NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004-2013.
| Chemical name: | (E)-4-(2-(6-(2-(2-(2-([18F]-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methyl benzenamine |
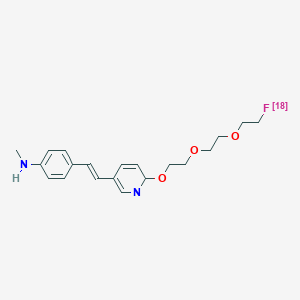
|
| Abbreviated name: | [18F]AV-45 | |
| Synonym: | [18F]Florbetapir | |
| Agent category: | Compound | |
| Target: | Amyloid-beta peptide | |
| Target category: | Acceptor | |
| Method of detection: | PET | |
| Source of signal: | 18F | |
| Activation: | No | |
| Studies: |
| Click on the above structure for additional information in PubChem. |
Background
[PubMed]
Alzheimer's disease (AD) is a form of dementia with a gradual memory loss and a progressive decline in mental functions overtime (1, 2). It is characterized pathologically by neuronal loss, extracellular senile plaques (aggregates of amyloid-beta peptides consisting of 40 to 42 amino acids) and intracellular neurofibrillary tangles (filaments of microtubule-binding hyper-phosphorylated protein tau) in the brain, especially in the hippocampus and associative regions of the cortex (3, 4). β-amyloid peptides and tau protein are implicated as the main causes of neuronal degeneration and cell death (5, 6).
Early diagnosis of AD is important for treatment consideration and disease management (7). Various β-amyloid imaging agents have been developed for magnetic resonance imaging (MRI), single photon emission computed tomography (SPECT), and positron emission tomography (PET) (8-13). The binding of different derivatives of Congo red, thioflavin, stibene, and aminonaphthalene has been studied in human post-mortem brain tissue and in transgenic mice. Out of these analogues, 2-(1-(6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl)ethylidene)malono nitrile ([18F]FDDNP) was studied in humans, showing more binding in the brains of patients with AD than in those of healthy people (14). However, [18F]FDDNP showed low signal-to-noise ratios for PET imaging, because it is highly lipophilic. N-methyl-[11C]-2-(4’-methylaminophenyl)-6-hydroxybenzothiasole, a β-amyloid binding compound based on a series of neutral thioflavin-T derivatives (15), was radiolabeled with the positron-emitting radionuclide 11C ([11C]6-OH-BTA-1 or [11C]PIB). [11C]6-OH-BTA-1 was found to be a promising imaging agent for the senile plaques in the brain (16). Zhang et al. (17) reported the development of a series of fluorinated polyethylene glycol (PEG) units (n = 2-5) for PET imaging of β-amyloid plaques in the brain. Two of them, [18F]{4-[2-(4-{2-(2[2-(2-Fluoro-ethoxy)-ethoxyl]-ethoxy}-phenyl)-vinyl]-phenyl}-methyl-amine ([18F]BAY94-9172, [18F]AV-1) (18) and (E)-4-(2-(6-(2-(2-(2-([18F]-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methyl benzenamine ([18F]AV-45) (19), are being evaluated in clinical trials.
Related Resource Links:
- Gene information in NCBI (Amyloid).
- Clinical trials (Amyloid)
- Drug information in FDA (Amyloid inhibitors)
Synthesis
[PubMed]
[18F]AV-45 was readily synthesized by standard 18F-fluorination of N-BOC-protected O-tosylated derivative with ([18F]KF/Kryptofix 2.2.2, 120°C for 10 min), followed by hydrolysis (HCl, 120°C for 5 min) (19). The radiochemical yield was 10-30% with the specific activity of 37-185 GBq/mmol (1-5 Ci/mmol) at the end of synthesis. Radiochemical purity was >99% as determined with high-performance liquid chromatography (HPLC). Total synthesis time was not reported.
Yao et al. (20) reported that [18F]AV-45 was prepared in 105 min using a tosylate precursor with Sumitomo modules for radiosynthesis under GMP-compliant conditions. The overall yield was 25.4 ± 7.7% with a HPLC purity of 95.3 ± 2.2% (n = 19). The specific activity of [18F]AV-45 was 470 ± 135 GBq/µmol (12.7 ± 3.6 Ci/µmol, n = 19).
In Vitro Studies: Testing in Cells and Tissues
[PubMed]
[18F]AV-45 has the binding affinity (Kd) value of 3.72 ± 0.3 nM for aggregated β-amyloid fibrils (postmortem AD brain homogenates, n = 4) and the receptor density (Bmax) value of 8.81 ± 1.64 pmol/mg protein (19). The Ki values for AV-45, PIB, and BAY94-9172 in competition with [18F]AV-45 were found to be 2.87 ± 0.17, 0.87 ± 0.18, and 2.22 ± 0.54 nM, respectively. [18F]AV-45 was bound to the cortex of postmortem AD brain slices but not to control brain slices as visualized by autoradiography studies.
Animal Studies
Rodents
[PubMed]
[18F]AV-45 (0.37 MBq, 10 μCi) was injected intravenously into normal mice (n = 3/group) to study its accumulation into the brain and other organs/tissues at 2, 60, 120 and 180 min after injection (19). [18F]AV-45 showed an initial rapid penetration into the brain with 6.23% injected dose (ID)/g at 2 min, with a quick washout (1.84% ID/g at 60 min) in female mice and 7.33% ID/g at 2 min and 1.88% ID/g at 60 min in male mice. The organs with the highest accumulation was the liver (11.07% ID/g) and intestine (12.46% ID/g), followed by the kidney (5.94% ID/g), stomach (4.39% ID/g), and lung (2.58% ID/g) at 60 min after injection. The radioactivity levels were 2.37% and 3.66% in the blood and bone, respectively. The bone radioactivity increased to 7.83% ID/g at 180 min. The rate of defluorination indicated by the bone uptake seems high. There was about 30% intact [18F]AV-45 in the plasma at 30 min after injection with two major metabolites, the N-demethylated and N-acetylated derivatives of [18F]AV-45. Both metabolites did not show any specific binding to β-amyloid fibrils in vitro. Ex-vivo autoradiography studies of the brain after injection of [18F]AV-45 in the transgenic Tg2576 mice overexpressing β-amyloid plaques were performed. The brain showed a distinctive labeling of β-amyloid plaques and co-staining with thioflavin-S. No blocking studies were performed.
Non-Human Primates
[PubMed]
PET imaging was performed in the brain of one healthy young female rhesus monkey after injection of 174 MBq (4.7 mCi) [18F]AV-45 (19). The radioactivity (4.4% ID) peaked at the cortex at 7 min after injection with quick washout to about the background level of the brain white matter at 20 min.
Human Studies
[PubMed]
Wong et al. (21) used dynamic PET imaging with [18F]AV-45 to study 16 patients with AD and 16 healthy elderly control patients. [18F]AV-45 binding was quantified by use of the standardized uptake value ratio (SUVR), which was calculated for the neocortex, with the cerebellum as reference region. Widespread neocortical binding was observed in all AD patients with a SUVR plateau at 50-60 min after injection with ~370 MBq (10 mCi) [18F]AV-45. A higher neocortical SUVR was observed in AD patients (1.67 ± 0.18) than in healthy controls (1.25 ± 0.18; P < 0.005) at 50-60 min. The effective dose was estimated to be 0.013 mSv/MBq (48.1 mrem/mCi). The organs with the greatest exposure were the gallbladder, liver, intestines, and urinary bladder. In a study of three patients with AD and three healthy elderly control patients, Lin et al. (22) reported also a higher SUVR in AD patients (1.51 ± 0.30) than in healthy controls (1.17 ± 0.11) at 50 min after injection with 382 MBq (10.3 mCi) [18F]AV-45.
NIH Support
ROI-AG022559, R43AG032206, K24DA000412, MH078175, AA012839
References
- 1.
- Forstl H., Kurz A. Clinical features of Alzheimer's disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288–90. [PubMed: 10653284]
- 2.
- Heininger K. A unifying hypothesis of Alzheimer's disease. IV. Causation and sequence of events. Rev Neurosci. 2000;11(Spec No):213–328. [PubMed: 11065271]
- 3.
- Mirra S.S., Heyman A., McKeel D., Sumi S.M., Crain B.J., Brownlee L.M., Vogel F.S., Hughes J.P., van Belle G., Berg L. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41(4):479–86. [PubMed: 2011243]
- 4.
- Hardy J.A., Higgins G.A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256(5054):184–5. [PubMed: 1566067]
- 5.
- Hardy J. The relationship between amyloid and tau. J Mol Neurosci. 2003;20(2):203–6. [PubMed: 12794314]
- 6.
- Brandt R., Hundelt M., Shahani N. Tau alteration and neuronal degeneration in tauopathies: mechanisms and models. Biochim Biophys Acta. 2005;1739(2-3):331–54. [PubMed: 15615650]
- 7.
- de Leon M.J., DeSanti S., Zinkowski R., Mehta P.D., Pratico D., Segal S., Clark C., Kerkman D., DeBernardis J., Li J., Lair L., Reisberg B., Tsui W., Rusinek H. MRI and CSF studies in the early diagnosis of Alzheimer's disease. J Intern Med. 2004;256(3):205–23. [PubMed: 15324364]
- 8.
- Bacskai B.J., Klunk W.E., Mathis C.A., Hyman B.T. Imaging amyloid-beta deposits in vivo. J Cereb Blood Flow Metab. 2002;22(9):1035–41. [PubMed: 12218409]
- 9.
- Nordberg A. PET imaging of amyloid in Alzheimer's disease. Lancet Neurol. 2004;3(9):519–27. [PubMed: 15324720]
- 10.
- Mathis C.A., Wang Y., Klunk W.E. Imaging beta-amyloid plaques and neurofibrillary tangles in the aging human brain. Curr Pharm Des. 2004;10(13):1469–92. [PubMed: 15134570]
- 11.
- Klunk W.E., Engler H., Nordberg A., Bacskai B.J., Wang Y., Price J.C., Bergstrom M., Hyman B.T., Langstrom B., Mathis C.A. Imaging the pathology of Alzheimer's disease: amyloid-imaging with positron emission tomography. Neuroimaging Clin N Am. 2003;13(4):781–9. [PubMed: 15024961]
- 12.
- Wang Y., Klunk W.E., Debnath M.L., Huang G.F., Holt D.P., Shao L., Mathis C.A. Development of a PET/SPECT agent for amyloid imaging in Alzheimer's disease. J Mol Neurosci. 2004;24(1):55–62. [PubMed: 15314250]
- 13.
- Kung M.P., Hou C., Zhuang Z.P., Skovronsky D., Kung H.F. Binding of two potential imaging agents targeting amyloid plaques in postmortem brain tissues of patients with Alzheimer's disease. Brain Res. 2004;1025(1-2):98–105. [PubMed: 15464749]
- 14.
- Shoghi-Jadid K., Small G.W., Agdeppa E.D., Kepe V., Ercoli L.M., Siddarth P., Read S., Satyamurthy N., Petric A., Huang S.C., Barrio J.R. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry. 2002;10(1):24–35. [PubMed: 11790632]
- 15.
- Bacskai B.J., Hickey G.A., Skoch J., Kajdasz S.T., Wang Y., Huang G.F., Mathis C.A., Klunk W.E., Hyman B.T. Four-dimensional multiphoton imaging of brain entry, amyloid binding, and clearance of an amyloid-beta ligand in transgenic mice. Proc Natl Acad Sci U S A. 2003;100(21):12462–7. [PMC free article: PMC218780] [PubMed: 14517353]
- 16.
- Klunk W.E., Engler H., Nordberg A., Wang Y., Blomqvist G., Holt D.P., Bergstrom M., Savitcheva I., Huang G.F., Estrada S., Ausen B., Debnath M.L., Barletta J., Price J.C., Sandell J., Lopresti B.J., Wall A., Koivisto P., Antoni G., Mathis C.A., Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19. [PubMed: 14991808]
- 17.
- Zhang W., Oya S., Kung M.P., Hou C., Maier D.L., Kung H.F. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Abeta aggregates in the brain. Nucl Med Biol. 2005;32(8):799–809. [PubMed: 16253804]
- 18.
- O'Keefe G.J., Saunder T.H., Ng S., Ackerman U., Tochon-Danguy H.J., Chan J.G., Gong S., Dyrks T., Lindemann S., Holl G., Dinkelborg L., Villemagne V., Rowe C.C. Radiation Dosimetry of {beta}-Amyloid Tracers 11C-PiB and 18F-BAY94-9172. J Nucl Med. 2009;50(2):309–15. [PubMed: 19164222]
- 19.
- Choi S.R., Golding G., Zhuang Z., Zhang W., Lim N., Hefti F., Benedum T.E., Kilbourn M.R., Skovronsky D., Kung H.F. Preclinical properties of 18F-AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med. 2009;50(11):1887–94. [PMC free article: PMC3065020] [PubMed: 19837759]
- 20.
- Yao C.H., Lin K.J., Weng C.C., Hsiao I.T., Ting Y.S., Yen T.C., Jan T.R., Skovronsky D., Kung M.P., Wey S.P. GMP-compliant automated synthesis of [(18)F]AV-45 (Florbetapir F 18) for imaging beta-amyloid plaques in human brain. Appl Radiat Isot. 2010;68(12):2293–7. [PubMed: 20638295]
- 21.
- Wong D.F., Rosenberg P.B., Zhou Y., Kumar A., Raymont V., Ravert H.T., Dannals R.F., Nandi A., Brasic J.R., Ye W., Hilton J., Lyketsos C., Kung H.F., Joshi A.D., Skovronsky D.M., Pontecorvo M.J. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F-AV-45 (florbetapir [corrected] F 18). J Nucl Med. 2010;51(6):913–20. [PMC free article: PMC3101877] [PubMed: 20501908]
- 22.
- Lin K.J., Hsu W.C., Hsiao I.T., Wey S.P., Jin L.W., Skovronsky D., Wai Y.Y., Chang H.P., Lo C.W., Yao C.H., Yen T.C., Kung M.P. Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent--a pilot study. Nucl Med Biol. 2010;37(4):497–508. [PubMed: 20447562]
- PMCPubMed Central citations
- PubChem SubstanceRelated PubChem Substances
- PubMedLinks to PubMed
- Review Trans-4-(N-Methylamino)-4´-{2-[2-(2-[(18)F]fluoro-ethoxy)-ethoxy]-ethoxy}-stilbene.[Molecular Imaging and Contrast...]Review Trans-4-(N-Methylamino)-4´-{2-[2-(2-[(18)F]fluoro-ethoxy)-ethoxy]-ethoxy}-stilbene.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 5-(5-(2-(2-(2-[(18)F]Fluoroethoxy)ethoxy)ethoxy)benzofuran-2-yl)-N-methylbenzenamine.[Molecular Imaging and Contrast...]Review 5-(5-(2-(2-(2-[(18)F]Fluoroethoxy)ethoxy)ethoxy)benzofuran-2-yl)-N-methylbenzenamine.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 5-(5-(2-(2-(2-[(18)F]Fluoroethoxy)ethoxy)ethoxy)benzofuran-2-yl)-N-methylpyridin-2-amine.[Molecular Imaging and Contrast...]Review 5-(5-(2-(2-(2-[(18)F]Fluoroethoxy)ethoxy)ethoxy)benzofuran-2-yl)-N-methylpyridin-2-amine.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review 2-(2-(2-(4-((4-(Methylamino)phenyl)ethynyl)phenoxy)ethoxy)[(18)F]fluoroethoxy)ethyl-4-methylbenzenamine.[Molecular Imaging and Contrast...]Review 2-(2-(2-(4-((4-(Methylamino)phenyl)ethynyl)phenoxy)ethoxy)[(18)F]fluoroethoxy)ethyl-4-methylbenzenamine.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- Review (E)-3-(4-(Dimethylamino)phenyl)-1-(4-(2-(([(18)F]fluoroethoxy)ethoxy)ethoxy)phenyl)-2-propen-1-one.[Molecular Imaging and Contrast...]Review (E)-3-(4-(Dimethylamino)phenyl)-1-(4-(2-(([(18)F]fluoroethoxy)ethoxy)ethoxy)phenyl)-2-propen-1-one.Leung K. Molecular Imaging and Contrast Agent Database (MICAD). 2004
- (E)-4-(2-(6-(2-(2-(2-([18F]-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-met...(E)-4-(2-(6-(2-(2-(2-([18F]-fluoroethoxy)ethoxy)ethoxy)pyridin-3-yl)vinyl)-N-methylbenzenamine - Molecular Imaging and Contrast Agent Database (MICAD)
- LOC117630924 [Prunus dulcis]LOC117630924 [Prunus dulcis]Gene ID:117630924Gene
Your browsing activity is empty.
Activity recording is turned off.
See more...
 In vitro
In vitro