbers for each SP scheduled for that day. This list can be used to verify the Daily Log Sheet and other forms. It also lists the number of SP’s that are expected for the session.
4.2. Daily Log
The automated system serves as a daily log to record information about each SP. However, if the system is malfunctioning, hardcopy logs will be maintained.
Daily log sheets are to be completed in addition to the automated system until instructed otherwise by the MEC manager (see Exhibit 4-1). The daily log sheet is located on a clipboard in the body measurement room. One page is used every session that examinations are being conducted during a stand. The technician completes the date, the session time, the stand number, and location.
For each SP examined, the technician records the SP’s ID number, age and sex, technician ID number and the time the exam started, the time it was completed and the assigned status code. Three status codes are used for the Status Code column. Code (C) indicates a "completed" exam, (PC) indicates a "partial complete" exam, and (NE) indicates "no exam." (PC) and (NE) both require reasons for the assigned codes. The comments sections is used to note reasons why the examination was not completed (PC or NE), or any unusual occurrences, etc. The pages of the log are kept in a file until the end of the stand. At that time, the log pages are sent to Westat with the other end of stand materials.
4.3. Equipment Calibration Log
Daily equipment checks and calibrations of the anthropometric equipment are recorded in the Calibration Log (see Exhibit 4-2). As indicated in Section 2, skinfold calipers, the infant measuring board, the Toledo weight scale, and the Stadiometer all require daily checks before the first examination session each day. Calibrations are performed at the times indicated in this manual.
The technician performing the checks completes the top of the log sheet entering the stand number and location. The technician’s ID number is recorded and the date of the check or calibrations should be recorded in the appropriate boxes. Calibration Log sheets are kept until the end of the stand. At that time, they are mailed with other required materials.
4.4. Shipment of Forms and Logs
At the end of each stand and at designated times during the stand, all materials are mailed to either Westat or NCHS. A standard transmittal form is to accompany any mailing of these materials. Transmittal forms are generated from the automated system.
Always make a copy of the transmittal form to keep for your records as well as recording any pertinent information about the mailing procedure (e.g., time of mailing, post office from which you mailed). If the packet with completed forms is lost in the mail, this information will enable the packet to be traced and you will have a record of everything that was contained in the packet.
For the body measurement procedure, you will be mailing the following forms, logs, and data material:
- Body measurement daily log sheets,
- Equipment calibration log,
- Stadiometer calibration pictures,
- Toledo scale readings,
- Quality control for body measurements form,
- SP standing height and sitting height pictures, and
- Hardcopy exam forms (if used).
Detailed procedures for shipment of materials is discussed in the Standardized Procedures section.
5. QUALITY CONTROL
Quality control procedures for body measurements are extremely important and must be observed. The most common errors in anthropometrics are body positioning, reading measurements and recording. In order to minimize these errors, standard procedures for obtaining measurements are described in this manual. The goal of the training session is to standardize all examiners to these procedures. Errors made in measuring technique are also minimized by the recorder’s role in assisting the examiner. The recorder assists the examiner with positioning of the SP and the examiner’s reading process. Reading errors frequently occur as a result of parallax, the phenomenon where an observer sees a different value on a measuring device depending on the angle from which it is viewed. Again, standardization in training will help alleviate this problem.
5.1. Examination Forms
The automated system is designed to function as a quality control measure by minimizing possible measuring and recording errors. Tolerance levels or ranges have been set for each measurement. If a measurement does not fall within these ranges, the system displays an "out of range" message and the examiner can recheck the measurement and enter the "correct" value. It is possible that some SP’s (i.e., very small or very large) will not be within the "normal" ranges. Therefore, the examiner and recorder would verify the original measurement value.
Measuring skinfolds often produces variability amongst examiners. It is extremely important to measure skinfolds accurately. Even after extensive practice it is possible to make errors due to slight misplacement of the caliper or misreading the dial. Therefore, all skinfold measurements are to be repeated on each SP. The automated system is programmed to restrict major discrepancies between technician skinfold measurements. Again, tolerance levels allow for some inter-observer differences, but discrepant measures which exceed the levels must be resolved.
The system also edits the data for placement of decimal points and length of the entry. For instance, if the number of positions entered for a measurement exceeds the number of positions allowed for a measurement, a message will be displayed and the cursor will not advance until the problem is resolved.
Hardcopy exam forms when used must be carefully edited for complete, accurate and legible data.
5.2. Examination Data Items
Standing height and sitting height photographs should be checked for clarity immediately after they developed. Unfocused or fuzzy photos should be retaken at that time. Photos should be stored in an orderly manner by date until the end of the stand. The automated system will have recorded the number of photos taken for each SP during the measurement procedure.
The photos will be shipped at the end of the stand to NCHS. Each shipment must include a transmittal sheet generated from the automated system.
5.3. Equipment Calibrations
Routine calibrations and checks of the body measurement equipment ensures that the equipment is standardized and producing accurate measures. Documentation of calibration activities is transmitted to NCHS at the end of the stand.
5.4. Review, Observations and Replication
Technicians will be periodically observed by the body measurement consultant to ensure standardization. The consultant will review any deviations from the protocol with the technicians.
Replication
Different types of replicates will be utilized in NHANES III as quality control measures:
- Partial Replicates - Replicates who will be selected on the same day of their examination to evaluate interexaminer variation. For example, body measurements performed the same day by different technicians.
- Complete Replicates - Replicates who will be scheduled from the pool of volunteers for a complete reexamination at the MEC.
- Home Replicates - Replicates who were examined at the MEC and will be reexamined at their home, scheduled from the pool of volunteers.
- "Expert" Replicates - These are the replications performed by experts, i.e., the body measurement consultant.
- Laboratory Replicates - These are the split duplicates produced in the MEC laboratory from "Dry runs" and "guest" specimens.
- Bench Replicates - These are the blind controls and split duplicates of the specimens created at the CDC laboratory.
5.5. Refresher Sessions
Refresher or retraining sessions will be scheduled when major changes in protocol are introduced or when a lack of standardization is observed amongst the technicians.
6. SAFETY PROCEDURES
6.1. Equipment Precautions
All equipment in the body measurement should be checked, maintained and cleaned on a regular basis to protect the equipment, the SP, and the technician. If any equipment is broken or starts to break, discontinue using it and notify the MEC manager. Broken equipment should be removed from the body measurement room and/or central areas in the MEC.
6.2. SP Movement and Positioning
The process of taking body measurements does not impose any physical harm or risk to the SP. However, there are certain precautions to be observed by the technicians due to specific positioning for the varied measurement components.
Body measurements which involve having the SP sit on the measurement table may require the assistance of the technicians. It is also recommended that the SP hold onto the edge of the counter for measurements which require them to maintain their balance on one leg.
Performing body measurements on children requires additional safety precautions and monitoring. Children in the body measurement room require constant supervision by the technicians. All anthropometric equipment should be placed out of reach of the smaller children. When using the baby board or the body measurement table, children must be carefully held by the technician to prevent any falls. Keep in mind that babies and small children tend to flip themselves over very quickly. Again, it is the technician’s responsibility to carefully explain and monitor the body measurement procedures to adequately protect the SP’s from any physical injury.
6.3. Emergency Procedures
Procedures for medical emergencies and other types of emergency situations are discussed in the Standardized Procedures.
"Performance-Based Measures of Physical Function for High-Function Populations"
Publication omitted
Curb JD, Ceria-Ulep CD, Rodriguez BL, Grove J, Guralnik J, Willcox BJ, Donlon TA, Masaki KH, Chen R. Performance-based measures of physical function for high-function populations. J Am Geriatr Soc. 2006 May;54(5):737-42. [PubMed ID: 16696737]
Gait Measurement Timer User's Guide
Framingham Heart Study
1/27/2009
This device is designed to measure the walking speed of a person over a fixed distance. It consists of two IR (infra-red) sensor boxes with companion reflectors and a central timer unit.

A diagram of the system is shown above. The two infrared (IR) sensors are placed on one side of the walking path pointing at right angle to the path. A reflector is mounted directly across from each sensor. A cable connects each sensor to the timer unit.
Operation
The unit may be powered by batteries or an AC power adapter. When the AC adapter is plugged in to the unit, the batteries are disconnected. To set up the system, plug a cable into each IR sensor, and plug the other end into one of the connectors (it doesn't matter which connector is chosen they are both equivalent) on the timer unit. Plug the AC adapter (if using) into a wall outlet and into the timer unit. Turn the power switch to “ON” (down). The red LED above the power switch should light and the LCD display light up.
Sensor Alignment
The IR sensors send a beam of infra-red (invisible) light to a reflector, which is received by a detector in the sensor box. Each sensor must be carefully aligned with a reflector before use.
Mount each IR sensor securely to a fixed object, with the sensor head (metal block with three holes) pointing directly across the walking path. Test the sensor by holding your hand or a piece of paper in front of the head a few inches away. The red LED light on the sensor should come on. This light indicates that the IR beam is actively being received.
Mount the reflector directly across from the sensor, and carefully position it such that the red LED is on. Move it around a bit to find the center of the region where the LED is lit, and attach the reflector securely to a fixed object.
Perform the same procedure with the other sensor. Double-check that the LEDs are lit on both sensors. Wave your hand between the sensor and reflector, and observe that the led goes off momentarily when the beam is interrupted.
Timer Operation
Turn the unit on. With both sensors connected, press the RESET button. The display should look like this:
Gait Timer V1.3
Ready 10:24:45
If the display shows changing numbers in place of “Ready” then one of the sensors is not properly aligned or the timer has been falsely triggered. The current time of day should be displayed. If the time is incorrect, it may be set by pressing the HOUR and MIN buttons.
Interrupt the one of the sensor beams (this is now the start sensor). The display should look like this:
Gait Timer V1.3
##.# 10:43:21
where ##.# shows the elapsed time. Interrupt the other sensor sensor beam.
The display should look like this:
04.425 10:45:20
Ready 10:43:55
The top line of the display shows that the elapsed time between start and stop was 4.425 seconds, and that the stop occurred at 10:45:20.
Interrupt the two beams beams again. The display should look line this:
04.425 10:45:20
02.361 10:48:37
This indicates that the elapsed time for the second measurement was 2.361 seconds and that the second stop occurred at 10:48:37.
If the time between start and stop exceeds 30 seconds, the display will show “30.0”. In this case, press RESET and start the measurements again.
After the first measurement is completed, the second measurement cannot start until two seconds have passed. This should prevent false triggers due to (for example) the subject's trailing foot interrupting the beam immediately after the leading foot. The display will momentarily show “Wait” during the delay.
Timer Operation Tips
- The operator should occupy a position where both sensor lights (red LED beam indicating) and the gait timer display is visible.
- The operator should ensure that the timer starts and stops as the patient crosses the two beams. To do this, monitor the timer display, and beam indicating lights on the sensors. The first sensor light will blink as the patient crosses the beam. This will initiate the timer. The second sensor light will blink as the patient crosses the beam. This will stop the timer. If something goes wrong, hit the RESET button and restart the trial.
- Note that the internal timer electronics operate at a much faster rate than that of the display. The display may seam sluggish to respond although the internal timing is still accurate.
Battery Replacement
The timer will operate on AA batteries if the AC power is unplugged. The unit requires 6 AA batteries, which are mounted inside the timer unit. To replace the batteries, remove the six screws around the lid of the unit and carefully remove the lid. Avoid disconnecting any of the wires if possible.
Replace the 6 batteries with new ones, making sure that the negative (-) terminal of each battery is against the spring in its holder.
Replace the lid, being careful that all the wires are completely inside and replace the six screws.
Service Contact Information
Mailing Address:
Boston University Electronics Design Facility
590 Commonwealth Avenue, Physics Room 255
Boston, MA 02215
Location:
The EDF is located at 3 Cummington Street (Physics Research Building) in Room 461. The building is behind Morse Auditorium, and across from the Metcalf Science Center (SCI) at 590 Commonwealth Ave.
Phone: (617) 353-4117
Fax: (617) 353-3331
Email: info/at/edf.bu.edu
Website: http://edf.bu.edu/
Detailed Project Documentation:
http://ohm.bu.edu/cgi-bin/edf/Gait_Measurement_Timer
"Television viewing, computer use, obesity, and adiposity in US preschool children"
Mendoza JA, Zimmerman FJ, Christakis DA. Television viewing, computer use, obesity, and adiposity in US preschool children. Int J Behav Nutr Phys Act. 2007 Sep 25;4:44. [PubMed ID: 17894878], [ID : PMC2131753]










"Peripheral Arterial Disease: Identification and Implications"
Publication omitted
Mohler ER. Peripheral arterial disease: identification and implications. Arch Intern Med. 2003 Oct 27;163(19):2306-14. [PubMed ID: 14581250]
"Physical Activity and Sedentary Behavior: A Population-Based Study of Barriers, Enjoyment, and Preference"
Publication omitted
Salmon J, Owen N, Crawford D, Bauman A, Sallis JF. Physical activity and sedentary behavior: a population-based study of barriers, enjoyment, and preference. Health Psychol. 2003 Mar;22(2):178-88. [PubMed ID: 12683738]
"Validation and Utility of a Self-report Version of PRIME-MD (Primary Care Evaluation of Mental Disorders) - The PHQ Primary Care Study"
Publication omitted
Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov 10;282(18):1737-44. [PubMed ID: 10568646]
"Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults"
Publication omitted
Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006 May;54(5):743-9. [PubMed ID: 16696738]
"Is Television Viewing Time a Marker of a Broader Pattern of Sedentary Behavior?"
Publication omitted
Sugiyama T, Healy GN, Dunstan DW, Salmon J, Owen N. Is television viewing time a marker of a broader pattern of sedentary behavior? Ann Behav Med. 2008 Apr;35(2):245-50. [PubMed ID: 18357498]
Short Physical Performance Battery
1. Balance Tests
- Side-by-Side Stand

Feet together side-by-side for 10 sec
< 10 sec (0 pt) → Go to 4-Meter Gait Speed Test
10 sec (1 pt) ↓
- Semi-Tandem Stand

Heel of one foot against side of big toe of the other for 10 sec
< 10 sec (+0 pt) → Go to 4-Meter Gait Speed Test
10 sec (+1 pt) ↓
- Tandem Stand

Feet aligned heel to toe for 10 sec
10 sec (+2 pt)
3-9.99 sec (+1 pt)
<3 sec (+0 pt)
2. Gait Speed Test
- Measures the time required to walk 4 meters at a normal pace (use best of 2 times)

<4.82 sec 4 pt
4.82-6.20 sec 3 pt
6.21-8.70 sec 2 pt
>8.7 sec 1 pt
Unable 0 pt
3. Chair Stand Test
- Pre-test

Participants fold their arms across their chest and try to stand up once from a chair
unable → Stop (0 pt)
able ↓
- 5 repeats

Measures the time required to perform five rises from a chair to an upright position as fast as possible without the use of the arms
<11.19 sec 4 pt
11.20-13.69 sec 3 pt
13.70-16.69 sec 2 pt
>16.7 sec 1 pt
>60 sec or unable 0 pt
"Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents"
Publication omitted
Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, Diebold MR, Morley JE. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005 Nov;82(5):1074-81. [PubMed ID: 16280441]
Informed Consent & Tracking Procedures
Informed Consent
An informed consent is administered to each participant by a trained interviewer prior to the collection of any research examination study data. The “consent form” is a two-part document. The first part is a narrative description of the studies goals, the content of the exam, the risks and benefits of participating, the confidentiality policies, the right to withdraw from the study, and what compensation is provided in the unlikely event that results in the need for medical care. The second part is the participants authorization page, which the participant signs. This documents the participant’s consent agreeing to (1) participate in an interview and clinical examination, (2) be contacted by study personnel in the future, (3) obtain medical records concerning information relevant to the study, (4) release their clinically relevant study data to their medical care provider, and (5) use of previously frozen blood samples for research. The documents core content complies with guidelines from the National Heart, Lung, and Blood Institute and is approved by BU Medical Center IRB.
A. Overview
Informed consent is the first data collection form administered during the FHS exam. Only updated versions of the informed consent form, approved by the BUMC IRB will be used. All study subjects will be provided with:
- A description of what data collection procedures will be followed and what is involved in each procedure;
- The benefits and risks of participating in a research study which includes genetic analysis;
- A description of what procedures are in place to protect confidentiality;
- Information on the right to withdraw from the study, to not participate in a procedure or to decline to answer a question(s) without penalty;
- An opportunity to document their preference for the use and disposition of their study data and genetic materials; and
- A record of and a mechanism for contacting the project director/principal investigator and the study coordinator.
B. Administration
As the FHS staff person obtaining informed consent, one must provide ample time for the participant to read the consent and answer any questions the participant may have. During the consent process the consenter must “…minimize the possibility of coercion or undue influence…”(46.116 Code of Federal Regulations). One does this by allowing the participant to make their decision to participate on their own, without rushing them during the consent process. Participants must be given “…sufficient opportunity to consider whether or not to participate…” and if the participant refuses the exam their wishes must be honored (46.116 Code of Federal Regulations).
Once the participant has agreed to participate in the current exam cycle, their consent must be documented. This is done by using “…a written consent form approved by the IRB and [the consent must be] signed and dated by the subject…” (50.27 Code of Federal Regulations). Note: Be sure to use the current version of the approved consent, if you have any question of what consent should be used please ask either Senior Secretary x411 or Administrative Manager x418.
Listed below is important information that must also be documented during the consent process.
Completing the Physician Checkbox
A participant should check yes in the following situations:
- If the participant has a doctor and would like us to send results to their doctor;
- If the participant does not have a doctor, but will be getting one within the next 4-6 weeks and would like us to send results to their new physician.
A participant should check no in the following situations:
- If the participant does not want their research exam results sent to their personal physician
- If the participant does not have a doctor and will not be getting one within 4-6 weeks
Visual Impaired Participants
For participants that are visually impaired, the consent form should be read to the participant. A witness must be present during the consent process. The witness must attest that the information in the consent form was accurately explained to and apparently understood by the participant. Therefore, the subject can either sign (“make their mark”) and date the consent form if they can or verbally agree to participate. The consenter signs the form as the person obtaining the consent and the witness will write on the consent form “consent witnessed by” and she/he also will sign and date the form.
If the participant refuses to have the consent form read to them (i.e., asks you to stop), a detailed summary of the exam contents must be provided to the participant. After the participant is informed of what is contained in the consent and they have indicated their agreement to participate, have them sign (“make their mark”) and date the consent form if they can, to indicate their willingness to participate or allow them to verbally agree. The consenter must also document on the consent the way he/she communicated this information and also have the witness sign and date.
Photocopying Consents
A photocopy of the participant’s signed consent must be given to the participant. According to the Code of Federal Regulation 21CFR 50.27 Documentation of Informed Consent “(a)…informed consent shall be documented by the use of a written consent form approved by the IRB and signed and dated by the subject…at the time of the consent. A copy shall be given to the person signing the form.”
Consent by Substituted Judgment
Consent by Substituted Judgment (CSJ) will be obtained for Generation 3 cohort participants who are cognitively impaired. Participants who required CSJ at examination 1 will be identified prior to scheduling the appointment for examination 2 so that appropriate consent for the exam can be obtained. Participants who do not have any prior history of a cognitive impairment but are identified as having a change in cognitive status during the recruitment process will be discussed with the Framingham Study Neurology/Dementia investigators to make a determination about capacity to provide consent.
To obtain Consent by Substituted Judgment, a proxy (an immediate family member, legal guardian, or person with Power of Attorney) will be identified and contacted prior to the exam. This person will be informed of the exam content and asked to consent on the participant’s behalf. The CSJ form will be mailed to this contact to complete and return in a pre-paid FHS envelope. When this form has been returned, the exam can be scheduled. At the time of the first examination (2002-2005) all participants without a cognitive impairment were asked to provide the name of a proxy and an alternate so that if in the future he/she was unable to provide medical information and consent for research examinations his/her designated FHS proxy could do so.
If at any time during the examination a staff member believes there is a question of cognitive impairment the FHS clinic physician will evaluate the participant prior to further testing. If it is determined that a CSJ is needed the examination will be aborted until CSJ can be obtained.
When Consent by Substituted Judgment should be administered:
- In cases where Cognitive Impairment is noted on the roster screen “Cog Imp”
- In cases where the consent status is a “3, 4, or 5”
- 3 = Likely to require consent by substituted judgment as well as own informed consent.
- 4 = Incompetent to give informed consent; has legally appointed guardian and needs consent by substituted judgment.
- 5 = Referred for assessment of competence to provide consent; status could not be determined.
- At any time in the recruitment process, in the admitting process, or during the exam if there should be even the slightest question of cognitive impairment, steps should be taken to determine whether consent by substituted judgment is needed.
HIPPA: Research Subject’s Authorization for Release of Health Information for Research Purposes
The HIPPA Privacy Rule, in effect April 14, 2003, protects the privacy of subject’s health information which is used in human research. For researchers to gain access to health information that is stored at any HIPPA “covered entity” investigators must provide the covered entity with written assurances covering how the health information will be used and protected.
The Framingham Heart Study is not a “covered entity”. However hospitals, nursing homes and physician offices from which the FHS collect medical records are covered by HIPPA rules. Therefore, in order for the FHS to retrieve medical records, participants must sign the HIPPA medical release form. If the participant chooses not to sign the form they will be able to participate in the exam but the FHS will not be able to obtain any outside medical records.
The following explanation of the form is to be given during the intake process:
We want to use your private health information in this research study. This will include both information we collect about you as part of this study as well as health information about you that is stored in your medical records. The law requires us to get your authorization (permission) before we can use your information or share it with others for research purposes. You can choose to sign or not sign this authorization. If you choose not to sign this authorization, you will still be able to take part in the research study.
The participant must also be given adequate time to read the release form. If they agree to sign the form, they must be given a copy of it with their signature. For offsite exams, a photocopy will be mailed with the Informed Consent to the participant.
For cognitively impaired participants: If the participant is cognitively impaired and have had their consent form waived, have the participants’ POA sign the HIPPA form and ask for copies of the POA documentation to go along with it. The POA documentation is necessary for medical records to obtain records from covered entities.
RESEARCH SUBJECT'S AUTHORIZATION FOR RELEASE OF HEALTH INFORMATION FOR RESEARCH PURPOSES
| Name of Research Study: | The Framingham Heart Study 73 Mt. Wayte Avenue, Suite #2, Framingham, MA 01702-5827 |
IRB Number: 1910G
Subject's Name: ____________________ Birth Date: _______________
We want to use your private health information in this research study. This will include both information we collect about you as part of this study as well as health information about you that is stored in your medical records. The law requires us to get your authorization (permission) before we can use your information or share it with others for research purposes. You can choose to sign or not to sign this authorization. If you choose not to sign this authorization, you will still be able to take part in the research study.
Section A:
I authorize the use or sharing of my heatlh information as described below:
Who will be asked to give us your health information:
- Hospitals and physicians you have identified as providing medical care for a reported health problem
Who will be able to use your heatlh information for research:
- The researchers and research staff conducting the Framingham Heart Study.
Section B: Description of information:
- The researchers need to collect information about you and your health. This will include information collected during the study as well as information from your existing medical records so we can review the health problem(s) you have reported to us. The information disclosed under this authorization will not be redisclosed to anyone but the researchers conducting this study except as required by law.
- I authorize (List name of hospital/physician or clinic) to release to the Framingham Heart Study the following information from my medical records. Disclose the following information for the dates ranging from __________ to __________.
Specific description of information we will collect may include:
- Face Sheet
- Discharge Summary
- ER Report
- Admission Notes
- Progress Notes
- Operative Report
- Pathology report
- Chest X-Rays
- EKGs (All)
- CT Scan (Head/Heart)
- MRI/MRA (Head/Neck)
- Lab Reports - Cardiac Enzymes
- Consults (Cardiology & Neurology)
- Cardiac Catheterization
- Exercise Tolerance Test
- Nursing Home Notes
- Notes near time of death
- Other: (for example: Echocardiogram, Arteriography, Venous Ultrasound, V/Q Scan, PA gram, etc.)
Section C: General
- Expiration:
This authorization expires atn the end of the study.
- Right to Revoke:
You may revoke (take back) this authorization at any time. To do this, you must ask the Framingham Heart Study for the names of the Privacy Officers at the institutions where we got your health information. You must then notify those Privacy Officers in writing that you want to take back your Authorization. If you do, we will still be permitted to use the information that we obtained before you revoked your authorization but we will only use your information the way the Informed Consent Form says.
- Your Access to the Information:
You have the right to see your Framingham Heart Study record only after the research study has been completed.
I have read this information, and I will receive a signed copy of this form.
| ________________________________________ Signature of research subject or personal representative | __________ Date |
Printed name of personal representative: ________________________________________
Relationship to research subject: ________________________________________
Please describe the personal representative's authority to act on behalf of the subject:
________________________________________________________________________
Call Backs/ Split Exams
Participants Name / I.D :
Clinic Exam Date:
Second Appointment Date:
*Check Box to indicate which test(s) will be completed on the second visit.
| TEST: | APPROXIMATE TIME: |
|---|---|
| ___ MD Questionnaire/ Physical Exam | 30 min. |
| ___ Anthropometry (Ht, Wt, Waist/Hip/Thigh girth, Sagittal Abdominal Diameter) | 10 min. |
| ___ Self-Administered Questionnaires | 10 min. |
| ___ Tech Administered Questions | 10 min. |
| ___ CERAD | 30 min. |
| ___ Urine | 5 min. |
| ___ Lab | 5 min. |
| ___ Glucose Tolerance Test | 2 hrs. |
| ___ ECG | 10 Min. |
| ___ Observed Performances | 10 min. |
| ___ Ankle/Arm Doppler | 15 min. |
| ___ Tonometry | 15 min. |
| ___ Bone Density | 20 min. |
| ___ PFT | 20-40* min. |
* Maximum Time is with Albuterol Testing Only.
- Why did this participant leave early?
- Which Technician did this participant work with most?
- Recruiters Initials:
Admitting Protocol
- If a participant does not bring his/her appointment letter then the admitter should provide a blank form to be completed by the
participant. The following information should be provided by the participant:
- Social security number
- Physician(s) if none please have them indicate none
- Brief medical history - Doctors visits etc. (to the best of your ability)
- A name and ID label should be placed on the sheet and this sheet stamped with the exam date and placed in the chart.
- MD Check box: If the participant does not have a doctor at the date of exam but plan to provide us with a name in the near future, please have them check the YES box.
- The following document should be given to the participant in admitting:
- One consent form with a copy of the signature page administered by admitter.
- One copy of the HIPPA form with the signature page attached administered by the admitter.
- Optional one copy of the CT consent with the signature page attached administered by the admitter.
Consenting Protocol
- Give participant Consent Form to read, encourage and offer to help if there are any questions.
- Check Consent for all boxes to be checked off, signature, name printed and correct date.
- Check list of doctors on Consent Form against appointment letter.
- Ask the participant if the listing of physicians being sent reports is complete.
- Sign and print your name on appropriate line of Consent Form and date.
- Explain the HIPPA form and ask them to sign the back page.
- Copy the checkbox and signature pages of the consent and the back page of the HIPPA form.
- Give the participant the photocopied pages for their records.
Consent Protocol for Third Generation Study
The Consent Form is administered in the Admitting Station at the time of the Clinic Exam. The participant is given the Consent Form and is instructed to read it carefully and to please check off the boxes on the last page, and date and sign it. If the participant has any questions regarding the Consent Form, they are encouraged to ask at this time. A duplicate copy of the Consent Form is given to the participant for his/her own records. Once the Consent has been read and signed it is reviewed by the person admitting, specifically checking for signature and that the boxes are checked. In the event that any of the boxes are checked “no, this should be reported to the Laboratory Director or the Patient Coordinator. The Consent should be place back in the chart with the signature page displayed.
Common questions that are generated are:
- What is DNA used for?
Answer: It is used for research to see which specific genes are linked to heart disease. Once those are identified, then the question remains, how do you turn those genes off to prevent heart disease.
- What is a Cell Line?
Answer: A Cell Line is a blood sample that is handled in a specific way to enable us to grow DNA forever.
- Are you going to clone me?
Answer: No.
- Will I be able to get any results from the DNA?
Answer: No, there will be no results from the DNA as the information is looked at as a group of information and not for the individual.
- Are you going to sell my blood? I don’t want my information to go to a forprofit company.
Answer: No we are not going to sell your blood.
- How much blood are you taking?
Answer: We take 12 tubes of blood which is 3 ½ ounces, which is less than onehalf cup of blood. When you give blood, they take one pint which is 2 cups of blood.
Dear___________________________:
We thank you for participating in the Framingham Heart Study. Your clinic appointment is scheduled for ___________at _____________.
The Framingham Heart Study’s address is 73 Mt. Wayte Avenue, in the Perini Building. The Framingham Heart Study offices are located in the wing at the Franklin Street side of the Building. There is reserved parking for participants behind the Franklin Street wing. Please see the enclosed map. The building is handicap accessible.
You should bring slippers and if you choose, bring your own robe. In order to perform certain tests, we ask that you NOT eat after 8:00 P.M. the previous evening. You may have water, decaffeinated black coffee or tea (no creamer, milk or sugar) that evening and again in the morning before your appointment. A urine sample will be collected when you arrive.
Please do not wear jewelry because of the Bone Density Scan.
Please take any prescription medications, as you normally would.
Using the enclosed MEDICATION BAG, please bring all prescription and nonprescription medications you currently take or have taken in the past month in their original containers.
ON THE BACK OF THIS SHEET, please list information regarding hospitalizations and major illnesses you have experienced since your last exam or health history with the Framingham Heart Study.
PLEASE BRING THIS LETTER WITH YOU TO THE CLINIC. If you need help completing this form, Clinic staff can assist you at the time of your appointment.
If you have any questions, please call, Project Coordinator at locally and for long distance at
Sincerely yours,
, MD
Director
Framingham Heart Study
OVER→
FHS REPORT GOES TO:
| Doctor’s Name | Doctor’s Address & Phone # |
| __________________________ | _______________________________________ |
| __________________________ | _______________________________________ |
| __________________________ | _______________________________________ |
Hospitalizations, Emergency Room Visits, or Day Surgery Since Your Last Clinic Visit
| Date | Reason | Hospital Name & Address | Doctor’s Name |
|---|---|---|---|
| _____ | _______________ | ________________________ | ____________________ |
| _____ | _______________ | ________________________ | ____________________ |
| _____ | _______________ | ________________________ | ____________________ |
| _____ | _______________ | ________________________ | ____________________ |
Doctor Office Visits:
| Date | Reason | Doctor’s Name |
|---|---|---|
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
| _____ | ________________________________ | _____________________________ |
Reminder Calls Script
Hello Mr/Mrs. Blank
This is _________ from the FHS. I am calling to remind you of the appt you have with us on Monday, January 2 at 7:30 a.m.
Please be sure to read the appt letter. There is important information you need to know about before you come in.
Please bring a list of your brothers and sisters, names, addresses, telephone numbers and a date of birth for each, as well information about a contact person.
If you have any questions, please be sure to call us at or, the number that is listed on your appt letter.
Thank you for your participation in the Framingham Heart Study.
NOTE: DO NOT USE THE STATEMENT BELOW UNTIL IT IS DECIDED TO DO SO.
*** We know you are aware how important it is to keep this appt so that everyone who would like to be a part of this famous/important Study will have an opportunity to do so.
Call Backs/ Split Exams
Participants Name / I.D. :
Clinic Exam Date:
Second Appointment Date:
*Check Box to indicate which test(s) will be completed on the second visit.
| TEST: | APPROXIMATE TIME: |
|---|---|
| ___ MD Questionnaire/ Physical Exam | 30 Min. |
| ___ Anthropometry (Ht, Wt, Waist/Hip/Thigh girth, Sagittal Abdominal Diameter) | 10 Min. |
| ___ Self-Administered Questionnaires | 10 Min. |
| ___ Tech Administered Questions | 10 Min. |
| ___ CERAD | 30 Min. |
| ___ Urine | 5 Min. |
| ___ Lab | 5 Min. |
| ___ Glucose Tolerance Test | 2 Hrs. |
| ___ ECG | 10 Min. |
| ___ Observed Performances | 10 Min. |
| ___ Ankle/Arm Doppler | 15 Min. |
| ___ Tonometry | 15 Min. |
| ___ Bone Density | 20 Min. |
| ___ PFT | 20-40* Min. |
| ___ Post Albuterol with Forced Vital Capacity | 30-35 Min. |
| ___ Post Albuterol Only | 15-20 Min. |
| ___ Accelerometer | 5 Min. |
* Maximum Time is with Post Albuterol Testing Only.
- Why did this participant leave early?
- Which Technician did this participant work with most?
- Estimated time for testing by tech?
- Recruiters Initials:
Protocol for Consent for Call Backs / Split Exams
If a participant returns after original consent date:
- The participant should date and initial the consent page next to the test being done.
- Someone will initial the page.
- Someone will staple a dated note to the consent explaining what was done.
Phlebotomy
Core Exam Components
Gen3 exam 2
core exam components
May 2008
| exam 1 | exam 2 | |
|---|---|---|
| cholesterol | yes | yes |
| HDL | yes | yes |
| triglycerides | yes | yes |
| insulin | yes | yes |
| proinsulin | yes | yes |
| fasting glucose | yes | yes |
| fibrinogen | yes | |
| serum creatinine | yes | yes |
| urine creatinine | yes | yes |
| urinalysis | yes | |
| CRP | yes | yes |
| PAI | yes | |
| urine albumin | yes | yes |
| uric acid | yes | |
| glucose tolerance test | yes | |
| GGT (gamma-glutamyltransferase) | yes | |
| ALT (alanine aminotransferase) | yes | |
| AST (aspartate aminotransferase) | yes | |
| calcium | yes | |
| phosphorus | yes | |
| total bilirubin | yes | |
| albumin | yes | |
| HbA1c | yes | |
| lipoprotein lipase | yes | |
| cystatin C | yes |
Phlebotomy Protocol
Blood samples are collected from an antecubital vein with participants in a supine position after a 12-hour fast. The following tubes are drawn.
- 5 x 10 ml lavender tops (EDTA)
- 1 x 15 ml red top (serum)
- 1 x 10 ml red top (serum)
- 1 x 4.5 ml blue top (citrate)
- 2 x 8.5 ml yellow top (ACD)
- 2 x 8 ml blue tiger top (CPT)
Total volume of blood drawn is 112.5 ml (3.8 ounces).
EDTA
- EDTA plasma used for cholesterol, HDL cholesterol, triglycerides and glucose measured fresh at the Heart Study.
- EDTA plasma sent to Tufts HNRC for other lipids.
- EDTA plasma and red blood cells sent to Tufts HNRC for homocysteine, vitamins B6, B12 and folate.
- EDTA plasma saved in several aliquots for future measurements. Stored at –80 C.
- Buffy coat samples saved from all 5 EDTA Vacutainers. To be sent to Framingham Genetics Laboratory at Boston Medical Center for extraction of DNA.
Serum
- Serum used for creatinine and uric acid, measured fresh at the Heart Study.
- Serum saved in several aliquots for future measurements. Stored at –80 C.
Citrate
- Citrate plasma saved for fibrinogen, stored at –80 C.
- Citrate plasma saved in several aliquots for future measurements. Stored at –80 C.
ACD
ACD whole blood shipped twice weekly to Framingham Genetics Laboratory at Boston Medical Center. Used for extraction of DNA.
CPT
CPT whole blood shipped daily to Fairview University Medical Center in Minneapolis, Minnesota. Lymphocytes are cryopreserved in preparation for future immortalization.
Urine
As part of the Gen 3 Exam 2 clinic visit participants are asked to provide a random urine sample. Samples are tested for pH, protein, glucose, ketone and blood with reagent test strips. Urine save in several aliquots for future measurements. Store at –80 C.
Clinical Measurements & Procedures - Part 2
Weight Measurement (Clinic)
- Ask participant to wear FHS gown for measurement if he/she brought a heavy gown from home ask them to remove it. The participant should remove slippers or shoes.
- Prior to asking participant to step onto the scale, if barefoot, put a piece of paper on the scales platform then lift the counter poise and position it at zero.
- Ask the participant to step onto the scale, facing measurement beam.
- Instruct the participant to stand in the middle of the scale platform with head erect and eyes looking straight ahead. Weight should be equally distributed on both feet and the participant should not touch or support him/herself.
- With the participant standing still in the proper position lift the counterweight (larger weight) and slide it to the right until the beam approaches balance.
- Adjust the top poise until the beam is evenly balanced.
- Have the participant step off the scale. The technician should stand directly in front of the scale and read the weight with eyes level to the point of measurement.
- Record the weight to the nearest pound; round up if ≥ 0.5, round down if < 0.5.
- If a participant exceeds 400 lbs Code as 400 and write a comment at bottom of page. *This will not be coded as a protocol modification only a comment.*
- If deviations from the protocol occur record it as a protocol modification.
Weight Measurement (Offsite)
- The participant should remove slippers or shoes.
- Prior to asking participant to step on the scale, if barefoot, put a piece of paper on scales platform then turn scale on and check to make sure it reads 0.0. The scale should be on a flat, hard surface.
- Ask the participant to step onto the scale.
- Instruct the participant to stand in the middle of the scale platform with head erect and eyes looking straight ahead. Weight should be equally distributed on both feet and the participant should not touch or support himself/herself.
- Read the digital display while participant is on the scale.
- Have the participant step off the scale.
- Record the weight to the nearest pound; round up if ≥ 0.5, round down if < 0.5.
- If a participant exceeds 350 lbs Code as 350 and write a comment at bottom of page. *This will not be coded as a protocol modification only a comment.*
- If participant is unable to stand for weight measurement at a nursing home record the last weight in nursing home chart and the date the weight was obtained. If the participant is unable to stand on a scale during a home visit, record the weight measurement as 999.
- Calibrate scale monthly with 50 lb. weight.
Standing Height Measurement (clinic only)
- Participant should be wearing thin socks if barefoot, put a piece of paper on the floor under the stadiometer. Ask participant to stand erect with his/her back centered on the vertical mounted stadiometer.
- Heels should be against the wall, both feet flat on the floor touching together with weight distributed evenly across both feet.
- Check to make sure both heels are back against the wall.
- Participant faces straight ahead with his/her head positioned in the Frankfort horizontal plane (see next page). The lower margin of the bony orbit (the socket containing the eye) should be on the same horizontal plane as the most forward point in the supratragal notch (the notch just above the anterior cartilaginous projections of the external ear).
- Ask participant to let arms hang freely by the sides of the trunk, palms facing the thighs. Ask participant to inhale deeply and maintain a fully erect position.
- Bring the level down snugly (but not tightly) on top of participant’s head.
- Record measurement to the nearest 1/4 inch, rounding down.
- If deviations from the protocol occur record it as a protocol modification.
- If a participant exceeds 83.5 inches code as 83.5 and write a comment at bottom of page. *This will not be coded as a protocol modification only a comment.*
Note: Measurement is not taken during offsite visits.
Standing Height Measurement

Neck Circumference
- Participant stands erect, arms hanging loosely at sides, weight equally distributed on both feet, head positioned in the Frankfort horizontal plane.
- Standing to face the left side of the participant, identify the thyroid cartilage by gentle palpation of the neck. Gently place your left index and second fingers on the front of the neck and ask the subject to swallow to help find the correct spot. You should feel a slight depression.
- Place the superior border of the anthropometric tape just inferior to the laryngeal prominence.
- Apply the tape snugly, but not tightly, perpendicular to the long axis of the neck, which is not necessarily in the horizontal plane at approximately a 90 degree angle.
- Record the neck circumference to the nearest 1/4 inch, rounding down.
- The pressure on the tape should be the minimum required to maintain skin contact.
Neck Girth

Waist Girth at Umbilicus
- Participant stands erect, arms hanging loosely at sides, weight equally distributed on both feet, head facing straight ahead.
- The technician will take the gown from the back and place it over the shoulder of the participant. The technician will ask the participant to bend their arms at the elbow and hold the gown in place.
- Apply anthropometric tape at the level of the umbilicus.
- Apply tape snugly but not tightly.
- Make sure the tape is horizontal and not twisted, checking from both the front and back by using 2 mirrors mounted to the wall.
- Before recording measurement ask the participant to fully relax their shoulders.
- Record measurement to the nearest 1/4 inch, rounding down.
For offsite visits the waist measurement will be done without using a mirror. Code 8 should be entered as a protocol modification to capture this.
Waist Girth

Hip Circumference Measurement
- Participant stands erect, arms hanging loosely at sides, feet together and weight equally distributed on both feet, facing straight ahead.
- If the participant is wearing a gown, ask them to hold up their gown above their waist with their elbows relaxed by their sides.
- The examiner stands on the participants left side and applies the measuring tape around the maximum extension of the buttocks (see figure on next page).
- The examiner should be squatting or kneeling so that their eyes are at the level of the maximum extension of the buttocks.
- Make sure the tape is horizontal and not twisted, checking from both the front and back by using 2 mirrors mounted on the wall.
- The zero end of the tape is held under the measurement value.
- Apply tape snugly but not tightly.
- Record measurement to the nearest ¼ inch, rounding down.
- If the participant is wearing baggy underpants then the examiner stands in back and gathers the side seams together and places the thumb in the fabric to make a fold.
Protocol from: National Health and Nutrition Examination Survey III – Body Measurements (Anthropometry)
http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/NCHS/MANUALS/ANTHRO.PDF

Thigh Circumference - from the Health ABC Study
Background and Rationale
- Thigh circumference
Thigh circumference may be a useful indicator of adiposity or lean body mass. Mid-thigh circumference will be measured using the right thigh at the midpoint between the inguinal crease and the proximal border of the patella.
- Equipment and Supplies
- A flexible inelastic fiberglass tape
- Grease pencil
- Chair
- Thigh Measurement Procedures
- Measure the thigh on the right side. If the right side cannot be used measure the left thigh and mark as a protocol modification and document reason for the modification. The measurement will be taken over bare skin. Participants should be dressed in a clinic gown so that appropriate landmarks can be located.
- The participant should start out sitting on the exam bed, with the knees flexed to about 90 degrees and the
thighs horizontal.
Script: “I am going to measure your right thigh circumference. In order to do that I first have to mark your thigh with a cosmetic pencil to determine where to do the measurement.”
- Mark the proximal border of the patella (knee cap). To help locate the landmark, ask the participant to straighten their leg to about a 120 degree angle while keeping their heel on the floor with the thigh muscles relaxed.
- Locate the midpoint of the inguinal crease (see figure below). This is easily located if the hips are flexed as they will be with the participant seated. To help locate the landmark, ask the participant to lift the thigh about 1 cm. The point where the muscle and tendon contract is the midpoint of the crease.
- With the participant seated and the thigh muscles relaxed, measure the distance between the inguinal crease
and the proximal border of the patella (rounding down to the nearest ¼ inch) and divide
by two to get the midpoint. Mark the location with a grease pencil.

- Ask the participant to stand up and place the heels about 20 cm apart.
- The weight should be evenly distributed over both feet, both feet flat on the floor. If balance is a problem, the participant may hold onto a chair or wall. The examiner should be squatting so that their eyes are at the level of the mid thigh.
- The circumference is measured at the marked midpoint with the measuring tape placed horizontally (that is, perpendicular to the long axis of the thigh) around the thigh. View the thigh from the front and both sides to check this.
- The tape should be in complete contact with the skin, without compressing the soft tissue.
- The midpoint mark should be visible in the gap made by the upper and lower wrap of the tape. Make sure that
the lower edge of the tape at the zero mark sits directly at the top of the midpoint mark. Make sure
that the top edge of the lower wrap of the tape sits directly below the midpoint mark. Read the
value directly below the zero mark (see example in figure on next page).

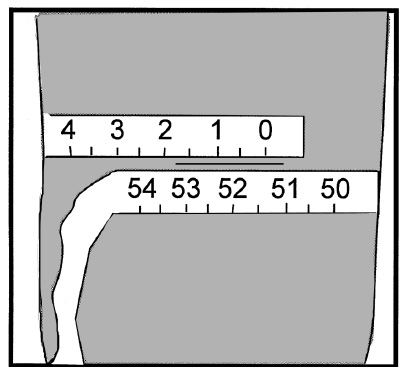
- Record the circumference to the nearest 1/4 inch (rounding down to the nearest 1/4 inch)
The same procedure will be used for off-site visits
- Quality Assurance
Training Requirements:
No special qualifications or experience are required to perform this assessment. Training should include:
- Read and study protocol
- Attend training session on techniques (or observe administration by experienced examiner)
- Practice on volunteers with a special emphasis on obese participants
- Compare measurements with those made by experienced colleagues
- Discuss problems and questions with clinic manager
Ongoing quality control:
- Inter-technician measurements
- Supervisor observations
- QC reports
Information on the Thigh Circumference Measurement found in this section was obtained through:
- Health ABC Operations Manual Vol. III Chapter 2K, pages 1-8 Version 1.0 8/1/02
- Health ABC Operations Manual Vol. III Chapter 3N, pages 1-7 Version 1.2 9/15/97
- Health ABC Study: http://www.grc.nia.nih.gov/branches/ledb/healthabc/index.htm
http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/NCHS/MANUALS/ANTHRO.PDF
Standard ECG & P-Hi Res Protocol
- Ask the participant to lie supine on the examination table.
- Inform them you will be performing an ECG and read the following script:
An ECG is made up of waves showing the electrical activity in different parts of the heart. This new research test is an ECG that looks at a specific wave, the P wave, which shows activity in the top part of the heart. In order to get an accurate test please try to lie still. The test takes approximately 10 minutes.
- Tell the participant you will be placing electrodes on their arms, legs, chest and back. Inform them you will be cleaning those areas with alcohol wipes as well as making marks on their chest with a cosmetic pencil.
- Before electrodes are placed on the participant, ask if he/she is known to be allergic to alcohol wipes. If yes, prepare the areas of electrode placement by rubbing with water and drying with a washcloth. If allergies are denied, prepare areas V1, E, V2-V6, RL, RA, LL, LA, and I by wiping with a Tens Cote Cleaner. Let dry.
- V2: The first intercostal space is palpated just below the clavicle. Count down and identify the 4th intercostal space just below the fourth rib. Point V2 is just to the left of the sternum in the fourth intercostal space. Make a small line with a marking pencil here to show where the ECG lead should be placed.
- V1: Is at the same level as Point V2 and immediately to the right of the sternum. Make a small line with a marking pencil to show where the ECG lead should be placed.
- E: To locate the horizontal reference level for electrodes (Point E), starting from V2, locate the fifth intercostal
space. Move your finger in the 5th intercostal space laterally to where the midclavicular (center of the chest where you feel a
bend in the clavicle) line intersects the fifth intercostal space. Make a horizontal line at this point.
Mark the exact transverse (horizontal) level at this spot with the midsternal line. It should be about one inch (1”) below V1 and V2 placements. This is point E.
- V6: Move the participant’s left elbow laterally away from the body. Mark the midaxillary line in the exact vertical center plane of the thorax down to the intersection of the horizontal plane marked by the location of E. This is the exact location of V6. (NOTE: It is a common mistake to locate the midaxillary line too far anteriorly, toward the V5 location).
- V4: Place the # arm of the Heart Square firmly across the lower sternum at the level of Point E (as you face the
participant, the writing on the Heart Square will appear upside down and backwards). Adjust the E and V6 arms of the Heart Square
so they are both perpendicular to the long axis of the thoracic spine at the level of the E position. The E arm should be exactly
horizontal. If the participant is lying flat, the V6 arm should be exactly vertical.
Slide the V6 arm so the 0 point (the arrow labeled V6) is at the marked location for V6. Double check that the E arm is still in the correct spot.
On the V6 arm (the slide) find the number corresponding to the E measurement. Following the corresponding 45 degree line to the surface (e.g. 16) and mark the location following the inside of the square. This is point V4. Make mark on TOP of the breast. Note: For women with smaller breasts the measurements may land directly below the breast tissue.
The participant may now place their left arm in a more comfortable position next to their body.
- V3: Exactly halfway between V2 and V4.
- V5: Exactly halfway between V4 and V6.
- I: While standing on the participant’s right side move the participant’s right elbow laterally away from the body.
Mark the midaxillary line in the exact vertical center plane of the thorax down to the intersection of the horizontal plane marked
by the location of E. This is the exact location of I.
The participant may now place their right arm in a more comfortable position next to their body.
- Place the electrodes on the participant in the following order: LA, LL, RA, RL, V1, E, V2-V6, I, H, M. The body of the
electrode is placed centrally at the pencil mark with the tab extending downward on the body.
Leg leads should be placed on the calf midway between the knee and ankle. If the participant has an amputation, the lead should be placed above this. The limb leads are placed on the body so the silver tab is facing outward.
- Place the Acquisition Module on the participants lap and ask them to hold it. Separate the leadwires. While holding wires 1 and 4 (the two longest wires) ask the participant to sit up. Move robe to expose the participant’s back. Be sure to keep the participants chest as covered as possible
- Ask the participant to sit up straight and prepare the skin by cleaning areas H and M with a Tens Cote Cleaner.
- Place electrode H on the back of neck tab facing downward, on the bony prominence. If necessary, have the participant put his/her head forward to identify the bony prominence
- Place electrode M at the center of spine tab facing downward, on the same horizontal level as lead E.
- Connect leads H and M
- Bring the wires over the participants left shoulder and ask the participant to lie down. Make sure the lead connections are not disrupted.
- The remaining leads should be connected in the following order: LA, LL, RA, RL, V1-V6, E, I

- Ask the participant to lie still and relax. In the computer, enter the participants Name, ID, Age, Height, Weight, and Gender. Enter the
Exam Cycle, Location (1=clinic) and Technician ID Number.
Note: When entering Height and Weight round up if the weight is ≥.5 or ≥ .50 for height.
- Double check that all leads are connected properly.
- Offer to cover the participant with a blanket or their robe. Note: If the participant is cold this could interfere with the quality of the ECG.
- When a quality ECG is displayed on the computer screen hit ECG to store.
- The ECG is reviewed and printed for errors
- Verify ECG Data Storage to Mac 5000
- Press File Manager
- Highlight the Participants Name
- Press Display
- View and Verify the Participants Name and ECG Recording
- If ECG needs to be run at 5 mmHg because of high voltage (if the standard 10 mmHg is beyond the lines of the ECG paper), highlight (yellow or orange highlighter) the 5 mmHg on the bottom of the printed ECG. On the top margin of the tracing write “1/2 STANDARD” using a bold magic marker.
- After the completion of the standard ECG again ask the participant to lay quietly and still during the acquisition of signal to decrease noise. Turn off the lights.
- Begin the ECG P Hi Res by following the steps below in the computer:
Hit Main → More → More (to get to P Hi-Res). Do NOT select Hi-Res.
Same patient
Press TEMPLATE
Computer responds that it is acquiring template data and prints it out.
Press AVERAGE
After 250 beats have been averaged the acquisition will end.
Press STORE - Leads are checked again for proper placement and disconnected after all leads are checked. Electrodes are carefully removed.
- Click back on the Main Menu and next on Resting ECG. When it asks you if you want to use the information for the last participant hit no. The machine will be ready for the next participant.
- Stamp both standard ECG and P Hi-Res ECG with #2.
- Place standard ECG in participant’s file for MD to review during exam.
- Place paper copy of P Hi-Res ECG in folder in room.
P-Wave Signal Averaged Electrocardiogram Notes:
Parameters: Keep on 250 qualified beats.
During testing keep noise level less than 1 mv (if possible) it is normal for the noise to start out high and then diminish during the averaging process.
Do not change the QRS complexes settings.
When and how to abort test:
A decision not to complete the test may be made when a participant has:
- 1=atrial fibrillation
- 2=paced rhythm
- 3=frequent PVCs or APCs
- 4=tremor
If the P Hi-Res ECG runs more than 10 minutes abort the test.
Computer instructions to abort test:
- Patient data
- Template
- Prints it
- Average
- Press “Pause” to abort test.
- STORE
If you press Pause by mistake, then press Resume.
Troubleshooting Tips
| Problems | Potential Causes | Approaches |
|---|---|---|
| Noisy signal | Cream on the body | Wash with washcloth |
| Hair on body | Separate hair | |
| Problem with leads | Check procedure & lead placement | |
| Cold; tremulous | Place blanket | |
| Feet dangling | Feet all the way on table | |
| Talking patient | Ask patient to lie still and not talk | |
| Environmental noise | Try to turn off the lights | |
| Doppler’s off | Check Doppler |
Adapted from Clinical Education and Development. MAC 5000 Resting ECG Analysis System Clinical Reference Guide, 030-CARD-MAC5000MT, Part No. 2015231-006
Exit Interview Procedures
The float staff member’s responsibility:
- Put the chart in order & check the chart for completeness.
- Using the Procedure Sheet on the back of the Numerical Data Sheet confirm that everything has been completed.
- Complete the top portion of the Procedure Sheet by filling in the number 1 when something has been done.
- If anything is missing flag the chart and make sure the procedure is completed prior to the participant having an exit interview.
- Put the Numerical Data Sheet and the Referral Tracking Form sticking sideways out of the chart in the correct order and obtain a button.
During the exit interview (all staff):
- Check the referral tracking sheet (complete with your ID number and any adverse events in clinic) and review with the participant any referral recommendations.
- Confirm with the participant that they have completed their Food Frequency Questionnaire and have given it to a FHS staff member.
- Ask for feedback from the participant on how they felt about their examination.
- Write in any comments that are made.
- Make sure the participant leaves the clinic area with all of their belongings; ESPECIALLY THEIR MEDICATION BAG WITH MEDICATIONS.
- Thank the participant for their time and willingness to participate.
- Remember that this is a very short Interview and should go no longer than 5 minutes.
NOTE: The chart does not have to be put in order to do an exit interview. If the clinic is busy and someone is unable to put it in order prior to the exit interview proceed as follows:
- Take the Numerical Data Sheet, Questionnaire packet & Referral tracking sheet in with you during the exit interview.
- Review with the participant that each procedure has been completed.
- Complete the top portion of the Procedure Sheet by filling in the number 1 when something has been done.
- Check the referral tracking sheet (complete with your ID number and any adverse events in clinic) and review with the participant any referral recommendations.
- Confirm with the participant that they have completed their Food Frequency Questionnaire and have given it to a FHS staff member.
- Ask for feedback from the participant on how they felt about their examination.
- Write in any comments that are made.
- Make sure the participant leaves the clinic area with all of their belongings; ESPECIALLY THEIR MEDICATION BAG WITH MEDICATIONS.
- Thank the participant for their time and willingness to participate
- Put the chart in order before it goes upstairs
COMMONLY ASKED QUESTIONS
Q. When will you call me back for my next exam?
A. We can’t say for sure right now. Investigators will begin planning for future exams as our current research contract with NHLBI is
completed.
Q. What will be in my report and when will I get it?
A. You will receive your report in roughly 4-6 weeks. Your report will have results of your blood work, your blood pressures, and a general
statement from the physician who saw you.
Chart Completion
After the clinic staff is finished with the participant’s charts for the day the charts are sent upstairs to the Statistical Technician. The Statistical Technician then proceeds as follows:
- The physician’s letter and the ankle arm blood pressure is typed.
- The consent form, cancer pages, the ECG, and the participant’s letter are photocopied.
- The exam is reviewed for any blanks or inconsistencies.
- Events are flagged for Medical Records.
- After editing is complete the chart waits for Dr. or Dr. to sign the physician letter.
- Once the letter is signed, the chart is put in a file drawer to wait for the lab results, PFT, and ECG card.
- Once all results are received, the physician letter with lab results, PFT, ECG, and ankle arm blood pressure, and the participant’s letter with lab results and ECG card are mailed.
- After the mailing, the chart goes to keying.
- Once the chart has been double keyed, it returns to the Statistical Technician to check off that it has been keyed.
- It then goes off to Medical Records for processing.
Instruction Manual: RESPIRONICS Actical Physical Activity Monitoring System
Instruction manual omitted -- Copyright © 2008 Respironics, Inc. and its affiliates. All rights reserved.
Actical Protocol (Use, Download and Creation of Data Sets)
06/10/08
CLINIC PROCEDURES
Identify Participants
- The clinic schedule lists the participants for following week.
- From the schedule determine number of clinic participants per day.
- Organize Actical devices into the corresponding number of piles per day.
- Assign an Actical device to each participant.
- Enter device serial number and id into the log book in the order of the pile.
- Label each pile with day of week
- Place devices into the blue bin located in room.
- DM gives clinic ID stickers on Friday, for the following week.
- For each participant ID, place one ID sticker on the device case and another in the log book.
Return Envelopes
- Envelopes are located across from the supply cabinet up stairs.
- Prepare the return envelopes the week before they are needed and include:
- Bubble wrap pouch
- Feedback form (Appendix D – Feedback Form.doc)
- Place envelope in room.
Initializing Device
- Each morning, take out the pile for that day from the blue bin.
- Initialize device during clinic (See Actical Software Protocol below).
- Initialize by entering the participants ID, Age, Sex, Height, Weight, Step Box will be checked, Epoch length set to .50, Start Time will be set to 15:00, and Start Date will be the date of exam.
- For participants who want a delay start, there is only a 4 day window at this time (Note the deviation on log sheet).
Mailing Device - Delayed Start
- Clinic staff to initialize the device at 15:00, the day the device is mailed out.
- Activation date is noted in the log book.
- Participant is instructed to press the event button at the time that the device is first worn.
Actical Software Protocol- Initialization
- Open the Actical software.
- Turn on the ActiReader (power indicated by red flashing light).
- From the main Actical window select 'File > Write'.
- At this point, a new window will open.
- Insert the 'Subject Identity'.
- Insert a Subject Age (can be any number and then modified at a later time).
- ‘Check’ the box for Step Counting as a data point (right side of the window).
- ‘Click’ inside the box to select the Epoch Length. You will need to select 0.50 (which is 30 seconds) to allow for data collection to be possible for up to 11 days. Be sure the data collection time-frame indicates 11 days. If not, 'click through' the epoch choices until you are back to 0.50. (As a side note, I let our software engineering team know that this needs to be addressed).
- If you would like to select a delayed start time, you can select that date and time by moving the date/time bars, clicking on the hours or clicking inside the bar area. That is your choice.
- Review all of the information you have selected for accuracy.
- Click the box ‘Send’ to send the data collection information to the Actical.
Distributing Device to Participant
- Ask participant if they want to wear the device for 8 days.
- If ‘yes’, explain the device using a formalized script (see Appendix A - Actical Script).
- Demonstrate how to wear the device.
- Provide feedback to the participant as they practices putting on the monitor.
- Log book checks and entries: (Note: The technician in charge of the participant is responsible for the following):
- Check the device number matches the ID.
- Writes the ID number in column ‘Tech ID ACC Hand Out’.
- If activation date is different from clinic date, enter the date in log book column ‘Date Activated if differs from Clinic mm/dd/yy’, otherwise leave blank.
- Ask if the participant will be wearing the device in their home community or outside their community and enter into column ‘Home (Yes) or Non-home (No)’.
- Write the log sheet observation number “OBS” on the return envelope and give it to the participant.
- Give the participant written instructions to take home (Appendix B – Device Instructions and Appendix C – Device Position).
- Explain instructions to them before they leave.
Return of Envelope – Device and Feedback Form
- The participant returns the device in the envelope provided.
- Front desk staff place return envelopes in black bin located in room 114.
- Check the envelope for the feedback form (Appendix D – Feedback Form.doc)
- Note in the comment section of the log book:
- No form – leave blank
- Negative comment – enter 0
- Positive comment – enter 1
- Other comments – enter 2
- Place feedback forms in ID order in folder marked FEEDBACK.
- Data Management will track this for the OSMB etc.
Download Data
- Download data to the following directory:
Process Complete
- Assigned technician will download the data.
- Recheck ID, height, weight, age, and gender for accuracy.
- After downloading:
- Check off the participant in the margin of the log book with a highlighter
- Enter the download date.
- Enter your TECH ID number in logbook column ‘Tech ID Downld Act.’.
Actical Software Protocol - Downloading
- Open the Actical software.
- Turn on the ActiReader (power indicated by red flashing light).
- From the main Actical window select 'Reader > Read'.
- At this point, a new window will open.
- Click ‘OK’ to start Reading.
- The data download will be shown by the red progress bar at the bottom of the window.
- A message is displayed to tell you when the download is complete.
- Take the Actical off the reader then click ‘Yes’ to Save Data.
- Saved file is automatic idtype-id.awc.
Export Statistics for Energy Expenditure
- Open Actical software
- Chose batch or individual download.
- Choose type of statistics i.e. _A_Stats.csv , W_Stats.csv etc.
- Save files to :
Export
- After imports into SAS move files to:
- Move downloaded AWC files into the following directory:
From:
To:
After Downloading
- Place device back in the box for reuse.
- Place belts in the laundry bind for cleaning.
Output Statistics and .CSV Files (Upgrade expected around 2008)
ASK JM AND VR WHAT TYPE OF DATA THEY WANT TO DOWNLOAD.
- Open Actical software.
- Select statistics you want to down load.
- Save all data to .csv file i.e. idtype-id_A_Stats.csv.
- Save whole data to .csv file i.e. idtype-id_W_Stats.csv.
Electronic Logsheet
- The electronic log file is located in the following directory:
.
- Designated clinic personnel, enter data from the log book into the electronic log i.e. ID sticker, serial number, date activated, and where they will be wearing the device.
- Save electronic file ACTICALMMYY.xls’.
- Each month, save the cumulative file as the new month and place the old file in ‘RETIRED’ folder.
DATA MANAGEMENT PROCEDURES
Importing Data into SAS
- Actical proprietary software dumps data from device and outputs into various files.
- Files are saved in
- Run programs in
- Create data sets and manuals that correspond to multiple participants with multiple records (WHOLE DATA SET, DAY, HOUR), idtype-id_W_Stats.csv and multiple participants with single records (WHOLE DATA SET) idtype-id_A_Stats.csv.
- Delete redundant variables.
Data Checks
- Check log date, clinic date and Actical date.
- Check ht and wt against Gen3 Exam2 data.
ACTICAL Training - Framingham Heart Study, May 5, 2008
Training slides omitted -- prepared by Respironics
Accelerometer Script:
We are asking everyone in your generation to wear this new device called an accelerometer which will capture you movement. We would like you to wear this device for 8 days, 24 hours a day. It is very small and will be attached to a belt worn around your waist. You may wear the belt over or under your clothing which ever is more comfortable for you. Is this something you are willing to do for the Framingham Heart Study?
(If yes)
Great! I will show you how to wear the belt and then you can put it on for me.
(Demonstrate and use the picture from the handout as a visual then watch them put it on.)
Here is an information sheet for you to take home. The only time you will be taking the belt off is when you shower. The device is waterproof but we are not asking anyone to wear it while showering.
Are you going to be going swimming or doing any water sports while wearing it? (If yes, give a second belt.)
Once you are done wearing the device place it into the return envelope sealed in the bubble bag with the belt.
Please put the device in the mail immediately after taking it off. We need these devices for the next participants that are coming in. Because these devices are expensive, we cannot keep a lot in stock.
Please read the instructions when you get home. There are some common questions on the back of the first page as well. If you have any questions or concerns just call (point out on sheet). Thank you very much.
(If No or hesitant)
Okay, would you be willing to just try it?
If a definite no then write in the Comments box an explanation as to why.
Physical Activity Monitor Instructions
Please read carefully:
- Wear the device for 8 days / 24 hours a day.
- Do NOT wear in the Shower (remember to put the device back on once you get out of the shower).
- Wear the device while sleeping.
- The device will be worn over the right hip resting on the iliac crest (Do NOT change sides).
- The device should be snug against the body but not tight.
- It can be worn underneath or on top of clothing.
- You may engage in activities that you normally do.
- Please return device and belt in the bubble pouch located inside the white return folder and write us any feedback you may have about your experience wearing the device.
You may take off the activity monitor and send it back in the return envelope given to you at your exam on: _______________
(Take off around 3 p.m.)
PLEASE RETURN THE DEVICE EVEN IF YOU ARE NOT ABLE TO WEAR IT FOR 8 DAYS. THE DEVICES ARE EXPENSIVE & FAILURE TO RETURN THE DEVICE MAY PROHIBIT FHS FROM COMPLETING THIS STUDY. AS ALWAYS, WE THANK YOU FOR YOUR PARTICIPATION.
FAQ
| 1. | What is the Actical physical activity monitor? The Actical is a small, waterproof device that measures activity. The Actical can not tell exactly what you are doing, just whether you are moving or are still. The Actical will also count the number of steps you take each day. |
| 2. | Is there anything I can not do while wearing the Actical? The Actical device is completely waterproof, so you can swim with the device. If you are planning a CT scan, MRI, or similar test, you may be instructed to remove the Actical for the scan. If left on, it will not harm you. You have no limitation of activity with the Actical device that you would not otherwise have. The Actical device will pass through airport security undetected. |
| 3. | Do I need to do anything to the Actical while I am wearing it? You do not need to do anything – just wear it and forget it. The Actical can not ‘turn off,’ so there is no need to worry that it is not capturing your activity. |
| 5. | Will I be getting any results from wearing the accelerometer? Yes, you will receive a report on the results of your activity. |
| 4. | Who do I contact if I have a question about the device I am wearing? For any questions you may contact: |
Actical® Instruction Manual
The belt should be mounted on the body so that the Actical device rests on the iliac crest of the hip. The iliac crest is the uppermost and widest of the three bones constituting either of the lateral halves of the pelvis.
Proper hip mount

Accelerometer feedback forms
If you have any feedback about the accelerometer, please note it below and return it with your accelerometer.
Thank you again for your participation.
Office Use: ID _____ - __________________
Initials:______________ Date Initialed: _______/___________/__________ Version:061708
If you have any feedback about the accelerometer, please note it below and return it with your accelerometer.
Thank you again for your participation.
Office Use: ID _____ - __________________
Initials:______________ Date Initialed: _______/___________/__________ Version:061708
Pulmonary Function Test Manual of Procedures
FVC chart
"Standardisation of spirometry"
Publication omitted
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005 Aug;26(2):319-38. [PubMed ID: 16055882]
Spirometry Quality Control Program
Spirometry Quality Control Program
FHS Third Generation Cohort - Examination 2
Review as of March 29, 2010
I. Overview
All qualified participants undergo baseline (pre-albuterol) spirometry, unless they are excluded on the basis of medical exclusion criterion or decline to participate. A limited number of these participants, i.e. those meeting criteria for a low FEV1 % predicted or low FEV1-to-FVC ratio % prediocted, also undergo post-albuterol spirometry to assess bronchodilator response and postbronchodilator lung function. The pre-albuterol data and the post-albuterol data are subjected to separate QC analyses.
II. Spirometry quality goals for QC analysis
The goal for every spirometry test session is to meet the American Thoracic Society (ATS) standard (Miller et al., Eur Respir J 2005) of obtaining at least three acceptable maneuvers that meet reproducibility goals. It should be noted that acceptability is an attribute of an individual spirometry maneuver whereas reproducibility is an attribute of an entire test session of multiple maneuvers.
Maneuver Acceptability Criteria:
An individual spirometry maneuver is considered acceptable if it meets to following criteria: Criteria set 1: Extrapolated Volume (EV) is less or equal to 0.15 L, total expiratory time (TET) is greater than 6 seconds, and ATS end-of-test (EOT) criterion is met (i.e. no change in expiratory volume (< 0.025) for ≥ 1 second). If TET exceeds 12 seconds, then the EOT criterion need not be met, in accordance with ATS guidelines to avoid multiple prolonged exhalations that may cause light headedness, syncope, undue fatigue and unnecessary discomfort.
Criteria set 2: Extrapolated Volume (EV) is less or equal to 5% of FVC, with TET and EOT criteria identical to criteria set 1.
Reproducibility Criteria:
Only maneuvers that are acceptable are considered in determining whether ATS reproducibility goals have been met.
A test session is considered to meet the ATS reproducibility goal if it meet the following criteria:
- At least three acceptable maneuvers obtained
- Among acceptable maneuvers, the highest forced vital capacity (FVC) and next highest FVC differ by 0.15 L or less
- Among acceptable maneuvers, the highest forced expiratory volume in one second (FEV1) and next highest FEV1 differ by 0.15 L or less
Summary:
To summarize, a test session is consider to meet the ATS standard if:
- Three acceptable maneuvers are obtained by Criteria Set 1 or Criteria Set 2, and
- Reproduciblity goals are met for both FVC and FEV1.
III. QC Program
Ms. Sims periodically does a QC analysis, calculating the number of spirometry test sessions that do and do not meet ATS criteria during a particular time window. These analyses include a breakdown stratified by technician. For each technician, the analysis shows the overall percentage of studies meeting ATS standards and also identifies the individual subjects studied by each technician that did not meet ATS standards. Each technician is then instructed to review each of their test sessions that did not meet ATS standard (the results of which they are able to visualize on the computer interfaced to the lung function system) and to determine what problem of participant and/or technician performance led to the failure to meet the standard. After these reviews have been completed, the technicians and Dr. O’Connor have a group meeting at which all cases of failure to meet ATS standard are reviewed. Dr. O’Connor and the group discuss how best to avoid a recurrence of any technician errors that are identified and how to enhance coaching to avoid problems in participant performance that are identified as having caused the test session to not meet the ATS standard.
While it is impossible for every test to meet ATS standards (for example, in the case of participants that perform one or two unacceptable maneuvers, find it taxing, and then refuse to continue with the testing), our goal is to achieve the highest rate possible of participants meeting the ATS standard.
IV. Rounding error issue in relation to reproducibility criteria
A limitation of our Collins CPL system is that during testing the FEV1 and FVC values are only displayed to two decimal places, e.g. an FEV1 of 3.21 L. The system does not automatically calculate and display the reproducibility of acceptable maneuvers, so our technicians subtract the highest FEV1 from the second highest to determine if reproducibility within 0.15 L has been met. While this generally works very well, we have discovered that in a small percent of cases a difference that appears to be 0.15 is actually slight above 0.15 (e.g. 0.156) when computer calculations are done that use the data for FVC and FEV1 that are measured to three decimal places. Rather than subject our participants to the extra burden of requiring reproducibility with 0.14 L (which would require additional maneuvers in many cases), we are considering these cases (less than 1% of participants tested) to meet reproducibility criteria. This will have negligible impact on the quality our data.
"Spirometric Reference Values from a Sample of the General U.S. Population"
Publication omitted
Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999 Jan;159(1):179-87. [PubMed ID: 9872837]
| Overview & Glossary of Terms | Staff Training | Subject Selection | Protocol Summary |
| Equipment & Tech Support | Supply List | Equipment procedures & Calibration | Participant Testing |
| Data Procedures | Supervisor Checklists | Data Back-up | Equipment Maint. Schedule |
| Appendix / Tracking Forms | Standardisation of Spirometry | J. Hankinson Reference Values | CPL Contract |
| Collins Instruction Manual |
PFT DAILY CALIBRATION
Read all prompts
- Minimize RaptorPlus (Patient Information)
- Shortcut to CPL
- Component = CPL
- Balloon check: check all boxes and click on Inflate
- Deflate
LEAK TEST
Put round weight on bell
- Leak Test
- Component = CPL
- S Delay = 20
- Duration = 60
- Start
- < -10 is ok. CANNOT HAVE ≥ -10. IF DO, REDO CALIBRATION
TOOLS (under RaptorPlus)
- (Follow prompts)
- Calibration
- *CPL (click on “+”)
- Barometric pressure (highlight and make drop-downs visible)
- Calibrate
- Calibrate
- Temp (leave temp that is shown on screen = machine temp)
- Enter
- Barometric pressure (leave barometric press that is shown on screen)
- Enter
- Continue
SPIROMETER
- Calibrate
- Calibrate (bell goes up)
- Attach syringe
- Press space bar
- Pull out syringe at constant pace
- Press space bar
- Push in at constant pace
- Press space bar
- All lines should have a green square = Valid. If not, repeat calibration
- Continue
PNEUMOTAC
- Calibrate
- Calibrate
- Continue
- Continue
- Press space bar
- Pull out syringe
- Press space bar
- Press space bar
- Push in syringe (push in before “4”)
- Space bar
- Continue
- Verify
- Close
Take off syringe
DL GAS ANALYZER
- Calibrate
- Next (all must be “Valid” in green)
- Next
- If “Failed” repeat DL Gas calibration ONLY
- If “Passed” press Finish
REPORT
- Check all boxes EXCEPT MOUTH PRESSURE
- Write initials at bottom of report
- Put printouts in white binder on low shelf in front of scale
END
- Minimize RaptorPlus
- Shortcut to CPL
- Component = CPL
- Close
- Enlarge Raptor
****On Fridays, please switch Collins to Nahanes (see instructions on wall below bulletin board)
Manual of Procedures for Pulmonary Function Testing – Generation 3 Cohort, Exam 2
FRAMINGHAM HEART STUDY
Pulmonary Function Testing
MANUAL OF PROCEDURES FOR GENERATION 3 EXAMINATION 2
Date: 4/1/09
1. Overview
Participants have undergone spirometry, which measures the ability to force air out of the lungs, at each exam cycle since the earliest days of the Original Cohort. Measurement of diffusion capacity, a measure of the lung’s ability to exchange oxygen and carbon dioxide, began with the first examination cycle of Generation 3 (Gen3).
A limited number of participants in each of the cohorts of the Framingham Heart Study will be undergoing post-bronchodilator spirometry, in addition to the pulmonary function testing that all participants undergo. Selection of participants to undergo postbronchodilator testing is based on evidence of airflow obstruction and will help discriminate between participants with reversible airflow obstruction (i.e., asthma) and those with fixed disease (i.e., chronic obstructive pulmonary disease).
For those undergoing post-bronchodilator testing, the time spent in the Pulmonary Function Testing station will be somewhat longer, as a result of the additional spirometry testing and additional time needed to allow onset of medication effect. Subjects not performing post-bronchodilator spirometry will proceed through the station as follows:
- spirometry
- diffusion effort #1
- questionnaire
- diffusion effort #2. At least 4 minutes should pass between diffusion maneuvers
Subjects performing post-bronchodilator spirometry will proceed through the station as follows:
- spirometry
- diffusion effort #1
- questionnaire
- diffusion effort #2 (at least 4 minutes should pass between diffusion maneuvers)
- completion of all remaining exam components
- administration of albuterol (at a time that ensures that post-bronchodilator spirometry will be able to be performed at least 10 minutes and no more than 15 minutes after administration)
- post-bronchodilator spirometry
The timeline below summarizes the time-lines for these two alternative test protocols:
| Start | 10 minutes | 15 minutes | 20 minutes | 25 minutes | 40 minutes | 50 minutes | |
|---|---|---|---|---|---|---|---|
| Those doing only prebronchodilator spirometry and diffusion | Prebronchodilator spirometry | First Diffusion Capacity | Respiratory Questionnaire | Second Diffusion Capacity | |||
| Those doing pre, post bronchodilator spirometry and diffusion | Prebronchodilator spirometry | First Diffusion Capacity | Respiratory Questionnaire | Second Diffusion Capacity | Bronchodilator Administration | 15 minute wait | Post- Bronchodilator spirometry |
2. Background and glossary of terms
Spirometry records the relationship between the rate at which air can be exhaled and the volume of air exhaled during a breathing maneuver called the FVC maneuver (forced vital capacity maneuver). Some common lung diseases reduce the rate at which air can be exhaled during a FVC maneuver. Such “obstructive” lung diseases include asthma, bronchitis and emphysema. The ratio of FEV1/FVC, a measure of how quickly air can be exhaled, is reduced in obstructive lung diseases (e.g. asthma and chronic obstructive lung disease) but may be normal in other conditions that reduce both FEV1 and FVC to the same degree (e.g. surgical removal of part of a lung, pulmonary fibrosis, or severe obesity).
A number of key terms are defined below:
- FEV1 is the Forced Expiratory Volume in one second, i.e. the total amount of air that a person can blow out in one second during a maximal forced exhalation. It is reduced in many conditions that cause impairment of lung function.
- FVC is the Forced Vital Capacity, the total volume of air that can be exhaled forcefully and as rapidly as possible from the point of maximal inhalation (lungs as full as possible) to the point of maximal exhalation (lungs as empty as possible). The subject takes as deep a breath as possible and then quickly exhales as much air as possible.
- FVC maneuver is the maneuver of exhaling as forcefully and as rapidly as possible from the point of maximal inhalation (lungs as full as possible) to the point of maximal exhalation (lungs as empty as possible).
- FEV1/FVC RATIO is the ratio of the FEV1 divided by the FVC. It is the proportion of total volume of air exhaled in an FVC maneuver that is exhaled in the first second of the maneuver. It is reduced in obstructive lung diseases such as asthma and chronic obstructive lung disease.
- PEF (or PEFR) is the Peak Expiratory Flow Rate, i.e. the highest flow measured during the FVC maneuver.
- PRED: is short for the “predicted value” of a pulmonary function parameter. It is determined on the basis of gender, age, and height from a regression equation derived from a large population study of apparently healthy people who have never smoked tobacco.
- PERCENT PREDICTED is the value of a given pulmonary function measurement, such as FEV1, when as expressed as a percentage of the value that would be predicted for a healthy never-smoker of the same gender, age, and height as the participant. For example, an FEV1 of 50 percent predicted means that the participant’s FEV1 is only 50 percent of what would be predicted for never-smokers of the same gender, age, and height.
- BACK EXTRAPOLATION: is the standard method used to determine “time zero” when
measuring the FEV1. The amount of slowly exhaled volume at the start of the maneuver excluded from the FEV1 by this
technique is called the back extrapolated volume (BEV or EV). The BEV is calculated as shown in the figure below:

http://erj.ersjournals.com/cgi/content/full/26/2/319/F2
The BEV should be less than 5% of the FVC or 0.15 L, whichever is greater, otherwise the maneuver is considered to have started too slowly.
- DIFFUSION CAPACITY OF CARBON MONOXIDE: is a measure of the lung’s ability to transfer gas into the bloodstream (volume of gas (carbon monoxide) transferred per minute per mmHg of mean pressure gradient). This volume is derived using the total lung capacity derived from a single breath dilution of an inert tracer gas (He, or CH4).
3. Staff training requirements
- Training requirements for new staff
- Read and understand the manual of procedures.
- Undergo pulmonary function testing.
- Training session with the physician investigator overseeing lung function testing at FHS.
- Perform unprompted pulmonary function testing on 5 non-participant volunteers while observed by certified technician.
- Perform pulmonary function testing on 5 FHS participants while supervised by certified technician.
- Perform unprompted pulmonary function testing on 1 non-participant volunteer while observed by the physician investigator overseeing lung function testing at FHS.
- Complete written quiz (and review of correct responses) administered by physician investigator overseeing lung function testing at FHS.
- Training requirements for staff previously certified to perform pulmonary function testing at FHS
- Read and understand the manual of procedures.
- Training session with the physician investigator overseeing lung function testing at FHS.
- Perform unprompted pulmonary function testing on 1 non-participant volunteer while observed physician investigator overseeing lung function testing at FHS.
- Complete written quiz (and review of correct responses) administered by physician investigator overseeing lung function testing at FHS.
4. Subject selection for bronchodilator testing
As noted above, in addition to the routine spirometry and diffusion capacity measurements obtained on all participants, some participants will have spirometry measured after inhaling a medication that may relax the airways of those with airflow obstruction. This will help discriminate between participants with reversible airflow obstruction (i.e., asthma) and those with fixed disease (i.e., chronic obstructive pulmonary disease).
All participants whose current pre-bronchodilator spirometry has EITHER a FEV1-to- FVC ratio less than 90 % of the predicted value OR a FEV1 less than 85 % of the predicted value will be asked to undergo post-bronchodilator testing.
The % predicted values used to assess eligibility for post-bronchodilator testing are those that are calculated by the Collins CPL pulmonary function system using the NHANES III prediction equations (Hankinson 1999). The NHANES III-Caucasian prediction equations should be used for Caucasian participants, i.e. for most Gen 3 participants. For any participants who report their race as black or African American, the NHANES III African-American equations should be used.
5. Pulmonary function protocol summary
This section provides of summary of the protocol. Detailed step-by-step instructions for specific procedures are provided in later sections.
- Subjects not undergoing bronchodilator testing
- Pre-bronchodilator spirometry
During the test, participants will be asked to take a deep breath and then to force the air out as hard and fast as possible. The spirometer will measure these maximal flow rates and also the volume of air that has been exhaled at particular time points. As the results the testing assumes that these values are the maximum levels a participant can do, it is imperative that participants are coached to blast the air out of their lungs as hard and fast as possible.
- Diffusion capacity
As mentioned, diffusion capacity measures the lungs ability to exchange oxygen and carbon dioxide. A gas that does not diffuse from the lung into the blood stream (a tracer gas, methane) and carbon monoxide (CO), which is quickly taken up by the blood, are inhaled at trace amounts. Participants will hold their breath for a fixed amount of time (10-12 seconds), and then exhale. The Collins CPL system will then measure the difference between the CO and tracer gas as they are exhaled. This difference is due to the diffusion of CO and, as the time interval is known, we can calculate the rate of transfer. It is important that the participants take a deep breath (at least 90% of their vital capacity). Ideally, at least 2 maneuvers should be performed and should agree within 10%. At least 4 minutes should be allowed between diffusion maneuvers to allow sufficient time for the CO and tracer gas to wash out. If the first two attempts do not produce two acceptable maneuvers with a value for the single-breath diffusion capacity (Dsb) within 10% of the higher value, then a third maneuver should be performed after an additional 4-minute wait.
- Respiratory Questionnaire
Technicians will administer a respiratory questionnaire. The questionnaire will help investigators to understand whether the participant has allergies, asthma, COPD, and other pulmonary diseases. Further, the questionnaire will capture information on recent inhaler use, which may affect the spirometry.
Table 2. Common bronchodilator inhalers, Brand names and Generic namesShort acting Intermediate 4-6 hours 12 hours Drug trade names Proventil, Proair, Ventolin, Maxair, Combivent, Maxair Xopenox, Volmax, Atrovent Serevent, Advair, Foradil, Sybmicort Brovana, Spiriva Generic drug names albuterol, levalbuterol, pirbuterol, iprapropium, salmeterol, fluticasone/salmeterol, formoterol arformoterol, budesonide/formoterol, tiotropium - Pre-bronchodilator spirometry
- Subjects undergoing bronchodilator testing
- Pre-bronchodilator spirometry
- Diffusion capacity
- Respiratory Questionnaire
- Post-bronchodilator spirometry - The post-bronchodilator spirometry should be performed no less than 10 minutes and no more than 15 minutes after administering the albuterol.
6. Pulmonary function equipment and technical support contact information
- nSpire Health 3 Litre Calibration Syringe Model #021156
- nSpire Health Vitalograph Linearity Syringe Serial #CS4769
- Contact person for PFT problems
Somerset Medical
7. Supply list
For use with the Collins Comprehensive Pulmonary Laboratory (CPL)
Collins 2000 Plus/SQL Software version 4.8
And the Hewlett-Packard Deskjet 845c Printer
| Item | Item Number | Vendor | |
|---|---|---|---|
| Lung Diffusion Mix (.3% CO, .3% CH4, 21% O2, BAL N2) Size 200 | Z04NI7852003060 | Salem, NH | |
| APC Smart-UPS Interruptible Power Supply | Mill City Connections | ||
| HP 840c Black Ink Cartridge Cartridge | W.B. Mason | ||
| CPLpf System Catalogue – No. 004010 | |||
| KoKoMo | 810050 | Somerset Medical | |
| Balloon Kit – Set of 4 (B1, B2, B3, B4) | K70085M | Somerset Medical | |
| Balloon Maintenance Kit | K710076 | Somerset Medical | |
| Disposable Hydrous Dessicator Columns | K021501UK | Somerset Medical | |
| PFT Adaptor for CPL | 212147 | Somerset Medical | |
| Roll of 100 Segmented Tubing | 001426 | Cardinal Health | |
| CPL System Catalogue – No. 004000 | |||
| Nafion Tubing | K381248 | Somerset Medical | |
| CPL Absorber PaK Package of 6 | 022556 | Somerset Medical | |
| Balloons w/o rings | K022356 | Somerset Medical | |
8. Pulmonary function equipment daily procedures
- Turn on: Spirometer switch.
- Open valves on gases. Turn counterclockwise all the way to open.
- Calibrate equipment.
- Print calibration report for log record.
- Date log book and put ID stickers for each expected participant.
- Record any/all comments if issues arise or if participant refuses test or has to stop test.
- Shutdown spirometer at the end of testing for the day.
- Close the gas valves clockwise all the way everyday after testing is done.
Note: the Spirometry computer should NOT be turned off at the end of the day.
9. Pulmonary function equipment calibration protocol
Read all prompts
- Minimize Raptor (Patient Information)
- Shortcut to CPL
- Component = CPL
- Balloon check: check all boxes and click on Inflate
- Deflate
LEAK TEST
Put round weight on bell
- Leak Test
- Component = CPL
- S Delay = 20
- Duration = 60
- Start
- < -10 is ok. CANNOT HAVE ≥ -10. IF DO, REDO CALIBRATION
- (20 ml in 2 min)
DYNAMIC AND PUT PLUG IN (KEEP WEIGHT ON)
- Click on Dynamic
- Keep same times
- Start
- < -10 is ok. CANNOT HAVE ≥ -10. IF DO, REDO CALIBRATION
- Close
- Take off weight and plug
TOOLS (under Raptor/Plus)
- (Follow prompts)
- Calibration
- *CPL (click on “+”)
- Barometric pressure (highlight and make drop-downs visible)
- Calibrate
- Calibrate
- Put in temp (≤ 25????)
- Enter
- Multiply barometric pressure X 25.4 and change number if necessary
- Enter
- Continue
SPIROMETER
- Calibrate
- Calibrate (bell goes up)
- Attach syringe
- Press space bar
- Pull out syringe at constant pace
- Press space bar
- Push in at constant pace
- Press space bar
- Continue
PNEUMOTAC
- Calibrate
- Calibrate
- Continue
- Continue
- Press space bar
- Pull out syringe
- Press space bar
- Press space bar
- Push in syringe (push in before “4”)
- Space bar
- Continue
- Verify
- Close
Take off syringe
DL GAS ANALYZER
- Calibrate
- Next (all must be “Valid” in green)
- Next
- If “Failed” repeat DL Gas calibration ONLY
- If “Passed” press Finish
REPORT
- Check all boxes EXCEPT MOUTH PRESSURE
- Put printouts in black binder on shelf
****On Fridays, please switch Collins to Nahanes (see instructions on wall below bulletin board)
10. Participant testing – detailed procedures
a. Exclusion criteria
Prior to all tests, the technician must ask the participant about pulmonary function testing exclusion criteria:
- Any of the following within 3 months: major surgery (chest, abdominal or brain), oral surgery, a heart attack, a stroke, or an aneurysm of the brain. If the participant has an aneurysm, ask where it is.
- Blood pressure today greater than or equal to 210/110. Check with the MD in clinic for the participant’s blood pressure reading. If the MD has not yet obtained a reading, take the participant’s blood pressure according to FHS protocol. If either the systolic or diastolic exceeds this limit, do not perform the PFT.
- Ask: Do you currently have any limitations on physical activity prescribed by your doctor? If the participant answers yes to this question the technician will discuss the limitation with the clinic physician. If the clinic physician determines the limitation will not be detrimental to the participant’s health if they perform the PFT, the PFT will be performed per protocol. If the limitation puts their health at risk if they perform the PFT the exam will be aborted and notes will be put in the computer.
On the following page is a flyer that should be printed out and placed on the wall at the pulmonary function testing station to remind the technician to ask about these exclusion criteria.
(To be placed in PFT testing room)
IN THE PAST 3 MONTHS HAVE YOU HAD:
- MAJOR SURGERY (Chest, abdominal, or brain, requiring hospitalization)?
- HEART ATTACK
- STROKE
- ANEURYSM OF THE BRAIN
IN THE PAST 1 MONTH HAVE YOU HAD:
- ORAL SURGERY (Surgery requiring tooth extraction, stitches, anything that can cause dry sockets)
DURING TODAY’S CLINIC EXAMINATION:
- BP ≥ 210/110 (Participant’s blood pressure MUST be taken prior to PFT Testing following the FHS protocol)
DO YOU CURRENTLY HAVE ANY LIMITATION ON PHYSICAL ACTIVITY PRESCRIBED BY YOUR DOCTOR? If YES~ STOP & Discuss with clinic MD
Note: Participants with chronic atrial fibrillation should not be excluded unless requested by participant.
b. Entering participant information
From the Windows desktop, double click on the “Plus 2000 Version 4.8” icon. On the Patient Information Screen tool bar click Search. Once the Patient Search box pops up enter the participant’s FHS ID Number and click search. Once the computer finds the participants information it will display on the right side of the search box. Click on the participant’s ID and hit Add to Cache. Close the search box and confirm that this is the correct participant (verify date of birth). Once this is confirmed click New Study. Update the participants Height & Weight and enter the tech number that is performing the test. Once the information card is appropriately completed, click on “Save” which will put the participant’s information in the “Cache” as seen in the navigation bar (top of the screen, next to “Notes”).
- Date - Before you update the information for the New Study, the computer will ask you (in a pop-up screen) for a date of the pulmonary function test- ensure that the date is correct.
- Name – If the participant’s name is not in all capital letters, retype the participant’s name. Enter the participant’s first name, tab to the next field then his last name. Use all capital letters.
- Date of birth -Confirm DOB
- Height - Enter the participant’s measured standing height in inches (including .25- .75)
- Weight – Enter the participant’s weight in pounds
- Gender - Does not need to be changed from exam 1
- Race - Does not need to be changed from exam 1
- Editing - If a mistake was made when entering information, use mouse to move the cursor to the error. Then begin typing the information.
- Saving the information - Once the data is satisfactorily entered, click on “Save.”
c. Spirometry
The technician is the critical part of the pulmonary function testing system, since the technician must guide the participant through breathing maneuvers that are highly dependent on participant effort. The technician must coach the participant to inhale maximally and then to exhale maximally and as rapidly as possible. The technician must also judge the quality of the participant’s effort. To obtain accurate results, the testing must be done in a standardized fashion.
Note: This manual refers to the participant as “he” or “him” for easy reading, although participants will be both male and female.
Position the Participant – Testing should be conducted in the sitting position in a chair without wheels. The participant should sit erect with chin slightly elevated.
Explain the Procedure – The technician should explain that the purpose of the next test is to determine how hard and fast he can exhale air, “Like blowing out dozens of candles on a birthday cake.” The technician should explain that he should take in as deep a breath as possible, and when his lungs are completely full, blow out all the air as hard and fast as possible, until told to stop.
Dentures, if they are loose, should be removed and placed in a clean cup, since they will prevent a tight seal from being formed around the mouthpiece. If dentures are not loose, leave them in place.
Always Demonstrate the Maneuver. The technician should ask the participant to watch them demonstrate the FVC maneuver. Again demonstrate correct placement of the mouthpiece. If the participant does not adjust well to using the mouthpiece (i.e. strong gag reflex) the participant can use just the neck of the filter for a mouthpiece. His lips must remain tightly sealed using this also. The technician should sit up straight. Take a deep breath, throw back their shoulders, and widen their eyes to emphasize the maximal depth of inhalation. Then dramatically BLAST out all of their air as hard and as fast as they can.
The technician’s vigorous demonstration will prevent time and effort from being wasted on unacceptable forced expiratory efforts that result from the participant’s failure to understand a verbal explanation of the procedure.
FVC Maneuver Test Steps
The technician should do the following:
- To begin doing the maneuvers, click on “Go to,” then on “Forced Vital Capacity.” This will bring up the testing page.
- Ensure that the participant has a clean filter and mouthpiece, but do not connect the participant until prompted by the computer. Click on “Start test.”
- The spirometer will fill the bell and prompt the technician- THEN have the participant connect to the mouthpiece and breathe normally.
- Ensure that the participant has a nose clip in place. If the nose clip is uncomfortable for a participant, then instruct the participant to tightly pinch his nostrils shut throughout each maneuver.
- Once the participant is connected to the spirometer, nose clip in place, and is breathing normally, press the space bar. This will prompt the computer to track the regular breathing of the participant.
- Once you are both ready, instruct the participant take in as deep a breath as possible and press the space bar while they are inspiring.
- Coach the participant through the FVC maneuver, encouraging him to blow out as hard as possible for at least 6 seconds (as seen at the red vertical line on the time axis on the screen) and until the red line tracking the participant’s maneuver (on the right hand graph) becomes flat. Watch the participant inspire deeply and then shout “BLAST OUT!!!” Lower your voice a bit and coach the participant by saying “keep going…keep on pushing out all that air…a little bit more…”
- Watch the body language of the participant as he attempts to follow your instructions. Pay attention to him, not the instrument.
- Once he has “pushed” for at least six seconds and the participant tracking line has become flat and the “Good Effort” message appears over graph, push the space bar again to end the test, have the participant come off the mouthpiece, remove the nose clip and breathe normally.
Example Graph of the Spirometry Maneuver:

To summarize the testing process:
- Once the participant is connected to the spirometer with a nose clip on, push the space bar.
- After a couple of normal breaths, have the participant take as deep a breath as possible.
- While the participant is inspiring, press the space bar.
- As soon as the participant has reached maximal inspiration, have him blast out all the air in their lungs.
- Once he has blown out for at least 6 seconds and the graph of his breathing has become flat and you see the “Good Effort” message, push the spacebar to end the test.
The quality of the effort is seen at the top of the right hand graph- the quality is graded on (1) the initial effort (Extrapolated Volume, or EV), (2) flatness of the line or reaching of RV, Residual Volume, (End of Test, as defined by flow of less than 30mL/sec, or EOT), and (3) total expiratory time (TET).
You can repeat testing by starting again (with the participant off the mouthpiece initially) by going back to #2.
If the participant fails to perform the maneuver correctly, again demonstrate both the error and the correct performance yourself. You may have to repeat the demonstration after every maneuver for some participants.
The participant will be coached to perform at least 3 FVC maneuvers and to perform additional maneuvers, up to a maximum of 8, until acceptability and reproducibility goals are met, as described below.
FVC Maneuver Acceptability
According to the ATS standards, the technician should coach every participant to obtain at least three maneuvers that are “acceptable.” FHS uses current ATS standards to determine acceptability. For each FVC maneuver, the Collins CPL system displays a “+” or a “-“ to indicate whether each of three acceptability criteria have been met. These acceptability criteria are:
- Extrapolated volume (EV): “+” = EV less than or equal to 0.15 L
- Total exhalation time (TET): “+” = TET 6 seconds or greater
- End of test criteria (EOT): “+” = expiratory flow is close to zero (less than 30 mL/sec), i.e. the plateau on the volume-time curve appears flat
To be considered acceptable, all three criteria must be met, i.e. three “+” signs (which will appear in green) must appear for the maneuver. If one of more criteria receives a “-“ sign (in which case they will appear in red), the maneuver will be considered unacceptable. There will be two exceptions to this rule:
- Some tall people can meet the alternative EV acceptability criterion of < 5% of FVC but not the criterion of ≤ 0.15 L. If a tall subject consistently does maneuvers that appear to be perfect but have an EV that just over 0.15L but less than 5% of FVC, then these maneuvers will be considered acceptable. This should apply to a small minority of subjects.
- Some people with significant airflow obstruction cannot reach the EOT criterion of a flat plateau on the volume-time curve within a reasonable exhalation time that avoids substantial discomfort and a risk of dizziness or even fainting. For this reason, any FVC maneuver that appears otherwise perfect but fails to reach meet the EOT criterion despite a TET of 12 seconds or greater will be considered acceptable.
Reproducibility goal of the test session
The technician should coach every participant to obtain at least three maneuvers that are “acceptable,” as defined above. Once three acceptable maneuvers have been performed, the technician must assess whether the reproducibility goal has been met. The reproducibility goal has two components:
- The two largest values of FVC must be within 0.150 L of each other
- The two largest values of FEV1 must be within 0.150 L of each other
Note that the largest FVC and the largest FEV1 may come from different maneuvers. If the reproducibility goal is met by the three acceptable maneuvers, then the test session is complete. If the reproducibility goal is not met, then additional maneuvers should be performed, up to a maximum of 8, until the reproducibility goal is met. Remember that ONLY acceptable maneuvers should be considered when assessing whether the reproducibility goal has been met. Also remember that the reproducibility goal requires that the largest two values (not any two values) of FVC or FEV1 be within 0.150 L of each other.
Maximum Number of Maneuvers
Don’t exhaust the participant by asking him to perform more than eight FVC maneuvers. If you haven’t obtained 3 acceptable maneuvers and met the reproducibility goal by the time you have done 8 maneuvers, it is unlikely that you will. Click on “Notes” which will bring you to a screen where you may add comments as to why the participant was not able to successfully complete testing.
Saving the Results
Once you have obtained three acceptable maneuvers and have met the reproducibility goal (or failed to do so after 8 tries and documented the why in the “notes” field), testing is complete. Now click on “Save.”
d. Diffusion capacity
Setting up
After completing the FVC maneuvers-
- Click on “Go to”
- Click on “Diffusion Capacity”
- Click on “START TEST”
Preparing the participant
While the machine prepares, explain to the participant that he will be asked to breathe normally and then to blow all his air out, just like the Vital Capacity maneuver. Once his lungs are as empty as possible, the participant will be asked to breathe in as deeply and quickly as possible and hold his breath for 10-12 seconds. The machine will close a valve, helping him to hold his breath and making it impossible for air to leak out- he will not be able to breathe while on the mouthpiece until the tester tells the participant to blow all his air out for the second time.
Starting the Test
- You will get a series of messages as the machine prepares. The machine includes the volume of the filter in the calculations.
- The computer will then display the following message- “OK.” Click OK and then once the next box pops up, have the participant connect to the mouthpiece. “Press the spacebar when the patient is connected to the mouthpiece and breathing normally.” Ensure that the participant’s lips are tightly sealed around the mouthpiece and that the nose clip is in place. Once the participant is attached and breathing normally, press the spacebar.
- The graph will show the participant’s tidal breathing. Once the participant is comfortable, have him breathe all the way out to Vital Capacity (the point at which the graph of his breathing becomes flat). Coach him, saying “Push, push, push” just as you would for the spirometry.
- Once he has pushed all the air out, press the spacebar and IMMEDIATELY instruct him to take as deep an inspiration as possible. Ideally, the deep inspiration should take one to two seconds.
- Once the graph of his breath has flattened out again at maximal inspiration, tell him to hold his breath.
- Push the “V” key, as soon as his breath has flattened out at maximal inspiration, to close the valve and keep air from escaping. He must hold his breath for 10-12 seconds for the maneuver.
- Once the participant’s graph crosses the vertical line on the screen, IMMEDIATELY instruct him to blow out all the air (if you closed the valve, it will open automatically at 12 seconds), just as though he was performing spirometry.
- Have the participant keep blowing until the red line becomes horizontal.
- Once the red line is horizontal, press the spacebar, ending the test.
- After each diffusion hit save and go back to FVC to deflate the balloons.
To summarize-
- Once in the Diffusion Capacity menu, Click on “Start Test” and prepare the participant
- Once the machine is set up and you clicked ok, ensure that the participant is comfortable on the mouthpiece, with a good seal, and with a nose-clip in place.
- Press the spacebar.
- After several breaths, have the participant blow out all the air he can.
- Once the graph flattens out horizontally, push the spacebar, then IMMEDIATELY have him breathe in as deeply and quickly as possible and hold his breath.
- Once the participant has taken as deep a breath as possible and the graph flattens out again, push the “V” key to keep him from breathing out.
- When the graph of the participant’s breath hold crosses the vertical line, IMMEDIATELY have him blow out all the air he can, much like with the spirometry maneuvers.
- Once the graph flattens out at maximal expiration, push the spacebar, ending the test.
Grading the Test
The screen will change, and the effort is graded at the top of the graph on the left. Three criteria are applied- Start of Test (SOT), Breath holding Time (BHT), and End of Test (EOT). If all three are acceptable, they will be displayed in green. If one criterion is not met, then all three appear in red. The failed criterion will have a (-) sign next to it. Review how to improve this result with the participant.
As with spirometry, maneuvers must be reproducible. For DLCO, two acceptable (all green effort marks) maneuvers must be within 10% of each other (i.e. 10% of the higher value).
Per ATS standards allow 4 minutes between tests. Note that the machine takes several minutes to set up. Use the clock on the computer to time the 4 minutes.
Repeat the maneuver from “Starting the Test” until you have two acceptable and reproducible maneuvers.
Limit the number of attempts for DLCO to 3 per participant.
Example Graph of the DICO Maneuver:

e. Respiratory questionnaire administration
The technician administers this questionnaire to the participant between the first and second diffusion maneuvers. Machine preparation for the second diffusion capacity maneuver should be started before the questionnaire is administered.
The questionnaire must be administered exactly as written. The answers are recorded in numbers, as indicated in the answer keys to the right of the questions. The technician follows the prompts on the questionnaire for the progression to follow, based on ‘Yes’, (“if yes fill in …”) and ‘No’ responses.
If a participant reports that he/she uses an inhaler “as needed” and it was last used more than 48 hours ago, code as 88.
f. Bronchodilator administration and post-bronchodilator spirometry
- Subject selection
The selection of subjects who will undergo bronchodilator administration and postbronchodilator spirometry is described earlier in section 4. Briefly, all participants whose current pre-bronchodilator spirometry has EITHER a FEV1-to-FVC ratio less than 90 % of the predicted value OR a FEV1 less than 85 % of the predicted value will be asked to undergo post-bronchodilator testing.
- Bronchodilator administration
- Albuterol information- We will administer 2 inhalations of albuterol HFA metered-dose inhaler 90 micrograms per actuation. Albuterol is a medication usually used to treat breathing problems like asthma or chronic obstructive pulmonary disease (COPD). The effects of albuterol last 3-4 hours. Participants with a history of adverse reactions to albuterol should not undergo this part of the protocol. At the doses we are using for FHS, only a small minority of participants would be expected to have side effects, and these side effects are listed in the “Consent Form.” The side effects include: lightheadedness, an increase in heart rate (pulse) or symptoms of jitteriness or shakiness (tremors).
- The administration of the albuterol will not take place until the participant has completed all other components of the exam. This will ensure that no other data will be affected by the possible side effects of albuterol.
- The participant will inhale two puffs of albuterol through a spacer. You should allow the participant to breathe normally for about a minute between inhalations, and there should be no less than 10 minutes and no more than 15 minutes between the administration of albuterol and the post-albuterol spirometry.
- Detailed step-by-step protocol for bronchodilator administration:
- Take the cap off the inhaler.
- Shake the inhaler.
- After shaking the inhaler, activate the inhaler in the air to check that it is operating adequately. Do this at arm’s length from the technician and not near a participant, such that neither technician nor participants inhales it.(Note: When a new inhaler is being used for the first time, or if an inhaler has not been used for 2 weeks, activate 4 times before testing the first participant.)
- Attach the inhaler to one end of a tube spacer. (Note: Each tube spacer, which is disposable and for single participant use, is a 6-inch length of segmented tubing cut from a 100-foot roll.)
- Have the participant breathe all the way out in a relaxed manner; there is no need to reach maximal exhalation (residual volume) by forcing out the maximum amount of air possible.
- Insert just the tip of the spacer into the participant’s mouth.
- Have the participant start to take a slow, deep breath in.
- Just as the participant starts to inhale, activate the inhaler once while encouraging the participant to keep inhaling all the way until he/she has reached the point of maximal inhalation (total lung capacity).
- At that point of maximum inhalation, have the participant hold his/her breath for about 10 seconds. After this, instruct the participant to exhale normally and relax.
- Wait 30 seconds and repeat for another inhalation.
- Following the second inhalation of albuterol, allow at least 10 minutes and no more than 15 minutes before doing post-bronchodilator spirometry.
g. Data Procedures after Participant Testing
Inserting Test Comments
At the end of testing, you may wish to insert comments regarding the ability of the participant to comply with testing. This is added at the end of testing, under the “Reports” menu.
- Click on “Notes”
- Type in your comments
- Save & close
“Notes” Option
There is a tab on the upper left portion of the “Patient Information” page. If the technician has a comment regarding a participant that may be helpful for clinical interpretation (e.g. “patient having pain with deep breath due to bruised ribs after fall yesterday”) or for quality review (“e.g. patient refused to go on after two efforts”), then this should be entered under “Technician Notes,” followed by clicking “Save and Exit.”
Examples of notes that should be put in computer:
- Reasons for unacceptable tests
- Reasons for aborted tests
- Refusals
- Physical symptoms affecting performance of PFT
Printing Reports
The PFT report is printed after the test is reviewed and graded by a FHS physician (pulmonologist). After grading the test, this physician will select the “File” tab and click on “Print Report”. The HP Deskjet 845c. is selected and 1 copy is printed.
Log Book
All participants are entered into the “PFT Daily Log, Comment, and Calibration” binder. Enter, by date, each participant name. An FHS generated sticker with the name and ID number can be used. An *A* is placed next to the name and sticker of all albuterol challenge participants (both pre-identified and clinic identified).
Participants Completing the PFT
Once the PFT is done, a white sheet labeled “PFT” is completed by attaching a participant label, writing the date onto the sheet and filing it in the participant’s chart.
Participants Not Having a PFT
Participants not having a PFT during their Clinic visit are also put in the “PFT Daily Log, Comment and Calibration” binder with the reason that the PFT was not done.
A sheet labeled “PARTICIPANT DID NOT HAVE PULMONARY FUNCTION TEST” is completed by selecting the appropriate reason that the participant did not have the PFT. The date and participant label are entered onto the sheet and the sheet is filed in the participant’s chart.
Participants Refusing Bronchodilator Response Testing
Occasionally a participant who is asked to participate in the post-bronchodilator test refuses to do so. This refusal is recorded next to the participant’s identifying sticker in the PFT Daily Log Book and the refusal reason is also noted under “technician notes” in the computer. Before the participant is asked to change and exited from the clinic, a second technician should ask the participant if he/she might be willing to perform the bronchodilator response testing. If the participant refuses a second time, the technician will also add the participant’s sticker, the date and the refusal reason to the sheet titled “ALBUTEROL REFUSALS”.
Participant and MD Notification – PFT
Once a week the FHS pulmonary specialist comes into FHS and interprets with pulmonary function tests performed in clinic. He types a clinical interpretation on the participant’s report and prints and signs one copy. He then delivers them to the Research Clinic Coordinator. The Research Clinic Coordinator makes a copy of each of the reports and gives them to the Statistical Technician.
The Statistical Technician files the original copy in the participant’s chart and the second copy is mailed to the participant’s physician.
Moving PFT studies from one participant ID to another
For incorrectly entered ID numbers:
*Pull up the participant information by using the incorrect ID number and add to cache.
*Then, enter the correct number and fill in all of the correct information. (name, birth date, height, weight, technician number, gender and race.) MAKE SURE TO ENTER THE ACTUAL TEST DATE.
*Click on the ‘Go To’ box. Click on ‘Database Utilities’. Four tabs will display. They are: Export Study, Import Study, Move Study and Predicted.
*Click on ‘Move Study’. There are 2 large boxes on this screen. On the left side you need to enter the ID number of the study that you want to move by clicking on ‘Search’ at the bottom and entering the number in the box that pops up. (This would be the incorrect number. If you don’t have it, click on the same ‘Search’ button at the bottom of the left side and do a search.)
*When the correct name appears, double click on it and then select the correct test date. All of the tests dates for a participant, if there are more than one, appear under the name after you double click.
*Then go over to the right side of screen and search for the new (correct) number. Highlight the name when it appears. Don’t double click.
*When you see the correct name, go to the column in-between the left and right sides. There is an arrow in a box in this space. Click on it. A message box appears asking you if you really want to transfer the test from one number to the other number. You have a chance to double check your information here. If all info is now correct, click on ‘Yes’. You will see a message that this move was successful.
*If you then do a “Search” again on the studies you moved, you will notice that there will be one with the correct time and date and one with just the correct date. The one with only the correct date does not contain any test data. To delete this date from the cache (which is just for housekeeping purposes) click on “Tools”, then Cache Management, then tab “FHS-DT-PFS101”. Scroll to the correct participant’s number and you can delete the item with JUST THE DATE. Do not delete the item with the correct date AND TIME. This is the actual test data.
11. Quality control observations of pulmonary function technicians by supervisor
Every three months, the clinic supervisor will observe each technician conduct a full pulmonary function test session on a participant. The supervisor will complete the following checklist for each observed session. Results will be reviewed with the technician and with physician-investigator overseeing pulmonary function testing at FHS. Constructive feedback will be provided as warranted. (Additional quantitative qualitycontrol assessment of all pulmonary function data will be performed by the physicianinvestigator, with ongoing monitoring of study quality for each technician. Technicianspecific feedback will be provided to optimize quality of all test procedures.)
Date: __________________ Tech ID#__________ Quarter:________
Supervisor:_________________ Participant Label_________________________
PFT Supervisor Checklist
Clinic
Check that each procedure is carried out correctly. Check yes if correct or no if incorrect. Provide an explanation of the incorrect item. Items are presented in the sequence of the examination procedure, but may require confirmation before or after the exam.
| Yes | No | PFT Instructions |
|---|---|---|
| ___ | ___ | Ask the participant: In the past 3 months have you had: major surgery (chest, abdominal, or brain, requiring hospitalization), heart attack, stroke, aneurysm of the brain, check BP (Participants blood pressure MUST be taken prior to PFT Testing ). |
| ___ | ___ | Ask the participant: Do you currently have any limitation on physical activity prescribed by your doctor? |
| ___ | ___ | If the participant is found to be ineligible due to the exclusion criteria the test is aborted and only the respiratory questions are completed & the reason is documented. |
| Yes | No | Spirometry/Forced Vital Capacity |
|---|---|---|
| ___ | ___ | Position the Participant – Testing should usually be conducted in the sitting position; however, obese participants (BMI >27) should stand. A chair (without wheels) should be positioned behind participants who stand for the test. Use the chair if the participant becomes light-headed or feels faint during testing. Ask the participant to sit erect with chin slightly elevated. |
| ___ | ___ | Explain the Procedure - Explain that the purpose of the next test is to determine how hard and fast he can exhale air, “Like blowing out dozens of candles on a birthday cake.” Explain that he should take in as deep a breath as possible, and when his lungs are completely full, blow out all the air as hard and fast as possible, until told to stop. Loose dentures should be removed. |
| ___ | ___ | Always Demonstrate the Maneuver. Ask the participant to watch you perform the FVC maneuver. Again demonstrate correct placement of the mouthpiece. If the participant does not adjust well to using the mouthpiece (i.e. strong gag reflex) the participant can use just the neck of the filter for a mouthpiece. His lips must remain tightly sealed using this also. Sit up straight. Take a deep breath, throw back your shoulders, and widen your eyes to emphasize the maximal depth of inhalation. Then dramatically BLAST out all of your air as hard and as fast as you can. |
| ___ | ___ | Have the participant connect to the spirometer with a nose clip on, push the space bar. |
| ___ | ___ | After a couple of normal breaths, have the participant take as deep a breath as possible. |
| ___ | ___ | While the participant is inspiring, press the space bar. |
| ___ | ___ | As soon as the participant has reached maximal inspiration, have them blast out all the air in their lungs. |
| ___ | ___ | Once s/he has blown out for at least 6 seconds and the graph of his breathing has become flat and you see the “Good Effort” message, push the spacebar to end the test. |
| ___ | ___ | If the participant fails to perform the maneuver correctly, again demonstrate both the error and the correct performance yourself. |
| ___ | ___ | The participant is not asked to perform more than eight FVC maneuvers |
| ___ | ___ | Once the participant has 3 acceptable tests, click Save. |
| ___ | ___ | The technician correctly assessed reversibility of the 3 acceptable tests. |
| ___ | ___ | Staff looks at the FEV1/FVC ratios and, if they are <70%, the staff member asks the participant if he would do a reversibility testing with albuterol and a brief explanation of this is given. |
| Yes | No | Diffusion Capacity |
|---|---|---|
| ___ | ___ | After completing the FVC maneuvers-
|
| ___ | ___ | Preparing the participant: While the machine prepares, explain to the participant that he will be asked to breathe normally and then to blow all his air out, just like the Vital Capacity maneuver. Once his lungs are as empty as possible, the participant will be asked to breathe in as deeply and quickly as possible and hold his breath for 12 seconds. The machine will close a valve, helping him to hold his breath and making it impossible for air to leak out- he will not be able to breathe while on the mouthpiece until the tester tells the participant to blow all his air out for the second time. |
| ___ | ___ | 1 Starting the Test: The box will pop up on the computer, hit ok. Do not have them attach to the mouthpiece until this done. The computer then will display the following message- “Press the spacebar when the patient is connected to the mouthpiece and breathing normally.” Ensure that the participant’s lips are tightly sealed around the mouthpiece and that the nose clip is in place. Once the participant is attached and breathing normally, press the spacebar |
| ___ | ___ | 2 The graph will show the participant’s tidal breathing. Once the participant is comfortable, have him breathe all the way out to Vital Capacity (the point at which the graph of his breathing becomes flat). Coach him, saying “Blow it out, blow it out” just as you would for the spirometry |
| ___ | ___ | 3 Once he has pushed all the air out, press the spacebar and IMMEDIATELY instruct him to take as deep an inspiration as possible. Ideally, the deep inspiration should take one to two seconds. |
| ___ | ___ | 4 Once the graph of his breath has flattened out again at maximal inspiration, tell him to hold his breath. He must hold his breath for 10-12 seconds for the maneuver. |
| ___ | ___ | Push the “V” key, as soon as his breath has flattened out at maximal inspiration, to close the valve and keep air from escaping. |
| ___ | ___ | Once the participant’s graph crosses the vertical line on the screen, IMMEDIATELY instruct him to blow out all the air (if you closed the valve, it will open automatically at 12 seconds), just as though he was performing spirometry. |
| ___ | ___ | Have the participant keep blowing until the red line becomes horizontal |
| ___ | ___ | Once the red line is horizontal, press the spacebar, ending the test. |
| ___ | ___ | Wait 4 minutes between each maneuver. Do not start the set up until 2 minutes have passed. |
| ___ | ___ | Repeat the maneuver from “Starting the Test” until you have two acceptable and reproducible maneuvers. |
| ___ | ___ | Grading the test: Confirm that both tests are acceptable, they will be displayed in green. If one criterion is not met, then all three appear in red. The failed criterion will have a (-) sign next to it. Review how to improve this result with the participant. Then do another maneuver. |
| ___ | ___ | Limit the number of attempts for DLCO to 3 per participant. |
| ___ | ___ | Once the participant has 2 acceptable test click save. |
| ___ | ___ | If there is a comment regarding a participant that is beneficial and should be saved, enter the comment under “Technician Notes” and then click on “Save and Exit.” |
| Yes | No | Albuterol Participants/Spirometry/FVC |
|---|---|---|
| ___ | ___ | Any participant that has a FEV1/FVC ratio of <70% (either preidentified or identified in clinic) is asked to participate in the albuterol challenge. |
| ___ | ___ | The administration of the albuterol is given after all of the other exam components have been completed. |
| ___ | ___ | Getting ready
|
| ___ | ___ | Using the MDI
|
| ___ | ___ | The spirometry/FVC protocol is performed according to the same protocol above. |
| Yes | No | PFT Completion |
|---|---|---|
| ___ | ___ | Respiratory questionnaire is administered. Questions are asked exactly as they are listed on the page. |
| ___ | ___ | All participants are entered into the “PFT Daily Log, Comment, and Calibration” binder. Enter, by date, each participant name. An FHS generated sticker with the name and ID number can be used. An *A* is placed next to the name and sticker of all albuterol challenge participants (both pre-identified and clinic identified). |
| ___ | ___ | Once the PFT is done, a white sheet labeled “PFT” is completed by attaching a participant label and the date onto the sheet and filing this in the participant’s chart. Check off whether the participant will be having Albuterol testing. |
| ___ | ___ | Participants not having a PFT during their Clinic visit are also put in the “PFT Daily Log, Comment and Calibration” binder with the reason that the PFT was not done. A sheet labeled “PARTICIPANT DID NOT HAVE PULMONARY FUNCTION TEST” is completed by selecting the appropriate reason that the participant did not have the PFT. The date and participant label are entered onto the sheet and the sheet is filed in the participant’s chart. |
| ___ | ___ | PARTICIPANTS REFUSING THE ALBUTEROL CHALLENGE: Occasionally a participant who is asked to participate in the post-bronchodilator test refuses to do so. This refusal is recorded next to the participant’s identifying sticker in the PFT Daily Log Book and the refusal reason is also noted. For tracking purposes, the technician will also add the participant’s sticker, the date and the refusal reason to the sheet titled “ALBUTEROL REFUSALS- - -CYCLE 8 OFFSPRING”. |
| ___ | ___ | PARTICIPANTS DISQUALIFIED FROM ALBUTEROL CHALLENGE: Occasionally a participant who is pre-identified for the albuterol challenge cannot participate because he is disqualified from performing the PFT maneuver based on the clinical PFT protocol. The tech will add this participant’s sticker to the sheet titled “PFT Disqualifications for Predetermined Albuterol Challenge: Offspring Cycle 8” |
| Yes | No | Technician Review |
|---|---|---|
| ___ | ___ | Did the technician introduce the set of questions with clear explanation? |
| ___ | ___ | Did the technician ask the questions exactly as written on the form? |
| ___ | ___ | Did the technician correctly clarify any questions the participant had? |
| ___ | ___ | Did the technician correctly use the answer key? |
| ___ | ___ | Did the technician score the participant’s responses correctly? |
| ___ | ___ | Did the technician review the form for completeness? |
| Yes | No | Deviations |
|---|---|---|
| ___ | ___ | Did the tech perform any minor deviations? |
| ___ | ___ | Did the tech perform any major deviations? |
| Comments/Corrections/Deviations: | ||
| Supervisor Signature: | ||
| Date: | ||
12. Pulmonary function data back-up
Cumulative back-up is performed once a week and monthly by the Systems Administrative Assistant.
Extracting the Database to a File:
- Within the Plus2000 program, click on Database Utilities. The program allows a search, so a single ID number can be backed up, or all of the data within a specific date range can be backed up.
- Set the date ‘From’ and ‘To’ be backed up and click ‘OK’.
- A list of all participants within that range will appear on the left side of the screen.
- Use the ‘Select All’ option or select only specific tests to back up. Whatever is selected will move to the right of the screen.
- Click on the ‘Extract’ option and enter a filename into the prompt.
- The status bar at the bottom of the screen will follow the status of the extraction process until it is complete. The more data there is, the longer this will take.
Backing up the Database:
- The software is equipped with a Database Backup/Restore Wizard.
- Go to: Start/All Programs/Plus2000.
- Once the program starts, click on ‘Backup’ and the prompt asks for a filename. Put in the filename and save to CD.
Storage:
- One copy of the weekly back-up disk is kept in the Systems Administrative Assistants office.
- Monthly back-up CDR’s are sent to Millennium for storage.
Millennium Contact Information:
Backup PFT Database
- Close the Plus 2000 program if it is open.
- Stop MS SQL Server.
- Go to Start – Programs – Startup – Service Manager.
- Click the Stop button.
- Double-click the shortcut on the Desktop named “PFT on array1”.
- Enter your domain username and password if requested. (Make sure to precede your username with “FHS\” – e.g. FHS\johndoe).
- In the PFT on array1 window, click “File” on the File menu.
- Select the “New” submenu.
- Click “Folder”.
- Name the folder “PFT Backup mmddyyyy”.
- Double-click the shortcut on the Desktop named “PFT Database (local)”.
- In the PFT Database (local) window, click Edit.
- Select “Copy”.
- Return to the “PFT on array1” window.
- Right-click the newly-created folder.
- Select “Paste”.
- When the copy process is done, you may close both folder windows.
- Return to the MS SQL Server program.
- Click “Start”, and close the MS SQL Server program window.
- Done – you may now use Plus 2000.
13. Pulmonary function equipment maintenance schedule
Maintenance information taken from:
The Instruction Manual for the Collins Comprehensive Pulmonary Laboratory (CPL)
No. 760096, Version August 2000
| Item | Frequency |
|---|---|
| Cleaning of CPL Covers and External Components | As Needed |
| Replacing CO2 Absorbent Cartridge | Every 3 Months |
| Replacing Desiccator Columns | When Blue Granules turn Pink |
| Replacing Balloon Cuffs in Valve* | As Needed |
| Cleaning of Balloon Valve | As Needed |
| Change Nafion Tubing | Every 4 months |
| 3.00 Liter Hans Rudolph | Annually |
Send to:
Calibration Syringe
nSpire Health
1830 Lefthand Circle
Longmont, CO 80501
*The balloons must be inflated and deflated 50 times before use. We have an extra balloon valve so that a full set of balloons are prepped and readied for use. The valves are switched with the prepped balloons already attached when needed.
14. Calibration syringe exchange
Once the replacement syringe is received at FHS, remove it from the shipping box for usage and immediately insert the syringe FHS has been using into the same box for return to nSpire Health via FHS carrier of choice. It is important that is written on the outside of this box and also insert the fax sheet into the box. Ship the syringe to: nSpire Health. File the enclosed calibration certificate in the FHS logbook or files.
Calibration Syringe Care
The 3.00 liter calibration syringe should be stored next to the spirometer so that it remains at the same temperature as the spirometer. Store the syringe with the plunger pushed all the way in. Take care not to drop the syringes.
15. Appendix
Tracking Forms
PATIENT LABEL:
DATE OF TEST:
PFT
COMPLETED_____ NOT COMPLETED_____
POST ALBUTEROL
(CIRCLE ONE) YES NO
PARTICIPANT DID NOT HAVE PULMONARY FUNCTION TEST
_____ UNABLE TO TEST DUE TO TEST CRITERIA
_____ PARTICIPANT STARTED TEST BUT WAS UNABLE TO FINISH
_____ PARTICIPANT REFUSED PFT
EACH TIME A NEW ALBUTEROL INHALER IS OPENED, PLEASE RECORD THE STOP DATE OF THE PREVIOUS ONE AND THE LOT NUMBER AND START DATE OF THE NEW ONE.
| LOT NUMBER | START DATE | STOP DATE |
|---|---|---|
| PFT PERIODIC MAINTENANCE 2008 | |||||||
|---|---|---|---|---|---|---|---|
| Cleaning CPL Covers (prn) | Replacing C02 Absorbent Cartridge (q.3mos) | Replacing Dessicator Columns (blue pink) | Replacing Balloons (and Prepping) (prn) | Cleaning Balloons Valve (prn) | Change Nafion Tubing (q.4mos) | Collins Syringe Calibration (annually) | Maintenance (annually) |
Tech-Administered Questionnaires
Frequency response card


Rosow-Breslau Questions
A. Rationale & Background
Respondents’ self-assessments of health may raise questions about the validity of such judgments. However, we are not interested in the literal details of people’s medical condition as much as in the behavioral consequences, their physical capacity for role fulfillment and social participation. We are primarily concerned with the functional health which old people report, i.e., the degree to which they claim they can manage adequately or are restricted in their activities because of their physical condition or capacity. Breslau, M, Rosow, I: A Guttman Health Scale for the Aged. 556-559
B. Methods
The method of assessing physical functioning is self-report. The questions assess the degree of difficulty that a person has performing a specific activity. This form has several important purposes:
- This data will enable us to assess the level of independence and function in the study population.
- It is hypothesized that impairments of physical function may be a risk factor for cardiovascular end points and progression of disease.
- It will measure loss of physical functioning as a consequence of cardiovascular disease.
C. Procedures
Questions:
- Coding
- 0 = No, unable to do
- 1 = Yes, independent
- 2 = Does not do
- 9 = Unknown
- Are you able to do heavy work around the house, like shovel snow or wash windows, walls, or floors without help? (Scrub floors, wash windows, rake leaves, and mow lawn). (Note: Code 2 if person does not do this activity).
- Are you able to walk half a mile without help? (Walk one half mile or 4-6 blocks without stopping for more than 5 minutes). (Note: Code 2 if person does not do this activity).
- Are you able to walk up and down one flight of stairs without help?
Fractures
1. Fractures
Ask the participant, “Since your last exam or medical history update have you broken any bones?”
- Coding
- 0 = No
- 1 = Yes
- 2 = Maybe
- 9 = Unknown
List the location of each fracture individually (3 spaces available)
CES-D Scale
A. Rationale and Background:
The Center for Epidemiologic Studies Depression Scale (CES-D) was developed for use in epidemiologic research of depressive symptomatology in the general population. It was designed as a screening instrument to elicit symptoms associated with depression. It is intended to document the presence and severity of depressive symptoms but is not intended to make clinical diagnosis. It assesses the current state of the subject by focusing on symptomatology in the past week.
Note: The depression questions used in the NHANES 1 survey were the 20-item set of the CES-D developed and validated by the Center for Epidemiologic Studies, National Institute of Mental Health (NIMH).
The scale is given at each exam. The scale is not given if the patient is: sedated, aphasic, non-English speaking, or uncooperative.
B. Procedure:
- Each question is read to the participant who responds with one of four answers.
- Response alternatives should be printed on paper which is placed in front of the participant for reference.
- Each category of response should be explained to the participant prior to administering the scale.
- If the participant is unable to read the response sheets, the interviewer should read each response as well as the question referring to their feelings in the past week.
- Be sure the participant understands that the questions refer to his/her feelings only during the past week.
C. CES-D Scoring:
Each item has a range of four response options which indicated how often the survey examinee had felt that way during the past week:
| Code | Response Option |
|---|---|
| 0 | Rarely or none of the time (less than 1 day) |
| 1 | Some or a little of the time (1-2 days) |
| 2 | Occasionally or a moderate amount of the time (3-4 days) |
| 3 | Most or all of the time (5-7 days) |
Questionnaire items 4, 8, 12, and 16 were worded in a positive (i.e., nondepressed) direction. The other 16 scale items were worded in a negative direction to elicit depressive symptomatology directly. To score the CES-D, the sense of the fours positive questionnaire items was reversed by subtracting their coded value (indicating the response option selected) from 3. Then the coded values for all 20 items were summed into a total score. The range of possible scores was 0-60. The final score is calculated by the computer.
D. Methods:
The CES-D Questionnaire consists of 20 questions. Since it is a scale for depression, it must be completed using responses by the participant, not a proxy.
SCRIPT: The questions below ask about your feelings. For each of the following statements, please say if you felt that way during the past week.
- Hand the response sheet to the participant and explain the response options. The following definitions should be given:
Code
- Rarely or none of the time (< one full day)
- Some or a little of the time (1 to 2 days in the past week)
- Occasionally or moderate amount of time (3 to 4 days in the past week, or about 1/2 the time)
- Most of the time (5 to 7 days in the past week)
If participant answers YES to a given statement, repeat the above responses to get a correct answer.
- Read each item as it is written on the form. Preface statements 1, 6 & 11 with the statement During the past
week, then continuing with the response categories. For example:
SCRIPT: During the past week I was bothered by things that usually don’t bother me. Did you feel that way rarely or none of the time, some or a little of the time, occasionally or moderate amount of time, or most or all of the time?
Question 1: During the past week I was bothered by things that usually don’t bother me.
Question 6: During the past week I felt depressed.
Question 11: During the past week my sleep was restless
**Note: Emphasize to the participant that it is only the past 7 days.**
- Discontinue reading the responses when the participant provides a response before you are finished. On the next item, however, again begin to read the entire set of responses.
- Code 9 = Refused or Do not know is used when:
- The question was asked, but the participant chooses not to answer. For example, response was I would rather not say, or Go on to the next question.
- The question was asked, but the participant does not know, does not remember, or does not understand the statement.
** If “unknown” is used more than 4 times on the questionnaire it is no longer considered valid for research. Do your best to have the participant give you an answer listed on the response key.**
- Circle the response on the form.
- When the participant asks about the meaning of any item or tries to qualify a statement, simply repeat the statement. For
example:
Participant: What do you mean by bothered?
Interviewer: I was bothered by things that usually don’t bother me. Did you feel that way rarely or none of the time, some or a little of the time, occasionally or moderate amount of time, or most or all of the time during the past week?
- When the participant still asks about the meaning or says he/she does not understand, check 9 = refused or do not know. Do not try to interpret the statement for the participant.
ADMINISTRATION INSTRUCTIONS
CERAD Word List Memory Task and the Victoria Stroop Test
General Instructions to Examiners
The CERAD is a test of memory, so interruptions should be avoided during testing. If someone interrupts the session, the examiner must note it on the “Factors Affecting the Validity of Data” form by coding it as “other” and providing an explanation in the Comments section of that form.)
Turn on the digital voice recorder and state the tech id, the participant’s FHS ID, the first name of the participant and the date of examination.
Position yourself so that the participant is not able to see the response sheets at any time. Use of a clipboard is recommended as this allows for easy recording without placing the response sheets on the table. Upon completion of one response sheet, it can be tucked under the others on the clipboard, or placed upside-down and out of sight of the participant.
Reassure participants that people are often not able to remember everything, and that’s ok; we would just like them to try their very best on all tasks. “In this next test you’re going to be asked to remember some words. Just to let you know, most people usually cannot remember everything, which is fine. Whatever you do will be great.”
If participants ask you for answers or feedback on their performance, respond with something neutral but encouraging. Inform them that you are not able to tell them whether their answers are correct. For example, if asked something like, “Is that right?” respond simply with, “You’re doing fine,” or “You did fine,” or “That was good.” If really pressed for feedback, say, “Unfortunately I can’t give you your score, I haven’t even calculated it yet, but you are doing fine.”
Offer neutral phrases of support during transitions from one condition to another. For example, when participants have provided all their responses on Trial 1 of the CERAD, a quick “great” or “good” before beginning the next trial is appropriate, although not mandatory. Nodding while the participant is responding is also a sign that seems to be reassuring. It is helpful especially with a participant who seems nervous, uncomfortable, or reluctant.
CERAD Word List Memory Task
Purpose
This test assesses participants’ ability to learn and remember new information. It is a memory test, not a reading test. Ten common nouns are used. To insure that participants are familiar with and attend to each word, they are asked to read the words printed on the pages of the CERAD "flip books." The ten words on the flip cards are presented at a constant rate and then the subject is immediately asked to recall as many as possible. There are three trials, each of which presents the words in a different order. After a delay, participants are asked to recall the words again. The final condition is a recognition task to see whether they can pick the correct words out of a group of target and non-target words.
Materials Needed
- CERAD Word List flip-book
- Stopwatch
- Data form
- Clipboard
- Pen
- Digital Voice Recorder**
**Record all test instructions and responses.
A. Learning Trials
Place the flipbook on the table in front of the participant so the title page faces him/her.
Trial 1
“Now I am going to show you ten printed words. Read each word out loud as I show it to you. Later I will ask you to recall all ten words.”
Show the words in the first set at the rate of one word every two seconds. If the participant cannot read the word, or misreads the word, or mispronounces the word say it for him/her and check the “target word is read incorrectly” column on the Response Sheet for that word. After the last word has been read, turn to the next (blank) page and say:
“Now, tell me as many as you can.” ** Start stopwatch immediately**
**Allow a maximum of 90 seconds for recall. If participant says s/he is done before 90 seconds, end trial and move on to next section. It is ok to confirm with the participant whether they are done if their nonverbal behavior leads you to believe they are. You can ask, "Are you finished?" **
Data Recording
- If the participant does not read the word correctly, place a check mark in the column with the header, “Check if target word is read incorrectly.”
- In the “Responses (verbatim)” column, write down each word the participant says in the exact order that the participant said them. Be sure to include words that are not on the list (intrusions) and words that are repeated (perseverations).
- Stop after the participant has given 35 responses.
- If the participant gives more than 20 responses photocopy the sheet in place in the CERAD folder outside of room 112.
Place the flipbook on the table in front of the participant so the title page faces him/her.
Trial 2
“I’m going to show you the same words again. As before, read them aloud as I show them to you.”
Show the words in the second set (Trial 2) at the rate of one word every two seconds. If the person mispronounces a word or misreads a word, do not correct them on Trials 2 and 3. The goal of the test is consistency between the trials, not reading or pronunciation. After the last word has been read, turn to the next (blank) page and say:
“Now, tell me as many as you can.” ** Start stopwatch immediately**
**Allow a maximum of 90 seconds for recall. If participant says s/he is done before 90 seconds, end trial and move on to next section. It is ok to confirm with the participant whether they are done if their nonverbal behavior leads you to believe they are. You can ask, "Are you finished?" **
Data Recording
- In the “Responses (verbatim)” column, write down each word the participant says in the exact order that the participant said them. Be sure to include words that are not on the list (intrusions) and words that are repeated (perseverations).
- Stop after the participant has given 35 responses.
- If the participant gives more than 20 responses photocopy the sheet in place in the CERAD folder outside of room 112.
Trial 3
“I’m going to show you the same words again. As before, read them aloud as I show them to you.”
Show the words in the second set (Trial 2) at the rate of one word every two seconds. After the last word has been read, turn to the next (blank) page and say:
“Now, tell me as many as you can.” ** Start stopwatch immediately**
**Allow a maximum of 90 seconds for recall. If participant says s/he is done before 90 seconds, end trial and move on to next section. It is ok to confirm with the participant whether they are done if their nonverbal behavior leads you to believe they are. You can ask, "Are you finished?" **
Data Recording
- In the “Responses (verbatim)” column, write down each word the participant says in the exact order that the participant said them. Be sure to include words that are not on the list (intrusions) and words that are repeated (perseverations).
- Stop after the participant has given 35 responses.
- If the participant gives more than 20 responses photocopy the sheet in place in the CERAD folder outside of room 112.
- Record the clock time (i.e., the time of day) in the space provided at the bottom of the page.
B. Word List Recall
Record the time in the “Clock time in “Start of Recall trial” box at the top of the form.
Say:
“A few minutes ago I asked you to learn a list of ten words which you read one at a time from pages in a book. Now I want you to try to recall those words. Go ahead and tell me as many of those ten words as you can remember.”
Allow the participant a maximum of 90 seconds.
Data Recording
- Record the clock time (i.e., the time of day) in the space provided.
- In the “Responses (verbatim)” column, write down each word the participant says in the exact order that the participant said them. Be sure to include words that are not on the list (intrusions) and words that are repeated (perseverations).
C. Word List Recognition
Say:
“Now I am going to show you a set of words printed on separate pages. Some of the words are from the list you saw earlier and some are words I haven't shown you before. I want you to tell me which words are from the list you saw earlier (show the first word). Is this one of the words you saw earlier?”
If participants lose track of the task, you can repeat the original instruction: “Is this one of the words you saw earlier?”
Urge subjects to give "Yes" or "No" responses, since "don't knows" cannot be scored. If a participant still does not wish to provide a response, record this in the “comments” section.
Data Recording
Circle (or check) 0 if the participant says “no,” or 1 if the participant says “yes”.
Victoria Stroop Test (VST)
Materials Needed:
- Stopwatch
- Data form
- Clipboard
- Pen
- Digital Voice Recorder
- Three stimuli pages: Dots, Words, and Colors
Trial 1: Dots
Place the “Dots” stimulus page in front of the participant. Say:
“Name the colors of the dots as quickly as you can. Begin here [point] and go across the rows from left to right.”
Direct the participant’s eyes across the rows from left to right. Clarify, using your own words, if necessary. Once the participant understands the task, say:
“Ready, begin.”
Start stopwatch.
Correct any errors immediately (if not self-corrected) by pointing to the incorrect color patch while saying the correct response. Then say, “Now continue as fast as you can.”If the technician and the participant make the correction at the same time and it is ambiguous on who made the correction first give it to them.
If the participant makes a partial self correction mark as correct. For example if they say “bl, green” mark as correct.
Record time to completion, to the 100th of a second.
Trial 2: Words
Place the “Words” stimulus page in front of the participant. Say:
“This time, name the colors of the words as quickly as you can. Begin here [point] and go across the rows from left to right.”
Direct the participant’s eyes across the rows from left to right. Clarify, using your own words, if necessary. Once the participant understands the task, say:
“Ready, begin.”
Start stopwatch.
Correct any errors immediately (if not self-corrected) by pointing to the incorrect color patch while saying the correct response. Then say, “Now continue as fast as you can.”If the technician and the participant make the correction at the same time and it is ambiguous on who made the correction first give it to them.
If the participant makes a partial self correction mark as correct. For example if they say “bl, green” mark as correct.
Record time to completion, to the 100th of a second.
Trial 3: Colors
Place the “Colors” stimulus page in front of the participant. Say:
“Again, name the colors in which the words are printed as quickly as you can. Do NOT read the word, tell me the color in which the word is printed.”
Direct the participant’s eyes across the rows from left to right. Clarify, using your own words, if necessary. Once the participant understands the task, say:
“Ready, begin.”
Start stopwatch.
Correct any errors immediately (if not self-corrected) by pointing to the incorrect color patch while saying the correct response. Then say, “Now continue as fast as you can.”If the technician and the participant make the correction at the same time and it is ambiguous on who made the correction first give it to them.
If the participant makes a partial self correction mark as correct. For example if they say “bl, green” mark as correct.
Record time to completion, to the 100th of a second.
Data Recording for all trials
- As participant is responding, follow along on the data page.
- If the participant makes a mistake but corrects it him/herself, circle the “1, Selfcorrected.”
- If the participant makes a mistake WITHOUT correcting, prompting the examiner to correct the error, circle the “2, Examiner-corrected.”
- Record the completion time, to the 100th of a second, in the appropriate box.
CERAD Word List Memory Task: Learning Trials
“In this next test you’re going to be asked to remember some words. Just to let you know, most people usually cannot remember everything, which is fine. Whatever you do will be great.”
Place flip book in front of participant.
Trial 1: “Now I am going to show you ten printed words. Read each word out loud as I show it to you. Later I will ask you to recall all ten words.”
Present the words. After last word is read, say, “Now, tell me as many as you can.” **Start stopwatch.**
(Maximum time = 90 seconds. If participant reports being done before 90 seconds, move on to next trial.)
Exposure Time
1 word every 2 seconds
Maximum Recall Time
90 seconds
Data Recording
Trial 1: check the appropriate box if the target word is read incorrectly.
All Trials: In the Responses column, record ALL words provided by the participant, in the exact order the participant says them.
| TRIAL 1 | ||
|---|---|---|
| Target Words | Check if target word is read incorrectly | Responses (verbatim) |
| Butter | 1. | |
| Arm | 2. | |
| Shore | 3. | |
| Letter | 4. | |
| Queen | 5. | |
| Cabin | 6. | |
| Pole | 7. | |
| Ticket | 8. | |
| Grass | 9. | |
| Engine | 10. | |
| 11. | ||
| 12. | ||
| 13. | ||
| 14. | ||
| 15. | ||
| 16. | ||
| 17. | ||
| 18. | ||
| 19. | ||
| 20. | ||
Trials 2 & 3: “I’m going to show you the same words again. As before, read them aloud as I show them to you.”
Present the words, one word every 2 seconds. Then say “Now, tell me as many as you can.” **Start Stopwatch**
In the Responses column, record ALL words provided by the participant, in the exact order the participant says them. Do not correct the participant on Trials 2 and 3.
(Maximum time = 90 seconds. If participant reports being done before 90 seconds, move on to next trial.)
| TRIAL 2 | TRIAL 3 | ||
|---|---|---|---|
| Target Words | Responses (verbatim) | Target Words | Responses (verbatim) |
| Ticket | 1. | Queen | 1. |
| Cabin | 2. | Grass | 2. |
| Butter | 3. | Arm | 3. |
| Shore | 4. | Cabin | 4. |
| Engine | 5. | Pole | 5. |
| Arm | 6. | Shore | 6. |
| Queen | 7. | Butter | 7. |
| Letter | 8. | Engine | 8. |
| Pole | 9. | Ticket | 9. |
| Grass | 10. | Letter | 10. |
| 11. | 11. | ||
| 12. | 12. | ||
| 13. | 13. | ||
| 14. | 14. | ||
| 15. | 15. | ||
| 16. | 16. | ||
| 17. | 17. | ||
| 18. | 18. | ||
| 19. | 19. | ||
| 20. | 20. | ||
| **** Clock time at end of third trial ____ : ____ | |||
***Note to Examiner: Turn off digital voice recorder until Recall test begins.***
Victoria Stroop Test (VST) – Parts 1-3
Part 1: Dots
Place the “Dots” stimuli page in front of participant.
“Name the colors of the dots as quickly as you can. Begin here and go across the rows from left to right.” Direct the participant’s eyes across the rows from left to right. Clarify, in your own words, if necessary. Once the participant understands the task say: “Ready, begin.”
**Examiner: Start stopwatch immediately after telling participant to begin.**
**Correct any errors immediately (if not self-corrected) by pointing to the incorrect color patch while saying the correct response. Then say “Now continue as fast as you can.”**
Data Recording: If an error is made, circle 1 if self-corrected or 2 if examiner-correct. If no error is made, leave blank. Record the total time in the box at the bottom of the page.
Dots
Total Time ___ (min) ___ ___ . ___ ___ (sec)
Part 2: Words
Place the “Words” stimuli page in front of participant.
“This time, name the colors of the words as quickly as you can. Begin here and go across the rows from left to right.” Direct the participant’s eyes across the rows from left to right. [Clarify if necessary with this instruction: “Name the colors in which the words are printed.”]
“Ready, begin.” Start stopwatch immediately after telling participant to begin**
**Correct any errors immediately (if not self-corrected) by pointing to the incorrect color patch while saying the correct response. Then say “Now continue as fast as you can.”**
Data Recording: If an error is made, circle 1 if self-corrected or 2 if examiner-correct. If no error is made, leave blank. Record the total time in the box at the bottom of the page.
Words
Total Time ___ (min) ___ ___ . ___ ___ (sec)
Part 3: Colors
Place the “Colors” stimuli page in front of participant.
“Again, name the colors in which the words are printed as quickly as you can. Do NOT read the word, tell me the color in which the word is printed.”
Direct the participant’s eyes across the rows from left to right. Clarify if necessary with this instruction: “Ready, begin”Start stopwatch immediately after telling participant to begin.
**Correct any errors immediately (if not self-corrected) by pointing to the incorrect color patch while saying the correct response. Then say, “Now continue as fast as you can.”**
Data Recording: If an error is made, circle 1 if self-corrected or 2 if examiner-correct. If no error is made, leave blank. Record the total time in the box at the bottom of the page.
Colors
Total Time ___ (min) ___ ___ . ___ ___ (sec)
Procedures administered during CERAD Word List delay
Please, check ALL that apply
| |__| | Anthropometry |
| |__| | Sociodemographic Questionnaire |
| |__| | SF-12 Health Survey |
| |__| | CES-D Scale |
| |__| | Physical Activity Questionnaire |
| |__| | Neurocognitive: Victoria Stroop Test |
| |__| | Urine Specimen |
| |__| | Blood Draw |
| |__| | Glucose tolerance test |
| |__| | ECG |
| |__| | Observed performance (Fast walk, hand grip, chair stands) |
| |__| | Tonometry |
| |__| | Ankle-brachial blood pressure by Doppler. (Participants > 40 years) |
| |__| | Spirometry |
| |__| | Post albuterol Spirometry |
| |__| | Diffusion Capacity |
| |__| | Physician visit |
| |__| | Bone density |
CERAD Word List Memory Task: Recall
**** Clock time at start of Recall trial ____ : ____
"A few minutes ago I asked you to learn a list of ten words which you read one at a time from pages in a book. Now I want you to try to recall those words. Go ahead and tell me as many of those ten words as you can remember."
(Maximum time = 90 seconds. If participant reports being done before 90 seconds, move on to Recognition trial.)
In the Responses column, record ALL words provided by the participant, in the exact order the participant says them.
Maximum Recall Time
90 seconds
CERAD Word List Memory Task: Recognition
Place flip-book on desk in front of participant.
”Now I am going to show you a set of words printed on separate pages. Some of the words are from the list you saw earlier and some are words I haven't shown you before. I want you to tell me which words are from the list you saw earlier.”
Show the first word.
Is this one of the words you saw earlier?
(Only YES or NO responses are acceptable.)
Data Recording: Circle 0 if participant says “No.” Circle 1 if participant says “Yes.”
Factors Affecting the Validity of Data
| CERAD | Victoria Stroop |
|---|---|
| Test completed _____ Test not completed _____ If test is not completed, circle the number below that corresponds to the reason:
| Test completed _____ Test not completed _____ If test is not completed, circle the number below that corresponds to the reason:
|
| It is possible that the test was completed, but something may have affected the validity of
the data. THIS IS A JUDGMENT CALL ON THE PART OF EXAMINER. Examples include, but are not limited to:
If yes, describe what happened in “Comments” section below. | It is possible that the test was completed, but something may have affected the validity of
the data. THIS IS A JUDGMENT CALL ON THE PART OF EXAMINER. Examples include, but are not limited to:
If yes, describe what happened in “Comments” section below. |
| Comments: | Comments: |
Were all portions of the CERAD tape recorded?
| Was the Victoria Stroop tape recorded?
|
Self-Administered Questionnaires
TV and Computer Use Time
In general, the items just seem to have face validity and people just ask “how many hours per week” or “how many hours in the past week” or whatever timeframe they want to use. In general, I think it is a better idea to use a concrete time period (“last week”) than to let the respondent come up with an “average,” which will tend to be more distorted by social desirability concerns.
Here are some examples:
- NHANES (self-report on day prior to survey)
From Mendoza, Zimmerman, & (D) Christakis
Television/video viewing was a categorical variable and was assessed similarly to previous reports from older releases of NHANES [2,25], by the following in which "SP" refers to sample person: "About how many hours did (SP) sit and watch TV or videos yesterday? Would you say less than 1 hour, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours or more, or none?" In order to test the AAP guidelines in the multivariate analyses, we dichotomized TV/video viewing into two categories: 2 hours or less/day and greater than 2 hours/day. Computer use was also a categorical variable and similarly assessed using the following: "About how many hours did (SP) use a computer or play computer games yesterday? Would you say less than 1 hour, 1 hour, 2 hours, 3 hours, 4 hours, 5 hours or more, or none?" - Some Health Psych studies use 2-week recall period (Salmon et al article)
- Australian study on TV viewing and other sedentary activities in Annals of Behavioral Med (Sugiyama et al):
Time spent in the following seven sedentary behaviors were examined in the study: television or video watching (TV viewing); computer/internet for leisure; video game use; reading; sitting and talking with friends or listening to music; talking on the telephone; and driving or riding in a car [11]. Participants were asked “On how many days did you do the activity in the past 7 days?” and “On average, how many minutes did you do the activity on the days that you did it?” for each of the above activities. The test– retest reliability of the TV viewing item was 0.82 [11]. The reliability for other items ranged from 0.6 to 0.8
Sociodemographics and Subjective Health
This is a self-reported form. The participant will fill this questionnaire out in clinic. Once the participant is done with the form a clinic staff member will review the form for completeness. If any blanks are left the question will be flagged and the participant will be asked to fill in the blank.
Question 1: What is your current marital status?
- 1=single/never married,
- 2=married/living as married/living with partner
- 3=separated
- 4=divorced
- 5=widowed
- 9=prefer not to answer
Question 2: Which of the following best describes you?
This is a two part question, first is asking the participant their ethnicity and the second their race. Be sure BOTH questions are answered.
Question 3: What is the highest degree or level of school you have completed? (if currently enrolled, mark the highest grade completed, degree received)
- 0= no schooling
- 1=grades 1-8
- 2=grades 9-11
- 3=completed high school (12th grade) or GED
- 4=some college but no degree
- 5=technical school certificate
- 6=associate degree (Junior college AA, AS)
- 7=Bachelor’s degree (BA, AB, BS)
- 8=graduate or professional degree (master’s, doctorate, MD, etc.)
- 9=prefer not to answer
Question 4: Please choose which of the following best describes your current employment status?
- 0=homemaker, not working outside the home
- 1=employed (or self-employed) full time
- 2=employed (or self-employed) part time
- 3=employed, but on leave for health reasons
- 4=employed, but temporarily away from my job
- 5=unemployed or laid off
- 6=retired from my usual occupation and not working
- 7= retired from my usual occupation but working for pay
- 8= retired from my usual occupation but volunteering
- 9=prefer not to answer
- 10=unemployed due to disability
- 11= full-time student
Question 5: What is your current occupation? Write in
____________________________________________
Question 6: Using the occupation coding sheet choose the code that best describes your occupation.
Note: the occupation coding sheet is on the clipboard that is given to the participant. Show this to the participant while explaining the questionnaire.
Question 7: Please select which income group best represents your combined family income for the past 12 months.
- 1=under $20,000
- 2 =$20,000 – $34,999
- 3 =$35,000 – $54,999
- 4 =$55,000 – $74,999
- 5 =$75,000 – $100.000
- 6 =over $100,000
- 99=prefer not to answer
Question 8: How many people are supported by this income?
Question 9: Do you have some form of health insurance? 0=No, 1=Yes
If the participant answers yes, make sure that the participant circles what type of insurance they have from the insurance plan list.
Question 10: Do you have prescription drug coverage
SF-12 Health Survey
The Quality of Life form has twelve questions and is designed to collect information about the participant's perceived physical and emotional health, as they relate to quality of daily living.
Q1 asks the participant to rank his/her general health on a five-point response scale: Excellent, Very Good, Good, Fair, Poor.
Q2 and Q3 address the extent to which a participant’s health limits his/her activities regarding moderate activities and climbing stairs, using a three-point response scale: No not limited, Yes- limited a little, Yes- limited a lot.
Q4-Q7 ask the participant to assess whether physical and emotional problems have impacted his/her ability to perform work tasks or regular daily activities during the previous four weeks. A Yes or No response is required.
Q8 asks the participant how much pain interfered with his/her normal work during the previous four weeks, using a five-point response scale: Not at all, A little bit, Moderately, Quite a bit, Extremely.
Q9-Q12 ask the participant how he/she has felt during the previous four weeks, using a six point response scale: All of the time, Most of the time, A good bit of the time, Some of the time, A little of the time, None of the time.
Simplified nutritional appetite questionnaire (SNAQ)
1. My appetite is
- very poor
- poor
- average
- good
- very good
2. When I eat
- I feel full after eating only a few mouthfuls
- I feel full after eating about a third of a meal
- I feel full after eating over half a meal
- I feel full after eating most of the meal
- I hardly ever feel full
3. Food tastes
- very bad
- bad
- average
- good
- very good
4. Normally I eat
- less than one meal a day
- one meal a day
- two meals a day
- three meals a day
- more than three meals a day
Eating Questions
5. Questions about eating.
- Do you often feel that you can't control what or how much you eat? ............... [ ] NO [ ] YES
- Do you often eat, within any 2-hour period, what most people would regard as an unusually large amount of food? ............................................... [ ] NO [ ] YES
If you checked 'NO' to either #5a or #5b, please skip questions #6-8.
6. Has this been as often, on average, as twice a week for the last 3 months? [ ] NO [ ] YES
(DO NOT DISTRIBUTE THIS PAGE TO THE PATIENT)
Scoring Instructions:
SNAQ Questions 1 through 4: Tally the results based upon the following numerical scale:
a = 1, b = 2, c = 3, d = 4, e = 5.
The sum of the scores for the individual items constitutes the SNAQ score. A SNAQ score ≤ 14 indicates significant risk of at least 5% weight loss within six months.
Reference: Wilson et al, Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr, 82:1074, 2005.
Eating Disorder Questions 5-8:
Bulimia Nervosa if #5a, 5b, 6 and #8 are all answered ‘Yes’;
Binge Eating Disorder: if #5a, 5b, and 6 are all answered ‘Yes’ but #8 is either ‘NO’ or left blank.
Reference: Spitzer RL et al, Validation and Utility of a Self-report Version of PRIMEMD: The PHQ Primary Care Study, JAMA, 282: 1737, 1999.
"Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents"
Publication omitted
Wilson MM, Thomas DR, Rubenstein LZ, Chibnall JT, Anderson S, Baxi A, Diebold MR, Morley JE. Appetite assessment: simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents. Am J Clin Nutr. 2005 Nov;82(5):1074-81. [PubMed ID: 16280441]
Physician-Administered Medical History
Elevated Blood Pressure
If, during a home visit the blood pressure is:
- > 200/110 a call is made to a FHS physician who will notify the participant’s personal physician. The chart will be marked “expedite” so that the letter to the personal physician is sent out ASAP.
- > 180/100 the chart is expedited
- The Referral sheet is completed to note that contact was made to an FHS MD during the exam.
- If a phone contact was made by an FHS MD to the participant’s personal physician, the FHS MD is to complete a “Record of Telephone Encounter” form.
If, during a nursing home visit the blood pressure is:
- > 140/90 inform the nurse caring for the participant or the charge nurse
- > 180/100 inform the nurse caring for the participant or the charge nurse. The chart will be marked “expedite” so that the letter to the personal physician is sent out ASAP.
Brachial Artery:
Located between the biceps and triceps, on the medial side of the elbow.

Radial Artery: Located on the thumb side of the wrist.

Referral Tracking
- Was further medical evaluation recommended for this participant?
This question is to be answered by the physician completing the chart. In addition to the physician writing in their physician ID number on the form, he/she will code this question using the following codes:
- Coding
- 0= No
- 1= Yes
- 9= Unknown
If No, go to the next section.
If Yes, the MD is to code the reason for further evaluation:
- Blood Pressure result ___/___
- Phone call > 200/110
- Expedite ≥180/100
- Elevated > 140/90
Write in abnormality or identified medical problem
- ECG abnormality ________________________________
- Clinical Physician ________________________________
- Other___________________________________________
- Method used to inform participants of need for further medical evaluation:
This information is to be coded by the physician completed the chart.
- Circle ALL that apply
- 1= Face-to-face in clinic
- 2= Phone call
- 3= Result letter
- 4= Other
- Method used to inform participant’s personal physician of need for further medical evaluation:
This information is to be coded by the physician completed the chart.
- Circle ALL that apply
- 1= Phone call
- 2= Results letter mailed
- 3= Results letter FAX’d
- 4= Other
Date referral made: ___--___--__ __ __ __ Use 4 digits for year
ID number of person completing this referral:________
Notes documenting conversation with participant or participant’s personal physician:
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
CRITERIA FOR EVENTS
1. Cardiovascular Disease
Cardiovascular disease is considered to have developed if there was a definite manifestation of coronary heart disease, intermittent claudication, congestive heart failure, or stroke or transient ischemic attack in the absence of a previous manifestation of any of these diseases. Criteria for all these events are given below. A person having more than one cardiovascular manifestation within the follow-up period is counted as an incident case only at the time of the first event.
2. Coronary Heart Disease
Subjects are diagnosed as having developed coronary heart disease (CHD) if upon review of the case a panel of three investigators (the Framingham Endpoint Review Committee) agrees on one of the following definite manifestations of CHD: myocardial infarction, coronary insufficiency, angina pectoris, sudden death from CHD, non-sudden death from CHD. Persons with pre-existing CHD at Exam 1 are excluded from the population at risk of developing CHD but may be eligible for studies of prevalent CHD. Pre-existing CHD at Exam 1 is identified by any one of the following diagnoses at Exam 1: definite angina pectoris, definite history of myocardial infarction, definite myocardial infarction by electrocardiogram, doubtful myocardial infarction by electrocardiogram, definite coronary insufficiency by electrocardiogram and history.
The various manifestations of CHD are these:
- Angina Pectoris
Brief recurrent chest discomfort of up to 15 minutes duration, precipitated by exertion or emotion and relieved by rest or by nitroglycerine is regarded as angina pectoris (AP) if two physicians interviewing the subject at a Framingham clinic visit or the Framingham Endpoint Review Committee, upon review of medical records, agree that this condition was definitely present. This diagnosis is based solely on evaluation of subjective manifestations. Abnormality of the resting or exercise electrocardiogram is not required for this diagnosis.
- Myocardial Infarction
Recent or acute myocardial infarction (MI) is designated when there were at least two of three findings:
- symptoms indicative of ischemia;
- changes in biomarkers of myocardial necrosis;
- serial changes in the electrocardiograms indicating the evolution of an infarction, including the loss of initial QRS potentials (that is, development of “pathologic” Q-waves of 0.04 second duration or greater).
An old or remote myocardial infarction is considered to be present when the electrocardiogram shows a stable pattern including a pathologic Q-wave of 0.04 second or greater or loss of initial QRS potential R-wave in those leads in which this would not be expected to occur. Also, an interim unrecognized MI is indicated when changes from a previous tracing show development of loss of Rwave potential or appearance of pathologic Q-waves not otherwise explained, in persons in whom neither the patient nor his physician considered the possibility of MI. If the patient was asymptomatic for chest pain or upper abdominal pain during the interval at which the unrecognized MI occurred, the event is classified as silent, unrecognized. More weight is given to this finding if a T-wave abnormality is also associated with Q-wave abnormality.
An autopsy report showing an acute, new, or recent infarction of the myocardium is accepted as evidence of an incident myocardial infarction. Because it is not possible to date an old infarction found on autopsy, such evidence is not used in the clinical diagnosis of a new event, unless there was an interim clinical event suspected of being an infarction.
- Coronary Insufficiency
The coronary insufficiency syndrome is designated when a history of prolonged ischemic chest pain (> 15 minutes duration) was accompanied by transient ischemic S-T segment and T-wave abnormality in the electrocardiographic tracing but not accompanied by development of Q-wave abnormality or by serum enzyme changes characteristic of myocardial necrosis.
- Coronary Heart Disease Death
Death from coronary heart disease is diagnosed as either sudden or nonsudden. For a detailed description of these diagnoses, see 6 below.
3. Stroke
The diagnosis of cerebrovascular disease is based on the occurrence of a clinically evident stroke documented by clinical records reviewed by at least two neurologists. Stroke is defined as the sudden or rapid onset of a focal neurologic deficit persisting for greater than 24 hours. Stroke is further categorized into infarction or hemorrhage.
- Hemorrhagic Stroke
The diagnosis of subarachnoid hemorrhage is based on a history suggestive of this process such as abrupt onset headache, with or without change in the level of consciousness, and signs of meningeal irritation with or without other localizing neurological deficits. Intracerebral hemorrhage is diagnosed clinically by the occurrence of abrupt focal neurologic deficit, often with altered level of consciousness and symptoms of increased intracranial pressure. Hemorrhages are confirmed by imaging.
- Ischemic Stroke
A diagnosis of cerebral embolism is made when an established source for embolus including atrial fibrillation, rheumatic heart disease with mitral stenosis, recent myocardial infarction, bacterial endocarditis or other known source is determined. A clinical course consistent with embolic infarction or evidence of other systemic embolism may be present. Symptoms are usually rapid with maximal severity at onset.
Antherothrombotic brain infarction is defined as the sudden onset of a focal neurologic deficit lasting longer than 24 hours, in the absence of:
- known source of embolism (atrial fibrillation, rheumatic heart disease with mitral stenosis, myocardial infarction within preceding six months, bacterial endocarditis);
- intracranial hemorrhage (intracerebral or subarachnoid);
- known hypercoagulable states;
- other disease processes causing focal neurologic deficits (brain tumor, subdural hematoma, hypoglycemia).
Confirmatory imaging supports the diagnosis.
Silent stroke may be documented at the stroke review sessions when a stroke event is determined and an incidental infarct is seen on brain imaging in the absence of a reported clinical event.
- Transient ischemic attack
A transient ischemic attack is defined as a focal neurologic deficit of sudden or rapid onset that fully resolves in less than 24 hours.
- Stroke Death
Death attributed to stroke is designated when a documented focal neurologic deficit of greater than 24 hours duration preceded death and was responsible for the fatality.
4. Intermittent claudication
Minimum criteria for the subjective diagnosis of intermittent claudication consists of a cramping discomfort in the calf clearly provoked by walking some distance with the pain appearing sooner when walking quickly or uphill and being relieved within a few minutes by rest. This diagnosis is designated if two physicians at a Framingham clinic visit or the Framingham Endpoint Review Committee, upon review of medical records, agree that this condition is definitely present. A diagnosis of intermittent claudication is based solely on evaluation of subjective manifestations.
5. Congestive heart failure
A definite diagnosis of congestive heart failure requires that a minimum of two major or one major and two minor criteria be present concurrently. The presence of other conditions capable of producing the symptoms and signs are considered in evaluating the findings.
- Major Criteria:
- Paroxysmal nocturnal dyspnea or orthopnea;
- Distended neck veins (in other than the supine position);
- Rales;
- Increasing heart size by x-ray;
- Acute pulmonary edema on chest x-ray;
- Ventricular S(3) gallop;
- Increased venous pressure > 16 cm H20;
- Hepatojugular reflux;
- Pulmonary edema, visceral congestion, cardiomegaly shown on autopsy;
- Weight loss on CHF Rx: 10 lbs./5days.
- Minor criteria:
- Bilateral ankle edema;
- Night cough;
- Dyspnea on ordinary exertion;
- Hepatomegaly;
- Pleural effusion by x-ray;
- Decrease in vital capacity by one-third from maximum record;
- Tachycardia (120 beats per minute or more);
- Pulmonary vascular engorgement on chest x-ray.
6. Coronary heart disease death
Death from coronary heart disease is diagnosed as either sudden or nonsudden.
- Nonsudden death from CHD
If the terminal episode lasted longer than one hour, if the available information implies that the cause of death was probably CHD, and if no other cause can be ascribed, this is called nonsudden death from CHD. In making this diagnosis, the review panel uses prior clinical information as well as information concerning the final illness.
- Sudden death from coronary heart disease
If a subject, apparently well, was observed to have died within a few minutes (operationally documented as under one hour) from onset of symptoms and if the cause of death cannot reasonably be attributed on the basis of the full clinical information and the information concerning death to some potentially lethal disease other than coronary heart disease, this is called sudden death and is attributed to coronary heart disease.
7. Cardiovascular disease death
This cause of death is designated when any disease of the heart or blood vessels is considered responsible.
8. All-cause mortality
The fact of death is supported by a death certificate. Additional information is obtained from records supplied by hospital, attending physician, pathologist, medical examiner, or family. The Framingham Endpoint Review Committee reviews all evidence to arrive at the cause of death.