By agreement with the publisher, this book is accessible by the search feature, but cannot be browsed.
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
Gilbert SF. Developmental Biology. 6th edition. Sunderland (MA): Sinauer Associates; 2000.

Developmental Biology. 6th edition.
Show detailsThe origin of epidermal cells
The cells covering the embryo after neurulation form the presumptive epidermis. Originally, this tissue is one cell layer thick, but in most vertebrates it shortly becomes a two-layered structure. The outer layer gives rise to the periderm, a temporary covering that is shed once the inner layer differentiates to form a true epidermis. The inner layer, called the basal layer (or stratum germinativum), is a germinal epithelium that gives rise to all the cells of the epidermis (Figure 12.32). The basal layer divides to give rise to another, outer population of cells that constitutes the spinous layer. These two epidermal layers together are referred to as the Malpighian layer. The cells of the Malpighian layer divide to produce the granular layer of the epidermis, so called because its cells are characterized by granules of the protein keratin. Unlike the cells remaining in the Malpighian layer, the cells of the granular layer do not divide, but begin to differentiate into epidermal skin cells, the keratinocytes. The keratin granules become more prominent as the keratinocytes of the granular layer age and migrate outward to form the cornified layer (stratum corneum) These cells become flattened sacs of keratin protein, and their nuclei are pushed to one edge of the cell.. The depth of the cornified layer varies from site to site, but it is usually 10 to 30 cells thick. Shortly after birth, the outer cells of the cornified layer are shed and are replaced by new cells coming up from the granular layer. Throughout life, the dead keratinized cells of the cornified layer are shed (humans lose about 1.5 grams of these cells each day*) and are replaced by new cells, the source of which is the mitotic cells of the Malpighian layer. Pigment cells (melanocytes) from the neural crest also reside in the Malpighian layer, where they transfer their pigment sacs (melanosomes) to the developing keratinocytes.

Figure 12.32
Diagram of the layers of the human epidermis. The basal cells are mitotically active, whereas the fully keratinized cells characteristic of external skin are dead and are continually shed. The keratinocytes obtain their pigment through the transfer of (more...)
The epidermal stem cells of the Malpighian layer are bound to the basal lamina by their integrin proteins. However, as these cells become committed to differentiate, they down-regulate their integrins and eventually lose them as they migrate into the spinous layer (Jones and Watt 1993).
Several growth factors stimulate the development of the epidermis. One of these is transforming growth factor-α (TGF-α). TGF-α is made by the basal cells and stimulates their own division. When a growth factor is made by the same cell that receives it, that factor is called an autocrine growth factor. Such factors have to be carefully regulated because if their levels are elevated, more cells are rapidly produced. In adult skin, a cell born in the Malpighian layer takes roughly 8 weeks to reach the cornified layer, and remains there for about 2 weeks. In individuals with psoriasis, a disease characterized by the exfoliation of enormous amounts of epidermal cells, a cell's time in the cornified layer is only 2 days (Weinstein and van Scott 1965; Halprin 1972). This condition has been linked to the overexpression of TGF-α (which occurs secondarily to an inflammation) (Elder et al. 1989). Similarly, if the TGF-α gene is linked to a promoter for keratin 14 (one of the major skin proteins) and inserted into the mouse pronucleus, the resulting transgenic mice activate the TGF-α gene in their skin cells and cannot down-regulate it. The result is a mouse with scaly skin, stunted hair growth, and an enormous surplus of keratinized epidermis over its single layer of basal cells (Figure 12.33B; Vassar and Fuchs 1991).
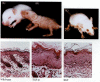
Figure 12.33
Growth factors and epidermal proliferation. (A) A wild-type mouse pup. (B) A littermate of (A) that is expressing high levels of TGF-α in its keratinocytes. It has scaly skin and very little hair. Below each mouse is a cross section through its (more...)
Another growth factor needed for epidermal production is keratinocyte growth factor (KGF; also known as fibroblast growth factor 7), a paracrine factor that is produced by the fibroblasts of the underlying (mesodermally derived) dermis. KGF is received by the basal cells of the epidermis and is thought to regulate their proliferation. If the gene encoding KGF is fused with the keratin 14 promoter, the KGF becomes autocrine in the resulting transgenic mice (Figure 12.33C). These mice have a thickened epidermis, baggy skin, far too many basal cells, and no hair follicles, not even whisker follicles (Guo et al. 1993). The basal cells are “forced” into the epidermal pathway of differentiation. The alternative pathway for basal cells leads to the generation of hair follicles.
Cutaneous appendages
The epidermis and dermis also interact at specific sites to create the sweat glands and the cutaneous appendages: hairs, scales, or feathers (depending on the species). In mammals, the first indication that a hair follicle primordium, or hair germ, will form at a particular place is an aggregation of cells in the basal layer of the epidermis. This aggregation is directed by the underlying dermal fibroblast cells and occurs at different times and different places in the embryo. It is probable that the dermal signals cause the stabilization of β-catenin in the ectoderm (Gat et al. 1998). The basal cells elongate, divide, and sink into the dermis. The dermal fibroblasts respond to this ingression of epidermal cells by forming a small node (the dermal papilla) beneath the hair germ. The dermal papilla then pushes up on the basal stem cells and stimulates them to divide more rapidly. The basal cells respond by producing postmitotic cells that will differentiate into the keratinized hair shaft (see Hardy 1992; Miller et al. 1993). Melanoblasts, which were present among the epidermal cells as they ingressed, differentiate into melanocytes and transfer their pigment to the shaft (Figure 12.34). As this is occurring, two epithelial swellings begin to grow on the side of thehair germ. The cells of the lower swelling may retain a population of stem cells that will regenerate the hair shaft periodically when it is shed (Pinkus and Mehregan 1981; Cotsarelis et al. 1990). The cells of the upper bulge form the sebaceous glands, which produce an oily secretion, sebum. In many mammals, including humans, the sebum mixes with the shed peridermal cells to form the whitish vernix caseosa, which surrounds the fetus at birth. Just as there is a pluripotent neural stem cell whose offspring become neural and glial cells, so there appears to be a pluripotent epidermal stem cell whose progeny can become epidermis, sebaceous gland, or hair shaft.

Figure 12.34
Development of the hair follicles in fetal human skin. (A) Basal epidermal cells become columnar and bulge slightly into the dermis. (B) Epidermal cells continue to proliferate, and dermal mesenchyme cells collect at the base of the primary hair germ (more...)
The first hairs in the human embryo are of a thin, closely spaced type called lanugo. This type of hair is usually shed before birth and is replaced (at least in part, by new follicles) by the short and silky vellus. Vellus remains on many parts of the human body usually considered hairless, such as the forehead and eyelids. In other areas of the body, vellus gives way to “terminal” hair. During a person's life, some of the follicles that produced vellus can later form terminal hair and still later revert to vellus production. The armpits of infants, for instance, have follicles that produce vellus until adolescence, then begin producing terminal shafts. Conversely, in normal masculine pattern baldness, the scalp follicles revert to producing unpigmented and very fine vellus hair (Montagna and Parakkal 1974).
WEBSITE
12.10 Normal variation in human hair production. The human hair has a complex life cycle. Moreover, some hairs grow short (such as those of our eyelashes) while other hairs (such as those of our scalp) grow long. The pattern of hair size and of baldness is determined by paracrine and endocrine factors. http://www.devbio.com/chap12/link1210.shtml
WEBSITE
12.11 Mutations of human hair production. In addition to normal variation, there are also inherited mutations that interfere with normal hair development. Some people are born without the ability to grow hair, while others develop hair over their entire bodies. These genetic conditions give us insights into the mechanisms of normal hair growth. http://www.devbio.com/chap12/link1211.shtml
Patterning of cutaneous appendages
It is obvious that cutaneous appendages such as hair, feathers, or scales do not grow randomly over the body. Rather, there are spaces between them, and these spaces (for instance, on the scalp) are very similar from region to region (Widelitz and Chuong 1998). Recent research suggests that a reaction-diffusionprocess may be responsible for this pattern (see Chapter 1). The activator is Sonic hedgehog, a paracrine factor that acts locally without much diffusion (Nohno et al. 1995). The inhibitor is believed to be BMP4 or BMP2, both of which are paracrine factors with a greater range of diffusion (Jung et al. 1998; Noramly and Morgan 1998). BMPs may prevent the dermal fibroblasts from aggregating, while Sonic hedgehog may support the formation and retention of the dermal papilla (see Figure 6.6).
Footnotes
- *
Most of this skin becomes “house dust” on furniture and floors. If you doubt this, burn some dust; it will smell like singed skin.
- The Epidermis and the Origin of Cutaneous Structures - Developmental BiologyThe Epidermis and the Origin of Cutaneous Structures - Developmental Biology
- Coryphaenoides filicauda[orgn] (0)Nucleotide
- IS607 family element RNA-guided endonuclease TnpB [Mycobacterium tuberculosis]IS607 family element RNA-guided endonuclease TnpB [Mycobacterium tuberculosis]gi|489996508|ref|WP_003899524.1|Protein
Your browsing activity is empty.
Activity recording is turned off.
See more...