How to register a clinical test in the GTR
OMB NO: 0925-0651
EXPIRATION DATE: 01/31/2025
Instructions on this page assume you have logged into the GTR submission site, have registered your laboratory, and are now ready to submit information about a clinical genetic test.
On the home page for your GTR submissions, under 'Tests in this lab', click on 'Submit tests'. To submit a test using the submission wizard, go to the section Submit a single test. The default selection is for a clinical genetic test. Click 'Add a new test'.
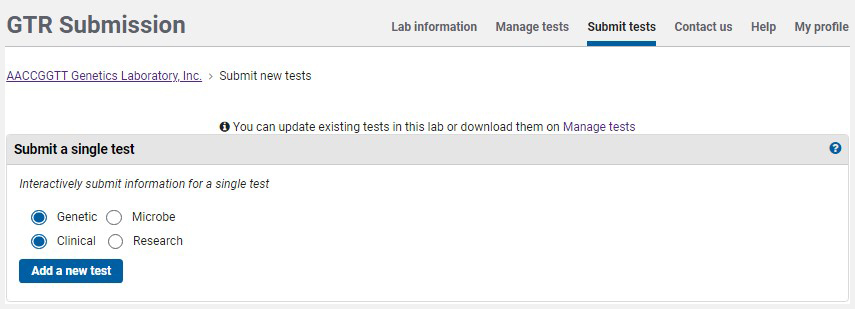
You will be presented with a sequence of pages in which to submit data. Each page has a distinct tab, as seen in the image below.

At the bottom of each page is the button Save & Continue. If your entries on that page validate, you will be taken to the next tab. If there is ever a problem with how you completed a value, you will remain on the same tab. Information will be provided to indicate what content needs to be corrected, and the type of error. If at any time you are unsure as to what information to provide in a field you can hover over the help icon (![]() ) to display help information, or review the help page for the tab you are on.
) to display help information, or review the help page for the tab you are on.
Your progress through the submission process is marked as follows. If you have not 'touched' a tab yet, text and tab color remain grey. The tab you are currently processing is shown enlarged. If you have touched a tab (blue text), you can go back to it at any time to update information (Remember click on Save & Continue before leaving the page.). So in the example above, the submitter is currently on Ordering.
The table below summarizes the content included under each tab. The names in the left column are linked to the details about how to submit the data elements in that section.
| Basics | Type of test, names licenses, etc. |
| Ordering | How to order |
| Indication | Condition(s) for which the test is appropriate |
| Methodology | Describe the methods used and what is measured (the test targets) |
| Interpretation | Describe how test results are interpreted |
| Performance | Where the test is performed, clinical validity, clinical utility, and more |
| Review & Submit | Review your data and submit |
