Manual 3 - Surveillance Component Procedures - Version 1.0
Table of Contents
- NOTICE TO READERS
- FOREWORD
- 1 INTRODUCTION
- 2 IDENTIFICATION OF EVENTS
- 3 EVENT INVESTIGATION
- 4 DIAGNOSTIC CRITERIA
- 5 EVENT DETERMINATION
- 6 MEDICAL CARE ASSESSMENT
- 7 LINKAGE OF MULTIPLE EVENTS
- 8 RELIABILITY AND VALIDITY OF COMMUNITY SURVEILLANCE PROCEDURES
- 9 REFERENCES
- APPENDIX I
- (1) ICD9 Codes for the Identification of Fatal CHD
- (2) ICD-9 Codes for the Identification of Hospitalized Myocardial Infarction
- ARIC SURVEILLANCE EVENT INVESTIGATION SUMMARY FORM (SEI)
- ARIC SURVEILLANCE EVENT ELIGIBILITY
- ARIC COHORT EVENT ELIGIBILITY FORM
- ARIC DEATH CERTIFICATE FORM
- ARIC INFORMANT INTERVIEW FORM
- ARIC PHYSICIAN QUESTIONNAIRE
- ARIC CORONER/MEDICAL EXAMINER FORM
- Washington County Medical Examiner Questions
- ARIC MMCC FINAL DIAGNOSIS FORM
- ARIC HOSPITAL RECORD ABSTRACTION FORM
- APPENDIX AA
- APPENDIX BB
- APPENDIX CC HOSPITAL CODES
- APPENDIX DD
- APPENDIX EE SOUNDEX
- APPENDIX FF
- APPENDIX GG ARIC SURVEILLANCE ECG SHIPPING INVENTORY
- APPENDIX II
- APPENDIX III
- FORMAT 1: SAMPLE LETTER TO INFORMANT: KNOWN TELEPHONE NUMBER
- FORMAT 2: SAMPLE LETTER TO INFORMANT: UNKNOWN TELEPHONE NUMBER
- FORMAT 3: REPLY POSTCARD FROM INFORMANT WITH TELEPHONE NUMBER
- FORMAT 4: SAMPLE LETTER TO A NEIGHBOR RE: LOCATION OF INFORMANT
- FORMAT 5: REPLY FORM ON THE LOCATION OF INFORMANT
- FORMAT 6: INFORMANT RELEASE OF INFORMATION FORM: NURSING HOME
- FORMAT 7: LETTER TO PHYSICIAN SIGNING DEATH CERTIFICATE
- FORMAT 8: LETTER TO ATTENDING PHYSICIAN OF DECEDENT
- FORMAT 9: INFORMANT RELEASE OF INFORMATION: PHYSICIAN
- FORMAT 10: INFORMANT RELEASE OF INFORMATION: OUT-OF-AREA HOSPITAL
For copies, please contact:
ARIC Coordinating Center
Department of Biostatistics
CB# 8030, Suite 203, NCNB Plaza
The University of North
Carolina
Chapel Hill, NC 27514
Version 1.0: February, 1988
NOTICE TO READERS
At the time Manual 3 was approved by the ARIC Steering Committee for release to the public, the following data collection forms have not been evaluated. Upon the completion of the revisions which resulted from pilot testing, these forms and their instructions will be added to the Appendices.
- Coroner/Medical Examiner Form
- Hospital Record Abstraction Form
- Stroke Form
FOREWORD
This manual entitled, Surveillance Component Procedures, is one of a series of protocols and manuals of operation for the Atherosclerosis Risk in Communities (ARIC) Study. The complexity of the ARIC Study requires that a sizeable number of procedures be described, thus this rather extensive set of materials has been organized into the set of manuals listed below. Manual 1 provides the background, organization, and general objectives of the ARIC Study. Manuals 2 and 3 describe the operation of the Cohort and Surveillance Components of the study. Detailed Manuals of Operation for specific procedures, including reading centers and central laboratories, make up Manuals 4 through 11. Manual 12 on Quality Assurance contains a general description of the study's approach to quality assurance as well as the details for quality assurance for the different study procedures.
The version status of each manual is printed on the title sheet. The first edition of each manual is Version 1.0. Subsequent modifications of Version 1 (pages updated, pages added, or pages deleted) are indicated as Versions 1.1, 1.2, and so on, and are described in detail in the Revision Log located immediately after the title page. When revisions are substantial enough to require a new printing of the manual, the version number will be updated (e.g., Version 2.0) on the title page.
ARIC Study Protocols and Manuals of Operation
| MANUAL | TITLE |
| 1 | General Description and Study Management |
| 2 | Cohort Component Procedures |
| 3 | Surveillance Component Procedures |
| 4 | Pulmonary Function Assessment |
| 5 | Electrocardiography |
| 6 | Ultrasound Assessment |
| a. Ultrasound Scanning | |
| b. Ultrasound B-mode Image Reading Protocol | |
| 7 | Blood Collection and Processing |
| 8 | Lipid and Lipoprotein Determinations |
| 9 | Hemostasis Determinations |
| 10 | Clinical Chemistry Determinations |
| 11 | Sitting Blood Pressure and Postural Changes in Blood Pressure and Heart Rate |
| 12 | Quality Assurance and Quality Control |
1. INTRODUCTION
Through community surveillance, the ARIC study enumerates and validates cases (events) of hospitalized myocardial infarction (MI) and coronary heart disease (CHD) deaths occurring during a specific time period in 35 through 74 year old male and female residents of the four ARIC study communities: Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland.
This manual details the procedures for ARIC community surveillance. Section 2 describes the procedures by which potential events in the community are identified (i.e., death registries, hospital discharge indexes). Section 3 details procedures for collecting the additional information needed once an event has been identified. Diagnostic criteria are documented in Section 4, and review and classification procedures are described in Section 5. The procedures for obtaining information on certain indicators of medical care are described in Section 6. Procedures for linkage of multiple events are described in Section 7.
CHD events occurring among ARIC cohort participants are ascertained through cohort follow-up as well as through the routine community wide surveillance. Cohort follow-up provides a limited validation of routine surveillance. Methods for coordinating cohort and surveillance procedures are described in Section 8.
2. IDENTIFICATION OF EVENTS
2.1. Introduction
The basic features of the community surveillance design are summarized in Table 1. Events surveyed in each of the four communities include fatal CHD and hospitalized MI (see Appendix I), beginning January, 1987.
| Criteria | Eligibility |
|---|---|
| Age | Between 35 and 74, inclusive |
| Race | All races |
| Place of residence | Within the defined boundaries of the ARIC communities |
| Date of discharge or death | January 1, 1987 - December 31, 1992 |
| ICD9 Codes for identification of CHD death | 250, 401, 402, 410–414, 427–429, 440, 518.4, 798, 799 |
| ICD9 Codes for identification of hospitalized MI | 402, 410–414, 427, 428, 518.4 |
Events meeting the eligibility criteria given in Table 1 are investigated for conformity with ARIC surveillance diagnostic criteria. Identification of hospitalized events is limited to acute care hospitals in the catchment area (Section 2.2.3); no systematic attempt is made to obtain events from records of nursing homes, mental hospitals, private physicians, or hospitals out of the catchment area.
Hospitalized MIs are documented by ARIC field staff trained to identify ECG Q waves using the Minnesota Code.
Out-of-hospital deaths (as defined in Section 3.1.2) are documented by means of informant interviews and certifying and family physician questionnaires. Coroner/medical examiner records are abstracted when available. Since Maryland laws prohibit the use of information found in death certificates as a means to contact relatives, the Washington County medical examiner adds relevant questions to his/her routine inquiry with family members of individuals deceased out-of-hospital.
Deaths occurring in acute care hospitals are documented by abstracting the medical record, as with all nonfatal events.
The elements of the diagnostic criteria for the various events are abstracted onto standardized data forms. For hospitalized events, the occurrence of MI is determined by computer analysis of the recorded diagnostic elements; for fatal events, cause of death is assigned by a Mortality and Morbidity Classification Committee (MMCC).
Quality control programs are carried out to assess reliability of abstracting medical records, both the reliability and validity of coding ECGs, and the reliability of MMCC procedures.
Two sources of identification of events are used: death certificates and hospital discharge indexes.
2.2. Identification of Hospitalized MI
2.2.1. Obtaining Access to Hospital Medical Records
A critical feature of surveillance is obtaining information from medical records. Without complete cooperation of hospitals, the usefulness of event rates in any community is limited. The following represents an approach for obtaining hospital cooperation which each Field Center adapts to suit the situation existing in its study community.
For the initial contact, a letter briefly describing the study is sent to the hospital administrator and the director of medical records. It is important to keep the director of medical records informed about the project and the progress of the research.
Following the initial letter, a detailed protocol is sent to each hospital administrator. Many hospitals have a medical ethics committee to review research protocols. Within two weeks of sending the protocol, the administrator and director of medical records are contacted to determine if there is difficulty in obtaining permission to review records.
Simultaneously with the submission of the protocol, the heads of cardiology are contacted. A face to face meeting with the cardiologists is important to enlist their cooperation. This may follow one of two formats. One is to arrange to meet individual cardiologists at their respective offices. The second consists of arranging a reception or dinner with the cardiologists in order to describe the study. The contact with the cardiologists is essential so that knowledge of the study is disseminated at each hospital. Moreover, if there is difficulty in obtaining permission to review records, the cardiologists can often assist in the review process.
It is also helpful to address the local medical records committees. Most require continuing education credits. A description of the epidemiologic research which provides the credits is beneficial to both the medical records librarians and the project.
If there appears to be difficulty in obtaining permission, it is important to recontact the hospital administrators, arrange to meet with the administrators and house staff, as well as to offer to meet with the review committee. A critical feature is to emphasize the scientific importance and the non-threatening nature of the research. An additional point for the hospital administrator is to indicate that the research will not impede normal hospital function. It may be helpful for a senior investigator to arrange to meet with the director of medical records to describe the study. The director of medical records often is of considerable help in obtaining permission. These individuals are professionals and recognize the importance of employing medical records in research.
An additional approach which is often successful is to have faculty from the medical school contact the cardiologists. Faculty on the medical school staff who either work in the hospitals or have trained the physicians on the staff can often be very helpful for contact.
A critical reason for refusal is confidentiality of medical records. It is important to be able to respond to this issue. It may help to obtain clarification from the individual States if questions of confidentiality arise.
After permission is received, a senior investigator and the staff who do the abstracting may arrange to meet with the directors of medical records. At this time, the study should be reviewed in addition to describing the role of the medical record departments in the research. It is important to schedule time for the ARIC staff to review the records at periods when the record rooms are not busy. It is essential that the ARIC research staff control the record review.
It is sometimes necessary to compromise with the hospital review committees and house staff. Again, the major consideration is confidentiality. Some hospitals will not permit the abstraction of a patient's name. It is important to obtain the name because this is the surest method to reduce redundancy in the records. However, less optimal procedures are available. The first is to seek permission to code the name and record addresses, social security numbers, and birth dates. If these are available, the likelihood of redundancy can be reduced by sorting lists of individuals by birth dates or social security numbers.
It is important to keep the medical records directors, hospital administrators and cardiologists informed concerning the progress of the project. A periodic newsletter and reprints of publications from the project may help demonstrate the significance of the research and the lack of threat to the hospitals. This is also important because there is turnover in staff both for the researchers and the hospitals, thus the newsletters serve as a reminder to the continuing staff and an introduction to the newly hired staff.
2.2.2. Hospital Discharge Index
Eligible hospitalized MIs are identified from the discharge index of each hospital surveyed. Discharge indices are obtained directly from the hospital or from an indexing service such as the Commission on Professional and Hospital Activities (CPHA).
When a person is discharged from a hospital, the physician must indicate the major illness from which the patient suffers. Usually one such diagnosis accounted for the hospitalization. This is the primary discharge diagnosis. Other old or new diagnoses may be listed as secondary discharge diagnoses. Discharge diagnoses are coded by the hospital medical records personnel according to the International Classification of Diseases (ICD). Most hospitals subscribe to a service which takes these diagnostic codes and produces an index of discharges classified by code.
The ICD was originally constructed to provide comparable international data on causes of death. It is now extended by many countries for use in coding hospital discharge diagnoses. The extension of the ICD currently being used by hospitals is called ICD9-CM (Clinical Modification). The hospital or "CM" modifications do not alter the basic three digit codes, but provide additional codes so that diagnoses may be classified with more detail. For instance, ICD9 uses the code 410 for acute MI: ICD9-CM adds a decimal point so that the location of the MI can be coded (e.g., an anterior wall MI is coded 410.1).
Using the discharge index for each hospital, hospitalized events are selected according to the following eligibility criteria.
- Age. ARIC examines cases only at ages 35 through 74 at time of discharge.
- Place of Residence. Patients must live within the boundaries of the ARIC community. The discharge index may give only Zip Code, in which case a determination of residence eligibility may require checking the address in the hospital records. If a review of the medical record indicates the person was only visiting the area or had two residences, the address where the person lived at least six months of the year is considered the place of residence for ARIC purposes. People residing in a local jail at the time of hospitalization are counted.
- Date. Time eligibility is determined from the date of discharge. Only cases discharged after January 1, 1987 are eligible.
- ICD9-CM Code. Cases with primary or secondary diagnoses with ICD9-CM codes 402, 410–414, 427, 428 and 518.4 are selected for documentation of hospitalized MI.
The number of cases meeting these four eligibility criteria is reduced by applying various sampling fractions to different classes of ICD9-CM codes.
- Code 410. 100% sample.
- Code 411. 50% sample, when the record does not have a 410 code. The 50% sample is selected by choosing only the events with discharges occurring on even days of the month, i.e., days 2, 4, 6, etc.
- Codes 412–414. 25% sample, when the record does not have a 410 or 411 code. The 25% sample is selected by choosing only the events with discharges occurring on days of the month divisible by 4, i.e., days 4, 8, 12, 16, 20, 24 and 28.
- Codes 402, 427, 428 and 518.4. 10% sample, when the record does not have a 410–414 code. The 10% sample is selected by choosing only the events with discharges occurring on days of the month divisible by 8, i.e, days 8, 16 and 24.
These sampling fractions are reassessed one year after community surveillance starts.
ICD codes listed on the hospital discharge index may not exactly correspond with those found in the corresponding hospital chart. Regardless, it is the codes listed on the discharge index which determine eligibility for selection.
2.2.3. MIs Occurring Outside the Study Community
Community residents with MI may be hospitalized out of the study area for the following reasons:
- A major hospital catchment area for the region exists outside of the study area (e.g., tertiary care hospital referral centers).
- Residents who work outside of the geographic area may be admitted to an out-of-area hospital if they have an MI at work.
- A resident may have an event while in transit outside of the geographic area for recreation or social activities.
In order to select hospitals outside of the study area to include in surveillance, the Field Center first identifies those hospitals which are located in the surrounding areas. Second, the center determines by checking with local physicians, cardiologists, hospital administrators and others whether patients with acute MI are usually hospitalized locally prior to admission to a tertiary care facility outside the study area. Third, 1984 and 1985 death certificates for study area residents are reviewed. Surveillance is carried out in any hospital outside of the geographic area that contributed at least six eligible in-hospital MI deaths (ICD9-CM 410–414) in the 1984–1985 period. Major medical centers or tertiary referral facilities some distance from the study area are not included in surveillance unless there is evidence that patients with acute MI from the study area are directly admitted to such hospitals without treatment at a within-area hospital. Selection of hospitals included in community surveillance is reassessed during the fourth year of the study.
Community residents hospitalized with acute MI while outside both the study area and the surrounding counties are not identified by routine surveillance. An estimate of the effect of this procedure is available from the surveillance for hospitalized events in cohort members.
2.2.4. Range of Facilities Covered in Surveillance
Hospitalized MI patients are identified by review of records only at acute care hospitals. Nursing homes, rehabilitation hospitals, long-term chronic disease hospitals, and psychiatric hospitals are excluded. A small number of MI patients hospitalized at these chronic care facilities for another disease, e.g., multiple sclerosis, peripheral vascular disease, diabetes, etc., may have an acute MI while in the chronic care facility and not be referred to an acute care facility or may die before referral. These individuals are lost to the surveillance system. Such an event is probably rare and would be difficult to identify from review of chronic care facility records.
Community surveillance does not identify nonfatal MI occurring outside of a hospital and for which the individual is not hospitalized (unrecognized MI).
2.3. Identification of CHD Deaths
2.3.1. Death Certificates
All deaths in the United States must be recorded on a death certificate which is filled out by a physician, medical examiner or coroner. The death certificate is a legally mandated, public document which is filed in the county of the decedent's residence. A copy is filed with the state. If a person dies away from his usual residence, a copy of the death certificate is (eventually) returned to the decedent's county of residence for filing and is also filed at the state health department. In each state, health department trained nosologists code the causes of death given on the death certificate according to the International Classification of Diseases (ICD). The 9th revision of the ICD (termed ICD9) is currently used.
Each of the four states containing the ARIC communities assigns the specific "underlying cause of death" from the nosologist's coding of the death certificate using the Automated Classification of Medical Entities (ACME) system. Computer files, which include the date of death, underlying cause, decedent's age and residence, are available. Each center obtains a monthly printout of potentially eligible cases based on the criteria listed below. The monthly printouts generally contain in-state deaths that occurred three to five months previously. In addition, all four centers annually obtain a final computer tape of eligible deaths to verify that ascertainment is complete and to provide numerators for rate calculations.
Fatal events are selected according to the following eligibility criteria.
- Age. ARIC examines deaths only at ages 35 to 74.
- Place of Residence. The decedent must have lived within the boundaries of the ARIC community. The residence at death determines eligibility. However, if it is found in event investigation that the decedent was only visiting the area, or had two residences, the place where the person lived at least six months out of the year is considered the residence for ARIC purposes. Do not count anonymous "John Doe" deaths. But do count people residing in a local jail at the time of death.
- Date. Only deaths occurring from January 1, 1987 through December 31, 1992 are eligible.
- ICD9 Code. Deaths whose underlying cause is coded (using ICD9) 250, 401, 402, 410–414, 427–429, 440, 518.4, 798 and 799 are selected for documentation of MI or CHD.
Copies of death certificates for the potential fatal events identified on the monthly printouts are obtained from the respective State Departments of Health. Abstractors review each certificate to confirm the death meets age, residency, date, and ICD9 code criteria. ARIC cohort members are identified by comparison with the cohort clinic roster and other criteria outlined in Section 1.10 of Manual 2. The remaining fatal cases are reduced in number by applying various sampling fractions to different classes of ICD9 codes. These constitute the deaths to be investigated:
- Codes 410-A14 and 429.2. 100% sample.
- Codes 250. 401. 402, 427–429 (except 429.2). 440. 518.4. 798 and 799. 25% sample. For a 25% sample of cases, only deaths occurring on days of the month divisible by 4 are selected for further investigation.
A Surveillance Event Eligibility Form (Appendix II) is completed for each event to help with sample selection. An ID number is assigned to each event, a Death Certificate Form (Appendix II) is completed (see Section 3.1), and the death certificate is filed locally.
2.3.2. CHD Deaths Occurring Outside the Study Community
For fatal hospitalized events, the address on the death certificate takes precedence over the address in the hospital record for determining eligibility.
Deaths outside of the study area, but within the state, are included on State Health Department monthly printouts, but some delay between the death and the transfer of the certificate to the place of residence file is expected. If the death certificate file is reviewed for ARIC prior to receipt of out-of-area certificates, subsequent review is undertaken to identify in-transfer deaths.
Deaths that occurred in other states are relatively few in the ARIC study areas which do not closely border another state. The out-of-state deaths cannot be identified in a timely fashion but can be identified on the annual mortality computer tapes provided by the State Health Departments. Access to identifiers for out-of-state deaths is restricted. For these reasons, out-of-state deaths will only be enumerated from vital records and not investigated further.
3. EVENT INVESTIGATION
For hospitalized MI, event investigation entails review of the hospital record. Investigation of fatal CHD includes review of the death certificate and hospital record where available, and, for out-of-hospital deaths in Forsyth County, Jackson, and Minneapolis, physician questionnaires, interviews with next-of-kin and collection of other information. In Washington County, the medical examiner adds relevant questions to his routine inquiry but other out-of-hospital investigations are prohibited.
Procedures for the identification and investigation of hospitalized and fatal events in members of the ARIC cohort differ from community surveillance procedures at certain stages and are described in detail in Manual 2, Section 3. In the following paragraphs, general differences between surveillance and the investigation of cohort events are noted. References to specific procedures within Manual 2, Section 3 are identified where appropriate.
3.1. Procedures for Fatal CHD
The Death Certificate (DTK) Form and the Surveillance Event Eligibility (SEL) Form are completed for all eligible fatal events. A worksheet, the Surveillance Event Investigation Summary (SEI) Form, is used locally to monitor completion of investigation forms. One or more of the following data forms may be completed: Hospital Record Abstraction (ERA) Form, Stroke (SIR) Form (for cohort members only), Informant Interview (IFI) Form, Physician Questionnaire (PHQ), and the Coroner/Medical Examiner Report (COR) Form. Autopsy reports for cohort members are copied. All forms and instructions are located in Appendix II.
For fatal events meeting ARIC eligibility criteria and sampling fractions, the Surveillance Event Eligibility form and the Death Certificate form are completed. Data from the Death Certificate form are entered into a computerized data base locally at each field center.
Some proportion of fatal events, either in-hospital or out-of-hospital, are coroner/medical examiner cases. This means that the county coroner or state medical examiner has performed an investigation of the circumstances of death in order to ascertain that the causes were natural. In this case, the coroner/ medical examiner signs the death certificate. In general, the coroner/medical examiner takes cases of unexpected death where no physician was in attendance during the 24 hours prior to death. During his investigation, the coroner/medical examiner may or may not perform an autopsy. Any death where a legal question is likely to arise (e.g., after surgery, during an automobile accident, etc.) will probably be a coroner/medical examiner case. If the death is certified by a coroner or medical examiner, the Coroner/Medical Examiner Report Form is completed, the data entered into the database at the field center, and later transferred to the Coordinating Center.
Medical Examiner and coroner reports are generally stored in their offices. The entirety of the documents generally require full review in order to complete the Coroner/Medical Examiner Report Form. Whatever can be retrieved from the records of these inquiries may be used to answer questions on the Coroner/Medical Examiner Form whether the document is called a report, an investigation, findings, or a summary.
Procedures for the investigation of fatal events in cohort members are described in Manual 2, Section 3.2.1. Briefly, a Cohort Eligibility Form and Death Certificate Form are completed for a fatal event occurring in a cohort member. A Coroner's Form is completed if the death is certified by a coroner/medical examiner, and the autopsy report copied if an autopsy is performed.
3.1.1. In-Hospital CHD Deaths
In-hospital deaths, which include deaths on the wards, in the ICU, CCU or operating room, may be identified by screening either the hospital discharges or the death certificates. Both the Hospital Record Abstraction Form and the Death Certificate Form are completed if the in-hospital death is eligible for study either as a hospitalized event (according to the discharge codes and sampling fractions specified in Section 2.2.2) or as a fatal event (according to the cause of death codes and sampling fractions specified in Section 2.3.1).
If the in-hospital death is initially identified from the death index, the hospital may occasionally lie outside the catchment area for the ARIC community. In this case, this fact is recorded on the Death Certificate Form and no attempt is made to obtain the hospital record.
Persons who upon record abstraction are found to have been admitted without vital signs are treated as out-of-hospital deaths (as defined in Section 3.1.2). Only the administrative data of the Hospital Record Abstraction Form are recorded in such cases. If the death is first identified from the death index and the death certificate indicates "dead on arrival", an attempt is made to find the hospital record to verify this information.
If the hospital record indicates that the person was transferred within the study area directly from another acute care hospital, the record for the other hospitalization is found and abstracted onto another Hospital Record Abstraction Form, provided the discharge occurred on an eligible date.
3.1.2. Out-of-Hospital CHD Deaths
CHD deaths occurring outside of regular acute care hospitals are categorized as "out-of-hospital CHD deaths". This includes deaths in nursing homes and other chronic care facilities. It also includes persons dead on arrival at acute care hospitals, dying in outpatient departments or emergency rooms, or admitted without vital signs. For purposes of defining out-of-hospital death, "no vital signs" means no pulse rate or no systolic blood pressure. A person admitted on a respirator who never had a pulse rate or a systolic blood pressure off the respirator is also considered an out-of-hospital death.
In Washington County, out-of-hospital deaths are investigated through the medical examiner only (Appendix II). For out-of-hospital deaths in the other three centers, information is sought from the decedent's family and physician(s) within 6 months after death. Prior to contacting the informant or the physician, it is ascertained whether the deceased was a member of the ARIC cohort. If the deceased was a cohort member, the cohort procedures for investigating deaths described in Manual 2, Section 3 and are followed instead of the community surveillance procedures.
The family member is contacted for an interview, the physician is sent a questionnaire. Whenever possible, the informant is the spouse or another family member of the decedent. On other occasions, the informant may be someone else who witnessed the death. Some death certificates contain the names of the spouse and a witness.
First an attempt is made to contact and interview the spouse or a first-degree relative (i.e., son, daughter, or sibling) of the decedent, or someone else who lived with the decedent. If another person witnessed the death, this person is interviewed as well. Using name and address information from the death certificate, an attempt is made to find the informant's telephone number in either the regular or the reverse ("criss-cross") telephone directory. If the telephone number is available, a Format 1 letter (Appendix III) is sent.
If a telephone number cannot be found, a Format 2 letter (Appendix III) is sent asking the informant to return a telephone number on an enclosed form in a self-addressed, stamped envelope (Format 3: Appendix III) to the Surveillance Supervisor at the Field Center. These letters include a request to the U.S. Post Office for address correction and are sent with both the interviewer and Field Center Principal Investigator's signatures.
After enough time for the Format 1 letter to arrive or upon receipt of a reply form, the interview is conducted over the telephone, or if necessary, in person using the Informant Interview Form. If a Format 2 letter is sent and no reply is received in two weeks, another such letter is sent by registered mail. If no reply is received, a Format A letter (Appendix III) is sent to next-door neighbor(s) (identified through the reverse telephone directory) to request information on the whereabouts of the potential informants. A reply is requested on a self-addressed, stamped postcard (Format 5: Appendix III) to the Surveillance Supervisor at the Field Center. Format 2 and Format 4 letters are also sent when a telephone number is initially available, but attempts at telephone contacts with informants are unsuccessful. If no reply is received from the neighbors, no further effort is needed.
When the death is witnessed by someone other than a member of the decedent's family, both a family member and the witness are interviewed. In such a case, the information from both interviews are recorded on separate Informant Interview Forms. Up to three (the three best) Informant Interview Forms may be completed for a given event.
Information is sought from physicians by sending the Physician Questionnaire. One questionnaire is sent to the physician who signed the death certificate, if he/she is not the medical examiner. From the informant interview, an attempt is made to identify the decedent's usual physician and/or a physician who attended the decedent for heart disease during the four weeks prior to death. A questionnaire is sent to these physicians (if any, and if different from the one signing the death certificate). Sample cover letters are provided in Appendix III for each of these physician contacts (Formats 7 and 8, respectively). Up to two (the two best) Physician Questionnaires may be entered into the ARIC database for a given event.
If there is no response after four weeks of the initial mailing to a physician, a follow-up letter and another copy of the Physician Questionnaire are sent. If there is no response after eight weeks of the initial mailing, the physician is contacted by telephone. On occasion, prior to returning the Physician Questionnaire (or prior to answering questions over the telephone), the physician requests a release form signed by the informant, which can be modeled after the Release-of-Information Form for physicians (Format 9: Appendix III) or for nursing homes (Format 6: Appendix III).
If the patient had no physician or no knowledgeable physician can be identified, and the patient's medical record or emergency room record is accessible, then it is permissible for an ARIC abstractor to complete the physician questionnaire using the record.
If the fatal event was a coroner's or medical examiner's case, his/her report is abstracted onto the Coroner Form. The medical examiner/coroner may require a Release of Information Form. If the decedent died in a nursing home, nursing home personnel are asked to complete a Physician Questionnaire based on the nursing home record. Centers may offer to assist with abstraction if this would be helpful. The nursing home may require the family informant to provide a Release-of-Information Form (Format 6, Appendix III).
If information provided by the informants or physicians indicates that a person who died out-of-hospital was hospitalized within 28 days prior to death for MI or heart surgery, an attempt is made to locate the hospital record. If the discharge diagnoses include an ARIC screening code, the chart is abstracted onto the Hospital Record Abstraction Form. Under these circumstances, for those hospitals which are not part of the community surveillance, the interviewer obtains the informant's permission (Format 10, Appendix III).
Procedures for the investigation of out-of-hospital deaths occurring in cohort members are described in Manual 2, Section 3.2.1.2. Procedures for contacting informants are similar to those described above, except that the letters refer to the decedent's participation in the ARIC Study (letters are in Manual 2, Appendix VII). A copy of the Release-of-Informtion forms signed by deceased accompany the Physician Questionnaire.
3.2. Procedures for Hospitalized MI
The Hospital Record Abstraction Form is used to abstract events meeting ARIC eligibility criteria for age, residence, date, hospital discharge code and sampling fraction (Section 2.2.2). If a patient was discharged alive without an ICD9 410 or 411 discharge code and with no ECGs taken and no cardiac enzymes measured, only the administrative information on the Hospital Record Abstraction Form is completed. Otherwise, the entire form is completed. There are a few cases in which the ICD9 code is recorded incorrectly, so that a code on the diagnostic index meets the ARIC criteria but none of the diagnoses recorded on the discharge summary of the medical record meet the study criteria. The HRA Form is still completed in such a case.
Prior to abstracting a record from a hospital for ARIC, information is collected on the normal ranges used for each of the cardiac enzymes abstracted. Many hospitals report use of more than one upper limit of normal for a particular enzyme, for example, when a different laboratory is used for determinations at night or on weekends.
If the hospital record indicates that the patient was transferred directly from another acute care hospital, or that the patient upon discharge is being transferred directly to another acute care hospital, the record for the other hospitalization is found and abstracted if the discharge occurred on an eligible date.
Procedures for investigation of hospitalized events with a discharge diagnosis code for MI or stroke occurring in cohort members are described in Manual 2, Section 3.2.2. Selection codes are listed in Section 3.1.1.2. A Hospital Record Abstraction Form and/or Stroke Form may be used. Cohort ECGs are coded by abstractors and copied and sent to the University of Minnesota to obtain full Minnesota Code (described in Manual 2, Section 3.3.1.7).
3.3. Summary of CHD Event. Investigations
The following scheme summarizes the forms completed for eligible surveillance events:
- Out-of-hospital CHD death, as defined in Section 3.1.2
- Death Certificate Form, Surveillance Event Eligibility Form
- Up to two Physician Questionnaires and Informant Interview Forms
- Coroner Form on all coroner/medical examiner's cases and Hospital Record Abstraction Form on cases hospitalized in past 28 days with heart conditions meeting screening codes.
- *Hospital CHD deaths, no vital signs in-hospital
- Surveillance Event Eligibility Form
- First part of Hospital Record Abstraction Form, then investigate as 1, above.
- *Hospital CHD death, vital signs sometime in hospital
- Surveillance Event Eligibility Form, Death Certificate Form, Hospital Record Abstraction Form.
- *Hospitalized CHD case, discharged alive
- Surveillance Event Eligibility Form, Hospital Record Abstraction Form.
*If a patient also transferred to or from a catchment area hospital, complete the additional Hospital Record Abstraction forms.
3.4. Correction of Erroneous Event Investigation Procedures
A fatal or hospitalized CHD event may be identified by surveillance procedures (death certificates or hospital discharge indices) and investigated as a surveillance event, then discovered at a later time to have occurred in a cohort member. In the case of a hospitalized event, a second Hospital Record Abstractor Form is completed independently by a. second abstractor (see Section 8.1). Twelve-lead ECG's have to be copied and sent to the Minnesota ECG Reading Center for coding. In addition, certain surveillance forms have to be replaced by cohort forms, certain items on other forms changed, and possibly additional forms appropriate for cohort members completed. Specifically, the Cohort Eligibility Form must replace the Surveillance Eligibility Form. Additional forms required for cohort members have to be indicated on the Death Certificate Form if the death occurred in an out-of-catchment area hospital. The Physician Questionnaire and Informant Interview Form remain unchanged.
If an eligible event has been investigated erroneously as a cohort event, the Cohort Eligibility Form must be deleted and replaced by the Surveillance Eligibility Form. If the event investigated was a stroke, the Stroke Form must be deleted.
4. DIAGNOSTIC CRITERIA
4.1. Fatal Coronary Heart Disease (CHD)
4.1.1. Confirmed Fatal Myocardial Infarction (MI)
Must meet criteria (1) AND (2) below:
- No known non-atherosclerotic or non-cardiac atherosclerotic process or event that was probably lethal.
- Confirmed hospitalized MI within four weeks of death; use criteria in Section 4.2.2 below for Confirmed Hospitalized MI.
4.1.2. Confirmed Fatal CHD
Must meet ALL of the following criteria:
- Lack of sufficient evidence to diagnose Confirmed Fatal MI according to the criteria given in Section 4.1.1.
- No known non-atherosclerotic or non-cardiac atherosclerotic process or event that was probably lethal.
- Presence of one or both of the following findings:
- A history of chest pain within 72 hours of death;
- A history of ever having had chronic ischemic heart disease such as definite or possible MI, coronary insufficiency, or angina pectoris in the absence of valvular disease or non-ischemic cardiomyopathy.
4.1.3. Possible Fatal CHD
Must meet ALL of the following criteria:
- Lack of sufficient evidence to diagnose Confirmed Fatal MI or Confirmed Fatal CHD according to the criteria in Sections 4.1.1 and 4.1.2.
- No known non-atherosclerotic or non-cardiac atherosclerotic process or event that was probably lethal.
- Death certificate with consistent underlying cause, i.e., ICD9 codes: 410-414, 427.5, 429.2, and 799.
4.1.4. Non-CHD Death
All deaths that do not meet the above criteria for Confirmed Fatal MI, Confirmed Fatal CHD, or Possible Fatal CHD.
4.1.5. Chronology of Death
All CHD deaths are classified, where possible, according to time interval from onset of acute symptoms to time of death.
4.1.6. Limitation of Activity
All out-of-hospital CHD deaths are classified according to whether the decedent's activity was limited in the month before death because of sickness or illness.
4.2. Hospitalized Myocardial Infarction (MI)
4.2.1. Introduction
The aim of the ARIC Study is to establish a well standardized process for the identification of hospitalized coronary disease of an acute nature, allowing for valid inter-community and longitudinal comparisons. Mild and chronic manifestations of ischemic heart disease, such as angina pectoris, congestive heart failure, and arrhythmias are not identified as target diagnoses in community surveillance but are included in the screening process to aid in the identification of acute MI. So-called silent infarctions are excluded. Transmural infarctions, based on the finding of Q waves, which are more reliably and accurately coded than ST and T wave changes, are sought in all hospital records abstracted. Non-transmural Mis are identified only if they meet specified chest pain and enzyme criteria. The criteria presented below are based on two source documents: the findings of the CCSP Pilot Study and the results of the Minnesota Heart Survey1–3, as well as other surveillance studies.
The diagnostic criteria presented here approximate those contained in the above mentioned documents. The differences are the lack of a duration requirement for cardiac pain, the use of Q waves only to define ECG categories for most records and the use of the more sensitive and specific CK-MB and LDH isoenzymes. The combinations of pain, ECG and enzyme categories required for each diagnosis belov are approximately the same as those contained in the above-mentioned documents.
It is recognized that aggressive treatment of early signs and symptoms of acute coronary events, such as coronary artery bypass graft or streptokinase infusion, may prevent the development of the full diagnostic syndrome. In such cases, it may be difficult to diagnose the event accurately. The use of such modalities are recorded and subject to data analysis, but are not employed in the criteria for diagnosis.
4.2.2. Confirmed Hospitalized MI
Must meet one or more of the following criteria:
- Evolving diagnostic Q wave (defined below);
OR
- Diagnostic Q wave and abnormal enzymes (both defined below);
OR
- Cardiac pain (defined below) and abnormal enzymes.
4.2.3. Possible Hospitalized MI
Must meet one or more of the following criteria in the absence of findings that meet the criteria for Confirmed Hospitalized MI:
- Equivocal enzymes.
AND
- Cardiac pain or diagnostic Q waves or equivocal Q waves.
- Equivocal enzymes.
- Abnormal enzymes.
OR
- Incomplete enzymes, diagnostic Q waves and cardiac pain.
The criteria for Confirmed and Possible Hospitalized MI are summarized in Table 2.
4.2.4. Definition of Cardiac Pain
Pain having both the following characteristics:
- It occurs anywhere in the anterior chest, left arm or jaw.
AND
- It has no definite non-cardiac cause.
4.2.5. Definitions of Electrocardiographic Criteria
The following hospital ECG tracings are abstracted:
- The first codable ECG recorded after admission
- The last codable ECG recorded before discharge; and
- The last codable ECG recorded on day 3 (or the first ECG thereafter) following admission or an in-hospital event.
The ECG series is assigned the highest category for which criteria are met, i.e., evolving diagnostic is greater than diagnostic is greater than equivocal is greater than other. The ECGs are coded using Minnesota Code (Manual 5, Electrocardiography, Appendix E).
4.2.5.1. Evolving Diagnostic Q Waves
An evolving pattern on serial ECGs of ECG changes within lead groups, i.e, anterior (V1-V5); lateral (I, aVL, V6) or inferior (II, III, aVF). Two or more ECG recordings during the hospitalization are needed for this classification. The following criterion must be met: No Q code in one ECG record followed by a record with a diagnostic Q code (see 4.2.5.2 Diagnostic Q Wave).
| CARDIAC PAIN | ECG FINDINGS | ENZYMES | DIAGNOSIS |
|---|---|---|---|
| Present | Evolving Diagnostic Q wave | Abnormal | Confirmed MI |
| Equivocal | Confirmed MI | ||
| Incomplete | Confirmed MI | ||
| Normal | Confirmed MI | ||
| Diagnostic Q wave | Abnormal | Confirmed MI | |
| Equivocal | Possible MI | ||
| Incomplete | Possible MI | ||
| Normal | No MI | ||
| Equivocal Q wave | Abnormal | Confirmed MI | |
| Equivocal | Possible MI | ||
| Incomplete | No MI | ||
| Normal | No MI | ||
| Absent, Uncodable, or other | Abnormal | Confirmed MI | |
| Equivocal | Possible MI | ||
| Incomplete | No MI | ||
| Normal | No MI | ||
| Not present | Evolving Diagnostic Q wave | Abnormal | Confirmed MI |
| Equivocal | Confirmed MI | ||
| Incomplete | Confirmed MI | ||
| Normal | Confirmed MI | ||
| Diagnostic Q wave | Abnormal | Confirmed MI | |
| Equivocal | Possible MI | ||
| Incomplete | No MI | ||
| Normal | No MI | ||
| Equivocal Q wave | Abnormal | Possible MI | |
| Equivocal | Possible MI | ||
| Incomplete | No MI | ||
| Normal | No MI | ||
| Absent, Uncodable, or other | Abnormal | Possible MI | |
| Equivocal | No MI | ||
| Incomplete | No MI | ||
| Normal | No MI |
4.2.5.2. Diagnostic Q Wave
Minnesota Code 1-1-1 through 1-2-5 or 1-2-7 for Q and QS patterns. These are indicated on the Prototype Surveillance ECG coding form (Manual 5, Appendix P) as C1, D1, or E1, where C, D, and E represent the three lead groups.
4.2.5.3. Equivocal Q Wave
Q and QS patterns 1-2-8 through 1-3-6. These are on the coding form as C2, D2, and E2.
4.2.5.4. Other ECG
All other findings, including normal. These are on the coding form as A1 or B2 (other findings) or C3, D3, and E3 (no Q-codes in any lead group).
4.2.5.5. Uncodable ECG
This is marked on the coding form as A2 only if all ECGs for an event are uncodable.
- Three or more missing leads.
- Muscle tremor artifact that produces possible false initial R's.
- Other technical errors making Q wave measurements impossible, such as extreme lack of centering, marked clipping, or no calibration mark.
- Other conditions defined as "uncodable" by the Minnesota Code.
4.2.6. Definitions of Cardiac Enzyme Criteria
All pertinent enzyme results (as defined below) recorded on days 1 through A after hospital admission or an in-hospital CHD event are abstracted. Information on non-ischemic cause for elevated enzymes is abstracted exclusively from the discharge summary on the medical chart.
4.2.6.1. Abnormal Cardiac Enzymes
Enzymes are classed as "abnormal" if any enzyme values recorded meet any of the following criteria:
- CK-MB is "present" (if laboratory uses the criterion of
"present" or "absent" without reporting a more specific value)
or CK-MB is twice the upper limits of normal (if the laboratory gives a
normal range) or, if no normal range is given, the CK-MB (heart fraction) is greater than or equal
to 10% of the total CK value,
AND
- There is no known non-ischemic cause (cardiac surgery, severe muscle trauma, rhabdomyolysis) for the elevated enzyme value.
- CK-MB is "present" (if laboratory uses the criterion of
"present" or "absent" without reporting a more specific value)
or CK-MB is twice the upper limits of normal (if the laboratory gives a
normal range) or, if no normal range is given, the CK-MB (heart fraction) is greater than or equal
to 10% of the total CK value,
- The ratioLDH1 :
LDH2 ≥ 1.
AND
- There is no evidence of hemolytic disease.
- The ratioLDH1 :
LDH2 ≥ 1.
- Total CK and LDH are both at least twice the upper limits of normal.
(These increases do not have to occur on the sane day.)
AND
- There is no known non-ischemic cause (cardiac surgery, severe muscle trauma, rhabdorayolysis) for the elevated enzyme value and no evidence of hemolytic disease.
- Total CK and LDH are both at least twice the upper limits of normal.
(These increases do not have to occur on the sane day.)
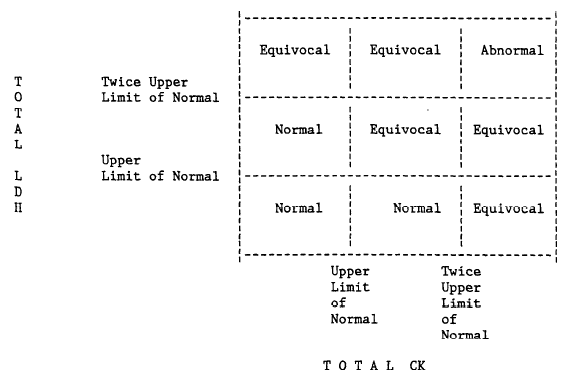
4.2.6.2. Equivocal Cardiac Enzymes
Enzymes are classed as "equivocal" if the criteria for abnormal enzymes are not met and if:
- Either total CK or total LDH are at least twice the upper limits of normal.
OR
- Both total CK and total LDH are between the upper limits of normal and twice the upper limits
of normal. (These increases do not have to occur on the same day.)
OR
- CK-MB is "weakly present" or between the upper limits of normal and twice the upper limits of normal or 5–9% of total CK.
A summary of the enzyme diagnostic criteria, as related to total CK and LDH is given in the following algorithm, Figure 1.
5. EVENT DETERMINATION
Hospitalized events are classified using a computer algorithm based on the criteria given in Section 4.2. An ARIC Morbidity and Mortality Classification Committee (MMCC) reviews all cohort hospitalized events to validate the computer diagnostic process. The MMCC also reviews a) hospital records in which the chest pain or elevated enzyme values recorded was classified as resulting from non-cardiac causes, b) cases coded to ICD 410 but diagnosed by the computer as "no MI" and c) eligible in-hospital deaths classified as no MI to determine if fatal CHD criteria are met.
All forms used to gather information on deaths investigated in community surveillance are used in the diagnostic process. Diagnostic criteria are given in Section 4. A proportion of the deaths is classified by computer as "Confirmed Fatal MI", "Confirmed Fatal CHD" or "Non-CHD Death" based on unequivocal coded information. The remainder of deaths requires review by the MMCC, using both the coded and the narrative information in the data collection forms. A convenient computer abstract of relevant coded information is used by the MMCC in reviewing each of these events. Relevant narrative information is reviewed from photocopies of the abstractor comments.
MMCC reviewers assign a diagnosis based on the diagnostic criteria but also record their "clinical impression." Confirmed and possible CHD deaths are classified by the duration from first symptoms to death.
For each event requiring an MMCC judgement, the process begins with two MMCC members independently reviewing the information. If they agree, adjudication by the full committee is not required. If they disagree, the Coordinating Center informs them that they have disagreed (without specifying the nature of the disagreement). If, after review, the two judges still disagree, adjudication by the MMCC chairman or at his discretion, the full MMCC is exercised. Selection of the two judges for each event is made by the Coordinating Center by a randomized process. The Coordinating Center also assigns specific tasks to the judges for each case (diagnosis, chronology of death, cause of elevated enzymes, etc.).
An important function of the MMCC is to maintain complete records of any clarifications of ARIC diagnostic criteria required to reach diagnostic decisions. Such "case law" is systematized for convenient reference purposes and, when appropriate, incorporated into the ARIC diagnostic protocol.
6. MEDICAL CARE ASSESSMENT
This section describes the assessment of medical care in community-wide surveillance. Medical care is also assessed in cohort members, as described in Manual 2, Section 4. Medical care elements which are recorded only in cohort members include the participant's access to and use of providers for routine and special care, use of all prescription and over-the-counter medications, records of all hospitalizations for all reasons, records of all cardiovascular procedures and all cardiovascular diagnoses received, and detailed information on hospitalizations for CHD and stroke.
Community surveillance for medical care includes information collected for out-of-hospital deaths, information abstracted from hospital records and information about the services provided in the community's acute care hospitals.
For out-of-hospital deaths, the Physician Questionnaire and Informant Interview Form allow collection of information about physician visits prior to the acute event, utilization of physician and emergency services during the acute event, history of hospital admission within one month prior to death, receipt of cardiopulmonary resuscitation, delay in receiving definitive care, use of nitrates and digitalis shortly before death and history of coronary bypass surgery. The potential for cardiopulmonary resuscitation is assessed by the information on whether death was witnessed and the location of death.
Demographic information and much of the information collected on out-of-hospital deaths is also available in hospital abstracting. In addition, information is collected on transportation to the hospital, time of arrival, receipt of cardiopulmonary resuscitation, use of a number of new procedures and medications for treatment of the cardiovascular event (such as angioplasty and streptokinase infusion), and the use of diagnostic procedures (such as cardiac catheterization and echocardiography).
7. LINKAGE OF MULTIPLE EVENTS
Since many deaths are listed on both the hospital discharge index and the state death index, survey personnel must compare these lists carefully to avoid duplicating the investigation of in-hospital deaths. If an eligible in-hospital death is found first from death certificate lists, the case is flagged and it is linked with the hospital chart when that record is found. The related forms are given the same event ID number. If an in-hospital death is found first using a hospital source, the hospital chart is abstracted, and the death certificate obtained as soon as possible. Again, only one event ID number is assigned.
If the hospital record indicates that the patient was transferred directly from another acute care hospital, or that the patient upon discharge is being transferred directly to another acute care hospital, the record for the other hospitalization is abstracted onto another HRA Form. The two forms initially have different hospitalization I.D. numbers. The procedures for identifying these as belonging to the same event will be established by the Coordinating Center.
Over the duration of the ARIC surveillance, increasing numbers of community residents are hospitalized for cardiovascular conditions more than once. Others are hospitalized and subsequently die of CHD. Sufficient information for correct identification of these patients is collected, where hospitals permit, and matching procedures based on the identifiers which can be recorded must be developed.
On occasion, it is difficult to differentiate between two or more successive admissions for the same event and two or more different events in the same person. As it is often difficult to make this distinction on the basis of ECG, enzyme or pain characteristics, a simple rule is followed: a hospital admission for MI occurring within 28 days of a previous discharge is regarded as the same event, for purposes of calculating rates.
8. RELIABILITY AND VALIDITY OF COMMUNITY SURVEILLANCE PROCEDURES
For cohort events, ascertainment must be as complete as possible and diagnoses as accurate as possible, in order to evaluate correctly the precursor status of each of the risk and medical care factors carefully measured at home interview, clinical examination and follow-up. Optimal cohort ascertainment and diagnoses also serve as a standard against which to compare the more routine ascertainment and diagnostic procedures used in community-wide surveillance.
Once data collection is complete for cohort events, a diagnosis, by computer or by the MMCC, is made both with and again without the additional information which is collected only for cohort members to compare the effect of the additional information.
For hospitalized events occurring among cohort members, diagnoses given by surveillance and cohort methods are compared directly using the same set of events in the same patients. For fatal events, cohort diagnoses in cohort members are compared with surveillance diagnoses in comparable non-cohort community residents. Additionally, for cohort decedents, diagnoses made on the basis of information available in surveillance are compared with diagnoses based on all the information available for cohort members.
8.1. Reliability
The extent to which cohort procedures can be used to confirm results of surveillance is limited, as described below, by the extent with which it is practical to undertake independent parallel investigations.
Hospital records are abstracted twice for cohort members whose hospital discharge codes are eligible for surveillance, once as a. part of routine community surveillance and again as part of quality control, on the same schedule as reabstraction of Surveillance records. The determination that the patient is a cohort member is achieved both by routine checking of the names of surveillance patients against cohort member lists and by routine cohort follow-up. The abstraction of the hospital record for the cohort member is undertaken either by an abstractor supervisor or by a different abstractor from the one who abstracted the record for surveillance. Differences between abstractors on key items of information (1) lead to a third record abstraction for adjudication of these items, (2) are recorded for quality assurance documentation, and (3) are used in the continuing training of abstractors. Abstraction of hospital records for cohort and routine surveillance differs only with respect to ECGs, which for cohort events receive full Minnesota coding by the ARIC ECG Center.
Unlike hospital record abstraction, death investigations are not conducted in a fully independent manner. No interviews with family informants or physician questionnaires are completed in surveillance until it is determined whether or not the decedent is a cohort member. If the decedent is a cohort participant, the special tracing information available for cohort members is used to establish contact with family members and physicians. The introduction to these informants includes reference to the permission which the decedent has given ARIC staff to undertake the investigation. If routine surveillance death investigation preceded use of the cohort approach, an early refusal or a reluctant level of cooperation might jeopardize an otherwise successful investigation.
Efficient methods for identifying surveillance residents who are cohort members and for appropriately scheduling the death investigations require direct assistance from the Coordinating Center to the Field Centers. These methods assure that where duplication of procedures is required for quality assurance, the duplication is appropriately blinded.
8.2. Validity
For cohort members, it is possible to validate information on selected variables obtained by community surveillance procedures, by using the more accurate information obtained by cohort procedures. An example of a variable for which the validity of surveillance approaches is assessed by using information available for cohort members is MI order (new vs. recurrent). Although community surveillance defines an MI as new when there is no mention in the medical chart of a past episode, additional information is available for cohort participants, including the clinic interview and a baseline ECG. Thus, the more accurate definitions of "new" and "recurrent" available for the cohort are used as a "gold standard" to determine sensitivity and specificity of surveillance procedures. Specifically, ECG findings at baseline are used to determine in cohort members to which extent a diagnostic Q wave, as classified in community surveillance, can be assumed to be either a "new" or an "old" Q wave.
"Ecologic" validation is also undertaken, by comparing event rates derived from community surveillance with those obtained from cohort follow-up. In addition to examining surveillance rates in population subgroups, the similarity of patterns of associations of rates with demographic variables between cohort and community surveillance is evaluated, using the former as the "gold standard".
9. REFERENCES
1. Gillum RF, Fortmann S, Prineas RJ, Kottke T. International Diagnostic Criteria for Myocardial Infarction and Acute Stroke. Prepared for the Committee on Criteria and Methods, Council on Epidemiology, American Heart Association. Am Heart J. 1984;108:150–158.
2. World Health Organization. Oct. 1981 Proposal for the Multinational Monitoring of Trends and Determinations of Cardiovascular Disease and Provisional Protocol.
3. CCSP Coordinating Center. Community Cardiovascular Surveillance Program: Final Report to the National Heart, Lung, and Blood Institute; June 1, 1984;
APPENDIX I
(1). ICD9 Codes for the Identification of Fatal CHD
| Code | Title |
|---|---|
| Event: Coronary Heart Disease | |
| 250 | Diabetes Mellitus |
| 401 | Essential Hypertension |
| 402 | Hypertensive Heart Disease |
| 410 | Acute Myocardial Infarction |
| 411 | Other Acute and Subacute Ischemic Heart Disease |
| 412 | Old Myocardial Infarction |
| 413 | Angina Pectoris |
| 414 | Other Chronic Ischemic Heart Disease |
| 427 | Cardiac Dysrhythmias |
| 428 | Heart Failure |
| 429 | Ill-Defined Descriptions and Complications of Heart Disease |
| 440 | Atherosclerosis |
| 518.4 | Acute Edema of Lung |
| 798 | Sudden Death, Cause Unknown |
| 799 | Other Ill-Defined and Unknown Causes of Morbidity and Mortality |
(2). ICD-9 Codes for the Identification of Hospitalized Myocardial Infarction
| Code | Title |
|---|---|
| Event: Myocardial Infarction | |
| 402 | Hypertensive Heart Disease |
| 410 | Acute Myocardial Infarction |
| 411 | Other Acute and Subacute Ischemic Heart Disease |
| 412 | Old Myocardial Infarction |
| 413 | Angina Pectoris |
| 414 | Other Chronic Ishcemic Heart Disease |
| 427 | Cardiac Dysrhythmias |
| 428 | Heart Failure |
| 518.4 | Acute Edema of Lung, Unspecified |
ARIC SURVEILLANCE EVENT INVESTIGATION SUMMARY FORM (SEI)


SURVEILLANCE EVENT INVESTIGATION SUMMARY FORM INSTRUCTIONS
I. GENERAL INSTRUCTIONS
The Surveillance Event Investigation Summary Form (SEI) is used to monitor the procedures of event investigation used in ARIC. It is intended to be used by the supervisor of Surveillance procedures at each field center. The SEI Form should be completed according to information collected in other forms for a given eligible event. This information is summarized in the "form flags" at the end of each form, which indicate what additional forms should be collected for an event.
II. INSTRUCTIONS FOR FORM COMPLETION
The form is divided into four parts: header information, procedure flowchart, form completion information, and contacting information for sources.
The header information at the top of page 1 should be completed as soon as an eligible event is identified by the Surveillance Event Eligibility Form (SEL). Complete the event ID number as assigned on the SEL Form, and record the current date and the name of the individual whose event is under investigation.
The flowchart on the left side of page 1 depicts the procedures used in event investigation. "Yes" and "No" paths emerge from each decision box and should be checked to indicate which path to follow. The sources of information for these decisions are detailed in the next section, but they essentially repeat the rules defined by the form flags in each form. If the designated path passes through a box containing three-letter form codes, these forms should be collected. As many as three Informant Interview (IFI) Forms and two Physician Questionnaires (PHQ) may be required. It is possible that evidence acquired later in the process may conflict with evidence acquired earlier. In such cases, a path may have to be changed from the one which was previously defined. For example, a death is defined as "in-hospital" on the death certificate. However, when the hospital records are reviewed, it is shown that the person died in the Emergency Room (ER). The path is then changed to indicate that the death is "out-of-hospital/DOA/ER."
The right-hand side of page 1 lists all the possible forms that could be scheduled for completion for an event. Based on the path which is followed in the flowchart, certain forms should be designated as "Needed" by circling the "Y" in that column. When the effort to complete this needed form is begun, the date should be recorded in the "Date Initiated" column. (For the Physician Questionnaire, this should reflect the date it is first sent to the physician. If additional mailings are made, record the date in the "Other Information" column. Dates of mailings for the Informant Interview attempts should be kept in the "Record of Calls" section of that form.) As each needed form is completed, the "C" under "Completed/Permanently Missing" should be circled. If, after exhaustive effort, a needed form cannot be completed, circle the "P" in this column. ALL FORM FLAGS MUST BE CAREFULLY REVIEWED IN COMBINATION WITH THESE PROCEDURES IN ORDER TO IDENTIFY EXACTLY WHICH FORMS SHOULD BE COLLECTED !
The second page of the SEI Form is used to record pertinent information for contacting of sources, such as names, addresses, and phone numbers. Space for additional comments is provided at the end. When the investigation is completed, i.e., all needed forms are designated either "completed" or "permanently missing," the date and code number are recorded at the end of the form.
The SEI form should be kept on file at the field center for all eligible events.
III. SOURCES OF INFORMATION
The following list indicates what forms and item numbers to use in the process of defining the appropriate path on the SEI Form:
| Decision | Answer | Source Form and Item, Response(s) |
|---|---|---|
| Death? | Yes if: | SEL 7 = Y |
| No if: | SEL 7 = N | |
| Out-of-hosp/DOA/ER? | Yes if: | DTH 12 = N or O or DTH 13 = A, B, or C |
| No if: | DTH 12 = A and DTH 13 = D, E, or F OR DTH 12 = B and DTH 13 = D, E, or F | |
| Hospital in catchment? | Yes if: | DTH 12 = A |
| No if: | DTH 12 = B | |
| Vital Signs? | HRA Form | |
| Hosp for MI within 28 days? | Yes if: | IFI 6a = Y or IFI 6b = Y |
| No if: | Otherwise | |
| Transfer? | HRA Form |
ARIC SURVEILLANCE EVENT ELIGIBILITY




SURVEILLANCE EVENT ELIGIBILITY FORM INSTRUCTIONS
I. GENERAL INSTRUCTIONS
The Surveillance Event Eligibility Form should be the first form to be completed for an identified event. The abstractor must be certified and should be familiar with the document titled "General Instructions For Completing Paper Forms" prior to completing this form. Event ID Number should only be completed for eligible events. Thus, it cannot be completed until the remainder of the form has been filled out.
The Surveillance Event Eligibility Form is used for all surveillance events regardless of the source of information (hospital records or death certificates). If a computer printout is used, it must contain the pertinent items.
SPECIAL NOTE ON HOSPITALIZED DEATHS: It is common to identify a death occurring in a hospital twice (once from the death index, and once from the hospital discharge index). In order to avoid duplication, all eligible deaths should be filed (or otherwise listed) BY DEATH CERTIFICATE NUMBER. Then, whenever a death is identified as eligible, it should be checked to make sure that it was not previously identified from another source. To do this, check the death certificate number for the event against the list/file of death certificate numbers for previously identified events.
II. DETAILED INSTRUCTIONS FOR VARIOUS QUESTIONS
- Name. Record the individual's first, middle, and last name, or as much as is available. Print clearly in capital letters.
- Address of Individual. Record the complete address, including the house number, the street name, and the apartment, box or lot number. This should be the place of residence for a patient or decedent. If the zip code is not available on the death certificate and is needed as an eligibility criterion, it must be located elsewhere and recorded. If it is not needed, it need not be recorded.
- Address Boundaries. Check to see that the address is eligible for the study community. If it is, record "Yes" and continue. If it is not, record "No" and indicate "not eligible" in item 19.
- Date of discharge or death. If the source of information is a hospital record, record the date of discharge. It will be found on the face sheet or the ER sheet. If the patient transferred from acute care to rehab or chronic care in the same hospital, count the date of transfer as the discharge date. If a computer listing is used, use the date on that list. If the patient died, or if the source of information is a death certificate, record the date of death. If the date recorded is not between 1/1/87 and 12/31/92 (inclusive), go to item 19 and indicate "not eligible." Otherwise, continue to complete the form.
- Birthdate. Birthdate is found on the death certificate or the face sheet of the medical record. Do not use the age given by the physician on the history and physical since it may be incorrect. If a computer printout is used, it must include this information.
- Age at discharge or death. If age at death is recorded and is within two years of eligibility cut-off (35
or 74 years), check by applying algorithm for computing age. Also, if age is not recorded, use this
algorithm to compute it. Record the correct age on form.
Compute the age at the date of discharge/death by subtracting the birthdate from the date of discharge/death. Do this as follows:
- Subtract the year of birth from the year of discharge/death.
- If the month and day of discharge/death fall before the month and day of birth (ignoring year), subtract one year from the above result.
Do not use an age given in the chart unless the birthdate cannot be found. If a computer printout is used, it must include this information. If the age is not between 35 and 74 (inclusive), go to item 19 and indicate "not eligible." Otherwise, continue to complete the form.
- Individual died? If the source is a death certificate/index or the hospital record indicates that the patient died, record "Yes." Otherwise, record "No."
- Cohort participant? Using name and address information, determine whether the individual was a participant in the Cohort Study. If "No," go to item 10.
- Visit 1 date. For cohort members, record the date of their baseline visit in contact year 1. If their date of baseline visit (item 9) is earlier than their date of discharge/death (item 4), go to item 19. (These events will be investigated as cohort events. Conversely, if the baseline visit is after the date of discharge/death, they are investigated as surveillance events.)
- Source. Indicate the source where the event was identified.
- Death Certificate Number. This number will be found stamped or typed on the death certificate. If a computer printout is used, it must include this information. Record the number starting in the leftmost box. Do not add zeroes to the right of the number.
- ICD code for underlying cause of death. Record the underlying cause of death by listing the ICD
code recorded on the death certificate. If it is not available, record the immediate cause. If the
first character is a letter, record that letter in the first box, followed by the 3-digit number
(right justified to the decimal point). Otherwise, record the 3-digit number with the first box left
blank. If a fourth digit (i.e. to right of decimal point) is not given, leave decimal field blank.
Do not zero fill. Examples:

- Is the code 410–414 or 429.2? Any code with a 3-digit body of 410 through 414 is accepted, regardless of decimal or prefix letter. A code of 429.2 is also accepted. If any of these, go to item 17 and indicate "eligible."
- Is the code 250, 401, 402, 427–429 (not 429.2), 440, 518.4, 798, or 799? Use the same rule as in (b). If "No," go to item 19 and indicate "not eligible."
- Date of death 4, 8, 12, 16, 20, 24, or 28. If the day of the month is one of those listed, record "Yes" and go to item 17 and indicate "eligible." If not, record "No", go to item 19 and indicate "not eligible."
- ICD code for underlying cause of death. Record the underlying cause of death by listing the ICD
code recorded on the death certificate. If it is not available, record the immediate cause. If the
first character is a letter, record that letter in the first box, followed by the 3-digit number
(right justified to the decimal point). Otherwise, record the 3-digit number with the first box left
blank. If a fourth digit (i.e. to right of decimal point) is not given, leave decimal field blank.
Do not zero fill. Examples:
- Hospital Name. Print clearly in capital letters.
- Hospital Record Number. This number will be found stamped or typed on almost every page of the hospital record. The easiest place to find it is both on the medical record folder and in the upper right/left hand corner of the face sheet. If a computer printout is used, it must include this information. Record the number starting in the leftmost box. Do not add zeroes to the right of the number.
- Hospital discharge diagnosis codes. Record the codes as listed on the hospital discharge index in the order listed. If a fourth digit (i.e. to right of decimal point) is not given, leave decimal field blank. Do not enter a zero there unless it appears on the chart. (See examples under item 11.a. above.) Be sure to include the primary diagnosis as designated by the M.D., as well as all secondary diagnoses. (If more than 16 codes are given, call the CC for prioritization.)
- Is a 410 code listed? If the 3-digit body of any code is 410, regardless of decimal or prefix, record "Yes" and go to item 17 and indicate "eligible."
- Is a 411 code listed? If the 3-digit body of any code is 411, regardless of decimal or prefix, record "Yes" and continue. If No, go to item 16.d.
- Is the date of discharge divisible by 2? If the day of the month is an even number, record "Yes" and go to item 17 and indicate "eligible." If not, record "No" and go to item 19 and indicate that event is "not eligible".
- Is a 412–414 code listed? If the 3-digit body of any code is in the specified range, regardless of decimal or prefix, record "Yes" and continue. If No, go to item 16.f.
- Date of discharge 4, 8, 12, 16, 20, 24, or 28. If the day of the month is one of those listed, record "Yes" and go to item 17 and indicate "eligible." If not, record "No" and go to item 19 and indicate event is "not eligible".
- Is a 402, 427, 428, or 518.4 code listed? If the 3-digit body of any code qualifies, regardless of decimal or prefix, record "Yes" and continue. If "No," go to item 19 and indicate "not eligible."
- Date of discharge 8, 16, or 24. If the day of the month is one of those listed, record "Yes" indicate "eligible" in item 17. If not, record "No" and go to item 19 and indicate "not eligible."
- Eligible event. Any path leading to this item indicates that the event is eligible. Record "Yes," the only possible response.
- Event Identification Number. Assign the next available event ID number at this point. Record the number here and in the header. Afterward, skip to item 20.
- Ineligible event. Any path leading to this item indicates that the event is not eligible. Record "No," the only possible response. Complete the remainder of this form, but no further action is necessary.
- Date of data collection. Record the date on which the form was completed.
- Code number of person completing this form. The field center staff member who has performed the abstraction and completed the form must enter his/her valid ARIC code number in the boxes provided.
Complete the form flags at the end of the form by placing an X in the appropriate box(es), indicating other forms that need to be collected for this event. Each form flag refers to one or more items on this form that define the criteria for that form flag.
The SEL form should be kept on file at the field center for all eligible events.
ARIC COHORT EVENT ELIGIBILITY FORM





COHORT EVENT ELIGIBILITY FORM INSTRUCTIONS
I. GENERAL INSTRUCTIONS
The Cohort Event Eligibility (CEL) Form should be the first form to be completed for an identified event in a cohort member. The abstractor must be certified and should be familiar with the document titled "General Instructions For Completing Paper Forms" prior to completing this form. Event ID Number should be completed for all deaths and hospitalizations regardless of eligibility.
There are several ways in which one may be led to fill out a Cohort Event Eligibility Form. Three common paths are: (1) an event is identified through Annual Follow-up procedures, (2) an event is identified through surveillance procedures, but is discovered to have occurred in a cohort participant, or (3) a hospitalized event is unexpectedly discovered while investigating another hospital admission of a cohort member. In each of these cases, a Cohort Event Eligibility Form is completed for the event. All hospitalizations regardless of eligibility should have a CEL form completed.
SPECIAL NOTE ON POSSIBLE DUPLICATION:
- Deaths found through Annual Follow-up must be cross-checked with the death certificate list and vice versa to avoid duplicate investigations.
- Hospitalizations in area hospitals found through annual follow-up need to be cross-checked against hospital discharge indices and vice versa.
- Hospitalized deaths identified using a hospital discharge index need to be cross-checked versus the death certificate index and vice versa.
II. DETAILED INSTRUCTIONS FOR VARIOUS QUESTIONS
| 1. | Name. Record the individual's last name and first and middle initials, or as much as is available. Print clearly in capital letters. |
| 2. | Participant ID. Record the individual's 7-digit cohort participant ID number. |
| 3. | Visit 1 date. Record the date of the participant's baseline visit in contact year 1. |
| 4. | Date of discharge or death. If the source of information is a hospital record, record the date of discharge. It will be found on the face sheet or the ER sheet. If the patient transferred from acute care to rehab or chronic care in the same hospital, count the date of transfer as the discharge date. If a computer listing is used, use the date on that list. If the patient died, or if the source of information is a death certificate, record the date of death. If the date of baseline visit (Item 3) is not earlier than the date of discharge/death (Item 4), go to item 16. Otherwise, continue to complete the form. If the source of information is Annual Follow-up, and the month, day, or year is not available, enter "=" in the appropriate boxes, ignore the skip following this item and continue with Item 5. |
| 5. | Source. Indicate the first source where the event was identified. |
| 6. | Is this event a death? If the event is a death, record "Yes." Otherwise, record "No" and go to item 8. |
| 7. | Out-of-hospital death or no hospitalization information? If the event is an out-of-hospital death (including DOA, ER), or if it is an in-hospital death for which no hospitalization information can be located after exhaustive efforts, record "Yes" and go to item 13. Otherwise, record "No" and continue. |
| 8. |
|
| 9. | Hospital Record Number. This number will be found stamped or typed on almost every page of the hospital record. The easiest place to find it is both on the medical record folder and in the upper right/left hand corner of the face sheet. Record the number starting in the leftmost box. Do not add zeroes to the right of the number. |
| 10. | Hospital discharge diagnosis codes. Record the codes as listed on the hospital discharge index in the order listed. If a fourth digit (i.e. to right of decimal point) is not given, leave decimal field blank. Do not enter a zero there unless it appears on the chart. (See examples under item 14.a. below.) Be sure to include the primary diagnosis as designated by the M.D., as well as all secondary diagnoses. (If more than 21 codes are given, call the CC for prioritization.) |
| 11. |
|
| 12. | In-hospital death? If the event is an in-hospital death, record "Yes" and continue with item 13. Otherwise, record "No" and skip to item 16. |
| 13. | Death Certificate Number. This number will be found stamped or typed on the death certificate. If a computer printout is used, it must include this information. Record the number starting in the leftmost box. Do not add zeroes to the right of the number. |
| 14. |
|
| 15. | Eligible event. Any path leading to this item indicates that the event is eligible. Record "Yes", the only possible response and continue with item 18. |
| 16. | Ineligible event. Any path leading to this item indicates that the event either occurred before visit one or is a non-fatal hospitalized event that is not eligible based on the discharge codes and discharge summaries. Record "No", the only possible response, and skip to item 19. |
| 17. | Ineligible death. Any path leading to this item indicates that the event is a death which is not eligible, but for which a DTH Form must be obtained. Record "No", the only possible response, and continue with the next question. |
| 18. | It is important to determine whether this cohort event would have been identified by the usual ARIC surveillance procedures. If this is a cohort death, check the death certificate list provided by the health department to determine whether the death appeared on the list. If this cohort event was a hospitalization, try to identify it (based on any identifiers available and date) on the list provided by that hospital. Be sure to check all lists provided, even supplements at the end of the year. If the event can be found through surveillance lists, indicate "Yes". If not, indicate "No". |
| 19. |
|
| 20. | Date of data collection. Record the date on which the form was completed. |
| 21. | Code number of person completing this form. The field center staff member who has performed the abstraction and completed the form must enter his/her valid ARIC code number in the boxes provided. |
| 22–28. | Complete the form flags at the end of the form by circling the corresponding letter, indicating other forms that need to be collected for this event. Each form flag refers to one or more items on this form that define the criteria for that form flag. If the criteria are not met for a particular form, do not circle the corresponding letter. |
ARIC DEATH CERTIFICATE FORM






DEATH CERTIFICATE FORM INSTRUCTIONS
I. GENERAL INSTRUCTIONS
The Death Certificate Form is completed for each eligible death as determined by the Surveillance Event Eligibility Form, and for all cohort deaths. The abstractor must be certified and should be familiar with the document titled "General Instructions For Completing Paper Forms" prior to completing this form. Event ID Number and Name (of decedent) should be completed in the form header section as described in that document.
II. DETAILED INSTRUCTIONS FOR VARIOUS QUESTIONS
- Decedent's Name. Enter the first, middle, and last name of the decedent. Begin each name in the leftmost box using capital letters.
- Death Certificate Number. This number will be found stamped or typed on the death certificate. If a computer printout is used, it must include this information. Record the number starting in the leftmost box. Do not add zeroes to the right of the number.
- Social Security Number. If the Social Security Number is on the death certificate, copy it exactly. If none, enter '=' in each field.
- Sex. Record the decedent's sex.
- Race. Circle the response that corresponds to the race specified on the death certificate. If missing, record "U". If the death certificate just indicates Hispanic origin, then record "U", and indicate appropriately in Item 6 below.
- Hispanic. Record "Y" (yes) if the death certificate clearly indicates that the decedent was of Hispanic origin. Record "N" (no) if the death certificate clearly indicates some other origin, i.e., "German", or "Scandinavian". If there is no information at all then record "U", i.e., if the death certificate merely indicates "White", then record "W" in Item 5 above, and record "U" in Item 6.
- Marital Status. Record as listed. If certificate just says "not married" or "S", record as "Single".
- Date of Birth. Enter as listed on the death certificate.
- Date of Death. Enter as listed on the death certificate.
- Age at death. If the age at death is recorded on the death certificate, check by using the following
algorithm. Also if age is not recorded, then use this algorithm to compute it.
- If the month and day of birth fall before the month and day of death, subtract the year of birth from the year of death.
- If the month and day of birth fall after the month and day of death, subtract (year of birth + 1) from the year of death.
Record the correct age on the form. If the age at death cannot be computed then enter "=" in each field.
- Time of Death. Convert all times to a 24 hr. clock and record. Enter unknown as '=' in each field.
- Location of Death. If in a hospital or other institution, its name will usually be listed; otherwise, a street address is usually provided. If a hospital location, refer to your center's list of catchment area hospitals, and indicate whether or not it is in the catchment area. If so, this would indicate the need for hospital record abstraction. If the decedent died in a nursing home, enter "N". If the decedent died at home or at another residence, or at a non-hospital institution enter "O" and then specify. If "other", transcribe as written on death certificate for the location of death. If obviously the home, indicate this in parentheses. Note the skip pattern.
- In Hospital Location. If an in-hospital death most death certificates will specify whether it was DOA, ER, inpatient, etc. If this information is not recorded then circle "F" (not recorded).
- Hospital. Enter the name and location of the hospital, including the city and state.
- Coroner's Case. Record "Y" or "N" as indicated on the death certificate. Coroners cases will be investigated using the COR Form, for out-of-hospital deaths only. Note the skip pattern.
- Coroner or Medical Examiner. Record the name and address of the medical examiner or coroner who signed the death certificate. Provide as much detail as is recorded on the death certificate.
- Autopsy. Record "Y" if the death certificate indicates that an autopsy was performed. If not recorded circle "N".
- ICD9 code for underlying cause of death. The underlying cause is the most important or primary cause that lead to this death. It may not be the the same as the first or "immediate" cause, and is assigned by a nosologist or a computer. Enter the ICD9 Code for the underlying cause of death recorded on the central death index computer listing. If the first character is a letter, record that letter in the first box, followed by the 3-digit number (right justified to the decimal point). Otherwise, record the 3-digit number with the first box left blank. If a fourth digit (i.e. to the right of decimal point) is not given, leave decimal field blank. Do not zero fill! See examples on the Surveillance Event Eligibility Form Instructions.
- All other listed ICD9 codes. Record all other ICD9 codes for the other causes of death, up to ten, exactly as they are listed on the death index computer listing. This list may include the code for the underlying cause of death recorded in Item 18. Enter the codes in the same way as for Item 18.
- Causes of death. Transcribe the causes of death exactly as recorded on the death certificate. Sometimes two causes will be listed on one line of the death certificate. Record them similarly on the form.
- Other significant conditions. Transcribe the other significant conditions contributing to the death, exactly as recorded on the death certificate.
- Interval between onset and death. Enter the shortest possible category for the immediate cause of death, as recorded on the death certificate. If this is missing, do not substitute the interval for another cause. Instantaneous should be recorded as "5 minutes or less."
- Informant. Host death certificates have a line for informant. Often this is the spouse, but it may be a co-worker, etc. Record the name and address. Provide as much detail as is recorded on the death certificate.
- Relationship of informant. Record as listed on the death certificate. If no information is provided then record "U". Note the skip pattern.
- Spouse. Record the name and address of the decedent's spouse if not the informant already listed in Item 23. If the death certificate does not contain any information on the spouse then leave blank.
- Certifying physician. Record the name and address of the certifying physician who signed the death certificate. In some cases this may also be the coroner or medical examiner.
- Date abstract completed. Record the date the death certificate abstract is completed.
- Code number of abstractor. The field center staff member who has performed the abstraction and completed the form must enter his/her valid ARIC code number in the boxes provided.
Complete the form flags at the end of the form by placing an X in the appropriate box(es), indicating other forms that need to be collected for this event. Each form flag refers to one or more items on this form that define the criteria for that form flag.
NOTE: Do not complete this section for cohort deaths that are not eligible by the criteria established in the Cohort Event Eligibility Form.
ARIC INFORMANT INTERVIEW FORM












INFORMANT INTERVIEW FORM INSTRUCTIONS
I. GENERAL INSTRUCTIONS
The purpose of the informant interview is to obtain information about possible CHD events in order to classify cause of death. The interview with next-of-kin is potentially difficult because of the sensitive nature of a relative's death and the difficulty recalling or understanding the events related to the death. Even if the informant initially claims no knowledge, begin the form to see if the questions can be answered.
The interviewer should enter the information required on the first page before the contact is made with the informant, though some of the informant data made need to be filled after contact, such as relationship to the decedent. In some cases the informant may change after calling, as in the case where a spouse is to be contacted but the actual informant is a son or daughter. A record of calls should be maintained for the attempts at contacting the informant. The interviewer should put the date and time of each call, any explanatory notes, a result code for each call, and the interviewer's assigned code number. Eight attempts to contact an informant should be made over a two-week period. If no contact is made, repeat in a month.
The questionnaire is divided into six sections. Section A is concerned with the decedent's medical history, including previous hospitalizations. Section B addresses the events immediately surrounding the fatal event, and Section C is concerned with the symptoms the deceased experienced prior to the event. Section D addresses emergency medical care that may have been provided at the time of death, and Section E asks for information on other potential informants. Section F asks the interviewer about the reliablity of the information obtained during the interview.
Almost all questions have multiple choices for answers; however, if necessary the interviewer can write any additional information or comments that may be important to understanding the response in the margins next to the question (or use a Note log when done by computer). A few questions require the interviewer to write out descriptions of the death or the decedent's state of health as related by the informant. For these questions, the interviewer should write word-for-word (in short phrases, abbreviating) the response of the informant. For questions asking the informant to specify names, if more than one answer is given, write all responses.
When reading questions to the informant, the interviewer should fill in the blanks with the name of the decedent. For example, "I'd like to start by asking about ______'s medical history" should be read "I'd like to start by asking about Mr. Smith's medical history."
The interviewer needs to know thoroughly the ARIC definition of death to complete the interview accurately. "Death" is defined as the point at which the decedent stops breathing on his/her own and never recovers. Thus, the onset of death for someone who is resuscitated or ventilated is the point at which he/she last breathes spontaneously. He/she may recover several tines after resuscitation, but the last cessation of breathing is considered "death". Death is not the time "pronounced dead". If someone is "found dead", timing of death may be estimable if the time since last seen alive was short. However, if long, timing of death may be unknown.
The interviewer should be familiar with skip patterns and nature of each question. Several questions are similar, with only subtle differences. The interviewer must make the distinction clear to the informant. Such questions may sound repetitive and are easier if clarified.
If informant contradicts a previous answer, probe to clarify and correct if obviously wrong.
If informant says at the start of the interview that he/she does not know anything about the death, coax the informant to start the interview and try to complete. If the informant is obviously not helpful, gracefully end the interview.
Ask for next-of-kin record during the interview if appropriate but get written permission only if needed. Written release need not be witnessed. Attached is an example of a release form for written permission.
Finally, the interviewer is responsible for reviewing and editing the Informant Interview Form thoroughly following the interview. Review every question and the skip patterns carefully. Every question must be answered unless skip patterns indicate otherwise. The description of the events preceding the death (Q2) is extermely important for diagnostic purposes. Make sure that the description includes the timing of events and the symptoms experienced.
II. DETAILED INSTRUCTIONS FOR VARIOUS QUESTIONS
- HISTORY
- This question asks for the relationship of the informant to the decedent. Make sure not to reverse this; for example, "She was my mother" should be answered "daughter/son". "Other relative" includes aunt, uncle, cousin, in-law, and grandparent.
- This question refers to any restriction from the decedent's usual day-to-day activities. It excludes the events at death.
- "Being cared for" refers to attendant medical care because of disability or sickness.
- Fill in as much information as is known by informant. If the informant asks why this is needed, explain that it may be important to get additional information from the nursing home, with permission, to understand the cause of death.
- Any hospitalization for any reason is "yes".
- Read the list and mark appropriate answer(s). If "other" is marked, specify in informant's own words.
- If decedent was hospitalized more than once or stayed in more
- than one hospital, record the most recent on the form, then list all dates, names, cities and states of other hospitalizations on a separate piece of paper. If exact days are unknown, fill in month and year. Missing values are indicated by "=" (equal sign) in appropriate field. (Note: an HRA form will need to be completed for each hospitalization for "heart attack" within 28 days of death, but these will have the same ID number, as they are considered the same event.)
- Refers to any encounter with a physician for any reason in the month preceding death including final symptoms.
- This should be the most recent. If more than one physician seen, provide names and addresses of most knowledgeable two.
- Record name and address of decedent's "usual" physician. If same as most recently seen (Q10), record "same".
- This question refers to chest pain from heart disease at any time before death. Angina or angina pectoris or a heart attack would be considered "yes" responses. Pain in the left arm or shoulder, jaw, or upper abdomen is considered equivalent to chest pain.
- Refer to list of names for nitroglycerin if informant hesitates. Nitroglycerin is usually administered as a small tablet placed under the tongue but may be taken as a pill, an ointment, or as "skin patch".
- Be aware that this refers to past history and does not include the fatal event under consideration (emphasize ever) and clarify to the interviewee, if required.
- Synonyms for heart attack are "myocardial infarction", "MI", coronary occlusion.
- Coronary bypass involves surgery bypassing the blocked coronary arteries with vessels removed from the arm or leg. "Balloon dilation" or "PTCA" are other terms for angioplasty. A cardiac catheterization, coronary angiography, or angiogram for diagnostic purposes without angioplasty should be answered "no".
- If yes, specify the condition in the informant's own words. Hypertension does not qualify here as a heart disease or condition. Conditions recorded in this section should not be conditions directly related to or resulting from past MI recorded in Q14. Probes may be needed.
- A stroke is a brain hemorrhage or ischemia (blockage of blood flow) also known as cerebrovascular attack, cerebral hemorrhage, or blood clot on brain.
- This includes the final, fatal event under consideration.
- CIRCUMSTANCES SURROUNDING DEATH
20. Narrative: Write out as close to word-for-word as possible, using short phrases. Probe neutrally for symptoms, order and timing of events, medical care, etc. Record these important items verbatim; try to limit the narrative to the space provided. When describing the events surrounding the death itself, be sure to differentiate between the onset of the last symptoms, the death (recalling definition of death), and being "pronounced dead". 21. "Present" is defined as being within sight or sound of the deceased at the time of death; for example, Present: lying next to in bed, in next room and could be heard, left decedent alone momentarily. Not Present: in another room out of eight and sound, outside out of sight and sound, left decedent alive and returned after 5 minutes, talked to on phone sometime right before. 22. These questions ask whether anyone was present at the time of 23. the decedent's death (defined above). If the decedent died in his/her sleep with someone nearby; Question 22 should be answered "yes". 24. Mark the shortest interval known to be reliable. If the informant hesitates, read the intervals in order starting with the shortest. 25. This question refers to the place where the decedent died, as defined in the general instructions. Read the question, wait for a response, and mark appropriate answer. If the informant needs prompting, read the list. If the informant says "in the hospital" ask if he/she died in the emergency room. If yes, mark appropriate response. - SYMPTOMS
26. We are primarily interested in acute symptoms, not chronic. Thus, if a person had been generally fatigued for a month and then had chest pain one hour before death, it is the chest pain that was the last episode. Similarly, if someone had a long history of angina but, not having acute pain, suddenly collapsed and stopped breathing, the onset of the final episode was the time of collapse. If the death occurred while sleeping or while someone was within hearing range of the decedent, the interval between onset and death is considered to be instantaneous. If the decedent was found dead (no one close enough to see or hear him/her), the onset may be unknown. Onset of last episode is defined as being at that point in time when new symptoms cause a change in activity. If the symptom is chronic (e.g., longstanding exertional chest pain), there must be a change in severity or frequency. Symptoms might be stepwise (e.g., one chest pain, then a more severe one a hour later). In this case it is the first pain, if it was new and caused a change, that is the onset of the final episode. The final episode for someone who collapses, is revived, and collapses again began at the first collapse. Interviewers will have to probe and define onset specifically for each informant.
The difference between Q12 and 26 is the time period referred to. In Q26, the time is specific: within 3 days of death. In 12, the decedent could have experienced pain at any time prior to death. If Q26 is answered "no", skip to Q30, as Q27–29 refer to an episode of pain with 3 days of death.
The location of the pain or discomfort referred to in Q12 and 26 is specific. If the pain was experienced at sites other than the chest, left arm or shoulder or jaw), the answer should be "no". If the informant is unsure, but is leaning toward a "yes", then proceed as with a "yes".
If decedent was found dead, Q26 must be answered either "yes" or "unknown". If the decedent was found dead, most of the answer will be "unknown". In this case, skip quickly through, verifying that the answers are unknown.
27. The option "yes" is checked if the pain occurred anywhere in chest within 3 days of death. 28. A list of names of "nitroglycerin" preparations is provided in the medication list and should be consulted if informant isn't sure or offers a brand name. 29. This is crucial question for timing of death. Use the definition provided above for death and onset of the final episode in order to clarify timing. Read the question, wait for response, and mark the shortest interval known to be true. If the informant hesitates, read the list and mark the appropriate response. The informant may have given a time interval when answering Q26. If so, the interviewer may want to preface the question stating the time interval and asking for confirmation (e.g. "You mentioned that ______ had chest pains two days before he died. Is that when the chest pain began?"). 30. This question asks about any symptoms other than pain or discomfort in the chest. The timing of onset of these "other" symptoms is crucial. After each "yes" answer, probe to make sure the onset was within 3 days, and that the condition was not longstanding or "usual". Read the list slowly and fill in the appropriate answers. - EMERGENCY MEDICAL CARE
Read the introductory statement. If an ambulance service (or other emergency medical service such as a fire department) was mentioned earlier, the interviewer may want to preface questions with a statement acknowledging that such information was given.
31. This question refers to calling for help. If no one was called, skip to Q35. 32. This question is to determine whether help was called for symptoms, or after informant knew decedent was already dead. 33. Read question, wait for response and mark the shortest interval known to be true. If informant hesitates, read the list. Timing is from the onset of the last episode. The following example would be coded E (24 hours or less): "decedent began having chest pain and nausea the night before, but ambulance was not called until the next morning." 34. Read the question, wait for response and mark appropriate answer. If the informant hesitates read the list and mark the shortest time known to be true. Be sure informant understands difference between Q33 and Q34. 35. The informant may not be familiar with CPR or the procedure of closed chest massage. If this is the case, tell the informant that CPR is a procedure used to resuscitate (restore breathing or revive) persons who are experiencing heart attacks and have no pulse or breath. It usually involves mouth-to-mouth resuscitation with compression of the chest to circulate the blood. 36. Read the questions, wait for response, and mark appropriate answer. 37. If informant hesitates, read the list and mark correct answer. 39. Fill in as much of the answer as is known. - ADDITIONAL INFORMATION
40. This question asks if there is any person who may be able to provide additional information about the events leading up to the death or the death itself. For example, a spouse may know most about the three days prior to death while a co-worker actually witnessed the death. (Note: If the answer is "yes", an interview will need to be carried out with this individual.) 41. Fill in as much information as is known. 42. Close the interview by thanking the informant and repeating how much the quality of our research depends on the cooperation of people like themselves. After closing the interview, fill in Section F (Reliability) and Section G (Administrative Information). - RELIABILITY
Complete this section immediately after completing the interview. Remember to fill in your code number.
47. The final script may ask the informant to provide consent in order that other sources of information may be contacted. Indicate the informant's response. For those who are not a next-of-kin enter the code for "Not applicable". - ADMINISTRATIVE INFORMATION
Complete the form flags at the end of the form by placing an X in the appropriate box(es), indicating other forms that need to be collected for this event. Each form flag refers to one or more items on this form that define the criteria for that form flag.
ARIC PHYSICIAN QUESTIONNAIRE




ARIC CORONER/MEDICAL EXAMINER FORM







Instructions for the Coroner/Medical Examiner Form (COR)
Version A 8/15/88
General Instructions
This form is completed for each eligible, out-of-hospital cohort or surveillance event which was a medical examiner's (coroner's) case. The medical examiner's (coroner's) report may consist of an investigation, an autopsy, or both. If this is a cohort case and an autopsy was done, Form AUT must also be completed. The abstractor should be familiar with ARIC Instructions for Completion of Paper Forms and the instructions for the Hospital Record Abstraction Form and Informant Interview Form. Whenever you have difficulty interpreting the medical information in the medical examiner's (coroner's) report, consult with your surveillance director.
Header
Record the event ID number as assigned on the CEL or SEL form, the participant's last name and initials. Before going to the coroner/medical examiner office, complete items 1–3.
| 1. | Date of Death: Transfer date from the death certificate or from the death printout. | ||||||
| 2. | Medical Examiner/Coroner's Office: Record the name of the office from the death certificate. | ||||||
| 3. | Cohort/Surveillance: Record C or S based on the SEL or CEL forms. | ||||||
| 4. | Report found? The report may be permanently lost. If so, indicate "no" and skip to Item 25. If "yes", complete the form. | ||||||
| 5. | Autopsy? Indicate whether an autopsy was done as part of the investigation. If an autopsy was done but not as part of the coroner's/medical examiner's investigation, record "No". | ||||||
| 6. | Examiner's findings: Review the examiner's report and diagnoses completely for conditions contributing to or present at death. To answer "yes" these do not have to be listed among the final diagnoses, but need only have been present. "Recent" here generally means in the last month and may require your judgement. If a condition (a-j) is specifically mentioned record as "yes". If definitely not present or not mentioned at all, record as "no". If equivocal, consult your surveillance director. Synonyms for the conditions are provided in the instructions for the HRA and STR forms. | ||||||
| 7. | Non-cardiac, non-stroke cause: Answer this question as described for Item 6. If you are uncertain about a condition, record in "specify" space and check with Surveillance Director. | ||||||
| 8. | Diagnoses: Record in order the listed final diagnoses and attach an ID label. This page will be duplicated for the MMCC. | ||||||
| 9. | Symptoms: This question seeks to determine if any acute symptoms (cardiac or non-cardiac) were known to have occurred. Indicate which one of the statements applies. If acute symptoms were not present, follow the skip. | ||||||
| 10. | Other symptoms: This question asks about symptoms other than pain or discomfort in the chest. The timing of onset of these "other" symptoms is critical. Make sure the onset was within 3 days, and that the condition was not long-standing or "usual". If not specified at all in the examiner's report, record as "U". If another prominent symptom occurred, specify under k. "Specify". | ||||||
| 11. |
| ||||||
| 12. | Location of death: Record location. | ||||||
| 13. |
| ||||||
| 14. | Timing: Estimate time from acute symptom onset to death (IFI definition) or if no
known acute symptoms, time from last known to be alive until death. Make an estimate to fit the
categories. If it cannot be determined, check "U". Acute symptom onset is defined as
that point in time when new symptoms caused a change in activity. If the symptom is chronic, there
must be a change in severity or frequency. Symptoms might be stepwise (e.g. one chest pain, then a
more severe one an hour later). In this case it is the first pain, if it was new and caused a
change, that is the onset of the episode. The final episode for someone who collapses, is revived
and collapses again began at the first collapse. Questions 15–23 refer to the patient's medical history before the onset of this event. Do not record the causes of death as "Yes" here, since they are listed in Question 8. | ||||||
| 15. |
| ||||||
| 16. | Previous angina or coronary insufficiency: Examine the record for mention of previous angina pectoris or coronary insufficiency prior to this event (i.e., > 72 hours before admission). This would include mention of chronic chest pain, ischemic pain, and "history of chest pain". Chest pain specified as being "of unknown origin" does not qualify. Answer "Yes" if the history includes any mention of the patient taking nitroglycerin for chest pain noted that the patient had "substernal pressure, pain, tightness, or burning distress precipitated by exercise or excitement, or both and is relieved by rest and/or nitroglycerin". Answer "No" if the history explicitly states that the patient has no history of any of the above. Answer U = unknown if none of the criteria for "Yes"/"No" responses apply. | ||||||
| 17. | Other IHD: History of other chronic ischemic heart disease may include CHF described as due to coronary disease or ASHD (Atherosclerotic Heart Disease). | ||||||
| 18. | Valvular disease or cardiomyopathy: History of valvular disease such as rheumatic heart disease, mitral valve prolapse, valvular stenosis or regurgitation. Other valvular diseases include: Aortic Valve diseases or disorders, aortic valve incompetence, insufficiency, regurgitation, or stenosis, aortic valve failure; Mitral valve diseases, disorders, mitral valve incompetence, insufficiency, regurgitation and stenosis or mitral valve failure; or Pulmonary valve diseases, disorders, incompetence, insufficiency, regurgitation and stenosis; and Tricuspid valve diseases, disorders, incompetence, insufficiency, regurgitation, stenosis and failure. In addition, any mention of valvular endocarditis warrants an answer of "Yes" to history of valvular disease. Not recorded is recorded as "U". | ||||||
| 19. and 20. | Coronary bypass or angioplasty: Has the patient had previous coronary bypass surgery or coronary angioplasty before this event (refer to HRA Form Appendix AA for definitions). Not recorded is "U". | ||||||
| 21. |
| ||||||
| 22. | Hypertension: If there is explicit mention of hypertension (high blood pressure) as being present, answer "Yes". If hypertension history is explicitly recorded as negative, answer "No". If no mention either way, record "U". Even if the patient is on a medication sometimes used for hypertension (e.g., beta-blocker), but hypertension is not mentioned, answer "U". | ||||||
| 23. | Heart medication: Refer to HRA Form Appendix BB (the list of Digitalis, Nitrates, Calcium Channel Blocking, and Beta-Blocking drugs). Does the history indicate the patient was taking any of these drugs? Record "Yes" if the general category, trade name, or generic name of the drug is listed. If no prior medications are listed, record "U". If meds are detailed but none of these heart medications were being taken, record "No". | ||||||
| 24. | Completed by Abstraction or Interview: Circle "A" for Abstraction or "I" for Interview. | ||||||
| 25. | Abstractor Number: Record your I.D. | ||||||
| 26. | Date abstract completed. | ||||||
| 27 – 30. | Form flags |
Be sure to complete. Circle the letter corresponding to the form(s) required. HRA and STR will be needed for cohort for any recent MI or stroke hospitalization and for surveillance if the MI or stroke hospitaliration occurred in a catchment area hospital.
Washington County Medical Examiner Questions

ARIC MMCC FINAL DIAGNOSIS FORM



ARIC HOSPITAL RECORD ABSTRACTION FORM





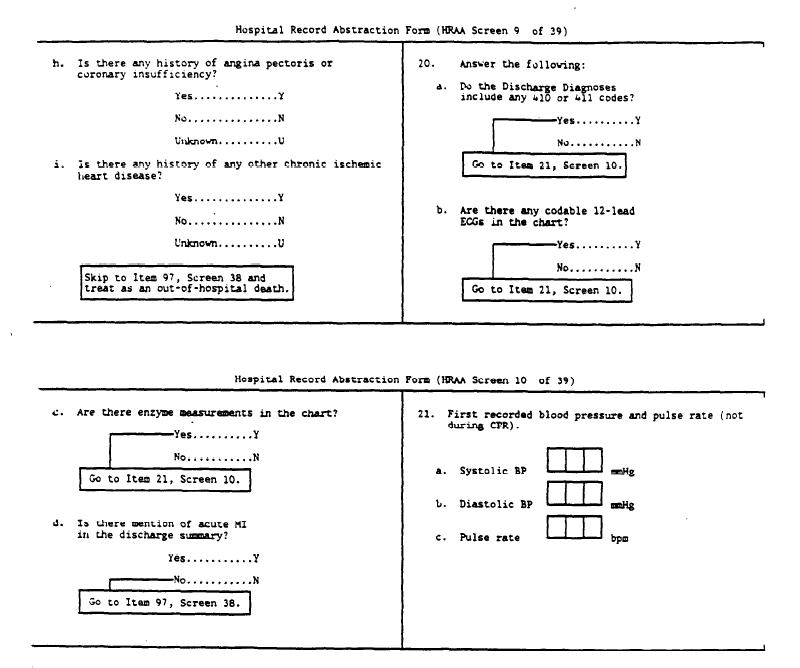















INSTRUCTIONS FOR ABSTRACTING ARIC HOSPITAL RECORD ABSTRACTION FORM (for HRAA, 5/11/88)
General Instructions
- The abstractor must be familiar with the ARIC Instructions for Completion of Paper forms.
- Write legibly. Use black or blue erasable pens. Press hard when filling in blanks with or circling numbers, letters, or X's. Pen will not photocopy if too light and will fade with time.
- Several types of responses are used:
Fill in the blank.
Fill in the number, such as a date, time or medical record number.
To answer most questions you will have several choices, the simplest of all being Yes = Y, No = N, or Unknown = U. In that case, "Yes" or "No" will be marked only if there is no doubt due to information in the hospital record. If nothing is written down that definitely answers the question, "U" should be circled. If the response categories are just Yes = Y or No = N, information not recorded is then marked as "No". In general, the following may be considered synonyms:
- Complete only the appropriate questions.
- Be sure to follow correct skip patterns, i.e., follow form logic.
- To record dates, fill in 2 digit numbers for month/day/year. Zero fill the left box for any single digit numbers (e.g., 03 for March and 06/08/45 for June 8, 1945). If part of the date is missing, record = for that part. For example, if the only information regarding date is June 1945, record 06/= =/45.
- To record times, use the 12-hour clock. If the time given in the chart is from the 24-hour clock, it should
be converted to the 12-hour clock before being recorded. Explanation for conversion:
If an exact time cannot be recorded (i.e., is not given in the chart), the best estimate should be given. If a time cannot be clearly estimated, the following guidelines for estimating times may be used in conjunction with the admission time. Use these only as a last resort For no mention of the time of day, please see xii.
- The middle of the night = 03:00 am
- Early morning = 08:00 am
- Morning = 09:00 am
- Late morning = 10:00 am
- Midday = 12 Noon = 12:00 pm
- Early afternoon = 02:00 pm
- Afternoon or midafternoon = 03:00 pm
- Late afternoon = 04:00 pm
- Early evening = 07:00 pm
- Evening = 09:00 pm
- Late evening = 10:00 pm
- No mention of time of day = 12:00 Noon = 12:00 pm
- To record other time frames, use the following guidelines:
Several days: ≥ 3 days
Few days: ≥ 1 day and < 3 days
Several hours: ≥ 4 hours and < 6 hours
Few hours: ≥ 2 hours and < 4 hours
- Whenever you have questions about the medical information recorded in the hospital record, consult with your surveillance director.
HEADER
Before going to the hospital, fill in event number as assigned on the CEL or SEL Form and patient name (if available).
DO NOT ANSWER QUESTIONS 60 - 69.
| 70. | If there are other codable ECGs in the chart answer "Yes". If not, answer "No" and skip. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 71. | Enter the date of the last codable ECG taken during the admission in Q71 and
ignore Q72–81. For a one day admit, if there are two EKGs done, use both EKGs, one for ECGF and the other for ECGL. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 82. | If the event began outside of hospital, day 3 is the third day after admission, regardless of time of day
of admission. For example, for a patient admitted at 12:01 a.m. on 8/25 day 3 is 8/27. Similarly, if
admitted 11:59 p.m. on 8/25, day 3 is 8/27. If the event began in the hospital, day 3 is the third
day after the event. If there are codable ECGs (other than ECGL) taken on or after day 3, pick the last codable one on day three or the first available ECG thereafter that is codable be sure to enter the date into Q83 and skip to Item 94. Examples on finding ECGT:
The following examples for choosing ECGF, ECGL, and ECGT include ones for a) no ECG until late in the hospital course, b) hospitalizations less than three days, or c) ECGs taken on less than three days but at least three ECGs are availalbe. General rule: Code up to three ECGs if available and codable, even if definitions do not always fit. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 94. | Circle the letter(s) corresponding to the 12-lead ECG(s) that will be duplicated and sent to the Minnesota ECG Reading Center for coding. For example, all three letters will be circled (F,L,T) if at least three ECGs in the chart were codable. Surveillance ECGs that need review should be handled locally by the field center. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Instructions for sending ECGs to Minneapolis ECG Reading Center for Coding
All ECGs are coded by the Minnesota ECG Reading Center. ECGs should be photocopied and the following protocol should be followed when sending ECGs to Minneapolis:
- Staple together the pages belonging to each individual ECG, and label the first page clearly as
follows:
- Surveillance ID label
- Last name
- "Cohort" or "Surveillance" as appropriate
- "ECGF" or "ECGL" or "ECGT" as appropriate
- Date of the ECG
- If an ID has more than one photocopied ECG to be sent, use a paper clip to bind these together.
- Send them to the Minnesota ECG Reading Center in batches, each batch containing only cases of one type(i.e., Surveillance or Cohort), and accompanied by a shipping inventory (see Appendix GG) that lists in order of batch contents the Surveillance ID and the number of ECG's sent for that ID. Lots can be any size (the Reading Center prefers large ones), but you should send them at least monthly. A sample shipping inventory is attached for you to copy and use. The batch ID should be of the form ARcEnnnn, where "c" is replaced by the capital letter corresponding to your Field Center and "nnnn" is the sequential batch number, counting all batches you have sent to the Reading Center since the start of the project. Be sure to keep a copy of each completed batch inventory for your records. Note that the inventory form provides for exactly 100 ID's, so larger batches can be accommodated by simply inserting the appropriate leading digit(s) in the position ("Pos") columns.
| 97. | Abstractor Number. This should be filled in, even when the chart proves to be ineligible. Double check that your code number has been written in on all the ineligibles since this is a common error. Include the date. |
| 98. | Date abstract completed. |
APPENDIX AA
Cardiac Catheterization - invasive procedure usually performed by a cardiologist to visualize heart chambers and contraction. This procedure is usually accompanied by coronary angiography and is generally not performed at the bedside. Include right-sided, left-sided, and both-sided catheterization, but not Swan-Ganz catheterization. (The ICD 9 Codes for cardiac cath include 37.2).
Coronary Angiography - invasive radiologic procedure to visualize coronary arteries. It is always done with cardiac catheterization, so "cardiac catheterization" should also be checked "Yes" when coronary angiography was present. Occasionally, drugs such as streptokinase are given. References to coronary angiography may be found in radiology reports, chart notes, cardiologist, or consult notes, etc. (88.5) This category also includes Digital Subtraction Angiography -also for visualizing coronary arteries. This procedure is noninvasive (not done by catheterization) and involves radioisotopes (88.57).
Coronary Angioplasty - dilation of coronaries via a balloon catheter or laser, sometimes done during an acute MI to reperfuse heart. (36.0)
Swan-Ganz Catheterization - insertion of balloon tipped (Swan-Ganz) catheter at the bedside into the right side of the heart which can be used to monitor pulmonary arterial pressure continuously and pulmonary capillary wedge pressure and oxygen of mixed venous blood intermittently. (89.64)
Echocardiography - a noninvasive procedure, often abbreviated as "echo", used to visualize heart valves and chambers. Synonyms are: Ultrasound of the Heart; M-Mode and Pulsed Doppler Echocardiography. (88.72)
Coronary Bypass Surgery - open-heart surgery in which a prosthesis or a section of blood vessel is grafted onto one of the coronary arteries and connected to the ascending aorta to bypass a narrowing or blockage in a coronary artery. The purpose of CABG is to improve blood supply to the heart, to reduce its workload and to relieve the pain of angina. (36.1)
Streptokinase, urokinase, or tissue plasminogen activator (TPA) coronary reperfusion. These are drugs given during the early stages of an MI to dissolve coronary thrombus or clots. The drugs may be administered intracoronary or in a peripheral intravenous solution. If given intracoronary, coronary angiography is almost always done, so item (b) should be marked. If given intravenously, answer (g) with "Yes", but do not mark (b). (99.29)
Aortic Balloon Pump - a mechanical procedure used in severe heart failure or shock or post surgery. Aortic balloon pumps are also called counterpulsation pumps, balloon counterpulsation pumps, intraaortic pumps, AVCO System 7, 10, Datascope System 80, IABP and Datscope, and Percor IABP. (37.6)
Radionucleide Scan of Heart - a radioisotope procedure to a) visualize heart contractility and estimate ejection fraction, b) estimate infarct size, or c) gauge myocardial perfusion. Radioisotopes include thallium (T1201), technetium pyrophosphate (Tc99m). Procedures include "heart scan", MUGA (multigated equilibrium blood pool imaging), radionucleide angiography, ejection fraction radionucleide scan, thallium scan, infarct (MI) scan, magnetic resonance imaging (MRI) of the heart, positron imaging, etc. Digital subtraction angiography is "No" and is instead recorded under coronary angiography, above. These tests would be recorded in radiology or nuclear medicine reports or as procedure codes. (92.05)
Other procedures might include aortic aneurismectomy, cardiac transplant, CT scan of the heart, pacemaker insertion, insertion of an automatic defibrillator, perxcardiocentesis, etc.
APPENDIX CC. HOSPITAL CODES
| Forsyth County | |
| 11 | North Carolina Baptist |
| 12 | Forsyth County Memorial |
| 96 | Hospital outside of study community |
| Jackson | |
| 21 | University of Mississippi Med Center |
| 22 | Veterans Administration Hospital |
| 23 | St. Dominic's Hospital |
| 24 | Hinds General Hospital |
| 25 | Mississippi Baptist Hospital |
| 97 | Hospital out of study community |
| Minneapolis | |
| 30 | Abbott-Northwestern |
| 31 | Riverside Medical Center |
| 32 | Fairview - Southdale |
| 33 | Fairview - Ridges |
| 34 | Hennepin County Medical Center |
| 35 | Mercy |
| 36 | Methodist |
| 37 | Metropolitan |
| 38 | Midway |
| 39 | Mt. Sinai |
| 40 | North Memorial |
| 41 | St. Paul Ramsey |
| 42 | St. John's, Northeast |
| 43 | St. Mary's |
| 44 | Unity |
| 45 | University of Minnesota Hospital |
| 46 | Veterans |
| 47 | Fairview - Minneapolis |
| 98 | Hospital outside of study area |
| Washington County | |
| 51 | Washington County Hospital |
| 52 | Western Maryland Center |
| 53 | VA Medical Center, WV |
| 54 | University of Maryland |
| 55 | Frederick Memorial Hospital |
| 99 | Hospital outside of study community |
APPENDIX DD
| Answers | ||||||
|---|---|---|---|---|---|---|
| Symptoms | Q23a | Q23b | Q25a | Q25b | ||
| A. | 9/1 | First episode of acute, severe chest pain, 8 AM | Y | H | Y | 9/2 |
| 9/2-4 | Many daily, less severe episodes of chest pain; none more prominent | |||||
| 9/5 | Admission, 10 AM | |||||
| Explanation: The first pain seems most prominent (use
judgement based on chart). Although onset of first pain was 9/1, the first within 72 hours was on 9/2. | ||||||
| B. | 9/1 | Collapse, no mention of pain, 8 AM | Y | B | N | – |
| 9/1 | Admission, 9 AM | |||||
| Explanation: No chest pain occurred. | ||||||
| C. | 9/1 | First acute, severe chest pain, 8 AM, resolved quickly | Y | A | Y | 9/5 |
| 9/2-4 | No symptoms | |||||
| 9/5 | Second acute, severe chest pain, 8 AM, did not resolve | |||||
| 9/5 | Admission, 8:30 AM | |||||
| Explanation: Second pain is most prominent and is the event. | ||||||
| D. | 9/1-4 | Chronic anginal pain, 8 AM every day 9/5 | Y | B | Y | 9/5 |
| 9/5 | Different, severe pain, 8 AM 9/5 | |||||
| 9/5 | Admission, 9:30 AM | |||||
| Explanation: 9/5 pain is the event. | ||||||
| E. | 9/1 | Admitted for hernia repair | N | - | Y | 9/3 |
| 9/3 | First acute chest pain | |||||
| Explanation: In-hospital event. | ||||||
| F. | 9/1 | Indigestion, 8 AM | Y | H | Y | 9/2 |
| Settles in chest. 10 AM | ||||||
| 9/2-4 | Stays in bed with vague chest pain which gradually gets worse | |||||
| 9/5 | Admission, 10 PM | |||||
| Explanation: Chearest onset is AM 9/1. If pain was
continous, first pain within 72 hours of admission was 9/2 at 10 PM. | ||||||
| G. | 9/1 | Marked fatigue and shortness of breath, 8 AM | Y | G | N | - |
| 9/2 | Admitted after MD does office ECG, 8 AM | |||||
| Explanation: Marked fatigue and shortness of breath can be
acute cardiac symptoms. Never had chest pain. | ||||||
APPENDIX EE. SOUNDEX
Soundex is a system of converting names and addresses to a short abbreviation. It is used in ARIC abstracting when the hospital, for confidentiality reasons, will not let the patient name or address be abstracted verbatim. In that case it is often acceptable to record the Soundex code for the patient's name and address.
The following section is arranged in three parts. Part A discusses Soundex coding for surnames. Part B discusses Soundex coding for given names. Part C discusses coding for address.
APPENDIX FF
SOLUTIONS AND RULES
| 1. | May use nurses physical exam findings when recorded. Interpret neurological signs with caution. |
| 2. | May use dates recorded in old chart if not recorded under PMH. |
| 3. | ER events are considered out of hospital. |
| 4. | In event of recurrent (multiple episodes) chest pain, use episode that prompted action. |
| 5. | Cannot use angiogram alone as evidence for MI. |
| 6. | In cases where a cardiac enzyme (LDH, CPK), is reported on a SMAC profile and as part of a specific isoenzyme battery, record the latter value for total enzyme. |
| *7. | Procedures do not need to be written out (Q17b). Make sure, however, all codes are listed. |
| *8. | Discharge codes should be taken from the face sheet or discharge summary. DRG codes may be limited as only 5 codes are recorded. It is important that all diagnoses are coded. It is important not to "mix and match" diagnoses and codes from different sources. |
| *9. | Q29D - refers to the episode of pain recorded in Part A. As long as pain persists and patient is given nitrates for pain relief, answer "4". If patient is placed on a nitro-drip. but not specifically for the episode of pain, record "N". |
| *10. | If CPK-MB enzymes are reported as both units and %, record units. |
| 11. | In the case of a patient who is transferred from another hospital with a Swan Ganz catheter, consider the SG as a monitoring procedure. Thus, although the catheter may have actually been inserted at the first hospital, if it remains in place for monitoring procedures answer Q29D "Yes". |
| 12. | In the case of cardiomyopathy, only answer Q34 "Y" re: chronic ischeaic heart disease if the etiology of the cardiomyopathy is specifically listed as atherosclerotic. All other types of cardiomyopathy including those where no specific etiology is listed would be indicated by answering Q35 "Y". |
| 13. | Chronic ischemic heart disease specifically relates to chronic illness from atherosclerotic cardiac disease. A pt. who is chronically ill may have had an MI or angina in the past, but the question is specifically asking about chronicity of symptoms and not referring to an acute event. |
| 14. | When a discrepancy exists between a discharge code and the code listed on the hospital index, the latter is used, even if it represents a transcription error. |
| 15. | In the event a cohort members is identified by the CEL form, abstract the entire chart, regardless of the final diagnosis. For instance, a pt. with chest pain who meets the criteria for abstraction would be abstracted even if the final diagnosis is pulmonary embolus. |
| 16. | Riverside Medical Center will be assigned a new code for those charts filed following the merger of St. Mary's and Riverside. Continue to use the codes for St. Mary's and Riverside for the charts abstracted prior to the merger. |
| 17. | Rales are considered present if they are ≥ "1/3 of the way up," noted by a physician or noted by a nurse on a day when a physician does not comment on the pulmonary exam. |
| 18. | For a 1 day admit, if there are 2 EKGs done, use both EKGs, one for ECCF and the other for ECGL. |
| 19. | If enzymes are drawn 3 times in a day and there is only room to record 2 sets, select the sets that have the highest CPK and highest LDH. Do not mix and match enzymes drawn at different times unless they are fairly close together (within 1–2 hours) and no cardiac procedure took place during that interval. |
| 20. | If a patient is transferred to another ward, but discharged (formally) and readmitted, consider this as 2 separate hospitalizations. This means 2 abstractions (if ICD eligible) and treating the second hospitalization as if the patient had gone home after the first hospitalization. |
| 21. | For Q24a, if patient is admitted for elective angioplasty, consider this an "other non-acute CHD evaluation (C)." |
| 22. | If angioplasty is attempted, but not performed successfully, answer Q29b and/or Q37 NO. This is different from an angioplasty that is successfully performed initially, but later followed by vessel reocclusion. |
| 23. | If infarct is interrupted and a person goes on to have a second episode, then you must decide a) if it is the same event or two separate events and b) which (if two events) is the more significant, in order to determine which one to "count." |
| 24. | If subacute symptoms, followed by definite acute changes, use acute symptoms for timing questions. |
| 25. | For 23a and 25a, if the patient comes to the emergency room complaining of acute onset of chest tightness, heaviness or discomfort, consider as chest pain. |
| 26. | If symptoms have been present for weeks, consider them nonacute. |
| 27. | Atherosclerosis and arteriosclerosis are frequently used interchangeably by physicians and may be abbreviated ASHD. |
| 28. | If symptoms are mild, but severe enough to prompt patient to seek medical attention right away, consider acute. |
| 29. | 42.a. (including any major surgery) |
| 30. | False aneurysm or aneuysms of peripheral vessels such as aorta, femoral artery and iliac artery are considered evidence of peripheral vasuclar disease. |
| 31. | Microcytic anemia without evidence of hemolysis = no for Q42d. |
| 32. | If there is an error on the index and the chart does not include 410 or 411, answer "No" to Q20. a. |
| 33. | If a patient has electrophysiologic studies (EPS) of the heart, answer "yes" to Q29. 1 and "no" to 29. a. and b. (unless patient also had cardiac cath or angio to visualize cardiac chambers or coronary arteries). |
| 34. | If there is slight, minimal, or mild pulmonary congestion on chest x ray, answer Q28b "no." |
| 35. | For Q35 regarding history of valvular disease, if 1+ mitral regurgitation is seen on echocardiogram, answer "no." |
UNANSWERED ISSUES/FORM CHANGES
| 1. | Reperfusion - meant to judge by angiography; will check if a change in symptoms alone is adequate. |
| *2. | Stroke symptoms - form may be changed; indicate which symptoms were present at event of onset. |
| *3. | If no enzymes were done on a given day, a new box labeled "not done" or "not recorded" will be marked. |
APPENDIX GG. ARIC SURVEILLANCE ECG SHIPPING INVENTORY

APPENDIX II
ARIC COHORT EVENT INVESTIGATION SUMMARY FORM (CEI)


COHORT EVENT INVESTIGATION SUMMARY FORM INSTRUCTIONS
I. GENERAL INSTRUCTIONS
The Cohort Event Investigation Summary Form (CEI) is used to monitor the procedures of event investigation used in ARIC. It is intended to be used by the supervisor of Surveillance procedures at each field center. The CEI Form should be completed according to information collected in other forms for a given eligible event. This information is summarized in the "form flags" at the end of each form, which indicate what additional forms should be collected for an event.
II. INSTRUCTIONS FOR FORM COMPLETION
The form is divided into four parts: header information, procedure flowchart, form completion information, and contacting information for sources.
The header information at the top of page 1 should be completed as soon as a death or an eligible nonfatal event is identified by the Cohort Event Eligibility Form (CEL). Complete the event ID number as assigned on the CEL Form, and record the current date and the name of the individual whose event is under investigation.
The flowchart on the left side of page 1 depicts the procedures used in event investigation. "Yes" and "No" paths emerge from each decision box and should be checked to indicate which path to follow. The sources of information for these decisions are detailed in the next section, but they essentially repeat the rules defined by the form flags in each form. If the designated path passes through a box containing three-letter form codes, these forms should be collected. As many as three Informant Interview (IFI) Forms and two Physician Questionnaires (PHQ) may be required. It is possible that evidence acquired later in the process may conflict with evidence acquired earlier. In such cases, a path may have to be changed from the one which was previously defined. For example, a death is defined as "in-hospital" on the death certificate. However, when the hospital records are reviewed, it is shown that the person died in the Emergency Room (ER). The path is then changed to indicate that the death is "out-of-hospital/DOA/ER."
The right-hand side of page 1 lists all the possible forms that could be scheduled for completion for an event. Based on the path which is followed in the flowchart, certain forms should be designated as "Needed" by circling the "Y" in that column. When the effort to complete this needed form is begun, the date should be recorded in the "Date Initiated" column. (For the Physician Questionnaire, this should reflect the date it is first sent to the physician. If additional mailings are made, record the date in the "Other Information" column. Dates of mailings for the Informant Interview attempts should be kept in the "Record of Calls" section of that form.) As each ***** form is completed, the "C" under "Completed/Permanently Missing" should be circled. If, after exhaustive effort, a needed form cannot be completed, circle the "P" in this column. ALL FORM FLAGS MUST BE CAREFULLY REVIEWED IN COMBINATION WITH THESE PROCEDURES IN ORDER TO IDENTIFY EXACTLY WHICH FORMS SHOULD BE COLLECTED!
The second page of the CEI Form is used to record pertinent information for contacting of sources, such as names, addresses, and phone numbers. Space for additional comments is provided at the end. When the investigation is completed, i.e., all needed forms are designated either "completed" or "permanently missing," the date and code number are recorded at the end of the form.
The CEI form should be kept on file at the field center for all eligible events.
III. SOURCES OF INFORMATION
The following list indicates what forms and item numbers to use in the process of defining the appropriate path on the CEI Form:
| Decision | Answer | Source Form and Item, Response(s) |
|---|---|---|
| Death? | Yes if: | CEL 6 = Y |
| No if: | CEL 6 = N | |
| Autopsy? | Yes if: | DTH 17 = Y |
| No if: | DTH 17 = N | |
| Eligible? | Yes if: | CEL 15 = Y |
| No if: | CEL 17 = Y | |
| Out-of-hosp/DOA/ER? | Yes if: | DTH 12 = N or O or DTH 13 = A, B, or C |
| No if: | DTH 12 = A and DTH 13 = D, E, or F OR DTH 12 = B and DTH 13 = D, E, or F | |
| HRA Eligible? | Yes if: | CEL 11a = Y or 11d = Y or CEL 12 = Y and 14b = Y |
| No if: | Otherwise | |
| Vital Signs? | HRA Form | |
| Hosp for MI within 28 days? | Yes if: | IFI 6a = Y or IFI 6b = Y |
| No if: | Otherwise | |
| STR Eligible? | Yes if: | CEL 11b = Y or 11e = Y |
| No if: | Otherwise | |
| Transfer? | HRA Form | |
| Event on area hosp. d/c/list? | Yes if: | On list |
| No if: | Not on list |
STROKE FORM AND STROKE FORM INSTRUCTIONS
are not available at this printing.
APPENDIX III
FORMAT 1: SAMPLE LETTER TO INFORMANT: KNOWN TELEPHONE NUMBER

FORMAT 2: SAMPLE LETTER TO INFORMANT: UNKNOWN TELEPHONE NUMBER

FORMAT 3: REPLY POSTCARD FROM INFORMANT WITH TELEPHONE NUMBER

FORMAT 4: SAMPLE LETTER TO A NEIGHBOR RE: LOCATION OF INFORMANT

FORMAT 5: REPLY FORM ON THE LOCATION OF INFORMANT

FORMAT 6: INFORMANT RELEASE OF INFORMATION FORM: NURSING HOME

FORMAT 7: LETTER TO PHYSICIAN SIGNING DEATH CERTIFICATE

FORMAT 8: LETTER TO ATTENDING PHYSICIAN OF DECEDENT

FORMAT 9: INFORMANT RELEASE OF INFORMATION: PHYSICIAN

FORMAT 10: INFORMANT RELEASE OF INFORMATION: OUT-OF-AREA HOSPITAL